Abstract
In order to impede the increase in pertussis incidence in the adolescent group, a school-leaving booster dose administered at the age of 14 to 16 years will be introduced in Sweden in 2016. Preceding this introduction, an open-label, randomized, multicenter, clinical trial without a control group and with blinded analysis was performed, investigating both safety and immunogenicity. Reported here are the memory B-cell and serological responses detected in a smaller cohort (n = 34) of the 230 subjects recruited to the study. All subjects had received primary vaccination consisting of three doses of diphtheria–tetanus–5-component pertussis (DTaP5) vaccine, at 3, 5, and 12 months of age, and a tetanus–low-dose diphtheria–5-component pertussis (Tdap5) vaccine booster at 5.5 years. In this study, the subjects were randomly assigned and received either a Tdap1 or Tdap5 booster. Of the 230 participants, 34 subjects had samples available for evaluation of IgG-producing memory B-cell responses. Both vaccine groups had significant increases in pertussis toxin-specific serum IgG levels, but only the 1-component group showed significant increases in pertussis toxin-specific memory B cells. The 5-component group had significant increases in filamentous hemagglutinin- and pertactin-specific memory B-cell and serum IgG levels; these were not seen in the 1-component group, as expected. In conclusion, this study shows that a 5th consecutive dose of an acellular pertussis vaccine induces B-cell responses in vaccinated adolescents. (This study has been registered at EudraCT under registration no. 2008-008195-13 and at ClinicalTrials.gov under registration no. NCT00870350.)
INTRODUCTION
Pertussis, or whooping cough, is caused by the bacterium Bordetella pertussis. It is a highly contagious disease that affects all ages, but infants are most vulnerable to severe and fatal infections. Two types of pertussis vaccines are currently available, i.e., whole-cell pertussis (Pw) and acellular pertussis (Pa) vaccines. Both types are given in combination with diphtheria and tetanus (i.e., diphtheria–tetanus–whole-cell pertussis [DTwP] and diphtheria–tetanus–acellular pertussis [DTaP] vaccines). The DTaP vaccines are available with 1 to 5 pertussis components, including pertussis toxoid, filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae serotypes 2 and 3 (Fim2/3).
The history of pertussis vaccination in Sweden differs from those of other countries. Due to low vaccine efficacy and reports of severe side effects, Pw vaccination was discontinued in Sweden in 1979. During the 17-year hiatus that followed, pertussis incidence increased in the population (1). Following the development of Pa vaccines (2), two large clinical trials of safety and efficacy were performed in Sweden (3, 4). These trials led to the introduction of Pa vaccination into the Swedish Childhood Vaccination Program in 1996. DTaP vaccination at 3, 5, and 12 months resulted in an 80 to 90% decrease in pertussis incidence in Sweden (5). Today, a DTaP booster at 5 to 6 years is also included in the Swedish vaccination scheme.
Traditionally, antigen-specific serum antibody levels are used as markers for vaccine immunogenicity and to evaluate correlates of protection (6). However, no single serological correlate of protection, on an individual level, has been found for pertussis. More-complex correlations have been reported on a group level, and antibodies to PRN, Fim, and pertussis toxin (PT), either singly or synergistically, have been shown to correlate with protection (7–9). T-cell-mediated protection is important in defeating pertussis infection (10, 11), and B cells have been shown to contribute to protection against pertussis in mouse studies (12, 13). Studies have also shown that antigen-specific memory B cells can be present despite waning antibody levels for both pertussis (14) and other pathogens (15, 16), indicating that inclusion of memory B-cell evaluations would broaden the understanding of vaccine-induced immunity and protection.
Despite multiple vaccine doses during childhood, the incidence of pertussis is increasing in the adolescent population (17, 18). This has led to the evaluation of an adolescent booster in many countries (19–23). In Sweden, a school-leaving booster at 14 to 16 years of age will be introduced in 2016. This booster consists of tetanus and reduced doses of diphtheria and pertussis (i.e., tetanus–diphtheria–acellular pertussis [Tdap]). Preceding this introduction, a trial of the safety and immunogenicity of an adolescent booster was performed. The safety data and serological responses from the trial will be reported elsewhere (data not shown). The aims of this study were (i) to analyze the memory B-cell responses after a 5th dose of either a 1-component or a 5-component acellular pertussis vaccine in 34 subjects and (ii) to compare the memory B-cell responses to the serological responses to see if there were any differences in the responses or if any correlation could be found. We report on the antigen-specific memory B-cell responses to PT, FHA, and PRN before and after booster vaccination in 34 subjects included in this study. The Fim2/3-specific responses were not evaluated because of methodological limitations.
MATERIALS AND METHODS
Ethics.
This study (registered at EudraCT under registration no. 2008-008195-13 and at ClinicalTrials.gov under registration no. NCT00870350) was approved by the regional ethical review board in Stockholm, Sweden (ref 2008/2014-31). Written informed consent was obtained from the participants and their parents or legal guardians.
Subjects and samples.
A total of 230 children (14 to 15 years of age) were recruited into the study. The trial was an open-label, randomized, multicenter study without a control group and with blinded analysis. All subjects had received primary vaccination consisting of three doses of DTaP5 vaccine (Connaught HCP4DT, lots 003-11 and 003-31), at 3, 5, and 12 months of age, followed by a booster dose of Tdap5 (Triaxis; Sanofi Pasteur MSD) at the age of 5.5 years. The subjects were randomized into two vaccine groups, receiving one dose of either Tdap1 (diTekiBooster; Statens Serum Institute) or Tdap5 (the same vaccine as used for the 5.5-year booster). The antigen contents of the two vaccines can be found in Table 1.
TABLE 1.
Antigen contents of the two study vaccines
| Antigen | Antigen content (in 0.5 ml) |
|
|---|---|---|
| Tdap5 (Triaxis) | Tdap1 (diTekiBooster) | |
| Tetanus toxoid (Lf)a | 5 | 6.25 |
| Diphtheria toxoid (Lf) | 2 | 6.25 |
| Pertussis toxoid (μg) | 2.5 | 20 |
| Filamentous hemagglutinin (μg) | 5 | |
| Pertactin (μg) | 3 | |
| Fimbriae 2/3 (μg) | 5 | |
Lf, limit of flocculation.
