Abstract
Few studies have evaluated the potential benefits of the topical application of probiotic bacteria or material derived from them. We have investigated whether a probiotic bacterium, Lactobacillus rhamnosus GG, can inhibit Staphylococcus aureus infection of human primary keratinocytes in culture. When primary human keratinocytes were exposed to S. aureus, only 25% of the keratinocytes remained viable following 24 h of incubation. However, in the presence of 108 CFU/ml of live L. rhamnosus GG, the viability of the infected keratinocytes increased to 57% (P = 0.01). L. rhamnosus GG lysates and spent culture fluid also provided significant protection to keratinocytes, with 65% (P = 0.006) and 57% (P = 0.01) of cells, respectively, being viable following 24 h of incubation. Keratinocyte survival was significantly enhanced regardless of whether the probiotic was applied in the viable form or as cell lysates 2 h before or simultaneously with (P = 0.005) or 12 h after (P = 0.01) S. aureus infection. However, spent culture fluid was protective only if added before or simultaneously with S. aureus. With respect to mechanism, both L. rhamnosus GG lysate and spent culture fluid apparently inhibited adherence of S. aureus to keratinocytes by competitive exclusion, but only viable bacteria or the lysate could displace S. aureus (P = 0.04 and 0.01, respectively). Furthermore, growth of S. aureus was inhibited by either live bacteria or lysate but not spent culture fluid. Together, these data suggest at least two separate activities involved in the protective effects of L. rhamnosus GG against S. aureus, growth inhibition and reduction of bacterial adhesion.
INTRODUCTION
The concept that probiotics are beneficial to gut health has been investigated for a number of years. Studies have demonstrated that probiotics improve gut function potentially through a number of mechanisms (1, 2), including increasing epithelial barrier function (3) and modulation of the immune response (4–6). There is also evidence that probiotics can prevent colonization of the gut by pathogens. This can be via mechanisms such as downregulation of virulence factors and inhibition of pathogen adherence to the epithelium (7–9). For example, Lactobacillus species inhibit the adhesion of Enterobacter sakazakii to intestinal mucus by competitive exclusion (8, 10). Other studies demonstrated that some probiotics increase the production of intestinal mucin, thus inhibiting pathogen adherence to intestinal epithelial cells (11). Probiotics are also able to produce antimicrobial peptides (bacteriocins) and acids. Collectively, there are numerous probiotic-mediated mechanisms that limit pathogen colonization (1, 12–14).
Since probiotics may have positive impacts on the gut, their potential effects on other systems, such as the mouth (15, 16) and the urogenital tract (17), have also been investigated. For example, a study in 2002 examined the impact of oral administration of Lactobacillus plantarum to patients who had abdominal surgery and showed that this bacterium lowered the incidence of postsurgical infection (18). Currently, research is also investigating the topical use of probiotics to augment the skin barrier function to promote skin health or prevent or treat disease (9, 19–24). The benefits of topical application of probiotics are still speculative, and researchers are now focusing on this area to improve conditions such as excessive skin sensitivity, atopic dermatitis, and psoriasis and to stimulate the wound healing process (19, 25–29). However, an important consideration will be the safety of using live bacteria, especially in situations where the skin barrier is breached. For this reason, many investigators have used bacterial lysates in their studies. Topical application of sonicated Streptococcus thermophilus strains to patients suffering from atopic dermatitis resulted in improved barrier function apparently through increasing the level of ceramides in the stratum corneum (26). The topical application of Lactobacillus plantarum lysate inhibited the pathogenic activity of Pseudomonas aeruginosa in infected burns (29). In vivo, L. plantarum lysate has also been shown to improve wound healing in burn patients (30).
Staphylococcus aureus is both a transient colonizer of skin and a major opportunistic skin pathogen, causing diseases ranging from impetigo to life-threatening conditions such as sepsis (31, 32). Previously, our laboratory demonstrated that the probiotic Lactobacillus reuteri or its lysate could protect epidermal keratinocytes from the toxic effects of S. aureus via competitive exclusion of the pathogen from keratinocyte binding sites (33). In the present study, we have identified Lactobacillus rhamnosus GG as a second probiotic with the ability to protect skin cells from the effects of S. aureus. The selection of L. rhamnosus GG was based on the results of a screening assay testing a range of probiotics for their ability to protect human keratinocytes from the effects of S. aureus (data not shown). In this assay, L. rhamnosus GG proved to be extremely efficacious either live or as a lysate and uses multiple mechanisms to protect against infection, including inhibition of S. aureus growth, competitive exclusion, and displacement of the pathogen from keratinocytes.
MATERIALS AND METHODS
Mammalian cell culture.
Normal human epidermal keratinocytes (NHEK) cultured in keratinocyte basal medium (Promocell, Heidelberg, Germany) containing a supplement mix (bovine pituitary extract, 0.004 mg/ml; epidermal growth factor [recombinant human], 0.125 ng/ml; insulin [recombinant human], 5 μg/ml; hydrocortisone, 0.33 μg/ml; epinephrine, 0.39 μg/ml; and holo-transferrin [human], 10 μg/ml) and 0.06 mM CaCl2 (Promocell) were used as a model system. These were cultured routinely at 37°C in a humid atmosphere of 5% CO2 in T-75 culture flasks as described previously (33).
Bacterial cell culture.
Lactobacillus rhamnosus Goldin and Gorbach (L. rhamnosus GG; ATCC 53103), Lactobacillus reuteri (ATCC 55730), and Lactobacillus salivarius (UCC118) (ATCC, Middlesex, United Kingdom) were grown routinely in Wilkins-Chalgren broth or agar (Oxoid, Basingstoke, United Kingdom) at 37°C in an anaerobic cabinet (atmosphere,10:10:80 H2-CO2-N2). Staphylococcus aureus was grown aerobically at 37°C in nutrient broth (Oxoid) as described previously (33).
Treatment of keratinocytes with bacteria.
