Abstract
In this study, we assessed the effects of the prevaccination titer and age on the immunogenicity of a low dose of influenza vaccine in children less than 4 years of age. A total of 259 children received two vaccine doses (0.1 ml for 0-year-olds and 0.2 ml for children 1 year of age or older) 4 weeks apart during the 2005/2006 season. The hemagglutination inhibition antibody titers were measured before vaccination and 4 weeks after the first and second doses. The geometric mean titer, mean fold rise, seroresponse proportion (≥4-fold rise in titer), and seroprotection proportion (titer ≥1:40) were calculated for the prevaccination titer and age categories. A multivariate logistic regression analysis was performed using the seroresponse and seroprotection proportions as dependent variables and the prevaccination titer and age as explanatory variables. As for the seroresponse against the H1 antigen after the first dose, the adjusted odds ratios of the prevaccination titers (versus <1:10) were 2.2 (95% confidence interval, 0.8 to 5.8) at 1:10 to 1:20 and 0.14 (0.04 to 0.49) at ≥1:40. The corresponding figures for ages were 0.03 (0.01 to 0.07) for the 0-year-olds and 0.17 (0.08 to 0.34) for the 1-year-olds compared with the 2- to 3-year-olds (Ptrend < 0.001). Similar results were also obtained for the H3 and B strains. Significantly elevated odds ratios for seroprotection were observed with greater prevaccination titers and older ages for all strains. The prevaccination titer and age were independently associated with the antibody response in young children. The immune response was weaker in the younger children and those without preexisting immunity.
INTRODUCTION
Influenza is a vaccine-preventable disease. The rate of seasonal influenza infection is highest among children, and children less than 2 years of age are at high risk of influenza-associated hospitalization (1, 2). The Advisory Committee on Immunization Practices routinely recommends that children 6 months to 8 years of age receive two doses of influenza vaccine during their first season of vaccination in order to optimize the immune response (3). This recommendation is based on data showing that vaccine effectiveness and immunogenicity are lower among young children treated with one dose of the vaccine, whereas two doses of vaccine provide substantial protection against influenza-like illness (ILI) (4–6) and induce a protective level of antibodies, even in young children (7–15).
The factors affecting low immune responses to the influenza vaccine among children are supposed to include immature immunity function due to age, infrequency of opportunity for exposure to influenza virus through vaccination and/or infection, thus resulting in a lack of induced priming, and a low-volume dose of the vaccine. As the subjects get older, it has been reported that their prevaccination titer (pretiter) increases (16–19), but there has been very little detailed research that considered the predictive factors in the immune response (20, 21).
In this report, we present the immunogenicity of the trivalent inactivated influenza vaccine (IIV3) in young children. More specifically, by using a thorough descriptive analysis and multivariate analysis, we performed a detailed evaluation of our preliminary 2005/2006 data (22), focusing on the mutual effects of age and the pretiter status, which are considered to be essential for evaluating the immunogenicity of young children.
MATERIALS AND METHODS
Study subjects and vaccination.
Healthy infants and children 6 months to 3 years of age were eligible for enrollment. The children were recruited from six pediatric practices in Japan. The exclusion criteria were fever or acute serious illness at the time of vaccination, a history of anaphylaxis to the vaccine components, and/or other conditions that rendered the subjects ineligible to receive vaccination. We attempted to register approximately 50 children in each age group (0-, 1-, 2-, and 3-year-olds); a total of 259 children were enrolled. The study protocol was approved by the ethics committee of the Osaka City University Faculty of Medicine, and written informed consent was obtained from the guardians of all children.
A single lot of licensed trivalent inactivated, thimerosal-free, unadjuvanted influenza HA vaccine (FLUBIK HA, lot HE01A; Biken, Japan) was used in this study. Each vaccine contained 15 μg/0.5 ml of each of the three hemagglutinin antigens recommended for the 2005/2006 influenza vaccine: the A/New Caledonia/20/99 (H1N1), A/NewYork/55/2004 (H3N2), and B/Shanghai/361/2002 strains.
The subjects received two subcutaneous injections of IIV3 in the arm, at a dose of 0.1 ml for 0-year-olds and 0.2 ml for children ≥1 year of age, in conformity with the Japanese influenza vaccine regulations at that time. All subjects received the first vaccine dose between 1 September and 31 October 2005, followed by the second dose 4 weeks later between 1 October and 31 November 2005. None of the children experienced physician-diagnosed influenza virus infection between the first and second dose or discontinued participation due to an adverse event and/or experienced any severe adverse events. Hence, all subjects were included in the analyses. According to the national infectious diseases surveillance, the 2005/2006 seasonal epidemic occurred in mid-December. This was at least 2 weeks after all children had received their second vaccination.
Information collection and antibody titer measurement.
The following information was collected via a self-administered questionnaire completed by the guardian: baseline characteristics, such as age, sex, body weight, and underlying medical conditions; previous influenza vaccination status within the past 3 years; and a history of ILI with a fever of ≥39°C during the last season.
A triplet serum sample was obtained before vaccination (S0), 4 weeks after the first dose (S1), i.e., immediately before the second dose, and 4 weeks after the second dose (S2). The sera were stored frozen at −70°C to −80°C until they were analyzed simultaneously. The hemagglutination inhibition (HI) antibody titers for each vaccine antigen were measured according to a standard assay using type O human erythrocytes (23).
Statistical analyses.
The outcome measurements of this study, which aimed to assess immunogenicity, were the geometric mean titer (GMT), mean fold rise (MFR), proportion of subjects with a ≥4-fold rise in the postvaccination titer (sR), and proportion of subjects achieving a titer of ≥1:40 (sP). For data processing, a titer of <1:10 was assigned a value of 1:5, and reciprocal antibody titers were handled after logarithmic transformation. Therefore, the use of 1:5 titers for lower censored values may lead to a reduced estimate of the variance. The results are presented in the original scale by calculating the antilogarithms. The data were categorized to examine the effects of the following factors considered to be medically important based on previous reports: pretiter (<1:10, 1:10 to 1:20, and ≥1:40), age, influenza vaccination within the past 3 years, and ILI history during the last season.