At two study sites included in the trial (Linköping and Stockholm), the subjects were given the possibility of providing an additional blood sample for evaluation of memory B-cell responses. Thirty-four subjects (Linköping, n = 26; Stockholm, n = 8) volunteered for this, of whom 18 subjects were from the Tdap1 group and 16 subjects were from the Tdap5 group. Samples were collected before (day 0) and after (days 28 to 42) vaccination.
Pertussis-specific serum IgG levels (PT, FHA, and PRN) were measured for all subjects, as this was the primary analysis of immunogenicity. For memory B-cell responses, the antigen-specific responses were prioritized as follows: PT > PRN > FHA. All 34 subjects were tested for PT-specific memory B cells but, due to low cell availability, PRN-specific responses were evaluated for 22 subjects (11 from each group) and FHA-specific responses were evaluated for 16 subjects (8 from each group).
Following laboratory analysis, three subjects with high prevaccination pertussis-specific serum IgG levels were identified (two in the 1-component group and one in the 5-component group). The high prevaccination levels could be an indication of a recent pertussis infection; therefore, the three subjects were excluded from the group analysis. The numbers of subjects per vaccine group were therefore adjusted to 16 for the 1-component group and 15 for the 5-component group. A flow chart of the inclusion of subjects for the antigen-specific analysis of memory B cells is shown in Fig. 1.
FIG 1.
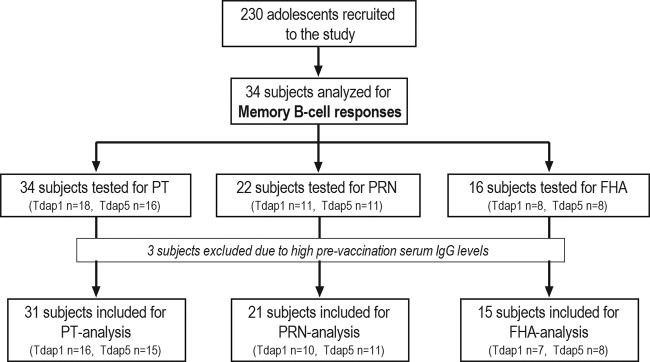
Flow chart of the subjects included in the antigen-specific memory B-cell ELISpot analysis.
Antigens.
For the memory B-cell enzyme-linked immunosorbent spot assay (ELISpot), PT (lot 042) and FHA (lot 039) were obtained from Kaketsuken (Japan). PRN (lot 180805 RS) was kindly provided by A. M. Buisman at the National Institute for Public Health and the Environment (RIVM) (the Netherlands). For the enzyme-linked immunosorbent assay (ELISA), PT (lot TOH 15) and FHA (lot TOH 15) were obtained from SmithKline Beecham (Rixensart, Belgium). PRN (SKA-QCDSCO4420) was obtained from Aventis Pasteur (Toronto, Canada).
Purification, cryopreservation, and thawing of PBMC.
Cells were sampled from two study sites using two slightly different protocols. For the Stockholm cohort (n = 8), peripheral blood mononuclear cells (PBMC) were purified from whole-blood samples collected in BD Vacutainer CPT tubes with sodium heparin (Becton, Dickinson, Franklin Lakes, NJ) and separated according to the manufacturer's instructions. Cryopreservation and thawing were performed as described previously (24), using freezing medium with 90% fetal calf serum (FCS) (Gibco Invitrogen, Paisley, United Kingdom) and 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO). For the Linköping cohort (n = 26), purification and cryopreservation were performed as described previously (25), using Ficoll (GE Healthcare, Uppsala, Sweden) and freezing medium with 10% DMSO (Sigma-Aldrich), 50% FCS, and 40% RPMI 1640 medium (both from Gibco Invitrogen). Thawing was performed as for the Stockholm cohort. The different protocols for purification and freezing of cells had no impact on cell viability following thawing.
IgG-specific memory B-cell ELISpot.
This method has previously been described in detail (26). In short, wells were coated with either 0.5 μg antigen/well or phosphate-buffered saline (PBS) (SVA, Uppsala, Sweden) for blank wells. Thawed PBMC were divided into two aliquots, one stimulated (1 μg/ml R848 plus 10 ng/ml interleukin 2 [IL-2]; Mabtech AB, Nacka Strand, Sweden) and one unstimulated. The cell concentration used was 2 × 106 PBMC/ml. Cells from the stimulated postvaccination time point were also added in 2-fold titrations, due to expected high numbers of antibody-secreting cells (ASC). Plates were analyzed with a CTL reader (Immunospot, Cleveland, OH). The lower cell concentration for the stimulated postvaccination samples was used only if the high concentration yielded too many spots to be counted. The plate data were processed as follows. The mean value of triplicates was enumerated as ASC/106 PMBC (an enumerated mean value of a triplicate is referred to as X). The number of antigen-specific memory B cells (nmemB) detected was calculated using the following formula: (Xstimulated − Xunstimulated) − Xblank = nmemB. The number of memory B cells should be seen as a relative number, however, since cell proliferation during stimulation is not accounted for. Subjects with ≥50 antigen-specific spots postvaccination and ≥100% increases in spot numbers in postvaccination samples versus prevaccination samples were considered to be vaccine responders. Total IgG was tested for all subjects and time points, as a positive control for the subjects. If no visible total IgG spots were detected, then the plate was retested. The IgG-producing cell line ARH77 (CRL-1621; LGC Standards) was included as a positive control for the assay. All plates with mean ARH77 triplicate values below 2 times the standard deviation were retested.
Serum IgG ELISA.
The serological responses of the 34 subjects with available memory B-cell samples were also included, for comparison. The serological method (ELISA) is described elsewhere (27). A positive antibody response was defined as (i) ≥4 times the minimum level of detection (MLD) in the postvaccination sample and (ii) ≥100% increase between the prevaccination sample and the postvaccination sample. The MLD values for the included antigens were 1 IU/ml for PT and FHA and 2 IU/ml for PRN.
Statistics.
All data were considered nonparametric. Comparisons between groups were performed with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test with the Dunn post hoc test. P values of <0.05 were considered statistically significant. Correlations were determined with Spearman's rank correlation coefficients.
RESULTS
Pertussis-specific IgG-producing memory B-cell responses after fifth dose of acellular pertussis vaccines.
