Abstract
The ficolins are a family of innate pattern recognition molecules that are known to bind acetylated compounds and activate complement through the association of mannose binding lectin (MBL)/ficolin-associated serine proteases (MASPs). Their importance has more recently become appreciated, as they have been shown to play a role in a variety of disease processes from infection to autoimmunity. While studying ficolin-2-mediated complement deposition on Streptococcus pneumoniae, we found that sera depleted of C1q or other complement components were also codepleted of ficolin-2 but not ficolin-1, ficolin-3, or MBL. MBL present in C1q-depleted sera was able to mediate complement deposition on Saccharomyces cerevisiae, suggesting the presence of MASPs. We found that complement was activated on pneumococci in C1q-depleted serum only after opsonization with exogenous recombinant ficolin-2 (rFicolin-2). Also, no complement deposition was observed in C1q-depleted serum when pneumococci were opsonized with rFicolin-2 mutated at its lysine-57 residue, where MASPs are known to associate. Thus, these depleted sera are a unique tool to study ficolin-2-mediated complement pathways; however, one should be aware that ficolin-2 is absent from complement component-depleted sera.
INTRODUCTION
The ficolins are a recently described family of innate opsonins, consisting of ficolin-1, -2, and -3 (also known as M-, L-, and H-ficolin, respectively). This family of proteins functions as pattern recognition molecules with an affinity for acetylated ligands (1). Ficolin-2 has been recently shown to be important during pneumococcal pathogenesis (2–4) and is known to bind to many other significant human pathogens (2, 5, 6). Moreover, ficolin-2 and -3 have been implicated in many different disease pathologies, such as transplant reperfusion injury (7), clearance of apoptotic cells (8, 9), and even preeclampsia (10).
The structure and function of these proteins are similar to those of the well-characterized mannose binding lectin (MBL). Like MBL, the ficolins activate the lectin pathway of complement through the association of their effector serine proteases called MBL/ficolin-associated serine proteases (MASPs) (11). The lectin pathway shares the same complement proteins as the classical pathway (12). Similar to C1q/r/s of the classical pathway, the ficolins and MBL activate their associated MASPs upon binding, which cleave C4 and C2 to create the C3 convertase (C4b2a) (11). Thus, results of lectin pathway studies can be confounded by the classical pathway. It is therefore necessary to reduce or eliminate complement activation through the classical pathway in order to understand the role of the lectin complement pathway.
As the classical pathway critically depends on C1q, the use of C1q-depleted serum can eliminate the complications of the classical pathway. However, while studying the role of the lectin pathway during pneumococcal disease, we discovered that commercially available C1q-depleted sera also lack ficolin-2, while other lectin pathway opsonins were still present. Here we describe our characterization of several commercially available complement component-depleted sera and how they can be exploited to study the lectin pathway of complement.
MATERIALS AND METHODS
Strains and reagents.
The serotype 11D (SSISP 11D/1) and 19C (SSISP 19C/2) strains used were purchased from Statens Serum Institut (SSI; Copenhagen, Denmark). The wcjE-null strain JC05 (serotype 11Dx) was previously described (3). The serotype 20A strain (AMB11) and serotype 20B strain (AMB12) were generated using ATCC 6320 (American Type Culture Collection, Manassas, VA) and CDC5931-06 (Centers for Disease Control and Prevention, Atlanta, GA), respectively, by introducing a streptomycin resistant rpsL allele using a previously described method (13). The wcjE-null strains AMB13 (serotype 20Ax) and AMB14 (serotype 20Bx) were generated from strains AMB11 and AMB12, respectively, using a previously described protocol (14). Saccharomyces cerevisiae used for MBL binding assays was a kind gift from the laboratory of Stephen Moser at the University of Alabama at Birmingham (Birmingham, AL). Bacteria were cultured at 37°C and 5% CO2 on blood agar plates (BD Biosciences, San Jose, CA) or in Todd-Hewitt broth (BD Biosciences, San Jose, CA) with 0.5% yeast extract (THY). Yeast was cultured in yeast extract-peptone-dextrose (YPD; 1% yeast extract, 2% peptone, 2% dextrose) broth or on YPD agar plates (YPD broth supplemented with 2% agar) at 30°C.
Ficolin binding buffer (FBB) contained 1× Hanks' buffered saline solution (Gibco, Life Technologies) with 2.2 mM CaCl2 and 0.5% bovine serum albumin (BSA). Gelatin Veronal buffer (GVB) contained 0.15 mM CaCl2, 141 mM NaCl, 0.5 mM MgCl2, 0.1% gelatin, and 5 mM barbital sodium C-IV at pH 7.3.
Complement component-depleted sera were purchased from three manufacturers: CalBiochem (Millipore, Billerica, MA), Quidel (San Diego, CA), and Complement Technologies (Tyler, TX), referred to as manufacturers A, B, and C, respectively, in Table 1. Normal human serum (NHS) was obtained from a consenting healthy adult volunteer donor at the University of Alabama at Birmingham (protocol approved by the Institutional Review Board, University of Alabama at Birmingham).
TABLE 1.
Opsonin concentrations in commercially available depleted sera
| Type of serum | Manufacturer | Concn (ng/ml) |
|||
|---|---|---|---|---|---|
| MBL | Ficolin-1 | Ficolin-2 | Ficolin-3 | ||
| Normal human serum | NAa | 36 | 255 | 3,600 | 30,000 |
| C1q-depleted serum | A | 36 | 280 | <64 | 16,800 |
| C1q-depleted serum | B | 56 | 300 | <64 | 15,900 |
| C1q-depleted serum | C | 44 | 265 | 64 | 18,600 |
| C2-depleted serum | C | 52 | 200 | <64 | 15,000 |
| C3-depleted serum | C | 44 | 175 | <64 | 16,800 |
| C4-depleted serum | C | 26 | 140 | <64 | 18,000 |
| fB-depleted serum | C | 16 | 25 | <64 | 15,900 |
NA, not applicable.
