Abstract
We have investigated two approaches to enhance and extend H2 photoproduction yields in heterocystous, N2-fixing cyanobacteria entrapped in thin alginate films. In the first approach, periodic CO2 supplementation was provided to alginate-entrapped, N-deprived cells. N deprivation led to the inhibition of photosynthetic activity in vegetative cells and the attenuation of H2 production over time. Our results demonstrated that alginate-entrapped ΔhupL cells were considerably more sensitive to high light intensity, N deficiency, and imbalances in C/N ratios than wild-type cells. In the second approach, Anabaena strain PCC 7120, its ΔhupL mutant, and Calothrix strain 336/3 films were supplemented with N2 by periodic treatments of air, or air plus CO2. These treatments restored the photosynthetic activity of the cells and led to a high level of H2 production in Calothrix 336/3 and ΔhupL cells (except for the treatment air plus CO2) but not in the Anabaena PCC 7120 strain (for which H2 yields did not change after air treatments). The highest H2 yield was obtained by the air treatment of ΔhupL cells. Notably, the supplementation of CO2 under an air atmosphere led to prominent symptoms of N deficiency in the ΔhupL strain but not in the wild-type strain. We propose that uptake hydrogenase activity in heterocystous cyanobacteria not only supports nitrogenase activity by removing excess O2 from heterocysts but also indirectly protects the photosynthetic apparatus of vegetative cells from photoinhibition, especially under stressful conditions that cause an imbalance in the C/N ratio in cells.
INTRODUCTION
Many species of cyanobacteria and eukaryotic microalgae are capable of water biophotolysis, a light-dependent photosynthetic reaction that results in water oxidation with concomitant generation of molecular H2 and O2. Among cyanobacteria, N2-fixing heterocystous species are the most promising candidates as potential H2 producers (1). In these species, H2 is generated mainly by the nitrogenase enzyme as an obligatory by-product of N2 fixation (2). Although nitrogenase is sensitive to inactivation by O2, N2 fixation in heterocyst-forming species is protected by localization in specialized heterocyst cells. The maintenance of microoxic conditions within heterocyst cells enables efficient N2 fixation, even under atmospheric levels of O2 (2, 3).
Hydrogen formed in heterocysts is usually recycled by uptake hydrogenase (Hup) (4, 5). The uptake of H2 may contribute to the decrease in partial pressure of oxygen inside the heterocyst cells via a Knallgas (oxyhydrogen) reaction, which is beneficial for sustained N2 fixation (6), and may also serve as an additional source of electrons for the nitrogenase enzyme and other processes (3). As a result, the vast majority of heterocystous strains isolated from the natural environment show very little net H2 photoproduction under ambient air conditions (1, 2). Under nitrogen deficiency, nitrogenase catalyzes solely the reduction of protons to H2 (2), thus improving H2 photoproduction yields (7). In addition to uptake hydrogenase, many heterocystous cyanobacteria contain bidirectional (Hox) hydrogenase. This enzyme may also participate in H2 recycling, though at substantially lower rates (4, 5, 8, 9). The primary function of the bidirectional hydrogenase in cyanobacteria is for regeneration of NAD(P)+ from NAD(P)H during dark fermentation with concomitant evolution of H2 (8). This enzyme, however, is also responsible for short-term H2 photoproduction and is believed to prevent overreduction of the photosynthetic electron transport chain at the onset of illumination, especially in anaerobic environments (9–11).
The real potential of cyanobacterial H2 photoproduction has yet to be fully explored. This is at least partly due to criticism of the high-energy requirement of nitrogenase-dependent H2 photoproduction, which requires at least 4 mol of ATP per mole of H2 produced (12). Heterocystous species are the most promising cyanobacteria for H2 photoproduction as they are the only organisms capable of driving the H2 photoproduction process under strictly autotrophic conditions, in the presence of atmospheric levels of O2, and for extended periods of up to a few months under solar light intensities and periodic light conditions (13, 14). While the efficiency of H2 photoproduction by any known species is currently far below requirements for commercial application, there are several promising approaches for significant improvement.
The H2 photoproduction yields in cultures of heterocystous cyanobacteria can be significantly enhanced via elimination of uptake hydrogenase activity in cells. A good example of this is the PK84 strain, a chemically induced mutant of Anabaena variabilis with impaired hydrogenases that, in contrast to the parental strain, is able to produce H2 in air containing 2% CO2 (15, 16). This mutant has also demonstrated H2 generation under outdoor conditions, where it has performed successfully for up to 40 days (14). There have been other mutant strains lacking HupL or HupS subunits of uptake hydrogenase which have also demonstrated more efficient H2 photoproduction (17–19). Among these are the ΔhupL and ΔhupL ΔhoxH mutants of Anabaena strain PCC 7120 (20) that were used in the current study. The inactivation of uptake hydrogenase in cyanobacteria not only affects H2 production but also results in a prominent loss of nonrecycled metabolic energy that leads to a significant change in the overall metabolic equilibrium. This change induces a cascade of compensatory mechanisms affecting both oxygen reduction mechanisms in heterocysts and processes providing reducing equivalents for different metabolic activities, including N2 fixation (21).
Another promising approach for improving H2 production yields in cyanobacteria is to screen for strains with naturally high H2 photoproduction activity. The H2 production capacity of promising strains can be further enhanced using genetic engineering techniques, including elimination of the hup genes (as described above), modification of the active center of nitrogenase (22, 23), and changes in the light-absorbing properties of both vegetative cells and heterocysts in a similar manner to approaches employed in green algae (24) and purple bacteria (25). Our recent screening of the University of Helsinki Cyanobacteria Collection (UHCC) revealed several cyanobacterial strains with promising H2 photoproduction rates (26). One of these strains, Calothrix strain 336/3, a filamentous N2-fixing cyanobacterium, showed efficient H2 photoproduction both in suspension cultures and immobilized films (26, 27). The yields and the rates of H2 photoproduction of Calothrix 336/3 were higher than in most other N2-fixing heterocystous species and were comparable to the rates in the ΔhupL mutant of Anabaena PCC 7120 (26, 27).
Future efforts into improving H2 yield should also target the development of better light utilization capacities in cyanobacterial cultures and the direction of absorbed light energy solely into H2 production rather than biomass accumulation. For better light utilization per surface area unit, future technology requires the entrapment of algae and cyanobacteria in thin films consisting of a few layers of photosynthetic cells. The concept of an artificial leaf has been recognized (28), and approaches for making such a leaf are under development. There are several different techniques that can be considered for further improvements, including natural biofilm formation on translucent matrices and latex coatings (29). Latex coatings (28, 30), including the wet coalescence approach (31), are promising methods for the entrapment of phototrophic organisms, but these methods are not usually as efficient as hydrogels for the immobilization of H2-producing microalgae and cyanobacteria. Recently, a technique for entrapment of eukaryotic microalgae within thin-layer alginate matrices has been developed, which has demonstrated 1.5% conversion efficiency of light energy to H2 (32). This same technique has also worked efficiently for immobilization of N2-fixing heterocystous cyanobacteria (27). The entrapment of cyanobacterial cells within thin alginate matrices under nitrogen-deficient conditions reduced the accumulation of cell biomass, improved H2 photoproduction yields, and prolonged the duration of the H2 production process.
In the present work, we evaluate possible routes to long-term and efficient H2 photoproduction by immobilized cells of N2-fixing heterocystous cyanobacteria by (i) supplementing CO2 periodically to the films producing H2 under an Ar atmosphere, (ii) investigating the effect of different gas atmospheres (Ar, air, and N2) on H2 production and cell fitness, and (iii) applying periodic short-term air treatments to the films producing H2 under an Ar atmosphere. We demonstrate that H2 photoproduction yield can be enhanced through the restoration of cell metabolism, particularly photosynthetic activity in N-starved immobilized cells, by periodic CO2 additions and air treatments. Our results also demonstrate the important role of uptake hydrogenase in photoprotection of the photosynthetic apparatus in the long-term process.
MATERIALS AND METHODS
Strains and growth conditions.
The wild-type (WT) Anabaena PCC 7120 strain was obtained from the Pasteur Culture Collection (Paris, France). The ΔhupL and ΔhupL ΔhoxH mutants of Anabaena PCC 7120 lacking uptake hydrogenase and both uptake and bidirectional hydrogenases, respectively, were kindly provided by H. Sakurai. The Calothrix 336/3 strain was selected from the UHCC as has been described previously (26). In this study, we always used cells grown under diazotropic conditions. Stock cultures of all strains were pregrown at room temperature in 150-ml Erlenmeyer flasks containing 50 ml of Z8 medium (33) without combined nitrogen (Z8x). The medium for growing stock cultures of the ΔhupL and ΔhupL ΔhoxH strains was supplemented with 25 μg ml−1 spectinomycin or 25 μg ml−1 spectinomycin plus 10 μg ml−1 neomycin, respectively. The flasks were illuminated from the top with cool-daylight fluorescence lamps (Lumilux T8 15W/865; Osram) with light intensity of about 30 μmol photons m−2 s−1 photosynthetic active radiation (PAR). Before immobilization, cultures were transferred into 500-ml Erlenmeyer flasks containing 300 ml of Z8x medium and grown under ∼50 μmol photons m−2 s−1 in a growth chamber at 22°C. During this stage, antibiotics were not added to the ΔhupL and ΔhupL ΔhoxH mutant cultures. All flasks were sparged continuously with sterile air.
