Abstract
The filamentous, nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120 accumulates sucrose as a compatible solute against salt stress. Sucrose-phosphate synthase activity, which is responsible for the sucrose synthesis, is increased by salt stress, but the mechanism underlying the regulation of sucrose synthesis remains unknown. In the present study, a response regulator, OrrA, was shown to control sucrose synthesis. Expression of spsA, which encodes a sucrose-phosphate synthase, and susA and susB, which encode sucrose synthases, was induced by salt stress. In the orrA disruptant, salt induction of these genes was completely abolished. The cellular sucrose level of the orrA disruptant was reduced to 40% of that in the wild type under salt stress conditions. Moreover, overexpression of orrA resulted in enhanced expression of spsA, susA, and susB, followed by accumulation of sucrose, without the addition of NaCl. We also found that SigB2, a group 2 sigma factor of RNA polymerase, regulated the early response to salt stress under the control of OrrA. It is concluded that OrrA controls sucrose synthesis in collaboration with SigB2.
INTRODUCTION
Cyanobacteria are a large group of bacteria characterized by oxygen-evolving photosynthesis. A photoautotrophic lifestyle has made cyanobacteria dependent only on light, water, and a few minerals, which enables them to inhabit almost all illuminated environments. Cyanobacteria have adapted to aquatic habitats with various salt concentrations. Halophilic strains, such as Aphanothece halophytica, are able to tolerate salinities five times as high as the salinity of seawater (equivalent to 3.0 M NaCl) (1). Salt-stressed cells accumulate compatible solutes to compensate for the difference in water potential (2). Among cyanobacteria, a correlation was found between the final salt tolerance limit and the major compatible solute (3). Low-halotolerance strains accumulate sucrose and trehalose as their major compatible solutes, while moderately halotolerant strains synthesize glucosylglycerol and glucosylglycerate. In halophilic strains, the main compatible solute is glycine betaine or glutamate betaine. Expression of genes involved in compatible solute synthesis is shown to be induced after salt shock treatments in some cyanobacteria (4, 5); however, molecular mechanisms of regulation of compatible solute synthesis have not been fully understood (6).
Cyanobacteria belonging to the genera of Anabaena and Nostoc accumulate sucrose as the only significant compatible solute under salt stress conditions (7). Anabaena and Nostoc are filamentous nitrogen-fixing cyanobacteria, in which nitrogen fixation is carried out within differentiated cells called heterocysts. Because heterocysts are unable to perform photosynthesis, vegetative cells supply carbohydrate, probably in the form of sucrose, to heterocysts, where sucrose is catabolized to provide carbon skeletons and reductant required for nitrogen fixation (8, 9). Thus, regulation of sucrose metabolism is also important for diazotrophic growth of heterocystous cyanobacteria. The metabolic pathways of sucrose have been well studied in Anabaena spp. (Fig. 1). Sucrose is mainly synthesized through sucrose-phosphate synthase (SPS) coupled to sucrose-phosphate phosphatase (10, 11). Sucrose synthase (SuS) is able to catalyze a reversible reaction of sucrose synthesis and cleavage, but SuS is involved in the cleavage of sucrose in vivo (12). Invertase catalyzes the irreversible hydrolysis of sucrose into hexoses. The invB gene is highly expressed in heterocysts and is essential for diazotrophic growth (13, 14).
FIG 1.
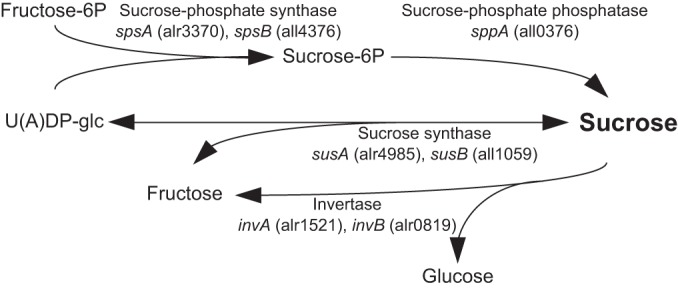
The metabolic pathways of sucrose in Anabaena PCC 7120.
In Anabaena sp. strain PCC 7120 (here, Anabaena PCC 7120), sucrose accumulation is also observed under dehydration stress, and DNA microarray analysis has shown that spsA, which encodes SPS, and genes encoding transcriptional regulators, orrA (alr3768), and sigB2 (alr3800), are induced by dehydration (15). OrrA is an NarL-type response regulator and is involved in salt induction of the lti2 gene (16). The lti2 gene has been originally isolated from Anabaena variabilis M3 as a low-temperature-induced gene (17). In Anabaena PCC 7120, expression of lti2 is also upregulated by salt and osmotic stresses, and its induction is eliminated by disruption of orrA (16). SigB2 is a group 2 sigma factor of RNA polymerase (18). In the unicellular cyanobacterium Synechocystis sp. strain PCC 6803, SigB regulates the salt acclimation response including upregulation of genes involved in compatible solute synthesis (19).
Cyanobacteria have recently drawn attention as promising organisms for sustainable production of biofuels or industrial feedstock (20, 21). Sucrose produced by cyanobacteria is also of interest as a sugar source for fermentative production of renewable fuels and chemicals. Because sucrose can be fermented directly, the costly pretreatment required for cellulosic biomass can be avoided. The sucrose accumulation capacities of model cyanobacteria, Synechocystis PCC 6803, Synechococcus elongatus PCC 7942, and Anabaena PCC 7120, was examined under salt stress conditions, and Anabaena PCC 7120 showed the highest sucrose accumulation among them (22). The sucrose productivities were improved by rerouting carbon fluxes to sucrose synthesis (22–24). The potential for sucrose production in genetically engineered cyanobacteria is estimated to be higher than that observed for sugarcane (23).
