Abstract
Salmonella enterica serovar Gallinarum is the etiological agent of fowl typhoid, which constitutes a considerable economic problem for poultry growers in developing countries. The vaccination of chickens seems to be the most effective strategy to control the disease in those areas. We constructed S. Gallinarum strains with a deletion of the global regulatory gene fur and evaluated their virulence and protective efficacy in Rhode Island Red chicks and Brown Leghorn layers. The fur deletion mutant was avirulent and, when delivered orally to chicks, elicited excellent protection against lethal S. Gallinarum challenge. It was not as effective when given orally to older birds, although it was highly immunogenic when delivered by intramuscular injection. We also examined the effect of a pmi mutant and a combination of fur deletions with mutations in the pmi and rfaH genes, which affect O-antigen synthesis, and ansB, whose product inhibits host T-cell responses. The S. Gallinarum Δpmi mutant was only partially attenuated, and the ΔansB mutant was fully virulent. The Δfur Δpmi and Δfur ΔansB double mutants were attenuated but not protective when delivered orally to the chicks. However, a Δpmi Δfur strain was highly immunogenic when administered intramuscularly. All together, our results show that the fur gene is essential for the virulence of S. Gallinarum, and the fur mutant is effective as a live recombinant vaccine against fowl typhoid.
INTRODUCTION
Salmonella enterica serovar Gallinarum biovar Gallinarum is a host-adapted pathogen that causes fowl typhoid, an important disease of poultry (1). Fowl typhoid is a septicemic disease that typically has a short course and significant morbidity and mortality, which can reach as high as 100% (2). The disease occurs primarily in mature flocks, although birds of all ages may be infected. Resistance to S. Gallinarum also varies with the species and breed. Among chickens, heavier breeds, such as Rhode Island Red, are more susceptible than lighter breeds, such as White Leghorns (1). Fowl typhoid has been eradicated from commercial poultry in many developed countries, including the United States and Canada, through the isolation and removal of contaminated flocks and the implementation of biosecurity and hygiene management (3). Nevertheless, it still constitutes a considerable economic problem for poultry growers, both small backyard farmers and larger commercial operations in many parts of the world, such as Central and South America, Africa, and Asia, where control measures are insufficient and the climate favors the spread of S. Gallinarum in the environment (1, 2).
The vaccination of chickens seems to be the most effective strategy to control fowl typhoid in developing countries where S. Gallinarum is endemic. The rough S. Gallinarum 9R strain is the most widely used vaccine. While somewhat effective, a number of drawbacks have been noted: its variability in protective efficacy between breeds, its persistence in immunized chickens leading to transmission through eggs, and the residual virulence in some breeds (4, 5). Moreover, the means of attenuation is not well defined genetically. Until recently, the attenuation of this strain was believed to be due solely to a defect in lipopolysaccharide (LPS) synthesis (5). However, a recent comparative analysis of its proteome and transcriptome showed that 9R may also be impaired in the regulation of several virulence factors (6).
In our efforts to develop safe and efficacious fowl typhoid vaccine candidates, we have been examining mutations in global virulence regulators and genes that affect O-antigen synthesis, with an emphasis on the genes required for virulence in S. enterica serovar Typhimurium (7). For example, the modification or deletion of the global regulator gene crp in S. Gallinarum results in a strain that is safe and efficacious against challenge with virulent S. Gallinarum (7, 8). Conversely, mutations in rfc (wzy), required for complete O-antigen synthesis, are attenuating in S. Typhimurium (9, 10) but have no effect on the virulence of S. Gallinarum when delivered by the oral route (7). In addition, an arabinose-regulated rfaH construction that results in arabinose-regulated O-antigen synthesis was partially attenuating in S. Typhimurium (11) but was not attenuating in S. Gallinarum (7). Based on these results, we decided to expand our approach and explore additional genes involved in global regulation or O-antigen synthesis.
The ferric uptake regulator (Fur) protein acts as a repressor of many genes whose products are involved in iron, zinc, and manganese acquisition and uptake (12, 13). One notable class of Fur-regulated proteins is the iron-regulated outer membrane proteins (IROMPs), which serve as receptors for iron-siderophore complexes. The genes for these proteins are repressed by Fur when iron is abundant and are upregulated when iron is limiting (12). Animal hosts restrict iron from invading bacteria during infection, a phenomenon known as nutritional immunity (14). Thus, the mechanisms for iron acquisition are crucial in the pathogenicity of many microorganisms, including Salmonella spp. Fur can also act as a transcriptional activator by enhancing RNA polymerase recruitment, regulating the production of small RNAs, or functioning as an antirepressor (12). In Salmonella, Fur also modulates the expression of genes involved in acid shock, adaptation (15, 16), and oxidative stress resistance (17, 18), and it plays a role in the regulation of the Salmonella pathogenicity island 1 (SPI-1) genes (e.g., hilA and hilD) necessary for invasion (19–21).
S. Typhimurium strains with an arabinose-regulated fur genotype (fur expressed in vitro in the presence of arabinose, not expressed in vivo where arabinose is not available) were partially attenuated and highly immunogenic in mice (22). The same study also showed that the attenuation of S. Typhimurium arabinose-regulated fur mutants is correlated with the level of fur expression. Furthermore, an S. enterica serovar Enteritidis Δfur strain was attenuated, and the immunization of mice with this strain resulted in a decrease in the bacterial load in systemic organs after challenge with the wild-type strain (23). A fur deletion was also employed to improve the safety of an S. Typhimurium ΔssaV mutant. The ΔssaV Δfur double mutant was safe and immunogenic in immunocompromised mice (24).
The pmi gene encodes phosphomannose isomerase, which facilitates the interconversion of fructose-6-phosphate into mannose-6-phosphate, which is subsequently converted into GDP-mannose, a substrate for incorporation into LPS O-antigen side chains. Thus, Δpmi mutants cannot produce O antigen unless an exogenous source of mannose is present. In the context of a vaccine, Δpmi strains are grown in vitro in the presence of mannose and synthesize a complete O antigen, a requirement for optimal host colonization (25). The O antigen is subsequently lost after several generations of growth in animal tissues, which are devoid of free nonphosphorylated mannose (25). S. Typhimurium pmi mutants are highly immunogenic and partially attenuated in mice (9).
