Abstract
The response of soil ammonia-oxidizing bacterial (AOB) and archaeal (AOA) communities to individual environmental variables (e.g., pH, temperature, and carbon- and nitrogen-related soil nutrients) has been extensively studied, but how these environmental conditions collectively shape AOB and AOA distributions in unmanaged agricultural soils across a large latitudinal gradient remains poorly known. In this study, the AOB and AOA community structure and diversity in 26 agricultural soils collected from eastern China were investigated by using quantitative PCR and bar-coded 454 pyrosequencing of the amoA gene that encodes the alpha subunit of ammonia monooxygenase. The sampling locations span over a 17° latitude gradient and cover a range of climatic conditions. The Nitrosospira and Nitrososphaera were the dominant clusters of AOB and AOA, respectively; but the subcluster-level composition of Nitrosospira-related AOB and Nitrososphaera-related AOA varied across the latitudinal gradient. Variance partitioning analysis showed that geography and climatic conditions (e.g., mean annual temperature and precipitation), as well as carbon-/nitrogen-related soil nutrients, contributed more to the AOB and AOA community variations (∼50% in total) than soil pH (∼10% in total). These results are important in furthering our understanding of environmental conditions influencing AOB and AOA community structure across a range of environmental gradients.
INTRODUCTION
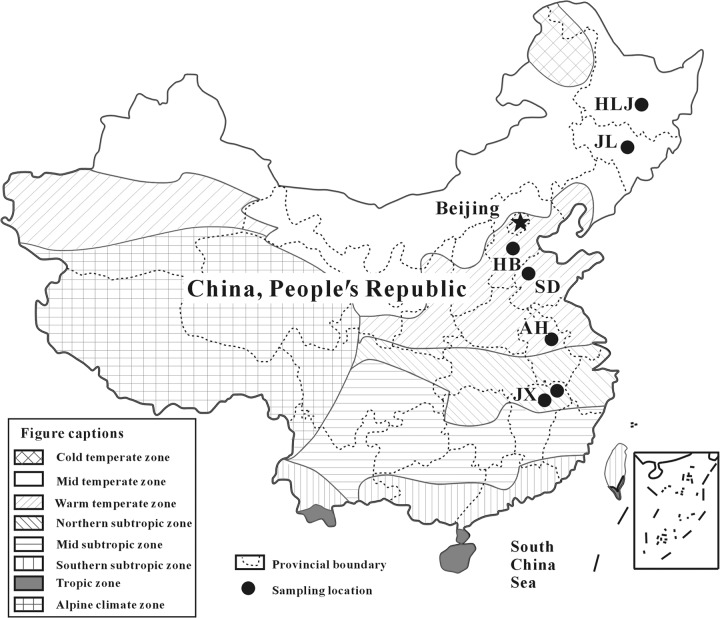
The eastern part of China is under East Asian monsoon climate. This region spans a latitude gradient of 33° and covers a wide range of monsoonal climate zones, from north to south by order, including midtemperate, warm temperate, northern subtropic, midsubtropic, southern subtropic, and tropic zones (Fig. 1). Due to variations in rainfall, solar irradiance, and the mineralogical composition of parent rocks, different types of soils are formed under these climatic conditions (1). The arable lands in eastern China are responsible for >70% of gross grain yield within China (2). In this region agricultural production relies heavily on inorganic fertilizers (e.g., urea and N substrates) to sustain crop production for decades (3).
FIG 1.
Map showing the sampling locations of the studied agriculture soils collected from eastern China.
Ammonia-oxidizing bacteria (AOB) and archaea (AOA) are two main groups of microorganisms affecting the utilization efficiency of inorganic nitrogen fertilizers (4). Both AOB and AOA possess amoA genes encoding the alpha subunit of ammonia monooxygenase. amoA gene-based phylogenetic analyses have shown that AOB and AOA are widely distributed in various environments (5).
Nitrosospira and Nitrosomonas of the Betaproteobacteria are two dominant genera of terrestrial AOB (6). The Nitrosospira-related AOB are abundant in different soils, and they can be grouped into clusters 0, 1, 2, 3a, 3b, 4, 9, 10, 11, and 12 (7, 8). The Nitrosomonas-related AOB are grouped into clusters 5, 6a, 6b, 7, and 8 (9), which are frequently found in freshwater environments (10), (hyper)saline lakes (11, 12), estuarine environments (13, 14), sewage sludge (15–17), sediments (9, 18), and flooded soils (19). Various environmental factors, such as vegetation type, nutrient level, microclimate (fertilizer, temperature, pH, water content, elevation), and management practice can affect AOB distribution in soils (20).
All known AOA fall into the archaeal phylum Thaumarchaeota (21, 22). They can be classified into the Nitrosopumilus, Nitrososphaera, Nitrosocaldus, Nitrosotalea, and Nitrososphaera sister clusters (23). The Nitrosocaldus cluster mainly consists of sequences from hot springs, whereas the Nitrosopumilus, Nitrosotalea, Nitrososphaera, and Nitrososphaera sister clusters are present in various environmental habitats (23). The distributions of AOA in these environments are influenced by multiple environmental conditions such as ammonium, dissolved oxygen and organic carbon levels, salinity, temperature, and pH (24–27).
Investigations of the AOA and AOB distributions and their response to environmental conditions are of great significance to the understanding of bioavailability and microbial transformation of nitrogen nutrients in agricultural soils. Previously, some amoA gene-based studies have been performed in manipulated Chinese agricultural soils (using either experimental stations or artificially managed soils) collected from the northern subtropic zone of central China (19, 28–33). These studies showed that pH was a major factor affecting AOB and AOA distributions: Nitrosospira clusters 10, 11, and 12 were the dominant AOB groups in acidic soils, whereas the Nitrosospira cluster 3a and Nitrosomonas clusters 6 and 7 were dominant in alkaline and neutral soils; for AOA communities, Nitrosopumilus and Nitrososphaera clusters were dominant AOA populations, and their relative abundances showed negative and positive correlations with soil pH, respectively (34).
In addition to pH, climatic conditions (i.e., temperature and precipitation) and soil properties have been shown to affect soil microbial communities under controlled conditions (35–37), but their effects on AOB and AOA are generally poorly known. Fierer et al. (20) showed that the AOB compositions in the soils across North America were strongly influenced by temperature, which was related to geographical locations, suggesting that biogeography controlled the AOB distribution. However, this study did not evaluate the relative importance of temperature and other environmental conditions in affecting AOB and AOA abundance and distribution in agricultural soils.
The objective of the present study was to quantitatively assess the relative importance of multiple environmental conditions in shaping AOA and AOB community structures in Chinese agricultural soils. We collected 26 agricultural soil samples from three different climatic zones from south to north in eastern China. AOB and AOA abundance and diversity were investigated using amoA gene-based quantitative PCRs (qPCRs) and bar-coded 454 sequencing techniques, respectively. Statistical analyses were performed to assess correlation between the AOB and AOA communities and environmental variables (e.g., geographic distance, mean annual temperature, mean annual precipitation, pH, and soil nutrients). These results are important to our understanding of the role of the microorganisms in transforming nitrogen nutrients in agricultural soils.
MATERIALS AND METHODS
Site description and sampling.
Soil samples were collected from arable lands across six provinces of different climatic zones in Eastern China (see for details Table S1). From north to south, the six provinces are Heilongjiang (HLJ), Jilin (JL), Hebei (HB), Shandong (SD), Anhui (AH), and Jiangxi (JX) (Fig. 1). Midtemperate climate dominates HLJ and JL provinces. The mean annual temperature (MAT) is 3.5 to 3.9°C, and the mean annual precipitation (MAP) is 480 to 700 mm. In HB and SD provinces, warm temperate prevails, and the MAT and MAP are 12.1 to 13.8°C and ∼620 mm, respectively. AH and JX provinces are dominated by northern subtropic climate, and the MAT and MAP are 15.5 to 17.2°C and ∼800 to 1,850 mm, respectively (see Table S1 in the supplemental material). Due to different climate and parent rock materials, different soil types developed in these provinces. Black soil, dark brown soil, medium loam soil, dark brown soil, paddy soil, and red soil are major soil types in HLJ, JL, HB, SD, AH, and JX provinces, respectively. These diverse soil types support many different crops (see Table S1 in the supplemental material).
