Abstract
We show here that oxidative stress is involved in both sclerotial differentiation (SD) and aflatoxin B1 biosynthesis in Aspergillus flavus. Specifically, we observed that (i) oxidative stress regulates SD, as implied by its inhibition by antioxidant modulators of reactive oxygen species and thiol redox state, and that (ii) aflatoxin B1 biosynthesis and SD are comodulated by oxidative stress. However, aflatoxin B1 biosynthesis is inhibited by lower stress levels compared to SD, as shown by comparison to undifferentiated A. flavus. These same oxidative stress levels also characterize a mutant A. flavus strain, lacking the global regulatory gene veA. This mutant is unable to produce sclerotia and aflatoxin B1. (iii) Further, we show that hydrogen peroxide is the main modulator of A. flavus SD, as shown by its inhibition by both an irreversible inhibitor of catalase activity and a mimetic of superoxide dismutase activity. On the other hand, aflatoxin B1 biosynthesis is controlled by a wider array of oxidative stress factors, such as lipid hydroperoxide, superoxide, and hydroxyl and thiyl radicals.
INTRODUCTION
Humans and animals are exposed to carcinogenic aflatoxins through contaminated food and feed, air, and drinking water (1, 2). Aspergillus flavus is the primary cause of aflatoxin-contaminated crops. A. flavus is a heterothallic fungus, and laboratory crosses produce ascospore-bearing ascocarps embedded within sclerotia. In the field, sclerotia are dispersed during crop harvest and require an additional incubation period on the soil for sexual reproduction (3). Despite the significant contribution of A. flavus to crop aflatoxin contamination, it is not yet known what the role of oxidative stress is for its sclerotial differentiation (SD) and aflatoxin B1 biosynthesis. Deciphering this relationship could contribute to the development of nontoxic antifungal means via the coinhibition of A. flavus SD and aflatoxin B1 biosynthesis.
Several toxigenic and phytopathogenic fungi spread and survive in nature through the formation of conidiophores and resistant sclerotia. It has been known that oxidative stress regulates the sclerotial differentiation of filamentous phytopathogenic fungi such as Rhizoctonia solani, Sclerotium rolfsii, Sclerotinia sclerotiorum, and Sclerotinia minor (4, 5). Moreover, it has been established that the regulation of morphogenesis in aspergilli and other fungi is genetically linked to secondary metabolism (6–9). In A. flavus, both SD and aflatoxin biosynthesis are governed by the regulatory protein VeA (10). Deletion of the veA gene in this fungus results in the inhibition of sclerotia formation and aflatoxin biosynthesis (10). However, it is not known whether SD in A. flavus is regulated by oxidative stress and whether the deletion of veA could alter its oxidative stress levels.
Previous reports have linked aflatoxin biosynthesis with oxidative stress in A. flavus and A. parasiticus both at the metabolic and transcriptional levels. Specifically, aflatoxin biosynthesis in both species is activated by high levels of oxidative stress-inducing factors (e.g., lipid hydroperoxides) (11–15), whereas it is inhibited by antioxidants (e.g., polyphenols and butyl hydroxy anisole) (16–19). At the transcriptional level, the activation of the gene cluster encoding the proteins for aflatoxin biosynthesis requires AflR expression. Moreover, transcription factors that control the expression of genes involved in the oxidative response (e.g., AtfB, AP1, MsnA, and Srr) also contribute to the modulation of the genes influencing aflatoxin biosynthesis (20–22).
In this context, the main objective of the present study is to elucidate (i) whether oxidative stress regulates sclerotial differentiation in A. flavus, (ii) whether SD and aflatoxin B1 biosynthesis are comodulated by oxidative stress, through comparative analysis with a ΔveA mutant strain that does not produce sclerotia and aflatoxin, and (iii) whether aflatoxin B1 biosynthesis and SD are controlled by different parameters of oxidative stress.
MATERIALS AND METHODS
Reagents.
Aminotriazole (ATR), ascorbic acid (ASC), N-acetylcysteine (NAC), bovine serum albumin, superoxide dismutase (SOD; bovine erythrocytes), catalase (CAT; bovine liver), butyl hydroxyl anisole (BHA), caffeine (CAF), Coomassie brilliant blue G-250, cumene hydroperoxide, cysteine (CSH), dithiothreitol (DTT), glutathione reductase (GR; from baker's yeast Saccharomyces cerevisiae), glutathione (GSH) and oxidized glutathione (GSSG), malonaldehyde bis(dimethyl acetal) (MDA), NADPH, N-ethylmaleimide (NEM), ninhydrin, o-dianisidine, o-phthalaldehyde (OPT), riboflavin, thiobarbituric acid, 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt (TIRON), tributyl phosphine, xylenol orange, and aflatoxins B1 and G1 were purchased from Sigma, St. Louis, MO. Acetonitrile, chloroform, absolute methanol, ethanol, isobutanol, ammonium ferrous sulfate, ammonium sulfate, hydrogen peroxide (H2O2), cyclohexane, diethyl oxide, dimethyformamide, dimethyl sulfoxide (DMSO), acetic acid, trifluoroacetic acid, and trichloroacetic acid (TCA) were purchased from Merck, Darmstadt, Germany.
Fungal strains and growth conditions.
The control A. flavus strain 70S(pSL82) and the ΔveA mutant strain described by Duran et al. (10) in 2007 were used. Spore suspensions were prepared from cultures grown on YGT agar medium consisting of 2% glucose, 0.5% yeast extract, 1.5% agar, and 1 ml/liter trace element solution (23). Medium pH was set at 4.0 in order to (i) minimize autoxidation of the thiol redox state (TRS) modulators GSH and NAC and (ii) maximize sclerotia and aflatoxin production of the control A. flavus (24). This acidic pH is normal for the A. flavus growth, since this fungus adjusts final pH of the growth medium at 4 (from initial pH between 3.8 and 6.4) (25, 26). Spores were collected in sterile deionized-distilled water (ddH2O) by gently scraping the colony surface, and were diluted to 200,000 spores ml−1. A 0.5-ml aliquot was spread onto the surface of a 9-cm-diameter sterile cellophane membrane disc (prepared as previously described [27]), which was then floated to the surface of 3 ml of YGT broth (in a 9-cm petri dish). The cultures were incubated for 24 h in the dark (an additional factor for maximizing sclerotium production [10]) and at 30°C (this was considered day 0). Then, 25 ml of YGT broth (with or without the investigated oxidative stress modulators) was added beneath the mycelium carrying cellophane membrane, and the cultures were further inoculated for a total of 3 days. Samples were collected daily. The following oxidative stress modulators were added fresh to the culture medium on day 2. Modulators of reactive oxygen species (ROS) and their parenthetical final concentrations were the mimetic of superoxide dismutase TIRON (100 mM), the irreversible inhibitor of catalase ATR (40 mM), the hydroxyl radical scavenger DMSO (5% [vol/vol]), the lipid antioxidant BHA (250 μM), and the general (nonspecific) antioxidants CAF (5 mM) and ASC (50 mM). Modulators of the thiol redox state were GSH (15 mM), NAC (15 mM), and DTT (15 mM). The same experiment was repeated with the ΔveA mutant strain but without the oxidative stress modulators. The maximum concentrations of the oxidative stress modulators in the growth medium were such that the fungal growth rate of the control A. flavus strain was not affected.
Fungal mycelium treatment.
Mycelia were separated from the cellophane membrane disc with sterile forceps, transferred to a porcelain mortar, and rinsed with cold sterile ddH2O. They were then immersed in liquid nitrogen and ground to a fine powder. The mycelial dry weight was determined after drying samples in an oven at 80°C for 1 h. One-fifth of the resulting mycelial powder (from the mycelium in one membrane disc) was used for aflatoxin B1 analysis. The remaining powder was homogenized in 2 volumes phosphate buffer (10 mM phosphate, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride [using a 0.33 M stock made in absolute ethanol], and 1 mM BHA [using a 0.66 M stock made in absolute ethanol]; pH 7.2). The protease inhibitors PMSE/EDTA and the lipid antioxidant BHA were added in the phosphate buffer to protect the antioxidant enzymes and eliminate the artificial auto-oxidation of the fungal lipids, respectively, during homogenization. The mycelial homogenate was centrifuged at 20,000 × g at 4°C for 15 min, the clear supernatant was collected, and its protein concentration was determined as previously described (28). This supernatant was used to determine lipid peroxidation, polyphenols, certain thiol redox state parameters, and also the activity of certain antioxidant enzymes.
Lipid peroxidation determination.
