Abstract
Acute hemorrhagic pneumonia caused by Streptococcus zooepidemicus has emerged as a major disease of shelter dogs and greyhounds. S. zooepidemicus strains differing in multilocus sequence typing (MLST), protective protein (SzP), and M-like protein (SzM) sequences were identified from 9 outbreaks in Texas, Kansas, Florida, Nevada, New Mexico, and Pennsylvania. Clonality based on 2 or more isolates was evident for 7 of these outbreaks. The Pennsylvania and Nevada outbreaks also involved cats. Goat antisera against acutely infected lung tissue as well as convalescent-phase sera reacted with a mucinase (Sz115), hyaluronidase (HylC), InlA domain-containing cell surface-anchored protein (INLA), membrane-anchored protein (MAP), SzP, SzM, and extracellular oligopeptide-binding protein (OppA). The amino acid sequences of SzP and SzM of the isolates varied greatly. The szp and szm alleles of the closely related Kansas clone (sequence type 129 [ST-129]) and United Kingdom isolate BHS5 (ST-123) were different, indicating that MLST was unreliable as a predictor of virulence phenotype. Combinations of conserved HylC and serine protease (ScpC) and variable SzM and SzP proteins of S. zooepidemicus strain NC78 were protectively immunogenic for mice challenged with a virulent canine strain. Thus, although canine pneumonia outbreaks are caused by different strains of S. zooepidemicus, protective immune responses were elicited in mice by combinations of conserved or variable S. zooepidemicus proteins from a single strain.
INTRODUCTION
Rarely isolated from normal healthy dogs, strains of the zoonotic Streptococcus zooepidemicus (Streptococcus equi subsp. zooepidemicus) have caused epizootics of highly fatal hemorrhagic pneumonia in racing greyhounds and dogs in shelters and research colonies in North America, the United Kingdom, and Korea (1–5). At least 2 shelter epizootics were shown to be caused by single clones of S. zooepidemicus (3, 4). Multilocus sequence typing (MLST) of isolates from persistent outbreaks in a United Kingdom shelter revealed the predominance of sequence type 10 (ST-10) from 1999 to 2002 and ST-62 from 2007 to 2010 (6). The clonality of shelter epizootics of S. zooepidemicus pneumonia in dogs contrasts with the situation usually observed in horses, in which a single serovar randomly derived from multiple serovars in the tonsillar complex is aspirated and infects the lower respiratory tract devitalized by virus infection or extended transportation (7). However, outbreaks of equine respiratory disease caused by specific clones of S. zooepidemicus are occasionally observed (8, 9). These clonal outbreaks differed from those observed in dogs in that equine mortality rates were very low. Disease in dogs is characterized by fibrinosuppurative necrotizing hemorrhagic changes in the lung, vascular damage with hemorrhage, pleural effusion, and bacterial emboli in blood vessels (4).
The clinical syndrome consisting of rapid onset of pyrexia, hypovolemia, and coagulopathy superficially resembles toxic shock syndrome in humans, wherein proinflammatory cytokines are released following the interaction of superantigenic pyrogenic exotoxins with T lymphocytes. Although tumor necrosis factor alpha (TNF-α), interleukin-8 (IL-8), and IL-6 mRNA levels are elevated in the lung tissue of dogs with S. zooepidemicus pneumonia, the majority (64%) of isolates from cases in the United Kingdom and the United States were negative for known superantigen genes and lymphocyte mitogenicity (5; C. Mérant and J. F. Timoney, unpublished data). Lungs of acutely affected dogs contain large numbers of extracellular cocci, indicating rapid proliferation and evasion of clearance mechanisms. Bacteremia/septicemia, as evidenced by ecchymotic hemorrhage in lymph nodes and parenchymatous organs including kidneys, is also present. Virulence factors of importance in pathogenesis and associated acquired immune responses are largely unknown. Studies of these aspects are complicated by the genetic diversity of the S. zooepidemicus population. The aims of the studies described in this report were (i) to genetically characterize isolates of S. zooepidemicus from outbreaks of canine respiratory disease in the United States, (ii) to identify S. zooepidemicus proteins present in diseased lungs and/or involved in acquired immune responses of convalescent dogs following natural infection, and (iii) to evaluate selected combinations of recombinant S. zooepidemicus proteins as protective immunogens in mice challenged with a virulent canine isolate from a shelter epizootic.
MATERIALS AND METHODS
Isolates of S. zooepidemicus.
Strains of S. zooepidemicus were isolated from the lungs of animals in 9 outbreaks of acute respiratory disease in greyhounds, shelter dogs, and other dogs in Texas (outbreaks 1 and 2), Kansas (outbreaks 3 and 4), Florida (outbreak 5), Nevada (outbreak 6), New Mexico (outbreaks 7 and 8), and Pennsylvania (outbreak 9). The years of isolation ranged from 1992 to 2011 (Table 1). All isolates were nonmucoid at 37°C, belonged to Lancefield group C, and fermented lactose and sorbitol but not trehalose.
TABLE 1.