Field sampling was performed in October 2010 after crops were harvested. In each of the provinces described above, soil samples cultivated with different crops were collected (see Table S1 in the supplemental material). At each chosen site, three separate soil subsamples (20 m apart from each other) were taken at the 5- to 10-cm depth with sterile spatulas and spoons. In the field, the three subsamples from each site were homogenized in a presterilized aluminum pan and were placed into 50-ml polypropylene tubes. The composite soil from one site was treated as one sample hereinafter, and all the downstream analyses were performed on the composite samples. Sample tubes were immediately stored in dry ice in the field and during transportation and then transferred to a −80°C freezer in the laboratory until further analysis.
Soil chemical analysis.
Soil pH was determined on a 1:2 soil-0.01 M CaCl2 suspension with a pH electrode (model 704; Mefrohm) (38). Contents of total organic carbon (TOC), total nitrogen, ammonium nitrogen (NH4+-N), nitrite nitrogen (NO2−-N), and nitrate-nitrogen (NO3−-N) were determined according to the established methods (38). The microbial carbon (Micro-C) concentration was determined by using a fumigation-incubation method described previously (39).
DNA extraction.
In order to avoid DNA extraction bias, DNA was extracted in triplicate from each composite soil sample using a FastDNA SPIN kit for soil (MP Biomedicals, Solon, OH, USA) according to the manufacturer's protocol. DNA quantity and quality were assessed by using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The resulting three DNA extractions per sample were pooled before further analysis.
qPCR of amoA and 16S rRNA genes.
qPCR was performed to determine the abundances of the bacterial and archaeal amoA and 16S rRNA genes in the investigated samples according to the method of Jiang et al. (12, 40). Briefly, the primer set amoA-1F/amoA-2R and Arch-amoAF/Arch-amoAR and the primer set Bac331F/Bac797R and Arch349F/Arch806R were used for qPCR of AOB/AOA amoA and bacterial/archaeal 16S rRNA genes, respectively (see Table S2 in the supplemental material). qPCRs were performed in a reaction volume of 20 μl, containing 10 μl of 2× SYBR Premix Ex Taq (TaKaRa, Japan), 0.2 μM concentrations of each primer, 0.4 μl of ROX Reference Dye II (50×), 20 μg of bovine serum albumin (TaKaRa), and 1 μl of soil DNA. qPCRs were performed in triplicate on an ABI7500 real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). Double-distilled H2O was used as a negative-control template. Purified plasmids of AOB and AOA amoA and bacterial and archaeal 16S rRNA gene clones from one soil sample were used as standard templates. The clone libraries of AOB and AOA amoA, as well as archaeal and bacteria 16S rRNA genes, were constructed with PCR products derived from the primer sets amoA-1F/amoA-2R, Arch-amoAF/Arch-amoAR, Arch21F/Univ1492R, and Bac27F/Univ1492R, respectively (see Table S2 in the supplemental material). Clone library construction and plasmids DNA extraction were performed according to the procedures described elsewhere (40). Standard curves were obtained by using serial dilutions of plasmids (pGEM-T) containing cloned amoA and 16S rRNA genes. The data were used to create standard curves correlating the threshold cycle (CT) values with the amoA gene copy numbers. Linear plots (not shown) between the CT value and log(copy number/reaction) for the AOB and AOA amoA and 16S rRNA genes were obtained with R2 correlation coefficients of >0.99. Melting-curve analysis was performed to determine the melting point of the amplification products and to assess the reaction specificity. After the qPCRs were complete, the temperature ramped up from 72°C to 95°C, rising by 0.1° per step, waiting for 45 s on the first step, and then 5 s for each subsequent step. The melting curve of each run had only one peak, a finding indicative of specific PCR amplification. The qPCR amplification efficiencies were in the range of 96 to 100%.
PCR amplification of AOB and AOA amoA genes.
The AOB and AOA amoA genes were amplified with the primer sets amoA-1F/amoA-2R and Arch-amoAF/Arch-amoAR, respectively (see Table S1 in the supplemental material). To pool multiple samples for one run of 454 pyrosequencing, a sample tagging approach was used (41). Special 5′-end-tagged primers for each sample were designed by combining primers with adaptors (“TC” and “CA” for forward and reverse primers, respectively) and a unique 8-mer tag (i.e., barcodes) (41). The PCR amplification procedure was as follows: an initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 40 s, and extension at 72°C for 40 or 60 s (for AOB and AOA amoA genes, respectively), followed by a final extension step at 72°C for 10 min.
To obtain enough amplicons for pyrosequencing, four PCRs were run for each sample. Each 25-μl PCR mix contained 10× PCR buffer (Mg2+ plus) (1× diluted; TaKaRa, Japan), deoxynucleoside triphosphate mixture (0.2 mM each; TaKaRa), primers (0.4 μM each), TaKaRa Taq DNA polymerase (1.5 U), bovine serum albumin (20 μg; TaKaRa), and 1 μl of template DNA. Reactions without template DNA were performed as negative controls in parallel for each sample. Amplicons from four PCRs were pooled for each sample. The PCR products were checked by agarose gel electrophoresis, and correct bands were incised. The incised PCR gels were then purified by using an AxyPrep DNA gel extraction kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer's protocol. The quantity and quality of the purified PCR products were assessed by using a NanoDrop ND-1000 UV-Vis spectrophotometer. Finally, the purified PCR amplicons were pooled in an equimolar concentration for further 454 pyrosequencing.
Pyrosequencing and data analysis.
The pyrosequencing was commercially performed on a GS FLX sequencer (454 Life Sciences, Branford, CT, USA) at the Chinese National Human Genome Center in Shanghai. The obtained sequences were extracted, trimmed, quality screened, and aligned with the use of the mothur 2.25.0 pipeline (42). Sequencing reads were assigned to each sample according to their unique barcodes, and low-quality sequences (quality score, <25; length, <150 bp; ambiguous bases, ≥1; homopolymer, ≥6) were removed. The sequences from the reverse primer (amoA-2R and Arch-amoAR for AOB and AOA, respectively) ends of the amplicons were used for downstream data analysis. Totals of 28,211 and 97,134 high-quality raw sequence reads were obtained for AOB and AOA, respectively.
The obtained high-quality raw sequences were clustered into operational taxonomic units (OTU) based on 97% sequence identity via the Qiime (quantitative insights into microbial ecology) pipeline (43, 44). To minimize potential deviant genotypes, OTU reads with a relative abundance of <0.5% were defined as rare sequences and were removed from downstream analysis. The remaining reads were checked against a local amoA gene database (downloaded from the Ribosomal Database Project FunGene [http://fungene.cme.msu.edu/index.spr]) and the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and then any chimera sequences (e.g., no hit in the database) were eliminated. The remaining quality-screened sequence reads were deposited into the NCBI Sequence Read Archive under accession number SRA082064.
One sequence read was randomly chosen as a representative sequence from each OTU with the use of the Qiime scripts. Phylogenetic assignment of AOB and AOA was made possible by aligning individual OTU sequences with those in the reference databases of AOB (7, 29, 45–47) and AOA (23), respectively. The cluster nomenclatures from the above references were adopted for the AOB and AOA amoA gene sequences, respectively.
Statistical analyses.