The significance of the MFR within a category was assessed according to the Wilcoxon signed-rank test, while intercategory comparisons of GMT and MFR values were made using either the Wilcoxon rank sum test or the Kruskal-Wallis test. The t test, analysis of variance, Mantel-extension method for trend test, and χ2 test were also employed where appropriate.
The independent effects of the pretiter status and age on antibody induction were evaluated using a multivariate logistic regression analysis. The models were constructed with sR or sP as a dependent variable and the pretiter status and age as explanatory variables. The odds ratios (ORs) and the 95% confidence intervals (CIs) are presented. The influenza vaccination history and ILI history were excluded from the final model after consideration of the correlations between these factors and age. In addition, if both factors were included together, we would have been forced to exclude 0-year-old infants who mostly did not have a vaccination history or ILI history (100% and 89%, respectively) from the analysis. This results in exclusion of children with a pretiter of <1:10, accounting for the majority of the subjects, and thus the validity of the multivariate analysis itself would have been compromised. Therefore, we excluded these parameters from the analysis to secure a sufficient number of subjects. A P value of <0.05 was considered to be statistically significant. All hypothesis tests were two-sided. The calculations were performed using the SAS version 9.2 software program (SAS Institute Inc., Cary, NC).
RESULTS
The baseline characteristics of the subjects are shown in Table 1. The mean and median ages were nearly the same (24.1 and 24.0 months). The subjects were distributed almost equally (64 to 66 subjects) among the four age groups. Asthma, urticaria, and atopic dermatitis were relatively frequent underlying diseases (5.0% to 6.6%).
TABLE 1.
Characteristics of study subjects
| Variable | Valuea |
|---|---|
| Total no. of subjects | 259 |
| Male sex | 142 (54.5) |
| Age at vaccination (mo) | |
| Mean (SD) | 24.1 (12.4) |
| Median (range) | 24.0 (45.0) |
| Age at vaccination | |
| 0 yr | 64 (25) |
| 1 yr | 65 (25) |
| 2 yr | 64 (25) |
| 3 yr | 66 (25) |
| Underlying illnesses | |
| Heart disease | 1 (0.4) |
| Renal disease | 1 (0.4) |
| Anemia | 2 (0.8) |
| Asthma | 14 (5.4) |
| Urticaria | 17 (6.6) |
| Atopic dermatitis | 13 (5.0) |
| Influenza vaccination within the past 3 yr | |
| Vaccinated | 114 (44) |
| 0 yr | 0 (0) |
| 1 yr | 17 (15) |
| 2 yr | 46 (40) |
| 3 yr | 51 (45) |
| Not vaccinated | 144 (56) |
| 0 yr | 64 (44) |
| 1 yr | 48 (33) |
| 2 yr | 17 (12) |
| 3 yr | 15 (10) |
| Influenza-like illness during the last season present | 122 (47) |
Values are expressed as no. (%) unless otherwise indicated.
Geometric mean titer and mean fold rise.
The GMT and MFR values in the subjects grouped according to the pretiter status, age, influenza vaccination history, and ILI history are summarized in Table 2 for each antigen. Approximately three-fourths of the children fell into the seronegative category (pretiter of <1:10), regardless of the type of test antigen (77%, 72%, and 73% for H1, H3, and B, respectively). The proportion of children with a pretiter of ≥1:40 was highest for the H3 antigen (24%) followed by the B (12%) and H1 (6%) antigens.
TABLE 2.
Geometric mean and mean fold rise
| Vaccine antigen and category | No. (%) of subjects | Mean age (yr) | GMTa |
MFRb |
||||
|---|---|---|---|---|---|---|---|---|
| S0 | S1 | S2 | S1/S0 | S2/S1 | S2/S0 | |||
| A/New Caledonia/20/99(H1N1) | ||||||||
| Entire sample | 259 (100) | 1.5 | 7 | 23 | 46 | 3.3 | 2.0 | 6.6 |
| Prevaccination titer | ||||||||
| <1:10 | 200 (77) | 1.2c | 5d | 15d | 36d | 3.0d,e | 2.4d,e | 7.1d,e |
| 1:10–1:20 | 44 (17) | 2.4 | 15 | 84 | 94 | 5.7e | 1.1 | 6.4e |
| ≥1:40 | 15 (6) | 2.6 | 66 | 153 | 176 | 2.3e | 1.1 | 2.6e |
| Age | ||||||||
| 0 yr | 64 (25) | 0.7c | 5d | 6d | 21d | 1.2d,e | 3.3d,e | 4.0d,e |
| 1 yr | 65 (25) | 1.4 | 5 | 14 | 39 | 2.7e | 2.7e | 7.3e |
| 2 yr | 64 (25) | 2.4 | 8 | 58 | 80 | 7.4e | 1.4e | 10.3e |
| 3 yr | 66 (25) | 3.4 | 11 | 54 | 70 | 5.0e | 1.3e | 6.4e |
| Influenza vaccination in the past 3 yr | ||||||||
| Unvaccinated | 144 (56) | 0.9c | 5d | 9d | 32d | 1.8d,e | 3.5d,e | 6.1e |
| Vaccinated | 114 (44) | 2.3 | 10 | 73 | 72 | 7.4e | 1.0 | 7.3e |
| Influenza-like illness during the last season | ||||||||
| Absent | 136 (53) | 1.2c | 7 | 16d | 37d | 2.2d,e | 2.3d,e | 5.2d,e |
| Present | 122 (47) | 1.9 | 7 | 35 | 59 | 5.1e | 1.7e | 8.7e |
| A/New York/55/2004(H3N2) | ||||||||
| Entire sample | 259 (100) | 13 | 37 | 71 | 2.8 | 2.0 | 5.5 | |
| Prevaccination titer | ||||||||
| <1:10 | 187 (72) | 1.2c | 5d | 12d | 29d | 2.3d,e | 2.5d,e | 5.9d,e |