Binding assays.
Ficolin-1 and -2 and MBL binding assays were performed as previously described (15). Briefly, bacteria or yeast was incubated with 5% (for ficolin-2 and MBL) or 15% (for ficolin-1) NHS or complement component-depleted sera in FBB for 1 h at 4°C. After a washing, bacteria were stained with an anti-ficolin-1 monoclonal antibody (MAb; 7G1, catalog number HM2196; Hycult), anti-ficolin-2 MAb (GN5, catalog number HM2091; Hycult), or anti-MBL MAb (3E7, catalog number MA1-40145; Pierce) for 30 min at 4°C, followed by a fluorescein-conjugated goat anti-mouse IgG antibody (Southern Biotech; catalog number 1030-09) for 30 min at 4°C. Stained bacteria were analyzed using FACSCalibur (BD Biosciences, San Jose, CA). Data were analyzed using FCS Express V3 (De Novo Software, Los Angeles, CA).
Determining MBL and ficolin-1, -2, and -3 concentrations.
Concentrations of MBL and ficolin-1, -2, and -3 in the complement component-depleted sera were determined using commercially available kits for each analyte according to the manufacturer's instructions. The MBL kit (catalog number HK323), ficolin-1 kit (catalog number HK357), ficolin-2 kit (catalog number HK336), and ficolin-3 kit (catalog number HK340) were purchased from Hycult.
Complement deposition on yeast.
Yeast were incubated in 100 μl of GVB containing 5% NHS or C1q-depleted serum with or without an inhibitor (100 mM d-mannose) for 1 h at 37°C, washed, and stained for 30 min at 4°C for C3 and C4 deposition using anti-human C3 Ab (Pierce; LF-MA0132), followed by phycoerythrin (PE)-labeled goat anti-mouse IgG secondary Ab (Southern Biotech; 1030-09) and fluorescein isothiocyanate (FITC)-labeled anti-human C4c/C4b antibody (Pierce; PA1-28407). Stained yeast was analyzed using FACSCalibur (BD Biosciences). Data were analyzed using FCS Express V3 (De Novo Software).
Western blotting of ficolin-2.
Five microliters of factor-depleted serum or NHS control serum in a total volume of 50 μl per well was electrophoresed on 1.5-mm SDS–12% polyacrylamide gels and blotted onto a nitrocellulose membrane. The membrane was blocked in 5% skim milk in Tris-buffered saline (TBS; 13.7 mM NaCl, 2.15 mM KCl, 10 mM Tris [pH 7.2]) supplemented with 0.05% Tween 20 (TBST) for 1 h. The membrane was then washed and probed with biotinylated anti-ficolin-2 antibody (R&D Systems) diluted 1:1,000 in TBST for 1 h at room temperature before washing and incubation with streptavidin-alkaline phosphatase (Zymed) diluted 1:1,000 in TBST for 30 min at room temperature. Blots were washed and developed in 1 M Tris (pH 8.8) with addition of 1 mg of Nitro Blue Tetrazolium and 5 mg of 5-bromo-4-chloro-3-indolylphosphate dissolved in 100 μl of dimethyl sulfoxide. Development was stopped by exchanging buffer with deionized water.
Generation of recombinant wild-type and mutant ficolin-2.
Wild-type ficolin-2 (FCN2) cDNA was purchased from Transomics (TCH1003) and cloned into pcDNA3.1(−) via NotI/EcoRI sites (FCN2-pcDNA3.1). To generate the K57 mutants, we performed site-directed mutagenesis. For the K57R construct, ficolin-2 was amplified from the FCN2-pcDNA3.1 using primer set T7 (5′-TAATACGACTCACTATAGGG-3′)/3571 (5′-GTGGTCCTGCCCTCCCAGGAG-3′) and 5571 (5′-CTCCTGGGAGGGCAGGACCAC-3′)/BGH (5′-TAGAAGGCACAGTCGAGG-3′) in individual reactions. The resulting products were mixed and amplified using primers T7 and BGH and then cloned into pcDNA3.1(−) via NotI/EcoRI sites (FCN2K57R-pcDNA3.1). The K57E construct was generated by the same procedure, substituting primers 3572 (5′-GTGGTCCTGCCTCCCCAGGAG-3′) and 5572 (5′-CTCCTGGGGAGGCAGGACCAC-3′) for 3571 and 5571 (FCN2K57E-pcDNA3.1). The FCN2-pcDNA3.1, FCN2K57R-pcDNA3.1, and FCN2K57E-pcDNA3.1 plasmids were transformed into competent Escherichia coli and selected for ampicillin resistance. Plasmids from resulting transformants were isolated and confirmed by sequencing (Heflin Center for Human Genetics, University of Alabama at Birmingham, Birmingham, AL) to verify the sequence or if the desired mutations had been incorporated. Purified plasmids were transfected into CHO-K1 using Lipofectamine 2000 (Invitrogen) per supplier protocol. Cells were cultured in Dulbecco's modified Eagle's medium–nutrient mixture Ham's F-12 liquid medium (Thermo Scientific) with 10% heat-inactivated fetal bovine serum (FBS) and selected using 750 mg/liter of Geneticin sulfate. Transfected cells were then subcloned.
The concentration of rFicolin-2 present in each of the culture supernatants was determined using a commercially available ficolin-2 enzyme-linked immunosorbent assay (ELISA) kit (catalog number HK336). Wild-type rFicolin-2 was present at 7.25 μg/ml, the K57R rFicolin-2 was present at 9.35 μg/ml, and the K57E rFicolin-2 was present at 2.83 μg/ml. These were comparable to the concentration of ficolin-2 in our NHS (3.6 μg/ml). The supernatant control had no detectable levels of ficolin-2 (<0.016 μg/ml).