Bacteria (108 CFU/ml of probiotics and 106 CFU/ml of S. aureus) were centrifuged at 15,000 × g, washed twice in 0.85% NaCl, and resuspended in keratinocyte basal medium. This suspension was added directly to 5 × 103 cells/cm2 of NHEK growing in 24-well plates. For experiments using a probiotic lysate, 100 ml of 108-CFU/ml L. rhamnosus GG was centrifuged, washed, resuspended in 25 ml of phosphate-buffered saline (PBS, pH 7.4; Invitrogen, Life Technologies Ltd., Paisley, United Kingdom), and lysed using an MSE Soniprep 150. Samples were filtered using a 0.22-μm-pore filter (Millipore, Billerica, MA) to remove any whole bacteria remaining. Approximately 100 μl of this lysate was used to treat keratinocytes (5 × 105 cells/cm2). In some experiments, cells were sedimented in a centrifuge at 15,000 × g for 5 min, and the cell-free supernatant (spent culture fluid) was collected and filtered using a 0.22-μm-pore filter (Millipore) to remove any whole bacteria remaining. In other experiments, keratinocyte monolayers were coinfected with pathogen plus probiotics or lysates simultaneously. In separate experiments, cells were exposed to L. rhamnosus GG lysate for 2, 4, 6, 8, and 12 h after S. aureus infection had commenced. In all experiments, keratinocytes were detached and cell viability was determined using trypan blue exclusion assays as described in reference 33. In other experiments using heated lysates, these were heat inactivated by placing them in a boiling water bath at 100°C for 5 min.
Measurement of S. aureus viability in cell culture.
To determine whether L. rhamnosus GG lysates or spent culture fluid was able to inhibit the growth of S. aureus in cell culture, keratinocytes were grown to confluence in a 24-well plate. These were exposed to 100 μl of 106-CFU/ml S. aureus alone or S. aureus plus 100 μl of L. rhamnosus GG lysates or 100 μl of spent culture fluid. In separate experiments, cells were exposed to L. rhamnosus GG lysates for 2, 4, 6, 8, and 12 h after infection with S. aureus. The total number of viable staphylococci was determined by counting the colonies as described previously (33).
Measurement of bacterial adhesion to keratinocytes.
Confluent keratinocytes were exposed to 106 CFU/ml of S. aureus and 108 CFU/ml of L. rhamnosus GG for 1 h. Cells were then washed three times in PBS (pH 7.4) to remove nonadherent bacteria. The cells were trypsinized and serial dilution plate counts performed to assess the number of adherent bacteria. Selective agar was used for growth of staphylococci. Additionally, keratinocytes were exposed to 106 log CFU/ml of S. aureus combined with 100 μl of lysate or spent culture fluid of L. reuteri or Lactobacillus salivarius UCC118. The experiment was carried out three times and results were taken as triplicates.
In separate experiments, cells were exposed to 100 μl of 108-CFU/ml probiotic bacteria or lysates or spent culture fluid for 1 h before the addition of 100 μl of 106-CFU/ml S. aureus at the same time or 2, 4, 6, 8, and 12 h after infection with S. aureus.
Determination of bacterial antagonism.
A 10-μl aliquot of an overnight culture of S. aureus was inoculated into 7 ml of soft agar medium (0.7% agar) and was added directly onto plates prepoured with agar base. A volume of 50 μl of live organisms or 50 μl of lysate extracted from 108 CFU/ml of L. rhamnosus GG or L. reuteri cultures was spotted onto an S. aureus lawn. The inhibition zone was evaluated after overnight incubation by measuring the diameter of the zone in millimeters using a ruler.
Determination of the outcome of coculture (competition assays).
Aliquots (100 μl) of L. rhamnosus GG lysates and 100 μl of 106-CFU/ml S. aureus were inoculated into 10-ml Wilkins-Chalgren broth. The pH and optical density of cultures were measured at 0 and 24 h. At regular intervals (indicated below), bacteria were counted by serial dilution plate counts using selective agar.
Statistical analyses.
All experiments were performed a minimum of three times, with three replicates within each experiment. Data generated were analyzed by one-way analysis of variance (ANOVA) and post hoc Tukey test using SPSS (IBM SPSS Statistics version 16.0) program. Results were considered significant if the P value was <0.05. Data are expressed as means ± standard errors of the means (SEM).
RESULTS
L. rhamnosus GG protects keratinocytes from the pathogenic effects of S. aureus.
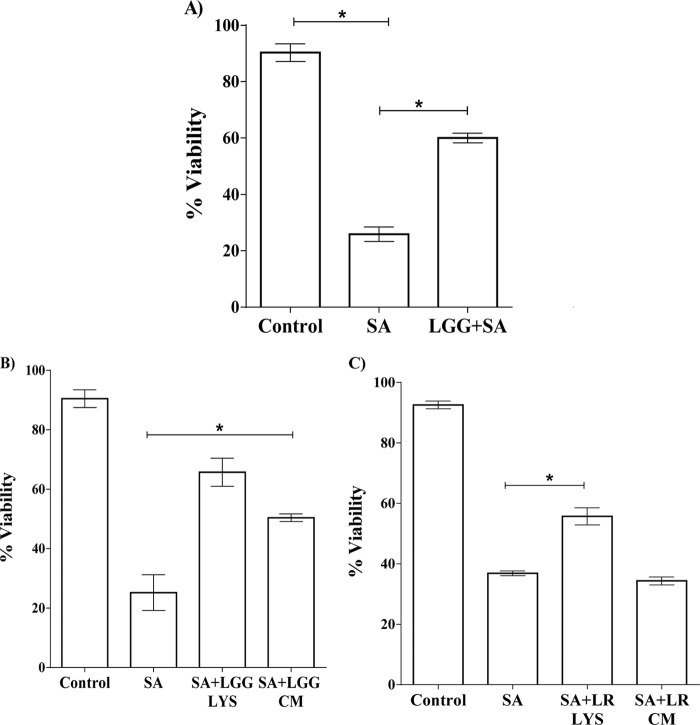
Initially, we investigated whether the viability of keratinocytes was affected by incubation with L. rhamnosus GG. However, following 24 h of incubation, there was no difference in the viability of keratinocytes incubated with the probiotic bacteria versus the control of untreated keratinocytes (data not shown). Next, the ability of L. rhamnosus GG to protect keratinocytes from the effects of S. aureus was investigated. In agreement with our previous findings (33), 24 h of exposure of keratinocytes to 106 CFU/ml of S. aureus resulted in significant keratinocyte death. However, keratinocytes incubated simultaneously with the pathogen and L. rhamnosus GG had a significantly higher viability (57%; P = 0.01) than monolayers infected with the pathogen alone (Fig. 1A).
FIG 1.