Isolates of Streptococcus zooepidemicus from outbreaks of pneumonia in dogs and cats
| Outbreak | Isolate | Place (yr) | MLST | Identity (%)a |
Comments | |
|---|---|---|---|---|---|---|
| SzP | SzM | |||||
| 1 | 2201b | Texas (1992) | ST-169 | 77.08 (N1HV1c, 8 PEPK) | 57.37 | Pneumonia, greyhound |
| 2 | 2295b | Texas (1993) | ST-162 | 77.49 (N1HV4, 10 PEPK) | 48.85 | Pneumonia, greyhound |
| 3 | 985, 997b | Kansas (2005) | ST-129 | 89.47 (N2HV2, 10 PEPK) | 23.92 | Hemorrhagic pneumonia, greyhound |
| 4 | 007, 782b | Kansas (2006) | ST-2 | 83.16 (N2HV3, 8 PEPK) | 24.96 | Hemorrhagic pneumonia, greyhound |
| 5 | 42, 43,b 44, 45 | Florida (2006) | ST-172 | 86.68 (N2HV4, 11 PEPK) | 30.68 | Hemorrhagic pneumonia, greyhound; influenza virus isolated |
| 6 | 1150 K,b L, M | Nevada (2007) | ST-173 | 83.95 (N2HV5, 9 PEPK) | 31.14 | Hemorrhagic pneumonia, shelter dogs; no virus isolated |
| 47Ab (cat) | Nevada (2007) | ST-316 | 84.21 (N2HV5, 9 PEPK) | 31.14 | Bacteremia, shelter cat | |
| 7 | 800,b 785b | New Mexico (2008) | ST-318 | 83.49 (N2HV2, 12 PEPK) | 30.99 | Pneumonia, service dogs |
| 8 | 566,b Sableb | New Mexico (2011) | ST-317 | 86.68 (N2HV4, 11 PEPK) | Pseudogene | Pneumonia, house dogs |
| 9 | 738-09,b Jack, Tiger,b Boomer,b 653,b 457b (cat), 629b (cat) | Pennsylvania (2009–2010) | ST-315 | 91.57 (N2HV2, 10 PEPK) | 31.51 | Hemorrhagic pneumonia, shelter dogs and cats |
| Dartb (cat) | Pennsylvania (2009–2010) | ST-315 | 82.63 (N2HV2, 3 PEPK) | 31.51 | Pneumonia, shelter cat | |
| BHS5 | United Kingdom (1999) | ST-123 | 100 (N2HV2, 11 PEPK) | 100 | Pneumonia, shelter dog | |
Amino acid identity shared with S. zooepidemicus strain BHS5.
The SzP and SzM sequences of the isolates were obtained.
N-terminal and hypervariable motifs.
Culture media.
Streptococci were cultured overnight at 37°C on colistin-nalidixic acid (CNA) blood agar and in Todd Hewitt broth (THB) with 0.2% yeast extract.
Sera.
Sera from 2 convalescent dogs (Angel and Lockjaw) and 23 unaffected dogs were collected from the Pennsylvania shelter in April 2010. Sera from Angel and Lockjaw were not cultured, but the dogs had clinical evidence of pneumonia and nasal hemorrhage similar to that for kennel mates that died and had positive cultures for S. zooepidemicus. Antisera to streptococcal antigens in the lungs of 2 dogs (Boomer and Jack) that died of acute hemorrhagic pneumonia caused by S. zooepidemicus (ST-315) were prepared separately in two 4-month-old goats. Affected lung tissue (5 g) was ground in phosphate-buffered saline (PBS) (pH 7.2) in a Tenbroeck grinder, and particulate material was removed by centrifugation at 5,000 × g for 20 min. The extracts were then heated at 56°C for 1 h to inactivate the remaining bacteria. Inactivation was confirmed by plating 100-μl aliquots on CNA blood agar. QuilA (60 μg/1.5 ml) and merthiolate (1:10,000) were then added. The extracts were administered subcutaneously in a dose volume of 2 ml. A second inoculation was given 14 days later, and sera were harvested at 28 days. The immunization protocol was approved by the University of Kentucky Animal Care Committee.
Extraction of surface proteins and precipitation of secreted proteins.
Surface-anchored proteins of S. zooepidemicus strain 653 (ST-315) cultured overnight in THB were obtained as described elsewhere (9). Proteins secreted into the culture supernatant were precipitated with the addition of an equal volume of saturated ammonium sulfate and overnight stirring at room temperature. After centrifugation at 10,000 × g for 30 min, the precipitate was dissolved in 200 μl of 20 mM Tris (pH 8.0).
Gel electrophoresis and immunoblotting.
Gels with proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were treated with 0.3% Coomassie brilliant blue R-250. Stained bands were excised and identified by tandem mass spectrometry (MS/MS) analysis, as described elsewhere (9). Briefly, gel slices were vacuum dried, alkylated by addition of 50 mM NH4HCO3 containing 50 mM iodoacetamide, and incubated for 30 min in the dark at room temperature. Proteins were digested in the gel on ice for 1 h by using proteomics-grade trypsin at a concentration of 20 ng/μl. Extracted peptides were then analyzed with an Applied Biosystems 4800 matrix-assisted laser desorption ionization–time of flight/time of flight (MALDI TOF/TOF) proteomics analyzer.
Separated proteins were transferred to nitrocellulose membranes as described previously (9). The membranes were then incubated with convalescent-phase serum (1:200) from Angel, following her recovery from pneumonia caused by a ST-315 strain in the Pennsylvania shelter. After washing, peroxidase-conjugated protein A (diluted 1:1,000) was added and incubated for 1 h at 37°C. Bound conjugate was detected by using 4-chloro-1-naphthol.
Recombinant proteins.
Recombinant proteins of S. zooepidemicus strain NC78, a clone (ST-307) responsible for an epizootic of equine respiratory disease in New Caledonia in 1997 and 1998, were prepared as described elsewhere (10). Clinical signs in affected horses included cough, mucopurulent nasal discharge, and abnormal lung sounds. His-tagged fusion proteins were purified, and their purity was checked by SDS-PAGE.
Enzyme-linked immunosorbent assay.
Levels of serum antibodies to secreted and surface-anchored proteins and to recombinant enolase, M-like protein (SzM), oligopeptide-binding protein (OppA), membrane-anchored protein (MAP), putative ferric siderophore receptor (FSR), InlA domain-containing cell surface-anchored protein (INLA), Sz115, MAC family protein IdeZ (SzMAC), serine protease (ScpC), protective protein (SzP), hyaluronidase (HylC), alkyl hydroperoxide reductase subunit C (AhpC), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and streptokinase (SKC) were measured as described elsewhere (10). Briefly, wells of 96-well polystyrene plates (Costar) were coated separately in triplicate with 1 μg of secreted, surface, or recombinant protein and blocked with 5% nonfat dry milk powder. Sera (1:200) were added in 100-μl aliquots and incubated for 2 h at 37°C. After washing, wells were incubated for 1 h with 100 μl peroxidase-conjugated protein A (1:4,000; Zymed, San Francisco, CA) and developed with 0.07% o-phenylenediamine (Sigma, St. Louis, MO). Absorbances were read at 490 nm. Wells without coating antigen and serum served as negative controls for background reactivity. Mouse sera were titrated to endpoints against the appropriate protein using serial dilutions of 1:50 to 1:102,400. Goat antisera to extracts of S. zooepidemicus-infected dog lungs were assayed at a dilution of 1:100.