In the present study, we investigated salt regulation of genes involved in sucrose metabolism in Anabaena PCC 7120. Salt-adapted Anabaena PCC 7120 cells accumulate sucrose as a compatible solute (7) and increase SPS activity (25). The transcript levels of spsA and SuS genes (susA and susB) were increased in response to addition of NaCl. This induction was abolished by disruption of the orrA gene. The orrA disruptant showed decreased sucrose accumulation under salt stress conditions, and overexpression of orrA increased expression of spsA, susA, and susB, followed by sucrose accumulation. It was also indicated that SigB2 was involved in the regulation of the early response to salt stress under the control of OrrA. It is concluded that OrrA controls sucrose synthesis in collaboration with SigB2 in Anabaena PCC 7120.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Anabaena sp. strain PCC 7120 and its derivatives were grown at 30°C under continuous illumination provided by a fluorescent lamp at 30 μE m−2 s−1 in nitrogen-free modified Detmer's medium (MDM0) (26) or the BG-11 medium containing nitrate as a nitrogen source (27). Liquid cultures were bubbled with air containing 1% (vol/vol) CO2. Salt stress conditions were set up by addition of 50 mM NaCl to cultures in the late logarithmic phase (optical density at 750 nm [OD750] of 0.5 to 0.7).
Mutant construction.
All primers used in this study (Table 1) were designed based on genome data from CyanoBase (28). A plasmid, pRorrAS, was constructed as follows. DNA fragments upstream and downstream of the orrA gene were amplified by PCR using the primer pair orrA-5F and orrA-5R and the pair orrA-3F and orrA-3R, respectively (Table 1). The upstream fragment was cloned between the XhoI and BamHI sites of pBluescript II KS+ (Agilent Technologies), and then the downstream fragment was cloned between the BamHI and SacI sites. A spectinomycin resistance cassette excised by digestion with BamHI from the plasmid pDW9 (29) was inserted into the BamHI site between the upstream and downstream fragments. The SacI-XhoI fragment was excised from the resultant plasmid and cloned between the SacI and XhoI sites of pRL271 (30) to construct pRorrAS. A plasmid, pRsigB2S, was constructed as described for pRorrAS using the primer pair sigB2-5F and sigB2-5R and the pair sigB2-3F and sigB2-3R (Table 1). pRorrAS and pRsigB2S were transferred by conjugation into Anabaena PCC 7120 according to the method of Elhai et al. (31) to construct the deletion mutants of orrA, DRorrAS, and sigB2, DRsigB2S, respectively.
TABLE 1.
Primers used in this study
A plasmid, pAorrA, for overexpression of the orrA gene was constructed as follows. A DNA fragment containing promoter and coding regions of orrA was amplified by PCR using the primer pair PorrA-F and OrrA-R and cloned into the EcoRV site of pBluescript II KS+. This fragment was excised by digestion with BamHI and SalI and cloned between the BamHI and SalI sites of shuttle vector pAM505 (32) to construct pAorrA.
Determination of sucrose contents.
Cells subjected to salt stress for the time indicated in Fig. 2B were filtered on mixed cellulose filter paper, and then low-molecular-mass compounds of cells were extracted with 80% ethanol for 3 h at 65°C (15). After centrifugation, supernatants were vacuum dried, and the residue was dissolved in 0.5 ml of water. The samples were treated with 3 U of invertase (Sigma) in 50 mM sodium acetate (pH 4.5) for 2 h at 37°C, and the concentration of released glucose was determined with a glucose assay kit (Biovision). The chlorophyll a (Chl a) content of cultures was determined by the method of Mackinney (33), and sucrose contents were normalized to the chlorophyll a content.
FIG 2.
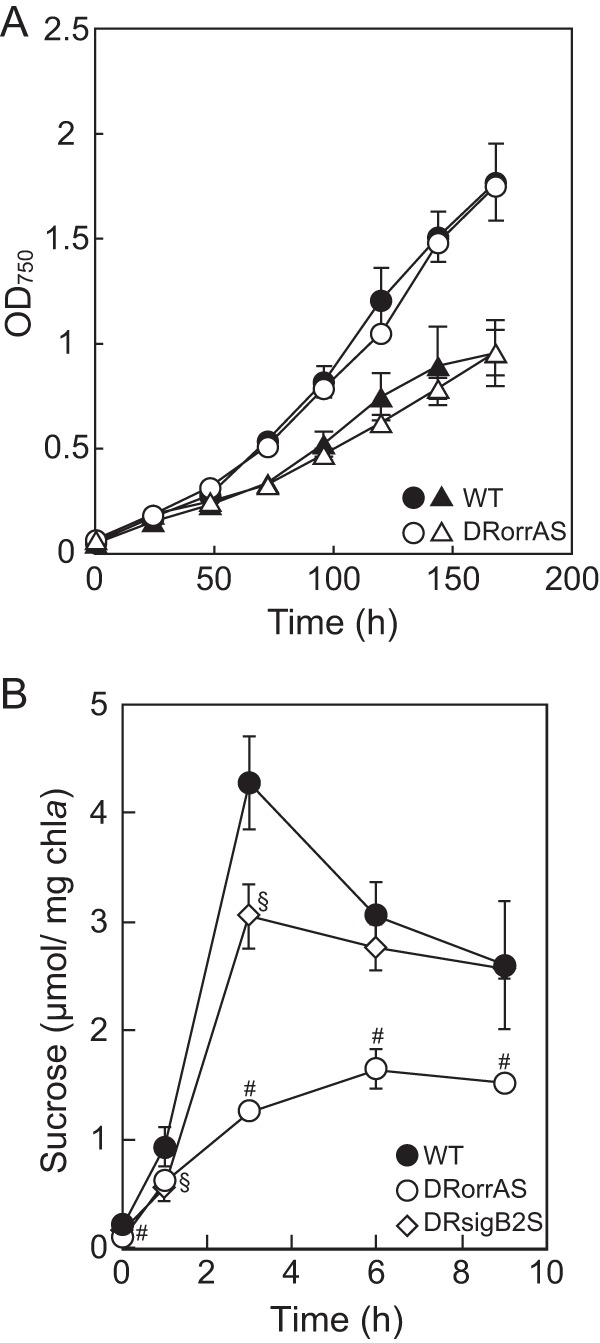
Growth and sucrose accumulation of Anabaena PCC 7120 under salt stress conditions. (A) Growth of the wild-type (WT) and the orrA disruptant DRorrAS with (triangles) or without (circles) 50 mM NaCl was measured as the OD750. (B) Cells in the logarithmic phase of the wild-type, DRorrAS, and sigB2 disruptant DRsigB2S strains were subjected to salt stress (50 mM NaCl) at time zero. Sucrose contents were determined at the indicated times. Means ± standard deviations (error bars) of at least three independent experiments are shown. Data that represent a significant difference (P < 0.05; t test) between the wild type and DRorrAS (#) or DRsigB2S (§) are indicated.
qRT-PCR.