(A) L. rhamnosus GG, lysate, or spent culture fluid protects keratinocytes from the toxic effects of S. aureus. A combination of S. aureus (SA) and L. rhamnosus GG (LGG+SA) resulted in a significantly higher (P = 0.01) percentage of viable keratinocytes after 24 h than in monolayers infected with S. aureus alone. The data were compared to those produced by uninfected control cells (control). (B) The viability of S. aureus-infected keratinocytes treated with L. rhamnosus GG lysate (SA+LGG LYS) or spent culture fluid (SA+LGG CM) was significantly increased compared to that of keratinocytes infected with S. aureus (SA) alone. (C) Monolayers exposed to S. aureus and a lysate of L. reuteri (SA+LR LYS) had a significantly higher percentage of viable keratinocytes than those infected with pathogen alone, but the same effect was not found with the spent culture fluid of L. reuteri (SA+LR CM). Data are representative of three individual experiments, and all values represent means ± SEM. *, P < 0.05.
We investigated whether viable bacteria were essential for the protective effect of L. rhamnosus GG by examining the effect of probiotic lysate and spent culture fluid on S. aureus-infected keratinocytes. Neither lysate nor spent culture fluid significantly affected the viability of keratinocytes (P > 0.05) (data not shown). However, both the lysate and spent culture fluid reduced the toxicity of S. aureus such that the viabilities of treated keratinocytes were 65% and 55.93%, respectively, compared to 25% in keratinocytes infected with S. aureus alone (P = 0.006 and P = 0.01, respectively) (Fig. 1B). This is in contrast to the effects observed with L. reuteri, which we showed previously to be protective to pathogen-infected keratinocytes (33). L. reuteri provides protection only when added either live or as a lysate, but the spent culture fluid has no ability to protect keratinocytes from the effects of S. aureus (Fig. 1C).
L. rhamnosus GG lysate but not spent culture fluid rescues keratinocytes from S. aureus toxicity.
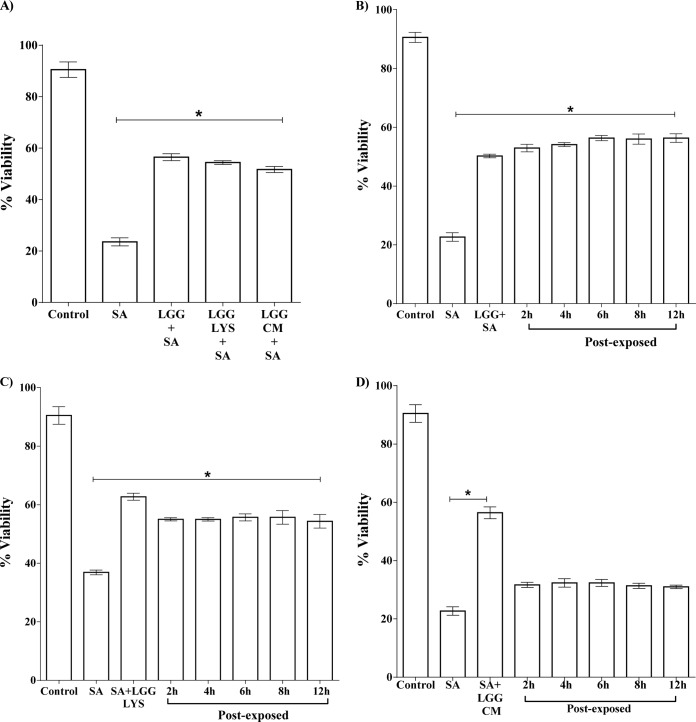
We next investigated the timing of the protective effect of L. rhamnosus GG by adding the live bacteria, the lysate, or the spent culture fluid either before or after infection of keratinocytes with S. aureus. The percentage of keratinocytes remaining viable was significantly greater in monolayers exposed to L. rhamnosus GG for 2 h prior to infection with S. aureus than in monolayers infected with S. aureus alone (P = 0.006). The lysate and spent culture fluid afforded similar levels of protection (P = 0.005 and P = 0.004) (Fig. 2A). In postexposure experiments, keratinocytes were exposed to S. aureus for 2 h, 4 h, 6 h, 8 h, and 12 h before addition of the live L. rhamnosus GG, lysate, or spent culture fluid. The viability of the keratinocytes was then measured at 24 h after infection with S. aureus. The data in Fig. 2B and C show that both live probiotic and its lysate could protect the keratinocytes when added after S. aureus. Even at 12 h after S. aureus infection, L. rhamnosus GG or lysate still afforded protection to the keratinocytes such that 58% and 55%, respectively, of cells remained viable, compared to 25% when exposed to S. aureus alone (P = 0.003 and P = 0.01, respectively). However, the spent culture fluid from L. rhamnosus GG had no protective effect on keratinocytes when added after S. aureus (Fig. 2D).
FIG 2.
L. rhamnosus GG protects and rescues keratinocytes from infection with S. aureus. (A) The percent viability of infected keratinocytes was significantly higher in cells that were preexposed to L. rhamnosus GG (LGG+SA), lysate (LGG LYS+SA), or spent culture fluid (LGG CM+ SA) than that of S. aureus (SA)-infected cells. (B) The viability of S. aureus-infected keratinocytes was significantly higher in cells exposed to L. rhamnosus GG 12 h after infection with S. aureus (“Post-exposed”). A similar effect was observed with lysate (C). However, cells postexposed to L. rhamnosus GG spent culture fluid (CM) did not have significant protection (D). Data are representative of three individual experiments, and all values represent means ± SEM (n = 3). *, P < 0.05.
L. rhamnosus GG lysate, but not spent culture fluid, inhibits the growth of S. aureus.
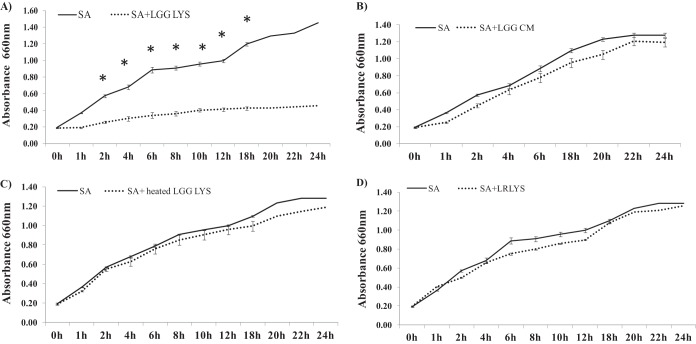
We investigated whether the probiotic lysate had direct effects on the growth of the pathogen by growing them simultaneously in culture. Competition assays showed a significant reduction in S. aureus growth over a period of 24 h in the presence of 100 μl of L. rhamnosus GG lysate compared to untreated cultures (P = 0.02) (Fig. 3A). This effect was specific to the lysate, because the spent culture fluid from L. rhamnosus GG had no effect on the growth of S. aureus (Fig. 3B). Furthermore, the ability of the lysate to inhibit pathogenic growth was negated by heating the lysate to 100°C for 10 min (Fig. 3C). Finally, this direct effect of L. rhamnosus GG on pathogenic growth appeared to be species specific, because the lysate from L. reuteri made in exactly the same way had no effect on the growth of S. aureus (Fig. 3D).