The primary focus of this work was to evaluate the virulence and immunogenicity of S. Gallinarum strains with deletions in the global regulatory gene fur and/or in pmi. We also examined the impact of the fur deletion in combination with several other mutations. Strains were screened for virulence and protective efficacy in two chicken breeds: Rhode Island Red and Brown Leghorn, which are differently susceptible to fowl typhoid (1). Our results show that immunization with an S. Gallinarum fur mutant provided excellent protection against challenge with virulent S. Gallinarum in both breeds.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli and S. Gallinarum strains were routinely cultured at 37°C in LB broth (26) or on LB agar. Cultures of S. Gallinarum mutants were supplemented with 0.05% mannose (Sigma-Aldrich, St. Louis, MO) (for Δpmi-2426), 0.2% arabinose (Sigma-Aldrich) (for ΔPrfaH178::TT araC PBAD rfaH, here ΔPrfaH178), or 15 μg/ml chloramphenicol (Sigma-Aldrich) (for Δfur-453::cam). Carbohydrate-free nutrient broth (NB) was used for growth when determining LPS profiles. The strains were grown in NB without mannose (for pmi strains) or arabinose (for ΔPrfaH178 strains) overnight and subcultured (1:100) into fresh NB with or without the appropriate sugar for a second passage. LB agar without sodium chloride and with 7.5% sucrose (Sigma-Aldrich) was employed for sacB-based counterselection. MacConkey plates with 1% mannose were used to indicate sugar fermentation.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli strains | ||
| χ7213 | thi-1 thr-1 leuB6 glnV44 fhuA21 lacY1 recA1 RP4–2-Tc::Mu[λpir] ΔasdA4 Δ(zhf-2::Tn10); used for conjugational transfer of suicide plasmids | 31 |
| χ7232 | endA1 hsdR17 (rK− mK+) glnV44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 λpir deoR (φ80dlac Δ(lacZ)M15); used for general cloning | 31 |
| S. Gallinarum strains | ||
| χ4173 | Wild-type challenge strain | 7 |
| 287/91 | Wild-type vaccine parent strain | 29 |
| χ11575 | Δfur-453::cam | 287/91 |
| χ11386 | ΔPrfaH178::TT araC PBAD rfaH | 287/91; 7 |
| χ11741 | Δpmi-2426 | 287/91 |
| χ11797 | Δfur-712 | 287/91 |
| χ11798 | Δpmi-2426 Δfur-712 | χ11741 |
| χ11820 | Δfur-712 Δpmi-2426 | χ11797 |
| χ11821 | ΔPrfaH178::TT araC PBAD rfaH Δfur-712 | χ11386 |
| χ11822 | ΔansB1235 | 287/91 |
| χ11823 | Δfur-712 ΔansB1235 | χ11797 |
| Plasmids | ||
| pKD46 | λ red expression vector | 30 |
| pRE112 | sacB mobRP4; R6K ori; Cmr | 53 |
| pYA3546 | Suicide vector for introduction Δpmi-2426 | 25, 32 |
| pYA5239 | Suicide vector for introduction Δfur-712 | pRE112 |
| pYA5272 | Suicide vector for introduction ΔansB1235 | pRE112 |
For the animal experiments, S. Gallinarum strains were cultured in LB broth with the appropriate supplements. Overnight cultures were diluted 1:100 and grown with shaking (200 rpm) to an optical density at 600 nm of ∼0.8. Next, bacteria were centrifuged at 5,000 × g for 15 min at room temperature and resuspended in phosphate-buffered saline (PBS) or buffered saline with 0.01% gelatin (BSG) (27). LB or Salmonella Shigella (SS) agar plates were used to enumerate S. Gallinarum organisms recovered from chicken tissues. Rappaport-Vassiliadis R10 (RV) broth was employed to enrich the samples for S. Gallinarum. All media were purchased from BD Difco (Franklin Lakes, NJ), unless otherwise indicated.
General DNA procedures.
DNA manipulations, including plasmid and genomic DNA isolation, restriction enzyme digestions, ligations, and other DNA-modifying reactions, were carried out as described by Sambrook and Russell (28) or were performed according to the manufacturers' instructions (New England BioLabs, Ipswich, MA; Qiagen, Valencia, CA; Promega, Madison, WI). The synthesis of primers (Table 2) and DNA sequencing were performed by Integrated DNA Technologies (Coralville, IA) and the DNA Laboratory at Arizona State University (Tempe, AZ), respectively. PCRs were carried out with Klentaq LA polymerase (DNA Polymerase Technology, St. Louis, MO), possessing proofreading activity. Recombinant plasmids were introduced into E. coli and S. Gallinarum cells by transformation or electroporation, respectively.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ to 3′)a | Orientation | Restriction siteb |
|---|---|---|---|
| AM-115 | TCTAATGAAGTGAATCGTTTAGCAACAGGACAGATTCCGCGTGTAGGCTGGAGCTGCTTC | Forward | ∅ |
| AM-116 | AAAAGCCAACCGGGCGGTTGGCTCTTCGAAAGATTTACACCATATGAATATCCTCCTTAG | Reverse | ∅ |
| fur-1F | TATAGAGCTCTCTGCCTGTTCTGCTATG | Forward | SacI |
| fur-1R | GGCGCAGATATAACGCTGCGCCGCATAAGATTAGGC | Reverse | ∅ |
| fur-2F | CAGCGTTATATCTGCGCCCTTTCGAAGAGCCAACCG | Forward | ∅ |
| fur-2R | TATAGGTACCGCCAGTTGTTCAGGTGTG | Reverse | KpnI |
| ansB-1F | TATAGAGCTCGCCGCTCATGCAGATTAC | Forward | SacI |
| ansB-1R | TTACTTCAGGCTGCCAACCAGCGCTTTGCGGCTATC | Reverse | ∅ |
| ansB-2F | GTTGGCAGCCTGAAGTAATGATAATGCCCCGGTCGG | Forward | ∅ |
| ansB-2R | TATAGGTACCCCAATACGCGTCCGCTTC | Reverse | KpnI |
Underlined nucleotides denote restriction enzyme sites used for cloning. Nucleotides in bold type are complementary to the S. Gallinarum 287/91 chromosome.
Ø, the primer does not encode an engineered restriction site.
Construction of S. Gallinarum vaccine strains.
All vaccine candidates were derived from S. Gallinarum strain 287/91 (29). The fur deletion/insertion mutation Δfur-453::cam was constructed via the λ red recombination method (30). The flanking sequences were based on the S. Gallinarum 287/91 genome using primers AM-115 and AM-116 (Table 2). All other gene replacements were introduced by the conjugational transfer of suicide plasmids using donor E. coli strain χ7213 (31).
To construct the Δfur-712 deletion, fur flanking regions were amplified from the S. Gallinarum 287/91 genome by two-step PCR. First, 644-bp and 663-bp DNA fragments flanking the fur gene were amplified with the fur-1F/-1R and fur-2F/-2R primer sets (Table 2), respectively. Thereafter, the mix of PCR products was used as a template in the next amplification reaction with fur-1F and fur-2R primers. The 1.3-kb DNA fragment was digested with SacI/KpnI restriction enzymes and cloned into suicide plasmid vector pRE112. The resulting suicide plasmid, pYA5239, carried a deletion of the entire fur gene, including a 251-bp promoter region. The Δfur-712 mutation was introduced by allelic exchange into S. Gallinarum strains 287/91, χ11741, and χ11386 to generate χ11797 (Δfur-712), χ11798 (Δpmi-2426 Δfur-712), and χ11821 (ΔPrfaH178::TT araC PBAD rfaH Δfur-712), respectively.
The ΔansB1235 deletion was constructed as described above using ansB-1F/-1R and ansB-2F/-2R primer pairs (Table 2). The resulting suicide plasmid, pYA5272, carried a deletion of the entire ansB gene, including the 188-bp promoter sequence. The ΔansB1235 mutation was introduced into S. Gallinarum strains 287/91 and χ11797 to generate χ11822 (ΔansB1235) and χ11823 (Δfur-712 ΔansB1235), respectively.
As S. Typhimurium and S. Gallinarum share >99% sequence similarity in the flanking region surrounding pmi, the previously constructed suicide plasmid pYA3546 carrying S. Typhimurium DNA sequences was used to create S. Gallinarum Δpmi-2426 mutants (25, 32). Plasmid pYA3546 was introduced by conjugation into S. Gallinarum strains 287/91 and χ11797 to generate χ11741 (Δpmi-2426) and χ11820 (Δfur-712 Δpmi-2426), respectively.
All mutations were verified by PCR. We confirmed Fur production, or lack thereof, by Western blotting. The Δpmi mutation was confirmed by white colony phenotype on mannose-MacConkey agar. LPS profiles were examined by silver staining in 12% polyacrylamide gels, as described previously (33).
Isolation of outer membrane proteins.
Outer membrane proteins (OMPs) were isolated using the Sarkosyl extraction method (34).
SDS-PAGE and Western blotting.
SDS-PAGE and Western blotting procedures were done by standard techniques. The blots were developed with Nitro Blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyl phosphate (Amresco, Solon, OH) as a substrate, using rabbit polyclonal anti-Fur serum (22) or anti-GroEL antibodies (Sigma-Aldrich) as primary antibodies, and mouse anti-rabbit IgG alkaline phosphatase conjugate (Sigma-Aldrich) as secondary antibodies.