Multilocus sequence, szp, and szm typing.
Multilocus sequence typing (MLST) based on arcC, nrdE, proS, spi, tdk, tpi, and yqiL was performed (11) on canine and feline S. zooepidemicus isolates from each outbreak, and sequence types (STs) were assigned (http://pubmlst.org/szooepidemicus). The szp and szm sequences were also amplified using the forward and reverse primers IGSzPF (5′-CTT GCT AAA GTA ATG GTT GAC-3′) and IGSzPR (5′-GTT TGT GAG CAA GGC TTA GTC-3′) (12) and SzMF (5′-ATA AAG TTC CTG TCA TTA-3′) and SzMR (5′-CAA CAG ACA GGA GAC TGT TGC-3′), respectively; amplicons (1,212 and 1,929 bp, respectively) were then sequenced (Eurofin MWG Operon, Huntsville, AL), and amino acid sequences were predicted.
Mouse immunization and challenge.
Three groups of 10 outbred, 8-week-old, ICR (CD-1):Hsd, female mice (12 to 14 g) bred in a University of Kentucky laboratory animal facility from breeding stock supplied by Harlan Laboratories (South Easton, MA) were immunized by subcutaneous inoculation with 20 μg of recombinant SzM, SzM plus SzP, or HylC plus ScpC in 0.15 ml PBS with 20 μg of QuilA (Desert King International, San Diego, CA), as described previously (10). Proteins in the three vaccines were selected because of demonstrated superior protective immunogenicity in mice and, with the exception of HylC, strong reactivity with convalescent-phase canine sera (10). The combinations were designed to test the hypothesis that combinations of conserved (HylC plus ScpC) or variable (SzM plus SzP) proteins would be protective. Two subcutaneous booster doses of 20 μg of each recombinant protein were given 2 weeks apart. Five mice immunized with sterile PBS plus QuilA served as controls.
Two weeks after the final booster inoculation, the vaccinated and control groups of mice were challenged with 3 × 106 CFU (female mice) or 3 × 105 CFU (male mice) of log-phase cultures of S. zooepidemicus strain 738-09, administered intraperitoneally in 0.15 ml PBS. Differences in challenge inocula reflected previously determined differences in the susceptibility of male and female mice to S. zooepidemicus challenges (10). Morbidity (depression and rough coat) and deaths in each group were recorded at 8-h intervals for 8 days following experimental infection. Mice were euthanized promptly after illness was apparent, and heart blood was cultured on CNA blood agar and assayed for antibodies to S. zooepidemicus proteins. Cumulative morbidity curves were prepared for vaccinated and control mice. The immunization-challenge protocol was approved by the University of Kentucky Animal Care Committee.
Statistical analyses.
Fisher's exact test was used to test the significance of differences in morbidity rates between groups of vaccinated and control mice. SigmaPlot 11.0 was used for box-and-whisker plots.
Nucleotide sequence accession numbers.
The full-length szm and szp gene sequences of representative canine and feline S. zooepidemicus isolates from each outbreak were submitted to the NCBI GenBank database under accession numbers KF214267 to KF214286 and KF768745 to KF768759.
RESULTS
Strains and clinical presentations.
Nine different S. zooepidemicus STs were identified in the study (Table 1). Isolates 2201 and 2295 from cases of pneumonia in greyhounds in Texas in 1992 and 1993 (outbreaks 1 and 2) were unrelated ST-169 and ST-162. Outbreaks 3 and 4, involving pneumonia in Kansas, were caused by isolates of ST-129 in 2005 and ST-2 in 2006. These isolates also expressed different SzP and SzM proteins. ST-2 isolates were from 5 greyhounds sampled in outbreak 4, involving coughing among 15 animals. An isolate of ST-2 (S. zooepidemicus strain 782) was also obtained from a single case of hemorrhagic pneumonia in a greyhound in a different kennel. Isolates 997 and 985 (ST-129) were from acute outbreak 3, which occurred in a greyhound kennel in 2005 and was characterized by epistaxis and death (Table 1). S. zooepidemicus strains 42, 43, 44, and 45 (ST-172) were cultured from greyhounds in Florida (in outbreak 5) that developed hemorrhagic bronchopneumonia and died shortly after coinfection with the H3N8 canine variant of equine influenza virus (13).
S. zooepidemicus strains 1150 K, L, and M (ST-173) were isolated in outbreak 6, which involved acute hemorrhagic pneumonia in more than 1,000 mixed-breed dogs in an animal shelter in Nevada (3). S. zooepidemicus strain 47A (ST-316) from a cat spleen was obtained from the same shelter and was a single-locus (proS) variant of ST-173 (Table 1). Serological and PCR investigations revealed no evidence of known viral pathogens during this shelter outbreak. S. zooepidemicus strains 800 and 785 (ST-318) were from outbreak 7, which involved coughing and pneumonia in service dogs on a horse ranch in New Mexico in 2008. Isolates 566 and Sable (ST-317) were from cases of pneumonia in companion housedogs in New Mexico in 2011 (outbreak 8). S. zooepidemicus isolates from Pennsylvania were from outbreak 9; this outbreak of hemorrhagic pneumonia in shelter dogs and cats began in spring 2009 and continued into late summer 2010. Isolates from multiple dogs in 2009 and from dogs and cats in 2010 were identified as ST-315 (Table 1).