FIG 3.
Effect of L. rhamnosus GG or L. reuteri lysates and spent culture fluid on S. aureus growth in a competition assay. The optical densities of cultures of S. aureus (SA) growing in the presence of L. rhamnosus GG lysate (LGG LYS) (A) or spent culture fluid (LGG CM) (B) or heated L. rhamnosus GG lysate (heated LGG LYS) (C) or L. reuteri lysate (LRLYS) (D) were determined every hour to monitor the growth of the bacteria. In the presence of the L. rhamnosus GG lysate, the growth of S. aureus was significantly lower than when it was grown alone (P = 0.02; n = 3), whereas the heated L. rhamnosus GG lysate or spent culture fluid had no significant effect (P > 0.05; n = 3). Furthermore, a lysate of L. reuteri had no effects on the growth of S. aureus. Data are representative of three individual experiments, and all values represent means ± SEM (n = 3). *, P < 0.05.
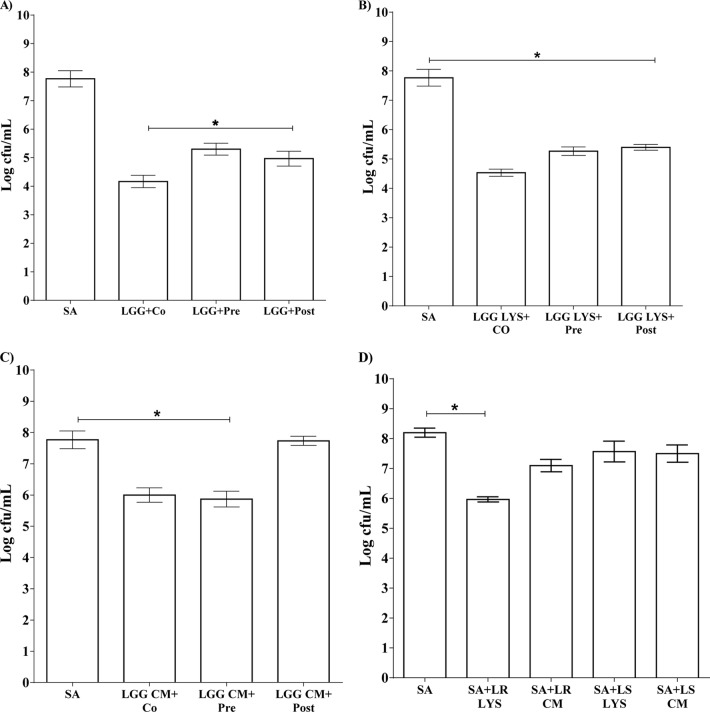
We determined the numbers of viable staphylococci following 24 h of incubation with keratinocytes in the presence or absence of the L. rhamnosus GG lysate. When S. aureus was added to keratinocytes at the same time as the L. rhamnosus GG lysate, the total number of viable staphylococci was also significantly reduced, to 5 log10 CFU/ml (Fig. 4), compared to 8 log10 CFU/ml for S. aureus alone (P = 0.02) (Fig. 5). Furthermore, when the L. rhamnosus GG lysate was added 12 h after infection of the keratinocytes, a reduction in number of viable S. aureus organisms was observed when these were counted 24 h later (Fig. 4). These effects were not seen with either the spent culture fluid from L. rhamnosus GG or a lysate from L. reuteri (data not shown). Since lactobacilli can produce organic acids, we measured the pH of keratinocyte media infected for 24 h with S. aureus, L. rhamnosus GG lysate, or both simultaneously. However, there was no significant difference in the pH between treatment groups (data not shown). We also measured the pH of lysate alone and found it be 7.2, thus suggesting that acid-mediated effects were not likely to be the mechanism underlying inhibition of pathogenic growth. The antimicrobial properties of L. rhamnosus GG and lysate were evaluated using a spot-on-lawn assay. This assay showed significant inhibition of S. aureus growth (as evidenced by the presence of zones of inhibition) by anaerobic live cultures or lysates of L. rhamnosus GG grown anaerobically (Table 1). In contrast, live L. reuteri or lysate did not induce zones of inhibition in this assay (Table 1).
FIG 4.
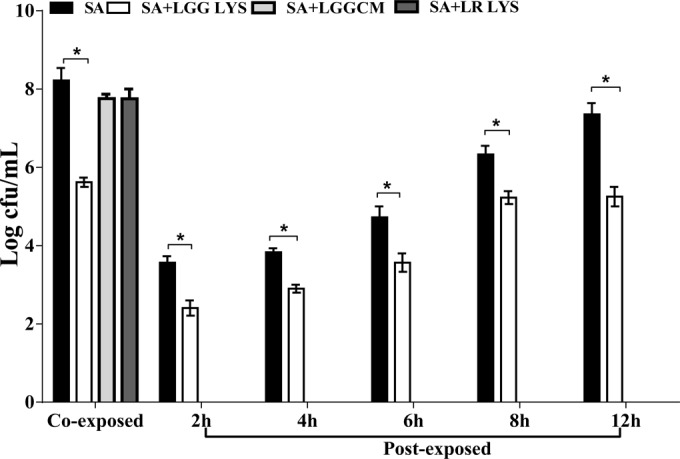
L. rhamnosus GG lysate, but not spent culture fluid, reduced the numbers of viable staphylococci. The number of viable S. aureus organisms (SA) was 8 log CFU/ml, whereas 5 log CFU/ml of S. aureus were viable in the presence of L. rhamnosus GG lysate (“Co-exposed”). Additionally, the total number of viable staphylococci in keratinocyte culture was reduced by the L. rhamnosus GG lysate when this was added 2, 4, 6, 8, and 12 h after infection of the keratinocytes with pathogen (“Post-exposed”; P = 0.05; n = 3). Data are representative of three individual experiments, and all values represent means ± SEM (n = 3). *, P < 0.05.