| 1:10–1:20 | 9 (4) | 2.1 | 15 | 235 | 296 | 16.0 | 1.3 | 20.2 |
| ≥1:40 | 63 (24) | 2.3 | 208 | 852 | 806 | 4.1e | 0.9 | 3.9e |
| Age | ||||||||
| 0 yr | 6d | 8d | 32d | 1.4d,e | 4.1d,e | 5.5e | ||
| 1 yr | 8 | 20 | 51 | 2.4e | 2.5e | 6.1e | ||
| 2 yr | 22 | 108 | 130 | 5.0e | 1.2e | 6.0e | ||
| 3 yr | 27 | 105 | 123 | 4.0e | 1.2e | 4.6e | ||
| Influenza vaccination in the past 3 yr | ||||||||
| Unvaccinated | 9d | 17d | 53d | 1.9d,e | 3.1d,e | 5.9e | ||
| Vaccinated | 20 | 97 | 105 | 4.8e | 1.1 | 5.2e | ||
| Influenza-like illness during the last season | ||||||||
| Absent | 10d | 23d | 54d | 2.4d,e | 2.3d,e | 5.6e | ||
| Present | 18 | 61 | 96 | 3.5e | 1.6e | 5.5e | ||
| B/Shanghai/361/2002 | ||||||||
| Entire sample | 259 (100) | 8 | 22 | 34 | 2.8 | 1.6 | 4.4 | |
| Prevaccination titer | ||||||||
| <1:10 | 188 (73) | 1.3c | 5d | 10d | 19d | 2.0d,e | 1.9d,e | 3.7d,e |
| 1:10–1:20 | 40 (15) | 2.2 | 13 | 126 | 121 | 9.5e | 1.0 | 9.2e |
| ≥1:40 | 31 (12) | 2.2 | 65 | 274 | 274 | 4.2e | 1.0 | 4.2e |
| Age | ||||||||
| 0 yr | 5d | 6d | 13d | 1.1d | 2.3d,e | 2.5d,e | ||
| 1 yr | 7 | 20 | 32 | 2.7e | 1.6e | 4.4e | ||
| 2 yr | 8 | 40 | 52 | 4.9e | 1.3e | 6.3e | ||
| 3 yr | 12 | 50 | 62 | 4.2e | 1.2 | 5.1e | ||
| Influenza vaccination in the past 3 yr | ||||||||
| Unvaccinated | 6d | 11d | 23d | 1.7d,e | 2.2d,e | 3.8d,e | ||
| Vaccinated | 11 | 55 | 56 | 5.1e | 1.0 | 5.2e | ||
| Influenza-like illness during the last season | ||||||||
| Absent | 7d | 14d | 24d | 2.0d,e | 1.8d,e | 3.6d,e | ||
| Present | 9 | 38 | 51 | 4.1e | 1.3e | 5.5e | ||
GMT, geometric mean titer.
MFR, mean fold rise.
P < 0.05 by t test or ANOVA.
P < 0.05 by the Wilcoxon rank sum test or Kruskal-Wallis rank test for intercategory comparisons.
P < 0.05 by the Wilcoxon signed-rank test for intracategory comparisons.
A higher pretiter against the H1 antigen was associated with a higher mean age and greater pre- and postvaccination GMT values (S0, S1, and S2) (P < 0.05 for each by analysis of variance [ANOVA] or the Kruskal-Wallis rank test). The MFR after the first dose (S1/S0) was higher in the 1:10 to 1:20 category (5.7-fold) than those in the <1:10 and ≥1:40 categories (3.0- and 2.3-fold, respectively). The S2/S1 values further increased 2.4-fold in the pretiter of <1:10 category, but not in the two higher pretiter categories (1.1-fold in both). After the second dose (S2/S0), a ≥6-fold rise was seen in the <1:10 and 1:10 to 1:20 categories compared to that in the ≥1:40 category (2.6-fold). Therefore, the subjects with a pretiter of ≥1:40 showed lower MFR values at both S1 and S2. The trends for GMT and MFR were similar for the H3 and B antigens, with substantially pronounced changes in H3. The prevaccination GMT against H3 was quite high in the ≥1:40 category (208 at S0), leading to far more elevated postvaccination GMT values (852 at S1 and 806 at S2). In addition, the GMT values in the 1:10 to 1:20 category also increased greatly after the first dose (235 at S1; S1/S0 = 16.0-fold).
When the data were examined according to age group, the pre- and postvaccination GMT values against H1 increased with increasing age (P < 0.05 at each time point for the Kruskal-Wallis rank test). A similar tendency was seen in the MFR S1/S0 and S2/S0 values (P < 0.05 at both time points for the Kruskal-Wallis rank test), with maximum values in the 2-year-olds (7.4- and 10.3-fold, respectively). An opposite trend was observed in the S2/S1 values, i.e., the MFR decreased with increasing age (P < 0.05 for the Kruskal-Wallis rank test). Comparable findings regarding GMT and MFR were also obtained for H3 and B, with distinctively elevated postvaccination GMT values against H3 in the older age groups. The pre- and postvaccination GMT values were consistently higher in the children with a history of vaccination or ILI than in those with no such history, at all time points for every strain.
The above findings can be summarized as follows. (i) Approximately 70% of the children were initially seronegative (pretiter of <1:10). (ii) A higher pretiter and older age were associated with elevated GMT values after the first and second doses, irrespective of the test antigen. (iii) The maximum titers varied with the antigens: the highest GMT value (exceeding 1:800) was attained for the high pretiter category against H3. (iv) In the pretiter of <1:10 category, the response after the first dose was weak, and an additional titer was induced by the second dose. On the other hand, in the pretiter of ≥1:10 category, the titer reached a plateau after the first dose, and no or little booster response was induced after the second dose. (v) The MFR values were lower after both the first and second doses in the pretiter of ≥1:40 category and the 3-year-olds.
Seroresponse proportion and seroprotection proportion.