Complement deposition with rFicolin-2.
Complement deposition assays were performed as previously described (3). Briefly, bacteria (1 × 107 CFU/ml) were preincubated in 100 μl of FBB containing 25% recombinant ficolin-2 (rFicolin-2) supernatant with or without inhibitors (10 mg/liter of acetylated BSA [acBSA] or 10 mg/liter of BSA) for 1 h at 4°C, washed, and then incubated in 100 μl of GVB containing 5% C1q-depleted serum and 25% rFicolin-2 supernatant with or without inhibitors (10 mg/liter of acBSA or 10 mg/liter of BSA).
RESULTS
Commercially available C1q-, C2-, C3-, C4-, and factor B-depleted sera lack ficolin-2 but not related opsonins.
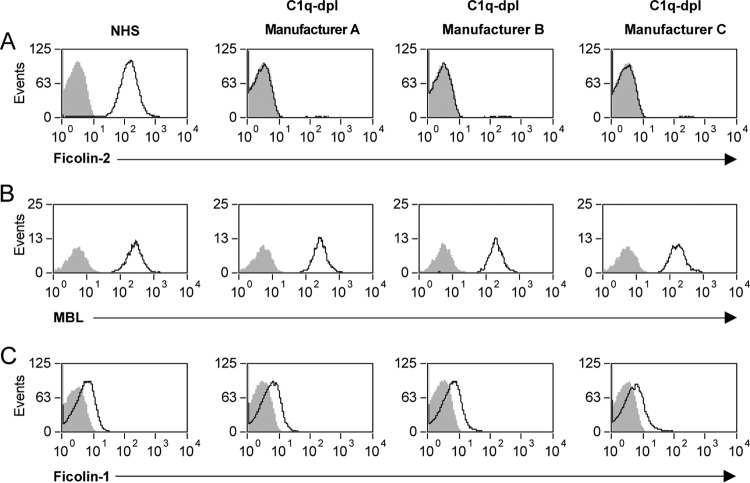
We have previously established flow cytometric binding assays to visualize ficolin-1, ficolin-2, and MBL binding to their natural microbial targets (serotype 19C pneumococci, serotype 11A pneumococci, and S. cerevisiae, respectively). We used these binding assays to investigate the activity of these opsonins in the C1q-depleted sera from three different vendors. Degrees of ficolin-1 binding to serotype 19C pneumococci were similar for the C1q-depleted sera and our NHS control (Fig. 1). Degrees of MBL binding to S. cerevisiae (yeast) were also similar for all the C1q-depleted sera and our NHS control (Fig. 1). However, we observed no binding of ficolin-2 to serotype 11A pneumococci in the C1q-depleted sera with all three preparations (Fig. 1).
FIG 1.
C1q-depleted sera lack ficolin-2 but not related opsonins. Flow cytometry histograms show binding of ficolin-2 to serotype 11A pneumococci (A), MBL to Saccharomyces cerevisiae (B), and ficolin-1 to serotype 19C pneumococci (C) using 5% normal human sera (NHS) or C1q-depleted serum (C1q-dpl) from manufacturers A, B, and C.
We used commercially available ELISA kits to determine the presence of ficolin-2 and related opsonins (ficolin-1, ficolin-3, and MBL) in C1q-depleted sera (Table 1). Similar to our binding results, we observed ficolin-2 at or below our detection limit (≤64 ng/ml) in the C1q-depleted sera. Ficolin-1 and MBL were present at concentrations similar to those in our NHS control. Ficolin-3 was present at concentrations lower than in our NHS control; however, the concentration of ficolin-3 in the C1q-depleted sera was similar to the median concentration previously reported for adults (16).
In order to determine if a similar phenomenon occurred in other commercially available complement component-depleted sera (C2-, C3-, C4-, and factor B-depleted sera), we determined the concentrations of ficolin-1, -2, and -3 and MBL in those sera (Table 1). We found that all complement component-depleted sera had little to no detectable ficolin-2 (below our detection limit of 64 ng/ml). However, ficolin-1, ficolin-3, and MBL were still present in all of these sera. The factor B-depleted sera had lower levels of ficolin-1, ficolin-3, and MBL than did NHS. Similar to the C1q-depleted sera, all of the depleted sera had lower levels of ficolin-3 than our NHS control; again, however, they were no different than the previously reported median concentration (16).
Complement component-depleted sera are codepleted of ficolin-2.
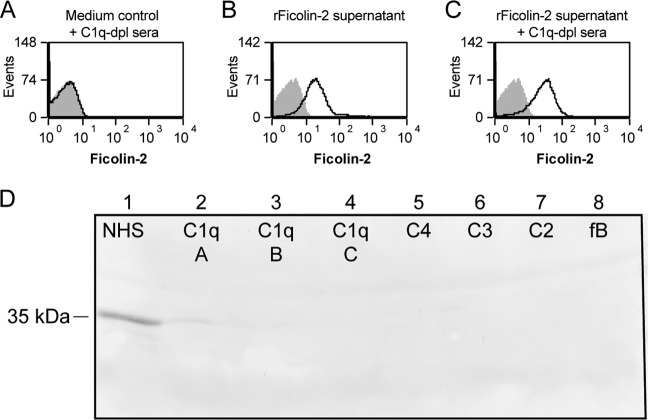
We previously found that plastic blood collection tubes contain a ficolin-2-specific inhibitor that sequesters ficolin-2, effectively inhibiting ficolin-2 binding to pneumococci (15). To investigate if C1q-depleted sera inhibited ficolin-2 binding, we mixed rFicolin-2 supernatant with C1q-depleted sera and studied ficolin-2 binding to serotype 11A bacteria using flow cytometry. Mixing of medium alone with C1q-depleted sera did not show any ficolin-2 binding, as expected (Fig. 2A). Incubation of bacteria with rFicolin-2 supernatant and C1q-depleted sera (Fig. 2C) showed binding similar to that with rFicolin-2 supernatant alone (Fig. 2B). Thus, C1q-depleted serum does not inhibit ficolin-2 binding in our assay.