FIG 5.
Live L. rhamnosus GG, lysate, or spent culture fluid inhibited S. aureus adhesion to keratinocytes. (A) Live L. rhamnosus GG (LGG) inhibited S. aureus adhesion when added at the same time (LGG+Co), before (LGG+Pre), or after (LGG+Post) infection of cells with S. aureus. (B) A similar effect was also observed with the lysate. (C) Spent culture fluid (LGG CM) reduced the adhesion of S. aureus but only when added at the same time or before infection with pathogen. (D) The L. reuteri lysate (SA+LR LYS) reduced the adhesion of S. aureus to keratinocytes when added simultaneously, but the L. reuteri spent culture fluid (SA+LRCM) did not. L. salivarius lysate (SA+LS LYS) or spent culture fluid (SA+LS CM) had no effect on the adhesion of S. aureus to keratinocytes. Data are representative of three individual experiments, and all values represent means ± SEM (n = 3). *, P < 0.05.
TABLE 1.
L. rhamnosus GG bacteria or lysate reduces the growth of S. aureus in a spot-on-lawn assaya
| Bacteria | Zone of inhibition (mm) |
|
|---|---|---|
| Aerobic condition | Anaerobic condition | |
| S. aureus + L. rhamnosus GG | No inhibition | 11 ± 1.3 |
| S. aureus + L. rhamnosus GG lysate | No inhibition | 18.38 ± 0.7 |
| S. aureus + L. reuteri | No inhibition | No inhibition |
| S. aureus +L. reuteri lysate | No inhibition | No inhibition |
Spot-on-lawn assay demonstrating zones of inhibition produced by L. rhamnosus GG and GG lysate under the anaerobic condition but not under the aerobic condition. However, neither live L. reuteri nor L. reuteri lysate inhibited S. aureus growth under either condition. The inhibition zone was evaluated after overnight incubation by measuring the diameter of zone sizes using a ruler. Results are expressed as the means ± SEM of three individual experiments.
L. rhamnosus GG inhibits adhesion of S. aureus to keratinocytes.
Another mechanism by which live bacteria, lysate, or spent culture fluid of L. rhamnosus GG may protect keratinocytes is by inhibition of pathogenic adhesion. Previously, we showed that agents that reduce adhesion of S. aureus to keratinocytes also reduce its toxicity (33). Hence, we considered that inhibition of adhesion may also be part of the protective mechanism of L. rhamnosus GG, lysate, or spent culture fluid. Adhesion assays were performed to determine whether inhibition was due to competition, exclusion, or displacement of pathogen from binding sites on keratinocytes. L. rhamnosus GG, either as viable cells or as lysate, was able to inhibit pathogen adhesion if keratinocytes were coinfected (competition; P = 0.03), preexposed (exclusion; P = 0.04), or applied 12 h after infection with S. aureus had begun (displacement; P = 0.01) (Fig. 5A and B). By comparison, and as shown previously, live L. reuteri or its lysate could reduce staphylococcal adhesion if it was added at the same time as the pathogen (33) (Fig. 5D). However, the spent culture fluid did not reduce S. aureus adhesion. Interestingly, the spent culture fluid from L. rhamnosus GG inhibited pathogen adhesion only if it was added to keratinocytes either before or at the same time as the pathogen, in keeping with the data on viability (Fig. 5C). Finally, L. salivarius, its lysate, or spent culture fluid did not affect the adhesion of S. aureus to keratinocytes (Fig. 5D).
DISCUSSION
This study explored whether an enteric probiotic, L. rhamnosus GG, could protect keratinocytes from the pathogenic effects of S. aureus. Our data indicate that L. rhamnosus GG, in the form of viable cells, a cell-free lysate, or spent culture fluid, enhanced keratinocyte viability in the presence of the pathogen.
The timing of application of L. rhamnosus GG cells or lysate did not affect the degree of protection conferred by the probiotic or lysate because keratinocytes pre-, post-, or coexposed to L. rhamnosus GG or lysate were protected from S. aureus-induced cell death. However, the probiotic spent culture fluid protected keratinocytes only if it was added either before or at the same time as the pathogen. These data contrast with those for L. reuteri and L. salivarius, since L. reuteri can protect as a live organism or lysate only when added before or at the same time as the pathogen and L. salivarius has no ability to protect keratinocytes (33).
The current investigation suggests that there are at least two, possibly separate, activities involved in the protective effects of L. rhamnosus GG. These are likely to be inhibition of pathogen adhesion and inhibition of pathogen growth. We showed previously that agents that reduce adhesion of S. aureus to keratinocytes also reduce its toxicity in our viability assay (33). In keeping with this, the ability of the lysate and spent culture fluid to enhance viability mirrors directly the ability of each to inhibit pathogen adhesion; i.e., while the L. rhamnosus GG lysate protects viability and inhibits adhesion when added pre- or postinfection, the spent culture fluid protects viability only when added before the pathogen and has no ability to inhibit adhesion or protect when added after the pathogen. Thus, we suggest that the live organism or the lysate protects against the effects of S. aureus by exclusion and displacement, whereas the spent culture fluid can only exclude pathogens. In contrast, L. salivarius, which cannot protect keratinocytes from S. aureus, does not inhibit adhesion as either a live organism, a lysate, or spent culture fluid. Taken together, all these data point to species-specific effects in the abilities of different lactobacilli to protect keratinocytes from the toxic effects of S. aureus. Our data may also suggest that the antiadhesive effects contained within the L. rhamnosus GG lysate and spent culture fluid are mediated by different molecules. However, we cannot rule out the possibility that the same molecule(s) may be involved but that the concentration in the spent culture fluid is too low for some of the effects to be observed.
The ability of species of Lactobacillus species to inhibit certain pathogens from binding to epithelial cells has been demonstrated previously in models of the gut epithelium (8, 10, 34). For example, in an in vitro study, probiotics (alone or in combinations), including L. rhamnosus NCC4007 and Lactobacillus paracasei NCC2461, were shown to inhibit E. sakazakii adhesion to intestinal mucus through competitive exclusion and displacement from the binding sites (8, 10, 35). Another study by Vesterlund and colleagues (36) showed that certain lactic acid bacteria, including L. rhamnosus GG, were able to reduce the adhesion of S. aureus to intestinal cells by as much as 44%. In keeping with our study, the mechanisms involved included competition, exclusion, and displacement. Interestingly, in the study of Vesterlund et al., the authors also noted reduced staphylococcal viability in the presence of some of the probiotic organisms (36).