Cell immobilization and H2 photoproduction experiments.
The details of the immobilization technique, which was originally developed for the entrapment of green algae in thin Ca2+-alginate films (32) and later adapted for cyanobacterial cells, are described in Leino et al. (27). After immobilization, the 3-cm2 Ca2+-alginate strips were transferred into 23-ml vials containing 5 ml of Z8x medium, purged with Ar for 20 min, supplemented with 6% CO2, and placed in a growth chamber at 26°C under continuous overhead illumination with cool-white fluorescent lamps (∼150 μmol photons m−2 s−1; Philips Master TL-D T8 15W/840). During long-term experiments, the medium in the vials was periodically changed to avoid general (nonnitrogen) nutrient deprivation.
At the beginning of each experiment, the headspace of all vials contained Ar supplemented with 6% CO2. This CO2 concentration was selected based on our previous data (27) that showed the optimal H2 photoproduction activities in Anabaena and Calothrix 336/3 cultures. Depending on the approach used, the gas phase in the headspace of the vials was replaced periodically (at the beginning of each incubation cycle) with the following: a new portion of Ar supplemented with 6% CO2 (Ar plus CO2), pure air (air), air supplemented with 6% CO2 (air plus CO2), or N2 supplemented with 6% CO2 (N2 plus CO2). In the case of air treatments, the headspace in the vial was regenerated using Ar with 6% CO2 after 16 to 20 h. This was performed to restore efficient H2 photoproduction by the films. The H2 and O2 contents in the headspace of vials were monitored once a day using a gas chromatograph (GC) (Clarus 500; PerkinElmer, Inc.) equipped with a thermal conductivity detector and a molecular sieve 5A column (60/80 mesh). All experiments were repeated several times with at least three vials for each individual set.
Cell fitness.
After 12 days of incubation under different atmospheres (as described above), cells were recovered from alginate by solubilization of the films in 50 mM Na-EDTA solution (pH 7.0). The cells were washed three times using Z8x medium and centrifuged (16,100 relative centrifugal force [RCF] for 2 min). The cells were diluted with Z8x to adjust the optical density at 750 nm (OD750) to values of 0.25, and 2 ml was transferred to the wells of a 24-well cell culture plate. The cells were then regrown under standard growth conditions at 50 μmol photons m−2 s−1 for 6 days, and the OD750 was measured daily to identify differences in cell fitness.
Nitrogenase activity assay.
Nitrogenase activity was determined by an acetylene reduction assay (34). The alginate films with entrapped cells were transferred into 23-ml vials containing 5 ml of Z8x medium, purged with Ar, supplemented with 10% acetylene, and placed in a growth chamber at 26°C under continuous overhead illumination with cool-white fluorescent lamps (∼150 μmol photons m−2 s−1; Philips Master TL-D T8 15W/840) for 18 h. For ethylene determination, 10-μl samples from the headspace were injected into a GC (PerkinElmer Autosystem) equipped with a flame ionization detector (FID) and a CP-CarboBond column (Varian). Helium was used as a carrier gas, and calibration was performed with 1% ethylene (AGA, Finland). Enzyme activity was calculated on the basis of the chlorophyll (Chl) a content of the cells and per film area.
Chl determination.
The Chl a content in the alginate films was assayed in randomly chosen strips after solubilization of the alginate matrices in 50 mM Na-EDTA solution (pH 7.0). The cells were washed once with Z8x medium by centrifugation. Then, Chl a was extracted from the pelleted cells with 90% methanol and determined either spectrophotometrically at 665 nm (35) or using high-performance liquid chromatography (HPLC). The data obtained spectrophotometrically were used for determination of the specific rates of H2 photoproduction (see Fig. 2A), while the data obtained using HPLC are presented in Fig. 5.
FIG 2.
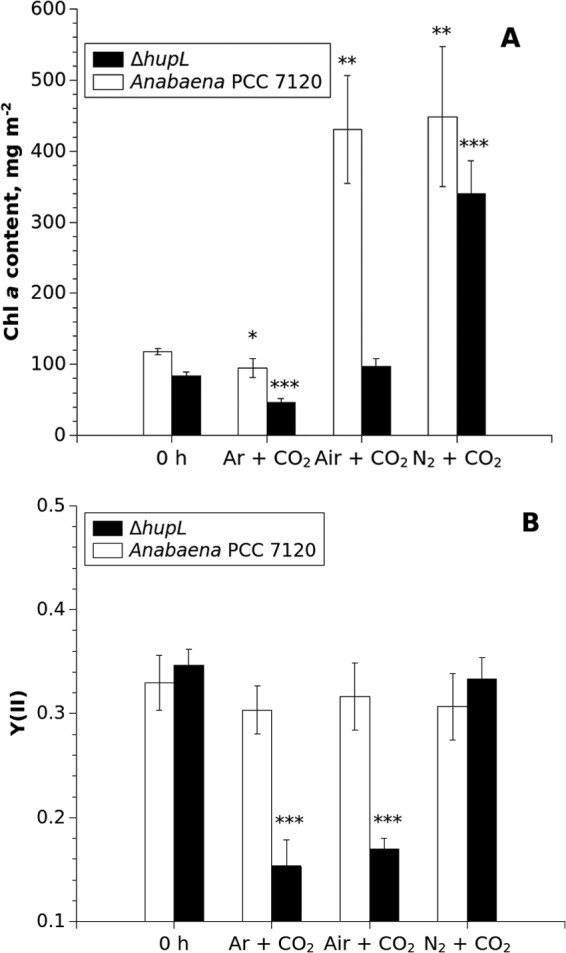
Chl a content (A) and effective PSII yield (B) in alginate films with entrapped Anabaena PCC 7120 and ΔhupL cells. The measurements were performed before (0 h) and after (12 days) the treatment of the samples under Ar, air, and N2 atmospheres supplemented with 6% CO2. The Chl a contents in films were measured spectrophotometrically (34). The average values from three films are presented ± standard deviations. Significant differences between the pre- and posttreated samples are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 5.
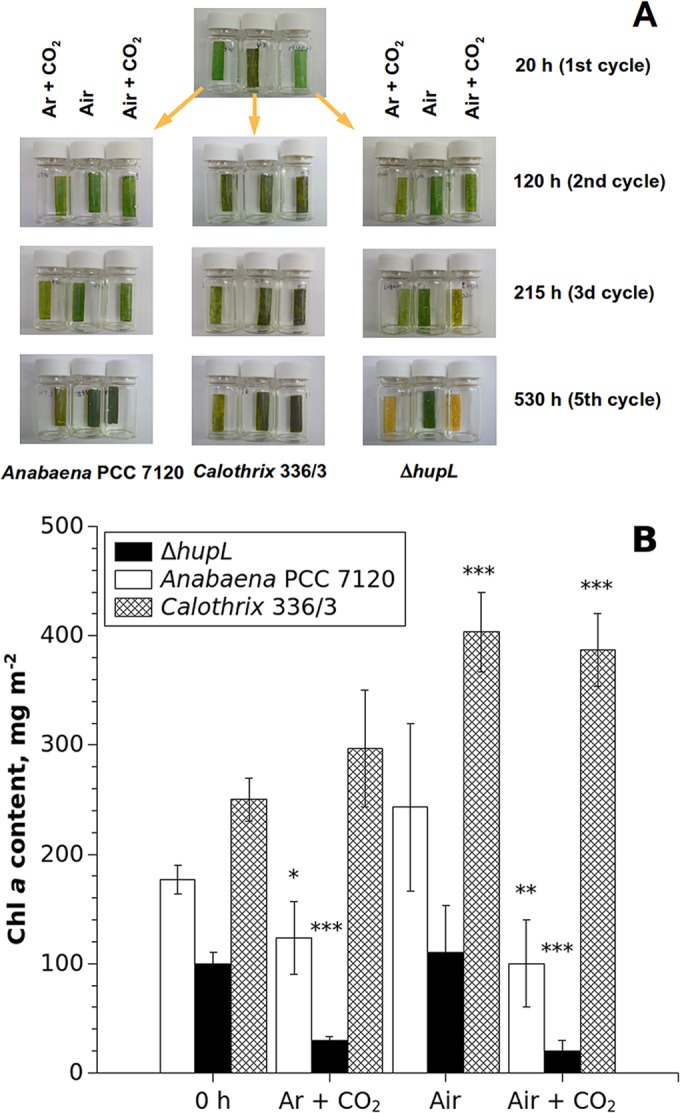
The visual changes in the pigment composition (A) and Chl a content (B) in alginate films with entrapped Calothrix 336/3, Anabaena PCC 7120, and ΔhupL cells during the long-term experiment. The experimental conditions are the same as described in the legend of Fig. 4. The Chl a contents in films were measured at the end of the experiment (∼600 h) by HPLC. The average values from three films are presented ± the standard deviations. Significant differences between the 0 h and posttreatment samples are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
For HPLC analysis, the harvested cell pellets were frozen in liquid nitrogen and stored at −80°C before processing. The pigments were separated from 100% methanol extracts by HPLC (model 1100; Agilent Technologies, Germany) equipped with a diode array detector and a reverse-phase C18 end-capped column (LiChroCART 125-4; Merck KGaA, Darmstadt, Germany). An acetonitrile-methanol-Tris buffer mixture (720:80:30) was used as a mobile phase. The Chl a standard was purchased from DHI Lab Products (Hørsholm, Denmark).