The top section of Fig. 1 shows the sR for each antigen. Comparing the three levels of the pretiter, the sR for <1:10 against H1 increased from 45% after the first dose (S1/S0) to 77% after the second dose (S2/S0). The corresponding values were 34% to 73% for H3 and 31% to 54% for B. In the 1:10 to 1:20 category, the sR reached nearly 90% (85% to 89%) with the first dose alone for all antigens. However, in the ≥1:40 category, the sR after one dose did not exhibit a large increase (33 to 62%) and instead reached a plateau even after the second dose (47 to 60%). An analysis of the sP (the bottom section of Fig. 1) was performed, excluding children with a pretiter of ≥1:40. The sP in the pretiter of <1:10 category was low, even with two doses (37 to 58%), whereas in the 1:10 to 1:20 category, more than about 90% of the subjects attained a seroprotective titer (≥1:40) with one dose alone.
FIG 1.
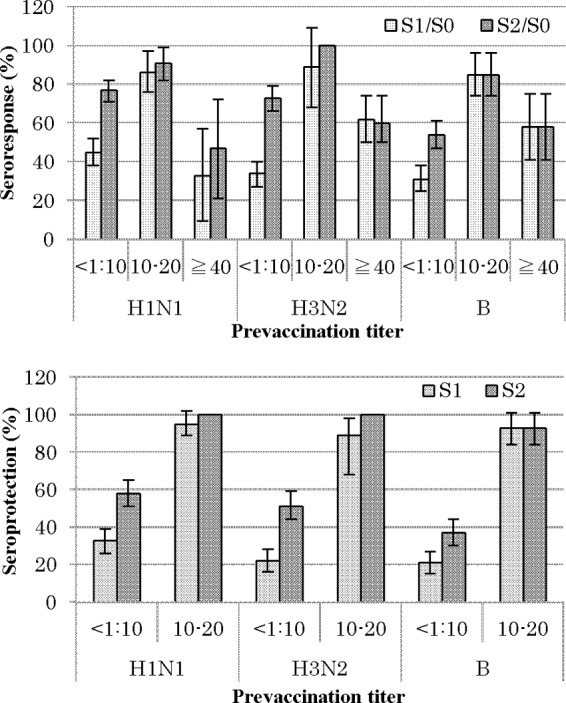
Seroresponse (≥4-fold rise) and seroprotection (HI titer of ≥1:40) proportion and 95% CIs. Subjects with prevaccination titers of ≥1:40 were excluded for the seroprotection analyses. S0, before vaccination. S1, after the first dose; S2, after the second dose.
Next, stratified analyses were conducted to examine the effects of the pretiter and age (Fig. 2 and 3). The age-specific sR and sP values were calculated after stratification for the three levels of the pretiter. Among those with a pretiter of <1:10, the S1/S0 sR was considerably lower in the younger children (0 years, 3 to 10%; 1 year, 20 to 36%) than in the 2- to 3-year-olds (61 to 82%) for each antigen, indicating an increase in sR with increasing age (P < 0.001 for each in the Mantel-extension method for trend test). The S2/S0 sR further increased in all age groups, maintaining a dose-response relationship similar to that observed for the S1/S0 sR. On the other hand, in the pretiter 1:10 to 1:20 group, all of the 1-year-olds achieved the sR level with one dose alone (100%), and slightly lower sR values were seen in the 2- to 3-year-olds (85 to 87%). However, in the pretiter ≥1:40 group, the S1/S0 sR for H3 decreased with increasing age (P < 0.03 in the Mantel-extension method for trend test), and a similar tendency was seen against H1 and B, although the skewed distribution of the subjects made it difficult to statistically confirm this finding. In addition, in the pretiter ≥1:40 group, no or little booster response to the second dose was induced for any antigen in any age category.
FIG 2.
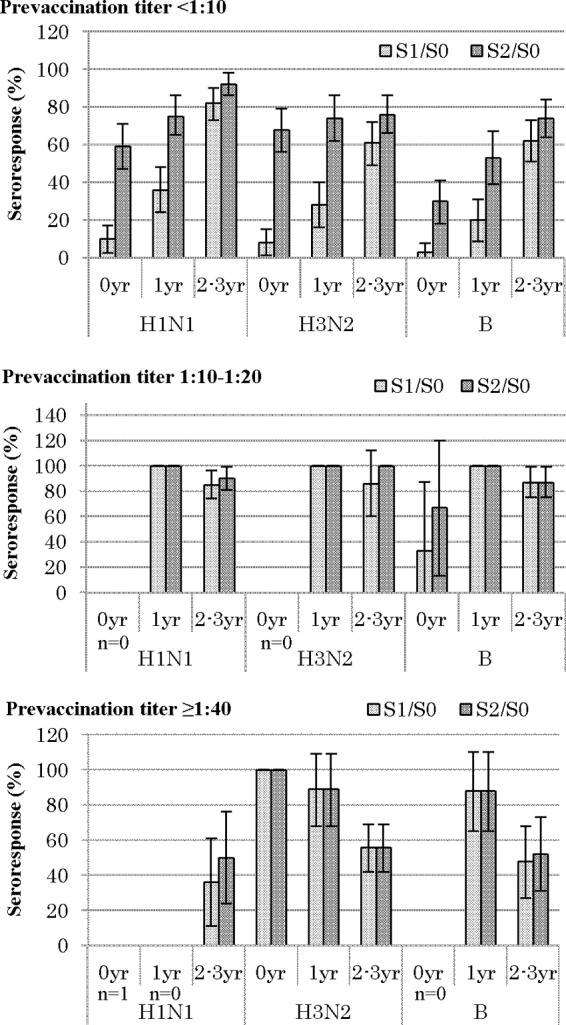
Age-specific seroresponse proportion and 95% CIs stratified by prevaccination titer. S0, before vaccination; S1, after the first dose; S2, after the second dose; n, total no. of subjects.
FIG 3.