The pertussis-specific IgG-producing memory B-cell responses before and after vaccination were evaluated in the two vaccine groups included in the study (Fig. 2 and Tables 2 and 3). The 1-component group had a significant increase (P < 0.05) in PT-specific memory B cells between the prevaccination and postvaccination measurements (Fig. 2A), with the median value increasing from 3 to 81 antigen-specific ASC/106 PBMC and with 11 of 16 subjects responding to the antigen. No responses to FHA or PRN were seen for the 1-component group (Fig. 2B and C and Table 2). Only one subject in the 5-component group responded with PT-specific memory B cells postvaccination (3 and 62 ASC/106 PBMC prevaccination and postvaccination, respectively). However, the 5-component group had significant increases in FHA- and PRN-specific memory B cells (P < 0.05). For FHA, the median value increased from 7 to 70 antigen-specific ASC/106 PBMC, and 5 of 8 subjects responded to the antigen. The median value for PRN-specific memory B cells increased from 3 to 232 antigen-specific ASC/106 PBMC, and 10 of 11 subjects responded to the antigen.
FIG 2.
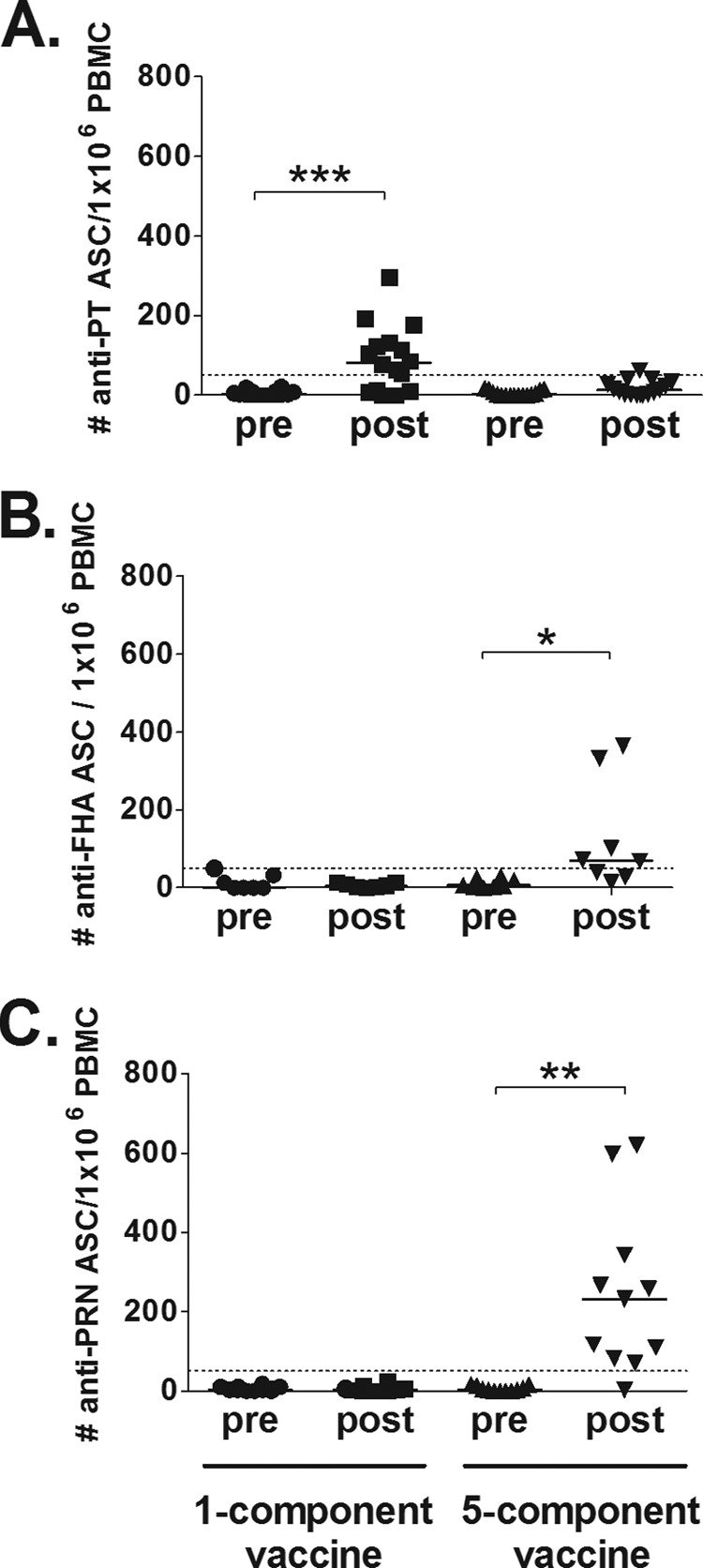
Pertussis-specific IgG-producing memory B-cell responses after a fifth dose of acellular pertussis vaccines. (A) A significant increase (P < 0.05, indicated by asterisks) in PT-specific memory B cells was detected in the 1-component group, with 11 of 16 subjects responding to the vaccination. No significant increase could be detected in the 5-component group. (B) FHA-specific memory B cells were significantly increased (P < 0.05) postvaccination in the 5-component group, with 5 of 8 subjects responding to the vaccination. No response was seen for the 1-component group. (C) Similar results were found for PRN-specific responses, with a significant increase (P < 0.05) in the 5-component group, in which 10 of 11 subjects responded. No response was detected in the 1-component group. Bars, median values; dotted lines, cutoff levels for positive vaccine responders.
TABLE 2.
Numbers of subjects responding to acellular pertussis booster with antigen-specific serum IgG and memory B cells
| Group and response | No. of responders/no. total for: |
||
|---|---|---|---|
| PT | FHA | PRN | |
| One-component group | |||
| Serum IgGa | 16/16 | 0/16 | 0/16 |
| Memory B cellsb | 11/16 | 0/7 | 0/10 |
| Five-component group | |||
| Serum IgGa | 14/15 | 15/15 | 15/15 |
| Memory B cellsb | 1/15 | 5/8 | 10/11 |
Responder criteria: ≥4 times the minimum level of detection and ≥100% increase between prevaccination and postvaccination samples.
Responder criteria: ≥50 antigen-specific cells and ≥100% increase between prevaccination and postvaccination samples.
TABLE 3.