Total RNA was extracted from whole filaments according to the method of Pinto et al. (34) and treated with DNase I (TaKaRa Bio). cDNA synthesis and quantitative reverse transcription-PCR (qRT-PCR) were performed as described previously using Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan) (35). Relative transcript levels were normalized to the value for 16S rRNA and are represented as means of duplicate measurements. Experiments were repeated at least three times.
Primer extension analysis.
Primer extension analysis of the 5′ end of the orrA transcripts was performed using the fluorescence-labeled primer IRD-orrA (Table 1) as described previously (36). DNA sequencing was carried out with a SequiTherm Excel II DNA Sequencing Kit-LC (Epicenter) using the plasmid pGEMorrAup containing the region from −574 to +280 with respect to the translation start site of the orrA gene as a template. The cDNA and sequencing products were analyzed with a model 4200 DNA sequencer (Li-Cor Biosciences).
RESULTS
Sucrose accumulation and induction of sucrose metabolism genes in response to salt stress.
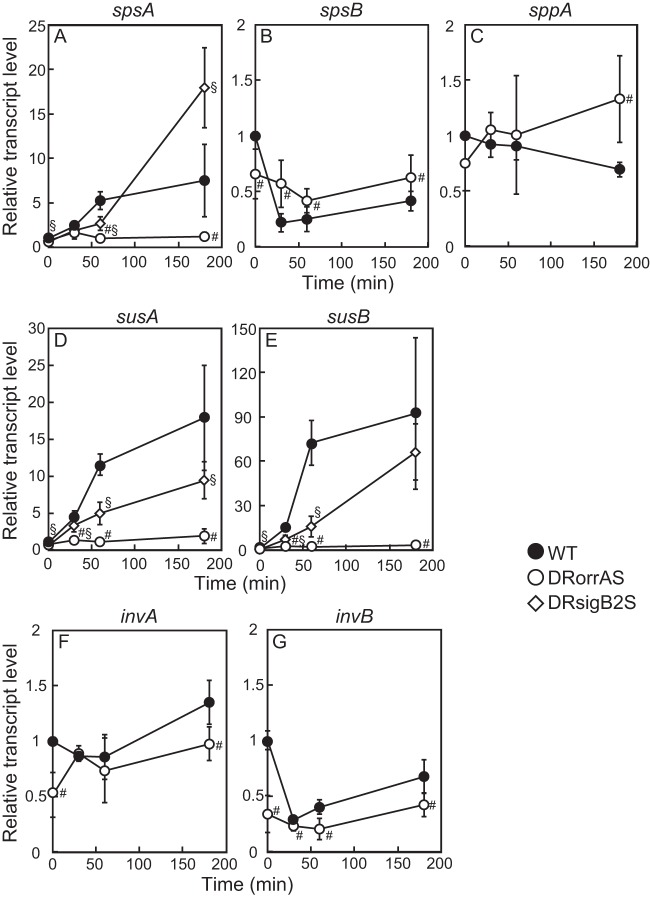
Diazotrophically grown Anabaena PCC 7120 showed a reduced growth rate in the presence of 50 mM NaCl (Fig. 2A) and accumulated sucrose in response to the addition of 50 mM NaCl (Fig. 2B). In the wild-type strain, the sucrose level was increased within 1 h after addition of NaCl and reached the highest level at 3 h, before gradually decreasing. The sucrose content of cells subjected to NaCl for 3 h was more than 20-fold higher than that of nontreated cells. Changes in expression of genes involved in sucrose metabolism by salt stress were determined by qRT-PCR in the wild-type strain. The transcript level of the spsA gene was upregulated within 30 min after salt stress and increased about 7-fold after 3 h (Fig. 3A). Expression of spsB was decreased by salt stress (Fig. 3B), and sppA was not affected (Fig. 3C). susA and susB were induced by salt stress (Fig. 3D and E). Particularly, induction of susB was remarkable. The transcript level of susB was increased more than 90-fold after 3 h (Fig. 3E). Expression of invA did not respond to salt stress (Fig. 3F), while invB was downregulated (Fig. 3G).
FIG 3.
Changes in the transcript levels of genes involved in sucrose metabolism in response to salt stress. The transcript levels of spsA (A), spsB (B), sppA (C), susA (D), susB (E), invA (F), and invB (G) before (0 h) and at 30, 60, and 180 min after the addition of 50 mM NaCl were determined by qRT-PCR in the wild-type, DRorrAS, and DRsigB2S strains. The transcript levels were determined in duplicate measurements using three independently grown cultures. The transcript level at 0 min in the wild type was taken as 1. Data that represent a significant difference (P < 0.05; t test) between the wild type and DRorrAS (#) or DRsigB2S (§) are indicated.
OrrA is necessary for the induction of sucrose metabolism genes by salt stress.
OrrA of Anabaena PCC 7120 and SigB of Synechocystis PCC 6803 are shown to be involved in regulation of the salt response (16, 19). We investigated expression of orrA and sigB2 under salt stress conditions. The transcript levels of both orrA and sigB2 were upregulated by NaCl (Fig. 4). Expression of orrA was drastically induced within 30 min after NaCl addition (Fig. 4A), and the sigB2 transcript level was gradually increased by salt stress (Fig. 4B). In Anabaena PCC 7120, there are three sigB2 paralogues, sigB, sigB3, and sigB4 (18). sigB2 is carried on the chromosome, whereas sigB, sigB3, and sigB4 are carried on plasmids (37). The transcript levels of the plasmid carrying sigB2 paralogues did not respond to the addition of NaCl (Fig. 4B).