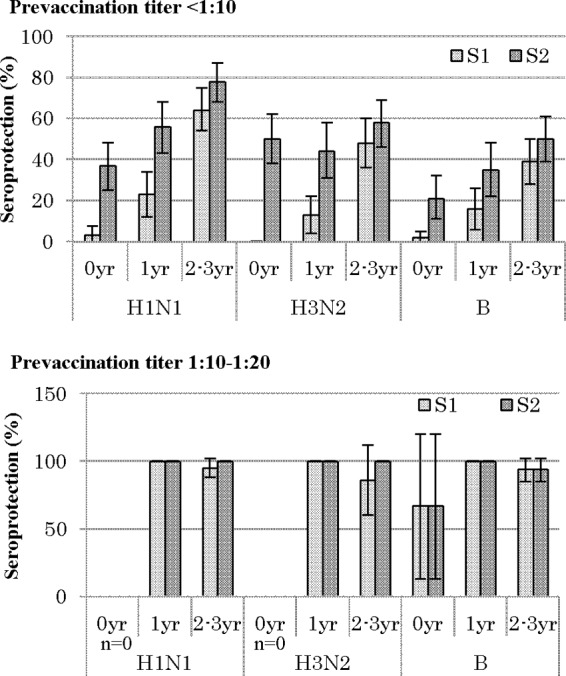
Age-specific seroprotection proportion and 95% CIs stratified by prevaccination titer. Subjects with prevaccination titers of ≥1:40 were excluded from the seroprotection analyses. S0, before vaccination; S1, after the first dose; S2, after the second dose; n, total no. of subjects.
The sP showed the same trend as the sR. In the pretiter of <1:10 group, the sP values improved with age for all antigens, and an additional antibody titer was induced by the second dose, although the sP value against H1 at S2 was at most 78% in the 2- to 3-year-olds. In contrast, in the 1:10 to 1:20 group, a substantial rise in titer was achieved with one dose alone, irrespective of the age category for all antigens, except in the 0-year-olds for B (67%).
Therefore, both the pretiter and age per se may mutually influence the antibody response. Hence, the independent effects of the pretiter and age on sR and sP were examined using a logistic regression model (Table 3).
TABLE 3.
Odds ratios for seroresponse and seroprotection proportions after the first vaccinationf
| Vaccine antigen and category | Seroresponse (S1/S0 ≥ 4) |
Seroprotection (S1 ≥ 1:40) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total no. of subjects | No. (%) with seroresponse | OR (95% CI)a |
Total no. of subjects | No. (%) with seroprotection | OR (95% CI)a |
|||
| Crude | Adjustedb | Crude | Adjustedb | |||||
| A/New Caledonia/20/99(H1N1) | ||||||||
| Prevaccination titer | ||||||||
| <1:10 | 200 | 90 (45) | 1.0 | 1.0 | 200 | 65 (33) | 1.0 | 1.0 |
| 1:10–1:20 | 44 | 38 (86) | 7.7 (3.1, 19.1) | 2.2 (0.8, 5.8) | 44 | 42 (95) | 43.6 (10.2, 186) | 5.4 (2.1, 13.6) |
| ≥1:40 | 15 | 5 (33) | 0.6 (0.2, 1.9) | 0.14 (0.04, 0.49) | ||||
| Ptrend | —c | |||||||
| Age | ||||||||
| 0 yr | 64 | 6 (9) | 0.01 (0.01, 0.08) | 0.03 (0.01, 0.07) | 63 | 2 (3) | 0.01 (0.003, 0.05) | 0.03 (0.01, 0.1) |
| 1 yr | 65 | 26 (40) | 0.2 (0.1, 0.4) | 0.17 (0.08, 0.34) | 65 | 18 (28) | 0.13 (0.06, 0.25) | 0.2 (0.1, 0.4) |
| 2–3 yr | 130 | 101 (78) | 1.0 | 1.0 | 116 | 87 (75) | 1.0 | 1.0 |
| Ptrend | —d | —d | —d | —d | ||||
| A/New York/55/2004 (H3N2) | ||||||||
| Prevaccination titer | ||||||||
| <1:10 | 187 | 63 (34) | 1.0 | 1.0 | 187 | 41 (22) | 1.0 | 1.0 |
| 1:10–1:20 | 9 | 8 (89) | 15.8 (1.9, 129) | 8.8 (1.1, 73.1) | 9 | 8 (89) | 28.5 (3.5, 234) | 16.3 (1.8, 148) |
| ≥1:40 | 63 | 39 (62) | 3.2 (1.8, 5.8) | 1.7 (0.9, 3.3) | ||||
| Ptrend | —d | |||||||
| Age | ||||||||
| 0 yr | 64 | 7 (11) | 0.08 (0.04, 0.19) | 0.11 (0.04, 0.26) | 62 | 0 | NA | NA |
| 1 yr | 65 | 29 (45) | 0.4 (0.2, 0.8) | 0.5 (0.3, 0.9) | 56 | 9 (16) | 0.18 (0.08, 0.42) | 0.18 (0.08, 0.44) |
| 2–3 yr | 130 | 78 (60) | 1.0 | 1.0 | 78 | 40 (51) | 1.0 | 1.0 |
| Ptrend | —d | —d | ||||||
| B/Shanghai/361/2002 | ||||||||
| Prevaccination titer | ||||||||
| <1:10 | 188 | 59 (31) | 1.0 | 1.0 | 188 | 39 (21) | 1.0 | 1.0 |
| 1:10–1:20 | 40 | 34 (85) | 12.4 (4.9, 31.1) | 9.2 (3.2, 26.6) | 40 | 37 (93) | 47.1 (13.8, 161) | 47.5 (11.5, 197) |
| ≥1:40 | 31 | 18 (58) | 3.0 (1.4, 6.6) | 1.5 (0.7, 3.4) | ||||
| Ptrend | —d | —e | ||||||
| Age | ||||||||
| 0 yr | 64 | 3 (5) | 0.03 (0.01, 0.09) | 0.03 (0.01, 0.11) | 64 | 3 (5) | 0.04 (0.01, 0.14) | 0.04 (0.01, 0.17) |
| 1 yr | 65 | 23 (35) | 0.3 (0.2, 0.5) | 0.4 (0.2, 0.7) | 65 | 23 (35) | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.8) |
| 2–3 yr | 130 | 85 (65) | 1.0 | 1.0 | 130 | 85 (65) | 1.0 | 1.0 |
| Ptrend | —d | —d | —d | —d | ||||
OR, odds ratio; CI, confidence interval.