Cell lipid peroxidation accumulates mainly lipid hydroperoxides (LOOH) and MDA, which are considered early and late peroxidation products, respectively (29). Mycelial LOOH and free malondialdehyde (FrMDA) were measured in the supernatant. These were fractionated after mixing the supernatant (adjusted to 20% TCA) with an equal volume chloroform-methanol (2:1), followed by incubation on ice for 30 min, and centrifugation at 15,000 × g at 4°C for 5 min. The bottom chloroform fraction was collected since it contains mycelial LOOH. The remaining middle (white protein disk) and upper (aqueous) fractions were re-extracted (for LOOH) by vortex mixing with 0.66 volumes of chloroform, followed by centrifugation at 15,000 × g for 5 min at 4°C. The resulting new bottom chloroform fraction was combined with the previous one for LOOH determination. The upper aqueous layer was also collected since it contains the extracted FrMDA. LOOH and FrMDA in the corresponding fractions were determined as described elsewhere (30).
Thiol redox state determination.
Mycelial supernatant was incubated in 10% TCA on ice-water bath for 30 min, followed by centrifugation at 15,000 × g at 4°C for 5 min and collection of the supernatant. The following thiol redox state markers were determined in the resulting deproteinized supernatant as previously described (31, 32): GSH, GSSG (or glutathione disulfide), CSH, and total oxidized CSH (CSHox; sum of cystine and CSH disulfides with other thiols). Briefly, deproteinized supernatant was washed three times with an equal volume diethyl ether to remove any TCA remnants. The resulting bottom aqueous phase was collected, and its volume was determined. GSH and GSSG were both quantified fluorometrically with OPT (at 20 μg ml−1): GSH at pH 8.0 and GSSG at pH 12 (after sample preincubation with 300 μM NEM to alkylate any GSH present) (33). Fluorescence (excitation/emission, 340/420 nm) was measured with a Shimadzu RF-1501 spectrofluorimeter (set at low sensitivity and with a slit width of 10 nm), using corresponding GSH and GSSG standard curves. CSH and CSHox (after reduction of the CSH-based mixed disulfides) were quantified as CSH (at 560 nm) by its reaction with ninhydrin under assay acidic conditions as previously described (34, 35).
Antioxidant enzyme activities.
The mycelial antioxidant enzymes SOD, CAT, glutathione peroxidase (GP), and glutathione reductase (GR) initially extracted in the supernatant were in very low concentrations. Thus, to measure their activity they were concentrated by salting out. Specifically, the supernatant was brought to 90% with solid ammonium sulfate and incubated overnight in an ice-water bath. Then, it was centrifuged at 15,000 × g for 20 min, and the resulting protein pellet was solubilized in a minimum volume (50 to 100 μl) of phosphate buffer (10 mM [pH 7.2], containing 1 mM EDTA and 0.5 mM phenylmethylsulfonyl fluoride). The resulting protein solubilizate was further diluted ∼100-fold before assaying for antioxidant enzyme activities. This minimizes the concentration of any remnant ammonium sulfate in the solubilizate and, thus, the need of eliminating by dialysis any possible assay interference due to ammonium sulfate. Nonetheless, ammonium sulfate does not interfere with the activities of the assayed enzymes; commercial preparations of superoxide dismutase, catalase, and glutathione reductase from various sources are actually stabilized as ammonium sulfate suspensions, whereas the purification of glutathione peroxidase involves ammonium sulfate fractionation. Enzyme activities were expressed per protein, the concentration of which was determined as previously described (28). The SOD specific activity was assayed as previously reported (36) and expressed as SOD units per mg of protein (using a standard curve made with pure bovine erythrocyte SOD). The CAT specific activity was measured with a Clark-type O2 electrode (controlled by Oxygraph Plus software [Hansatech Instruments Co., England] provided by Hansatech) by monitoring the decomposition of H2O2 to O2 (in a 1-ml assay solution containing 0.3 mM H2O2 in 10 mM phosphate buffer [pH 7.0] with or without a 1 mM concentration of the CAT inhibitor ATR). The net slope of oxygen production rate was converted to catalase specific activity, which was expressed as units (1 U equals 1 nmol of oxygen min−1 ml−1 released by bovine liver CAT) per mg of protein. GR and GP specific activities were determined as previously described (37).
Aflatoxin determination by LC-MS.
Aflatoxin (e.g., in the powdered mycelial homogenate) was extracted with methanol (22 ml per g dry weight of mycelium) by incubation at 70°C for 15 min, followed by mixing with ddH2O (22 ml per g dry weight mycelium) and centrifugation (at 20,000 × g, 4°C) for 10 min (38). The resulting supernatant was evaporated, and the dry pellet was solubilized in ddH2O-methanol (90:10) and injected onto a reversed-phase Luna C18 column (3-μm particle size, 100-Å pore size; Phenomenex) connected to a liquid chromatography-mass spectrometry (LC-MS) apparatus (LCQ Advantage mass spectrometer [Thermo Scientific, USA]) operated for the positive electrospray ionization. The measurement of aflatoxin concentration was performed by the use of eluent A (0.01% trifluoroacetic acid in ddH2O) and eluent B (0.01% trifluoroacetic acid in methanol) mixed in a gradient method with three intermediate isocratic steps, starting from 2% B–98% A up to 85% B–15% A at a total duration of 68 min (flow rate, 0.5 ml/min; column temperature, 37°C). Aflatoxin B1 was the main aflatoxin (mycelial and exported) observed in the chromatographs; it was identified compared to pure aflatoxin B1 standard and quantified by its ions 313 (M+1), 335 (M+Na), and 647 (2M+Na). Norleucine was used as internal standard for normalization purposes.
Statistical analysis.
Measurements were analyzed using the SPSS statistical package (release 11.0.0, 2001 [SPSS, Inc., USA]) and depicted as their means (for at least four independent experiments) ± the standard deviations. Comparisons of multiple groups were performed using the one-way analysis of variance, followed by Bonferroni's post hoc test when variances across groups were equal or by Dunnett's T3 post hoc test when variances were not equal. The same software tested variance equality. Differences were considered significant when the P value was <0.05.
RESULTS
The oxidative stress and aflatoxin B1 levels (mycelial and exported) were measured in both the undifferentiated (control-UD) and the sclerotium differentiated (control-SD) stages of a control A. flavus strain. The quantification of oxidative stress was based on the measurement of accumulative markers of oxidative damage such as the lipid hydroperoxides (LOOH). The FrMDA was also measured, but it cannot be considered accumulative, since it is highly reactive (29). Other oxidative stress markers included natural antioxidants (such as polyphenols [PLP]) and the main antioxidant enzymes SOD, CAT, GP, and GR. The latter two are also modulators of the TRS. TRS was evaluated by measuring key representative markers such as GSH, GSSG, CSH, and CSHox (sum of cystine and CSH disulfides with other thiols). For comparative analysis, the aflatoxin B1 production and the same oxidative stress markers were also measured in a ΔveA mutant strain that is unable to produce sclerotia and aflatoxin B1 (10).
The yield of aflatoxin B1 (mycelial and exported) and the sclerotial differentiation (expressed as number of sclerotia per cm2 colony) were also measured in the presence of oxidative stress modulators. These were selected for their ability to regulate two different parameters of oxidative stress; the ROS and the TRS. The ROS modulators included specific antioxidants such as a mimetic of superoxide dismutase activity (TIRON), an irreversible inhibitor of catalase (ATR), a hydroxyl radical scavenger (DMSO), and a lipid antioxidant (BHA). The CAF and ASC were used as nonspecific (or general) ROS antioxidants. The TRS modulators were GSH (a substrate of GP), NAC (a precursor of GSH), and DTT (a powerful thiol reductant and general ROS antioxidant). Figure 1 shows the developmental stages of the control A. flavus and the ΔveA mutant, and the phenotypic effect of oxidative stress modulators on the sclerotial differentiation (SD) of the control A. flavus. Tables 1 to 4 and Fig. 2 compare the patterns of the oxidative stress markers between the control A. flavus and the ΔveA mutant strains. Tables 1 to 4 also show how the oxidative stress modulators change the oxidative stress markers in relation to the aflatoxin B1 yield and the SD in the control A. flavus.
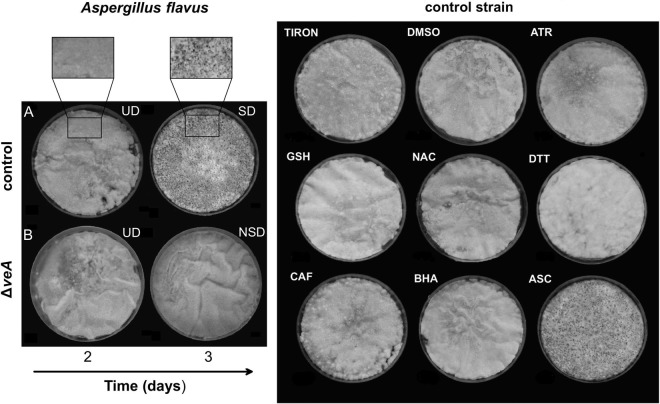
FIG 1.
