Abstract
Ninety-one serum samples from 51 hematology patients with bacteremia infections were tested for (1,3)-β-d-glucan (BG). Eleven samples (15%) from 7 patients (14%) were positive for BG. Of these 7 patients with positive BG results, 4 (8%) had invasive aspergillosis and 3 (6%) had no invasive fungal disease. Bacteremia was an unlikely cause of the false-positive BG results.
TEXT
A major cell wall component of various clinically important fungi is (1,3)-β-d-glucan (BG). Serum levels of BG are included in the updated European Organization for the Research and Treatment of Cancer-Mycoses Study Group (EORTC/MSG) diagnostic criteria for invasive fungal disease (IFD) in immunocompromised patients (1). One of the limitations reported for the BG assay is the high number of potential causes of false-positive results, including bacterial bloodstream infections (BSIs), particularly those due to Pseudomonas aeruginosa, Streptococcus pneumoniae, or Alcaligenes faecalis (2–5). However, existing data on the frequency of false-positive BG results during BSI with the aforementioned bacteria are inconsistent (6–12). While older studies reported up to 66% (10/15) of patients with a BSI due to Gram-positive cocci having more than one sample positive for BG, a more recent analysis found only one positive BG result among 70 patients with BSI and no IFD (7, 12). The aim of this study was to investigate the rate of BG positivity in patients with hematological malignancies and BSI in whom the BG assay was used for IFD screening twice per week.
All the patients who were admitted to the hematology units of San Martino University Hospital and who developed a BSI between January 2011 and December 2013 were retrospectively identified. In cases of common skin contaminants, two consecutive positive blood cultures were required to diagnose a BSI. Patients who had at least one BG measurement within 48 h after a positive blood culture were included in the study. The data on the concomitant IFD (diagnosed according to 2008 EORTC/MSG criteria), amoxicillin treatment, surgery, and immunoglobulin administration were recorded. BG testing was performed using the Fungitell assay (Associates of Cape Cod, Inc., Cape Cod, MA) according to the manufacturer's instructions. A BG value of >80 pg/ml was considered positive.
Overall, 66 BSIs in 51 patients were included in the study (due to P. aeruginosa [26], Escherichia coli [12], coagulase-negative Staphylococcus spp. [8], viridans group Streptococcus spp. [5], Enterococcus spp. [4], Enterobacter cloacae [3], Klebsiella pneumoniae [3], Acinetobacter spp. [2], Klebsiella oxytoca [1], Serratia odorifera [1], or Staphylococcus aureus [1]).
A total of 91 serum samples were analyzed for BG (median number of samples per patient, 1 [range, 1 to 4]). The median time between the blood draws for culture and for the BG assay was 24 h. The BG measurement was performed at the same time as the blood culture in 27 BSI episodes, within 24 h after BSI in 30 episodes, and between 24 and 48 h in 9 episodes. In 21 BSI episodes, 2 consecutive samples were tested.
BG results were positive in 11 samples (12%) from 7 patients (14%). Among these, 3 patients (6%) were diagnosed with probable invasive aspergillosis (IA) and 1 patient (2%) with possible IA. The median value of BG in these patients was 214 pg/ml. The other 3 patients (6%) showed no evidence of IFD, including having no pneumocystosis and receiving no surgery or amoxicillin or immunoglobulin therapy, and the median BG value was 146 pg/ml. In patients with BSI and no IFD, the rate of BG positivity was 6% (3/47) in the per-patient analysis and 4% (3/79) in the per-sample analysis.