Clinical presentations included rapid onset of cough, pyrexia, respiratory distress, hemorrhagic nasal discharge, lethargy, and rapid deterioration, with death in 1 or 2 days. Deaths in shelter outbreaks continued for months and involved many hundreds of dogs. Affected cats exhibited respiratory signs similar to those observed in dogs. Necropsy findings included hemothorax and hemorrhagic pneumonia. Some dogs had acute necrotizing sinusitis and rhinitis. Histologically, lesions were those of peracute fibrinosuppurative pneumonia, with obliteration of alveolar spaces with neutrophils. Gram-positive cocci were found in large numbers in alveolar spaces and in macrophages. Affected lungs, spleens, and heart blood yielded pure cultures of nonmucoid S. zooepidemicus. Attempts to implicate a viral etiology by culture, PCR, or serology were unsuccessful, with the exception of greyhounds in the Florida outbreak.
Streptococcal proteins in infected dog lung.
Streptococcal proteins recognized by antibodies in goat antisera raised against extracts of acutely infected lungs from Boomer and Jack are shown on Table 2. The objectives were to detect immunogenic S. zooepidemicus proteins expressed in vivo in acutely infected lung tissue and to correlate the results with those obtained using convalescent-phase sera from recovered dogs. Antibody responses in one or both goat antisera included all proteins except SKC and FSR. Responses to lung tissue from Boomer were of greater magnitude and broader specificity. This might have been due to differences in the levels of proteins in each lung or to differences in the responsiveness of each goat. The background activity of preimmune sera for some proteins in the panel suggested nonspecific binding or, less likely, prior exposure of the goats to S. zooepidemicus.
TABLE 2.
Specificities of antibodies raised in 2 goats against separate lung extracts from dogs (Boomer and Jack) that died of hemorrhagic pneumonia caused by S. zooepidemicus ST-315 in Pennsylvania
| Protein | OD at 490 nma |
|
|---|---|---|
| Boomer | Jack | |
| OppA | 0.32 | 0.08 |
| INLA | 0.34 | 0.00 |
| SKC | 0.05 | 0.05 |
| FSR | 0.05 | 0.05 |
| Sz115 | 0.41 | 0.05 |
| IdeZ (SzMAC) | 0.02 | 0.16 |
| ScpC | 0.29 | 0.21 |
| SzM | 0.30 | 0.06 |
| SzP | 0.26 | 0.15 |
| GAPDH | 0.15 | 0.00 |
| AhpC | 0.11 | 0.12 |
| MAP | 0.25 | 0.51 |
| HylC | 0.50 | 0.10 |
OD values are means of 3 replicates, following subtraction of the OD values of sera harvested prior to immunization.
Reactivities of canine convalescent-phase serum.
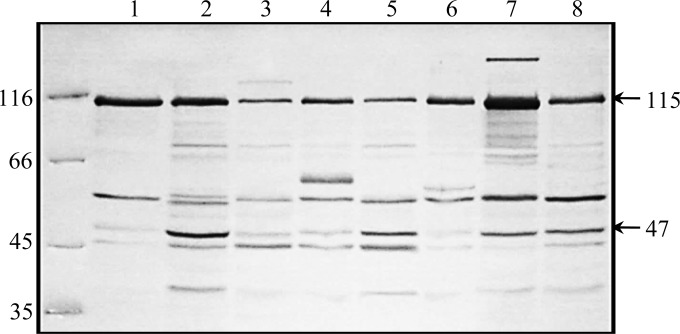
The immunoblot in Fig. 1 shows the reactivity of secreted proteins of canine isolates of S. zooepidemicus with convalescent-phase serum from Angel from the Pennsylvania epizootic. Peak lists from the MS/MS spectra of peptides in tryptic digests of a reactive band at 115 kDa revealed the amino acid sequence of a putative metalloprotease with mucinase activity (Sz115) (see Table S1 in the supplemental material) common to all 8 STs. The 47-kDa band was identified as enolase (see Table S1 in the supplemental material).
FIG 1.
Immunoreactive secreted proteins from clones of S. zooepidemicus from outbreaks of pneumonia in dogs and greyhounds in different regions of the United States. Proteins secreted in overnight cultures in THB were precipitated with 50% ammonium sulfate, dissolved in PBS, and transferred to nitrocellulose membranes following SDS-PAGE (12%). The blot was probed with convalescent-phase serum (1:200) from Angel (Pennsylvania), infected with ST-315. Lane 1, S. zooepidemicus strain 007 (Kansas); lane 2, S. zooepidemicus strain 985 (Kansas); lane 3, S. zooepidemicus strain 43 (Florida); lane 4, S. zooepidemicus strain 2201 (Texas); lane 5, S. zooepidemicus strain 2295 (Texas); lane 6, S. zooepidemicus strain 800 (New Mexico); lane 7, S. zooepidemicus strain 1150K (Nevada); lane 8, S. zooepidemicus strain 653 (Pennsylvania). Molecular masses (kDa) are shown on the left. The positions of metalloproteinase/mucinase (115 kDa) and enolase (47 kDa) are shown on the right. The positions of metalloproteinase/mucinase (115 kDa) and enolase (47 kDa) are shown on the right.