Developmental stages of the control A. flavus (A; left image) and its ΔveA mutant (B; left image) and the effect of oxidative stress modulators on the differentiation of the control A. flavus (right image). The left images show the undifferentiated (UD) stage of the control A. flavus on day 2, and its sclerotial differentiation (SD) stage on day 3 (sclerotia are seen as black dots in the magnified picture); the stage of no sclerotial development (NSD) of the ΔveA mutant on day 3 is also shown (the control strain is at SD stage on the same day). The right side of the figure shows the effect of certain oxidative stress modulators on the SD stage of the control A. flavus (on day 3). Oxidative stress modulators (defined in Materials and Methods): TIRON (a superoxide dismutase activity mimetic), DMSO (hydroxyl radical scavenger), ATR (an irreversible inhibitor of catalase), GSH (a thiol redox state modulator), NAC (a thiol redox state modulator and precursor of GSH), DTT (a thiol redox state modulator, an -SS- reductant, and a nonspecific antioxidant), CAF (a nonspecific antioxidant), BHA (butyl hydroxy toluene; a lipid antioxidant), ASC (a nonspecific antioxidant).
TABLE 1.
Effect of oxidative stress modulators on sclerotial differentiation and aflatoxin B1 production in A. flavus
| Marker | Stage | Value with no modulatora | Value with indicated ROS modulatora |
Value with indicated TRS modulatora |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specific |
General |
||||||||||
| TIRON | DMSO | BHA | ATR | ASC | CAF | GSH | NAC | DTT | |||
| SDb | Control-SD | 656 ± 60 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 625 ± 55 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Control-UD | 0 (0) | NAd | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 0 (0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 0 (0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| AFB1c | Control-SD | (0.3) | (0.15) | (0.2) | (0.6) | (0.5) | (0.4) | (1) | (1) | (0.03) | |
| Mycelial | 407 ± 47 (1) | 123 ± 18 | 61 ± 3 | 78 ± 10 | 255 ± 19 | 200 ± 24 | 176 ± 26 | 480 ± 77 | 417 ± 66 | 13 ± 2 | |
| Exported | 3,760 ± 412 | 1,200 ± 132 | 670 ± 57 | 690 ± 55 | 2,300 ± 250 | 1,990 ± 211 | 1,490 ± 150 | 4,010 ± 385 | 3,700 ± 310 | 150 ± 20 | |
| Control-UD | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Mycelial | 341 ± 34 (0.8) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Exported | 3,100 ± 305 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Mycelial | 0 (0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Exported | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Mycelial | 0 (0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Exported | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
Values in parentheses depict the fold change in the marker concentration that was measured at the particular developmental stage of the control A. flavus or the ΔveA mutant strain or at the differentiated stage of the control A. flavus strain on day 3 (control-SD) after the addition of the depicted oxidative stress modulator to the growth medium on day 2, with respect to the “normal” marker concentration at the control-SD stage. The designations “control-UD,” “ΔveA-UD,” and “ΔveA-NSD” indicate, respectively, the undifferentiated stage (on day 2) of the control A. flavus strain, the vegetative growth stage of the ΔveA mutant, and the day 3 developmental stage of the ΔveA mutant, at which time sclerotia are absent (nonsclerotial development), while they are present in the control A. flavus strain. Abbreviations: TIRON (4,5-dihydroxy-1,3-benzenedisulfonic acid; mimetic of superoxide dismutase), DMSO (dimethyl sulfoxide; hydroxyl radical scavenger), ATR (aminotriazole; inhibitor of catalase), BHA (butyl hydroxyl anisol; lipid antioxidant), ASC (ascorbic acid; nonspecific antioxidant), CAF (caffeine; nonspecific antioxidant), GSH (reduced glutathione; thiol redox state modulator), NAC (N-acetyl cysteine; thiol redox state modulator, precursor of GSH), DTT (dithiothreitol; -SS- bond reductant, thiol redox state modulator, nonspecific antioxidant).
Number of sclerotia per cm2 colony.
The concentration (ppm [μg/g mycelial dry weight]) of exported aflatoxin B1 was normalized to the corresponding g mycelial dry weight.
NA, not applicable.
TABLE 4.
Effect of oxidative stress modulators on antioxidant enzymes in A. flavus
| Marker | Stage | Value with no modulatora | Value with indicated ROS modulatora |
Value with indicated TRS modulatora |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specific |
General |
||||||||||
| TIRON | DMSO | BHA | ATR | ASC | CAF | GSH | NAC | DTT | |||
| SODb | Control-SD | 4.9 ± 1.8 (1) | 0 (0) | 12.8 ± 1.4 (2.6) | 4.2 ± 0.9 (1) | 25.2 ± 4 (5.1) | 4.3 ± 0.8 (1) | 17.9 ± 1.1 (3.7) | 17 ± 0.7 (3.5) | 17.4 ± 0.5 (3.6) | 13 ± 2.5 (2.7) |
| Control-UD | 15.7 ± 1.6 (3.2) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 18.2 ± 2.1 (3.7) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 19.8 ± 1.8 (4) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| CATb | Control-SD | 42 ± 2.2 (1) | 86.6 ± 11 (2.1) | 34.8 ± 5.7 (1) | 29 ± 3.2 (0.7) | 12.9 ± 1.2 (0.3) | 72.7 ± 6.3 (1.7) | 36.2 ± 2.9 (1) | 40.5 ± 3.9 (1) | 34 ± 4.4 (1) | 37.6 ± 3.5 (1) |
| Control-UD | 38.5 ± 4.6 (0.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 20.6 ± 3.5 (0.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 24.7 ± 3.5 (0.6) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| GPc | Control-SD | 30 ± 5 (1) | 36 ± 4 (1) | 77 ± 8 (2.6) | 36 ± 5 (1) | 65 ± 6.5 (2.2) | 44 ± 5 (1.5) | 59 ± 5 (2) | 57 ± 5.5 (1.9) | 32 ± 4 (1) | 60 ± 6.5 (2) |
| Control-UD | 47 ± 4.5 (1.6) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 30 ± 2.9 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 47 ± 4 (1.6) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| GRc | Control-SD | 140 ± 18 (1) | 140 ± 16 (1) | 260 ± 27 (1.9) | 180 ± 15 (1.3) | 270 ± 30 (1.9) | 145 ± 14 (1) | 250 ± 20 (1.8) | 150 ± 13 (1) | 200 ± 17 (1.4) | 300 ± 27 (2.1) |
| Control-UD | 260 ± 25 (1.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 170 ± 12 (1.2) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 340 ± 20 (2.4) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
See footnote a of Table 1.
SOD (superoxide dismutase) and CAT (catalase) are measured in units/mg total fungal protein.
GP (glutathione peroxidase) and GR (glutathione reductase) are measured in mU/mg total fungal protein.
FIG 2.
Aflatoxin B1 (AFB1) yield, sclerotial differentiation (SD), and oxidative stress in A. flavus. (A) Aflatoxin B1 biosynthesis and SD in the control A. flavus and the ΔveA mutant strain are regulated by different oxidative stress levels; an oxidative stress increase or decrease in these strains is designated by open upward or downward arrows, respectively. Oxidative stress levels are assigned as low or high compared to the normal level, defined by the concentrations of the oxidative stress markers LOOH and SOD in the undifferentiated (UD) stage of the control A. flavus (on day 2). The upward and downward arrows designate an increase or decrease in these markers, respectively. (B) Effect of certain oxidative stress modulators on the aflatoxin B1 yield and SD in the control A. flavus, and their correlation with certain oxidative stress markers. A concentration increase and decrease in these markers is designated by the “+” and “−” signs, respectively (the actual concentration values are given in Tables 2 to 4). Abbreviations: TIRON, DMSO, ATR, GSH, NAC, DTT, CAF, BHA, and ASC are as described in the legend of Fig. 1 and as defined in Materials and Methods, as are LOOH (lipid hydroperoxides), FrMDA, and PLP (polyphenols), GSSG, SOD, CAT, GP, and GR.
Effect of oxidative stress modulators on sclerotial differentiation and aflatoxin B1 yield.