Photochemical activity.
The photochemical performance of cyanobacteria entrapped in alginate films was evaluated using a Dual-PAM 100 system (Walz, Effelrich, Germany). The films were removed from vials and placed in the center of a leaf holder (Dual-BA; Walz). The steady-state Chl a fluorescence level (Ft) was determined during the application of actinic red light intensity of ∼50 or 150 μmol photons m−2 s−1 for 5 min. Saturating light pulses of 300-ms duration and light intensity of 3,000 μmol photons m−2 s−1 were applied to determine a maximum fluorescence level at light (Fm′). The effective photosystem II (PSII) yield, Y(II), was calculated as (Fm′ − Ft/Fm′).
Statistical testing.
Student t tests were applied for significance at 95% using GraphPad Prism, version 5, for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
The effect of CO2 supplementation cycles on long-term H2 photoproduction activity.
Efficient H2 photoproduction in N2-fixing heterocystous cyanobacteria is only possible in the absence of N2, where nitrogenase catalyzes solely the reduction of protons to H2 (2). This activity also requires the presence of CO2 (27). Therefore, in the first approach to achieving long-term H2 photoproduction, the alginate films with entrapped wild-type and ΔhupL cells of Anabaena PCC 7120 were subjected to a number of CO2 supplementation cycles, where each cycle was initiated by the change of gas phase in the headspace of vials to Ar containing 6% CO2 (Fig. 1). A lack of CO2 has previously resulted in a significant attenuation of H2 photoproduction starting from the first cycle and loss of H2 evolution on the conclusion of the next cycle (27). As shown in Fig. 1, CO2 supplementation cycles allowed extension of the overall H2 photoproduction period of immobilized ΔhupL cells to at least 600 h. Nevertheless, both strains demonstrated a gradual loss of H2 photoproduction activity over the time of the experiment, whereby supplementation of 6% CO2 could not completely recover earlier production rates (Fig. 1 and Table 1).
FIG 1.
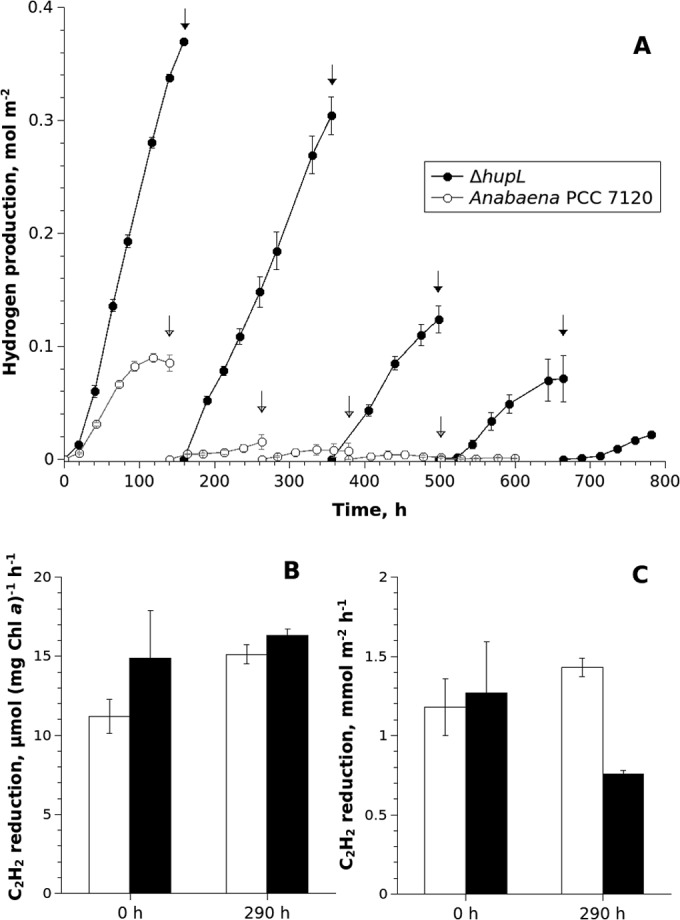
Kinetics of long-term H2 photoproduction (A) and changes in specific (B) and per area (C) rates of acetylene reduction by Anabaena PCC 7120 and its ΔhupL mutant entrapped in thin Ca2+-alginate films. For H2 photoproduction, arrows indicate the points where the atmosphere of the vials was replaced with Ar supplemented with 6% CO2. For acetylene reduction, white bars indicate Anabaena PCC 7120, and black bars indicate the ΔhupL mutant. Error bars show the standard deviations of triplicate samples. In the case of acetylene reduction, the difference between two strains was significant (P < 0.001) only for the 290-h point, when activities were calculated on a per area basis (C).
TABLE 1.
Summary of kinetic parameters of O2 evolution and H2 photoproduction in the wild-type Anabaena PCC 7120 and the ΔhupL mutant entrapped in thin Ca2+-alginate films
| Cycle no. | Strain/genotype | Kinetic profilea |
|||
|---|---|---|---|---|---|
| Maximum specific rate of H2 production (μmol/mg Chl a/h) | Net H2 photoproduction yield (mol/m2) | Maximum specific rate of O2 evolution (μmol/mg Chl a/h) | Net O2 evolution yield (mol/m2) | ||
| 1 | Anabaena PCC 7120 | 7.1 ± 3.0*** | 0.07 ± 0.02*** | 60.0 ± 8.8*** | 0.19 ± 0.01** |
| ΔhupL | 31.9 ± 9.1 | 0.31 ± 0.08 | 78.1 ± 10.0 | 0.33 ± 0.06 | |
| 2 | Anabaena PCC 7120 | 2.9 ± 2.1*** | 0.03 ± 0.02** | 18.2 ± 8.3 | 0.15 ± 0.01 |
| ΔhupL | 16.6 ± 4.5 | 0.23 ± 0.09 | 21.4 ± 8.2 | 0.24 ± 0.09 | |
| 3 | Anabaena PCC 7120 | 1.8 ± 0.8** | 0.01 ± 0.01* | 19.4 ± 9.4 | 0.16 ± 0.04 |
| ΔhupL | 10.9 ± 5.2 | 0.08 ± 0.05 | 22.3 ± 5.1 | 0.19 ± 0.03 | |
| 4 | Anabaena PCC 7120 | 1.5 ± 1.1* | 0.002 ±.0.001 | 15.6 ± 9.3 | 0.13 ± 0.06* |
| ΔhupL | 9.2 ± 7.0 | 0.05 ± 0.04 | 12.8 ± 7.2 | 0.07 ± 0.01 | |
| 5 | Anabaena PCC 7120 | 1.1 ± 0.8 | 0.001 ± 0.001* | 13.5 ± 9.1 | 0.12 ± 0.07 |
| ΔhupL | 4.9 ± 3.8 | 0.02 ± 0.01 | 14.4 ± 4.4 | 0.11 ± 0.06 | |
| Total | Anabaena PCC 7120 | 0.12 ± 0.02*** | 0.74 ± 0.11* | ||
| ΔhupL | 0.70 ± 0.18 | 0.93 ± 0.14 | |||
The long-term H2 photoproduction activities in immobilized cells were maintained by periodic purging of the headspaces of the vials with Ar supplemented with 6% CO2. Values represent an average of three independent experiments (with three vials in each) ± the standard deviations. Significant differences between the two strains are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As expected, H2 photoproduction activity was more pronounced in the ΔhupL mutant due to the lack of uptake hydrogenase in this strain (20). Indeed, in the beginning of the first cycle, the nitrogenase activity measured as C2H2 reduction and calculated on the basis of Chl (specific nitrogenase activity) or per area unit was only slightly higher in the ΔhupL mutant than in the wild-type strain (Fig. 1B and C). After 290 h, nitrogenase activity calculated per area unit declined about 2-fold in the ΔhupL mutant but increased slightly in the wild-type strain (Fig. 1C). However, no significant changes were observed in specific rates of C2H2 reduction between the two strains (Fig. 1B, 290-h samples).
The maximum specific H2 production rates for both strains (Table 1) were highest in the first cycle, with the ΔhupL strain demonstrating a significantly higher maximum rate (32 μmol H2 mg Chl a−1 h−1) than Anabaena PCC 7120 (∼7 μmol H2 mg Chl a−1 h−1). In five cycles (three independent experiments) (Table 1), ΔhupL cells entrapped in alginate film produced a total 0.7 mol m−2 of H2 in 780 h (∼33 days), while the films with Anabaena PCC 7120 produced only around 0.12 mol m−2 of H2 in 600 h (25 days).
As was observed for H2 photoproduction, immobilized cells also demonstrated a decline in O2 evolution over the course of experimental cycles (Table 1). The most pronounced change in the O2 production rate occurred after the first cycle, where a decrease of about 70% was observed for both strains. In later cycles, the maximum O2 production activity stabilized, and the gap between rates and yields of the two strains decreased (Table 1). The sum of final O2 yields was higher in vials with ΔhupL mutant films than in vials containing Anabaena PCC 7120 films (0.93 and 0.74 mol m−2 after 5 cycles, respectively). The reason for this is not clear since both O2 evolution by photosystem II (PSII) and its consumption in respiratory pathways are responsible for the final O2 yields.