Acid shock assay.
Acid resistance was evaluated essentially as previously described, with a few modifications (35). Strains were grown aerobically in LB broth with appropriate supplements until they reached an optical density of ∼0.4. The culture aliquots were centrifuged (10 min at 5,000 × g) at room temperature, and bacterial pellets were washed with E medium (pH 7.0). Thereafter, the cells were centrifuged again and resuspended at a density of ∼0.5 × 109 CFU/ml in E medium (pH 3.0). Acid challenge was conducted at 37°C, and samples were collected immediately after resuspension and at 30-min intervals. The samples were serially diluted and plated onto LB agar to assess bacterial viability.
Animal supply and housing.
Animal experiments were performed using two breeds of chickens: Rhode Island Reds and Brown Leghorns. Straight run Rhode Island Red chicks were obtained from Randall Burkey Co. (Boerne, TX) or Murray McMurray Hatchery (Webster City, IA) 1 or 2 days after hatch. The birds were housed in separate cages for each group and given water and feed ad libitum. All animal experiments were carried out in compliance with the Institutional Animal Care and Use Committee (IACUC) and the Animal Welfare Act at Arizona State University.
Female Brown Leghorn chickens were hatched in-house. The chickens were feed Purina Lab Chow 5065, and water and feed were available ad libitum. Six-week-old chickens were distributed among several isolators and tagged.
Determination of LD50.
The strains were grown and harvested as described above in “Bacterial strains, plasmids, media, and growth conditions.” Bacterial pellets were resuspended in PBS or BSG and adjusted to achieve a dose of ∼102 to ∼108 CFU in a volume of 100 μl for orally inoculating chicks. The virulence of the wild-type strain, 287/91, and its derivatives were assessed in 3- or 5-day-old Rhode Island Red chicks. The birds were observed for fowl typhoid symptoms for 3 weeks postinoculation. Deaths were recorded daily. The 50% lethal dose (LD50) was calculated using the Reed and Muench method (36).
Immunization and challenge regimen.
For Rhode Island Reds, 3- or 5-day-old chicks were inoculated orally with 100 μl of PBS containing ∼1 × 108 CFU of the appropriate S. Gallinarum strain and boosted with the same strain and dose 2 weeks later. No food was provided for ∼5 to 6 h prior to immunizations or challenge. Groups of birds inoculated with buffer (PBS or BSG) served as controls. At 4 weeks of age (i.e., 2 weeks after the booster), all birds were orally challenged with ∼1 × 107 CFU of heterologous S. Gallinarum strain χ4173. Note that in the case of fur::cam deletion/insertion strain χ11575, all chicks survived the virulence study described above. They were then treated as vaccinated chicks, boosted with ∼1 × 108 CFU of χ11575, and challenged.
The chickens were observed for fowl typhoid symptoms for 3 weeks postchallenge. Deaths were recorded daily. At the end of the observation period, each surviving bird was euthanized, and its organs were inspected for lesions. Spleens and livers were collected and homogenized. Dilutions of the homogenate were made in BSG and plated onto LB agar plates for enumeration of the Salmonella organisms present in each tissue. Enrichment with RV broth and subsequent plating onto SS agar plates was carried out for organ samples in which no Salmonella organisms were detected by direct plating.
For experiments with Brown Leghorns, groups of 15 or 16 seven-week-old pullets were immunized orally (∼2 × 107 CFU) or intramuscularly (∼2 × 104 or ∼2 × 107 CFU) with the appropriate S. Gallinarum strain. A group of nonvaccinated birds was used as a control. At 10 weeks of age (i.e., 3 weeks postimmunization), all birds were orally challenged with ∼2 × 108 CFU of homologous S. Gallinarum strain 287/91. The birds were monitored for 3 weeks postchallenge. Next, each surviving bird was euthanized, and necropsies were performed to determine the presence of tissue lesions.
Statistical analyses.
All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA). The significance of differences between the obtained values was appraised using two-way analysis of variance (ANOVA), followed by Tukey's or Dunnett's tests. P values of <0.05 were considered significant.
RESULTS
Screening for S. Gallinarum immunogenic mutants.
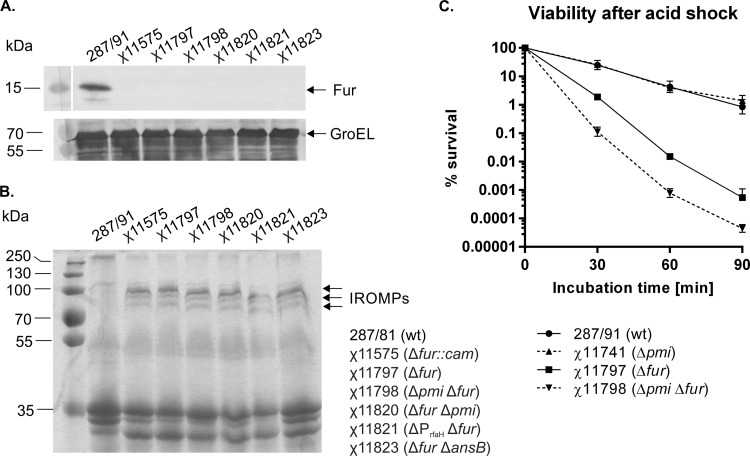
To evaluate the impact of a fur deletion in S. Gallinarum, we constructed strain χ11575, harboring the Δfur-453::cam deletion/insertion. As expected, Fur was not detected in χ11575 by Western blot analysis (Fig. 1A). Next, we screened for the production of IROMPs after growth in LB, a medium in which iron is not limiting (∼7.6 μM iron [37]). Under these conditions, IROMPs were not detected in parent strain 287/91 but were easily detectable in χ11575 (Fig. 1B). The three distinct bands with approximate molecular masses of 83, 78, and 74 kDa correspond to the predicted molecular masses of the Fur-regulated IROMPs FepA, IroN, and Cir, respectively (38) (Fig. 1B). The protein pattern is in an agreement with previous observations of S. Typhimurium outer membrane preparations from wild-type cells grown under iron-limiting conditions (38) or from a fur mutant grown in the relatively iron-rich medium NB (25).
FIG 1.
Phenotype characterization of S. Gallinarum Δfur mutants. (A) Fur production in S. Gallinarum wild-type (287/91), Δfur-453::cam (χ11575), and Δfur-712 vaccine strains (χ11798, χ11820, χ11821, and χ11823). Whole-cell lysates were obtained from overnight cultures, electrophoresed on a 12% SDS-PAGE gel, transferred onto nitrocellulose, and probed with anti-Fur serum (top). The blot was also probed with anti-GroEL antibodies (bottom) to serve as a loading control. (B) IROMP production in S. Gallinarum vaccine strains. OMPs were obtained by Sarkosyl extraction from overnight cultures, electrophoresed on a 10% SDS-PAGE gel, and stained with Coomassie blue. wt, wild type. (C) Effect of acid shock on viability of S. Gallinarum fur mutants. Strains 287/91 (wt), χ 11741 (Δpmi-2426), χ11797 (Δfur-712), and χ11798 (Δpmi-2426 Δfur-712) were grown in LB to early logarithmic phase, washed in E medium (pH 7.0), and then challenged with E medium (pH 3.0). Survival was monitored by plating samples on LB agar. The data shown are the mean and standard error of the mean (SEM) values from four independent experiments. A statistical analysis was carried out using two-way ANOVA, followed by Tukey's multiple-comparison test. All possible pairs of data within each time point except 287/91 versus χ11741 were significantly different (P < 0.01).