FIG 4.
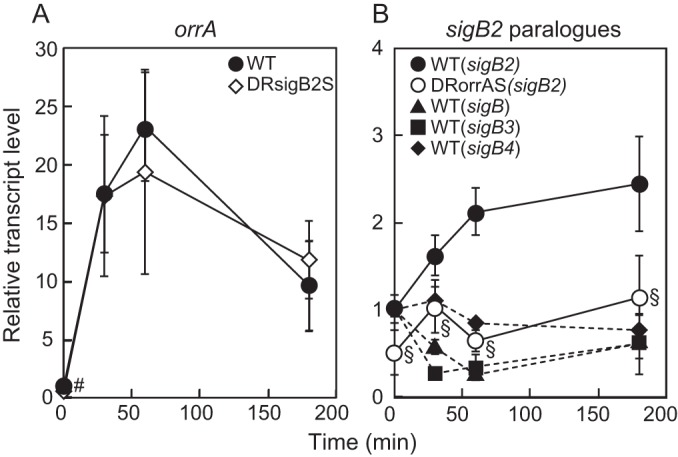
Changes in the transcript levels of orrA and sigB2 paralogues in response to salt stress. (A) The transcript levels of orrA in the wild-type and DRsigB2S strains after addition of 50 mM NaCl were determined by qRT-PCR. (B) The transcript levels of sigB2 paralogues, sigB, sigB2, sigB3, and sigB4, in the wild type and sigB2 in the DRorrAS strain were determined. The transcript levels were determined in duplicate measurements using three independently grown cultures. The transcript level at 0 min in the wild type was taken as 1. Data that represent a significant difference (P < 0.05; t test) between the wild type and DRorrAS (#) or DRsigB2S (§) are indicated.
To examine the role of OrrA and SigB2 on the salt response of sucrose metabolism genes, the orrA and sigB2 genes were inactivated by deletion from the chromosome of Anabaena PCC 7120. Disruption of orrA decreased the transcript level of sigB2 and diminished the upregulation of sigB2 by salt stress (Fig. 4B), while sigB2 disruption did not affect the upregulation of orrA (Fig. 4A). In addition to sigB2, OrrA was necessary for salt induction of sucrose metabolism genes. Before NaCl addition, the transcript levels of spsA, susA, and susB in the orrA disruptant were equivalent to those in the wild type, but induction of spsA, susA, and susB by NaCl was completely abolished in the orrA disruptant (Fig. 3). Expression of spsA, susA, and susB was also regulated by SigB2. The transcript levels of spsA, susA, and susB in the sigB2 disruptant were lower than those in the wild type 60 min after NaCl addition though expression of these genes was upregulated by NaCl (Fig. 3). Thus, the effects of sigB2 disruption were limited to the beginning of salt stress. It was concluded that OrrA controls the salt induction of spsA, susA, susB, and sigB2 and that SigB2 is involved in the early response of spsA, susA, and susB to salt stress.
OrrA activates sucrose synthesis.
Sucrose accumulation of the orrA disruptant was investigated under salt stress conditions. Sucrose was gradually increased in the orrA disruptant in contrast to drastic accumulation in the wild type (Fig. 2B). The sucrose content of the orrA disruptant reached 1.6 ± 0.2 μmol/mg Chl a at 6 h after NaCl addition, which was 40% of the highest level of the wild type (4.3 ± 0.4 μmol/mg Chl a) (Fig. 2B). Although the sucrose content of the orrA disruptant was decreased, its growth rate was comparable to that of the wild type even in the medium supplemented with 50 mM NaCl (Fig. 2A). These results support the idea that sucrose is more important as a general stress protectant than salt stress-specific protectants (38). The sucrose content was also reduced by the sigB2 disruption during the first 3 h after NaCl addition (Fig. 2B). The sucrose contents of the sigB2 disruptant at 1 and 3 h were decreased to 60% and 71% of those of the wild type, respectively, while differences in sucrose contents after 6 h were not significant (Fig. 2B).
To further investigate the regulation of sucrose synthesis by OrrA, the orrA gene was overexpressed from a multicopy plasmid. The transcription initiation site of orrA was situated at position −73 with respect to the translation start site of the orrA gene (Fig. 5). A plasmid, pAorrA, which carries the orrA gene with 240 bp of the promoter region, was transferred into Anabaena PCC 7120. We obtained a number of strains bearing pAorrA, but diazotrophic growth of these strains was unsettled; i.e., some strains were able to grow diazotrophically, but others were not. Since growth levels of all strains in nitrate-containing medium were similar, experiments were carried out in the presence of nitrate using a representative strain. The orrA transcript level was more than 10-fold higher in the strain bearing pAorrA than in the control strain (Fig. 6A). Overexpression of orrA increased the transcript levels of spsA, susA, and susB, while the increase was less than that of the orrA transcript, suggesting the presence of translational or posttranslational regulation of OrrA (Fig. 6A). The sucrose level of the control strain in the presence of nitrate was seven times as high as that of the wild type under diazotrophic conditions, but the sucrose content of the strain overexpressing orrA was nevertheless twice as much as that of the control strain (Fig. 6B). It is concluded that increase in expression of the orrA gene causes accumulation of sucrose irrespective of salt stress.
FIG 5.