Adjusted for prevaccination titer and age.
P < 0.05.
P < 0.001.
P < 0.01.
Subjects with prevaccination titer of ≥1:40 were excluded from the seroprotection analyses.
The crude ORs (95% CIs) of the pretiter for sR after the first dose were significantly high at 1:10 to 1:20 (versus <1:10): 7.7 (3.1 to 19.1) for H1, 15.8 (1.9 to 129) for H3, and 12.4 (4.9 to 31.1) for B. These values shifted toward null when the effect of age was simultaneously considered: the adjusted ORs (95% CIs) were 8.8 (1.1 to 73.1) for H3 and 9.2 (3.2 to 26.6) for B, with statistical significance. However, the pretiter of ≥1:40 category demonstrated lower ORs for all test antigens than the pretiter of 1:10 to 1:20 category in both the univariate and multivariate analyses. For sP, significantly elevated ORs were observed in the pretiter of 1:10 to 1:20 category, although this trend was unstable, as indicated by the wide CIs. The adjusted ORs (95% CIs) were 5.4 (2.1 to 13.6) for H1, 16.3 (1.8 to 148) for H3, and 47.5 (11.5 to 197) for B.
Age was found to be positively associated with sR and sP for all antigens. Against H1, the adjusted ORs (95% CIs) for sR after the first dose were 0.17 (0.08 to 0.34) in the 1-year-olds and 0.03 (0.01 to 0.07) in the 0-year-olds, with a clear dose-response relationship, compared with the 2- to 3-year-olds as the reference (Ptrend < 0.001). Similar results were obtained for H3 and B (Ptrend < 0.001 for both). In addition, significantly elevated ORs for sP after the first dose were observed in association with an older age for all strains (Ptrend < 0.001 for H1 and H3). For both sR and sP, the adjusted ORs were quite similar to the crude values, irrespective of the type of antigen, which suggests a low combined effect of age with the pretiter.
DISCUSSION
Previous research has proven that, in many cases, the immune response to the influenza vaccine is low among children. Although most of this research indicates the extent of the immune response, very few studies have delved into the predictive factors regarding this observation. In this study, we considered the response to the HI antibody, an immune correlate of protection against influenza infection induced after vaccination, along with the predictive factors thereof in young children up to 4 years of age.
Regarding seronegative children (pretiter of <1:10), a trend was noticed wherein the antibody titer rose postvaccination in line with age, but at the same time, regardless of the strain of vaccine, the immune response was low. A booster effect was obtained by vaccination twice, but the sP reached ≥70% only for the H1 antigen, which is one of the international criteria for approval (24, 25), following the second vaccination, and this was only achieved in the oldest children. These results were inconsistent with those of previous studies, which indicated that even young children can reach protective levels of antibody values if the vaccination is carried out twice (7–15). We think the reason that in this study, the vaccination dose used was smaller than the regulated volume used in Europe and the United States. The regulated IIV3 vaccination dose in Japan was lower than that used in Europe and the United States up to the 2010/2011 season (Japan: 0.1 ml for 0-year-olds, 0.2 ml for 1- to 5-year-olds, 0.3 ml for 6- to 12-year-olds, and 0.5 ml for 13-year-olds and older; Europe and the United States: 0.25 ml for children of 6 to 36 months of age and 0.5 ml for those older than 36 months). Although these dosage levels have been widely discussed for many years, they were maintained during the H1N1pdm09 pandemic. It was therefore assumed that if the current group were vaccinated twice with the high vaccine doses, even seronegative children with no antibody prior to vaccination would acquire an antibody titer that would cover them.
That said, seropositive children (pretiter of 1:10 to 1:20) demonstrated an excellent immune response, regardless of the strain of vaccine. In this group, minimum values of 85% sR and sP were achieved in children aged 1 year or older who were vaccinated with all strains with one dose alone. Children exposed to the influenza virus prior to vaccination, due either to an earlier vaccination or to becoming infected with influenza, who retained a certain level of antibodies have been demonstrated as being capable of achieving a sufficient antibody value with only a single vaccination, even at a young age. These results support the current recommendation that children aged between 6 months and 8 years who have completed a course of two vaccinations in the previous year require only one vaccination in the following season.
Children with a minimum pretiter of 1:40, however, regardless of the strain of vaccine, demonstrated a slightly lower sR than seropositive children, a tendency which was particularly noticeable in the older group (the 2- to 3-year-olds). Furthermore, no booster effect was apparent upon a second vaccination. A phenomenon in which the antibody titer reaches a certain level but then plateaus (negative feedback) has been reported, suggesting a risk that if the immune response is evaluated without stratification of the pretiter, the immunogenicity of the vaccine may be underestimated (26–28). The reason that this is noted more strongly in older children is that within the same pretiter of ≥1:40 category, older children had even higher pretiters.
In previous research, the immune response to IIV3, along with predictive factors such as the pretiter and age, was evaluated by multivariate analysis. Walter et al. used a multivariate logistic model adjusted for multiple factors and reported that in young children aged between 6 and 23 months, increasing age is a significant predictive factor of sP (20). Unfortunately, however, no adjustment using the pretiter was made. In this study, we adjusted for the pretiter and, furthermore, gave older age a significant association with immune response. While the OR of the pretiter adjusted for age differed from the crude OR, the OR of age adjusted for the pretiter gave values extremely close to those of the crude OR. This suggests that the effect of age is certain and that the OR of the pretiter is strongly affected by age. Furthermore, Neuzil et al. performed a multivariate analysis after adjusting for both factors, pretiter and age, and reported that the strongest predictive indicator for immune response after a single vaccination was pretiter and that age was not an independent predictive factor (21). The age range of subjects in their study, however, was between 5 and 8 years, which is older than the ages of the subjects in the present study. Comparing these results with the results of this study leads us to consider that even in young children less than 8 years of age, immune function develops up to the age of 4 to 5 years and that the effect of age on the immune response in less significant.