In order to calculate Chao1 (i.e., the predicted number of OTU), Shannon, and evenness indices, three AOB amoA gene libraries with fewer than 240 reads were removed. The AOA and remaining AOB libraries were normalized to 610 and 240 reads (the number of the smallest libraries), respectively. Chao1 (the predicted number of OTU), Shannon, and evenness indices were calculated at the 97% cutoff level. Coverage was calculated as the ratio of the observed OTU to Chao1 (48), and this index was used to evaluate the sequencing depth.
Pearson correlation was performed to test any associations between the AOA and AOB amoA gene abundances and the environmental variables. Significance levels were adjusted by using the Holm correction for each environmental variable (49). Any significance levels lower than 0.05 were chosen (50). Mantel tests were performed to assess any correlation between AOA and AOB community structures and the measured environmental variables (51). The measured environmental variables were first normalized to zero means and unit standard deviations. Euclidean distances were then calculated and used to construct dissimilarity matrices of communities and environmental variables. All analyses were performed by functions in the vegan package (v.1.15-1) in the R software (52).
UPGMA clustering was performed to compare similarity of AOB (or AOA) communities among the samples based on the Bray-Curtis matrix. Analysis of similarity (ANOSIM) was performed to further confirm any significant similarity in community composition between the samples as identified by the UPGMA (unweighted pair-group method with arithmetic averages) clustering. SIMPER (similarity percentage) analysis was performed to rank the relative contribution of AOB and AOA clusters or subclusters to the differences revealed by the UPGMA cluster analysis.
To determine the relative importance of the geographic distance and the measured environmental factors in shaping AOA and AOB communities, a canonical correspondence analysis (CCA)-based variation partitioning analysis (VPA) was implemented according to the methods described previously (53). First, a spatial decomposition method, principal coordinates of neighbor matrices (PCNM), was applied to the geographic coordinates of the samples (54). This method separates sample geographic coordinates into multiple spatial variables. Then for each community data set, CCA test with 1,000 permutations was used to select the significant (P < 0.05) spatial variables, which were then retained for VPA. All of these analyses were carried out using the functions in the vegan package in the R software (52).
RESULTS
Soil geochemical characteristics.
The mean annual temperatures and precipitations (MAT and MAP) of the sampling sites varied latitudinally from north to south by order: MAT ranged from 3.5°C in HLJ (Heilongjiang Province) to 17.5°C in JX (Jiangxi Province), and MAP ranged from 481.5 mm in HLJ (Heilongjiang Province) to 1,852.1 mm in JX (Jiangxi Province). The contents of TOC, microbial carbon, and total nitrogen in the samples of HLJ and JL (Jilin Province) were much higher than most soil samples from HB (Hebei Province), SD (Shandong Province), AH (Anhui Province), and JX. However, the contents of NH4-N, NO3-N, and NO2-N did not vary significantly among the investigated samples (see Table S2 in the supplemental material). In addition, the HB and SD soils were slightly alkaline (pH 8.5 to 9.0), whereas those from other provinces were slightly acidic to circumneutral (pH 5.9 to 7.7) (see Table S2 in the supplemental material).
Quantification of archaeal and bacterial 16S rRNA and AOA and AOB amoA genes.
The archaeal and bacterial 16S rRNA gene abundances were 2.9 × 107 to 4.5 × 108 and 1.5 × 108 to 7.5 × 109 copies per g of soil (dry weight), respectively. The AOA and AOB amoA gene abundances were 2.9 × 106 to 9.9 × 107 and 1.2 × 106 to 4.0 × 107 copies per g of soil (dry weight), respectively. The ratio of AOA amoA gene abundance to archaeal 16S rRNA gene abundance ranged from 0.006 to 0.735, whereas the ratio of AOB to bacterial 16S rRNA gene abundance ranged from < 0.001 to 0.067 (see Table S3 in the supplemental material). The ratio of AOA amoA gene to AOB was much greater than 1 (1.1 to 85.6) with the exception of the JL3 sample (0.3) (Fig. 2). The Pearson correlation analysis showed that the AOA abundance was significantly (P < 0.05) correlated with climatic (e.g., altitude, MAT, and MAP) and nutritional (Micro-C and NH4-N) factors; in contrast, the AOB abundance did not show any significant correlation with the measured environmental conditions (see Table S4 in the supplemental material).
FIG 2.
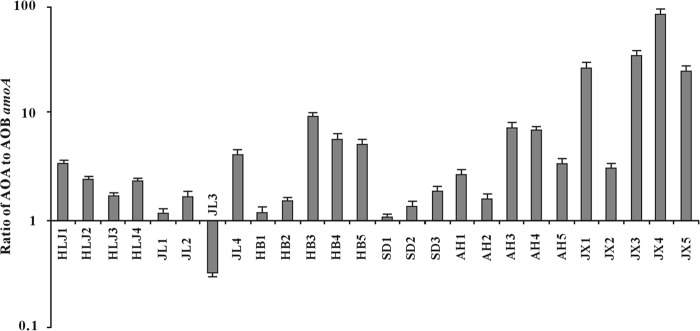
Ratios of AOA to AOB amoA gene copies in the studied soils. The error bars were from three analytical replicates.
Diversity of AOB amoA gene and its correlation to environmental conditions.
A total of 23,451 AOB amoA gene sequence reads remained for 23 soil samples (three samples that had fewer than 240 sequence reads were removed) after removal of low-quality, chimeric, and low-abundance (<0.5% in all samples) OTU sequences. These sequence reads were clustered into 20 to 77 observed and 21 to 98 predicted OTU (based on Chao1) at the 97% cutoff with coverage values ranging from 77.3% to 100% (see Table S5 in the supplemental material). The obtained AOB amoA gene OTU could be grouped into Nitrosospira (clusters 1, 2, 3, 4, 9, 10, 11, and 12), Nitrosomonas (clusters 6 and 8), and Nitrosovibrio clusters, with Nitrosospira being the dominant (94.3%, 22,122 out of 23,451) (Fig. 3A).
FIG 3.
