Abstract
Compounds of natural origin are increasingly used as adjuncts to oral hygiene. We have adopted four distinct approaches to assess the antibacterial activity of dentifrices containing natural active ingredients against oral bacteria in several test systems. Corsodyl Daily (CD), Kingfisher Mint (KM), and Parodontax fluoride (PF) were compared to a dentifrice containing fluoride (Colgate Cavity Protection [CCP]) and one containing triclosan (Colgate Total [CT]). The growth inhibitory and bactericidal potency of the formulations were determined for 10 isolated oral bacteria. Effects of single exposures of simulated supragingival plaques were then determined by epifluorescence and confocal microscopy, while the effects of repeated exposures were quantified by viable counting. Additionally, dense plaques, maintained in continuous culture, were repeatedly dosed, and the outcome was assessed by viable counting and eubacterial DNA profiling. The test dentifrices exhibited variable specificity and potency against oral bacteria in axenic culture. Of the herbal formulations, KM caused the largest viability reductions in simulated supragingival plaques, with CT causing the greatest reductions overall. Following single exposures, CD caused moderate reductions, while PF had no effect. After multiple dosing, all formulations significantly reduced numbers of total, facultative, and Gram-negative anaerobes, but only KM and CT caused greater reductions than the fluoride control. KM also reduced counts of streptococci (rank order of effectiveness: CT > KM > CCP > PF > CD). Marked changes in eubacterial DNA profiles were not detected for any herbal formulation in dense plaques, although KM markedly reduced viable counts of streptococci, in agreement with supragingival data. While both nonherbal comparators displayed antibacterial activity, the triclosan-containing formulation caused greater viability reductions than the herbal and nonherbal formulations.
INTRODUCTION
Tooth brushing is an important oral hygiene measure which reduces the accumulation of oral biofilms that may otherwise lead to the development of dental caries, gingivitis, and periodontal disease. The continued global prevalence of such oral diseases indicates that optimal oral hygiene is often not maintained, which suggests that chemotherapeutic adjuncts to routine brushing may be of benefit (1). Thus, a variety of oral hygiene formulations have been developed which contain antimicrobial agents, including chlorhexidine (2), cetylpyridinium chloride, (3), metal salts (4), and triclosan (5). The addition of antimicrobial agents to dentifrice and mouthwash formulations has proven efficacious; for example, the use of triclosan in toothpastes has been shown to reduce bacterial viability in vivo and to reduce gingival and plaque index scores (6, 7). Extracts derived from herbal or botanical origins are attracting renewed interest as potential adjuncts in toothpastes. If such ingredients, alone or in combination, exhibit antiplaque activity or other beneficial activities, they may be of use as alternative or adjunctive active ingredients in oral hygiene regimes. There is, however, a lack of reliable data on the antibacterial efficacy of formulations containing such adjuncts in comparison to more widely used products (8).
Examples of antibacterial agents derived from natural sources that have been formulated into oral hygiene products include chamomile, echinacea, sage, myrrh, rhatany, and peppermint oil. Sage oil has previously been shown to have antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Candida albicans (9). Myrrh extract is a natural antimicrobial, and the extract of Mentha piperita is anti-inflammatory and antimicrobial (10). Chamomile extract has also been shown to possess anti-inflammatory properties (11). Echinacea is claimed to stimulate the immune response and to activate leukocytes (12). Reports attribute analgesic, anti-inflammatory, and antimicrobial properties to peppermint oil (13). Lime and fennel extracts have also been formulated into dentifrices. Lime is a source of flavonoids, which are known to have diverse pharmacodynamic abilities and have been reported to exhibit antimicrobial activity against a wide range of microorganisms (14). These antimicrobial effects are a result of the ability of flavonoids to form complexes with proteins, as well as bacterial cell membranes (14, 15). Fennel contains the essential oils transanethole, fenchone, and estragole. It also contains phenolic compounds, including flavonoids phenolic acids, hydroxycinnamic acids, coumarins, and tannins (16). These essential oils have previously been shown to be potent antifungals, including activity against C. albicans (17) and antibacterial activity against Enterococcus faecalis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica serovar Typhi, Salmonella enterica serovar Typhimurium, and Shigella flexneri. These studies have demonstrated various degrees of antibacterial activity of such oils in isolation, although this has not necessarily led to many follow-up studies demonstrating efficacy of dental product formulations.