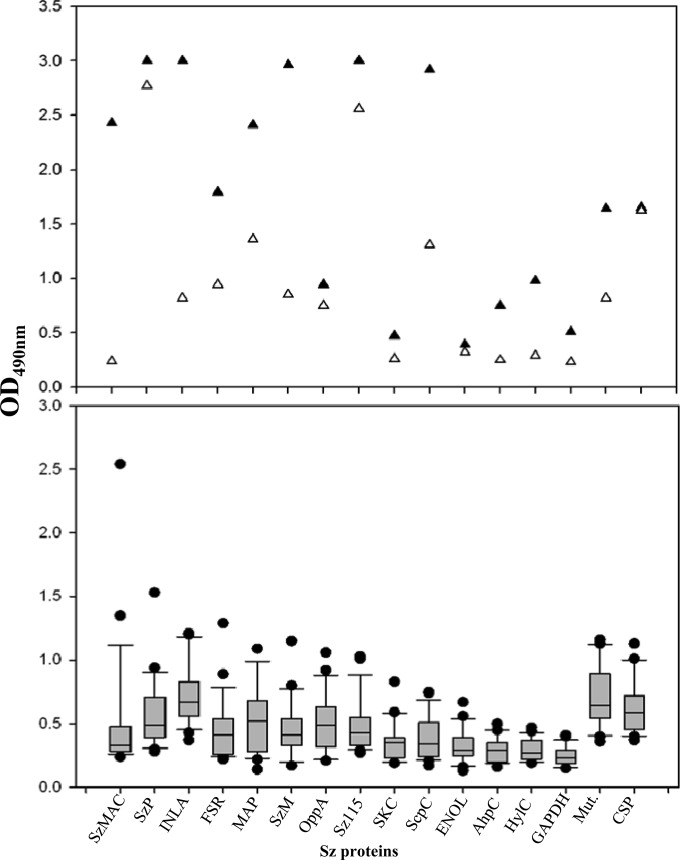
Figure 2 shows the specificities of antibodies in a set of sera from 23 unaffected dogs and in convalescent-phase sera from Angel and Lockjaw from the Pennsylvania shelter. Twelve of 14 recombinant proteins were strongly reactive with convalescent-phase serum from Angel. Both convalescent-phase sera reacted strongly with secreted and mutanolysin-extracted proteins of S. zooepidemicus strain 653 (ST-315), which was responsible for the Pennsylvania epizootic. Sera from 23 healthy dogs residing in the Pennsylvania shelter between August 2009 and July 2010 were generally unreactive with proteins in the panel, although some sera reacted strongly (enzyme-linked immunosorbent assay [ELISA] optical density [OD] values of >1.0) with 3 or more proteins, suggesting exposure to S. zooepidemicus. Serum from one of the 23 unaffected dogs reacted strongly with preparations of both secreted and mutanolysin-extracted surface proteins of S. zooepidemicus strain 653. It also reacted with recombinant Sz115 and FSR but reacted weakly or undetectably with 10 other S. zooepidemicus proteins.
FIG 2.
(Upper) Levels of antibodies (ELISA OD values) reactive with each of 16 recombinant proteins of S. zooepidemicus strain NC78 in 1:200 dilutions of convalescent-phase sera from Angel (▲) and Lockjaw (△), which survived infection in the Pennsylvania shelter outbreak. Each symbol represents the mean OD value of 3 replications of the ELISA for that protein. (Lower) Antibody levels for 23 dogs that remained healthy in the shelter for 11 months during the pneumonia epizootic. Mut., mutanolysin-extracted surface proteins of S. zooepidemicus; CSP, culture supernatant secreted proteins of S. zooepidemicus. ●, outlier values. Horizontal lines, median values; error bars, 90th and 10th percentiles.
Sequences of SzP and SzM.
The SzP amino acid sequences of isolates of each S. zooepidemicus sequence type varied greatly. All 5 hypervariable (HV) regions were represented and, with the exception of the Texas isolates, were combined with N2 N-terminal sequences (Table 1). PEPK (proline-glutamic acid-proline-lysine) repeat numbers ranged from 4 to 12. An isolate from a cat in the Pennsylvania outbreak that was otherwise identical to the clone from affected dogs showed a reduction in the number of PEPK repeats from 10 to 3. SzP and SzM sequences in isolates from specific outbreaks were identical, consistent with clonality of the S. zooepidemicus strains involved. Comparison of the SzM sequences of isolates from each outbreak with the SzM sequence of S. zooepidemicus strain BHS5 isolated in the United Kingdom revealed identities that ranged from 23.92 to 57.37%. The BHS5 strain was chosen for identity comparisons because of access to its genomic sequence (14). Interestingly, although its sequence type (ST-123) included 6 loci common to isolates 985 and 997 (ST-129) from greyhounds from the outbreak in Kansas in 2005, the SzP and SzM sequences of these isolates were not shared. Moreover, the Kansas clone lacked szeF, which was found in BHS5.
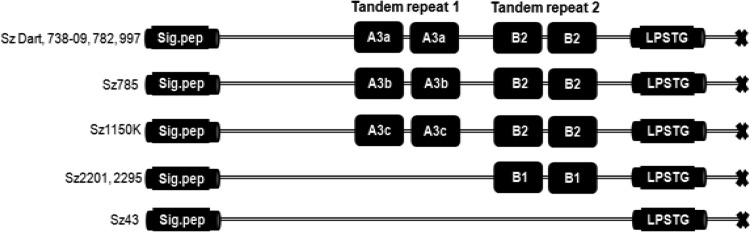
Figure 3 shows the diversity of tandem repeat sequences in SzM (15). Texas isolates from different outbreaks in 1992 and 1993 shared the same tandem repeat structure; szm from the 2011 New Mexico isolate was a pseudogene and lacked recognizable tandem repeats.
FIG 3.
Tandem repeats in SzM proteins of S. zooepidemicus clones from outbreaks of canine pneumonia. Sig.pep, N-terminal signal peptide.
Protective efficacy of SzM, SzM plus SzP, and HylC plus ScpC.
Mice immunized subcutaneously with a combination of HylC plus ScpC or SzM plus SzP were significantly (P < 0.02) resistant to intraperitoneal challenge with S. zooepidemicus strain 738-09 (ST-315), compared with unimmunized controls (Table 3). Only 50% of mice immunized with SzM alone were resistant (P < 0.1). Titers of serum antibodies from groups of immunized mice 7 days after challenge ranged from 1:12,800 for SzP to 1:51,200 for SzM and ScpC. Titers of HylC-specific antibodies were 1:25,600 (Table 3), which is evidence that the immunization protocol was highly effective. ICR (CD-1):Hsd mice were used because this strain had been shown to be susceptible to challenge with different strains of S. zooepidemicus (10).