Strain χ11575 was then screened for virulence in Rhode Island Red chicks. The birds were given orally graded doses of bacteria and monitored for 3 weeks. The strain was fully attenuated, with no deaths occurring at the highest dose tested (LD50, >∼1 × 108 CFU) (Table 3). Encouraged by these results, we evaluated the ability of χ11575 to confer protection against challenge with virulent S. Gallinarum. The same chicks used in the virulence assay were boosted 2 weeks after the first inoculation with ∼1 × 108 CFU of χ11575 and challenged 2 weeks later with ∼1 × 107 CFU of heterologous wild-type strain χ4173. All the birds, even those primed with the lowest dose (∼1 × 102 CFU) of χ11575, survived challenge with a virulent S. Gallinarum strain (Table 4), suggesting that an S. Gallinarum fur mutant is a viable vaccine candidate. However, strain χ11575 contains a chloramphenicol resistance cassette in the chromosome, precluding its use as a vaccine. Thus, we constructed S. Gallinarum strain χ11797, carrying the unmarked Δfur-712 deletion (Table 1). We confirmed the absence of detectable Fur in this strain (Fig. 1A), and the production of IROMPs following growth in LB was indistinguishable from that in χ11575 (Fig. 1B).
TABLE 3.
Attenuation of S. Gallinarum mutants in Rhode Island Red chicks
| Strain | Genotype | LD50 (CFU) |
|---|---|---|
| 287/91 | Wild type | 6.7 × 104 |
| χ11575 | Δfur-453::cam | >∼1 × 108 |
| χ11797 | Δfur-712 | >0.9 × 108 |
| χ11741 | Δpmi-2426 | 1.0 × 107 |
| χ11822 | ΔansB1235 | 1.3 × 104 |
| χ11821 | ΔPrfaH178 Δfur-712 | >1.2 × 108 |
TABLE 4.
Protective efficacy of S. Gallinarum fur mutants in Rhode Island Red chickensa
| Strain | Genotype | Expt | Prime (CFU) | Boost (CFU) | No. with condition/total no. (%) for: |
No. of chickens alive/total no. | % survival | ||
|---|---|---|---|---|---|---|---|---|---|
| Hepatomegaly | Splenomegaly | Heart lesions/pericarditis | |||||||
| Single fur | |||||||||
| χ11575 | Δfur-453::cam | 1 | Variousb | ∼1 × 108 | NTc | NT | NT | 20/20 | 100d |
| χ11797 | Δfur-712 | 2 | 1.0 × 108 | 1.2 × 108 | NT | NT | NT | 10/11 | 91d |
| 3 | 1.2 × 108 | 1.1 × 108 | 1/13 (8)d | 2/13 (15)d | 5/13 (38) | 12/13 | 92d | ||
| fur combined with other mutations | |||||||||
| χ11798 | Δpmi-2426 Δfur-712 | 2 | 1.0 × 108 | 1.0 × 108 | NT | NT | NT | 2/9 | 22 |
| χ11820 | Δfur-712 Δpmi-2426 | 3 | 1.2 × 108 | 1.3 × 108 | 10/12 (83) | 9/12 (75) | 3/12 (25) | 4/12 | 33 |
| χ11821 | ΔPrfaH178 Δfur-712 | 3 | 1.1 × 108 | 1.1 × 108 | 9/12 (75) | 8/12 (67) | 3/12 (25) | 4/12 | 33 |
| χ11823 | Δfur-712 ΔansB1235 | 3 | 1.0 × 108 | 0.9 × 108 | 7/12 (58) | 8/12 (67) | 3/12 (25) | 6/12 | 50 |
| Controls | |||||||||
| BSG | 1 | NT | NT | NT | 2/20 | 10 | |||
| PBS | 2 | NT | NT | NT | 2/11 | 18 | |||
| PBS | 3 | 10/12 (83) | 10/12 (83) | 1/12 (8) | 2/12 | 17 | |||
Three- or 5-day-old chicks were immunized orally with the indicated dose of S. Gallinarum and boosted 2 weeks later. At 4 weeks of age, all birds were challenged with ∼1 × 107 CFU of heterologous S. Gallinarum wild-type strain (χ4173).
These birds were survivors of the virulence assay, so the chicks received 1 × 102, 104, 106, or 108 CFU as a priming dose. The boost was 1 × 108 CFU for all birds.
NT, not tested.
P < 0.01 compared to control.
In S. Typhimurium, fur mutants display an acid-sensitive phenotype (39). To determine the acid resistance of S. Gallinarum fur mutants, χ11797 (Δfur-712) and parent strain 287/91 were cultured in LB to the early logarithmic phase of growth and then challenged at pH 3.0. The percentage of viable cells during low-pH challenge declined more rapidly for χ11797 than for 287/91 (Fig. 1C). After 30 min of pH 3.0 exposure, the survival of the mutant was significantly lower (2.0%) than that of the wild type (25.1%; P < 0.01). After 90 min of challenge, only 0.001% of the χ11797 cells survived compared to 0.847% of the wild-type cells, corresponding to an ∼880-fold (2.9-log) difference in the number of viable cells (P < 0.0001).
Virulence and protective efficacy of S. Gallinarum Δfur-712 mutant in Rhode Island Red chickens.
We determined the virulence of S. Gallinarum χ11797 (Δfur-712) in 5-day-old Rhode Island Red chicks. As expected, strain χ11797 was fully attenuated (LD50, >0.9 × 108 CFU) (Table 3). In contrast, parent strain 287/91 was highly virulent, with an LD50 of 6.7 × 104 CFU, consistent with previous results (7).
Strain χ11797 was then evaluated for its ability to induce protective immunity against challenge with a virulent S. Gallinarum strain. Two independent protection experiments were performed on 5-day-old Rhode Island Red chickens. The birds were primed and boosted orally 2 weeks later with identical doses of ∼1 × 108 CFU of χ11797. In each study, a control group was given a sterile buffer instead of vaccine. At 4 weeks of age, all birds were challenged with ∼1 × 107 CFU of heterologous virulent strain χ4173. In both studies, immunization with strain χ11797 provided significant protection compared to nonimmunized control birds (Table 4). In both experiments, >90% of the vaccinated chickens survived compared to only 17 to 18% survival in the control groups (P < 0.001).