The aim of the current study was to evaluate the in vitro antimicrobial efficacies of three herbal toothpaste formulations containing natural extracts against oral bacteria in pure culture, as well as in previously validated microcosm systems (18), using mixed oral bacteria derived from human saliva as inocula, which simulate the species complexity found in dental plaques. In order to benchmark the activities of the herbal pastes against commonly used nonherbal formulations, two dentifrices that have been extensively evaluated in previous preclinical and clinical studies were included as comparators. These comprised a fluoride-containing product (19, 20) with no additional antibacterial active ingredients and a product containing 0.3% triclosan and fluoride (21, 22).
MATERIALS AND METHODS
Test dentifrices.
Three formulations, containing different combinations of natural active ingredients (see Table 1), were evaluated. These were Parodontax fluoride (PF), Corsodyl Daily (CD; GlaxoSmithKline), and Kingfisher Mint (KM; Kingfisher Natural Toothpastes). A fluoride-containing product, Colgate Cavity Protection (CCP), and a product containing 0.3% triclosan and 2% copolymer, Colgate Total (CT) (Colgate-Palmolive Company), were included as comparators. All dentifrices were prepared as slurries (10%, wt/vol) in sterile distilled water.
TABLE 1.
Constituents of test and control dentifrices, as listed on respective product labels
| Product and company | Ingredients |
|---|---|
| Colgate Cavity Protection (CCP; negative control), Colgate-Palmolive Company | Dicalcium phosphate dehydrate, water, glycerin, sodium lauryl sulfate, cellulose gum, flavor, tetrasodiumpyrosphate, sodium saccharin, sodium monofluorophosphate |
| Colgate Total (CT; positive control), Colgate-Palmolive Company | Water, hydrated silica, glycerin, sorbitol, PVM/MA copolymer, sodium lauryl sulfate, cellulose gum, flavor, sodium hydroxide, propylene glycol, carrageenan, sodium saccharin, titanium dioxide |
| Parodontax fluoride (PF), GlaxoSmithKline | Sodium bicarbonate, water, glycerin, cocamidopropylbetaine, alcohol, Krameria triandra extract, Echinacea purpura juice, xanthan gum, Chamomilla recutita extract, Commiphora myrrha extract, sodium fluoride, sodium saccharine, sodium benzoate, Salvia officinalis oil, Mentha piperita oil, Mentha arvensis oil, limonene, iron oxide |
| Corsodyl Daily gum and toothpaste (CD), GlaxoSmithKline | Sodium bicarbonate, water, glycerin, cocamidopropylbetaine, alcohol, Krameria triandra extract, Echinacea purpura juice, alcohol denat., xanthan gum, Chamomilla recutita extract, Commiphora myrrha extract, sodium fluoride, sodium saccharine, sodium benzoate, Salvia officinalis oil, Mentha piperita oil, Mentha arvensis oil, limonene, iron oxide |
| Kingfisher Mint, natural with fluoride (KM), Kingfisher Natural Toothpaste | Calcium carbonate, glycerin, water, sodium lauryl sulfate, hydrated silica, cellulose gum, sodium monofluorophosphate, Mentha piperita oil, Citrus limonum, Foeniculum vulgare, limonene |
MICs.
MICs and minimum bactericidal concentrations (MBCs) of test formulations were carried out against selected oral bacteria (Actinomyces naeslundii, Fusobacterium nucleatum, Lactobacillus rhamnosus, Neisseria subflava, Porphyromonas gingivalis, Prevotella oralis, Streptococcus mutans, Streptococcus oralis, Streptococcus sanguis, and Veillonella dispar) as previously described (23). Testing was performed in 96-well microtiter plates (Becton Dickinson, New Jersey, USA). Overnight cultures were diluted 1:100 and delivered to each test well (100 μl). Stock solutions of test dentifrices (100 μl) were added to the first column of test organism and mixed. Two-fold serial dilutions were then carried out across the plate using a multichannel pipette, changing the tips at each dilution step. Although the addition of dentifrices increased turbidity at high concentrations, this effect became negligible at MIC values.