The prevaccination GMT for the H3 strain was far higher than those for the other strains, and the postvaccination GMT was also high as a result. This finding is believed to be related to the fact that the H3 strain was the predominant circulating strain during the past four seasons. These findings agree with the results of other studies conducted during the four seasons (17, 18). However, children with a pretiter of <1:10 accounted for 55% even in 2- to 3-year-olds who had a chance of prevaccination exposure, and these children may represent the subpopulation in which the titer does not increase well after exposure. In fact, sR and sP after the first and second vaccine administrations were lower in 2- to 3-year-old children with a pretiter of <1:10 against the H3 strain compared to those with a pretiter of <1:10 against the H1 or B strain (Fig. 2 and 3).
Some limitations associated with this study include the fact that the vaccination dose used was smaller than that regulated for the United States and Europe, along with the fact that the dose used for children between 6 months and 1 year was different from that used for children more than 1-year-old (0.1 ml and 0.2 ml, respectively). It is possible that the seronegative children (pretiter of <1:10) may have had a lower immune response even after the second vaccination as a result of this. Additionally, a simple comparison of the immune responses of children 6 months to 1 years of age with those of older children cannot be made. Despite this, however, within the seronegative group there was a clear difference in the responses between 1-year-olds and those aged 2 to 3 years old, who received the same dose of vaccine, confirming the effect of age. The group with a pretiter of ≥1:10 had almost no children aged between 6 months and 1 year within its distribution, while increases in antibodies in children aged 1 year and older similar to those noted in other studies were seen, thus leading us to believe that the impact of the low dosage is small. Second, with regard to covariate adjustment, given their correlation to age and the sample size, influenza vaccination history and ILI history were not included in the final multivariate analysis in order to construct a stable model. In fact, an adjusted analysis was carried out using both factors; however, since the overall trends remained consistent, we deemed that there was no significant impact on the results.
The viruses causing influenza epidemics differ, depending on the time and place, and as such, the vaccine strain also changes from season to season. In the same manner, the proportion of people possessing antibodies varies, depending on the time, place, and population within which the virus occurs. As a result, just as it is difficult to evaluate effectiveness, it is similarly difficult to evaluate efficacy with immune markers such as antibody titers as a substitute endpoint. Research on adult subjects by Hobson et al. (29) indicates that an HI antibody titer of 1:40 is determined to confer 50% protection against infection (protective level). However, among young children, an HI titer of 1:110 is suggested to be the threshold value for achieving 50% prevention, leading to other reports questioning whether or not a 50% protective effect is in fact sufficient in terms of public health policy (30). An immunological correlation is considered to have been established between the HI antibody titer and the protective effect of influenza; however, this threshold cannot be said to have been firmly established as of yet, particularly with regard to young children.
In summary, we demonstrated in the present study that the pretiter and age are mutually independent predictive factors of the immune response to IIV3 in young children less than 4 years of age. The immunogenicity of the vaccine was low in the young children without prevaccination antibodies and in young children generally. We therefore hope that future studies will evaluate the immune response of vaccine-naive young children to various types of vaccines, including high-dose vaccines other than the split virus type and adjuvant-added vaccines, to improve the immunogenicity of vaccines in this age group. Furthermore, in order to correctly evaluate the immunogenic potential of influenza vaccine in young children, not only the stratification of the HI value 1:40 but also a more detailed stratification, along with stratification according to age are considered important. Additionally, further considerations are required with regard to the HI antibody titer thresholds for immune correlates of vaccine-induced protection in children.
ACKNOWLEDGMENTS
Members of the Fukuoka Pediatricians Group for Vaccine Efficacy include Yohio Takasaki, Shizuo Shindo, Takashi Yokoyama, Yuji Yamashita, and Keigo Shibao.
We thank Hideki Koyanagi for his valuable contribution to the data collection.
This study was supported by a research grant from Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare, Japan (grant H17-SHIKO-010).
We declare no conflicts of interest.