TABLE 3.
Resistance of mice vaccinated with recombinant proteins of S. zooepidemicus strain NC78 (ST-307) to intraperitoneal challenge with S. zooepidemicus strain 738-09 (ST-315) from outbreak of hemorrhagic pneumonia in shelter dogs in Pennsylvania
| Protein(s) | Challenge (CFU/mouse)a | No. of survivors/total no.b | Resistance (%) | Pc | Antibody titer(s) |
|---|---|---|---|---|---|
| SzM | 106 (female) | 5/10 | 50 | <0.1 | >1:51,200 |
| SzM + SzP | 105 (male) | 8/10 | 80 | <0.02 | SzM, >1:25,600; SzP, 1:12,800 |
| HylC + ScpC | 106 (female) | 9/10 | 90 | ≤0.01 | HylC, 1:25,600; ScpC, 1:51,200 |
| Control | 106 (female) | 0/5 | 0 |
Challenge CFU administered to male mice were lower than those administered to female mice based on the previously determined greater susceptibility of male mice to intraperitoneal inoculation of S. zooepidemicus (10).
Eight days postchallenge.
For difference in morbidity between vaccinated and control mice.
DISCUSSION
Sporadically recognized in the late 1970s as a serious and highly fatal disease of racing greyhounds in the United States, acute fatal pneumonia with septicemia caused by S. zooepidemicus has recently emerged as a major disease in shelter dogs in North America and elsewhere (1, 3, 4, 16). Epizootics involving hundreds of animals in shelters in Nevada, Pennsylvania, New York, and Wisconsin have required intensive control measures, including complete depopulation and disinfection. Transmission is rapid under conditions of crowded housing, the stress of regrouping, and confinement in unfamiliar surroundings. S. zooepidemicus is rarely isolated from healthy dogs (6, 17, 18) but is a normal commensal organism of the tonsillar complex of horses (19). The sources of S. zooepidemicus clones for canine epizootics are unknown. Possibilities include encounters of dogs, prior to admission to the shelter, with infected wild animals or birds, since deaths caused by S. zooepidemicus have been reported for chickens, foxes, and mink (20–22).
The clonal nature of outbreaks of S. zooepidemicus pneumonia in shelters was first recognized in Nevada and was confirmed in a Korean outbreak (3, 4). The MLST, SzP, and SzM data based on multiple randomly selected isolates from the Nevada and Pennsylvania outbreaks are consistent with these earlier conclusions. Outbreaks in greyhound kennels in Kansas and Florida and among service dogs on a ranch in New Mexico also appeared to be clonal, although findings were based on small numbers of isolates. Only one isolate was submitted from each of 2 separate outbreaks among greyhounds in Texas in 1992 and 1993. Based on MLST, SzP, and SzM analyses (Table 1), the S. zooepidemicus clones responsible for each of the US outbreaks were different from each other and from BHS5, a United Kingdom isolate (14). S. zooepidemicus strains 985 and BHS5 have been reported to be closely related, yet their SzM sequences show low levels of identity (14). Moreover, the szeF sequence found in S. zooepidemicus strain BHS5 is missing from S. zooepidemicus strain 985. These observations highlight both the limitations of the use of MLST alone in epidemiological studies and the importance of characterizing virulence genes in defining clones involved in epizootics. SzM has been shown to be a protective antigen in mice and to activate equine plasminogen. SzeF shares 59% amino acid identity with streptococcal pyrogenic exotoxin H (SPEH) of S. equi and was found in 31% of equine S. zooepidemicus isolates in the United Kingdom. Its role in virulence is unknown.
The clinical features and lesions of fibrinosuppurative necrotizing pneumonia common to all outbreaks indicate that S. zooepidemicus clones of varied genetic backgrounds express factors in common that directly or indirectly cause acute blood vessel damage, necrosis, erythrocyte exudation, and fibrin deposition in canine lungs. Direct necrotizing effects on nasal and sinus mucosal surfaces have also been reported (3). The large numbers of extracellular streptococci in affected lungs suggest rapid proliferation and evasion of innate immune responses. Heavy infiltration of neutrophils, many with intracellular bacteria, suggests that this aspect of the innate immune response is ineffective and potentially deleterious because of bystander injury from the release of proteases. A recent study of genes transcribed by S. zooepidemicus in infected porcine lung supports the hypothesis of rapid proliferation (23). S. zooepidemicus genes associated with metabolic pathways, cell wall synthesis, and nutrient transport are upregulated, as are genes for proteases such as clpP and clpB, which play roles in resistance to stress conditions (23).
Confinement in crowded shelter environments and intense physical activity have the potential to reduce resistance to microbial invasion by triggering the release of stress hormones such as cortisol and norepinephrine (NE). Cortisol reduces the bactericidal activity of neutrophils and downregulates the interleukin-2 receptor of T helper lymphocytes (24, 25). NE, synthesized in the lung, has the ability to release iron from transferrin and lactoferrin, thereby making it available for bacterial proliferation (26–28). The involvement of stress hormones theoretically could indirectly explain the large numbers of streptococci located extracellularly and in neutrophils in acutely infected lungs (3).
The reactivities of convalescent-phase sera and of antisera to infected lung tissue provide some limited insight into proteins expressed during infection. Convalescent-phase antibodies (Fig. 1 and 2) showed varying reactivities with a metalloprotease/mucinase secreted by all S. zooepidemicus clones tested. The roles of mucinase in pathogenesis include degradation of the mucin barrier lining the airways, with loss of glycocalyx barrier function. This may facilitate invasion and provide nutrients for bacterial growth (29, 30). Bacterial metalloproteases are known contributors to the virulence of Streptococcus pneumoniae, Staphylococcus aureus, and Listeria monocytogenes (31).