The plates were then incubated for 48 h in either an anaerobic or standard incubator at 37°C. Growth was detected as turbidity (495 nm), relative to that of an uninoculated well, using a microtiter plate reader (Powerwave XS; BioTek, Bedfordshire, United Kingdom). MICs were expressed as the lowest concentration of dentifrice at which growth did not occur, i.e., that which inhibited continued growth in the presence of the dentifrice. Each MIC determination was carried out in quadruplicate. Negative (sterile broth) and positive (overnight culture with no added dentifrice) controls were also included.
MBCs.
MBCs were determined using the microtiter plates that had been set up for the MIC determinations. Aliquots (10 μl) taken from each well up to and including the MIC endpoint were transferred and spot-plated onto the appropriate agar. MBCs were expressed as the lowest concentration of dentifrice at which growth was not observed after 5 days of incubation, i.e., that which inactivated all bacteria in the original inoculum.
Viable staining of dosed plaques.
Glass slides were partially submerged in artificial saliva medium (40 ml) (24, 25) supplemented with cysteine (26). The broth was inoculated with saliva from healthy volunteers, mixed briefly, and incubated statically at 37°C in an anaerobic cabinet for 48 h. Slides were then immersed in 1% slurries of test dentifrices (50 ml) for 60 s and gently agitated. Dosed plaques were then removed into fresh medium and gently agitated as described above to remove excess dentifrice and loosely attached organisms. Excess liquid was drained from the slides without allowing the biofilms to dry. For the negative control, slides were placed into fresh medium and gently agitated as described above for 5 min. Following exposure, plaques were imaged immediately or replaced into cell-free culture supernatant and incubated for a further 12 h before imaging. For viable staining, a working solution of BacLight LIVE/DEAD stain (Invitrogen Ltd., Paisley, United Kingdom) was prepared according to the manufacturer's instructions (20 μl) and applied directly to the biofilm and covered with a glass coverslip. Slides were incubated at room temperature in the dark for 15 min, according to standard BacLight staining protocols. Biofilms were visualized with an Axioskop 2 fluorescence microscope (Carl Zeiss Ltd., Rugby, United Kingdom) using a 10× objective lens, and images were captured using an AxioCam CM1 camera controlled by a PC running AxioVision 4.8 (Carl Zeiss Ltd., Rugby, United Kingdom) and exported as JPEG files. Bacterial cells incubated in the presence of both stains fluoresce either green (viable) or red (dead). ImageJ (NIH) was used to quantify the percentage of viable biomass by calculating the proportion of red fluorescence as a percentage of total fluorescence. Means were calculated from two biological repeats, each including 5 technical repeats. Statistical significance between treatments was determined using the unpaired Student t test.
Viability profiling of dosed plaques.
Optical sectioning of viability distributions in plaque was carried out using techniques originally described by Netuschil et al. (27) and developed extensively by Hope and coworkers (28). In the current study, plaques were cultivated and exposed as described above using glass discs as substrata. Discs were then immersed in a working solution of BacLight LIVE/DEAD stain and incubated at room temperature in the dark for 15 min before being transferred to a shallow dish and covered in distilled water. Biofilms were visualized with an SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) with a water immersion objective lens (×10 magnification), ensuring the entire biofilm depth was captured. Imaris (Bitplane, Zurich, Switzerland) was used to process images.
Hydroxyapatite biofilm reactors.