FIG 2.
Complement component-depleted sera are codepleted of ficolin-2. (A to C) Flow cytometry histograms show ficolin-2 binding to serotype 11A pneumococci in 5% C1q-depleted sera with medium control (A), rFicolin-2 supernatant (B), or C1q-depleted sera with rFicolin-2 supernatant (C). Shaded gray areas represent staining with secondary antibody only as a negative control. (D) Western blot for ficolin-2 in normal human sera and depleted sera. Lane 1, NHS; lane 2, C1q-depleted sera from manufacturer A (C1q A); lane 3, C1q-depleted sera from manufacturer B (C1q B); lane 4, C1q-depleted sera from manufacturer C (C1q C); lane 5, C4-depleted sera from manufacturer C (C4); lane 6, C3-depleted sera from manufacturer C (C3); lane 7, C2-depleted sera from manufacturer C (C2); lane 8, factor B-depleted sera from manufacturer C (fB).
Though the inhibitor present in plastic blood collection tubes affects detection of ficolin-2 by ELISA and flow cytometry, sequestered ficolin-2 is still visible by Western blotting (15). Therefore, we assayed NHS and all of the depleted sera for ficolin-2 by Western blotting to determine if the absence of ficolin-2 in the complement component-depleted sera may be due to the type of tube used during blood collection. Unlike what was observed with sera collected using plastic blood collection tubes (15), Western blotting revealed significantly reduced ficolin-2 compared to our NHS control for all of the depleted sera (Fig. 2D). Taken together, these data show that ficolin-2 is depleted in complement component-depleted sera and is likely not due to the type of tube used during blood collection.
C1q-depleted sera can be used to study MBL-mediated complement deposition.
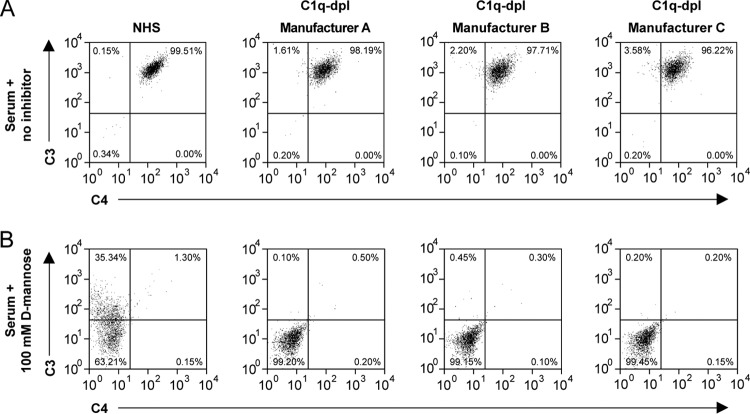
Upon binding, MBL and the ficolins activate the lectin pathway of complement activation through the association of MASPs. To investigate whether MASPs were still present in the C1q-depleted sera at concentrations sufficient to support lectin pathway of complement activation, we investigated MBL-initiated C3 and C4 deposition on yeast using C1q-depleted sera or NHS. We observed a high degree of C3 and C4 deposition on yeast incubated in all three C1q-depleted sera, and this deposition was similar to C3 and C4 deposition observed on yeast incubated in NHS (Fig. 3A). Addition of 100 mM d-mannose, which selectively inhibits MBL binding to yeast, inhibited C3 and C4 deposition on yeast in all sera (Fig. 3B). Thus, functional MASPs are still present in the C1q-depleted sera and are capable of activating complement deposition.
FIG 3.
C1q-depleted sera can activate complement through MBL and therefore still contain MASPs. Shown are flow cytometry dot plots of C4 (x axis) and C3 (y axis) deposition on S. cerevisiae using 5% normal human serum (NHS) or C1q-depleted serum (C1q-dpl) from manufacturers A, B, and C. (A) Complement deposition using serum without inhibitor. (B) Complement deposition using serum with 100 mM d-mannose to inhibit MBL binding.
C1q-depleted serum supplemented with recombinant ficolin-2 mediates complement deposition on wcjE-containing serotypes of S. pneumoniae.
Because MASPs appear to be present in sufficient amounts to activate complement, we next questioned whether rFicolin-2-opsonized bacteria could mediate complement deposition in C1q-depleted sera. We have recently shown that ficolin-2 binds to many pneumococcal serotypes that express WcjE-mediated O-acetylation (Oac) on their capsular polysaccharides, and loss of WcjE-mediated Oac in these serotypes leads to loss of ficolin-2 binding (3). Moreover, we found that ficolin-2 mediates complement deposition on serotype 11A pneumococci (which express WcjE-mediated Oac) but not on serotype 11E pneumococci (which lack WcjE-mediated Oac) (3). Because these previous studies were performed with only one wcjE-containing/wcjE-null pair (3), we studied ficolin-2-mediated complement deposition on three other wcjE-containing/wcjE-null pairs (serotypes 11D and 11Dx [SSISP 11D/1 and JC08, respectively], serotypes 20A and 20Ax [AMB11 and AMB13, respectively], and serotypes 20B and 20Bx [AMB12 and AMB14, respectively]) in the absence of C1q.