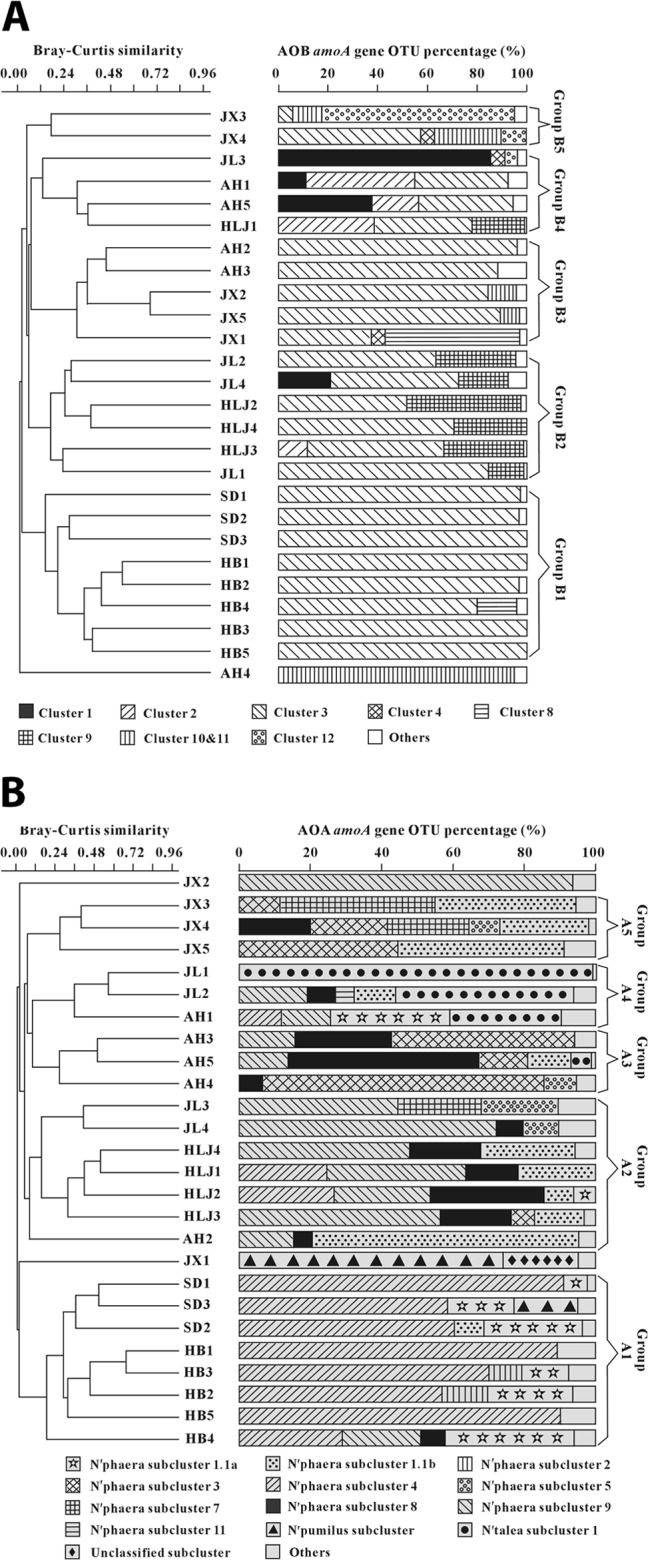
UPGMA cluster tree constructed based on the 97% cutoff level-based unweighted Unifrac matrix. Microbial groups with an abundance greater than 2% are displayed. Groups with abundances lower than 2% were included as “Others,” which include Nitrosovibrio and Nitrosomonas clusters 6 and 8. (A) AOB; (B) AOA. For AOB, the previously published nomenclatures of Nitrosospira clusters 0, 1, 2, 3a, 3b, 4, 9, 10, 11, and 12 (7, 8) and Nitrosomonas clusters 5, 6a, 6b, 7, and 8 (9) were used; for AOA, the previously published nomenclatures of Nitrosopumilus, Nitrososphaera, Nitrosocaldus, and Nitrosotalea subclusters and Nitrososphaera sister cluster (23) were used.
Based on the Bray-Curtis similarity matrix, the amoA gene libraries were classified into five significantly (ANOSIM, R > 0.71, P < 0.01) different groups, and these groups were approximately distributed according to geographic location (Fig. 3A). Group B1 included all SD and HB samples from central-eastern China, and all sequences were mainly affiliated with Nitrosospira cluster 3, within which only 31 OTU were shared between the SD and HB samples (accounting for ∼40 and 25% of the cluster 3 OTU for the SD and HB samples, respectively) (see Fig. S1 in the supplemental material). This analysis indicated that even though both SD and HB samples were dominated by cluster 3, the composition of cluster 3 was very different in the SD and HB samples. Group B2 contained samples from JL (JL2 and JL4) and HLJ (HLJ2, HLJ3, and HLJ4) from northeastern China, and the sequences were predominantly affiliated with Nitrosospira clusters 3 and 9. AH2, AH3, JX1, JX2, and JX5 samples from southeastern China constituted group B3, which was mainly composed of Nitrosospira clusters 3, 4, 8, 10, and 11. Unlike the previous three groups, group B4 was somewhat more diverse and included samples from northeastern China (HLJ1 and JL3) and southeastern China (AH1 and AH5 samples), and the dominant AOB were Nitrosospira clusters 1, 2, 3, and 4. Two samples from JX (JX1 and JX4) formed group B5, but this small group was fairly diverse, consisting of Nitrosospira clusters 3, 10, 11, and 12 (Fig. 3A).
Among the five groups (B1 to B5), some Nitrosospira clusters were present in multiple groups but their compositions were greatly different among the groups. For example, cluster 3 was a major component in B1, B2, and B3 and was present in some samples of the B4 and B5 groups (Fig. 3A). However, only 16 OTU were shared between the B1 and B2 groups (accounting for ∼8% and ∼21% of the OTU within cluster 3, respectively) (see Fig. S2 in the supplemental material); the samples in the B2 and B3 groups shared 14 OTU, accounting for ∼18 and ∼33% of the total cluster 3 OTU in these two groups, respectively. The B1 and B3 groups shared 13 OTU, which accounted for ∼7% and 3% of the cluster 3 OTU in these two groups (see Fig. S2 in the supplemental material).
The CCA results showed that the AOB community structures were correlated with soil pH, NO2−-N, and Micro-C for the HLJ, JL, HB, and SD samples, but NO3−-N, NH4+-N, and geography-related environmental variables (MAP, PCNM1, PCNM13, and PCNM14) were important factors for the AH and JX samples (Fig. 4A). More than a half (54.1%) of the observed variations in the AOB community structures (at the OTU level) could be explained by the measured environmental variables, among which the Grp3 of variables (e.g., TOC, Micro-C, Micro-C/TOC, total N, NH4-N, NO3-N, and NO2-N) were more important (∼30%, including direct and indirect) than the other two groups of environmental variables (soil pH, geographic distance, MAT, and MAP) (Fig. 5A).
FIG 4.
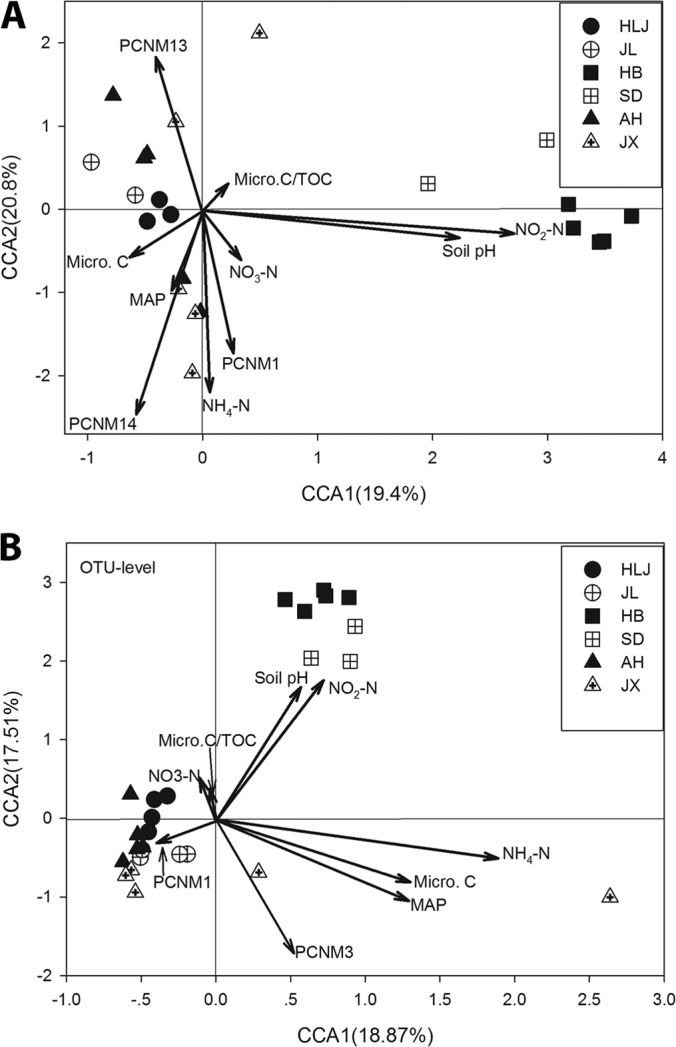
Canonical correspondence analysis (CCA) of the AOB and AOA communities (at the OTU level) and measured environmental variables. (A) AOB; (B) AOA.