Simulated supragingival plaques were maintained in a hydroxyapatite disc model (HDM) as previously described (18). Fresh saliva (0.5 ml) from a healthy volunteer (male, age 32) and sterile artificial saliva (0.5 ml) were dispensed into a sterile 24-well tissue culture plate. Sterile discs were aseptically placed in the wells, and inoculated plates were incubated in a Mark 3 anaerobic work station (Don Whitley Scientific, Shipley, United Kingdom) at 37°C (gas mix: 80% N2, 10% CO2, and 10% H2) for plaque formation. Biofilms were allowed to form for 24 h prior to the following dosing regimen. Dentifrice slurries (10%, wt/vol) or sterile water (control discs) was delivered to each appropriate well of the HDM daily for 5 min. Discs were either immediately processed for sampling as described below or transferred to a fresh plate containing artificial saliva (0.5 ml) and fresh salivary inoculum (0.5 ml). This dosing regimen continued for 5 days. Three biological replicates were included, each analyzed in triplicate for viable organisms (as described below). For sampling, discs were aseptically removed, gently immersed in sterile phosphate-buffered saline (PBS) to remove excess dentifrice and loosely attached organisms, added to prereduced, half-strength thioglycolate medium, and vortexed thoroughly for 1 min. Samples were serially diluted, and appropriate dilutions (0.1 ml) were plated in triplicate onto a variety of selective and nonselective media. These media were as follows: Wilkins-Chalgren agar (total anaerobes), Wilkins-Chalgren agar with Gram-negative supplement (total Gram-negative anaerobes), Trypticase yeast extract, cysteine, sucrose agar (Streptococcus spp.), and nutrient agar (total aerobes and facultative anaerobes). Inoculated agars were incubated in an anaerobic chamber at 37°C for up to 5 days, except for nutrient agar, which was incubated aerobically for 3 days.
Multiple Sorbarod devices.
Dense plaques were maintained as previously described (29). The model comprised a perfused, inline, cellulose filter-based fermentation system enabling replicate (n = 5) plaques to be established and sampled. Filters were conditioned in situ with artificial saliva medium continuously supplied by a peristaltic pump (Minipulse 3; Gilson, Villiers-Le-Bel, France) for ca. 2 h and similarly fed throughout the study (flow rate, 7.0 ± 0.1 ml/h). Biofilm formation in multiple Sorbarod devices (MSDs) was initiated by depositing saliva from healthy volunteers twice (at 4-h intervals) into the upper equilibration chamber. MSDs were treated twice daily with slurries of each dentifrice, as described above (2 ml). Biofilms and perfusates (PA) (representing cells released in the eluted medium) were sampled daily (for 5 days) to enumerate viable organisms. Perfusate (spent culture fluid) samples (ca. 5 ml) were collected in sterile plastic Universal bottles as described previously (29). Biofilm samples were obtained by aseptically removing the filter and replacing it with a new sterile filter. The removed filter or perfusate sample (1 ml) was added to prereduced, half-strength thioglycolate medium and vortexed thoroughly for 1 min. Samples were diluted, plated, and incubated as described above. Previous validation studies have optimized these procedures (29).
RESULTS
MIC and MBC values for the test dentifrices.
The lowest and highest MIC values (g/liter) for each dentifrice were as follows: CCP, 1.9 and 31.3; CT, <0.2 and 7.8; PF, 0.9 and 13.7; CD, 0.5 and 62.5; KM, 0.2 and 31.3 (Table 2). The lowest and highest MBC values (g/liter) for each dentifrice were as follows: CCP, 1.9 and 31.3; CT, <0.2 and 7.8; PF, 0.9 and 15.6; CD, 0.5 and 125; KM, 1.9 and 31.3.
TABLE 2.