The effects of different gas compositions (Ar, air, and N2) in CO2 supplementation cycles on cell fitness.
To study the possible negative effects of nitrogen deficiency on H2 photoproduction activity in immobilized cyanobacteria and to clarify the role of uptake hydrogenase in adaptation processes, films with entrapped cells of Anabaena PCC 7120 and its ΔhupL mutant were placed under different atmospheres (Ar, air, or N2). All atmospheres were supplemented with 6% CO2, and the gas phase in the headspace of vials was regenerated to its initial composition every fourth day.
Under our experimental conditions, the ΔhupL mutant significantly outperformed the wild-type strain in H2 production (see Fig. S1 in the supplemental material). In contrast to the wild-type strain, which produced H2 only under the Ar atmosphere, the ΔhupL mutant also produced H2 under atmospheres both with N2 plus 6% CO2 and air plus 6% CO2, but at substantially reduced rates compared to the samples with Ar plus 6% CO2. The loss of H2 photoproduction yields in the presence of N2 is a well-known phenomenon for N2-fixing cyanobacteria and is caused by the change in the stoichiometry of H+ reduction by the nitrogenase enzyme (2). Nevertheless, these experiments allowed us to compare cell fitness of immobilized Anabaena PCC 7120 and ΔhupL cells under different experimental conditions.
Despite a very high volumetric cell density in the films (24), the alginate matrix did not completely prevent cell growth in the presence of high levels of N2 and CO2, where significant increases in Chl a content (Fig. 2A, air and N2 samples) and OD750 (data not shown) were determined. However, under the Ar atmosphere, where immobilized cells were subject to N deficiency, both Anabaena PCC 7120 and ΔhupL strains demonstrated a decline in Chl a over the course of the experiment. This was more pronounced in the films containing ΔhupL mutant cells, where Chl a decreased by ∼35% (Fig. 2A). Interestingly, under the atmosphere of air plus 6% CO2, the Chl a content of the ΔhupL mutant did not change, but a significant increase was observed for the wild-type strain. Both conditions led to a significant inhibition of the effective PSII yield in the entrapped ΔhupL cells, where Y(II) declined from ∼0.34 to 0.15 (Ar plus 6% CO2) and 0.17 (air plus 6% CO2) after 284 h (∼12 days) of continuous treatments with respective gas compositions (Fig. 2B). The replacement of air with N2 in the headspace of vials improved both Chl a content (Fig. 2A) and Y(II) (Fig. 2B) in the ΔhupL mutant.
After 12 days, the alginate-entrapped cells were recovered from the films to suspensions and equilibrated to the same cell density. Cell fitness was then monitored as regrowth under standard conditions for another 6 days (Fig. 3). Cells that were recovered from the films immediately after immobilization (i.e., not undergoing pretreatment) were used as a control. The results of the regrowth experiment confirmed that the cells recovered from the ΔhupL mutant films were indeed adversely affected under Ar and air atmospheres supplemented with 6% CO2, with final OD750 values that were significantly less than those of the respective control. The wild-type strain grew slowly only after pretreatment with Ar plus 6% CO2 but still recovered considerably faster than the ΔhupL mutant. Indeed, following a 3-day lag period under standard growth conditions, the wild type pretreated with Ar plus 6% CO2 finally reached an OD750 that was not significantly different from that of the control. The pretreatment of the ΔhupL films under N2 plus 6% CO2 did not significantly affect cell fitness compared to the control ΔhupL cells which were grown under standard conditions without any pretreatment. It is worth mentioning that the control ΔhupL cells without any pretreatment regrew significantly more slowly (one-sided t test, P < 0.001) than the control wild-type Anabaena PCC 7120 (Fig. 3).
FIG 3.
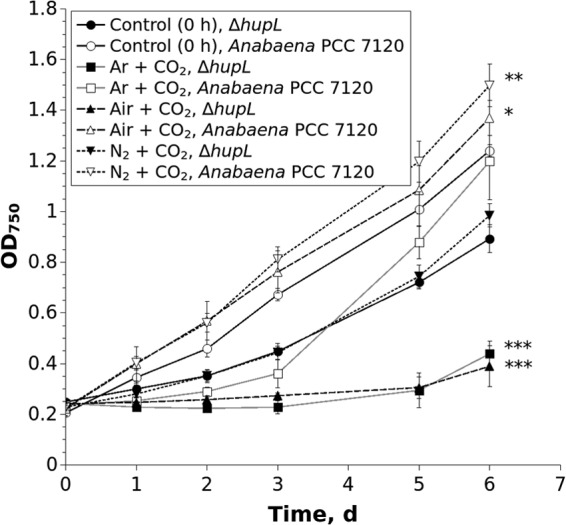
Regrowth (OD750) of the cells recovered from alginate films. Control films did not undergo any treatment (0 h), whereas treated films were subject to 12 days under an Ar, air, or N2 atmosphere supplemented with 6% CO2. The suspension cultures were regrown diazotrophically in 24-well plates under 50 μmol m−2 s−1 and an air atmosphere. Error bars indicate the means ± standard deviations from three biological repetitions. Significant differences between the treated and nontreated samples are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. d, days.
To further determine the possible impact of deletion of HupL on growth, we inoculated the control cells at two different values of OD750 (0.1 and 0.5) and cultivated them under standard growth conditions (air with light intensity of 50 μmol photons m−2 s−1). When the cells were inoculated at an OD750 of 0.5, only a very small difference was observed between the growth of wild-type Anabaena PCC 7120 and the ΔhupL mutant cells after the second day (see Fig. S2 in the supplemental material). The highest difference in the growth of the WT and ΔhupL cells was observed when the cells were inoculated at the lowest OD750 of 0.1, indicating a possible high light sensitivity of the ΔhupL mutant cells.
The effects of periodic short-term treatments with air and air plus 6% CO2 on long-term H2 photoproduction activities in immobilized cultures.
The results obtained from the experiments described above indicated that long-term and efficient H2 photoproduction might be achieved by protecting the fitness of immobilized cells through periodic and short-term exposure of films to a N2-containing atmosphere. It was decided that these treatments should be applied between CO2 supplementation cycles (Ar plus 6% CO2) and for a time that is short enough to exclude significant cell growth inside the films but long enough to ensure the recovery of photosynthetic and nitrogenase activities in immobilized cyanobacteria. This condition was achieved by changing the gas phase to air for a short treatment of 16 to 20 h.
Since the continuous treatment with air plus 6% CO2 applied in the cell fitness experiments adversely affected the ΔhupL mutant (Fig. 2 and 3), our next approach involved two conditions: periodically (every 3 to 4 days) changing the gas phase of the vials to contain (i) air with ambient levels of CO2 (air sample) or (ii) air supplemented with 6% CO2 (air plus CO2). After about 16 to 20 h of either treatment to restore some level of cell fitness (Fig. 4, insets), the headspace was returned to Ar supplemented with 6% CO2 in order to achieve maximal H2 photoproduction capacity. In this experiment, the effect of treatments with air or air plus CO2 on H2 photoproduction was evaluated for four different strains: Anabaena PCC 7120, Calothrix 336/3, and ΔhupL and ΔhupL ΔhoxH mutants of Anabaena PCC 7120. Since the ΔhupL ΔhoxH strain demonstrated very similar results as the ΔhupL mutant, the data are not shown.
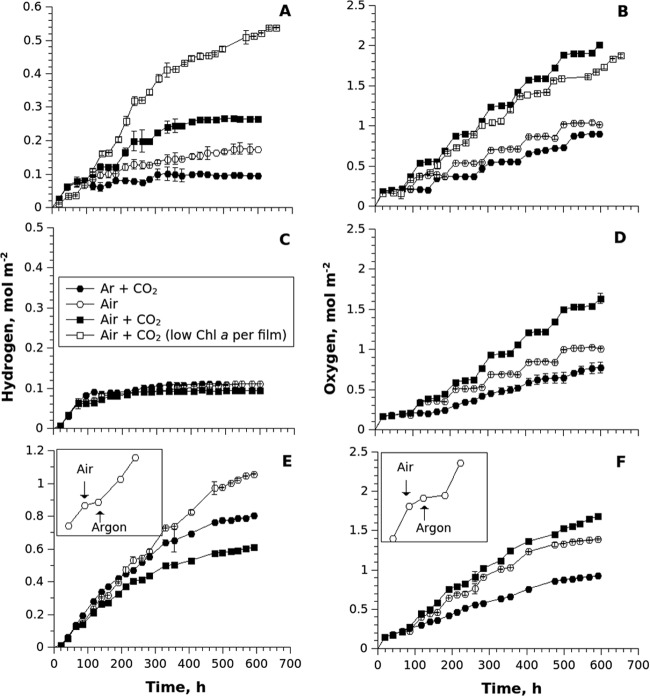
FIG 4.
Long-term H2 (A, C, and E) and O2 (B, D, and F) photoproduction yields from Calothrix 336/3 (A and B), Anabaena PCC 7120 (C and D), and the ΔhupL mutant of Anabaena PCC 7120 (E and F) entrapped in alginate films. In contrast to the experiment shown in Fig. 1, the cumulative yields are presented. The gas phase in the headspace of the vials was renewed periodically with Ar supplemented with 6% CO2 (Ar + CO2), air (air), or air supplemented with 6% CO2 (air + CO2). In the case of Calothrix 336/3, the kinetic curves from the films treated with air plus CO2 with lower Chl a content per area unit are also presented. The insets represent the typical H2 (inset E) and O2 (inset F) photoproduction curves during a single cycle of air treatment.