The protective efficacies of the combinations of SzM plus SzP and HylC plus ScpC for mice challenged with S. zooepidemicus strain 738-09 from the Pennsylvania outbreak suggest that combinations of conserved or variable proteins from S. zooepidemicus may each have vaccine potential in a heterologous challenge model. It is important to note that the SzM and SzP proteins used for vaccination were produced in another study using DNA sequences of S. zooepidemicus strain NC78 and so share limited amino acid identity (79.95 and 24.05%, respectively) with these proteins of S. zooepidemicus strain 738-09 (10). SzMNC78 by itself stimulated protection in 5/10 mice against S. zooepidemicus strain 738-09, suggesting that antibodies specific for conserved epitopes on this protein contribute to protection. Further support for the vaccine potential of variable proteins was the reactivity of sera from Angel and Lockjaw with both SzMNC78 and SzPNC78 (Fig. 2). In addition, the highly conserved HylC and ScpC were previously shown to be separately protective, in a mouse model, against a lethal challenge with 3 × 104 CFU of S. zooepidemicus strain NC78 (10). These 2 antigens together protected mice against 3 × 106 CFU of S. zooepidemicus strain 738-09. The protective efficacy of HylC in mice was unexpected not only because of the moderate elevation of HylC-specific antibody levels in convalescent-phase sera from Angel and Lockjaw but also because equine convalescent-phase responses to this protein tend to be less consistent than those to other proteins (10).
Isolates 457, 629, 653, and Dart from cats with pneumonia and bacteremia in the Pennsylvania shelter outbreaks were identical to S. zooepidemicus clones from dogs, an indication that these clones were not host restricted and that a primary viral agent was unlikely. Interestingly, feline isolate 47A (ST-316) is a single-locus (proS) variant of ST-173, which was isolated from dogs and environmental samples at the Nevada shelter. It is also a single-locus variant of an isolate (ST-18) from dogs with respiratory disease in the United Kingdom in 2008 (MLST database). The proS locus of ST-173 differs from that of ST-316 by 23 single-nucleotide polymorphisms, suggesting recent lateral gene transfer and recombination rather than mutation in the locus (32).
Outbreaks of hemorrhagic pneumonia in shelter and kenneled dogs and greyhounds are each caused by clones of nonmucoid S. zooepidemicus that differ in MLST, SzP, and SzM sequences. S. zooepidemicus proteins detected in infected lung tissue and/or reactive with convalescent-phase antibodies include Sz115, HylC, SKC, SzP, SzM, SzMAC, INLA, FSR, MAP, and ScpC. Recombinant HylC plus ScpC or SzM plus SzP from SzNC78 were protectively immunogenic for mice challenged with a virulent dog S. zooepidemicus strain, suggesting that combinations of conserved or variable proteins may be effective vaccine components.
Supplementary Material
ACKNOWLEDGMENT
Endowment income from the Keeneland Chair of Infectious Diseases supported this research.
Footnotes
Published ahead of print 2 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00222-14.
REFERENCES
- 1.Sundberg JP, Hill D, Wyand DS, Ryan MJ, Baldwin CH. 1981. Streptococcus zooepidemicus as the cause of septicemia in racing greyhounds. Vet. Med. Small. Anim. Clin. 76:839–842 [PubMed] [Google Scholar]
- 2.Garnett NL, Eydelloth RS, Swindle MM, Bonderfecht SL, Strandberg JD, Luzarraga MB. 1982. Hemorrhagic streptococcal pneumonia in newly procured research dogs. J. Am. Vet. Med. Assoc. 181:1371–1374 [PubMed] [Google Scholar]
- 3.Pesavento PA, Hurley KF, Bannasch MJ, Artiushin S, Timoney JF. 2008. A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs by Streptococcus equi subsp. zooepidemicus. Vet. Pathol. 45:51–53. 10.1354/vp.45-1-51 [DOI] [PubMed] [Google Scholar]
- 4.Byun JW, Yoon SS, Woo GH, Jung BY, Joo YS. 2009. An outbreak of fatal hemorrhagic pneumonia caused by Streptococcus equi subsp. zooepidemicus in shelter dogs. J. Vet. Sci. 10:269–271. 10.4142/jvs.2009.10.3.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priestnall SL, Erles K, Brooks HW, Cardwell JM, Waller AS, Paillot R, Robinson C, Darby AC, Holden MTG, Schöniger S. 2010. Characterization of pneumonia due to Streptococcus equi subsp. zooepidemicus in dogs. Clin. Vaccine Immunol. 17:1790–1796. 10.1128/CVI.00188-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalker J, Waller A, Webb K, Spearing E, Crosse P, Brownlie J, Erles K. 2012. Genetic diversity of Streptococcus equi subsp. zooepidemicus and doxycycline resistance in kenneled dogs. J. Clin. Microbiol. 50:2134–2136. 10.1128/JCM.00719-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anzai T, Walker JA, Blair MB, Chambers TM, Timoney JF. 2000. Comparison of phenotypes of Streptococcus zooepidemicus isolated from tonsils of healthy horses and specimens obtained from foals and donkeys with pneumonia. Am. J. Vet. Res. 61:162–166. 10.2460/ajvr.2000.61.162 [DOI] [PubMed] [Google Scholar]
- 8.Lindahl S, Aspán A, Båverud V, Paillot R, Pringle J, Rash NL, Söderlund R, Wright NL, Waller AS. 2013. Outbreak of upper respiratory disease in horses caused by Streptococcus equi subsp. zooepidemicus ST-24. Vet. Microbiol. 166:281–285. 10.1016/j.vetmic.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 9.Velineni S, Desoutter D, Perchec AM, Timoney JF. 2014. Characterization of a mucoid clone of Streptococcus zooepidemicus from an epizootic of equine respiratory disease in New Caledonia. Vet. J. 200:82–87. 10.1016/j.tvjl.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 10.Velineni S, Timoney JF. 2013. Identification of novel immunoreactive proteins of Streptococcus zooepidemicus with potential as vaccine components. Vaccine 31:4129–4135. 10.1016/j.vaccine.2013.06.100 [DOI] [PubMed] [Google Scholar]