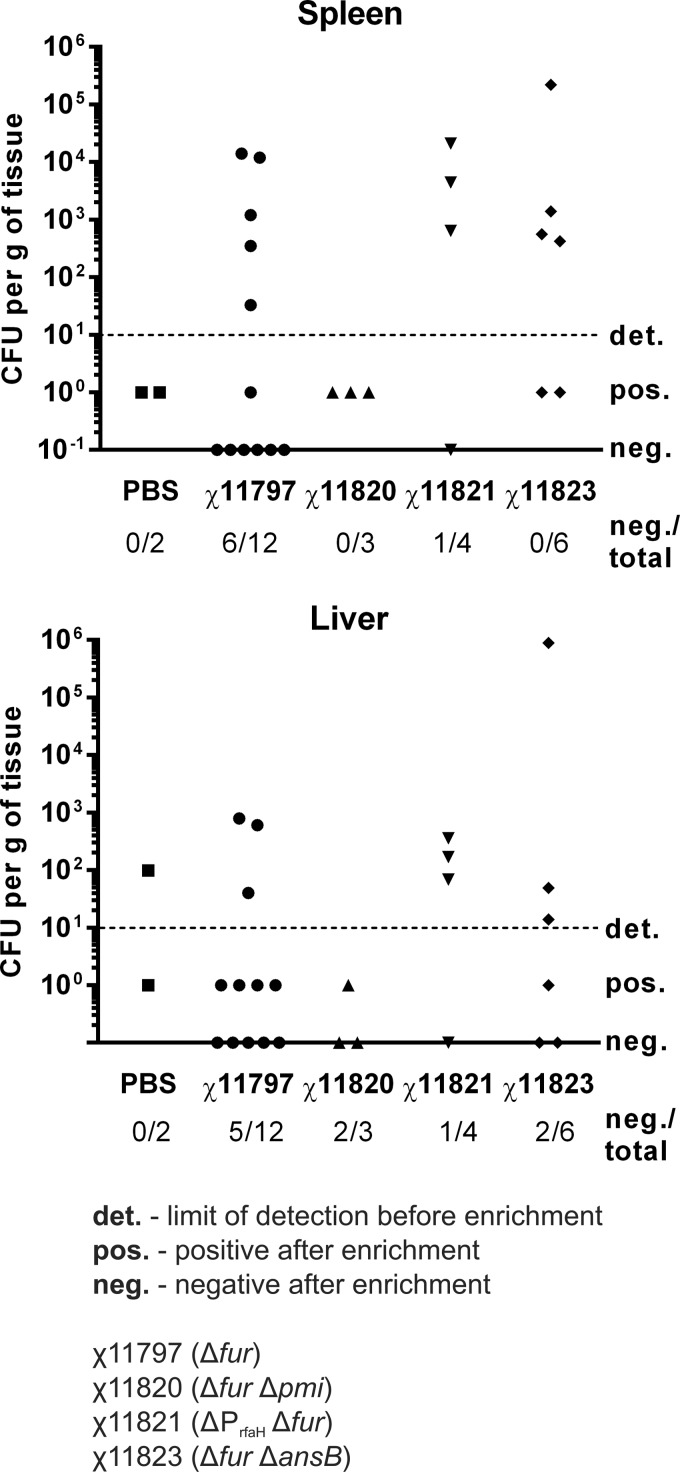
Additionally, in one of the protection studies (Table 4, experiment 3), the internal organs from all animals were inspected for lesions and bacterial loads after challenge. The birds that died from challenge were necropsied immediately, and each survivor was euthanized 3 weeks postchallenge, with necropsies performed at that time. In Rhode Island Reds that died of fowl typhoid, characteristic lesions included splenomegaly and hepatomegaly and, in some animals, some bronzing of the liver was noted. No other gross lesions were detected in chickens that did not survive the challenge. In contrast, spleens and livers in birds vaccinated with χ11797 were, for the most part, not enlarged or congested (Table 4). However, we found nodules in the hearts and observed acute pericarditis in 38% of the immunized birds. Furthermore, 19 days postchallenge, spleen and liver samples were collected from all surviving birds to enumerate S. Gallinarum colonization in each tissue (Fig. 2). In birds vaccinated with χ11797 (Δfur-712), the S. Gallinarum challenge strain was not detectable in 50% of the spleen and 42% of the liver samples. The bacterial loads were significantly lower (maximum [max], 1.4 × 104 CFU/g for spleen; 7.9 × 102 CFU/g for liver) in the remaining S. Gallinarum-positive tissues than in the nonvaccinated birds that succumbed to the infection, where counts were typically around 1 × 106 CFU per g of tissue (data not shown).
FIG 2.
Colonization of spleen and liver in birds that survived the challenge with wild-type S. Gallinarum. Survivors from experiment (expt.) 3 (Table 4) were euthanized 19 days postinfection, and spleens and livers were collected to recover viable S. Gallinarum from each tissue. The organs were homogenized, diluted, and plated on LB agar. Negative samples were additionally enriched using RV broth and plated on SS agar.
Immunogenicity of S. Gallinarum double mutants.
We next examined two distinct genetic strategies for enhancing the immunogenicity of χ11797. It is well established that Salmonella O antigen is required for efficient colonization of the chicken host (40, 41). Mutations that result in the gradual loss of O antigen in vivo can be used in Salmonella vaccine strains to enhance the induction of high-antibody titers to outer membrane proteins (25). Thus, we investigated the possibility that the introduction of a Δpmi or an arabinose-regulated rfaH mutation might enhance the immunogenicity of χ11797.
It is likely that all successful pathogens have various means to suppress host immune responses. An example of this in S. Typhimurium is ansB. The product of this gene, l-asparaginase II, suppresses host T-cell responses important for clearance of a S. Typhimurium infection, and S. Typhimurium ΔansB mutants are attenuated for virulence in mice (42). Thus, as an alternative approach to enhance immunogenicity, we examined the effect of ΔansB on virulence and immunogenicity alone or when combined with a Δfur mutation.
We constructed χ11741 (Δpmi-2426) and χ11822 (ΔansB1235) single mutant strains and χ11798 (Δpmi-2426 Δfur-712), χ11820 (Δfur-712 Δpmi-2426), χ11821 (ΔPrfaH178::TT araC PBAD rfaH Δfur-712), and χ11823 (Δfur-712 ΔansB1235) double mutant strains. The absence of detectable Fur was verified in the double mutants (Fig. 1A), and IROMP synthesis was not affected by combining Δpmi-2426, ΔPrfaH178, or ΔansB123 with Δfur-712 (Fig. 1B). An analysis of the LPS profiles of both pmi mutants (χ11741 and χ11798) and the ΔPrfaH178 χ11821 double mutant strain indicated that full-length O antigen was produced by both pmi strains and the ΔPrfaH178 mutant only when mannose or arabinose, respectively, was added to the growth medium (data not shown).
We also evaluated the acid resistance of the Δpmi mutant strains. Interestingly, strain χ11798 (Δpmi-2426 Δfur-712) was more sensitive to low pH than χ11797 (Δfur-712), even though it was grown in the presence of mannose prior to challenge (Fig. 1C). At every time point during challenge, the survival rate of strain χ11798 was significantly lower than that of strain χ11797 (P < 0.001). It is unlikely that the addition of mannose to strain χ11798 was responsible for the increase in acid sensitivity because strain χ11741 (Δpmi-2426), when grown in LB with mannose, displayed a survival profile identical to that of wild-type strain 287/91, and we observed no change in survival to acid challenge when mannose was added during the growth of χ11797 (Δfur-712) (data not shown).
Since an adequate level of attenuation is critical for designing safe and efficacious vaccines, we examined the virulence of S. Gallinarum strains χ11741 and χ11822, harboring single Δpmi or ΔansB mutations, respectively. The Δpmi χ11741 mutant was partially attenuated, similar to the phenotype observed for S. Typhimurium (9), while ΔansB χ11822 mutant was fully virulent (Table 3). Strain χ11821 (ΔPrfaH178 Δfur-712), a derivative of hypervirulent strain χ11386 (ΔPrfaH178) (7), was also tested. The introduction of Δfur-712 into χ11386 resulted in a complete loss of virulence.
The S. Gallinarum double mutants were then tested for protective efficacy in Rhode Island Reds. Note that while strains χ11820 and χ11798 have the same genotype, the mutations were introduced in a different order, with the Δfur-712 mutation introduced first, and then Δpmi-2426, in strain χ11820 and second in strain χ11798. Birds immunized with either χ11820 (Δfur-712 Δpmi-2426) or χ11798 (Δpmi-2426 Δfur-712) were not protected (33% and 22% survival, respectively) (Table 4). A lack of protection was also observed for birds vaccinated with χ11821 (ΔPrfaH178 Δfur-712) (33% survival). Vaccination with χ11823 (Δfur-712 ΔansB1235) resulted in 50% protection, but this result was not significantly different from that with the nonvaccinated controls.
Protective efficacy of S. Gallinarum vaccine strains in Brown Leghorn chickens.