The molecules mediating the inhibitory effects of probiotics against pathogens have been investigated in a number of studies. In some cases, the molecules mediating antiadhesive activity are largely associated with other functions, i.e., the so-called “moonlighting proteins” (37–49). For example, enolase from Lactobacillus crispatus can bind to laminin and collagen I, which reduces the adhesion of S. aureus to epithelial cell lines through these binding sites (50). Similarly, enolase from L. plantarum has been reported as binding to fibronectin to prevent S. aureus adhesion to epithelial cell lines (7, 51). Other moonlighting proteins contributing to bacterial adhesion have been found in lactobacilli. For example, triosephosphate isomerase (TPI) from L. plantarum plays a role in the adhesion of lactobacilli to Caco-2 cells and has the ability to compete with pathogens such as Clostridium sporogenes and Enterococcus faecalis by excluding and displacing them from the cell-binding sites (12, 52, 53). However, thus far, the molecules mediating the effects preventing adhesion of L. rhamnosus GG to keratinocytes remain to be identified.
L. rhamnosus GG lysate may also protect keratinocytes is via inhibition of S. aureus growth. Two lines of evidence suggest that this is the case: first, a reduction in the total number of viable staphylococci in the presence of the L. rhamnosus GG lysate and inhibition assays demonstrating zones of inhibition when S. aureus was challenged with lysates from the probiotic grown anaerobically (Table 1). This could be due to the presence of a toxic molecule(s) within the probiotic that is able to directly inhibit S. aureus growth and/or viability. It is possible that this molecule(s) may be synthesized but not secreted because there was no effect of L. rhamnosus GG spent culture fluid on the viability of S. aureus. However, again, we cannot rule out the possibility that such molecules may be secreted but diluted once contained in the spent culture fluid. If L. rhamnosus GG contains bacteriostatic substances, then this may also, at least partially, explain the protective effect of the probiotic in keratinocyte survival assays. Probiotics, especially lactobacilli, have previously been shown to exert a strong inhibitory effect on S. aureus growth. Certain Lactobacillus strains have been reported to be highly antagonistic to biofilm-forming S. aureus (3). Other studies have reported that probiotics can improve gut health by inhibiting growth of pathogens through production of bacteriocins or lactic acid (36, 54–56). However, in the present study, we could find no evidence of the involvement of acid production as part of the protective effects of L. rhamnosus GG. Indeed, the lysate from this organism was neutral (pH 7.2) but was still able to inhibit S. aureus growth. Furthermore, neither L. reuteri nor L. salivarius showed any inhibitory activity on the growth of S. aureus even though both these bacteria are also able to produce acid (57, 58).
In conclusion, we have shown that L. rhamnosus GG uses multiple mechanisms to protect keratinocytes from S. aureus. These include exclusion of pathogens, inhibition of pathogen growth, and displacement of pathogen from keratinocytes. Of course, it is possible that this displacement activity may be related to the ability of L. rhamnosus GG to inhibit growth, and further studies will be required to clarify this point. A number of studies have suggested the utility of probiotic species of lactobacilli for use topically. In keeping with these studies, we suggest that L. rhamnosus GG is a potential new agent to inhibit the pathogenicity of S. aureus to keratinocytes. Furthermore, our data show that the utility of L. rhamnosus GG on skin will not be limited by whether it can grow and survive on skin, because a lysate of the organisms is just as efficacious at preventing S. aureus colonization as live bacteria. We suggest that the use of bacterial lysates will enhance the utility of lactobacilli since the need to produce formulations that maintain bacterial viability is negated. Furthermore, lysates potentially offer a safer option than live bacteria for treatment of damaged skin.
ACKNOWLEDGMENT
This work was supported by a Scholarship from Ministry of Higher Education of Saudi Arabia to Walaa Mohammedsaeed.
Footnotes
Published ahead of print 11 July 2014
REFERENCES
- 1.Rolfe RD. 2000. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 130:396S–402S [DOI] [PubMed] [Google Scholar]
- 2.Sanders ME. 1999. Probiotics. Food Technol. 53:67–77 [Google Scholar]
- 3.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. 2008. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295:G1025–G1034. 10.1152/ajpgi.90227.2008 [DOI] [PubMed] [Google Scholar]
- 4.Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. 2001. Probiotics: effects on immunity. Am. J. Clin. Nutr. 73:444S–450S [DOI] [PubMed] [Google Scholar]
- 5.Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. 1992. Enhancement of the circulating antibody secreting cell response in human diarrhoea by a human Lactobacillus strain. Pediatr. Res. 32:141–144. 10.1203/00006450-199208000-00002 [DOI] [PubMed] [Google Scholar]
- 6.Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, Ryan AF, Di Nardo A, Gallo RL. 2010. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Invest. Dermatol. 130:2211–2221. 10.1038/jid.2010.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Cagno R, De Angelis M, Calasso M, Vincentini O, Vernocchi P, Ndagijimana M, De Vincenzi M, Dessi MR, Guerzoni ME, Gobbetti M. 2010. Quorum sensing in sourdough Lactobacillus plantarum DC400: induction of plantaricin A (PlnA) under co-cultivation with other lactic acid bacteria and effect of PlnA on bacterial and Caco-2 cells. Proteomics 10:2175–2190. 10.1002/pmic.200900565 [DOI] [PubMed] [Google Scholar]