We first opsonized our bacteria with rFicolin-2 or supernatant control and then incubated them in C1q-depleted sera to allow MASP association and complement deposition to occur. We then stained the bacteria for C3 and C4 deposition and analyzed them using flow cytometry. We observed C3 and C4 deposition on serotype 11D and 20A bacteria but little to no deposition on serotype 11Dx and 20Ax bacteria (Fig. 4A and B). Bacteria incubated with supernatant control had no C4 and minimal C3 deposition. Though serotype 20B contains WcjE-mediated Oac of its capsular polysaccharide, we have previously shown that this serotype is not bound by ficolin-2 (3). The structure of serotype 20B polysaccharide differs from that of serotype 20A polysaccharide by the addition of an extra glucose residue (17), which may sterically hinder ficolin-2 recognition of WcjE-mediated O-acetylation on this polysaccharide. Serotype 20Bx (wcjE-null variant of serotype 20B) has a disrupted wcjE and is also not bound by ficolin-2 (data not shown). Thus, serotypes 20B and 20Bx serve as good negative controls for ficolin-2-mediated complement deposition, as neither is bound by ficolin-2. Accordingly, we observed little to no C3 deposition and no C4 deposition on either serotype 20B or 20Bx following the addition of rFicolin-2 (Fig. 4C). We have previously shown that acBSA inhibits ficolin-2 binding (3). As expected, acBSA inhibited C3 and C4 deposition on serotypes 11D and 20A, while BSA alone did not affect complement deposition (Fig. 4A and B).
FIG 4.
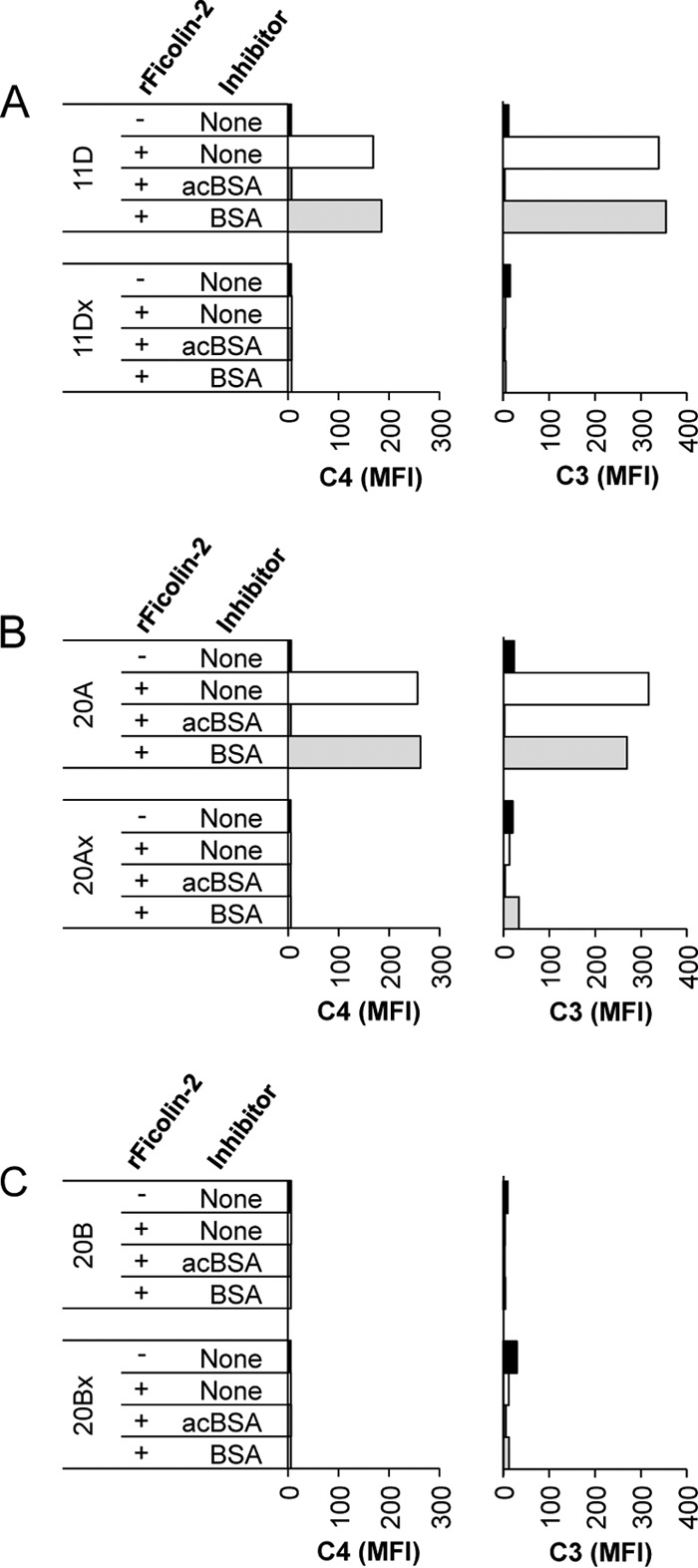
Complement deposition using C1q-depleted sera supplemented with rFicolin-2. Shown is complement deposition on wcjE-containing/wcjE-null isogenic strain pairs in 5% C1q-depleted serum following opsonization with rFicolin-2 in the absence or presence of inhibitors: acBSA (10 mg/liter) or BSA (10 mg/liter). C4 (left column) and C3 (right column) deposition on serotypes 11D (wcjE containing) and 11Dx (wcjE null) (A), 20A (wcjE containing) and 20Ax (wcjE null) (B), and 20B (wcjE containing) and 20Bx (wcjE null) (C).
C1q-depleted sera supplemented with lysine-57 ficolin-2 mutants can be used to study the lectin pathway of complement.