Two vaccine strains, χ11797 (Δfur-712) and χ11798 (Δpmi-2426 Δfur-712), were also tested for protection immunity in Brown Leghorn chickens. In this study, 7-week-old female chickens were vaccinated with a single dose of vaccine by intramuscular (∼2 × 104 or ∼2 × 107 CFU) or oral (∼2 × 107 CFU) routes and challenged 3 weeks later with the virulent wild-type vaccine parent strain 287/91. As shown in Table 5, intramuscular immunization with a high dose (2.6 × 107 CFU) of strain χ11797 provided protection to all vaccinated birds. Moreover, enlargement of the spleen or liver was observed only in 6 and 12% of vaccinated birds postchallenge, respectively. Interestingly, a single low dose (2.6 × 104 CFU) of χ11797 delivered intramuscularly was also highly protective (100% survival; splenomegaly and hepatomegaly detected in 0 and 19% of birds, respectively). In comparison, only 38% of the nonvaccinated birds survived the challenge, and spleen and liver lesions were observed in most of them (73%). On the other hand, when delivered orally, strain χ11797 did not protect Brown Leghorns from wild-type challenge in this model. A survival rate of 50% for Brown Leghorns vaccinated with this route was not significantly different from that of nonvaccinated birds. Moreover, the percentage of birds in this group with organ lesions was similar to that in the control. In contrast, when administered intramuscularly, strain χ11798 provided significant protection against fowl typhoid (100% survival, P < 0.001; lower percentage of birds with lesions relative to control; P < 0.01).
TABLE 5.
Protective efficacy of S. Gallinarum fur mutants in female Brown Leghorn chickensa
| Strain | Genotype | Route | Prime (CFU) | No. with condition/total no. (%) for: |
No. of chickens alive/total no. | % survival | ||
|---|---|---|---|---|---|---|---|---|
| Hepatomegaly | Splenomegaly | Heart lesions/pericarditis | ||||||
| χ11797 | Δfur-712 | i.m. | 2.6 × 104 | 0/16 (0)b | 3/16 (19)b | 2/16 (13) | 16/16 | 100b |
| i.m. | 2.6 × 107 | 1/16 (6)b | 2/16 (12)b | 3/16 (19) | 15/16 | 100b | ||
| Oral | 2.6 × 107 | 14/16 (88) | 15/16 (94) | 2/16 (13) | 8/16 | 50 | ||
| χ11798 | Δpmi-2426 Δfur-712 | i.m. | 2.2 × 107 | 1/16 (6)b | 4/16 (25)b | 5/16 (31) | 16/16 | 100b |
| No vaccine | 11/15 (73) | 11/15 (73) | 5/16 (31) | 6/16 | 38 | |||
Seven-week-old birds were immunized orally or intramuscularly (i.m.) with the indicated dose of S. Gallinarum vaccine strain. Three weeks later, all birds were challenged orally with 2 × 108 CFU of S. Gallinarum strain 287/91. The health of the birds was monitored for 3 weeks postchallenge.
P < 0.01 compared to control.
DISCUSSION
In our study, we found that deletion of the fur gene in S. Gallinarum resulted in a completely avirulent strain that is highly efficacious as a live vaccine and can protect chickens against fowl typhoid when delivered orally in Rhode Island Red chickens or intramuscularly in Brown Leghorn chickens. These results differ from observations of an S. Typhimurium mutant, which is commonly used as a model for typhoid fever-like infections. While S. Typhimurium Δfur mutants were attenuated when delivered orally (43) or intraperitoneally (44) in mice, they were not found to be highly immunogenic (45). However, the level of attenuation conferred to S. Typhimurium by a fur mutation appears to be strain dependent. When delivered orally, derivatives of S. Typhimurium SL1344 are attenuated only about 10-fold (43, 44), while S. Typhimurium strain UK-1 fur mutants are attenuated 1,000-fold (43) or more (22). Differences in rpoS alleles can influence the acid tolerance response of both wild-type and fur mutants of Salmonella (46) and may therefore affect other phenotypic aspects of fur mutants. Alternatively, it is possible that undefined differences between strains may also affect virulence and immunogenicity. In addition, differences in immunogenicity between fur mutants of S. Typhimurium and S. Gallinarum may also be explained by the fact that the disease caused by S. Typhimurium in mice is not exactly the same as that caused by S. Gallinarum in chickens. Support for this view comes from observations that mutations that completely attenuate S. Typhimurium are often insufficiently attenuating for S. enterica serovar Typhi in humans (47, 48). Since S. Gallinarum and S. Typhi are strictly host-adapted serovars, the mechanisms of their pathogenesis are different from those of the broad-host range S. Typhimurium. Unfortunately, the molecular basis of host specificity and the mechanisms determining which type of disease is caused in which animal species are still poorly understood (49).
Adequate balance between the level of attenuation and immunogenicity is crucial for designing effective live vaccines but is often difficult to achieve. As we suggest above, the same means of attenuation may result in different levels of attenuation, reactogenicity, and/or immunogenicity, depending on the serovars or strains used for their construction. Protection from disease may also be influenced by the route of administration as well as the genetic properties or age of particular breeds, such as Rhode Island Red or Brown Leghorn chickens. Deletions of fur have been introduced into the fish pathogens Pseudomonas fluorescens (50) and Edwardsiella ictaluri (51) to generate live attenuated vaccines. A fur mutant of P. fluorescens was attenuated and able to elicit protection in Japanese flounders against P. fluorescens, as well as cross-protection against Aeromonas hydrophila (50). The authors of that study suggest that the observed cross-protection was related, at least in part, to constitutive production of IROMPs by the P. fluorescens fur mutant. Similarly, arabinose-regulated fur mutants of S. Typhimurium induce antibodies that recognize the IROMPs present in the outer membranes of a number of S. enterica and E. coli serovars (25). Since our S. Gallinarum fur mutants constitutively synthesize IROMPs (Fig. 1B), it will be interesting to determine how well an S. Gallinarum Δfur mutant, such as χ11797, protects chickens against other Salmonella serovars, in particular S. Enteritidis and S. Typhimurium. This is a topic for future study.
The Δpmi mutant strain χ11741 was moderately attenuated, with an oral LD50 about 2.5 logs higher than that of its wild-type parent, 287/91 (Table 3). This modest reduction in virulence is similar to a situation seen in S. Typhimurium, in which a 3.3-log increase in oral LD50 (for mice) was observed for a pmi mutant grown with mannose (9). The partial virulence of χ11741 makes this mutant unsuitable for use as a stand-alone vaccine strain. The idea behind combining Δpmi and Δfur in the same strain was that the loss of O antigen over time would enhance the presentation of the IROMPs to the host immune system. Because it has been argued that the lack of immunogenicity of S. Typhimurium fur mutants is due to an inability to colonize the gut-associated lymphoid tissue (GALT) (45), we considered it a plus that the pmi mutant was not fully attenuated and should therefore have a minimal impact on its immunogenicity. We felt that this would reduce the possibility that the double mutant would be overattenuated. However, while the χ11798 and χ11820 double mutants were attenuated, neither strain was protective when administered orally (Table 4). We used a similar strategy by combining fur with the arabinose-regulated rfaH mutation, ΔPrfaH178, which is not attenuating and in fact appears to be hypervirulent (7). Once again, this combination was also not protective (Table 4).
In contrast to our results in Rhode Island Red chicks, the S. Gallinarum Δpmi Δfur χ11798 mutant was highly immunogenic when used to intramuscularly immunize 7-week-old Brown Leghorns (Table 5). Thus, it may be that because the double mutant is more sensitive to low pH than the Δfur strain (Fig. 1C), it does not survive as well during passage through the low-pH environment of the proventriculus. If this is the case, pH sensitivity may also help to explain our conflicting results with the fur χ11797 mutant, which was protective when orally administered to chicks (Table 4) but was less effective when orally administered to older layers (Table 5). A recent study showed that the proventricular pH in chickens changes during the first few weeks of life, ranging from a pH of about 5 at 2 days of age to about 3 to 3.5 by 15 days of age (52). Thus, it is possible that the survival of strain χ11797 was greater in chicks than in the older birds used in our study. When we bypassed the gastric compartment by intramuscular injection, the χ11797 mutant was able to elicit a protective response (Table 5). The increased acid sensitivity of χ11798 might account for its lack of immunogenicity in chicks. An alternative interpretation of these results is that because fur, pmi, and rfaH all affect outer membrane structure/composition, the overexpression of outer membrane proteins (e.g., IROMPs) in the absence of complete O antigen has a negative influence on immunogenicity, perhaps due to the destabilization of outer membrane integrity in vivo. Of course, it is possible that other factors, including possible iron toxicity, have played a role (45).