Footnotes
Published ahead of print 2 July 2014
REFERENCES
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333–1340. 10.1001/jama.292.11.1333 [DOI] [PubMed] [Google Scholar]
- 2.Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K. 2000. Influenza and the rated of hospitalization for respiratory disease among infants and young children. N. Engl. J. Med. 342:232–239. 10.1056/NEJM200001273420402 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). 2013. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm. Rep. 62(RR-07):1–43 [PubMed] [Google Scholar]
- 4.Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. 2005. Effectiveness of the-2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics. 116:153–159. 10.1542/peds.2005-0049 [DOI] [PubMed] [Google Scholar]
- 5.Shuler CM, Iwamoto M, Bridges CB, Marin M, Neeman R, Garqiullo P, Keyserling HL, Terebuh PD. 2007. Vaccine effectiveness against medically attended, laboratory-confirmed influenza among children aged 6 to 59 months, 2003-2004. Pediatrics. 119:e587–e595. 10.1542/peds.2006-1878 [DOI] [PubMed] [Google Scholar]
- 6.Allison MA, Daley MF, Crane LA, Barrow J, Beaty BL, Allred N, Berman S, Kempe A. 2006. Influenza vaccine effectiveness in healthy 6- to 21-month-old children during the 2003-2004 season. J. Pediatr. 149:755–762. 10.1016/j.jpeds.2006.06.036 [DOI] [PubMed] [Google Scholar]
- 7.Skowronski DM, Hottes TS, Chong M, De Serres G, Scheifele DW, Ward BJ, Halperin SA, Janjua NZ, Chan T, Sabaiduc S, Petric M. 2011. Randomized controlled trial of dose response to influenza vaccine in children aged 6 to 23 months. Pediatrics. 128:e276–e289. 10.1542/peds.2010-2777 [DOI] [PubMed] [Google Scholar]
- 8.Englund JA, Walter EB, Gbadebo A, Monto AS, Zhu Y, Neuzil KM. 2006. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics. 118:e579–e585. 10.1542/peds.2006-0201 [DOI] [PubMed] [Google Scholar]
- 9.Englund JA, Walter EB, Fairchok MP, Monto AS, Neuzil KM. 2005. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics. 115:1039–1047. 10.1542/peds.2004-2373 [DOI] [PubMed] [Google Scholar]
- 10.Walter EB, Neuzil KM, Zhu Y, Fairchok MP, Gagliano ME, Monto AS, Englund JA. 2006. Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming? Pediatrics. 118:e570–e578. 10.1542/peds.2006-0198 [DOI] [PubMed] [Google Scholar]
- 11.Donatelli I, Zannolli R, Fuiano L, Biasio LR. 1998. Influenza vaccine in immunogenically naive healthy infants. Eur. J. Pediatr. 157:949–950. 10.1007/s004310050974 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell DK, Ruben FL, Gravenstein S. 2005. Immunogenicity and safety of inactivated influenza virus vaccine in young children in-2004. Pediatr. Infect. Dis. J. 24:925–927. 10.1097/01.inf.0000180978.66362.d9 [DOI] [PubMed] [Google Scholar]
- 13.Della Cioppa G, Vesilari T, Sokal E, Lindert K, Nicolay U. 2011. Trivalent and quadrivalent MF59-adjuvanted influenza vaccine in young children: a dose- and schedule-finding study. Vaccine 29:8696–8704. 10.1016/j.vaccine.2011.08.111 [DOI] [PubMed] [Google Scholar]
- 14.King JC, Jr, Cox MM, Reisinger K, Hedrick J, Graham I, Patriarca P. 2009. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6–59 months. Vaccine 27:6589–6594. 10.1016/j.vaccine.2009.08.032 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez M, Pirez MC, Ward E, Dibarboure H, García A, Picolet H. 2000. Safety and immunogenicity of a paediatric presentation of an influenza vaccine. Arch. Dis. Child. 83:488–491. 10.1136/adc.83.6.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuzil KM, Dupont WD, Wright PF, Edwards KM. 2001. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, to 1985-1990: the pediatric experience. Pediatr. Infect. Dis. J. 20:733–740. 10.1097/00006454-200108000-00004 [DOI] [PubMed] [Google Scholar]
- 17.Nolan T, Richmond PC, McVernon J, Skeljo MV, Hartel GF, Bennet J, Basser RL. 2009. Safety and immunogenicity of an inactivated thimerosal-free influenza vaccine in infants and children. Influenza Other Respir Viruses. 3:315–325. 10.1111/j.1750-2659.2009.00108.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt-Ott R, Schwarz T, Haase R, Sander H, Walther U, Fourneau M, Htun-Myint L, Sänger R, Schuster V. 2007. Immunogenicity and reactogenicity of a trivalent influenza split vaccine in previously unvaccinated children aged 6–9 and 10–13 years. Vaccine 26:32–40. 10.1016/j.vaccine.2007.10.049 [DOI] [PubMed] [Google Scholar]
- 19.Kumagai T, Nagai K, Okui T, Tsutsumi H, Nagata N, Yano S, Nakayama T, Okuno Y, Kamiya H. 2004. Poor immune responses to influenza vaccination in infants. Vaccine 22:3404–3410. 10.1016/j.vaccine.2004.02.030 [DOI] [PubMed] [Google Scholar]
- 20.Walter EB, Rajagopal S, Zhu Y, Neuzil KM, Fairchok MP, Englund JA. 2010. Trivalent inactivated influenza vaccine (TIV) immunogenicity in children 6 through 23 months of age: do children of all ages respond equally? Vaccine 28:4376–4383. 10.1016/j.vaccine.2010.04.058 [DOI] [PubMed] [Google Scholar]
- 21.Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, Dunn J, DeStefano F, Shay D. 2006. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8-year-old children. J. Infect. Dis. 194:1032–1039. 10.1086/507309 [DOI] [PubMed] [Google Scholar]
- 22.Irie S, Fujieda M, Ito K, Ishibashi M, Takamizawa T, Ishikawa T, Takasaki Y, Shindo S, Yokoyama T, Yamashita Y, Shibao K, Koyanagi H, Maeda A, Hirota Y. 2007. Immunogenicity of trivalent-inactivated influenza vaccine among children less than 4 years old. Kansenshogaku Zasshi. 81:284–290 (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. 2002. WHO manual on animal influenza diagnosis and surveillance. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/influenza/en/whocdscsrncs20025rev.pdf [Google Scholar]
- 24.European Committee for Proprietary Medicinal Products. 1997. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). European Agency for the Evaluation of Medicinal Products, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf [Google Scholar]
- 25.U.S. Department of Health and Human Services, Food and Drug Administration Center for Biologics Evaluation and Research. 2007. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. Office of Communication, Training and Manufacturers Assistance, Rockville, MD: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091985.pdf [Google Scholar]
- 26.Hirota Y, Kaji M, Ide S, Goto S, Oka T. 1996. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine 14:1597–1602. 10.1016/S0264-410X(96)00153-3 [DOI] [PubMed] [Google Scholar]
- 27.Chiu SS, Peiris JS, Chan KH, Wong WH, Lau YL. 2007. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics. 119:1076–1082. 10.1542/peds.2006-3176 [DOI] [PubMed] [Google Scholar]
- 28.Seidman JC, Richard SA, Viboud C, Miller MA. 2012. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses. 6:52–62. 10.1111/j.1750-2659.2011.00268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobson D, Curry RL, Beare AS, Ward-Gardner A. 1972. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond.) 70:767–777. 10.1017/S0022172400022610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Tsai T, Clemens R, Rappuoli R. 2011. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 30:1081–1085. 10.1097/INF.0b013e3182367662 [DOI] [PubMed] [Google Scholar]