FIG 5.
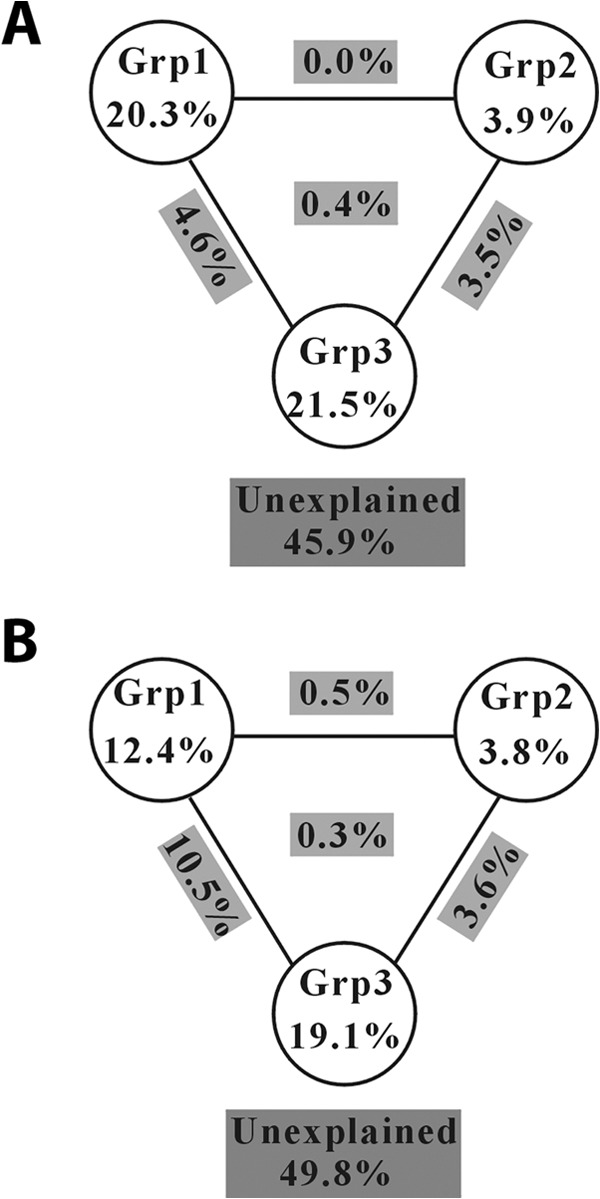
Variation partition analysis of the effects geographic distance and environmental variables on the phylogenetic structure (at the OTU level) of ammonia-oxidizing microbial populations. “Grp1” contains geographic distance and climatic factors (mean annual temperature and precipitation); “Grp2” denotes soil pH; “Grp3” contains TOC, Micro-C, Micro-C/TOC, Total N, NH4-N, NO3-N, and NO2-N. (A) AOB; (B) AOA.
Diversity of AOA amoA gene and its correlation with environmental factors.
A total of 85,930 AOA amoA gene sequence reads remained for the 26 soil samples after removal of low-quality, chimeric and low-abundance (<0.5% in all samples) OTU sequences. The remaining sequence reads were clustered into 7 to 77 observed and 8 to 86 predicted OTU (based on Chao1) at the 97% cutoff with coverage values ranging from 83.7 to 99.3% (see Table S5 in the supplemental material). The AOA amoA gene OTU could be grouped into Nitrososphaera (including subclusters 1.1a and 1.1b and subclusters 2 to 11), Nitrosopumilus, Nitrosotalea subcluster 1, and two unclassified clusters, with Nitrososphaera being the predominant (84.3% [72,047 of 85,930]) (Fig. 3B).
Based on the Bray-Curtis similarity matrix, the AOA amoA gene libraries could be classified into five significantly (ANOSIM, R > 0.63, P < 0.009) different groups, again approximately according to geographic location (Fig. 3B). Similar to group B1 of AOB, all of the SD and HB samples formed a tight distinct cluster that was composed of Nitrososphaera subclusters 1.1a and 4. Within group A1, the SD and HB samples shared 21 OTU, accounting for ∼60 and ∼40% of the SD and HB OTU that were affiliated with the Nitrososphaera subcluster 4, respectively) (see Fig. S3 in the supplemental material). Similar to group B2 of AOB, group A2 consisted of all HLJ samples, some JL samples (JL3 and JL4) and one AH sample. The sequences in this group were diverse and mainly affiliated with Nitrososphaera subclusters 1.1b, 4, 5, 7, 8, and 9. Within group A2, the HLJ and two JL samples (JL3 and JL4) shared five OTU, accounting for ∼24 and ∼28% of the HLJ and JL OTU that were affiliated with Nitrososphaera subcluster 9 (see Fig. S3 in the supplemental material). Group A3 consisted of three AH samples (AH2, AH3, and AH5), which were mainly composed of Nitrososphaera subclusters 3, 8, and 9. Again, similar to group B4, group A4 consisted of samples from JL (JL1 and JL2) and AH (AH1) but no HLJ samples, and the sequences in this group were mainly composed of Nitrososphaera subclusters 1.1a, 4, and 9 and Nitrosotalea subcluster 1. Similar to group B5, the JX3, JX4, and JX5 samples formed group A5, and they were mainly composed of Nitrososphaera subclusters 1.1b, 3, 7, and 8 (Fig. 3B). Similar to the AOB groups, some AOA groups (A1 to A5) were dominated by the same Nitrososphaera subclusters, but the OTU compositions of these subclusters were greatly different among the groups. For example, the Nitrososphaera subcluster 8 only had one OTU in common (10%) between the A2 and A3 groups (see Fig. S4 in the supplemental material).
The CCA results showed that the AOA community structures were correlated with soil pH and NO2−-N for the HB, SD, JL, and JX samples but with NH4+-N and geography-related environmental variables (MAP and PCNM1) for the AH and JX samples (Fig. 4A). Approximately one-half (50.2%) of the observed variations in the AOA community structures (at the OTU level) could be explained by the measured environmental variables, among which the Grp3 of variables (e.g., TOC, Micro-C, Micro-C/TOC, Total N, NH4-N, NO3-N, and NO2-N) contributed the most (∼43.5%, including direct and indirect) to the variation of AOA distribution patterns (Fig. 5B).
DISCUSSION
Predominance of AOA over AOB amoA genes in the eastern Chinese agricultural soils.
The predominance of AOA over AOB in the investigated soils was consistent with previous observations in various agriculture soils (30, 34, 55). The ratio of AOA to AOB amoA gene abundance was not correlated with latitude, MAT, and MAP, suggesting that latitudinal variations (i.e., climatic change) might not be important factors influencing the relative abundance between AOA and AOB in agricultural soils. This was in contrast with one previous observation in North American arid lands: AOA were more abundant than AOB in the warmer, more southern deserts, but the relative abundance between AOA and AOB is reversed in the samples from colder, more northern deserts (56). Such inconsistency may be ascribed to the difference of sample types: nonagricultural desert soils (56) versus mature agricultural soils (the present study).
Correlation between AOB and AOA community structure and environmental variables.