Periodic treatments of immobilized cells with air, or air containing 6% CO2, significantly increased H2 production yield in Calothrix 336/3 compared to the N-deprived control cells (Fig. 4A, Ar + CO2). With periodic air treatments, entrapped Calothrix 336/3 cells produced 0.17 mol H2 m−2 (600 h), and the addition of CO2 to air treatments increased the yield to 0.26 mol H2 m−2. The effect of air treatments was even more pronounced in thin films of Calothrix 336/3 with a lower Chl a content per surface area unit (113 versus 215 mg Chl m−2 in the original films). When treated with air supplemented with 6% CO2, the low-Chl a thin films with entrapped Calothrix 336/3 cells reached more than 0.5 mol H2 m−2 by 650 h (Fig. 4A).
In contrast to the Calothrix strain, no significant changes were observed in Anabaena PCC 7120 under any treatments (Fig. 4C). For this strain, the rate of H2 production declined dramatically after ∼70 h in all samples, but cells continued producing H2 at very low rates (similar to the Ar-treated curves presented in Fig. 1 and in Fig. S1 in the supplemental material) to a final value of approximately 0.1 mol H2 m−2. As expected, H2 photoproduction yields were significantly higher in the vials with the ΔhupL mutant (Fig. 4E). The ΔhupL cells entrapped in alginate produced the highest level of H2 (1.06 mol m−2) when treated with air. In line with the effects observed during extended H2 production under CO2 supplementation treatments (Fig. 2 and 3), the supplementation of 6% CO2 during the periodic 16- to 20-h air treatments actually decreased the H2 photoproduction yield of this ΔhupL strain (0.61 mol m−2) (Fig. 4E). However, this yield was still higher than that of both wild-type strains.
Cyanobacterial cells entrapped in films did not produce any (both wild-type strains) or only negligible amounts (ΔhupL mutant) of H2 during periods of air treatment (Fig. 4E, inset). These results were independent of the presence or absence of introduced CO2. Indeed, during exposure to air, cyanobacterial cells entrapped in the films actively fixed N2 (as detected by GC) (data not shown) and therefore demonstrated significantly diminished stoichiometry of H2 production (2, 4, 5). Interestingly, during the periods of air treatment (no CO2 supplementation), all strains also demonstrated decreased O2 evolution (Fig. 4F, inset). The inhibitory effect often remained noticeable during the first ∼24 h after the return of the gas to Ar. After this lag period, the O2-evolving ability of the cells recovered and led to accumulation of O2 in the headspace of vials.
Although air treatments, through restoring the fitness of immobilized cells, improved overall H2 photoproduction yields of Calothrix 336/3 and ΔhupL cells, this approach could not drive continuous H2 photoproduction. All strains finally stopped producing H2 although this endpoint was reached at different times for the different strains (Fig. 4A, C, and E). Importantly, the periodic treatments of cells with air supplemented with 6% CO2 significantly improved the O2 production yields in all strains (Fig. 4B, D, and F), but the effect was less pronounced in the ΔhupL mutant films (Fig. 4F). The air-only treatments slightly improved O2 yields in both wild-type strains compared to untreated cells (Fig. 4B and D), with the ΔhupL mutant (Fig. 4F) demonstrating a more pronounced increase. These results demonstrate the potential benefit of periodic air treatments for the recovery of photosynthetic apparatus and cell fitness in N-starved cells. However, we cannot directly link the O2 yield determined from the headspace with the photosynthetic activity of the cells due to difficulties in distinguishing between photosynthetic O2 evolution and O2 consumption by terminal oxidases.
Changes in chlorophyll content and photochemical activity of immobilized cells during the long-term H2 photoproduction experiment.
For a more sensitive study of photosynthetic machinery, we measured Chl a content and effective PSII yield, Y(II), of the samples during long-term H2 photoproduction experiments where treatments with air or air plus CO2 were applied. Changes in the pigment composition of immobilized cyanobacteria during experiments were easily seen in the color of the films (Fig. 5A). The first noticeable change in the pigment composition occurred soon after the second cycle and progressed thereafter. To quantify Chl a more accurately, we used HPLC (Fig. 5B).
Under N deprivation, both the Anabaena PCC 7120 strain and its ΔhupL mutant demonstrated not only progressive decreases in H2 and O2 production activities but also significant degradation of Chl a in the films (Fig. 5B, Ar + CO2). The most noticeable degradation of Chl a was detected in the ΔhupL mutant, in which Chl a degraded by 70% compared to the same culture in the beginning of the experiment (0 h). Interestingly, this mutant also showed significant Chl bleaching when the cells were regularly treated by air supplemented with 6% CO2 (Chl a degraded by almost 80% on average), replicating the effect observed for the continuous treatment with air plus 6% CO2 (Fig. 2). The treatments of the films with air only supported the Chl a contents in both Anabaena PCC 7120 and its ΔhupL mutant, with that of the ΔhupL mutant remaining approximately constant and that of the wild type slightly increasing (Fig. 5B, air). In the control (Ar plus CO2) films with entrapped Calothrix 336/3, the Chl a level actually increased from the beginning of the experiment by approximately 20%. Air treatments further increased the Chl content in the immobilized Calothrix 336/3 films by around 50 and 60% in samples treated with air plus 6% CO2 and air, respectively. Therefore, it is possible that some minor growth of Calothrix 336/3 occurred in the alginate films, although at a considerably lower rate than would occur in suspension cultures.
Measurements of photochemical activity from the surface of the films using the Dual-PAM 100 system were performed at the beginning (1 h) and in the middle (∼455 h) of long-term experiments. These measurements demonstrated that photosynthetic activity was significantly impaired in all studied strains under long-term N deprivation conditions (Fig. 6, Ar + CO2 films). In these films, the effective PSII yield, Y(II), declined from 0.33 to 0.16 for Anabaena PCC 7120, from 0.29 to 0.18 for Calothrix 336/3, and from 0.34 to 0.06 the ΔhupL mutant after ∼455 h of the experiment. The periodic treatments of all three entrapped strains with air, which brought nitrogen back to the system, considerably restored PSII yield Y(II) to levels of 0.32 in Anabaena PCC 7120, 0.38 in Calothrix 336/3, and 0.39 in the ΔhupL mutant. Periodic changes of the medium during long-term experiments did not impart a noticeable effect on the restoration of photosynthetic apparatus (data not shown), thus eliminating the possible effects of a shortage of other nutrients. Interestingly, the entrapped cells periodically treated by air supplemented with 6% CO2 adjusted their photochemical activity differently: Anabaena PCC 7120 demonstrated a similar PSII yield (0.30), and Calothrix 336/3 showed an even higher activity (0.44), whereas the ΔhupL mutant demonstrated significantly decreased PSII yield (0.12) compared to the cells treated with only air (Fig. 6).
FIG 6.
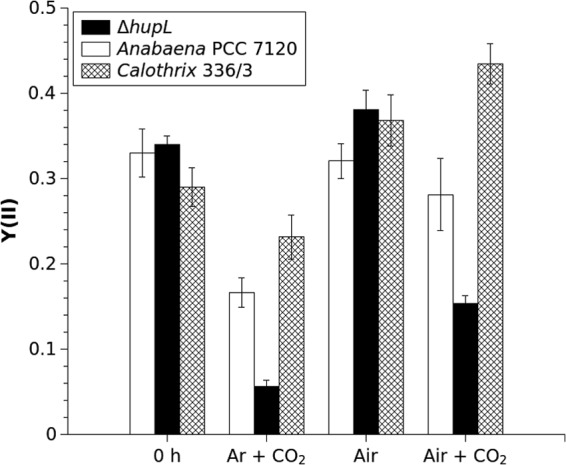
The effect of different treatments on the PSII yield, Y(II), in alginate films with entrapped Calothrix 336/3, Anabaena PCC 7120, and ΔhupL mutant cells. Measurements of photochemical activity from the surface of the films were performed after 1 h and in the middle (∼455 h) of the long-term experiment. The experimental conditions are the same as described in the legend of Fig. 4.
DISCUSSION
Supplementation of CO2 prolongs H2 production in immobilized N2-fixing heterocystous cyanobacteria under an Ar atmosphere.
H2 photoproduction in heterocystous, filamentous N2-fixing cyanobacteria is mediated mainly by the nitrogenase enzyme located in the heterocyst cells, where H2 is coproduced in the reaction of N2 fixation. It is a highly energy-demanding process requiring 16 mol of ATP for every mole of N2 fixed and H2 produced. Although the efficiency of the reaction toward H2 evolution improves significantly in the absence of N2 (see Fig. S1 in the supplemental material), where nitrogenase exclusively catalyzes ATP-dependent reduction of H+ to H2, 4 mol of ATP is still required for every mole of H2 evolved. Consequently, under photoautotrophic conditions, where CO2/HCO3− is the only carbon source, H2 photoproduction in heterocystous cyanobacteria depends solely on the photosynthetic activity of vegetative cells that fix CO2 and provide heterocysts with energy in the form of sucrose (36). Thus, long-term H2 photoproduction by cyanobacteria requires CO2 supply into the cultures (37, 38). Indeed, periodic supplementations of 6% CO2 into vials containing Ca2+-alginate films with entrapped Anabaena PCC 7120 and ΔhupL mutant cells under an Ar atmosphere allowed us to sustain H2 photoproduction for 600 h and 780 h, respectively (Fig. 1 and Table 1). The effect of 6% CO2 supplementations was similar to that observed previously by our group for Calothrix 336/3 (27), where supplementations prolonged H2 photoproduction for even longer periods (936 h) than the ΔhupL mutant in the present work, but at lower maximum H2 yields per cycle.