Recently, it was shown that S. Typhimurium utilizes a product of ansB gene, l-asparaginase II, to inhibit host T-cell responses essential for the clearance of Salmonella infection (42). A canonical function of l-asparaginase II is hydrolyzing l-asparagine to l-aspartate and ammonia. However, beyond the metabolic function, the enzyme plays a role in virulence. The production of l-asparaginase II by Salmonella leads to the depletion of exogenous l-asparagine, a metabolite required for T-cell proliferation. While an S. Typhimurium ansB mutant was attenuated for virulence in mice, this was not the case for S. Gallinarum in chicks (Table 3), and the introduction of an ΔansB mutation into the Δfur χ11797 mutant strain abrogated, rather than enhanced, its immunogenicity (Table 4). Thus, our study did not identify a role for ansB in S. Gallinarum infection or pathogenicity in chicks.
One of the primary goals of our research was to develop a safe and effective orally administered fowl typhoid vaccine for birds. Oral administration of vaccines is, in general, easier to perform than injection and more likely to induce mucosal responses. The results of this study indicate that the Δfur χ11797 mutant is safe (Table 3). It is effective in chicks (Table 4) but is unsuitable for use as an oral vaccine in older birds (Table 5), though it is highly immunogenic when delivered by the intramuscular route. It is possible that the problem of efficacy in older birds can be rectified by the introduction of a mutation that allows for regulated delayed fur expression, as has been demonstrated to be effective in the S. Typhimurium mouse model (25). We used a similar strategy to regulate the expression of crp in S. Gallinarum, with promising results (7). If this strategy is effective with fur, it may allow us to take advantage of a second mutation in pmi, as we described above.
In conclusion, this study demonstrates that the fur gene is essential for the virulence of S. Gallinarum, and a fur deletion resulted in the complete attenuation of S. Gallinarum. Further, a Δfur mutant is protective against fowl typhoid when used as a live recombinant vaccine following intramuscular administration, or by the oral route in young birds.
ACKNOWLEDGMENTS
We thank Crystal Willingham and Jacquelyn Kilbourne for their expert technical assistance. We also thank Julie Pugsley, Nathan Lawyer, and the Arizona State University Department of Animal Care and Technology for taking outstanding care of the animals used in this study.
This work was funded by BREAD grant IOS-0965511 from the National Science Foundation and NIH R01 AI60557.
Footnotes
Published ahead of print 2 July 2014
REFERENCES
- 1.Shivaprasad HL. 2000. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 19:405–424 [DOI] [PubMed] [Google Scholar]
- 2.Barrow PA, Freitas Neto OC. 2011. Pullorum disease and fowl typhoid–new thoughts on old diseases: a review. Avian Pathol. 40:1–13. 10.1080/03079457.2010.542575 [DOI] [PubMed] [Google Scholar]
- 3.Desin TS, Köster W, Potter AA. 2013. Salmonella vaccines in poultry: past, present and future. Expert Rev. Vaccines 12:87–96. 10.1586/erv.12.138 [DOI] [PubMed] [Google Scholar]
- 4.Silva EN, Snoeyenbos GH, Weinack OM, Smyser CF. 1981. Studies on the use of 9R strain of Salmonella Gallinarum as a vaccine in chickens. Avian Dis. 25:38–52. 10.2307/1589825 [DOI] [PubMed] [Google Scholar]
- 5.Kwon HJ, Cho SH. 2011. Pathogenicity of SG 9R, a rough vaccine strain against fowl typhoid. Vaccine 29:1311–1318. 10.1016/j.vaccine.2010.11.067 [DOI] [PubMed] [Google Scholar]
- 6.Kang MS, Kwon YK, Kim HR, Oh JY, Kim MJ, An BK, Shin EG, Kwon JH, Park CK. 2012. Comparative proteome and transcriptome analyses of wild-type and live vaccine strains of Salmonella enterica serovar Gallinarum. Vaccine 30:6368–6375. 10.1016/j.vaccine.2012.08.048 [DOI] [PubMed] [Google Scholar]
- 7.Mitra A, Loh A, Gonzales A, Laniewski P, Willingham C, Curtiss R, III, Roland KL. 2013. Safety and protective efficacy of live attenuated Salmonella Gallinarum mutants in Rhode Island Red chickens. Vaccine 31:1094–1099. 10.1016/j.vaccine.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 8.Rosu V, Chadfield MS, Santona A, Christensen JP, Thomsen LE, Rubino S, Olsen JE. 2007. Effects of crp deletion in Salmonella enterica serotype Gallinarum. Acta Vet. Scand. 49:14. 10.1186/1751-0147-49-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins LV, Attridge S, Hackett J. 1991. Mutations at rfc or pmi attenuate Salmonella Typhimurium virulence for mice. Infect. Immun. 59:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong Q, Liu Q, Jansen AM, Curtiss R., III 2010. Regulated delayed expression of rfc enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Vaccine 28:6094–6103. 10.1016/j.vaccine.2010.06.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong Q, Liu Q, Roland KL, Curtiss R., III 2009. Regulated delayed expression of rfaH in an attenuated Salmonella enterica serovar Typhimurium vaccine enhances immunogenicity of outer membrane proteins and a heterologous antigen. Infect. Immun. 77:5572–5582. 10.1128/IAI.00831-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troxell B, Hassan HM. 2013. Transcriptional regulation by ferric uptake regulator (Fur) in pathogenic bacteria. Front. Cell. Infect. Microbiol. 3:59. 10.3389/fcimb.2013.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido ME, Bosch M, Medina R, Llagostera M, Pérez de Rozas AM, Badiola I, Barbé J. 2003. The high-affinity zinc-uptake system znuACB is under control of the iron-uptake regulator (fur) gene in the animal pathogen Pasteurella multocida. FEMS Microbiol. Lett. 221:31–37. 10.1016/S0378-1097(03)00131-9 [DOI] [PubMed] [Google Scholar]
- 14.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10:525–537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall HK, Foster JW. 1996. The role of fur in the acid tolerance response of Salmonella Typhimurium is physiologically and genetically separable from its role in iron acquisition. J. Bacteriol. 178:5683–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baik HS, Bearson S, Dunbar S, Foster JW. 1996. The acid tolerance response of Salmonella Typhimurium provides protection against organic acids. Microbiology 142(Pt 11):3195–3200 [DOI] [PubMed] [Google Scholar]
- 17.Leclerc JM, Dozois CM, Daigle F. 2013. Role of the Salmonella enterica serovar Typhi Fur regulator and small RNAs RfrA and RfrB in iron homeostasis and interaction with host cells. Microbiology 159:591–602. 10.1099/mic.0.064329-0 [DOI] [PubMed] [Google Scholar]
- 18.Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. 2007. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol. Microbiol. 63:1495–1507. 10.1111/j.1365-2958.2007.05600.x [DOI] [PubMed] [Google Scholar]
- 19.Teixido L, Carrasco B, Alonso JC, Barbé J, Campoy S. 2011. Fur activates the expression of Salmonella enterica pathogenicity island 1 by directly interacting with the hilD operator in vivo and in vitro. PLoS One 6:e19711. 10.1371/journal.pone.0019711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troxell B, Sikes ML, Fink RC, Vazquez-Torres A, Jones-Carson J, Hassan HM. 2011. Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J. Bacteriol. 193:497–505. 10.1128/JB.00942-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellermeier JR, Slauch JM. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J. Bacteriol. 190:476–486. 10.1128/JB.00926-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtiss R, III, Wanda SY, Gunn BM, Zhang X, Tinge SA, Ananthnarayan V, Mo H, Wang S, Kong W. 2009. Salmonella enterica serovar Typhimurium strains with regulated delayed attenuation in vivo. Infect. Immun. 77:1071–1082. 10.1128/IAI.00693-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karasova D, Sebkova A, Vrbas V, Havlickova H, Sisak F, Rychlik I. 2009. Comparative analysis of Salmonella enterica serovar Enteritidis mutants with a vaccine potential. Vaccine 27:5265–5270. 10.1016/j.vaccine.2009.06.060 [DOI] [PubMed] [Google Scholar]