Susceptibilities of selected oral bacteria to test dentifrices
| Bacterium | Value (SD) (g/liter)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCP |
CT |
PF |
CD |
KM |
||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Actinomyces naeslundii | 15.6 | 15.6 | 3.9 | 7.8 | 7.8 | 15.6 | 7.8 | 7.8 | 3.9 | 3.9 |
| Fusobacterium nucleatum | 1.9 | 1.9 | 0.9 | 7.8 | 0.9 | 7.8 | 0.5 | 0.5 | 3.9 | 3.9 |
| Lactobacillus rhamnosus | 7.8 | 7.8 | 0.5 | 1.9 | 3.9 | 7.8 | 1.5 (0.2) | 2 | 0.9 | 1.9 |
| Neisseria subflava | 15. (1.2) | 15. | <0.2 | <0.2 | 7.8 | 5.8 (0.2) | 15.6 | 2 | 7.8 | 15.6 |
| Porphyromonas gingivalis | 1.9 | 1.9 | 3.9 | 3.9 | 3.9 | 3.9 | 0.5 | 0.5 | 15.6 | 15.6 |
| Prevotella oralis | 3.9 | 3.9 | 0.5 | 0.5 | 3.9 | 3.9 | 7.8 | 7.8 | 3.9 | 3.9 |
| Streptococcus mutans | 3.9 | 7.8 | 3.9 | 3.9 | 13.7 (3.9) | 7.8 | 15.6 | 15.6 | 3.9 | 3.9 |
| Streptococcus oralis | 31.3 | 31.3 | 7.8 | 7.8 | 7.8 (3.1) | 7.8 | 62.5 | 125 | 31.3 | 31.3 |
| Streptococcus sanguis | 7.8 | 11.7 (4.5) | 2.6 (1.0) | 5.2 (1.8) | 0.9 | 0.9 | 11.7 (0.5) | 16 | 3.9 | 3.9 |
| Veillonella dispar | 1.9 | 3.9 | <0.2 | <0.2 | 0.9 | 1.9 | 0.9 | 0.9 | 0.2 | 3.9 |
Data were determined by broth dilution endpoint. Where data varied between replicates (n = 4), standard deviations are given in parentheses.
Effects of single exposures on simulated supragingival plaques.
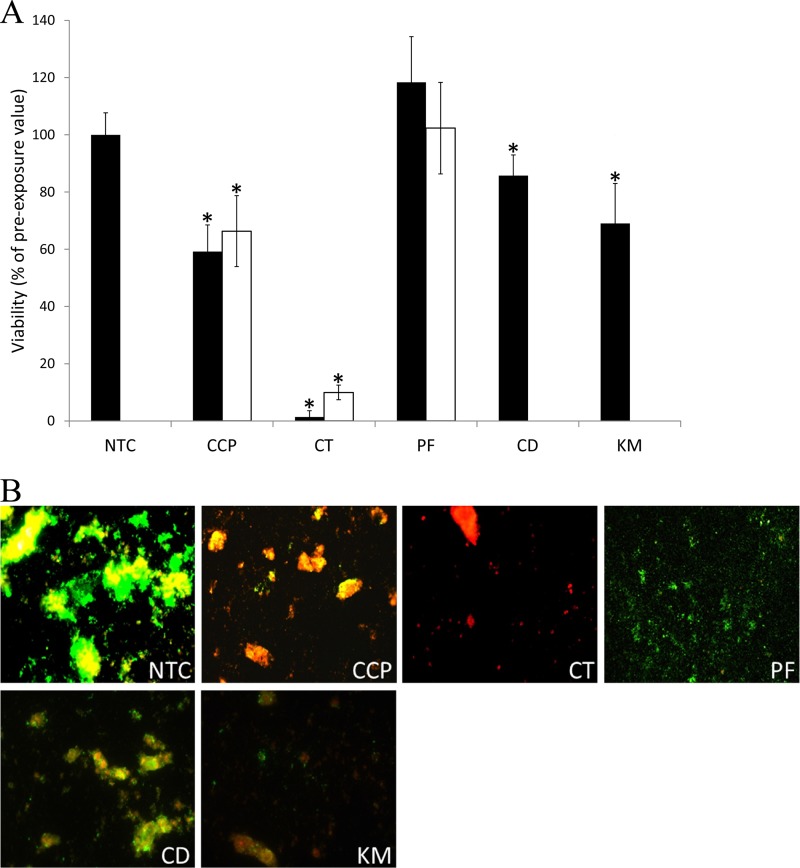
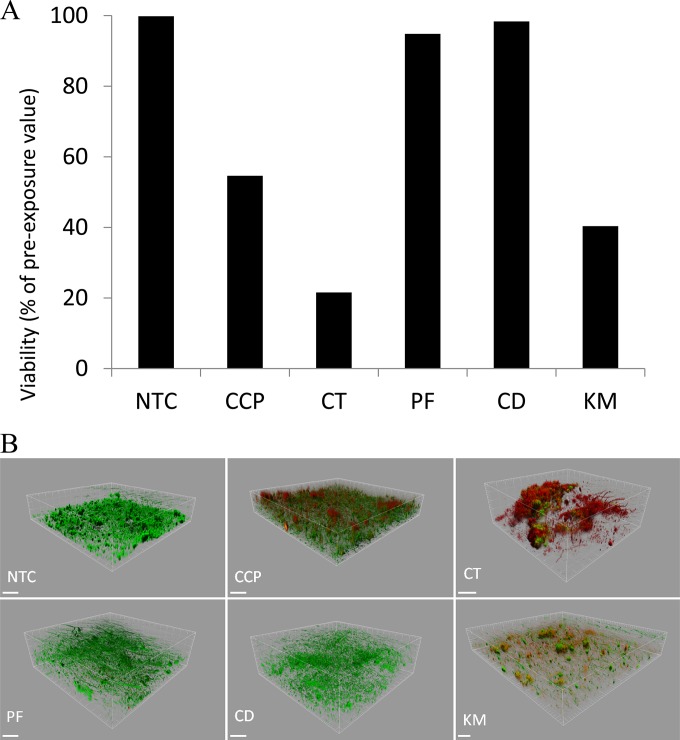
Fig. 1 shows reductions in the viability of plaques following a single dentifrice exposure as determined by viable staining. CD and KM caused significant reductions in plaque viability (P < 0.05) compared to that of the untreated control but not compared to that of the control fluoride toothpaste (CCP). Overall, CT was the most efficacious dentifrice, while PF demonstrated no apparent effect. Viability profiling of treated plaques broadly corroborated these findings (Fig. 2). This technique, which utilizes confocal microscopy to image the biofilm in three dimensions, indicated that, of the herbal formulations tested, KM caused the largest reductions