Although supplementation of the cells with CO2 does prolong H2 production in heterocystous cyanobacteria, this is clearly not the only factor influencing the long-term performance of this process. Indeed, under an Ar atmosphere, we observed H2 photoproduction yields to steadily decline in each subsequent cycle of CO2 supplementation for both Anabaena strains (Fig. 1). Since the ΔhupL mutant does not have uptake hydrogenase, the decline of H2 photoproduction yields for this strain could not be linked to an increase of H2 uptake activity. Additionally, the ΔhupL ΔhoxH mutant of Anabaena PCC 7120, deficient in both hydrogenases, showed approximately equivalent kinetics of H2 photoproduction as the ΔhupL strain (data not shown), indicating that the bidirectional hydrogenase is not involved either. The specific nitrogenase activity of the wild-type and ΔhupL strains did not show significant changes during treatments (Fig. 1B). Moreover, there was no significant difference in the specific nitrogenase activity between the wild-type and ΔhupL strains, which agrees well with previous data that Masukawa and coauthors obtained for suspension cultures (20). However, when the activity was calculated per area of film (Fig. 1C), a decrease in nitrogenase activity was observed in the ΔhupL mutant. This decrease could be due to the lysis of ΔhupL cells under N-deprived conditions. Thus, the decline of H2 photoproduction yields in each subsequent cycle is likely caused by nitrogen, rather than carbon, deficiency.
Long-term nitrogen deprivation, but not anaerobiosis, affects cell fitness of immobilized cells under conditions suitable for efficient H2 photoproduction.
Comparison of cell fitness of Anabaena PCC 7120 and its ΔhupL mutant after 12 days under Ar and N2 atmospheres supplemented with 6% CO2 clearly showed that Anabaena cultures entrapped in alginate films do not suffer from microoxic conditions since the immobilized cells continue growing under an atmosphere with N2 plus 6% CO2 (Fig. 2). These cells also showed the fastest recovery of growth in suspension culture after treatment (Fig. 3). The situation changed dramatically under the atmosphere of Ar plus 6% CO2, where the cells suffer from nitrogen deficiency leading to imbalance in the C/N ratio. The incubation of both wild-type and ΔhupL cells under an Ar atmosphere in the presence of high CO2 was harmful to cell fitness, as determined by regrowth experiments (Fig. 3). The situation was more dramatic in the ΔhupL mutant films, where the cell fitness was affected not only under Ar but also under air atmospheres supplemented with 6% CO2 (Fig. 3). It should be noted that the decrease of O2 partial pressure in vials, achieved by changing the headspace atmosphere to N2 plus 6% CO2, recovered the Chl a content (Fig. 2A), Y(II) (Fig. 2B), and cell fitness (Fig. 3) of this strain. These results indicate that the ΔhupL mutant might be more sensitive to excess O2 than the wild-type strain.
Long-term nitrogen deprivation decreases photosynthetic yield and H2 production activity in immobilized cyanobacteria, particularly in the ΔhupL strain.
Prolonged cultivation of alginate-entrapped cyanobacterial cells in N-deprived conditions caused a significant loss of photochemical activity in Calothrix 336/3 and in both Anabaena cell types, with a more pronounced effect observed in the ΔhupL mutant (Fig. 2B and 6, Ar plus CO2 films). Under these conditions, presumably due to impaired protein biosynthesis, cyanobacteria could not efficiently repair the photosynthetic apparatus. A distinct loss of Chl a was also observed in both Anabaena cell lines under the atmosphere with Ar plus 6% CO2 (Fig. 2 and 5). Contrasting this, the Chl a content in the Calothrix 336/3 strain was not affected by N deficiency. Moreover, this strain demonstrated a very small amount of growth, even under an Ar atmosphere (Fig. 5, Calothrix films with Ar plus CO2).
The prolonged (Fig. 2B) or periodic (Fig. 6) treatments of two wild-type and ΔhupL cells with air, which brought nitrogen back to the system, allowed the cells to restore the photosynthetic apparatus. Interestingly, treatments with air supplemented with 6% CO2 had different effects on the different species, whereby the treatment further improved (Calothrix 336/3), did not change significantly (Anabaena PCC 7120), or negatively affected (ΔhupL mutant), PSII yield compared to that of air-treated cells (Fig. 6). In the case of the ΔhupL mutant, this negative effect was accompanied by a pronounced bleaching of the films (Fig. 2 and 5). Under prolonged incubation with air plus 6% CO2, the ΔhupL cells were undergoing lysis, which was confirmed by the extremely slow recovery of the cells during the regrowth experiment (Fig. 3).
The response of H2 production to periodic 16- to 20-h air treatments was strain specific. For example, the ΔhupL mutant produced more H2 when treated with air only (Fig. 4E), Calothrix 336/3 required the continuous presence of CO2 in the headspace of the vials for improved production (Fig. 4C), and the wild-type Anabaena strain did not improve H2 production under any condition tested (Fig. 4A). It has previously been shown that continuous supplementation of cells with N2 suppressed the H2-producing activity of Anabaena PCC 7120 and its hydrogenase(s)-deficient mutants (38). In that experiment, cultures were supplemented with 1% N2 every day, which could have led to inhibition of H2 production due to allocation of electrons to N2 fixation and/or caused a metabolic shift in the C/N balance of microoxic cultures. A similar suppression was observed in our experiments under N2 and air atmospheres supplemented with 6% CO2 (see Fig. S1 in the supplemental material), whereas periodic 16- to 20-h air treatments, which were employed to provide nitrogen to the cultures for restoration of photosynthetic activity and cell metabolism, enabled some high H2 photoproduction yields in ΔhupL and Calothrix 336/3 strains (Fig. 4).
While declines in photochemical activity were observed under nitrogen deficiency, the increase of H2 uptake capacity over the time period of experiments could also influence H2 production levels for the wild-type strains. It is known that the alginate matrix significantly restricts diffusion of gas both to and from immobilized cells (32, 39), thus increasing intracellular levels of H2 and O2. Such elevations in H2 may enhance the activity (40, 41) and expression level of the uptake hydrogenase enzyme (42), while O2 accumulated inside the films may favor the oxyhydrogen or Knallgas reaction (5, 6). However, not all heterocystous cyanobacteria show positive regulation of hupSL genes by H2 (43). Since the nitrogenase activity (both specific and per area basis) in the films with the entrapped wild-type strain of Anabaena PCC 7120 did not change significantly by the time of the sharp decline in net H2 photoproduction yield at around 100 h (Fig. 1 and 4C; see also Fig. S1 in the supplemental material), the decrease could only be caused by an increase in the rate of H2 consumption. Clearly, more investigations are required for resolving the role of hydrogenase(s) in this process.
Uptake hydrogenase protects filaments during long-term N deprivation.
Comparison of net H2 and O2 yields in the wild-type strain of Anabaena PCC 7120 and its uptake hydrogenase mutant (Table 1) demonstrates a direct link between H2 uptake and respiration in these strains. Over five cycles, the ΔhupL mutant produced a total of 0.58 mol m−2 more H2 than the wild-type strain. Assuming 100% consumption of H2 in the oxyhydrogen reaction with a molar ratio of 2 to 1 H2/O2, the absence of this process would bring an additional 0.29 mol m−2 O2 into the final O2 yields for the wild-type strain, yielding almost equivalent O2 production in both strains (0.93 versus 1.03, respectively). However, this link between H2 uptake and respiration was not as pronounced in air-treated samples (Fig. 4) due to a high sensitivity of the H2 production and H2 uptake enzymes to O2. Similar results have been observed for Anabaena variabilis ATCC 29413 and its hydrogenase-impaired PK84 mutant (44). Thus, the decline in the rate of H2 photoproduction in the wild-type strain over time is partly caused by the recycling of H2 through the oxyhydrogen reaction. The other wild-type strain evaluated in our study, Calothrix 336/3, also demonstrated H2 uptake activity in vials treated with Ar plus CO2 by the end of each CO2 supplementation cycle (Fig. 4A). This cyanobacterium possesses the genes encoding the uptake hydrogenase enzyme and demonstrates in vitro H2 uptake activity (45). Therefore, it is likely that H2 uptake in Calothrix 336/3 is also connected to the respiratory chain although the oxyhydrogen reaction in this strain might be less pronounced, leading to higher H2 photoproduction yields (Fig. 4A) than in the Anabaena PCC 7120 wild-type strain (Fig. 4C).