- 11.Webb K, Jolly KA, Mirchell Z, Robinson C, Newton JR, Maiden MC, Waller AS. 2008. Development of an unambiguous and discriminatory multilocus sequence typing scheme for the Streptococcus zooepidemicus group. Microbiology 154:3016–3024. 10.1099/mic.0.2008/018911-0 [DOI] [PubMed] [Google Scholar]
- 12.Ijaz M, Velineni S, Timoney JF. 2011. Selective pressure for allelic diversity in SeM of Streptococcus equi does not affect immunoreactive proteins SzPSe or Se18.9. Infect. Genet. Evol. 11:1159–1163. 10.1016/j.meegid.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Crawford PC, Dubovi EJ, Castleman WL, Stephenson I, Gibbs EP, Chen L, Smith C, Hill RC, Ferro P, Pompey J, Bright RA, Medina M-J, Johnson CM, Olsen CW, Cox NJ, Klimov AI, Katz JM, Donis RO. 2005. Transmission of equine influenza virus to dogs. Science 310:482–485. 10.1126/science.1117950 [DOI] [PubMed] [Google Scholar]
- 14.Paillot R, Darby AC, Robinson C, Wright NL, Steward KF, Anderson E, Webb K, Holden MTG, Efstratiou A, Broughton K, Jolley KA, Priestnall SL, Marotti Campi MC, Hughes MA, Radford A, Erles K, Waller AS. 2010. Identification of three novel superantigen-encoding genes in Streptococcus equi subsp. zooepidemicus, szeF, szeN, and szeP. Infect. Immun. 78:4817–4827. 10.1128/IAI.00751-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velineni S, Timoney JF. 2013. Characterization and protective immunogenicity of the SzM protein of Streptococcus zooepidemicus NC78 from a clonal outbreak of equine respiratory disease. Clin. Vaccine Immunol. 20:1181–1188. 10.1128/CVI.00069-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyand DS, Sherman BA. 1978. Streptococcal septicaemia in racing greyhounds. J. Am. Anim. Hosp. Assoc. 14:399–401 [Google Scholar]
- 17.Biberstein EL, Brown C, Smith T. 1980. Serogroups and biotypes among beta-hemolytic streptococci of canine origin. J. Clin. Microbiol. 11:558–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamm CG, Ferguson AC, Lehenbauer TW, Love BC. 2010. Streptococcal infection in dogs: a retrospective study of 393 cases. Vet. Pathol. 47:387–395. 10.1177/0300985809359601 [DOI] [PubMed] [Google Scholar]
- 19.Kasai K, Nobata R, Rya E. 1944. On the incidence of Streptococcus hemolyticus in the normal tonsils of horse and the typing of equine tonsillar streptococci. Jpn. J. Vet. Sci. 6:116–123 [Google Scholar]
- 20.Edwards PR. 1934. Characters of haemolytic streptococci isolated from pathological conditions in fowls. J. Comp. Pathol. Ther. 47:152–158. 10.1016/S0368-1742(34)80014-8 [DOI] [Google Scholar]
- 21.Thal E, Moberg K. 1958. Serological grouping of β hemolytic streptococci from animals. Nord. Vet. Med. 5:835–846 [Google Scholar]
- 22.Peckham MC. 1966. An outbreak of streptococcosis (Apoplectiform septicemia) in White Rock chickens. Avian Dis. 10:413–421. 10.2307/1588248 [DOI] [PubMed] [Google Scholar]
- 23.Yi L, Wang Y, Ma Zhe Zhang H, Xie H, Yang Y, Lu C, Fan H. 2013. Identification of genes transcribed by Streptococcus equi subsp. zooepidemicus in infected porcine lung. Microb. Pathog. 59:7–12. 10.1016/j.micpath.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 24.Mandell GL, Rubin W, Hook WE. 1970. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J. Clin. Invest. 49:1381–1388. 10.1172/JCI106355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palacios R, Sugawara I. 1982. Hydrocortisone abrogates proliferation of T cells in autologous mixed lymphocyte reaction by rendering the interleukin-2 producer T cells unresponsive to interleukin-1 and unable to synthesize the T-cell growth factor. Scand. J. Immunol. 15:25–31. 10.1111/j.1365-3083.1982.tb00618.x [DOI] [PubMed] [Google Scholar]
- 26.Esler M, Jennings G, Korner P, Blomberg P, Sacharias N, Leonard P. 1984. Measurement of total and organ-specific norepinephrine kinetics in humans. Am. J. Physiol. 247:E21–E28 [DOI] [PubMed] [Google Scholar]
- 27.Freestone PP, Lyte M, Neal CP, Maggs AF, Haigh RD, Williams PH. 2000. The mammalian neuroendocine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J. Bacteriol. 182:6091–6098. 10.1128/JB.182.21.6091-6098.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandrini SM, Shergill R, Woodward J, Muralikuttan R, Haigh RD, Lyte M, Freestone PP. 2010. Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin. J. Bacteriol. 192:587–594. 10.1128/JB.01028-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Hoeven JS, van den Keiboom CWA, Camp PJM. 1990. Utilization of mucin by oral Streptococcus species. Antonie Van Leeuwenhoek 57:165–172. 10.1007/BF00403951 [DOI] [PubMed] [Google Scholar]
- 30.Govindarajan B, Menon BB, Spurr-Michaud S, Rastogi K, Gilmore MS, Argüeso P, Gipson IK. 2012. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS One 7:e32418. 10.1371/journal.pone.0032418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Häse CC, Finkelstein RA. 1993. Bacterial extracellular zinc-containing metalloproteases. Microbiol. Rev. 57:823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velineni S, Breathnach CC, Timoney JF. 2014. Evidence of lateral gene transfer among strains of Streptococcus zooepidemicus in weanling horses with respiratory disease. Infect. Genet. Evol. 21:157–160. 10.1016/j.meegid.2013.11.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.