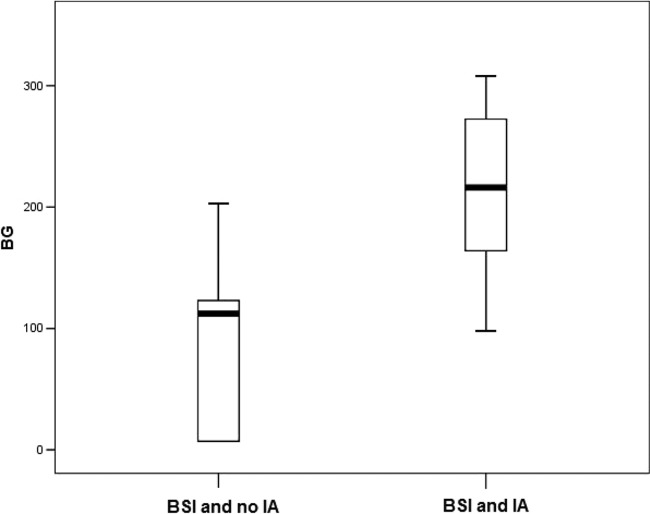
Considering 13 samples from patients with a positive BG result, the median value was lower in patients with BSI than in those with BSI and IA (112 pg/ml [range, 7 to 203 pg/ml] versus 216 pg/ml [range, 98 to 308 pg/ml], respectively; P = 0.04 [Mann-Whitney two-sided test]) (Fig. 1).
FIG 1.
Box plot comparison between BG values in patients with bloodstream infection (BSI) and invasive aspergillosis (IA) and in those with BSI and no IA. The thick bars represent the median values, and the lines extended below and above the boxes represent the lowest and highest values, respectively.
With 2 consecutive positive samples regarded as indicating BG positivity, only 4 patients, all with IA, were categorized as positive. None of the patients with BSI were positive for BG, although 1 patient with 1 sample produced an indeterminate result.
This study reported a low rate (4%) of BG positivity in patients with BSI and no IFD. All the patients with BSI and IFD had two consecutive positive BG results. In contrast, those patients who had BSI without IFD and positive BG results had only one positive result. However, multiple samples were tested for 2 out of 3 patients with BSI without IFD.
Contrary to older reports, no bacterial BSI in our study was consistently associated with serum BG positivity. Only 1 of 18 (5.5%) patients with BSI caused by Gram-positive cocci and 1 of 12 (8%) patients with BSI caused by E. coli were found to be BG positive in our cohort, compared to 66% and 38%, respectively, in other studies (7, 9). Note that none of the 23 patients with P. aeruginosa BSI without IFD had a positive BG result. In a more recent study, Metan et al. reported a positivity rate of 2% (1 patient with E. coli BSI among 58 other subjects with various pathogens but no IFD) (12), while in the study by Racil et al., 2 out of 26 patients with bacterial BSI were positive for BG, but these 2 patients had an IFD (11). Therefore, our results confirm the more recent findings of a low rate of BG positivity in the absence of IFD. The retrospective nature of our study did not allow for the testing of supernatants of bacterial colonies for BG in order to confirm or reject the hypothesized bacterial origin of the BG found.
Additionally, the kinetics of BG differed in patients with IFD and BSI compared to those with BSI and no IFD. Patients with IFD and BSI had two consecutive serum samples positive for BG, while those with BSI and no IFD had only one positive BG result, although in 2 out of 3 patients two consecutive serum samples were tested. There were no means to determine if a single BG-positive result in these patients was due to the BSI or to any other cause, including environmental contamination of a tested sample, but abrupt rises and falls of BG levels have been associated with false positivity (13, 14). In fact, a recent meta-analysis reported that specificity and positive predictive value were improved when two consecutive positive samples were used to defined a positive BG result (15). Finally, in this cohort, the median BG value was significantly lower in patients with BSI and no IFD than in those with BSI and IA (146 versus 216 pg/ml). There are data to indicate that the optimal cutoff for BG positivity may be >80 pg/ml, as increased specificity and positive predictive value were observed for a value of 120 pg/ml in one study, and values of >160 pg/ml were considered highly indicative of candidemia in another study (14–17).
In conclusion, false-positive BG results are infrequent in patients with BSI and no IFD but could not be excluded. In these patients, BG positivity was not confirmed by the second blood sample and the BG value was lower in them than in those with a BSI and a fungal infection.