Median values for antigen-specific serum IgG and memory B-cell responses in Pa-booster-treated adolescents
| Response, group, and time | PT |
FHA |
PRN |
|||
|---|---|---|---|---|---|---|
| n | Median (25th–75th percentiles) | n | Median (25th–75th percentiles) | n | Median (25th–75th percentiles) | |
| Serum IgG level (IU/ml) | ||||||
| 1-component group | 16 | 16 | 16 | |||
| Prevaccination | 2.0 (1.0–8.3) | 12.0 (3.8–28.8) | 35.4 (14.0–48.5) | |||
| Postvaccination | 80.5 (24.0–132.5) | 13.0 (3.3–25.3) | 30.3 (12.7–51.7) | |||
| 5-component group | 15 | 15 | 15 | |||
| Prevaccination | 2.0 (0.5–5.0) | 9.0 (5.0–21.0) | 33.4 (15.8–42.8) | |||
| Postvaccination | 18.0 (10.0–26.0) | 93.0 (62.0–136.0) | 437.9 (392.5–689.6) | |||
| Memory B-cell level (no. of ASC/106 PBMC) | ||||||
| 1-component group | 16 | 7 | 10 | |||
| Prevaccination | 3 (0–10) | 0 (0–32) | 3 (2–10) | |||
| Postvaccination | 81 (12–130) | 5 (2–15) | 3 (0–7) | |||
| 5-component group | 15 | 8 | 11 | |||
| Prevaccination | 2 (0–13) | 7 (2–25) | 3 (0–13) | |||
| Postvaccination | 14 (3–33) | 70 (31–275) | 232 (82–343) | |||
Serological pertussis-specific IgG responses after fifth dose of acellular pertussis vaccines.
All subjects (Tdap1, n = 16; Tdap5, n = 15) were tested for serological IgG responses to PT, FHA, and PRN prevaccination and postvaccination (Fig. 3 and Table 3). The 1-component group had significantly increased levels (P < 0.05) of PT-specific IgG postvaccination (80.5 IU/ml) versus prevaccination (2.0 IU/ml) (Fig. 3A), with all 16 subjects responding to the antigen. As expected, no increase in serum IgG levels was observed for FHA or PRN in the 1-component group (Fig. 3B and C). The 5-component group had significantly increased levels (P < 0.05) of antigen-specific serum IgG for all included antigens (Fig. 3). The PT response was lower than in the 1-component group (2.0 and 18.0 IU/ml prevaccination and postvaccination, respectively) (Fig. 3A), but 14 of 15 subjects responded to PT. All subjects in the 5-component group responded to FHA (9.0 and 93.0 IU/ml prevaccination and postvaccination, respectively) and PRN (33.4 and 437.9 IU/ml prevaccination and postvaccination, respectively), indicating broad responses to the vaccine.
FIG 3.
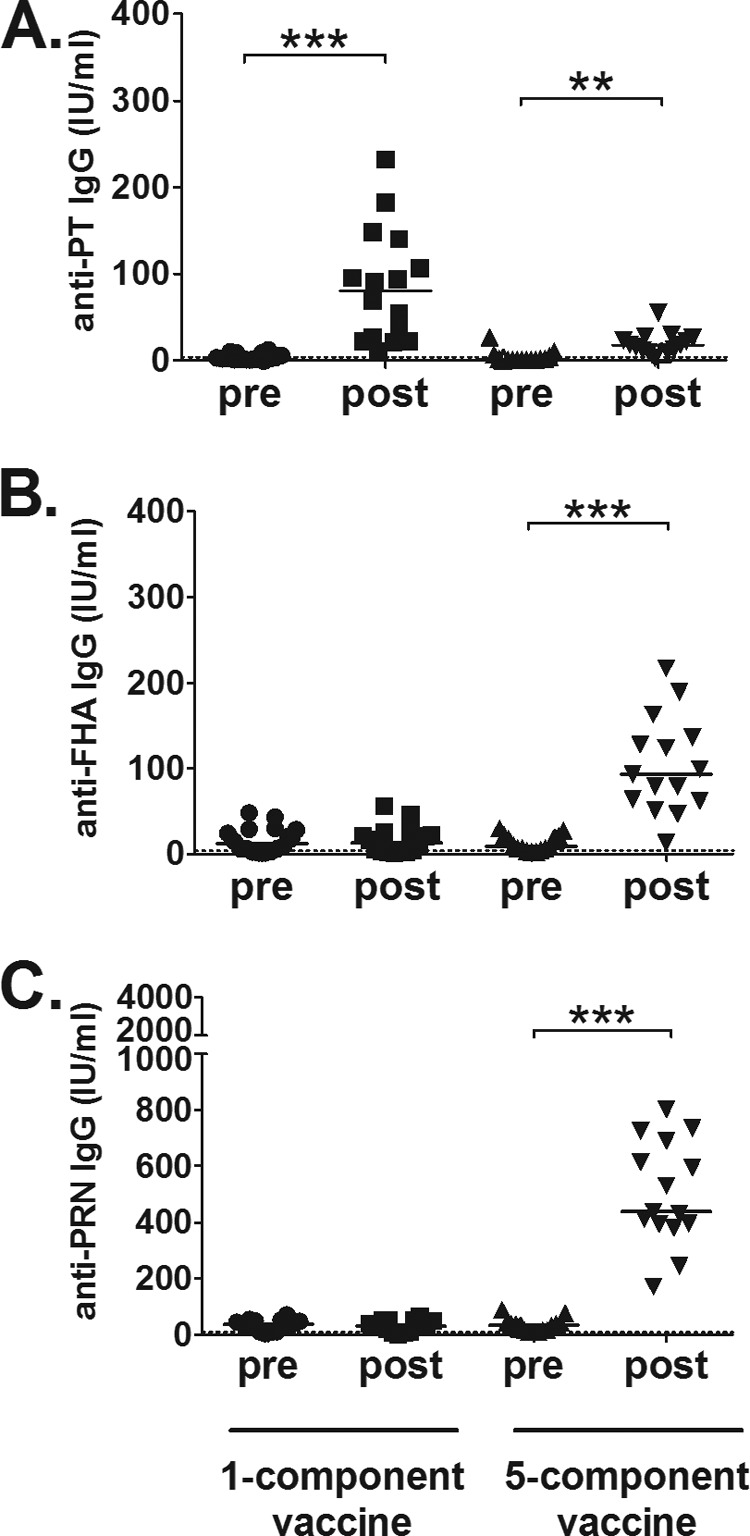
Serological pertussis-specific IgG responses after a fifth dose of acellular pertussis vaccines. (A) Significant increases (P < 0.05, indicated by asterisks) in PT-specific serum IgG levels were detected in both groups, albeit at higher levels in the 1-component group than in the 5-component group. All 16 subjects in the 1-component group and 14 of 15 subjects in the 5-component group responded. (B and C) The 5-component group had significant increases (P < 0.05) in the responses to FHA (B) and PRN (C), with 15 of 15 subjects responding to both antigens. The 1-component group did not mount any serological responses to FHA or PRN. Bars, median values; dotted lines, cutoff levels for positive vaccine responders.