- 8.Coconnier MH, Bernet MF, Kerneis S, Chauvière G, Fourniat J, Servin AL. 1993. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 110:299–306. 10.1111/j.1574-6968.1993.tb06339.x [DOI] [PubMed] [Google Scholar]
- 9.Gan Bingâ S, Kim J, Reid G, Cadieux P, Howard JC. 2002. Lactobacillus fermentum RC-14 inhibits Staphylococcus aureus infection of surgical implants in rats. J. Infect. Dis. 185:1369–1372. 10.1086/340126 [DOI] [PubMed] [Google Scholar]
- 10.Collado MC, Isolauri E, Salminen S. 2008. Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol. Lett. 285:58–64. 10.1111/j.1574-6968.2008.01211.x [DOI] [PubMed] [Google Scholar]
- 11.Mack D, Michail S, Wei S, Mcdougall L, Hollingworth MA. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 276:G941–G950 [DOI] [PubMed] [Google Scholar]
- 12.Ohnemus U, Kohrmeyer K, Houdek P, Rohde H, Wladykowski E, Vidal S, Horstkotte MA, Aepfelbacher M, Kirschner N, Behne MJ, Moll I, Ouwehand AC, Kirijavainen PV, Short C, Salminen S. 1999. Probiotics: mechanisms and established effects. Int. Dairy J. 9:43–52. 10.1016/S0958-6946(99)00043-6 [DOI] [Google Scholar]
- 13.Resta-Lenert S, Barrett KE. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988–997. 10.1136/gut.52.7.988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarika AR, Lipton AP, Aishwarya MS. 2010. Bacteriocin production by a new isolate of Lactobacillus rhamnosus GP1 under different culture conditions. Adv. J. Food Sci. Technol. 2:291–297 [Google Scholar]
- 15.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. 2010. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346–349. 10.1038/nature09074 [DOI] [PubMed] [Google Scholar]
- 16.Mcbain AJ, Madhwani T, Eatough J, Ledder R. 2009. An introduction to probiotics for dental health. Food Sci. Technol. Bull. 6:5–29. 10.1616/1476-2137.15748 [DOI] [Google Scholar]
- 17.Reid G, Beuerman D, Heinemann C, Bruce AW. 2001. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med. Microbiol. 32:37–41. 10.1111/j.1574-695X.2001.tb00531.x [DOI] [PubMed] [Google Scholar]
- 18.Rayes N, Seehofer D, Muller AR, Hansen S, Bengmark S, Neuhaus P. 2002. Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery—results of a prospective trial. Z. Gastroenterol. 40:869–876 (In German.) 10.1055/s-2002-35259 [DOI] [PubMed] [Google Scholar]
- 19.Cinque B, La Torre C, Melchiorre E, Marchesani G, Zoccali G, Palumbo P, Di Marzio L, Masci A, Mosca L, Mastromarino P, Giuliani M, Cifone MG. 2011. Use of probiotics for dermal applications. Microbiol. Monogr. 21:221–241. 10.1007/978-3-642-20838-6_9 [DOI] [Google Scholar]
- 20.Gueniche A, Castiel I. 2009. Probiotic lysate applied to skin: in vitro evidences of beneficial effects of Bifidobacterium longum sp on sensitive skin and aging. J. Invest. Dermatol. 129:S64. [Google Scholar]
- 21.Guéniche A, Bastien P, Ovigne JM, Kermici M, Courchay G, Chevalier V, Breton L, Castiel-Higounenc I. 2010. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 19:e1–e8. 10.1111/j.1600-0625.2009.00932.x [DOI] [PubMed] [Google Scholar]
- 22.Gueniche A, David P, Achachi A, Vocanson M, Buyukpamukcu A, Bastien P, Nicolas JF, Castiel I. 2009. Oral supplementation with Lactobacillus johnsonii reinforces skin immune homeostasis following UV exposure and limits UV-induced damages. J. Invest. Dermatol. 129:S34. [Google Scholar]
- 23.Gueniche A, Delattre C, Winstall E, Bastien P, Bernard D, Castiel-Higounec I. 2010. An original topical probiotic related ingredient for dry skin: efficacy evaluated in a clinical trial with the help of bioinstrumental measurements and proteomic tools. J. Invest. Dermatol. 130:S65. [Google Scholar]
- 24.Sultana R, Mcbain AJ, O'Neill CA. 2013. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl. Environ. Microbiol. 79:4887–4894. 10.1128/AEM.00982-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Ghazzewi FH, Tester RF. 2010. Effect of konjac glucomannan hydrolysates and probiotics on the growth of the skin bacterium Propionibacterium acnes in vitro. Int. J. Cosmet. Sci. 32:139–142. 10.1111/j.1468-2494.2009.00555.x [DOI] [PubMed] [Google Scholar]
- 26.Di Marzio L, Centi C, Cinque B, Masei S, Giuliani M, Arclei A, Zicari L, Simone CD, Cifone MG. 2003. Effect of lactic acid bacterium Streptococcus thermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Exp. Dermatol. 12:615–620. 10.1034/j.1600-0625.2003.00051.x [DOI] [PubMed] [Google Scholar]
- 27.Nasrabadi HM, Ebrahimi TM, Banadaki DS, Kajousangi TM, Zahedi F. 2011. Study of cutaneous wound healing in rats treated with Lactobacillus plantarum on days 1, 3, 7, 14 and 21. Afr. J. Pharm. Pharmacol. 5:2395–2401 [Google Scholar]
- 28.Rodrigues KL, Caputo LR, Carvalho JC, Evangelista J, Schneedorf JM. 2005. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 25:404–408. 10.1016/j.ijantimicag.2004.09.020 [DOI] [PubMed] [Google Scholar]
- 29.Valdéz JC, Peral MC, Rachid M, Santana M, Perdigon G. 2005. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 11:472–479. 10.1111/j.1469-0691.2005.01142.x [DOI] [PubMed] [Google Scholar]
- 30.Peral MC, Martinez MA, Valdez JC. 2009. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 6:73–81. 10.1111/j.1742-481X.2008.00577.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanger P, Holzer J, Schleucher R, Scherbaum H, Schittek B, Gabrysch S. 2010. Severity of Staphylococcus aureus infection of the skin is associated with inducibility of human beta-defensin 3, but not human beta-defensin 2. Infect. Immun. 78:3112–3117. 10.1128/IAI.00078-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanger P, Holzer J, Schleucher R, Steffen H, Schittek B, Gabrysch S. 2009. Constitutive expression of the antimicrobial peptide RNase 7 is associated with Staphylococcus aureus infection of the skin. J. Infect. Dis. 200:1907–1915. 10.1086/648408 [DOI] [PubMed] [Google Scholar]