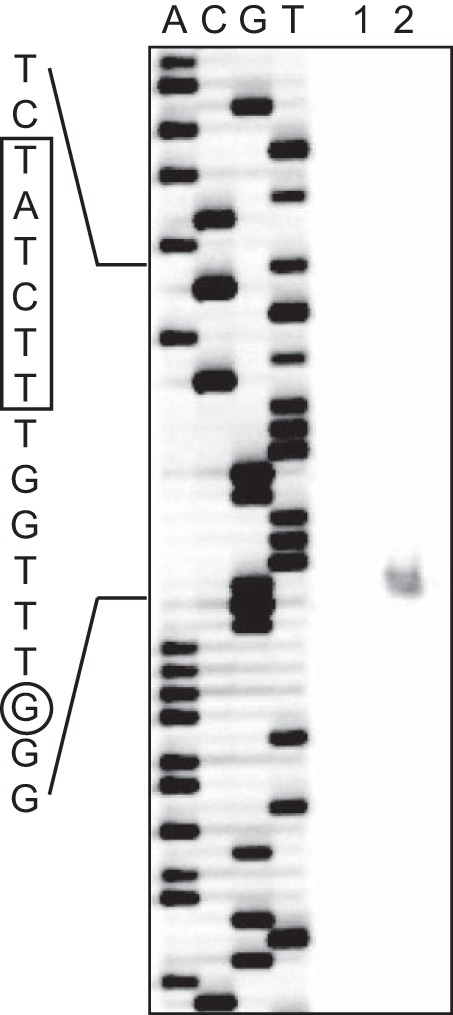
Primer extension analysis of the 5′ end of the orrA transcript. Primer extension was carried out with primer IRD-orrA, and total RNA was isolated from the wild-type cells grown without NaCl addition (lane 1) or from cells subjected to salt stress for 60 min (lane 2). A sequencing ladder of the same DNA region is shown in lanes A, C, G, and T.
FIG 6.
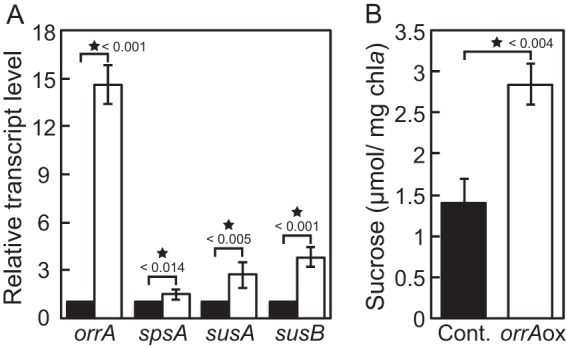
Overexpression of the orrA gene. Anabaena PCC 7120 having extra copies of the orrA gene on a plasmid (white bars) or the control plasmid (black bars) was grown in the presence of combined nitrogen. The transcript levels of orrA, spsA, susA, and susB (A) and sucrose contents (B) in the mid-logarithmic phase were determined without adding NaCl. Means ± standard deviations (error bars) of three independent experiments are shown. Data that represent a significant difference by applying the t test are marked with asterisks and P values.
DISCUSSION
In the present study, we demonstrated that the response regulator OrrA controls sucrose synthesis in Anabaena PCC 7120. Disruption of the orrA gene resulted in abolishment of salt induction of the genes involved in sucrose metabolism (Fig. 3) and reduced accumulation of sucrose under salt stress conditions (Fig. 2B). Moreover, in Anabaena PCC 7120 overexpressing the orrA gene, the transcript levels of genes involved in sucrose metabolism and the cellular sucrose level were increased without addition of salt (Fig. 6). Thus, OrrA plays a pivotal role in the regulation of sucrose synthesis. Metabolic engineering using a transcriptional regulator that regulates sugar catabolism has succeeded in accelerating production of bioplastics in Synechocystis PCC 6803 (39, 40). OrrA should be applicable to metabolic engineering of sucrose production by cyanobacteria.
Two-component systems that contribute to the perception and transduction of salt stress signals have been identified in Synechocystis PCC 6803. The histidine kinase/response regulator pairs Hik33/Rre31, Hik34/Rre1, Hik2/Rre1, Hik16/Hik41/Rre17, and Hik10/Rre3 are involved in the upregulation of different sets of genes (41). However, expression of many salt-inducible genes, including genes involved in compatible solute synthesis, was not controlled by any of these two-component systems. Four two-component systems, Hik33/Rre31, Hik34/Rre1, Hik2/Rre1, and Hik10/Rre3, are also present in Anabaena PCC 7120, but OrrA is not found in Synechocystis PCC 6803 (42). OrrA is conserved in cyanobacteria belonging to the genera of Anabaena and Nostoc. Four genes (all3767-all3764) of two-component systems are located upstream of the orrA gene on the genome of Anabaena PCC 7120 (Fig. 7). All3767 is a histidine kinase with two PAS domains, All3766 is a response regulator without output domain, and All3765 and All3764 are histidine kinases with a receiver domain (43). PAS (Per-ARNT-Sim) domains perform a variety functions within sensory proteins by promoting protein-protein interaction or by directly binding different substrates, such as cofactors, ligands, and ions (44). Orthologues of all3765 and all3764 are also found in the vicinity of orrA in the genomes of Anabaena and Nostoc species (Fig. 7). A genetic locus containing orrA and all3767-all3764 could be involved in the perception and transduction of salt stress signals in Anabaena PCC 7120.
FIG 7.
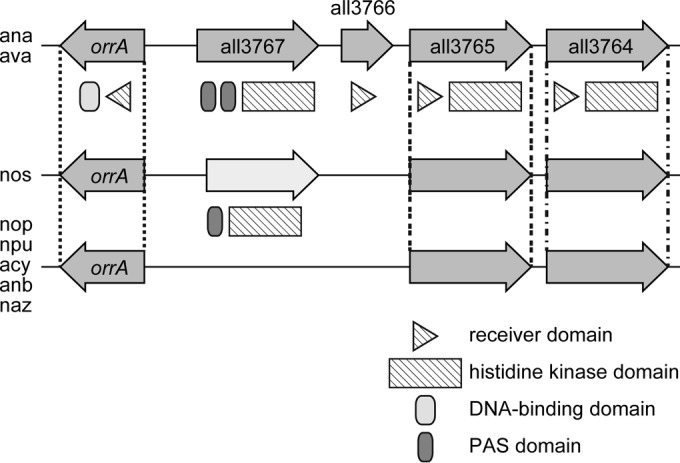
The genetic organization around the orrA gene in Anabaena and Nostoc species. ana, Anabaena PCC 7120; ava, Anabaena variabilis ATCC 29413; nos, Nostoc strain PCC 7107; nop, Nostoc strain PCC 7524; npu, Nostoc punctiforme PCC 73102; acy, Anabaena cylindrica PCC 7122; anb, Anabaena strain 90; naz, Anabaena azollae 0708. The domain structure of each gene product is schematically shown.