When linked to the respiratory chain, uptake hydrogenase removes excess O2 in heterocysts and provides the cells with ATP via the oxyhydrogen reaction. Both conditions are beneficial for the nitrogenase enzyme (3, 5). The importance of this reaction becomes clear in our long-term experiments, especially during periods of air treatment, when cyanobacterial cells experience an excess of O2. Indeed, the ΔhupL mutant lacking uptake hydrogenase showed significant degradation of Chl a and photochemical activity not only in the films treated with Ar plus CO2 but also, most importantly, in the films that were treated periodically with air supplemented with 6% CO2 (Fig. 5 and 6). After prolonged incubation under an atmosphere with air plus 6% CO2, the immobilized ΔhupL mutant cultures showed reduced cellular fitness (Fig. 3). In contrast, the cell fitness of the Anabaena PCC 7120 strain was affected only under the atmosphere with Ar plus 6% CO2, while still recovering significantly faster than the ΔhupL strain. The reduction of O2 partial pressure by placing the films under the atmosphere with N2 plus 6% CO2 was the only treatment able to fully recover the cell fitness of the mutant (Fig. 3).
We suggest that an increased level of O2 inside the vegetative cells of the ΔhupL mutant, occurring due to efficient photosynthesis (in air plus CO2) and to limited gas exchange through the alginate matrix, led to the penetration of a significant amount of O2 into the heterocysts via narrow terminal pores that connect heterocysts with vegetative cells (46). In this case, the ΔhupL mutant was unable to fix N2 efficiently during the treatment with air plus 6% CO2 and thus failed to restore the photosynthetic apparatus. Subsequently, a dramatic shift in the intracellular C/N ratio would have caused a strong metabolic imbalance for ΔhupL cells. Under this condition, the ΔhupL mutant demonstrates significant chlorosis (Fig. 5) and pronounced inactivation of the photosynthetic apparatus (Fig. 6) in contrast to both wild-type strains, which possess active uptake hydrogenase. A similar protective role of uptake hydrogenase has been demonstrated in the unicellular cyanobacterium Cyanothece strain PCC 7822, where its involvement in the protection of nitrogenase is clear due to a single compartmentalization whereby, under diazotrophic conditions, the ΔhupL strain failed to grow even under atmospheric air (47).
In the absence of 6% CO2 during air treatments, films with entrapped ΔhupL cells do not produce O2 efficiently (Fig. 4F, inset) and can maintain microoxic conditions, probably through the upregulation of alternative pathways (21). This may include the expression of heterocyst-specific flavodiiron proteins, which are known to be involved in the protection of nitrogenase by redirecting excess electrons to O2 (48–50). It is also possible that under low CO2 levels, highly inducible Flv1A and Flv3A flavodiiron proteins, localized to the vegetative cells of Anabaena PCC 7120 (48) and an active photorespiratory pathway (51, 52), could contribute to lowering intracellular O2 levels in vegetative cells, thereby also protecting heterocyst nitrogenase when uptake hydrogenase is absent. As a result, short-term, air-only treatments of ΔhupL mutant films resulted in the highest H2 photoproduction yields in immobilized cultures (Fig. 4E).
We propose that the uptake hydrogenase in heterocystous cyanobacteria is not only important for supporting the high activity of the nitrogenase system by removing excess O2 in heterocysts and providing cells with ATP via the oxyhydrogen reaction but also indirectly involved in protecting the photosynthetic apparatus of vegetative cells, especially under N-deprived conditions. Indeed, the diazotrophic filament heterocysts and vegetative cells are completely interdependent, and changes within one cell type affect dramatically another cell type. Although the absence of uptake hydrogenase in heterocysts can be partly compensated for by the upregulation of alternative mechanisms, these may fail to satisfy cyanobacteria in long-term culture, especially immobilized cultures, where the matrix polymer significantly restricts diffusion of gases and nutrients in both directions. In this context, the elimination of uptake hydrogenase in cells, while increasing H2 photoproduction yield and rate, may significantly decrease the duration of the process. Clearly, more detailed studies are required for understanding the role of the H2 uptake mechanism in immobilized strains.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by the Kone Foundation (Y.A.), Academy of Finland mobility grant 267409 (Y.A.), Maj and Tor Nessling Foundation grant 2014050 (S.K.), FCoE projects 118637 and 271832 (E.-M.A.), and the Nordic Energy Research AquaFEED project.
Footnotes
Published ahead of print 11 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01776-14.
REFERENCES
- 1.Schütz K, Happe T, Troshina O, Lindblad P, Leitao E, Oliveira P, Tamagnini P. 2004. Cyanobacterial H2 production—a comparative analysis. Planta 218:350–359. 10.1007/s00425-003-1113-5 [DOI] [PubMed] [Google Scholar]
- 2.Tsygankov AA. 2007. Nitrogen-fixing cyanobacteria: a review. Appl. Biochem. Microbiol. 43:279–288. 10.1134/S0003683807030040 [DOI] [PubMed] [Google Scholar]
- 3.Bothe H, Schmitz O, Yates MG, Newton WE. 2010. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol. Mol. Biol. Rev. 74:529–554. 10.1128/MMBR.00033-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamagnini P, Axelsson R, Lindberg P, Oxelfelt F, Wunschiers R, Lindblad P. 2002. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol. Mol. Biol. Rev. 66:1–20. 10.1128/MMBR.66.1.1-20.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamagnini P, Leitao E, Oliveira P, Ferreira D, Pinto F, Harris D, Heidorn T, Lindblad P. 2007. Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol. Rev. 31:692–720. 10.1111/j.1574-6976.2007.00085.x [DOI] [PubMed] [Google Scholar]
- 6.Rao KK, Hall DO. 1996. Hydrogen production by cyanobacteria: potential, problems and prospects. J. Mar. Biotechnol. 4:10–15 [Google Scholar]
- 7.Weissman JC, Benemann JR. 1977. Hydrogen production by nitrogen-starved cultures of Anabaena cylindrica. Appl. Environ. Microbiol. 33:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrieri D, Wawrousek K, Eckert C, Yu J, Maness P-C. 2011. The role of the bidirectional hydrogenase in cyanobacteria. Bioresour. Technol. 102:8368–8377. 10.1016/j.biortech.2011.03.103 [DOI] [PubMed] [Google Scholar]
- 9.Appel J, Schulz R. 1998. Hydrogen metabolism in organisms with oxygenic photosynthesis: hydrogenases as important regulatory devices for a proper redox poising? J. Photochem. Photobiol. B Biol. 47:1–11. 10.1016/S1011-1344(98)00179-1 [DOI] [Google Scholar]
- 10.Appel J, Phunpruch S, Steinmuller K, Schulz R. 2000. The bidirectional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch. Microbiol. 173:333–338. 10.1007/s002030000139 [DOI] [PubMed] [Google Scholar]
- 11.Cournac L, Guedeney G, Peltier G, Vignais PM. 2004. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. J. Bacteriol. 186:1737–1746. 10.1128/JB.186.6.1737-1746.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benemann J. 1996. Hydrogen biotechnology: progress and prospects. Nat. Biotechnol. 14:1101–1103. 10.1038/nbt0996-1101 [DOI] [PubMed] [Google Scholar]
- 13.Borodin VB, Tsygankov AA, Rao KK, Hall DO. 2000. Hydrogen production by Anabaena variabilis PK84 under simulated outdoor conditions. Biotechnol. Bioeng. 69:478–485. [DOI] [PubMed] [Google Scholar]
- 14.Tsygankov AA, Fedorov AS, Kosourov SN, Rao KK. 2002. Hydrogen production by cyanobacteria in an automated outdoor photobioreactor under aerobic conditions. Biotechnol. Bioeng. 80:777–783. 10.1002/bit.10431 [DOI] [PubMed] [Google Scholar]
- 15.Mikheeva LE, Schmitz O, Shestakov SV, Bothe H. 1995. Mutants of the cyanobacterium Anabaena variabilis altered in hydrogenase activities. Z. Naturforsch. C Biosci. 50:505–510 [Google Scholar]
- 16.Tsygankov AA, Borodin VB, Rao KK, Hall DO. 1999. H2 photoproduction by batch culture of Anabaena variabilis ATCC 29413 and its mutant PK84 in a photobioreactor. Biotechnol. Bioeng. 64:709–715. [DOI] [PubMed] [Google Scholar]
- 17.Happe T, Schütz K, Böhme H. 2000. Transcriptional and mutational analysis of the uptake hydrogenase of the filamentous cyanobacterium Anabaena variabilis ATCC 29413. J. Bacteriol. 182:1624–1631. 10.1128/JB.182.6.1624-1631.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg P, Schütz K, Happe T, Lindblad P. 2002. A hydrogen producing, hydrogenase-free mutant strain of Nostoc punctiforme ATCC 29133. Int. J. Hydrogen Energy 27:1291–1296. 10.1016/S0360-3199(02)00121-0 [DOI] [Google Scholar]