- 33.Prince T, Mcbain AJ, O'Neill CA. 2012. Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion. Appl. Environ. Microbiol. 78:5119–5126. 10.1128/AEM.00595-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collado M, Meriluoto J, Salminen S. 2008. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 226:1065–1073. 10.1007/s00217-007-0632-x [DOI] [Google Scholar]
- 35.Vélez MP, De Keersmaecker SC, Vanderleyden J. 2007. Adherence factors of Lactobacillus in the human gastrointestinal tract. FEMS Microbiol. Lett. 276:140–148. 10.1111/j.1574-6968.2007.00908.x [DOI] [PubMed] [Google Scholar]
- 36.Vesterlund S, Karp M, Salminen S, Ouwehand AC. 2006. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology 152:1819–1826. 10.1099/mic.0.28522-0 [DOI] [PubMed] [Google Scholar]
- 37.Agarwal S, Kulshreshtha P, Bambah Mukku D, Bhatnagar R. 2008. Alpha-enolase binds to human plasminogen on the surface of Bacillus anthracis. Biochim. Biophys. Acta 1784:986–994. 10.1016/j.bbapap.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 38.Aleljung P, Paulsson M, Emödy L, Andersson M, Naidu AS, Wadström T. 1991. Collagen binding by lactobacilli. Curr. Microbiol. 23:33–38. 10.1007/BF02092306 [DOI] [Google Scholar]
- 39.Jeffery CJ. 2003. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 19:415–417. 10.1016/S0168-9525(03)00167-7 [DOI] [PubMed] [Google Scholar]
- 40.Jeffery CJ. 2003. Multifunctional proteins: examples of gene sharing. Ann. Med. 35:28–35 [DOI] [PubMed] [Google Scholar]
- 41.Jeffery CJ. 2009. Moonlighting proteins—an update. Mol. Biosyst. 5:345–350. 10.1039/b900658n [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita H, Uchida H, Kawai Y, Kawasaki T, Wakahara N, Matsuo H, Watanabe M, Kitazawa H, Ohnuma S, Miura K, Horii A, Saito T. 2008. Cell surface Lactobacillus plantarum LA 318 glyceraldehyde-3-phosphate dehydrogenase (GAPDH) adheres to human colonic mucin. J. Appl. Microbiol. 104:1667–1674. 10.1111/j.1365-2672.2007.03679.x [DOI] [PubMed] [Google Scholar]
- 43.Kinoshita H, Uchida H, Kawai Y, Kitazawa H, Miura K, Shiiba K, Horii A, Saito T. 2007. Quantitative evaluation of adhesion of lactobacilli isolated from human intestinal tissues to human colonic mucin using surface plasmon resonance (BIACORE assay). J. Appl. Microbiol. 102:116–123. 10.1111/j.1365-2672.2006.03061.x [DOI] [PubMed] [Google Scholar]
- 44.Kinoshita H, Wakahara N, Watanabe M, Kawasaki T, Matsuo H, Kawai Y, Kitazawa H, Ohnuma S, Miura K, Horii A, Saito T. 2008. Cell surface glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of Lactobacillus plantarum LA 318 recognizes human A and B blood group antigens. Res. Microbiol. 159:685–691. 10.1016/j.resmic.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 45.Marco ML, Pavan S, Kleerebezem M. 2006. Towards understanding molecular modes of probiotic action. Curr. Opin. Biotechnol. 17:204–210. 10.1016/j.copbio.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 46.Morita H, Toh H, Oshima K, Murakami M, Taylor TD, Igimi S, Hattori M. 2009. Complete genome sequence of the probiotic Lactobacillus rhamnosus ATCC 53103. J. Bacteriol. 191:7630–7631. 10.1128/JB.01287-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramiah K, Van Reenen CA, Dicks LMT. 2007. Expression of the mucus adhesion genes Mub and MapA, adhesion-like factor EF-Tu and bacteriocins gene plaA of Lactobacillus plantarum 423, monitored with real-time PCR. Int. J. Food Microbiol. 116:405–409. 10.1016/j.ijfoodmicro.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 48.Villamón E, Villalba V, Nogueras MM, Tomas JM, Gozalbo D, Gil ML. 2003. Glyceraldehyde-3-phosphate dehydrogenase, a glycolytic enzyme present in the periplasm of Aeromonas hydrophila. Antonie Van Leeuwenhoek 84:31–38. 10.1023/A:1024435612550 [DOI] [PubMed] [Google Scholar]
- 49.Winram SB, Lottenberg R. 1996. The plasmin-binding protein plr of group A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase. Microbiology 142:2311–2320. 10.1099/13500872-142-8-2311 [DOI] [PubMed] [Google Scholar]
- 50.Antikainen J, Anton L, Sillanpää J, Korhonen TK. 2002. Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Mol. Microbiol. 46:381–394. 10.1046/j.1365-2958.2002.03180.x [DOI] [PubMed] [Google Scholar]
- 51.Castaldo C, Vastano V, Siciliano RA, Candela M, Vici M, Muscariello L, Marasco R, Sacco M. 2009. Surface displaced alfa-enolase of Lactobacillus plantarum is a fibronectin binding protein. Microb. Cell Fact. 8:14. 10.1186/1475-2859-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirjavainen PV, Ouwehand AC, Isolauri E, Salminen SJ. 1998. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 167:185–189. 10.1111/j.1574-6968.1998.tb13226.x [DOI] [PubMed] [Google Scholar]
- 53.Ramiah K, van Reenen CA, Dicks LM. 2008. Surface-bound proteins of Lactobacillus plantarum 423 that contribute to adhesion of Caco-2 cells and their role in competitive exclusion and displacement of Clostridium sporogenes and Enterococcus faecalis. Res. Microbiol. 159:470–475. 10.1016/j.resmic.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 54.Kirjavainen PV, Tuomola EM, Crittenden RG, Ouwehand AC, Harty DW, Morris LF, Rautelin H, Playne MJ, Donohue DC, Salminen SJ. 1999. In vitro adhesion and platelet aggregation properties of bacteremia-associated lactobacilli. Infect. Immun. 67:2653–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu Z-R, Hooper LV, Schmidt RR, Von Aulock S, Radek KA, Huang C-M, Ryan AF, Gallo RL. 2009. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 15:1377–1382. 10.1038/nm.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Todorov SD, Dicks LMT. 2005. Growth parameters influencing the production of Lactobacillus rhamnosus bacteriocins ST461BZ and ST462BZ. Ann. Microbiol. 55:283–289 [Google Scholar]
- 57.Axelsson LT, Chung TC, Dobrogosz WJ, Lindgren SE. 1989. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 2:131–136 [Google Scholar]
- 58.Flynn S, Van Sinderen D, Thornton GM, Holo H, Nes IF, Collins JK. 2002. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology 148:973–984 [DOI] [PubMed] [Google Scholar]