Ficolin-2 is known to associate with MASP-1, -2, and -3 through the lysine-57 (K57) residue of its collagen-like domain, and mutation of this residue alters interaction with the MASPs (18). Mutation of K57 to an alanine or glutamic acid strongly inhibits the interaction of ficolin-2 with MASP-1, -2, and -3 and impaired lectin complement pathway activation. Mutation of K57 to an arginine abolished interaction of ficolin-2 with MASP-2 but allowed MASP-1 and -3 to associate (18). To investigate the role of MASPs during ficolin-2-mediated complement deposition in the C1q-depleted sera, we performed complement deposition experiments using ficolin-2 mutants.
We opsonized serotype 11D and 11Dx bacteria with wild-type or either mutant form of rFicolin-2 (K57E or K57R) and then incubated them in C1q-depleted sera. As previously seen (Fig. 4), serotype 11D and 11Dx bacteria incubated with medium only had little to no complement deposition, indicating that C1q-depleted serum alone did not activate complement (Fig. 5). Also, opsonization of serotype 11D with wild-type rFicolin-2 mediated C4 and C3 deposition that could be inhibited by acBSA but not BSA (Fig. 5A). Accordingly, opsonization of serotype 11D bacteria with the rFicolin-2 K57E mutant, which should not associate with any MASPs, did not mediate complement deposition. Similarly, opsonization of serotype 11D bacteria with the K57R mutant, which should associate only with MASP-1 and MASP-3, did not mediate complement deposition (Fig. 5A). No C4 deposition and little to no C3 deposition were observed on serotype 11Dx bacteria following opsonization with any of the rFicolin-2 forms (Fig. 5B).
FIG 5.
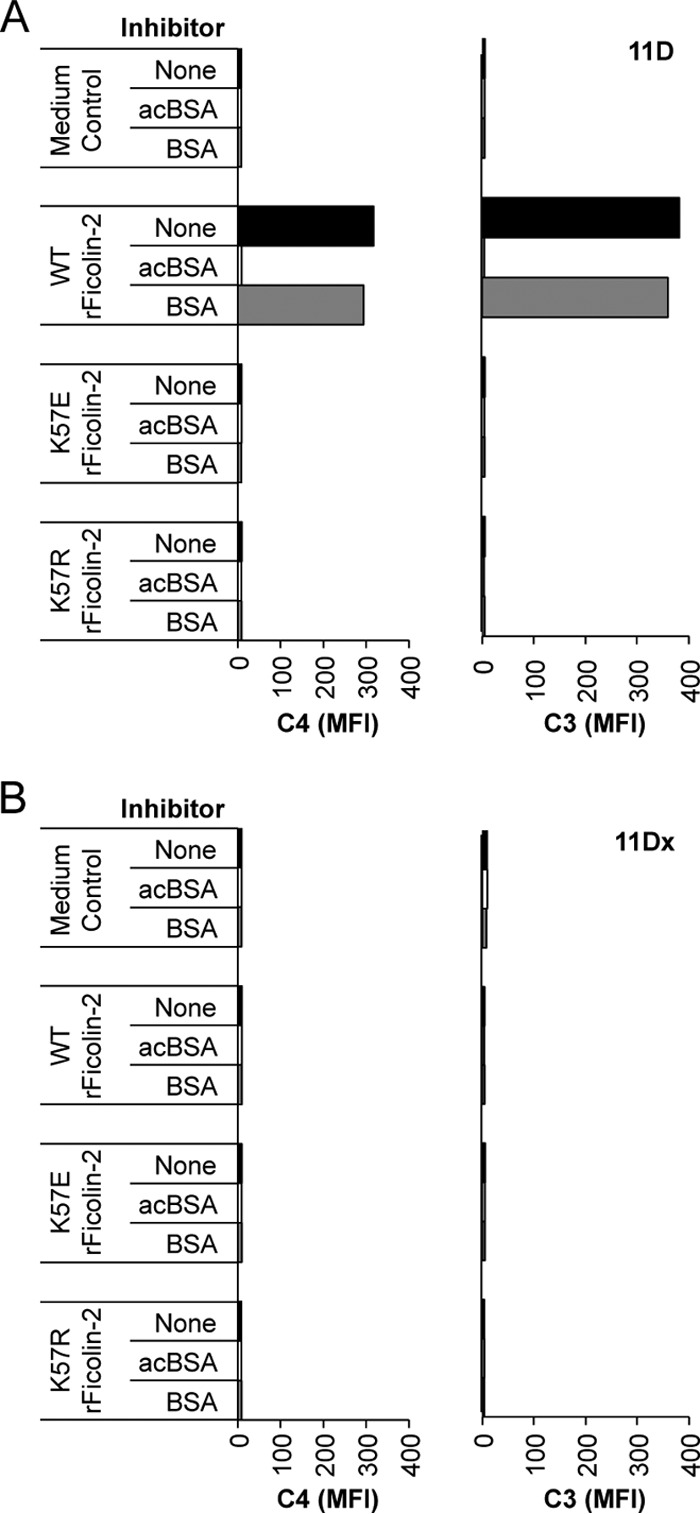
Complement deposition using C1q-depleted sera supplemented with rFicolin-2 lysine-57 mutants. Shown is deposition of C4 (left) and C3 (right) on serotypes 11D (wcjE containing) (A) and 11Dx (wcjE null) (B) in 5% C1q-depleted serum following opsonization with medium control, wild-type rFicolin-2 (WT rFicolin-2), or mutant rFicolin-2 (K57E or K57R) in the absence or presence of inhibitors acBSA (10 mg/liter) and BSA (10 mg/liter).
DISCUSSION
Studying the lectin pathway of complement activation can prove difficult, as it shares common proteins with the classical pathway. Previous studies have used sera from persons or mice deficient in specific complement pathway components (4, 19). However, clinically deficient serum samples are not readily available, and deficient mouse sera are not a good surrogate, as mouse lectin pathway components evolved separately from humans (20). Thus, C1-depleted serum should be very useful for separating the human classical and lectin pathways. However, we show here that C1q-depleted sera also lack ficolin-2.