The salt-responsive promoter of orrA was identified by primer extension analysis (Fig. 5). The promoter sequence was compared with that of the lti2 gene, which was also induced by salt stress (16, 17). We found a conserved sequence, TCTN6AGA, upstream of the −35 promoter region (Fig. 8A). The sequences are centered 77.5 and 74.5 nucleotides (nt) upstream from the transcription initiation sites of orrA and lti2, respectively, indicating that a transcriptional regulator that binds to this sequence activates transcription by the class I mechanism (45). The conserved sequence was also found within regions upstream of spsA and susA (Fig. 8B), suggesting a common regulatory mechanism of orrA, lti2, spsA, and susA. OrrA belongs to the NarL family, and response regulators of this family generally recognize inverted repeat sequences (46). Thus, OrrA is a candidate for the transcriptional regulator that binds to the conserved sequence.
FIG 8.
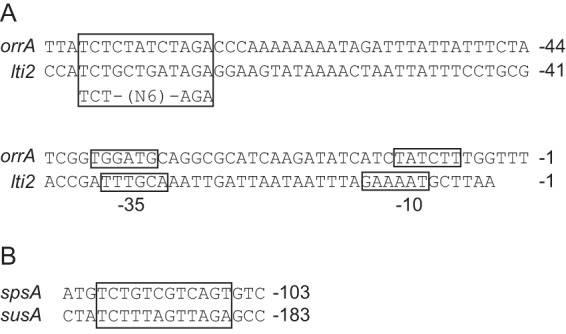
Promoter sequences of the salt-inducible genes. (A) Nucleotide sequences of the promoter regions of the orrA and lti2 genes are indicated. Numbers indicate the distance from the transcription initiation site. The −10 and −35 promoter sequences and the conserved inverted repeat sequence are boxed. (B) The conserved sequences found within upstream regions of the spsA and susA genes are boxed. Numbers indicate the distance from the translational start codon.
A group 2 sigma factor, SigB2, which was upregulated by salt stress under the control of OrrA (Fig. 4B), was involved in the early response to salt stress. Disruption of sigB2 decreased the transcript levels of spsA, susA, and susB and the sucrose level during the first 1 and 3 h after salt stress, respectively (Fig. 2B and 3). However, decreases in the expression of spsA, susA, and susB and the sucrose level were prominent in the orrA disruptant even in the beginning of salt stress (Fig. 2B and 3), indicating that OrrA or a transcription factor(s) regulated by OrrA was also involved in the early response of these genes. It is indicated that SigB regulates the early salt acclimation processes, including glucosylglycerol synthesis in Synechocystis PCC 6803 (19). Inactivation of sigB retards the salt induction of ggpS encoding glucosylglycerol-phosphate synthase but does not totally prevent the induction. Thus, SigB homologues of cyanobacteria could regulate the salt response in collaboration with other transcriptional regulators, such as OrrA, especially in early phases of salt stress.
The SPS activity of Anabaena PCC 7120 cells increases by salt treatment (25), and the spsA gene has been induced by salt stress (Fig. 3A). Since expression of spsB was decreased by salt stress (Fig. 3B), induction of spsA is likely to contribute to the salt-dependent increase of SPS activity. Although induction of spsA by salt stress was completely abolished in the orrA disruptant (Fig. 3A), the sucrose level was increased (Fig. 2B), indicating posttranslational activation of SpsA. SPS activities of Synechococcus sp. strain PCC 6301 and Synechocystis PCC 6803 were stimulated by the addition of NaCl to the assay buffer, while with Anabaena PCC 7120 NaCl addition did not change the SPS activity (25, 47). As the activation of SpsA of Synechocystis PCC 6803 by NaCl is concentration dependent (47), the concentration of NaCl (171 mM) used in the assay of SPS of Anabaena PCC 7120 might be inappropriate. In Synechocystis PCC 6803, salt-induced sucrose accumulation is achieved by SpsA (25), and overexpression of spsA results in increased sucrose accumulation under salt stress conditions (22). In the orrA-overexpressing strain, expression of spsA and the sucrose level were increased without the addition of NaCl (Fig. 6). Addition of NaCl would further increase the expression of spsA and the activity of SPS, followed by enhanced accumulation of sucrose. Thus, the orrA-overexpressing strain is a promising strain for sucrose production using cyanobacteria. The susA and susB genes were also induced by NaCl (Fig. 3), and SuS is also capable of synthesizing sucrose (10). However, SuS functions mostly to catalyze the cleavage of sucrose in vivo. In Anabaena PCC 7119, sucrose levels were markedly increased by susA disruption, and sucrose could not be detected in an susA-overexpressing strain (12). Thus, in salt-treated Anabaena cells, an increase in sucrose synthesis is accompanied by an enhancement in sucrose degradation. Although sucrose degradation is activated by NaCl, the transcriptional and posttranslational regulation of SPS activity by NaCl could result in a net accumulation of sucrose. Sugar cycles are also observed in trehalose accumulation during dehydration stress in Anabaena PCC 7120 (15). These sugar cycles may have physiological functions, such as control of respiration, sugar accumulation, and sugar signaling, as suggested in plants (48).
ACKNOWLEDGMENTS
This work was supported by PRESTO of the Japan Science and Technology Agency and Grant-in-aid for Scientific Research (C) 23603005 from the Japan Society for the Promotion of Science to S.E.