- 19.Khetkorn W, Lindblad P, Incharoensakdi A. 2012. Inactivation of uptake hydrogenase leads to enhanced and sustained hydrogen production with high nitrogenase activity under high light exposure in the cyanobacterium Anabaena siamensis TISTR 8012. J. Biol. Eng. 6:19. 10.1186/1754-1611-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masukawa H, Mochimaru M, Sakurai H. 2002. Disruption of the uptake hydrogenase gene, but not of the bidirectional hydrogenase gene, leads to enhanced photobiological hydrogen production by the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120. Appl. Microbiol. Biotechnol. 58:618–624. 10.1007/s00253-002-0934-7 [DOI] [PubMed] [Google Scholar]
- 21.Ekman M, Ow SY, Holmqvist M, Zhang X, van Wagenen J, Wright PC, Stensjö K. 2011. Metabolic adaptations in a H2 producing heterocyst-forming cyanobacterium: potentials and implications for biological engineering. J. Proteome Res. 10:1772–1784. 10.1021/pr101055v [DOI] [PubMed] [Google Scholar]
- 22.Masukawa H, Inoue K, Sakurai H, Wolk CP, Hausinger RP. 2010. Site-directed mutagenesis of the Anabaena sp. strain PCC 7120 nitrogenase active site to increase photobiological hydrogen production. Appl. Environ. Microbiol. 76:6741–6750. 10.1128/AEM.01056-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weyman PD, Pratte B, Thiel T. 2010. Hydrogen production in nitrogenase mutants in Anabaena variabilis. FEMS Microbiol. Lett. 304:55–61. 10.1111/j.1574-6968.2009.01883.x [DOI] [PubMed] [Google Scholar]
- 24.Kosourov SN, Ghirardi ML, Seibert M. 2011. A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int. J. Hydrogen Energy 36:2044–2048. 10.1016/j.ijhydene.2010.10.041 [DOI] [Google Scholar]
- 25.Eltsova Z, Vasilieva L, Tsygankov A. 2010. Hydrogen production by recombinant strains of Rhodobacter sphaeroides using a modified photosynthetic apparatus. Appl. Biochem. Microbiol. 46:487–491. 10.1134/S0003683810050042 [DOI] [PubMed] [Google Scholar]
- 26.Allahverdiyeva Y, Leino H, Saari L, Fewer D, Shunmugam S, Sivonen K, Aro E-M. 2010. Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int. J. Hydrogen Energy 35:1117–1127. 10.1016/j.ijhydene.2009.12.030 [DOI] [Google Scholar]
- 27.Leino H, Kosourov SN, Saari L, Sivonen K, Tsygankov AA, Aro E-M, Allahverdiyeva Y. 2012. Extended H2 photoproduction by N2-fixing cyanobacteria immobilized in thin alginate films. Int. J. Hydrogen Energy 37:151–161. 10.1016/j.ijhydene.2011.09.088 [DOI] [Google Scholar]
- 28.Gosse JL, Engel BJ, Hui JC-H, Harwood CS, Flickinger MC. 2010. Progress toward a biomimetic leaf: 4,000 h of hydrogen production by coating-stabilized nongrowing photosynthetic Rhodopseudomonas palustris. Biotechnol. Prog. 26:907–918. 10.1002/btpr.406 [DOI] [PubMed] [Google Scholar]
- 29.Tsygankov A, Kosourov S. 2014. Chapter 14. Immobilization of photosynthetic microorganisms for efficient hydrogen production, p 321–347 In Zannoni D, De Philippis R. (ed), Microbial bioenergy: hydrogen production. Springer, Dordrecht, The Netherlands [Google Scholar]
- 30.Gosse JL, Engel BJ, Rey FE, Harwood CS, Scriven LE, Flickinger MC. 2007. Hydrogen production by photoreactive nanoporous latex coatings of nongrowing Rhodopseudomonas palustris CGA009. Biotechnol. Prog. 23:124–130 [DOI] [PubMed] [Google Scholar]
- 31.Gosse JL, Chinn MS, Grunden AM, Bernal OI, Jenkins JS, Yeager C, Kosourov S, Seibert M, Flickinger MC. 2012. A versatile method for preparation of hydrated microbial-latex biocatalytic coatings for gas absorption and gas evolution. J. Ind. Microbiol. Biotechnol. 39:1269–1278. 10.1007/s10295-012-1135-8 [DOI] [PubMed] [Google Scholar]
- 32.Kosourov SN, Seibert M. 2009. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol. Bioeng. 102:50–58. 10.1002/bit.22050 [DOI] [PubMed] [Google Scholar]
- 33.Kótai J. 1972. Instructions for preparation of modified nutrient solution Z8 for algae, publication B-11/69. Norwegian Institute for Water Research, Oslo, Norway [Google Scholar]
- 34.Dilworth MJ. 1966. Acetylene reduction by nitrogen fixing preparations from Clostridium pasteurianum. Biochim. Biophys. Acta 127:285–294. 10.1016/0304-4165(66)90383-7 [DOI] [PubMed] [Google Scholar]
- 35.Meeks JC, Castenholz RW. 1971. Growth and photosynthesis in an extreme thermophile, Synechococcus lividus (Cyanophyta). Arch. Mikrobiol. 78:25–41. 10.1007/BF00409086 [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Igual R, Flores E, Herrero A. 2010. Inactivation of a heterocyst specific invertase indicates a principal role of sucrose catabolism in heterocysts of Anabaena sp. J. Bacteriol. 192:5526–5533. 10.1128/JB.00776-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markov SA, Bazin MJ, Hall DO. 1995. Hydrogen photoproduction and carbon dioxide uptake by immobilized Anabaena variabilis in a hollow-fiber photobioreactor. Enzyme Microb. Technol. 17:306–310. 10.1016/0141-0229(94)00010-7 [DOI] [Google Scholar]
- 38.Marques AE, Barbosa AT, Jotta J, Coelho MC, Tamagnini P, Gouveia L. 2011. Biohydrogen production by Anabaena sp. PCC 7120 wild-type and mutants under different conditions: light, nickel, propane, carbon dioxide and nitrogen. Biomass Bioenergy 35:4426–4434. 10.1016/j.biombioe.2011.08.014 [DOI] [Google Scholar]
- 39.Sabra W, Zeng AP, Lunsdorf H, Deckwer WD. 2000. Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl. Environ. Microbiol. 66:4037–4044. 10.1128/AEM.66.9.4037-4044.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houchins JP, Burris RH. 1981. Physiological reactions of the reversible hydrogenase from Anabaena 7120. Plant Physiol. 68:717–721. 10.1104/pp.68.3.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oxelfelt F, Tamagnini P, Salema R, Lindblad P. 1995. Hydrogen uptake in Nostoc strain PCC 73102: effects of nickel, hydrogen, carbon and nitrogen. Plant Physiol. Biochem. 33:617–623 [Google Scholar]
- 42.Axelsson R, Lindblad P. 2002. Transcriptional regulation of Nostoc hydrogenases: effects of oxygen, hydrogen and nickel. Appl. Environ. Microbiol. 68:444–447. 10.1128/AEM.68.1.444-447.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weyman PD, Pratte B, Thiel T. 2008. Transcription of hupSL in Anabaena variabilis ATTC 29143 is regulated by NtcA and not by hydrogen. Appl. Environ. Microbiol. 74:2103–2110. 10.1128/AEM.02855-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsygankov AA, Serebryakova LT, Rao KK, Hall DO. 1998. Acetylene reduction and hydrogen photoproduction by wild-type and mutant strains of Anabaena at different CO2 and O2 concentrations. FEMS Microbiol. Lett. 167:13–17. 10.1111/j.1574-6968.1998.tb13201.x [DOI] [Google Scholar]
- 45.Leino H, Shunmugam S, Isojarvi J, Oliveira P, Mulo P, Saari L, Sivonen K, Battchikova N, Lindblad P, Aro E-M, Allahverdiyeva Y. 2014. Characterization of ten highly H2 producing cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int. J. Hydrogen Energy 39:8983–8991. 10.1016/j.ijhydene.2014.03.171 [DOI] [Google Scholar]
- 46.Walsby AE. 2007. Cyanobacterial heterocysts: terminal pores proposed as sites of gas exchange. Trends Microbiol. 15:340–349. 10.1016/j.tim.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Sherman DM, Sherman L. 2014. The uptake hydrogenase in the unicellular diazotrophic cyanobacterium Cyanothece sp. strain PCC 7822 protects nitrogenase from oxygen toxicity. J. Bacteriol. 196:840–849. 10.1128/JB.01248-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ermakova M, Battchikova N, Allahverdiyeva Y, Aro E-M. 2012. Novel heterocyst-specific flavodiiron proteins in Anabaena sp. PCC 7120. FEBS Lett. 587:82–87. 10.1016/j.febslet.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 49.Ermakova M, Battchikova N, Richaud P, Leino H, Kosourov S, Isojärvi J, Peltier G, Flores E, Cournac L, Allahverdiyeva Y, Aro EM. 2014. Heterocyst-specific flavodiiron protein Flv3B enables oxic diazotrophic growth of the filamentous cyanobacterium Anabaena sp. PCC 7120. Proc. Natl. Acad. Sci. U. S. A. 10.1073/pnas.1407327111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Batchikova N, Cournac L, Aro EM. 2013. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. U. S. A. 110:4111–4116. 10.1073/pnas.1221194110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allahverdiyeva Y, Ermakova M, Eisenhut M, Zhang P, Richaud P, Hagemann M, Cournac L, Aro EM. 2011. Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J. Biol. Chem. 286:24007–24014. 10.1074/jbc.M111.223289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauwe H, Hagemann M, Kern R, Timm S. 2012. Photorespiration has a dual origin and manifold links to central metabolism. Curr. Opin. Plant Biol. 15:269–275. 10.1016/j.pbi.2012.01.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.