- 24.Vishwakarma V, Pati NB, Chandel HS, Sahoo SS, Saha B, Suar M. 2012. Evaluation of Salmonella enterica serovar Typhimurium TTSS-2 deficient fur mutant as safe live-attenuated vaccine candidate for immunocompromised mice. PLoS One 7:e52043. 10.1371/journal.pone.0052043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtiss R, III, Zhang X, Wanda SY, Kang HY, Konjufca V, Li Y, Gunn B, Wang S, Scarpellini G, Lee IS. 2007. Induction of host immune responses using Salmonella-vectored vaccines, p 297–313 In Brogden KA, Minion FC, Cornick N. (ed), Virulence mechanisms of bacterial pathogens, 4th ed. ASM Press, Washington, DC [Google Scholar]
- 26.Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtiss R., III 1965. Chromosomal aberrations associated with mutations to bacteriophage resistance in Escherichia coli. J. Bacteriol. 89:28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 29.Thomson NR, Clayton DJ, Windhorst D, Vernikos G, Davidson S, Churcher C, Quail MA, Stevens M, Jones MA, Watson M, Barron A, Layton A, Pickard D, Kingsley RA, Bignell A, Clark L, Harris B, Ormond D, Abdellah Z, Brooks K, Cherevach I, Chillingworth T, Woodward J, Norberczak H, Lord A, Arrowsmith C, Jagels K, Moule S, Mungall K, Sanders M, Whitehead S, Chabalgoity JA, Maskell D, Humphrey T, Roberts M, Barrow PA, Dougan G, Parkhill J. 2008. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 18:1624–1637. 10.1101/gr.077404.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roland K, Curtiss R, III, Sizemore D. 1999. Construction and evaluation of a delta cya delta crp Salmonella Typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis. 43:429–441. 10.2307/1592640 [DOI] [PubMed] [Google Scholar]
- 32.Gunn BM, Wanda SY, Burshell D, Wang C, Curtiss R., III 2010. Construction of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine vector strains for safety in newborn and infant mice. Clin. Vaccine Immunol. 17:354–362. 10.1128/CVI.00412-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hitchcock PJ, Brown TM. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlone GM, Thomas ML, Rumschlag HS, Sottnek FO. 1986. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J. Clin. Microbiol. 24:330–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenneman KE, Willingham C, Kong W, Curtiss R, III, Roland KL. 2013. Low-pH rescue of acid-sensitive Salmonella enterica serovar Typhi strains by a rhamnose-regulated arginine decarboxylase system. J. Bacteriol. 195:3062–3072. 10.1128/JB.00104-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed LJ, Muench LH. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27:493–497 [Google Scholar]
- 37.Lee JY, Passalacqua KD, Hanna PC, Sherman DH. 2011. Regulation of petrobactin and bacillibactin biosynthesis in Bacillus anthracis under iron and oxygen variation. PLoS One 6:e20777. 10.1371/journal.pone.0020777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabsch W, Methner U, Voigt W, Tschäpe H, Reissbrodt R, Williams PH. 2003. Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect. Immun. 71:6953–6961. 10.1128/IAI.71.12.6953-6961.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster JW. 1991. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J. Bacteriol. 173:6896–6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craven SE. 1994. Altered colonizing ability for the ceca of broiler chicks by lipopolysaccharide-deficient mutants of Salmonella Typhimurium. Avian Dis. 38:401–408. 10.2307/1592059 [DOI] [PubMed] [Google Scholar]
- 41.Turner AK, Lovell MA, Hulme SD, Zhang-Barber L, Barrow PA. 1998. Identification of Salmonella Typhimurium genes required for colonization of the chicken alimentary tract and for virulence in newly hatched chicks. Infect. Immun. 66:2099–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kullas AL, McClelland M, Yang HJ, Tam JW, Torres A, Porwollik S, Mena P, McPhee JB, Bogomolnaya L, Andrews-Polymenis H, van der Velden AW. 2012. l-Asparaginase II produced by Salmonella Typhimurium inhibits T cell responses and mediates virulence. Cell Host Microbe 12:791–798. 10.1016/j.chom.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riesenberg-Wilmes MR, Bearson B, Foster JW, Curtiss R., III 1996. Role of the acid tolerance response in virulence of Salmonella Typhimurium. Infect. Immun. 64:1085–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-del Portillo F, Foster JW, Finlay BB. 1993. Role of acid tolerance response genes in Salmonella Typhimurium virulence. Infect. Immun. 61:4489–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curtiss R., III 2005. Antigen delivery systems: development of live recombinant attenuated bacterial antigen and DNA vaccine delivery vector vaccines, p 1009–1037 In Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W. (ed), Mucosal immunology, 3rd ed. Academic Press, Waltham, MA [Google Scholar]
- 46.Lee IS, Lin J, Hall HK, Bearson B, Foster JW. 1995. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella Typhimurium. Mol. Microbiol. 17:155–167. 10.1111/j.1365-2958.1995.mmi_17010155.x [DOI] [PubMed] [Google Scholar]
- 47.Bumann D, Hueck C, Aebischer T, Meyer TF. 2000. Recombinant live Salmonella spp. for human vaccination against heterologous pathogens. FEMS Immunol. Med. Microbiol. 27:357–364. 10.1111/j.1574-695X.2000.tb01450.x [DOI] [PubMed] [Google Scholar]
- 48.Frey SE, Bollen W, Sizemore D, Campbell M, Curtiss R., III 2001. Bacteremia associated with live attenuated chi8110 Salmonella enterica serovar Typhi ISP1820 in healthy adult volunteers. Clin. Immunol. 101:32–37. 10.1006/clim.2001.5088 [DOI] [PubMed] [Google Scholar]
- 49.Suar M, Jantsch J, Hapfelmeier S, Kremer M, Stallmach T, Barrow PA, Hardt WD. 2006. Virulence of broad- and narrow-host-range Salmonella enterica serovars in the streptomycin-pretreated mouse model. Infect. Immun. 74:632–644. 10.1128/IAI.74.1.632-644.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang HR, Hu YH, Zhang WW, Sun L. 2009. Construction of an attenuated Pseudomonas fluorescens strain and evaluation of its potential as a cross-protective vaccine. Vaccine 27:4047–4055. 10.1016/j.vaccine.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 51.Santander J, Golden G, Wanda SY, Curtiss R., III 2012. Fur-regulated iron uptake system of Edwardsiella ictaluri and its influence on pathogenesis and immunogenicity in the catfish host. Infect. Immun. 80:2689–2703. 10.1128/IAI.00013-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rynsburger JM, Classen HL. 2007. Effect of age on intestinal pH of broiler chickens. International Poultry Scientific Forum, Atlanta, GA [Google Scholar]
- 53.Edwards RA, Keller LH, Schifferli DM. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149–157. 10.1016/S0378-1119(97)00619-7 [DOI] [PubMed] [Google Scholar]