Manufacturer labeling of these depleted sera suggests that the lectin pathway of complement activation is still intact. It is unclear what specific methods companies use to deplete the sera of specific complement components; however, we speculate that they use some form of affinity chromatography. Interestingly, ficolin-2 has been previously found to bind to cyanogen bromide-activated Sepharose beads (6); thus, it is possible that this may be the reason ficolin-2 is codepleted. We did find that the MBL-mediated lectin pathway was still functional in these sera, and presumably the ficolin-1 and ficolin-3 pathways are still intact, as their effector proteins are still present. However, we found that the ficolin-2-mediated lectin pathway is not functional, as these sera lack ficolin-2. Moreover, it is unclear if these complement component-depleted sera are also deficient in complement regulators, like C1 inhibitor (21). Thus, manufacturers should improve their product labeling. Researchers should be cautious when using these reagents, as their protein of interest or related proteins may have been unexpectedly removed. Also, previous studies performed using these sera should be reevaluated, as their conclusions were drawn under the assumption that these depleted sera were sufficient in all other components necessary for complement activation.
We have shown that complement component-depleted sera are also codepleted of ficolin-2 but not other related opsonins, like MBL, ficolin-1, and ficolin-3. Thus, these depleted sera (C2-, C3-, C4-, and factor B-depleted sera) provide a unique tool for studying ficolin-2-mediated complement pathways, as the other necessary factors (i.e., MASPs) appear to be present. For instance, C2-depleted sera may be used to determine whether ficolin-2 can mediate the C2 bypass pathway like MBL (22, 23), or factor B-depleted sera could be used to determine the relative contribution of the alternative pathway during ficolin-2-mediated complement activation.
Activation of MASPs is required for ficolin-2-mediated complement deposition to occur. Previous studies have shown that MASP-2 is activated by MASP-1 (24, 25). Following activation, MASP-2 can cleave C4 and C2 to generate the C3 convertase (consisting of C4bC2a) (11). MASP-1 and MASP-3 have been suggested to play a role in alternative pathway activation (26). However, others reported that neither MASP-1 nor MASP-3 is necessary for alternative pathway activation (27). We observed no C3 activation on whole live bacteria with our K57R mutant, which should be able to associate with MASP-1 and MASP-3 but not MASP-2 (18), in C1q-depleted sera by flow cytometry (Fig. 5). Although additional studies should be performed, we speculate, based on our findings (Fig. 5), that MASP-1/-3 association is not sufficient to activate the alternative pathway. Ficolin-2 is also known to associate with both calreticulin and CD91 through the same residue as the MASPs (18, 28), and the interaction of ficolin-2 and CD91 can mediate clearance of apoptotic cells (28). Moreover, calreticulin and CD91 are also known to associate and function as a cell receptor (29, 30). Thus, complement component-depleted sera along with rFicolin-2 mutants may be useful tools to study ficolin-2 complement pathways or ficolin-2-mediated phagocytosis (via calreticulin and/or CD91).
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grant numbers T32 AI007051 to A.M.B. and R56 AI31473 to M.H.N.).
We declare no competing financial interests.
Footnotes
Published ahead of print 16 July 2014
REFERENCES
- 1.Matsushita M. 2010. Ficolins: complement-activating lectins involved in innate immunity. J. Innate Immun. 2:24–32. 10.1159/000228160 [DOI] [PubMed] [Google Scholar]
- 2.Krarup A, Sorensen UB, Matsushita M, Jensenius JC, Thiel S. 2005. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect. Immun. 73:1052–1060. 10.1128/IAI.73.2.1052-1060.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady AM, Calix JJ, Yu J, Geno KA, Cutter GR, Nahm MH. 2014. Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation. J. Infect. Dis. 10.1093/infdis/jiu195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali YM, Lynch NJ, Haleem KS, Fujita T, Endo Y, Hansen S, Holmskov U, Takahashi K, Stahl GL, Dudler T, Girija UV, Wallis R, Kadioglu A, Stover CM, Andrew PW, Schwaeble WJ. 2012. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 8:e1002793. 10.1371/journal.ppat.1002793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoyagi Y, Adderson EE, Rubens CE, Bohnsack JF, Min JG, Matsushita M, Fujita T, Okuwaki Y, Takahashi S. 2008. L-Ficolin/mannose-binding lectin-associated serine protease complexes bind to group B streptococci primarily through N-acetylneuraminic acid of capsular polysaccharide and activate the complement pathway. Infect. Immun. 76:179–188. 10.1128/IAI.00837-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilpatrick DC, Chalmers JD. 2012. Human L-ficolin (ficolin-2) and its clinical significance. J. Biomed. Biotechnol. 2012:138797. 10.1155/2012/138797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan QQ, Ye QF, Zhou JD. 2013. Mannose-binding lectin 2 and ficolin-2 gene polymorphisms influence the susceptibility to bloodstream infections in kidney transplant recipients. Transplant. Proc. 45:3289–3292. 10.1016/j.transproceed.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 8.Jeannin P, Jaillon S, Delneste Y. 2008. Pattern recognition receptors in the immune response against dying cells. Curr. Opin. Immunol. 20:530–537. 10.1016/j.coi.2008.04.013 [DOI] [PubMed] [Google Scholar]