Footnotes
Published ahead of print 7 July 2014
REFERENCES
- 1.Laloknam S, Tanaka K, Buaboocha T, Waditee R, Incharoensakdi A, Hibino T, Tanaka Y, Takabe T. 2006. Halotolerant cyanobacterium Aphanothece halophytica contains a betaine transporter active at alkaline pH and high salinity. Appl. Environ. Microbiol. 72:6018–6026. 10.1128/AEM.00733-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AD. 1976. Microbial water stress. Bacteriol. Rev. 40:803–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagemann M. 2011. Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol. Rev. 35:87–123. 10.1111/j.1574-6976.2010.00234.x [DOI] [PubMed] [Google Scholar]
- 4.Cumino AC, Perez-Cenci M, Giarrocco LE, Salerno GL. 2010. The proteins involved in sucrose synthesis in the marine cyanobacterium Synechococcus sp. PCC 7002 are encoded by two genes transcribed from a gene cluster. FEBS Lett. 584:4655–4660. 10.1016/j.febslet.2010.10.040 [DOI] [PubMed] [Google Scholar]
- 5.Marin K, Huckauf J, Fulda S, Hagemann M. 2002. Salt-dependent expression of glucosylglycerol-phosphate synthase, involved in osmolyte synthesis in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 184:2870–2877. 10.1128/JB.184.11.2870-2877.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagemann M. 2013. Genomics of salt acclimation: Synthesis of compatible solutes among cyanobacteria, p 27–55 In Chauvat F, Cassier-Chauvat C. (ed), Genomics of cyanobacteria. Academic Press, Amsterdam, The Netherlands [Google Scholar]
- 7.Reed RH, Richardson DL, Warr SRC, Stewart WDP. 1984. Carbohydrate accumulation and osmotic stress in cyanobacteria. Microbiology 130:1–4. 10.1099/00221287-130-1-1 [DOI] [Google Scholar]
- 8.Wolk CP, Ernst A, Elhai J. 1994. Heterocyst metabolism and development, p 769–823 In Bryant D. (ed), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 9.Cumino AC, Marcozzi C, Barreiro R, Salerno GL. 2007. Carbon cycling in Anabaena sp. PCC 7120. Sucrose synthesis in the heterocysts and possible role in nitrogen fixation. Plant Physiol. 143:1385–1397. 10.1104/pp.106.091736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porchia AC, Salerno GL. 1996. Sucrose biosynthesis in a prokaryotic organism: presence of two sucrose-phosphate synthases in Anabaena with remarkable differences compared with the plant enzymes. Proc. Natl. Acad. Sci. U. S. A. 93:13600–13604. 10.1073/pnas.93.24.13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumino A, Ekeroth C, Salerno GL. 2001. Sucrose-phosphate phosphatase from Anabaena sp. strain PCC 7120: isolation of the protein and gene revealed significant structural differences from the higher-plant enzyme. Planta 214:250–256. 10.1007/s004250100608 [DOI] [PubMed] [Google Scholar]
- 12.Curatti L, Flores E, Salerno G. 2002. Sucrose is involved in the diazotrophic metabolism of the heterocyst-forming cyanobacterium Anabaena sp. FEBS Lett. 513:175–178. 10.1016/S0014-5793(02)02283-4 [DOI] [PubMed] [Google Scholar]
- 13.López-Igual R, Flores E, Herrero A. 2010. Inactivation of a heterocyst-specific invertase indicates a principal role of sucrose catabolism in heterocysts of Anabaena sp. J. Bacteriol. 192:5526–5533. 10.1128/JB.00776-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas WA, Nishi CN, Giarrocco LE, Salerno GL. 2011. Differential roles of alkaline/neutral invertases in Nostoc sp. PCC 7120: Inv-B isoform is essential for diazotrophic growth. Planta 233:153–162. 10.1007/s00425-010-1288-5 [DOI] [PubMed] [Google Scholar]
- 15.Higo A, Katoh H, Ohmori K, Ikeuchi M, Ohmori M. 2006. The role of a gene cluster for trehalose metabolism in dehydration tolerance of the filamentous cyanobacterium Anabaena sp. PCC 7120. Microbiology 152:979–987. 10.1099/mic.0.28583-0 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz SH, Black TA, Jäger K, Panoff JM, Wolk CP. 1998. Regulation of an osmoticum-responsive gene in Anabaena sp. strain PCC 7120. J. Bacteriol. 180:6332–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato N. 1992. Cloning of a low-temperature-induced gene lti2 from the cyanobacterium Anabaena variabilis M3 that is homologous to alpha-amylases. Plant Mol. Biol. 18:165–170. 10.1007/BF00018474 [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura H, Okamoto S, Tsumuraya Y, Ohmori M. 2007. Group 3 sigma factor gene, sigJ, a key regulator of desiccation tolerance, regulates the synthesis of extracellular polysaccharide in cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 14:13–24. 10.1093/dnares/dsm003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikkinen H-L, Hakkila K, Gunnelius L, Huokko T, Pollari M, Tyystjärvi T. 2012. The SigB σ factor regulates multiple salt acclimation responses of the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 158:514–523. 10.1104/pp.111.190058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana N, Van der Kooy F, Van de Rhee MD, Voshol GP, Verpoorte R. 2011. Renewable energy from cyanobacteria: energy production optimization by metabolic pathway engineering. Appl. Microbiol. Biotechnol. 91:471–490. 10.1007/s00253-011-3394-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducat DC, Way JC, Silver PA. 2011. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 29:95–103. 10.1016/j.tibtech.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 22.Du W, Liang F, Duan Y, Tan X, Lu X. 2013. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab. Eng. 19:17–25. 10.1016/j.ymben.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 23.Ducat DC, Avelar-Rivas JA, Way JC, Silver PA. 2012. Rerouting carbon flux to enhance photosynthetic productivity. Appl. Environ. Microbiol. 78:2660–2668. 10.1128/AEM.07901-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Guerra LT, Li Z, Ludwig M, Dismukes GC, Bryant DA. 2013. Altered carbohydrate metabolism in glycogen synthase mutants of Synechococcus sp. strain PCC 7002: cell factories for soluble sugars. Metab. Eng. 16:56–67. 10.1016/j.ymben.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Hagemann M, Marin K. 1999. Salt-induced sucrose accumulation is mediated by sucrose-phosphate-synthase in cyanobacteria. J. Plant Physiol. 155:424–430. 10.1016/S0176-1617(99)80126-6 [DOI] [Google Scholar]