Table 1 shows that the control A. flavus produced a large number of sclerotia and high levels of aflatoxin B1 (mycelial and exported, at exported ratio ∼0.9, calculated from data in Table 1). The aflatoxin exported ratio is defined as the ratio of exported aflatoxin over the sum of exported and mycelial (39). The ΔveA mutant did not produce any sclerotia and aflatoxin B1, as expected. The yield of aflatoxin B1 was 20% higher in the differentiated (control-SD) than in the undifferentiated (control-UD) stage of the control A. flavus. Table 1 also shows that the SD was inhibited in the control A. flavus by the ROS modulators, with the exception of the ASC. Moreover, the aflatoxin B1 (mycelial and exported) production was inhibited 1.7- to 7-fold by all ROS modulators, with the highest inhibition observed when the hydroxyl radical scavenger DMSO was used. Similarly, all of the TRS modulators inhibited SD in the control A. flavus, but only the very potent antioxidant DTT inhibited (almost completely) aflatoxin B1 production. This result differentiates the oxidative stress levels at which the inhibition of SD and aflatoxin B1 production occur. The TRS modulators GSH and NAC decreased the oxidative stress to a level sufficient to inhibit the SD but not the production of aflatoxin B1. On the other hand, the higher antioxidant potential of DTT decreased the oxidative stress to a lower level at which both the SD and the aflatoxin B1 production are inhibited.
Effect of oxidative stress modulators on lipid peroxidation and polyphenol content.
Table 2 shows that the levels of the lipid hydroperoxides (LOOH) are two times higher in the sclerotial differentiated (control-SD) stage of the control A. flavus compared to its undifferentiated (control-UD) stage. The opposite trend is observed for the natural antioxidants polyphenols (PLP). These findings are in agreement with previous studies on the differentiation of other filamentous sclerotiogenic fungi (4). The control-UD represents an early stage of the A. flavus development at which time nutrients of the growth medium are not exhausted. In contrast, the control-SD stage corresponds to a late developmental stage and takes place under high oxidative stress. Of particular importance is also the finding that throughout development of the ΔveA mutant strain the LOOH levels are 2.5 times lower even than those in the control-UD stage of the A. flavus. This suggests that the veA also causes a significant decrease in the “normal” oxidative stress levels in the A. flavus, along with the inhibition of the production of the aflatoxin B1 and sclerotia production. No significant change in the levels of the FrMDA and PLP markers was observed between the control and the mutant strains. Table 2 also shows that the LOOH was decreased (1.25- to 10-fold) in the control A. flavus by both the ROS (except for the DMSO) and TRS modulators. As expected, the largest LOOH decrease was caused by the DTT. Moreover, most ROS and TRS modulators decreased (1.4- to 2-fold) FrMDA in the control A. flavus. In contrast, most ROS modulators increased the natural antioxidant polyphenols (PLP) by 1.8- to 25-fold; the highest increase in PLP was caused by the superoxide dismutase mimetic TIRON. From the TRS modulators, only DTT increased the PPL levels by 1.8-fold, as it also acts as a general ROS antioxidant; the GSH and NAC decreased PLP by ∼2-fold. This finding confirms once more the difference in the antioxidant potential and roles between the thiol groups of the GSH/NAC and DTT.
TABLE 2.
Effect of oxidative stress modulators on lipid peroxidation and polyphenol content in A. flavus
| Marker | Stage | Value with no modulatora | Value with indicated ROS modulatora |
Value with indicated TRS modulatora |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specific |
General |
||||||||||
| TIRON | DMSO | BHA | ATR | ASC | CAF | GSH | NAC | DTT | |||
| LOOHb | Control-SD | 1.7 ± 0.09 (1) | 1.3 ± 0.12 (0.8) | 1.52 ± 0.15 (1) | 1.26 ± 0.1 (0.7) | 1.37 ± 0.09 (0.8) | 1.42 ± 0.1 (0.8) | 1.14 ± 0.13 (0.7) | 1.22 ± 0.12 (0.7) | 0.63 ± 0.09 (0.4) | 0.22 ± 0.08 (0.1) |
| Control-UD | 0.89 ± 0.16 (0.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 0.49 ± 0.05 (0.3) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 0.32 ± 0.03 (0.2) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| FrMDAc | Control-SD | 373 ± 30 (1) | 360 ± 48 (1) | 398 ± 43 (1) | 270 ± 23 (0.7) | 261 ± 22 (0.7) | 333 ± 39 (1) | 291 ± 30 (1) | 255 ± 22 (0.7) | 270 ± 21 (0.7) | 168 ± 30 (0.5) |
| Control-UD | 320 ± 32 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 380 ± 20 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 361 ± 28 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| PLPd | Control-SD | 446 ± 49 (1) | 10,921 ± 640 (25) | 2,145 ± 190 (4.8) | 440 ± 38 (1) | 2,391 ± 200 (5.4) | 2,197 ± 240 (5) | 788 ± 54 (1.8) | 245 ± 19 (0.6) | 241 ± 15 (0.5) | 818 ± 64 (1.8) |
| Control-UD | 1,096 ± 75 (2.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 422 ± 38 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 431 ± 42 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
See footnote a of Table 1.
Lipid hydroperoxides (LOOH) in nmol cumene hydroperoxide equivalents/mg total fungal protein.
Free malondialdehyde (FrMDA) in pmol/mg total fungal protein
Polyphenols (PLP) in nmol caffeic acid equivalents/mg total fungal protein.
Effect of oxidative stress modulators on thiol redox state.
Table 3 evaluates the TRS in the control A. flavus and the ΔveA mutant, as it is estimated based on certain representative markers; GSH, GSSG, CSH, and CSHox (the sum of cystine and CSH disulfides with other thiols). This table also shows the effect of the ROS and TRS modulators on the studied TRS markers. As a general trend, the levels of these markers are not different between the control A. flavus and the ΔveA mutant. An exception is the increase of GSSG by 1.7-fold in the ΔveA-NSD (undifferentiated) stage compared to the corresponding (in terms of developmental time) control-SD (differentiated) stage of the control A. flavus. Table 3 also shows that among the ROS modulators, the superoxide dismutase mimetic TIRON and the inhibitor of catalase ATR increased the TRS markers by 1.4- to 7.9-fold in the control A. flavus; an exception was the decrease in CSHox by ∼2-fold. Similarly, the TRS modulators GSH and NAC increased the levels of the TRS markers by 2.2- to 9.1-fold. In contrast, DTT increase only CSH (by 5.8-fold), possibly by releasing it by reduction from the CSH-based disulfides. This finding also supports the differentiation between the antioxidant role of the thiol group of DTT from the thiol groups of GSH and NAC. The antioxidant role of the NAC/GSH pair is mainly associated with the antioxidant enzyme GP, which uses GSH as the substrate (29).
TABLE 3.
Effect of oxidative stress modulators on thiol redox state in A. flavus
| Markerb | Stage | Value with no modulatora | Value with indicated ROS modulatora |
Value with indicated TRS modulatora |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specific |
General |
||||||||||
| TIRON | DMSO | BHA | ATR | ASC | CAF | GSH | NAC | DTT | |||
| GSH | Control-SD | 6.5 ± 0.7 (1) | 51 ± 4.5 (7.9) | 7.7 ± 2 (1) | 6.8 ± 1.6 (1) | 38.7 ± 3.8 (6) | 9 ± 1.9 (1) | 5.15 ± 1.3 (1) | 20 ± 3.4 (3.1) | 5 ± 1.1 (1) | 11.1 ± 3.2 (1.7) |
| Control-UD | 6.4 ± 0.9 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 7.7 ± 1.2 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 7.1 ± 0.8 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| GSSG | Control-SD | 16 ± 2.4 (1) | 92 ± 9.7 (5.8) | 34 ± 5 (2.1) | 25 ± 4.2 (1) | 44 ± 7.6 (2.8) | 37 ± 6.4 (2.3) | 22 ± 5 (1) | 145 ± 15 (9.1) | 38 ± 9.6 (2.4) | 17 ± 2.6 (1) |
| Control-UD | 18 ± 2.2 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 27 ± 3.4 (1.7) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 20 ± 2 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| CSH | Control-SD | 2.6 ± 0.4 (1) | 4.3 ± 0.5 (1.7) | 4.5 ± 0.8 (1.7) | 4.2 ± 0.5 (1.6) | 3.7 ± 0.5 (1.4) | 10 ± 1.2 (3.9) | 2.5 ± 0.5 (1) | 5.9 ± 0.6 (2.3) | 13 ± 1.2 (5) | 15 ± 1.1 (5.8) |
| Control-UD | 2.1 ± 0.2 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 2.5 ± 0.3 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 2.4 ± 0.4 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| CSHox | Control-SD | 37 ± 3 (1) | 22 ± 3 (0.6) | 41 ± 5 (1) | 46 ± 6 (1) | 54 ± 6 (1.5) | 40 ± 5 (1) | 32 ± 4 (1) | 121 ± 10 (3.3) | 81 ± 9 (2.2) | 37 ± 4 (1) |
| Control-UD | 39 ± 4 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-NSD | 48 ± 6 (1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ΔveA-UD | 55 ± 5 (1.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
See footnote a of Table 1.