Subjects with high prevaccination serum IgG levels.
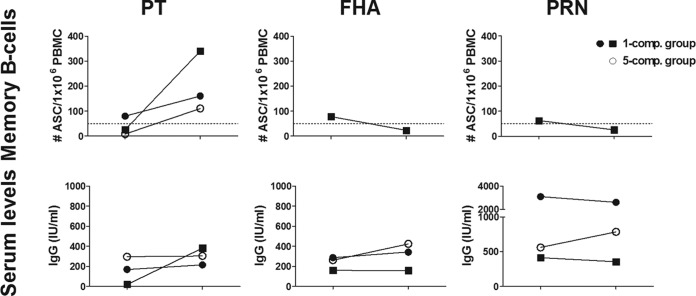
The three subjects with high prevaccination serum levels were analyzed separately for memory B-cell and serum IgG responses following vaccination. Two of the subjects had high antibody levels for all included antigens, and one subject had high levels for FHA and PRN (Fig. 4) and did not respond with serum IgG against these antigens following the booster. One subject from the 1-component group with low PT-specific prevaccination levels did respond with PT-specific serum IgG following the booster, however.
FIG 4.
Subjects with high prevaccination serum IgG levels. High pertussis-specific serum IgG levels were detected in three of the subjects. All subjects had high antibody levels against all antigens tested, except for one subject who did not have high PT-specific serum levels prevaccination but did respond to that antigen following booster vaccination. The high prevaccination levels were not detected in the peripheral memory B-cell responses. All three subjects responded with PT-specific memory B cells, whereas the only subject with samples available for assessment of FHA and PRN responses showed decreased levels postvaccination versus prevaccination. Dotted lines, cutoff levels for positive vaccine responders.
The high prevaccination levels were not seen in the memory B-cell responses detected in peripheral blood (Fig. 4), and all three subjects increased their levels of PT-specific memory B cells following the booster. Only one of the three subjects (1-component group) was tested against PRN and FHA, with declining levels of antigen-specific memory B cells postvaccination versus prevaccination.
Correlations between antigen-specific humoral and memory B-cell responses.
Correlations between serum and memory B-cell responses were evaluated for the postvaccination sample; PT was analyzed for both groups, and FHA and PRN were analyzed only for the 5-component group. The 1-component group showed significant correlation (Spearman r = 0.638, P = 0.001) between the PT-specific antibody levels and the memory B cell levels (Fig. 5). No correlation was found in the 5-component group for any of the antigens (PT, Spearman r = 0.354, P = 0.195; FHA, Spearman r = −0.024, P = 0.977; PRN, Spearman r = −0.100, P = 0.776).
FIG 5.
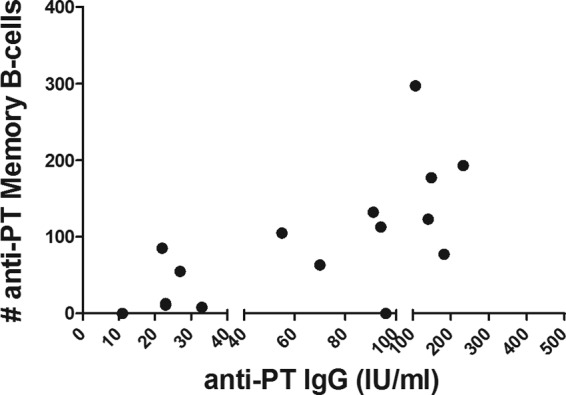
Correlation between antigen-specific humoral and memory B-cell responses in the 1-component group. A significant correlation between the IgG-specific humoral and memory B-cell responses was detected only for the pertussis toxin-specific responses in the 1-component group (Spearman r = 0.638, P = 0.001). The x axis was segmented for better view of the data for individual subjects.
DISCUSSION
Today there are no available vaccines offering long-lasting protection to pertussis. Therefore, booster doses are an important and efficient strategy to maintain pertussis-specific immunity in the population. Several studies have already shown the efficacy and safety of an adolescent Pa booster (23, 28–31). However, most of them did not include children entirely vaccinated with a 5-component Pa vaccine, as in this study. The 17-year hiatus in pertussis vaccination in Sweden is also expected to have influenced the immunity of the general population, making it not directly comparable to other countries. Therefore, we decided to perform an additional study among Swedish adolescents, preceding the introduction of the school-leaving booster dose in 2016.
Adolescent pertussis boosters have been shown to induce T-cell-mediated immunity (23, 32–34), but reports of B-cell immunity are scarce. However, Hendrikx et al. (35) studied the impact of a second Pa booster on memory B cells in 9-year-old children who had received four doses of Pw vaccine in their first year. They reported memory B-cell kinetics similar to those in this study, with increased levels of memory B cells at day 28 versus day 0.
Serum antibody levels are maintained by long-lived plasma blast cells resident in the bone marrow. The continuous secretion and circulation of pathogen-specific antibodies enable rapid neutralization of reinfecting pathogens. If a pathogen causes reinfection, then the memory B-cell pool is readily available for a recall response and rapidly differentiates, amplifying the antibody-mediated protection (36, 37). As reviewed by Yoshida et al. (37), the two B-cell responses most likely represent two forms of independently controlled immunological memory. This idea is supported by studies finding only low or moderate correlations between humoral and memory B-cell responses (38–40), as well as by mouse studies showing that serum antibody levels are not affected by depletion of the memory B-cell pool (41, 42). This study reports a correlation for the 1-component group for the PT-specific responses, but no correlation was detected for the 5-component group for any of the antigens. The low PT-specific memory B-cell response and the small number of subjects included in the FHA and PRN analyses could be a possible explanation for this. These responses are detected rather shortly after vaccination (4 to 6 weeks postvaccination), however, and thus more likely represent the acute versus the long-term relationship between the two B-cell responses (43). The Dutch booster study in 9-year-old children detected a correlation between memory B-cell levels 1 month postbooster and serum IgG levels 1 year postbooster (35), illustrating that the true relationship between the B-cell responses is not yet established.