- 26.Watanabe A. 1960. List of algal strains in collection at the Institute of Applied Microbiology, University of Tokyo. J. Gen. Appl. Microbiol. 6:283–292. 10.2323/jgam.6.283 [DOI] [Google Scholar]
- 27.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61. 10.1099/00221287-111-1-1 [DOI] [Google Scholar]
- 28.Fujisawa T, Okamoto S, Katayama T, Nakao M, Yoshimura H, Kajiya-Kanegae H, Yamamoto S, Yano C, Yanaka Y, Maita H, Kaneko T, Tabata S, Nakamura Y. 2014. CyanoBase and RhizoBase: databases of manually curated annotations for cyanobacterial and rhizobial genomes. Nucleic Acids Res. 42:D666–D670. 10.1093/nar/gkt1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golden JW, Wiest DR. 1988. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science 242:1421–1423. 10.1126/science.3144039 [DOI] [PubMed] [Google Scholar]
- 30.Black TA, Cai Y, Wolk CP. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77–84. 10.1111/j.1365-2958.1993.tb01670.x [DOI] [PubMed] [Google Scholar]
- 31.Elhai J, Vepritskiy A, Muro-Pastor AM, Flores E, Wolk CP. 1997. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 179:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon H, Golden JW. 1998. Heterocyst pattern formation controlled by a diffusible peptide. Science 282:935–938. 10.1126/science.282.5390.935 [DOI] [PubMed] [Google Scholar]
- 33.Mackinney G. 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140:315–322 [Google Scholar]
- 34.Pinto FL, Thapper A, Sontheim W, Lindblad P. 2009. Analysis of current and alternative phenol based RNA extraction methodologies for cyanobacteria. BMC Mol. Biol. 10:79. 10.1186/1471-2199-10-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehira S, Ohmori M. 2014. NrrA directly regulates expression of the fraF gene and antisense RNAs for fraE in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. Microbiology 160:844–850. 10.1099/mic.0.076703-0 [DOI] [PubMed] [Google Scholar]
- 36.Ehira S, Ohmori M. 2006. NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 59:1692–1703. 10.1111/j.1365-2958.2006.05049.x [DOI] [PubMed] [Google Scholar]
- 37.Kaneko T, Nakamura Y, Wolk CP, Kuritz T, Sasamoto S, Watanabe A, Iriguchi M, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kohara M, Matsumoto M, Matsuno A, Muraki A, Nakazaki N, Shimpo S, Sugimoto M, Takazawa M, Yamada M, Yasuda M, Tabata S. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205–213. 10.1093/dnares/8.5.205 [DOI] [PubMed] [Google Scholar]
- 38.Klähn S, Hagemann M. 2011. Compatible solute biosynthesis in cyanobacteria. Environ. Microbiol. 13:551–562. 10.1111/j.1462-2920.2010.02366.x [DOI] [PubMed] [Google Scholar]
- 39.Osanai T, Oikawa A, Numata K, Kuwahara A, Iijima H, Doi Y, Saito K, Hirai MY. 2014. Pathway-level acceleration of glycogen catabolism by a response regulator in the cyanobacterium Synechocystis species PCC 6803. Plant Physiol. 164:1831-1841. 10.1104/pp.113.232025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osanai T, Numata K, Oikawa A, Kuwahara A, Iijima H, Doi Y, Tanaka K, Saito K, Hirai MY. 2013. Increased bioplastic production with an RNA polymerase sigma factor SigE during nitrogen starvation in Synechocystis sp. PCC 6803. DNA Res. 20:525-535. 10.1093/dnares/dst028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Los DA, Suzuki I, Zinchenko VV, Murata N. 2008. Stress responses in Synechocystis: regulated genes and regulatory systems, p 117–158 In Herrero A, Flores E. (ed), The cyanobacteria—molecular biology, genomics and evolution. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 42.Ashby MK, Houmard J. 2006. Cyanobacterial two-component proteins: Structure, diversity, distribution, and evolution. Microbiol. Mol. Biol. Rev. 70:472–509. 10.1128/MMBR.00046-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohmori M, Ikeuchi M, Sato N, Wolk P, Kaneko T, Ogawa T, Kanehisa M, Goto S, Kawashima S, Okamoto S, Yoshimura H, Katoh H, Fujisawa T, Ehira S, Kamei A, Yoshihara S, Narikawa R, Tabat S. 2001. Characterization of genes encoding multi-domain proteins in the genome of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:271–284. 10.1093/dnares/8.6.271 [DOI] [PubMed] [Google Scholar]
- 44.Henry JT, Crosson S. 2011. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu. Rev. Microbiol. 65:261–286. 10.1146/annurev-micro-121809-151631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Browning DF, Busby SJ. 2004. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2:57–65. 10.1038/nrmicro787 [DOI] [PubMed] [Google Scholar]
- 46.de Been M, Bart MJ, Abee T, Siezen RJ, Francke C. 2008. The identification of response regulator-specific binding sites reveals new roles of two-component systems in Bacillus cereus and closely related low-GC Gram-positives. Environ. Microbiol. 10:2796–2809. 10.1111/j.1462-2920.2008.01700.x [DOI] [PubMed] [Google Scholar]
- 47.Desplats P, Folco E, Salerno GL. 2005. Sucrose may play an additional role to that of an osmolyte in Synechocystis sp. PCC 6803 salt-shocked cells. Plant Physiol. Biochem. 43:133–138. 10.1016/j.plaphy.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 48.Roby C, Cortès S, Gromova M, Le Bail J-L, Roberts JKM. 2002. Sucrose cycling in heterotrophic plant cell metabolism: First step towards an experimental model. Mol. Biol. Rep. 29:145–149. 10.1023/A:1020309309045 [DOI] [PubMed] [Google Scholar]