- 9.Jensen ML, Honore C, Hummelshoj T, Hansen BE, Madsen HO, Garred P. 2007. Ficolin-2 recognizes DNA and participates in the clearance of dying host cells. Mol. Immunol. 44:856–865. 10.1016/j.molimm.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 10.Halmos A, Rigo J, Jr, Szijarto J, Fust G, Prohaszka Z, Molvarec A. 2012. Circulating ficolin-2 and ficolin-3 in normal pregnancy and pre-eclampsia. Clin. Exp. Immunol. 169:49–56. 10.1111/j.1365-2249.2012.04590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaer TR, Thiel S, Andersen GR. 2013. Toward a structure-based comprehension of the lectin pathway of complement. Mol. Immunol. 56:413–422. 10.1016/j.molimm.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 12.Walport MJ. 2001. Complement. Second of two parts. N. Engl. J. Med. 344:1140–1144. 10.1056/NEJM200104123441506 [DOI] [PubMed] [Google Scholar]
- 13.Calix JJ, Saad JS, Brady AM, Nahm MH. 2012. Structural characterization of Streptococcus pneumoniae serotype 9A capsule polysaccharide reveals role of glycosyl 6-O-acetyltransferase wcjE in serotype 9V capsule biosynthesis and immunogenicity. J. Biol. Chem. 287:13996–14003. 10.1074/jbc.M112.346924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calix JJ, Brady AM, Du VY, Saad JS, Nahm MH. 2014. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J. Clin. Microbiol. 52:758–765. 10.1128/JCM.02695-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brady AM, Spencer BL, Falsey AR, Nahm MH. 2014. Blood collection tubes influence serum ficolin-1 and ficolin-2 levels. Clin. Vaccine Immunol. 21:51–55. 10.1128/CVI.00607-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallenbach S, Thiel S, Aebi C, Otth M, Bigler S, Jensenius JC, Schlapbach LJ, Ammann RA. 2011. Serum concentrations of lectin-pathway components in healthy neonates, children and adults: mannan-binding lectin (MBL), M-, L-, and H-ficolin, and MBL-associated serine protease-2 (MASP-2). Pediatr. Allergy Immunol. 22:424–430. 10.1111/j.1399-3038.2010.01104.x [DOI] [PubMed] [Google Scholar]
- 17.Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, Nahm MH. 2012. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J. Biol. Chem. 287:27885–27894. 10.1074/jbc.M112.380451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacroix M, Dumestre-Perard C, Schoehn G, Houen G, Cesbron JY, Arlaud GJ, Thielens NM. 2009. Residue Lys57 in the collagen-like region of human L-ficolin and its counterpart Lys47 in H-ficolin play a key role in the interaction with the mannan-binding lectin-associated serine proteases and the collectin receptor calreticulin. J. Immunol. 182:456–465. 10.4049/jimmunol.182.1.456 [DOI] [PubMed] [Google Scholar]
- 19.Ma YJ, Doni A, Hummelshoj T, Honore C, Bastone A, Mantovani A, Thielens NM, Garred P. 2009. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J. Biol. Chem. 284:28263–28275. 10.1074/jbc.M109.009225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo Y, Liu Y, Kanno K, Takahashi M, Matsushita M, Fujita T. 2004. Identification of the mouse H-ficolin gene as a pseudogene and orthology between mouse ficolins A/B and human L-/M-ficolins. Genomics 84:737–744. 10.1016/j.ygeno.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 21.Keizer MP, Kamp AM, Brouwer N, van de Wetering MD, Wouters D, Kuijpers TW. 2014. Plasma-derived mannose-binding lectin shows a direct interaction with C1-inhibitor. Mol. Immunol. 58:187–193. 10.1016/j.molimm.2013.11.022 [DOI] [PubMed] [Google Scholar]
- 22.Tateishi K, Matsushita M. 2011. Activation of the alternative complement pathway by mannose-binding lectin via a C2-bypass pathway. Microbiol. Immunol. 55:817–821. 10.1111/j.1348-0421.2011.00378.x [DOI] [PubMed] [Google Scholar]
- 23.Selander B, Martensson U, Weintraub A, Holmstrom E, Matsushita M, Thiel S, Jensenius JC, Truedsson L, Sjoholm AG. 2006. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J. Clin. Invest. 116:1425–1434. 10.1172/JCI25982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M, Iwaki D, Kanno K, Ishida Y, Xiong J, Matsushita M, Endo Y, Miura S, Ishii N, Sugamura K, Fujita T. 2008. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J. Immunol. 180:6132–6138. 10.4049/jimmunol.180.9.6132 [DOI] [PubMed] [Google Scholar]
- 25.Møller-Kristensen M, Thiel S, Sjoholm A, Matsushita M, Jensenius JC. 2007. Cooperation between MASP-1 and MASP-2 in the generation of C3 convertase through the MBL pathway. Int. Immunol. 19:141–149 [DOI] [PubMed] [Google Scholar]
- 26.Iwaki D, Kanno K, Takahashi M, Endo Y, Matsushita M, Fujita T. 2011. The role of mannose-binding lectin-associated serine protease-3 in activation of the alternative complement pathway. J. Immunol. 187:3751–3758. 10.4049/jimmunol.1100280 [DOI] [PubMed] [Google Scholar]
- 27.Degn SE, Jensen L, Hansen AG, Duman D, Tekin M, Jensenius JC, Thiel S. 2012. Mannan-binding lectin-associated serine protease (MASP)-1 is crucial for lectin pathway activation in human serum, whereas neither MASP-1 nor MASP-3 is required for alternative pathway function. J. Immunol. 189:3957–3969. 10.4049/jimmunol.1201736 [DOI] [PubMed] [Google Scholar]
- 28.Duus K, Thielens NM, Lacroix M, Tacnet P, Frachet P, Holmskov U, Houen G. 2010. CD91 interacts with mannan-binding lectin (MBL) through the MBL-associated serine protease-binding site. FEBS J. 277:4956–4964. 10.1111/j.1742-4658.2010.07901.x [DOI] [PubMed] [Google Scholar]
- 29.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781–795. 10.1084/jem.194.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. 2002. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 169:3978–3986. 10.4049/jimmunol.169.7.3978 [DOI] [PubMed] [Google Scholar]