Previous studies have shown that AOB and AOA community structures in soils can be shaped by multiple environmental conditions, including geography (20, 56), soil type (19), vegetation type (57), temperature (26, 27), pH (27, 58), and nutrient level (e.g., ammonium, dissolved oxygen, and organic carbon levels) (27, 59, 60). However, little is known about the relative contributions of these environmental conditions to the overall AOB and AOA community structures. In the present study, canonical correspondence analysis (CCA)-based VPA results suggested that environmental factors influencing AOB and AOA distribution across different soil types were complicated, and nearly half of AOB and AOA community structure variability could not be explained by our measured environmental variables. Thus, geography, climatic conditions (i.e., MAT and MAP) and carbon/nitrogen-related soil nutrients were all important factors in shaping the AOB and AOA community structures in the studied soils. The importance of geography and climatic conditions were also apparent in the UPGMA clustering pattern (Fig. 3). In general, soil samples from adjacent provinces were clustered together but distinct from other provinces. These observations were consistent with previous studies in showing geographic distribution of AOB and AOA in North American soils (20, 56). Different temperatures from distinct geographic locations were believed to lead to the development of differential geographic niches and to result in distinct AOB and AOA community structures (20, 56). In our case, in addition to temperature, MAP and soil parent materials were also different latitudinally. In the JX province, due to high MAP and MAT, parent rocks undergo strong physical, chemical, and biological weathering, and extensive leaching leads to enrichment of aluminum and iron in acidic soils. Extensive leaching also results in lower levels of soil nutrients and fertility. However, due to the local tradition of adding plant ash before each season, the pH of the JX soils was not as acidic as other soils under similar climatic conditions (61, 62). In the AH, SD, and HB provinces, soils are mainly developed from loess and sandy aeolian sediments and, due to relatively lower temperature and precipitation, chemical leaching is not important and erosion is unlikely. As a result soils with moderate fertility and circumneutral to slightly alkaline pH have developed. In contrast, black or dark brown soils in the JL and HLJ provinces develop due to low MAT and plenty of organic input (63), leading to high levels of soil nutrients and high soil fertility. These distinct climatic and lithological conditions may have led to the formation of different soil types, which collectively contribute to the development of “geographic niches” to host distinct AOB and AOA communities.
Compared to soil type, vegetation type appeared to be less important in accounting for the observed differences in AOB and AOA communities in the studied soils, in contrast to previous studies that have shown predominant control of vegetation type on microbial distributions (64) and ammonia oxidizers (57) in soils. Such inconsistency may be ascribed to our single-season samples. In the present study, soils were sampled in one season (after all crops were harvested). Thus, it is reasonable to observe less importance of vegetation types to AOB and AOA communities than other measured environmental factors.
Compared to geography, climatic conditions, and soil nutrients, the contribution of soil pH to AOB and AOA community structures was minor. Theoretically, pH can affect the AOB and AOA communities through changing the availability of ammonia (6, 27, 65, 66). In two previous studies, among the seven measured environmental variables (i.e., pH, carbon, nitrogen, and organic matter content, C:N ratio, soil moisture, and vegetation), pH was shown to be a determinant in shaping the AOA community (58, 67). Such an inconsistency may be ascribed to the different pH gradients of the soil samples between previous studies and in the present study. The pH ranged from 4.5 to 7.5, while keeping other physicochemical conditions nearly constant, for the soils in a study by Nicol et al. (58). The soils of the Gubry-Rangin et al. (67) study included samples used by Nicol et al. (58) and those collected from the countryside of the United Kingdom, and the pH spanned from 3.5 to 8.7, which was much larger than our soil pH range (pH 5.9 to 9.0). Any pH effect from a small pH range of the soils in the present study may be masked by other more important effects such as climatic conditions, soil types, and nutrient availability.
AOB and AOA community composition in Chinese agricultural soils.
The AOB and AOA communities were mainly composed of Nitrosospira and Nitrososphaera in the studied agricultural soils, a finding consistent with other studies that have shown the dominance of these two groups of ammonia-oxidizing microbes in soils (6, 7, 23, 29, 47, 65, 68–70). However, some samples contained different AOB and AOA genera. For example, more than half of the AOB and AOA sequence reads in JX1 were affiliated with Nitrosomonas cluster 8 and the Nitrosopumilus subcluster, respectively. Previous studies have shown that the Nitrosomonas cluster 8 was composed of Nitrosomonas species and related sequences of miscellaneous origins (including soils) (9, 71). So it was not unexpected to observe the dominance of and Nitrosomonas-related AOB in the JX1 sample. In contrast, the Nitrosopumilus subcluster has previously been observed in aquatic environments (23) and is usually not abundant in soils (23, 58, 70). However, because only one sample (JX1) was predominated by Nitrosopumilus-related sequences, the exact reason for its predominance in this sample could not be determined at this time.
It is notable to observe that the circumneutral-pH JL1 sample was almost completely composed of Nitrosotalea-related AOA. In addition, Nitrosotalea AOA sequences were also one of the major AOA populations in two circumneutral-pH samples (JL2 and AH1). Nitrosotalea-related AOA was absent in the acidic sample (JX5 with pH 5.9). These data were in contrast to previous studies, in which Nitrosotalea-related AOA have been observed predominant in acidic environments. For example, the Nitrosotalea subcluster included the first obligate acidophilic AOA isolate from one acidic soil (72) and was subsequently found to be dominant in another acidic arable soil (23). Thus, these data suggested that Nitrosotalea-related AOA may not be limited to acidic soils.
In conclusion, this study represents a large effort to investigate the latitudinal patterns of AOB and AOA in the agricultural soils of eastern China. We showed that geographic distance, MAP, MAT, pH, and carbon- and nitrogen-related soil nutrients were important factors in shaping the AOB and AOA community structures. This is first study to quantitatively assess the relative contributions of each measured environmental parameter to the observed AOB and AOA distribution pattern. Our measured environmental factors accounted for more than a half of observed variations of the AOB and AOA community structures. Among the measured environmental factors, geographic distance and climatic conditions (MAP and MAT) and carbon- and nitrogen-related soil nutrients were important factors that influenced the AOB and AOA distributions across the agricultural soils of eastern China. These observations provide baseline data for future investigations of the functional response of microbial groups to climate-induced environment changes.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Program on Key Basic Research Project (973 Program; 2012CB822000), the Scientific Research Funds for the 1000 “Talents” Program Plan from China University of Geosciences—Beijing, the Program for New Century Excellent Talents in University (NCET-12-0954), and the Fundamental Research Funds for National University (China University of Geosciences—Wuhan).
Jizheng He from Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, provided helpful suggestions and comments on the manuscript. We are grateful to three anonymous reviewers, whose constructive criticisms significantly improved the quality of the manuscript.
Footnotes
Published ahead of print 7 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01617-14.