GSH (glutathione), GSSG (oxidized glutathione), CSH (cysteine), and CSHox (total oxidized CSH; the sum of values for cystine and CSH disulfides with other thiols) are measured in nmol/mg total fungal protein.
Effect of oxidative stress modulators on antioxidant enzymes.
Table 4 presents the activities of the antioxidant enzymes SOD, CAT, GP, and GR during the development of the control A. flavus and the ΔveA mutant. The table also shows the effect of the ROS and TRS modulators on the activities of these enzymes in the differentiated (control-SD) stage of the control A. flavus. The SOD activity is 3.2-fold higher in the control-UD stage compared to the control-SD stage of this fungus. Of particular importance is the finding that throughout the development of the ΔveA mutant, the SOD activity is ∼4-fold higher than its activity in the differentiated (control-SD) stage of the control A. flavus. Moreover, the SOD activity in the ΔveA mutant is ca. 25% higher, even with respect to the undifferentiated (control-UD) stage of the control A. flavus. Concerning the activity of the other important antioxidant enzyme, CAT, it remained constant throughout the development of both the control A. flavus and the ΔveA mutant, but it was half in the ΔveA mutant. The latter observation shows that there is an opposite change in the CAT and SOD activities in the ΔveA mutant (in reference to control A. flavus). No changes in the GR and GP activities were observed between the control A. flavus and the ΔveA mutant.
Table 4 also shows that among the ROS modulators, the superoxide dismutase mimetic (TIRON) and the inhibitor of catalase (ATR) exhibit opposite effects on the SOD and the CAT activity at the differentiation (control-SD) stage of the control A. flavus. Specifically, TIRON eliminated the SOD activity (as expected of a SOD mimetic) but increased the CAT activity by 2.1-fold. On the other hand, the ATR increased the SOD activity by 5.1-fold but decreased CAT activity by 3.3-fold (as expected by an irreversible inhibitor of CAT). It should be noted that the potent antioxidant DTT increased the SOD activity by 2.7-fold without affecting the CAT activity. This confirms the association of an increased SOD activity with low oxidative stress. A similar effect on SOD and CAT was noted for the other TRS modulators GSH and NAC and also by the ROS modulators DMSO (hydroxyl radical scavenger) and CAF (a general antioxidant). In contrast, ASC, the other general antioxidant tested, increased only the activity of CAT (by 1.7-fold), whereas the specific lipid antioxidant butyl hydroxyl toluene (BHA) decreased it by 1.4-fold. Having previously associated a decreased CAT activity with low oxidative stress (confirmed also by the decrease of the CAT activity by BHA), the increase in the CAT activity caused by the ASC suggests that this ROS nonspecific modulator acts as an oxidant at its tested concentration. Most ROS and TRS modulators increased GP and GR activities by 1.3- to 2.6-fold.
DISCUSSION
This study investigated the modulation of sclerotial differentiation (SD) and aflatoxin B1 production by oxidative stress in A. flavus. Oxidative stress was evaluated in control A. flavus and ΔveA mutant strains by determining the concentration levels of certain oxidative stress markers: the lipid peroxidation products lipid hydroperoxides (LOOH) and FrMDA; the natural antioxidant polyphenols (PLP); the TRS markers GSH, GSSG, CSH, and CSHox (total oxidized CSH); and the antioxidant enzymes SOD, CAT, GP, and GR. The “normal” oxidative stress levels in the control A. flavus were defined based on the concentration of the key marker of oxidative damage LOOH (29) at the undifferentiated (control-UD) stage (on day 2). This is an early developmental stage at which the growth medium nutrients are still adequate to maintain a pro-oxidant/antioxidant balance in the control A. flavus.
The modulation of SD and aflatoxin B1 production in the control A. flavus was studied at various levels of oxidative stress. These were experimentally achieved via the different antioxidant potentials of certain oxidative stress modulators; i.e., the SOD mimetic TIRON (40), the inhibitor of CAT ATR (41, 42), the hydroxyl radical scavenger DMSO (43), the lipid antioxidant butyl hydroxyl toluene (BHA) (44), the nonspecific antioxidants CAF and ASC (29), and the TRS modulators GSH and NAC (29) in combination with the general -SS- group reductant, which is also a general antioxidant, dithiothreitol (45). An observed decrease or increase in the LOOH concentration compared to its “normal” value at the control-UD stage in the control A. flavus characterizes a low or high oxidative stress level, respectively, whereas no change refers to the “normal” oxidative state. In particular, the modulators TIRON and ATR induce an increase in the intracellular levels of H2O2 via either the dismutation of superoxide radicals to H2O2 (TIRON) or the inhibition of CAT (ATR). This induction enables the indirect investigation of the role of intracellular H2O2 on SD and aflatoxin B1 production in A. flavus.
Although the LOOH concentration is an unequivocal marker of oxidative stress, measurements such as the CAT and SOD activities are less certain if interpreted alone. Any observed changes in the SOD and CAT activities induced by oxidative stress modulators can be associated with increased or decreased oxidative stress levels (and, consequently, to a corresponding change in the SD and aflatoxin B1 production) compared to the control strain only when a similar change in the LOOH concentration has also been observed. The interpretation of the observed results was also based on the facts that in other phytopathogenic fungi (i) SD is inhibited by low oxidative stress and (ii) an increase in the intracellular levels of H2O2 has been observed to concur with low oxidative stress and undifferentiated stage and sclerotium initiation (4, 46–48). In the present study, similar morphological mycelial developmental stages occurred in the control A. flavus when its development was modulated by antioxidants (Fig. 1). In addition, we observed that the aflatoxin B1 production was induced or inhibited by high or low oxidative stress modulators, respectively (Table 1). These observations are in agreement with previous experiments in which A. flavus was grown under high oxidative stress levels (11, 14, 49). The detection of aflatoxin B1 as the main aflatoxin produced by the control A. flavus is also in agreement with previous studies (14). Mycelial and exported aflatoxin B1 levels were almost unchanged throughout development (Table 1). Exported aflatoxin B1 levels were of the same magnitude with those measured when the fungus had been grown in a different version of the YES medium (25). Interestingly, A. flavus mycelial and exported aflatoxin B1 levels were similar to those in A. parasiticus (50).
The transition of the control A. flavus from the undifferentiated (control-UD) to the sclerotial differentiated (control-SD) stage coincided with a 2-fold increase in the LOOH concentration. This increase in the oxidative stress level was also confirmed by the 2.5-fold decrease in the intracellular antioxidant polyphenols (Table 2). The 2-fold increase in the oxidative stress level was responsible for the SD induction (Table 1, Fig. 1), a finding in agreement with observations in other filamentous fungi (4, 51), and concurred with a 20% increase in the aflatoxin B1 concentration (Table 1). The latter observation is also in agreement with previous experiments in A. flavus and A. parasiticus, which have shown that an increase in oxidative stress is required for the onset of aflatoxin biosynthesis (12, 18, 52). Also consistent with this interpretation was the almost 50% increase in the aflatoxin B1 production when the oxidative stress level of A. flavus was increased by the addition of the tertiary-butyl hydroperoxide (14), which is an LOOH analogue.
Transition from the undifferentiated stage of the control A. flavus to the SD stage, which is characterized by high oxidative stress, concurred with a 3.2-fold decrease in the SOD activity (Table 4). This observation associates the decrease in the SOD activity with high oxidative stress. In agreement with this interpretation was the observation that the SOD activity did not change in the ΔveA mutant strain, the oxidative stress of which was lower than in the control A. flavus. Moreover, the day 3 concentration of the GSSG, which is a thiol redox state marker, was 1.7-fold greater in the ΔveA strain than in the control A. flavus strain (Table 3, Fig. 2A). Given that the mutant strain operates at low oxidative stress, this observation implies that an increase in the GSSG concentration may be indicative of a low oxidative stress state. This implied characteristic, however, is the opposite of what has been traditionally believed for GSSG (29).
Effect of oxidative stress modulators on sclerotial differentiation and aflatoxin B1 production.
Two classes of oxidative stress modulators were studied: ROS and TRS modulators. SD in the control A. flavus was inhibited by the antioxidant action of ROS modulators, with the exception of ASC (Table 1, Fig. 1). This observation supports the general hypothesis that in sclerotiogenic filamentous fungi high oxidative stress levels induce SD (4). Similarly, all ROS modulators reduced the production of aflatoxin B1(Table 1) to various degrees. It should be noted that SD and aflatoxin B1 production were fully inhibited only by DTT, which acts both as a strong ROS antioxidant and as a TRS modulator. The use of DTT was also associated with the largest decrease in the oxidative stress. As shown in Table 2, the intracellular concentration of LOOH was 5-fold lower in the control A. flavus when DTT was used compared to its absence. Moreover, the inhibition of aflatoxin B1 production and SD by the hydroxyl radical scavenger DMSO is of particular interest (Table 2). This finding suggests that the hydroxyl radicals may be implicated in both the aflatoxin B1 biosynthesis and the SD in A. flavus.