Footnotes
Published ahead of print 2 July 2014
REFERENCES
- 1.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawazu M, Kanda Y, Nannya Y, Aoki K, Kurokawa M, Chiba S, Motokura T, Hirai H, Ogawa S. 2004. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-β-d-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J. Clin. Microbiol. 42:2733–2741. 10.1128/JCM.42.6.2733-2741.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199–205. 10.1086/421944 [DOI] [PubMed] [Google Scholar]
- 4.Mennink-Kersten MA, Ruegebrink D, Verweij PE. 2008. Pseudomonas aeruginosa as a cause of 1,3-beta-d-glucan assay reactivity. Clin. Infect. Dis. 46:1930–1931. 10.1086/588563 [DOI] [PubMed] [Google Scholar]
- 5.Stone BA, Clarke AE. 1992. Chemistry and biology of (1→3)beta-d-glucans. La Trobe University Press, Melbourne, Australia [Google Scholar]
- 6.Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D. 2003. Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin. Diagn. Lab. Immunol. 10:882–885. 10.1128/CDLI.10.5.882-885.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. 2005. Evaluation of a (1–3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957–5962. 10.1128/JCM.43.12.5957-5962.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Racil Z, Kocmanova I, Lengerova M, Weinbergerova B, Buresova Toskova M, Winterova J, Timilsina S, Rodriguez I, Mayer J. 2010. Difficulties in using 1–3-{beta}-d-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies—high frequency of false-positive results and their analysis. J. Med. Microbiol. 59:1016–1022. 10.1099/jmm.0.019299-0 [DOI] [PubMed] [Google Scholar]
- 9.Albert O, Toubas D, Strady C, Cousson J, Delmas C, Vernet V, Villena I. 2011. Reactivity of (1→3)-β-d-glucan assay in bacterial bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 30:1453–1460. 10.1007/s10096-011-1244-8 [DOI] [PubMed] [Google Scholar]
- 10.Koya J, Nannya Y, Kobayashi H, Okugawa S, Moriya K, Kurokawa M. 2013. Simultaneous increase in 1,3-β-d-glucan and procalcitonin levels in Pseudomonas aeruginosa infection. J. Infect. 67:164–166. 10.1016/j.jinf.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 11.Racil Z, Kocmanova I, Toskova M, Winterova J, Lengerova M, Timilsina S, Mayer J. 2013. Reactivity of the 1,3-β-d-glucan assay during bacteraemia: limited evidence from a prospective study. Mycoses 56:101–104. 10.1111/j.1439-0507.2012.02210.x [DOI] [PubMed] [Google Scholar]
- 12.Metan G, Koc AN, Ağkuş Ç Kaynar LG, Alp E, Eser B. 2012. Can bacteraemia lead to false positive results in 1,3-beta-d-glucan test? Analysis of 83 bacteraemia episodes in high-risk patients for invasive fungal infections. Rev. Iberoam. Micol. 29:169–171. 10.1016/j.riam.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 13.Kelaher A. 2006. Two non-invasive diagnostic tools for invasive aspergilosis: (1–3)-beta-d-glucan and the galactomannan assay. Clin. Lab. Sci. 19:222–224 [PubMed] [Google Scholar]
- 14.Pazos C, Ponton J, Del Palacio A. 2005. Contribution of (1–3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 43:299–305. 10.1128/JCM.43.1.299-305.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O, Third European Conference on Infections in Leukemia (ECIL-3) 2012. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin. Infect. Dis. 54:633–643. 10.1093/cid/cir897 [DOI] [PubMed] [Google Scholar]
- 16.Ellis M, Al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, Klingspor L. 2008. Assessment of the clinical utility of serial beta-d-glucan concentrations in patients with persistent neutropenic fever. J. Med. Microbiol. 57:287–295. 10.1099/jmm.0.47479-0 [DOI] [PubMed] [Google Scholar]
- 17.Del Bono V, Delfino E, Furfaro E, Mikulska M, Nicco E, Bruzzi P, Mularoni A, Bassetti M, Viscoli C. 2011. Clinical performance of the (1,3)-β-d-glucan assay in early diagnosis of nosocomial Candida bloodstream infections. Clin. Vaccine Immunol. 18:2113–2117. 10.1128/CVI.05408-11 [DOI] [PMC free article] [PubMed] [Google Scholar]