One of the exclusion criteria in this study was previous clinical or bacteriological diagnosis of pertussis, as stated by the participants. Interestingly, three of the subjects did display pertussis-specific antibody levels indicative of a recent infection, demonstrating the unrecognized presence of pertussis in the adolescent population. The high prevaccination levels were not seen in the peripheral memory B-cell response and, following vaccination, all three subjects responded with PT-specific memory B cells. Only one of the three subjects was analyzed for FHA- and PRN-specific memory B cells and was shown to have decreased levels of antigen-specific memory B cells postvaccination versus prevaccination. This subject received the 1-component vaccine and was therefore not boosted against the antigens. Following vaccination, the serum IgG levels of the three subjects showed only minor fluctuations, except for one subject whose PT-specific IgG serum levels were low prevaccination but increased following the booster. Interestingly, both the pertussis memory B-cell levels and the serum IgG levels in the three subjects were higher than the group's median values. Although these comparisons are based on a very small number of subjects, this could support the idea that natural infection induces a stronger immune response than the two vaccines included in the study.
The rationale behind introducing an adolescent booster is to decrease pertussis incidence in that age group and also to impede transmission to infants who are not fully vaccinated. The benefits of an adolescent booster are under debate, however. As discussed by Hallander et al. (44), adolescents might benefit more from natural infection, which would induce long-lasting immunity, than from a booster vaccination. A natural infection during adolescence could provide immunity sustainable into the childbearing years and thus reduce the risk of parent-child transmission. Lavine et al. (18) have shown that the yearly peak of adolescent pertussis does not coincide with those for infant and adult groups, indicating a more-adult source of infant pertussis transmission. The effects of an adolescent booster on infant pertussis have been evaluated in two separate studies. One study showed that the overall pertussis incidence was not affected by an adolescent booster (45), whereas the other study indicated that the adolescent booster reduced the number of hospitalizations due to severe infant pertussis (46). This indicates that the impact an adolescent booster would have on infant pertussis is yet to be determined. However, further development of acellular pertussis vaccines, e.g., with additional antigens (47, 48) or adjuvants (49, 50), could lead to better efficacy of the adolescent booster and thus induction of sustainable protection into the childbearing years. Studies have shown that whole-cell pertussis vaccines seem to offer better protection than acellular vaccines (51, 52). The greater reactogenicity of whole-cell vaccines is a concern and must be reduced, however. A novel intranasal attenuated pertussis whole-cell vaccine, BPZE1, has shown promising results in a clinical study (53, 54) and, with optimization, could offer a nonreactogenic whole-cell vaccine in the future. Another benefit of this vaccine is that it is designed to mimic natural nonpathological infection, likely inducing immunity similar to that seen after natural infection.
In this study, two different acellular pertussis vaccines were evaluated, as an adolescent booster dose, for their immunogenicity with regard to antigen-specific memory B-cell and serum IgG levels. We could see that both vaccines were immunogenic but antigen contents and concentrations influenced the responses. The 1-component vaccine induced higher levels of PT-specific memory B cells than did the 5-component vaccine, which most likely is explained by the higher antigen concentration in the 1-component vaccine. The 5-component vaccine, on the other hand, produced broader responses, with increases in both FHA- and PRN-specific memory B cells. Similar profiles were seen for the serum IgG responses. Establishing the optimal antigen contents and concentrations that should be included in a booster dose is important, as we have shown here that these factors influence the magnitude of the vaccine response. However, the short follow-up time in this study is not sufficient to allow any conclusions regarding the optimal adolescent booster vaccine. This study does indicate, however, that the choice of vaccine is dependent on whether a strong or broad pertussis-specific response is sought. In conclusion, a 5th consecutive dose of a Pa vaccine has been shown to be immunogenic in Swedish adolescents and induces significant increases in pertussis-specific B-cell responses.
ACKNOWLEDGMENTS
We thank Kicki Helander and Kerstin von Segebaden for dedicated work in sample collection, Anne-Marie Fornander for skillful sampling and handling of cells, and all of the children participating in the study.
This work was supported by Statens Serum Institute and Sanofi Pasteur MSD (Sweden).