The use of most ROS modulators leads to a decrease in the production of aflatoxin B1 with a concurrent decrease in the LOOH concentration and an increase in the polyphenol levels. This result is in accordance with the reported antioxidant role of polyphenols assisting in the inhibition of aflatoxin biosynthesis (18, 19). However, polyphenols seem not to act in the same way as the lipid antioxidants. It was observed that the use of the lipid antioxidant butyl hydroxyl toluene (BHA) was associated with a 33% decrease in the LOOH concentration, but the intracellular polyphenol levels remained constant (Table 2). It has long been known that the decrease in the lipid peroxidation due to BHA in A. flavus is associated with inhibition of the aflatoxin production and SD (16, 17).
As already mentioned, apart from the dual ROS and TRS modulator, DTT, which inhibited both the aflatoxin B1 biosynthesis and SD, the typical TRS modulators GSH and NAC inhibited only SD. Specifically, the use of GSH and NAC was associated with a decrease in oxidative stress to half the “normal” levels, as shown by the corresponding LOOH concentration, leading to the inhibition of SD (Tables 1 and 2). The differential effect of GSH and NAC on SD and aflatoxin B1 production has also been observed in other sclerotiogenic filamentous fungi (53–55). The fact that the use of GSH and NAC, a precursor of GSH, was not associated with the inhibition of aflatoxin B1 biosynthesis implies that the halving of the oxidative stress level due to GSH or NAC is sufficient to inhibit SD, but it is not of adequately high antioxidant capacity to also inhibit the production of aflatoxin B1, as was the case with the 5-fold reduction in the oxidative stress level due to DTT. This is true because GSH is not as direct and as broad spectrum an antioxidant as DTT (45). Instead, GSH reaction with ROS could generate highly oxidative thiyl radicals (29). The antioxidant action of GSH is exerted only when it is used as the substrate of glutathione peroxidase. The latter has a limited antioxidant role since it only detoxifies the cells from both hydrogen peroxide and organic hydroperoxides (29).
The use of GSH and NAC was also associated with an increase in the intracellular concentration of all markers of the thiol redox state (i.e., GSH, GSSG, CSHox, and CSH). Among the antioxidant TRS enzymes, GSH and NAC increased the GP and GR (the enzyme that regenerates GSH by reduction of GSSG), respectively (Tables 3 and 4). The observed increase in all markers of the thiol redox state and GR suggests that (i) GSSG could act as a pool for GSH as the substrate of GR (29), as previously shown (31, 55), and (ii) the CSHox may act as a pool for CSH via the recycling action of the cystine reductase (56).
Modulation of aflatoxin B1 biosynthesis and sclerotial differentiation by superoxide dismutase and catalase: the role of H2O2.
The SOD mimetic TIRON and the CAT inhibitor ATR were used to modulate aflatoxin B1 and sclerotial differentiation by intracellular H2O2. TIRON is expected to replace SOD and dismutate the superoxide radical to H2O2, while the ATR would maintain a high intracellular H2O2 concentration by inhibiting CAT. As expected, TIRON eliminated the intracellular SOD activity, while it almost doubled the CAT activity (Table 4) to potentially counterbalance the overproduction of H2O2. The observed decrease in CAT activity due to ATR had also been anticipated. Most importantly, the use of ATR was associated with an increase in the SOD activity (Table 4). These enzyme activity changes occurred while the oxidative stress level decreased, as shown by the decrease in the LOOH concentration and confirmed by the 5- to 25-fold increase in the polyphenol levels (Table 2). These findings indicate that the inhibition of aflatoxin B1 biosynthesis and SD are related to the simultaneous increase in the SOD and decrease in the CAT activity, both being an outcome of a low oxidative stress level. This conclusion is further corroborated by the higher SOD and lower CAT activity in the ΔveA mutant strain, which is characterized by low oxidative stress.
Moreover, we observed that the SOD activity in the ΔveA mutant strain is ca. 25% higher compared to the undifferentiated (control-UD) stage of the control A. flavus. This result, in combination with the 2.5-fold decrease in the LOOH concentration in the ΔveA mutant with respect to the UD stage of the control A. flavus (Table 2), suggests that (i) the higher SOD activity and the lower CAT activity in the ΔveA mutant concur with a decrease of oxidative stress to such a low level that both SD and the production of aflatoxin B1 are inhibited and (ii) inhibition of the aflatoxin B1 production is induced at a lower level of oxidative stress than that at which inhibition of sclerotial differentiation is initiated. The latter is a significant result that reveals significant aspects of the regulatory mechanisms underlying SD and aflatoxin B1 production.
The simultaneous inhibition of both SD and aflatoxin B1 production in the ΔveA mutant could be explained based on the fold difference between the activity fold increase (2.9-fold) of SOD and the activity fold decrease (2-fold) of CAT in the ΔveA mutant compared to the control A. flavus on day 3. This difference is ∼6-fold higher (i.e., 2 × 2.9) in the ΔveA mutant. The larger the particular difference is, the higher the intracellular concentration of H2O2 would be in the mutant compared to the control strain. A high intracellular H2O2 could justify the nondifferentiation of the ΔveA mutant. Based on previous studies in sclerotiogenic fungi, high levels of H2O2 inhibit SD because H2O2 promotes intense cell proliferation throughout the whole mycelial colony. Such extensive cell proliferation surpasses the localized cell proliferation that is required for the generation of sclerotial initials (that are developed to mature sclerotia) (4, 36, 46–48). Therefore, our data suggest that the increase in the SOD activity, combined with the decrease in CAT activity in the mutant strain compared to the control and the consequent increase in the concentration of H2O2, could contribute to the prevention of SD in the ΔveA mutant strain.
Pro-oxidant and antioxidant modulation of ascorbic acid.
The effect of the ROS modulator ASC on SD and the aflatoxin B1 production is of particular interest. ASC can exhibit antioxidant and pro-oxidant action based on its intracellular concentration and the availability of transition metals (29). As a pro-oxidant, it is involved in the redox cycling of transition metals of biological importance (such as Fe and Cu) to their reduced state, which could be implicated in the conversion of H2O2 to hydroxyl radicals (29). Considering the dual role of ascorbic acid as pro-oxidant and antioxidant could assist in the elucidation of antioxidant mechanisms that regulate SD and aflatoxin B1 production in A. flavus. Based on our results, the administration of ASC partly inhibited (i.e., by ca. 50%) the production of aflatoxin B1 through its general antioxidant action. The decrease in the oxidative stress level in A. flavus was shown by the decrease in the intracellular LOOH concentration and the simultaneous increase in polyphenols (Tables 1 and 2, Fig. 2B). This observation suggests that aflatoxin B1 biosynthesis may be controlled by a wide spectrum of endogenous oxidants/antioxidants. This is also supported by the observed various degrees of inhibition in aflatoxin B1 production caused by the tested ROS modulators (Table 1). However, the administration of ASC was not associated with inhibition of SD. This may be explained by the observed increase in the activity of the CAT and GP enzymes (Table 4, Fig. 1 and 2B), and the consequent decrease in the intracellular H2O2 concentration, followed by the induction of SD.
How does oxidative stress modulate aflatoxin B1 and sclerotial differentiation?
As discussed above, the inhibition of aflatoxin B1 biosynthesis in A. flavus is initiated at a lower oxidative stress level than that of SD. Thus, inhibition of aflatoxin biosynthesis requires a larger decrease in the oxidative stress level compared to the normal to be initiated compared to the inhibition of SD. It was shown that SD but not aflatoxin B1 biosynthesis was inhibited when the level of oxidative stress was half the normal level upon administration of the TRS modulations GSH and NAC. Inhibition of aflatoxin B1 biosynthesis in A. flavus requires an oxidative stress level at least equal to that of the ΔveA mutant. This level is 2.5-fold lower than the normal level in the undifferentiated A. flavus. Of course, both aflatoxin B1 and SD were inhibited upon administration in the control A. flavus of the potent antioxidant DTT, which caused a 5-fold decrease in the oxidative stress level compared to the normal level. The fact that the low oxidative stress levels at which both the SD and aflatoxin B1 biosynthesis are inhibited is observed in the ΔveA strain implies a relationship of the veA gene with oxidative stress levels. In general, there seems to be a genetic link between stress response and secondary metabolism in endosomes, potentially suggesting that aflatoxin biosynthesis is a consequence of fungal oxidative stress exposure (Fig. 3). At least five transcription factors (AflR, AtfB, AP1, MsnA, and SrrA) participate in the regulatory network that induces aflatoxin biosynthesis as part of the cellular response to oxidative stress in aspergilli. Furthermore, sequence analysis reveals a conserved motif in gene promoters of aflatoxin biosynthesis and the stress-response genes CAT and SOD (21, 22).