REFERENCES
- 1.Wang Y, Fang X, Bai Y, Xi X, Zhang X, Wang Y. 2007. Distribution of lipids in modern soils from various regions with continuous climate (moisture-heat) change in China and their climate significance. Sci. China (Ser. D) Earth Sci. 50:600–612. 10.1007/s11430-007-2062-9 [DOI] [Google Scholar]
- 2.Meng Z. 2009. Economics analysis on the agronomic modeling of food production with ENSO impacts in the mid-eastern China. Ocean University of China, Qingdao, China [Google Scholar]
- 3.Peng L. 2000. Progressive process, prospects and distribution of fertilizer use and grain production in China. Res. Agric. Modern. 21:14–18 (In Chinese.) [Google Scholar]
- 4.Stark C, Condron LM, Stewart A, Di HJ, O'Callaghan M. 2007. Influence of organic and mineral amendments on microbial soil properties and processes. Appl. Soil Ecol. 35:79–93. 10.1016/j.apsoil.2006.05.001 [DOI] [Google Scholar]
- 5.Stahl DA, de la Torre JR. 2012. Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 66:83–101. 10.1146/annurev-micro-092611-150128 [DOI] [PubMed] [Google Scholar]
- 6.Kowalchuk GA, Stephen JR. 2001. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu. Rev. Microbiol. 55:485–529. 10.1146/annurev.micro.55.1.485 [DOI] [PubMed] [Google Scholar]
- 7.Avrahami S, Conrad R. 2003. Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl. Environ. Microbiol. 69:6152–6164. 10.1128/AEM.69.10.6152-6164.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avrahami S, Liesack W, Conrad R. 2003. Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ. Microbiol. 5:691–705. 10.1046/j.1462-2920.2003.00457.x [DOI] [PubMed] [Google Scholar]
- 9.Freitag TE, Prosser JI. 2003. Community structure of ammonia-oxidizing bacteria within anoxic marine sediments. Appl. Environ. Microbiol. 69:1359–1371. 10.1128/AEM.69.3.1359-1371.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laanbroek HJ, Speksnijder AGCL. 2008. Niche separation of ammonia-oxidizing bacteria across a tidal freshwater marsh. Environ. Microbiol. 10:3017–3025. 10.1111/j.1462-2920.2008.01655.x [DOI] [PubMed] [Google Scholar]
- 11.Hu A, Yao T, Jiao N, Liu Y, Yang Z, Liu X. 2010. Community structures of ammonia-oxidizing archaea and bacteria in high-altitude lakes on the Tibetan Plateau. Freshw. Biol. 55:2375–2390. 10.1111/j.1365-2427.2010.02454.x [DOI] [Google Scholar]
- 12.Jiang H, Dong H, Yu B, Lv G, Deng S, Berzins N, Dai M. 2009. Diversity and abundance of ammonia-oxidizing archaea and bacteria in Qinghai Lake, northwestern China. Geomicrobiol. J. 26:199–211. 10.1080/01490450902744004 [DOI] [Google Scholar]
- 13.Bernhard AE, Donn T, Giblin AE, Stahl DA. 2005. Loss of diversity of ammonia-oxidizing bacteria correlates with increasing salinity in an estuary system. Environ. Microbiol. 7:1289–1297. 10.1111/j.1462-2920.2005.00808.x [DOI] [PubMed] [Google Scholar]
- 14.Santoro AE, Francis CA, de Sieyes NR, Boehm AB. 2008. Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ. Microbiol. 10:1068–1079. 10.1111/j.1462-2920.2007.01547.x [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Sun Q, Wen D, Tang X. 2012. Abundance of ammonia-oxidizing bacteria and archaea in industrial and domestic wastewater treatment systems. FEMS Microbiol. Ecol. 80:323–330. 10.1111/j.1574-6941.2012.01296.x [DOI] [PubMed] [Google Scholar]
- 16.Urakawa H, Maki H, Kawabata S, Fujiwara T, Ando H, Kawai T, Hiwatari T, Kohata K, Watanabe M. 2006. Abundance and population structure of ammonia-oxidizing bacteria that inhabit canal sediments receiving effluents from municipal wastewater treatment plants. Appl. Environ. Microbiol. 72:6845–6850. 10.1128/AEM.00807-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Ye L, Tong A, Shao M-F, Lok S. 2011. Ammonia-oxidizing archaea and ammonia-oxidizing bacteria in six full-scale wastewater treatment bioreactors. Appl. Microbiol. Biotechnol. 91:1215–1225. 10.1007/s00253-011-3408-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urakawa H, Kurata S, Fujiwara T, Kuroiwa D, Maki H, Kawabata S, Hiwatari T, Ando H, Kawai T, Watanabe M, Kohata K. 2006. Characterization and quantification of ammonia-oxidizing bacteria in eutrophic coastal marine sediments using polyphasic molecular approaches and immunofluorescence staining. Environ. Microbiol. 8:787–803. 10.1111/j.1462-2920.2005.00962.x [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Zhang L-M, Shen J-P, Xu Z, He J-Z. 2010. Soil type determines the abundance and community structure of ammonia-oxidizing bacteria and archaea in flooded paddy soils. J. Soils Sediments 10:1510–1516. 10.1007/s11368-010-0256-9 [DOI] [Google Scholar]
- 20.Fierer N, Carney K, Horner-Devine M, Megonigal J. 2009. The biogeography of ammonia-oxidizing bacterial communities in soil. Microb. Ecol. 58:435–445. 10.1007/s00248-009-9517-9 [DOI] [PubMed] [Google Scholar]
- 21.Pester M, Schleper C, Wagner M. 2011. The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology. Curr. Opin. Microbiol. 14:300–306. 10.1016/j.mib.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spang A, Hatzenpichler R, Brochier-Armanet C, Rattei T, Tischler P, Spieck E, Streit W, Stahl DA, Wagner M, Schleper C. 2010. Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol. 18:331–340. 10.1016/j.tim.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 23.Pester M, Rattei T, Flechl S, Gröngröft A, Richter A, Overmann J, Reinhold-Hurek B, Loy A, Wagner M. 2012. amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ. Microbiol. 14:525–539. 10.1111/j.1462-2920.2011.02666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biller SJ, Mosier AC, Wells GF, Francis CA. 2012. Global biodiversity of aquatic ammonia-oxidizing archaea is partitioned by habitat. Front. Microbiol. 3:252. 10.3389/fmicb.2012.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao H, Auguet J-C, Gu J-D. 2013. Global ecological pattern of ammonia-oxidizing archaea. PLoS One 8:e52853. 10.1371/journal.pone.0052853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W. 2009. Environmental factors shaping the ecological niches of ammonia oxidizing archaea. FEMS Microbiol. Rev. 33:855–869. 10.1111/j.1574-6976.2009.00179.x [DOI] [PubMed] [Google Scholar]
- 27.Hatzenpichler R. 2012. Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl. Environ. Microbiol. 78:7501–7510. 10.1128/AEM.01960-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X-P, Zhu Y-G, Xia Y, Shen J-P, He J-Z. 2008. Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ. Microbiol. 10:1978–1987. 10.1111/j.1462-2920.2008.01613.x [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Zhang L-M, Shen J-P, Wei W-X, He J-Z. 2011. Abundance and community structure of ammonia-oxidizing archaea and bacteria in an acid paddy soil. Biol. Fertil. Soils 47:323–331. 10.1007/s00374-011-0542-8 [DOI] [Google Scholar]
- 30.He J-Z, Shen J-P, Zhang L-M, Zhu Y-G, Zheng Y-M, Xu M-G, Di H. 2007. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 9:2364–2374. 10.1111/j.1462-2920.2007.01358.x [DOI] [PubMed] [Google Scholar]
- 31.Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ. 2008. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol. 10:1601–1611. 10.1111/j.1462-2920.2008.01578.x [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Ke X, Wu L, Lu Y. 2009. Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as affected by nitrogen fertilization. Syst. Appl. Microbiol. 32:27–36. 10.1016/j.syapm.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 33.Yao H, Gao Y, Nicol GW, Campbell CD, Prosser JI, Zhang L, Han W, Singh BK. 2011. Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl. Environ. Microbiol. 77:4618–4625. 10.1128/AEM.00136-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen J-P, Zhang L-M, Di HJ, He J-Z. 2012. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 3:296. 10.3389/fmicb.2012.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries FT, Manning P, Tallowin JRB, Mortimer SR, Pilgrim ES, Harrison KA, Hobbs PJ, Quirk H, Shipley B, Cornelissen JHC, Kattge J, Bardgett RD. 2012. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol. Lett. 15:1230–1239. 10.1111/j.1461-0248.2012.01844.x [DOI] [PubMed] [Google Scholar]