Footnotes
Published ahead of print 9 July 2014
REFERENCES
- 1.Nilsson L, von Segebaden K, Bergström J, Nuru N. 2013. Pertussis surveillance in Sweden: fifteen year report. Swedish Institute for Communicable Disease Control, Solna, Sweden [Google Scholar]
- 2.Sato Y, Kimura M, Fukumi H. 1984. Development of a pertussis component vaccine in Japan. Lancet 1(8369):122–126 [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 334:349–355. 10.1056/NEJM199602083340602 [DOI] [PubMed] [Google Scholar]
- 4.Olin P, Rasmussen F, Gustafsson L, Hallander HO, Heijbel H. 1997. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Lancet 350:1569–1577 [DOI] [PubMed] [Google Scholar]
- 5.Olin P, Gustafsson L, Barreto L, Hessel L, Mast TC, Rie AV, Bogaerts H, Storsaeter J. 2003. Declining pertussis incidence in Sweden following the introduction of acellular pertussis vaccine. Vaccine 21:2015–2021. 10.1016/S0264-410X(02)00777-6 [DOI] [PubMed] [Google Scholar]
- 6.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherry JD, Gornbein J, Heininger U, Stehr K. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901–1906. 10.1016/S0264-410X(98)00226-6 [DOI] [PubMed] [Google Scholar]
- 8.Storsaeter J, Hallander HO, Gustafsson L, Olin P. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907–1916. 10.1016/S0264-410X(98)00227-8 [DOI] [PubMed] [Google Scholar]
- 9.Storsaeter J, Hallander HO, Gustafsson L, Olin P. 2003. Low levels of antipertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine 21:3542–3549. 10.1016/S0264-410X(03)00407-9 [DOI] [PubMed] [Google Scholar]
- 10.Mascart F, Verscheure V, Malfroot A, Hainaut M, Pierard D, Temerman S, Peltier A, Debrie AS, Levy J, Del Giudice G, Locht C. 2003. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J. Immunol. 170:1504–1509. 10.4049/jimmunol.170.3.1504 [DOI] [PubMed] [Google Scholar]
- 11.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills KH. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 175:1246–1250. 10.1086/593682 [DOI] [PubMed] [Google Scholar]
- 12.Leef M, Elkins KL, Barbic J, Shahin RD. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841–1852. 10.1084/jem.191.11.1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahon BP, Brady MT, Mills KH. 2000. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J. Infect. Dis. 181:2087–2091. 10.1086/315527 [DOI] [PubMed] [Google Scholar]
- 14.Buisman AM, de Rond CG, Ozturk K, Ten Hulscher HI, van Binnendijk RS. 2009. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 28:179–186. 10.1016/j.vaccine.2009.09.102 [DOI] [PubMed] [Google Scholar]
- 15.Bauer T, Jilg W. 2006. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine 24:572–577. 10.1016/j.vaccine.2005.08.058 [DOI] [PubMed] [Google Scholar]
- 16.West DJ, Calandra GB. 1996. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine 14:1019–1027. 10.1016/0264-410X(96)00062-X [DOI] [PubMed] [Google Scholar]
- 17.Hallander H, Andersson M, Advani R, Brytting M, Lepp T, Ljungman M, Netterlid E, Norder H. 2007. Vaccinationsuppföljningen: seroepidemiologisk tvärsnittstudie 2007. Smittskyddsinstitutet, Solna, Sweden [Google Scholar]
- 18.Lavine J, Broutin H, Harvill ET, Bjornstad ON. 2010. Imperfect vaccine-induced immunity and whooping cough transmission to infants. Vaccine 29:11–16. 10.1016/j.vaccine.2010.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang S, O'Connor PM, Slade BA, Woo EJ. 2013. U.S. postlicensure safety surveillance for adolescent and adult tetanus, diphtheria and acellular pertussis vaccines: 2005–2007. Vaccine 31:1447–1452. 10.1016/j.vaccine.2012.10.097 [DOI] [PubMed] [Google Scholar]
- 20.Edelman K, He Q, Makinen J, Sahlberg A, Haanpera M, Schuerman L, Wolter J, Mertsola J. 2007. Immunity to pertussis 5 years after booster immunization during adolescence. Clin. Infect. Dis. 44:1271–1277. 10.1086/514338 [DOI] [PubMed] [Google Scholar]
- 21.Hara M, Okada K, Yamaguchi Y, Uno S, Otsuka Y, Shimanoe C, Nanri H, Horita M, Ozaki I, Nishida Y, Tanaka K. 2013. Immunogenicity and safety after booster vaccination of diphtheria, tetanus, and acellular pertussis in young adults: an open randomized controlled trial in Japan. Clin. Vaccine Immunol. 20:1799–1804. 10.1128/CVI.00490-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prelog M, Almanzar G, Rieber N, Ottensmeier B, Zlamy M, Liese J. 2013. Differences of IgG antibody avidity after an acellular pertussis (aP) booster in adolescents after a whole cell (wcP) or aP primary vaccination. Vaccine 31:387–393. 10.1016/j.vaccine.2012.10.105 [DOI] [PubMed] [Google Scholar]
- 23.Rieber N, Graf A, Belohradsky BH, Hartl D, Urschel S, Riffelmann M, Wirsing von Konig CH, Liese J. 2008. Differences of humoral and cellular immune response to an acellular pertussis booster in adolescents with a whole cell or acellular primary vaccination. Vaccine 26:6929–6935. 10.1016/j.vaccine.2008.09.064 [DOI] [PubMed] [Google Scholar]
- 24.Nilsson C, Aboud S, Karlen K, Hejdeman B, Urassa W, Biberfeld G. 2008. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin. Vaccine Immunol. 15:585–589. 10.1128/CVI.00161-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenmalm MC, Bjorksten B, Macaubas C, Holt BJ, Smallacombe TB, Holt PG. 1999. Allergen-induced cytokine secretion in relation to atopic symptoms and immunoglobulin E and immunoglobulin G subclass antibody responses. Pediatr. Allergy Immunol. 10:168–177. 10.1034/j.1399-3038.1999.00016.x [DOI] [PubMed] [Google Scholar]
- 26.Jahnmatz M, Kesa G, Netterlid E, Buisman AM, Thorstensson R, Ahlborg N. 2013. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J. Immunol. Methods 391:50–59. 10.1016/j.jim.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Reizenstein E, Hallander HO, Blackwelder WC, Kuhn I, Ljungman M, Mollby R. 1995. Comparison of five calculation modes for antibody ELISA procedures using pertussis serology as a model. J. Immunol. Methods 183:279–290. 10.1016/0022-1759(95)00067-K [DOI] [PubMed] [Google Scholar]
- 28.Halperin SA, Scheifele D, Mills E, Guasparini R, Humphreys G, Barreto L, Smith B. 2003. Nature, evolution, and appraisal of adverse events and antibody response associated with the fifth consecutive dose of a five-component acellular pertussis-based combination vaccine. Vaccine 21:2298–2306. 10.1016/S0264-410X(03)00173-7 [DOI] [PubMed] [Google Scholar]