FIG 3.
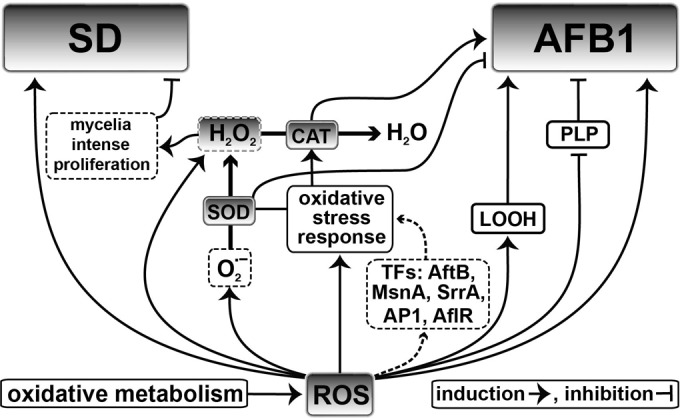
Proposed regulatory mechanism of aflatoxin B1 (AFB1) production and sclerotial differentiation (SD) in A. flavus.
According to our data, SD is mainly inhibited by factors that increase the intracellular H2O2 concentration, as is the increase in the SOD enzyme activity or the decrease (or no change) in CAT activity. On the other hand, the biosynthesis of aflatoxin B1 appears to be controlled by a wider array of oxidative stress factors, including the SOD and CAT enzymes, possibly acting in combination. It has been known that aflatoxins are produced in the peroxisomes and aflatoxisomes, colocalizing with enzymes associated with response to oxidative stress, such as the SOD and CAT, along with important sources of ROS generation (57). The new insights into the association of oxidative stress with SD and the biosynthesis of aflatoxin B1 in A. flavus reported here could contribute to the development of control strategies to decrease aflatoxin B1 contamination of agricultural commodities, such as maize, cotton, peanuts, tree nuts, and other oil seed crops (15). These can be based on the antioxidant inhibitors of SD and aflatoxin B1 biosynthesis that were identified and discussed here.
ACKNOWLEDGMENTS
We thank Ana M. Calvo (Department of Biological Sciences, Northern Illinois University, DeKalb, IL) for providing the A. flavus strains used in the present study and Maria Panagiotonakou for text language editing.
K.G. thanks the Bodossaki Foundation (Athens, Greece) for a Ph.D. fellowship and FORTH/ICE-HT internal funds for the execution of the aflatoxin LC-MS quantification experiments.
Footnotes
Published ahead of print 7 July 2014
REFERENCES
- 1.Mehl HL, Jaime R, Callicott KA, Probst C, Garber NP, Ortega-Beltran A, Grubisha LC, Cotty PJ. 2012. Aspergillus flavus diversity on crops and in the environment can be exploited to reduce aflatoxin exposure and improve health. Ann. N. Y. Acad. Sci. 1273:7–17. 10.1111/j.1749-6632.2012.06800.x [DOI] [PubMed] [Google Scholar]
- 2.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. 2007. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677–1692. 10.1099/mic.0.2007/007641-0 [DOI] [PubMed] [Google Scholar]
- 3.Horn B, Sorensen R, Lamb M, Sobolev V, Olarte R, Worthington C, Carbone I. 2014. Sexual reproduction in Aspergillus flavus sclerotia naturally produced in corn. Phytopathology 104:75–85. 10.1094/PHYTO-05-13-0129-R [DOI] [PubMed] [Google Scholar]
- 4.Georgiou CD, Patsoukis N, Papapostolou I, Zervoudakis G. 2006. Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr. Comp. Biol. 46:691–712. 10.1093/icb/icj034 [DOI] [PubMed] [Google Scholar]
- 5.Bishop DC, Erezyilmaz FD, Flatt T, Georgiou CD, Hadfield GM, Heyland A, Hodin J, Jacobs WM, Maslakova AS, Pires A, Reitzel MA, Santagata S, Tanakay K, Youson HJ. 2006. What is metamorphosis? Integr. Comp. Biol. 46:655–661. 10.1093/icb/icl004 [DOI] [PubMed] [Google Scholar]
- 6.Bayram O, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, Braus-Stromeyer S, Kwon NJ, Keller NP, Yu JH, Braus GH. 2008. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320:1504–1506. 10.1126/science.1155888 [DOI] [PubMed] [Google Scholar]
- 7.Bayram O, Braus GH. 2012. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol. Rev. 36:1–24. 10.1111/j.1574-6976.2011.00285.x [DOI] [PubMed] [Google Scholar]
- 8.Calvo AM, Wilson RA, Bok JW, Keller NP. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447–459. 10.1128/MMBR.66.3.447-459.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo AM. 2008. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45:1053–1061. 10.1016/j.fgb.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 10.Duran RM, Cary JW, Calvo AM. 2007. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 73:1158–1168. 10.1007/s00253-006-0581-5 [DOI] [PubMed] [Google Scholar]
- 11.Fabbri AA, Fanelli C, Panfili G, Passi S, Faseila P. 1983. Lipoperoxidation and aflatoxin biosynthesis by Aspergillus parasiticus and A. flavus. J. Gen. Microbiol. 129:3447–3452 [Google Scholar]
- 12.Jayashree T, Subramanyam C. 2000. Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Radic. Biol. Med. 29:981–985. 10.1016/S0891-5849(00)00398-1 [DOI] [PubMed] [Google Scholar]
- 13.Narasaiah KV, Sashidhar RB, Subramanyam C. 2006. Biochemical analysis of oxidative stress in the production of aflatoxin and its precursor intermediates. Mycopathologia 162:179–789. 10.1007/s11046-006-0052-7 [DOI] [PubMed] [Google Scholar]
- 14.Mahoney N, Molyneux RJ, Kim JH, Campbell BC, Waiss AC, Hagerman AE. 2010. Aflatoxigenesis induced in Aspergillus flavus by oxidative stress and reduction by phenolic antioxidants from tree nuts. World Mycotoxin J. 3:49–57. 10.3920/WMJ2009.1185 [DOI] [Google Scholar]
- 15.Yu J. 2012. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 4:1024–1057. 10.3390/toxins4111024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung DYC, Taylor S, Kahan J. 1977. Effects of butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) on growth and aflatoxin production of Aspergillus flavus. J. Food Saf. 1:39–51. 10.1111/j.1745-4565.1977.tb00258.x [DOI] [Google Scholar]
- 17.Thompson DP. 1996. Effect of butylated hydroxyanisole on intracellular components of aflatoxigenic strains of Aspergillus flavus and Aspergillus parasiticus. Mycol. Res. 100:1256–1258. 10.1016/S0953-7562(96)80189-4 [DOI] [Google Scholar]
- 18.Kim JH, Yu J, Mahoney N, Chan KL, Molyneux RJ, Varga J, Bhatnagar D, Cleveland TE, Nierman WC, Campbell BC. 2008. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 122:49–60. 10.1016/j.ijfoodmicro.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 19.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Chang P-K. 2011. Aflatoxins: mechanisms of inhibition by antagonistic plants and microorganisms, p 285–304 In Guevara-Gonzalez RG. (ed), Aflatoxins: biochemistry and molecular biology. InTech, New York, NY [Google Scholar]
- 20.Chang PK, Scharfenstein LL, Luo M, Mahoney N, Molyneux RJ, Yu J, Brown RL, Campbell BC. 2011. Loss of msnA, a putative stress regulatory gene, in Aspergillus parasiticus and Aspergillus flavus increased production of conidia, aflatoxins, and kojic acid. Toxins (Basel) 3:82–104. 10.3390/toxins3010082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roze LV, Hong SY, Linz JE. 2012. Aflatoxin biosynthesis: current frontiers. Annu. Rev. Food Sci. Technol. 4:293–311. 10.1146/annurev-food-083012-123702 [DOI] [PubMed] [Google Scholar]
- 22.Hong S-Y, Roze LV, Wee J, Linz JE. 2013. Evidence that a transcription factor regulatory network coordinates oxidative stress response and secondary metabolism in aspergilli. Microbiologyopen 2:144–160. 10.1002/mbo3.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kafer E. 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19:33–131. 10.1016/S0065-2660(08)60245-X [DOI] [PubMed] [Google Scholar]
- 24.Cotty PJ. 1988. Aflatoxin and sclerotical production by Aspergillus flavus: influence of pH. Phytopathology 78:1250–1253. 10.1094/Phyto-78-1250 [DOI] [Google Scholar]