- 36.Groffman P, Hardy J, Fisk M, Fahey T, Driscoll C. 2009. Climate variation and soil carbon and nitrogen cycling processes in a northern hardwood forest. Ecosystems 12:927–943. 10.1007/s10021-009-9268-y [DOI] [Google Scholar]
- 37.Slaughter LC. 2012. Soil microbial community response to climate change: results from a temperate Kentucky pasture. M.S. thesis University of Kentucky, Lexington, KY [Google Scholar]
- 38.Wilke B-M. 2005. Determination of chemical and physical soil properties, p 47–95 In Margesin R, Schinner F, Wilke B-M. (ed), Monitoring and assessing soil bioremediation, vol 5 Springer, Berlin, Germany [Google Scholar]
- 39.Vance ED, Brookes PC, Jenkinson DS. 1987. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19:703–707. 10.1016/0038-0717(87)90052-6 [DOI] [Google Scholar]
- 40.Jiang H, Dong H, Yu B, Liu X, Li Y, Ji S, Zhang CL. 2007. Microbial response to salinity change in Lake Chaka, a hypersaline lake on Tibetan plateau. Environ. Microbiol. 9:2603–2621. 10.1111/j.1462-2920.2007.01377.x [DOI] [PubMed] [Google Scholar]
- 41.Binladen J, Gilbert MTP, Bollback JP, Panitz F, Bendixen C, Nielsen R, Willerslev E. 2007. The use of coded PCR primers enables high-throughput sequencing of multiple homolog amplification products by 454 parallel sequencing. PLoS One 2:e197. 10.1371/journal.pone.0000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunin V, Engelbrektson A, Ochman H, Hugenholtz P. 2010. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ. Microbiol. 12:118–123. 10.1111/j.1462-2920.2009.02051.x [DOI] [PubMed] [Google Scholar]
- 45.Huson DH, Mitra S, Ruscheweyh H-J, Weber N, Schuster SC. 2011. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 21:1552–1560. 10.1101/gr.120618.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen X-Y, Zhang L-M, Shen J-P, Li L-H, Yuan C-L, He J-Z. 2011. Nitrogen loading levels affect abundance and composition of soil ammonia oxidizing prokaryotes in semiarid temperate grassland. J. Soils Sediments 11:1243–1252. 10.1007/s11368-011-0375-y [DOI] [Google Scholar]
- 47.Zhang L-M, Wang M, Prosser JI, Zheng Y-M, He J-Z. 2009. Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiol. Ecol. 70:52–61. 10.1111/j.1574-6941.2009.00775.x [DOI] [PubMed] [Google Scholar]
- 48.Hou W, Wang S, Dong H, Jiang H, Briggs BR, Peacock JP, Huang Q, Huang L, Wu G, Zhi X, Li W, Dodsworth JA, Hedlund BP, Zhang C, Hartnett HE, Dijkstra P, Hungate BA. 2013. A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS One 8:e53350. 10.1371/journal.pone.0053350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6:65–70 [Google Scholar]
- 50.He Z, Xu M, Deng Y, Kang S, Kellogg L, Wu L, Van Nostrand JD, Hobbie SE, Reich PB, Zhou J. 2010. Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2. Ecol. Lett. 13:564–575. 10.1111/j.1461-0248.2010.01453.x [DOI] [PubMed] [Google Scholar]
- 51.He Z, Piceno Y, Deng Y, Xu M, Lu Z, DeSantis T, Andersen G, Hobbie SE, Reich PB, Zhou J. 2012. The phylogenetic composition and structure of soil microbial communities shifts in response to elevated carbon dioxide. ISME J. 6:259–272. 10.1038/ismej.2011.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixon P, Palmer MW. 2003. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14:927–930. 10.1111/j.1654-1103.2003.tb02228.x [DOI] [Google Scholar]
- 53.Zhou J, Xue K, Xie J, Deng Y, Wu L, Cheng X, Fei S, Deng S, He Z, Van Nostrand JD, Luo Y. 2012. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Climate Change 2:106–110. 10.1038/nclimate1331 [DOI] [Google Scholar]
- 54.Ramette A, Tiedje JM. 2007. Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Proc. Natl. Acad. Sci. U. S. A. 104:2761–2766. 10.1073/pnas.0610671104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. 10.1038/nature04983 [DOI] [PubMed] [Google Scholar]
- 56.Marusenko Y, Bates S, Anderson I, Johnson S, Soule T, Garcia-Pichel F. 2013. Ammonia-oxidizing archaea and bacteria are structured by geography in biological soil crusts across North American arid lands. Ecol. Processes 2:9. 10.1186/2192-1709-2-9 [DOI] [Google Scholar]
- 57.Liang Y, He X, Liang S, Zhang W, Chen X, Feng S, Su Y. 2013. Community structure analysis of soil ammonia oxidizers during vegetation restoration in southwest China. J. Basic Microbiol. 54:180–189. 10.1002/jobm.201300217 [DOI] [PubMed] [Google Scholar]
- 58.Nicol GW, Leininger S, Schleper C, Prosser JI. 2008. The influence of soil pH on the diversity, abundance, and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 10:2966–2978. 10.1111/j.1462-2920.2008.01701.x [DOI] [PubMed] [Google Scholar]
- 59.Huang L, Dong H, Wang S, Huang Q, Jiang H. 2013. Diversity and abundance of ammonia-oxidizing archaea and bacteria in diverse Chinese paddy soils. Geomicrobiol. J. 31:12–22 [Google Scholar]
- 60.Kelly JJ, Policht K, Grancharova T, Hundal LS. 2011. Distinct responses in ammonia-oxidizing archaea and bacteria after addition of biosolids to an agricultural soil. Appl. Environ. Microbiol. 77:6551–6558. 10.1128/AEM.02608-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu L, Han W, Zhang J, Wu Y, Wang B, Lin X, Zhu J, Cai Z, Jia Z. 2012. Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J. 6:1978–1984. 10.1038/ismej.2012.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu L, Jia Z. 2013. Urease gene-containing Archaea dominate autotrophic ammonia oxidation in two acid soils. Environ. Microbiol. 15:1795–1809. 10.1111/1462-2920.12071 [DOI] [PubMed] [Google Scholar]
- 63.Zhang J. 1996. Soil in China. The Commercial Press Co, Ltd, Beijing, China: (In Chinese.) [Google Scholar]
- 64.Chu H, Neufeld JD, Walker VK, Grogan P. 2011. The influence of vegetation type on the dominant soil bacteria, archaea, and fungi in a low Arctic tundra landscape. Soil Sci. Soc. Am. J. 75:1756–1765. 10.2136/sssaj2011.0057 [DOI] [Google Scholar]
- 65.Carney KM, Matson PA, Bohannan BJM. 2004. Diversity and composition of tropical soil nitrifiers across a plant diversity gradient and among land-use types. Ecol. Lett. 7:684–694. 10.1111/j.1461-0248.2004.00628.x [DOI] [Google Scholar]
- 66.Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C. 2011. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. U. S. A. 108:8420–8425. 10.1073/pnas.1013488108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gubry-Rangin C, Hai B, Quince C, Engel M, Thomson BC, James P, Schloter M, Griffiths RI, Prosser JI, Nicol GW. 2011. Niche specialization of terrestrial archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. U. S. A. 108:21206–21211. 10.1073/pnas.1109000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avrahami S, Conrad R. 2005. Cold-temperate climate: a factor for selection of ammonia oxidizers in upland soil? Can. J. Microbiol. 51:709–714. 10.1139/w05-045 [DOI] [PubMed] [Google Scholar]
- 69.Pratscher J, Dumont MG, Conrad R. 2011. Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc. Natl. Acad. Sci. U. S. A. 108:4170–4175. 10.1073/pnas.1010981108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wessén E, Soderstrom M, Stenberg M, Bru D, Hellman M, Welsh A, Thomsen F, Klemedtson L, Philippot L, Hallin S. 2011. Spatial distribution of ammonia-oxidizing bacteria and archaea across a 44-hectare farm related to ecosystem functioning. ISME J. 5:1213–1225. 10.1038/ismej.2010.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops H-P. 2003. 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int. J. Syst. Evol. Microbiol. 53:1485–1494. 10.1099/ijs.0.02638-0 [DOI] [PubMed] [Google Scholar]
- 72.Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. 2011. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci. U. S. A. 108:15892–15897. 10.1073/pnas.1107196108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.