- 29.Pichichero ME, Casey JR, Francis AB, Marsocci SM, Murphy M, Hoeger W, Cleary C. 2006. Acellular pertussis vaccine boosters combined with diphtheria and tetanus toxoid boosters for adolescents: safety and immunogenicity assessment when preceded by different 5-dose DTaP/DTwP schedules. Clin. Pediatr. (Phila.) 45:613–620. 10.1177/0009922806289593 [DOI] [PubMed] [Google Scholar]
- 30.Tran Minh NN, He Q, Ramalho A, Kaufhold A, Viljanen MK, Arvilommi H, Mertsola J. 1999. Acellular vaccines containing reduced quantities of pertussis antigens as a booster in adolescents. Pediatrics 104:e70. 10.1542/peds.104.6.e70 [DOI] [PubMed] [Google Scholar]
- 31.Zepp F, Habermehl P, Knuf M, Mannhardt-Laakman W, Howe B, Friedland LR. 2007. Immunogenicity of reduced antigen content tetanus-diphtheria-acellular pertussis vaccine in adolescents as a sixth consecutive dose of acellular pertussis-containing vaccine. Vaccine 25:5248–5252. 10.1016/j.vaccine.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 32.Cassone A, Ausiello CM, Urbani F, Lande R, Giuliano M, La Sala A, Piscitelli A, Salmaso S. 1997. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. Arch. Pediatr. Adolesc. Med. 151:283–289. 10.1001/archpedi.1997.02170400069013 [DOI] [PubMed] [Google Scholar]
- 33.Guiso N, Njamkepo E, Vie le Sage F, Zepp F, Meyer CU, Abitbol V, Clyti N, Chevallier S. 2007. Long-term humoral and cell-mediated immunity after acellular pertussis vaccination compares favourably with whole-cell vaccines 6 years after booster vaccination in the second year of life. Vaccine 25:1390–1397. 10.1016/j.vaccine.2006.10.048 [DOI] [PubMed] [Google Scholar]
- 34.Meyer CU, Zepp F, Decker M, Lee M, Chang SJ, Ward J, Yoder S, Bogaert H, Edwards KM. 2007. Cellular immunity in adolescents and adults following acellular pertussis vaccine administration. Clin. Vaccine Immunol. 14:288–292. 10.1128/CVI.00364-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendrikx LH, Felderhof MK, Ozturk K, de Rond LG, van Houten MA, Sanders EA, Berbers GA, Buisman AM. 2011. Enhanced memory B-cell immune responses after a second acellular pertussis booster vaccination in children 9 years of age. Vaccine 30:51–58. 10.1016/j.vaccine.2011.10.048 [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. 2010. From vaccines to memory and back. Immunity 33:451–463. 10.1016/j.immuni.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida T, Mei H, Dorner T, Hiepe F, Radbruch A, Fillatreau S, Hoyer BF. 2010. Memory B and memory plasma cells. Immunol. Rev. 237:117–139. 10.1111/j.1600-065X.2010.00938.x [DOI] [PubMed] [Google Scholar]
- 38.Amanna IJ, Carlson NE, Slifka MK. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903–1915. 10.1056/NEJMoa066092 [DOI] [PubMed] [Google Scholar]
- 39.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969–4973. 10.4049/jimmunol.171.10.4969 [DOI] [PubMed] [Google Scholar]
- 40.Leyendeckers H, Odendahl M, Lohndorf A, Irsch J, Spangfort M, Miltenyi S, Hunzelmann N, Assenmacher M, Radbruch A, Schmitz J. 1999. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur. J. Immunol. 29:1406–1417. [DOI] [PubMed] [Google Scholar]
- 41.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. 2008. Maintenance of the plasma cell pool is independent of memory B cells. Proc. Natl. Acad. Sci. U. S. A. 105:4802–4807. 10.1073/pnas.0800555105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. 2008. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol. 180:361–371. 10.4049/jimmunol.180.1.361 [DOI] [PubMed] [Google Scholar]
- 43.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. 2009. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood 114:4998–5002. 10.1182/blood-2009-03-211052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hallander HO, Nilsson L, Gustafsson L. 2011. Is adolescent pertussis vaccination preferable to natural booster infections? Expert Rev. Clin. Pharmacol. 4:705–711. 10.1586/ecp.11.55 [DOI] [PubMed] [Google Scholar]
- 45.Skoff TH, Cohn AC, Clark TA, Messonnier NE, Martin SW. 2012. Early impact of the US Tdap vaccination program on pertussis trends. Arch. Pediatr. Adolesc. Med. 166:344–349. 10.1001/archpediatrics.2011.1093 [DOI] [PubMed] [Google Scholar]
- 46.Auger KA, Patrick SW, Davis MM. 2013. Infant hospitalizations for pertussis before and after Tdap recommendations for adolescents. Pediatrics 132:e1149–e1155. 10.1542/peds.2013-1747 [DOI] [PubMed] [Google Scholar]
- 47.Cherry JD, Heininger U, Richards DM, Storsaeter J, Gustafsson L, Ljungman M, Hallander HO. 2010. Antibody response patterns to Bordetella pertussis antigens in vaccinated (primed) and unvaccinated (unprimed) young children with pertussis. Clin. Vaccine Immunol. 17:741–747. 10.1128/CVI.00469-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung GY, Xing D, Prior S, Corbel MJ, Parton R, Coote JG. 2006. Effect of different forms of adenylate cyclase toxin of Bordetella pertussis on protection afforded by an acellular pertussis vaccine in a murine model. Infect. Immun. 74:6797–6805. 10.1128/IAI.01104-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gracia A, Polewicz M, Halperin SA, Hancock RE, Potter AA, Babiuk LA, Gerdts V. 2011. Antibody responses in adult and neonatal BALB/c mice to immunization with novel Bordetella pertussis vaccine formulations. Vaccine 29:1595–1604. 10.1016/j.vaccine.2010.12.083 [DOI] [PubMed] [Google Scholar]
- 50.Sugai T, Mori M, Nakazawa M, Ichino M, Naruto T, Kobayashi N, Kobayashi Y, Minami M, Yokota S. 2005. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine. Vaccine 23:5450–5456. 10.1016/j.vaccine.2004.09.041 [DOI] [PubMed] [Google Scholar]
- 51.Sheridan SL, Ware RS, Grimwood K, Lambert SB. 2012. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308:454–456. 10.1001/jama.2012.6364 [DOI] [PubMed] [Google Scholar]
- 52.Witt MA, Katz PH, Witt DJ. 2012. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin. Infect. Dis. 54:1730–1735. 10.1093/cid/cis287 [DOI] [PubMed] [Google Scholar]
- 53.Mielcarek N, Debrie AS, Raze D, Bertout J, Rouanet C, Younes AB, Creusy C, Engle J, Goldman WE, Locht C. 2006. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2:e65. 10.1371/journal.ppat.0020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorstensson R, Trollfors B, Al-Tawil N, Jahnmatz M, Bergstrom J, Ljungman M, Torner A, Wehlin L, Van Broekhoven A, Bosman F, Debrie AS, Mielcarek N, Locht C. 2014. A phase I clinical study of a live attenuated Bordetella pertussis vaccine — BPZE1: a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One 9:e83449. 10.1371/journal.pone.0083449 [DOI] [PMC free article] [PubMed] [Google Scholar]