- 25.Davis ND, Diener UL, Eldridge DW. 1966. Production of aflatoxins B1 and G1 by Aspergillus flavus in a semisynthetic medium. Appl. Microbiol. 14:378–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clevström G, Ljunggren H, Tegelström S, Tideman K. 1983. Production of aflatoxin by an Aspergillus flavus isolate cultured under a limited oxygen supply. Appl. Environ. Microbiol. 46:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgiou CD, Sideri M. 2000. Colorimetric method for determining hydrogen peroxide production in liquid media by filamentous fungi. Mycologia 92:835–840. 10.2307/3761578 [DOI] [Google Scholar]
- 28.Georgiou CD, Grintzalis K, Zervoudakis G, Papapostolou I. 2008. Mechanism of Coomassie brilliant blue G-250 binding to proteins: a hydrophobic assay for nanogram quantities of proteins. Anal. Bioanal. Chem. 391:391–403. 10.1007/s00216-008-1996-x [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B, Gutteridge CMJ. 1999. Free radicals in biology and medicine, 3rd ed. Oxford University Press, Oxford, England [Google Scholar]
- 30.Grintzalis K, Zisimopoulos D, Grune T, Weber D, Georgiou CD. 2013. Method for the simultaneous determination of free/protein malondialdehyde and lipid/protein hydroperoxides. Free Radic. Biol. Med. 59:27–35. 10.1016/j.freeradbiomed.2012.09.038 [DOI] [PubMed] [Google Scholar]
- 31.Patsoukis N, Georgiou CD. 2004. Determination of the thiol redox state of organisms: new oxidative stress indicators. Anal. Bioanal. Chem. 378:1783–1792. 10.1007/s00216-004-2525-1 [DOI] [PubMed] [Google Scholar]
- 32.Patsoukis N, Georgiou CD. 2005. Fluorometric determination of thiol redox state. Anal. Bioanal. Chem. 383:923–929. 10.1007/s00216-005-0095-5 [DOI] [PubMed] [Google Scholar]
- 33.Hissin PJ, Hilf R. 1976. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 74:214–226. 10.1016/0003-2697(76)90326-2 [DOI] [PubMed] [Google Scholar]
- 34.Ogwu V, Cohen G. 1998. A simple colorimetric method for the simultaneous determination of N-acetylcysteine and cysteine. Free Radic. Biol. Med. 25:362–364. 10.1016/S0891-5849(98)00024-0 [DOI] [PubMed] [Google Scholar]
- 35.Gaitonde KM. 1967. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 104:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papapostolou I, Georgiou CD. 2010. Superoxide radical induces sclerotial differentiation in filamentous phytopathogenic fungi: a superoxide dismutase mimetics study. Microbiology 156:960–966. 10.1099/mic.0.034579-0 [DOI] [PubMed] [Google Scholar]
- 37.Munkres DK, Rana SR, Goldstein E. 1984. Genetically determined conidial longevity is positively correlated with superoxide dismutase, catalase, glutathione peroxidase, cytochrome c peroxidase, and ascorbate free radical reductase activities in Neurospora crassa. Mech. Ageing Dev. 24:83–100. 10.1016/0047-6374(84)90177-5 [DOI] [PubMed] [Google Scholar]
- 38.Vernardis SI, Goudar CT, Klapa MI. 2013. Metabolic profiling reveals that time related physiological changes in mammalian cell perfusion cultures are bioreactor scale independent. Metab. Eng. 19:1–9. 10.1016/j.ymben.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 39.Chanda A, Roze LV, Kang S, Artymovich KA, Hicks GR, Raikhel NV, Calvo AM, Linz JE. 2009. A key role for vesicles in fungal secondary metabolism. Proc. Natl. Acad. Sci. U. S. A. 106:19533–19538. 10.1073/pnas.0907416106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacKenzie A, Martin W. 1998. Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: restoration by superoxide dismutase mimetics. Br. J. Pharmacol. 124:719–728. 10.1038/sj.bjp.0701899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholls P. 1962. The reaction between aminotriazole and catalase. Biochim. Biophys. Acta 59:414–420. 10.1016/0006-3002(62)90191-9 [DOI] [PubMed] [Google Scholar]
- 42.Darr D, Fridovich I. 1986. Irreversible inactivation of catalase by 3-amino-1,2,4-triazole. Biochem. Pharmacol. 35:3642. 10.1016/0006-2952(86)90639-8 [DOI] [PubMed] [Google Scholar]
- 43.Lomonosova EE, Kirsch M, de Groot H. 1998. Calcium versus iron-mediated processes in hydrogen peroxide toxicity to L929 cells: effects of glucose. Free Rad. Biol. Med. 25:493–503. 10.1016/S0891-5849(98)00080-X [DOI] [PubMed] [Google Scholar]
- 44.Liochev IS, Fridovich I. 1997. How does superoxide dismutase protect against tumor necrosis factor: a hypothesis informed by effect of superoxide on “free” iron. Free Radic. Biol. Med. 23:668–671. 10.1016/S0891-5849(97)00060-9 [DOI] [PubMed] [Google Scholar]
- 45.Cadenas E. 1989. Biochemistry of oxygen toxicity. Annu. Rev. Biochem. 58:79–110. 10.1146/annurev.bi.58.070189.000455 [DOI] [PubMed] [Google Scholar]
- 46.Sideri M, Georgiou CD. 2000. Differentiation and hydrogen peroxide production in Sclerotium rolfsii are induced by the oxidizing growth factors, light and iron. Mycologia 92:1033–1042. 10.2307/3761468 [DOI] [Google Scholar]
- 47.Papapostolou I, Georgiou CD. 2010. Hydrogen peroxide is involved in the sclerotial differentiation of filamentous phytopathogenic fungi. J. Appl. Microbiol. 109:1929–1936. 10.1111/j.1365-2672.2010.04822.x [DOI] [PubMed] [Google Scholar]
- 48.Papapostolou I, Sideri M, Georgiou CD. 2013. Cell proliferating and differentiating role of H2O2 in Sclerotium rolfsii and Sclerotinia sclerotiorum. Microbiol. Res. 169:527–532. 10.1016/j.micres.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 49.Hamid A. 1989. Effects of carbon tetrachloride (CCl4) on aflatoxin biosynthesis by Aspergillus flavus. MARDI Res. J. 17:29–35 [Google Scholar]
- 50.Chiou CH, Lee LW, Owens SA, Whallon JH, Klomparens KL, Townsend CA, Linz JE. 2004. Distribution and subcellular localization of the aflatoxin enzyme versicolorin B synthase in time-fractionated colonies of Aspergillus parasiticus. Arch. Microbiol. 182:67–79. 10.1007/s00203-004-0700-6 [DOI] [PubMed] [Google Scholar]
- 51.Georgiou CD. 1997. Lipid peroxidation in Sclerotium rolfsii: a new look into the mechanism of sclerotial biogenesis in fungi. Mycol. Res. 101:460–464. 10.1017/S0953756296002882 [DOI] [Google Scholar]
- 52.Reverberi M, Fabbri AA, Zjalic S, Ricelli A, Punelli F, Fanelli C. 2005. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 69:207–215. 10.1007/s00253-005-1979-1 [DOI] [PubMed] [Google Scholar]
- 53.Patsoukis N, Georgiou CD. 2007. Effect of glutathione biosynthesis-related modulators on the thiol redox state enzymes and on sclerotial differentiation of filamentous phytopathogenic fungi. Mycopathologia 163:335–347. 10.1007/s11046-007-9008-9 [DOI] [PubMed] [Google Scholar]
- 54.Patsoukis N, Georgiou CD. 2007. Effect of thiol redox state modulators on oxidative stress and sclerotial differentiation of the phytopathogenic fungus Rhizoctonia solani. Arch. Microbiol. 188:225–233. 10.1007/s00203-007-0237-6 [DOI] [PubMed] [Google Scholar]
- 55.Patsoukis N, Georgiou CD. 2008. The role of thiols on sclerotial differentiation of filamentous phytopathogenic fungi. Open Mycol. J. 2:1–8. 10.2174/1874437000802010001 [DOI] [Google Scholar]
- 56.Kasatkina ID, Zeltova ET. 1963. Cystine reductase activity in Aspergillus niger. Mikrobiologiia 32:973–980 [PubMed] [Google Scholar]
- 57.Linz JE, Chanda A, Hong SY, Whitten DA, Wilkerson C, Roze LV. 2012. Proteomic and biochemical evidence support a role for transport vesicles and endosomes in stress response and secondary metabolism in Aspergillus parasiticus. J. Proteome Res. 11:765–775. 10.1021/pr2006389 [DOI] [PMC free article] [PubMed] [Google Scholar]