in viability and, overall, CT caused the greatest reduction. To further examine the apparent inability of PF to alter plaque viability, and to identify any possible longer-term effect, plaques treated with PF (as well as the comparators CCP and CT) were reincubated for a further 12 h. Despite modest regrowth in CCP- and CT-treated plaques, viability remained significantly reduced. Conversely, treatment with PF caused no apparent reduction in plaque viability (Fig. 1).
FIG 1.
Viability of plaques following exposure to Parodontax fluoride (PF), Corsodyl Daily (CD), and Kingfisher Mint (KM). Also included are the comparators Colgate Cavity Protection (CCP) and Colgate Total (CT), as well as a no-treatment control (NTC). (A) Data show the proportion of viable biomass in oral biofilms 15 min after (black bars) a single 60-s exposure to test dentifrices. White bars show the proportion of viable biomass in oral biofilms treated with PF and the controls (CCP and CT) 12 h after exposure, as explained in the text. Values are means from two separate experiments, each including 5 technical replicates. Error bars show standard deviations (n = 10). Asterisks indicate significant difference (P < 0.001). (B) Example images show viability maps of treated plaques. Green, viable biomass; red, nonviable biomass; yellow, overlapping green and red signal; black, uncolonized glass.
FIG 2.
Viability of plaques following exposure to Parodontax fluoride (PF), Corsodyl Daily (CD), and Kingfisher Mint (KM). Also included are the comparators Colgate Cavity Protection (CCP) and Colgate Total (CT), as well as a no-treatment control (NTC). (A) Example data show proportion of viable biomass in oral biofilms immediately after a single exposure to test dentifrices. Values are means from three separate experiments, each including 5 technical replicates. Error bars show standard deviations (n = 10). (B) Example images show viability maps of treated plaques. Green, viable biomass; red, nonviable biomass. Scale bars represent 100 μm.
Effects of multiple exposures on simulated supragingival plaques.
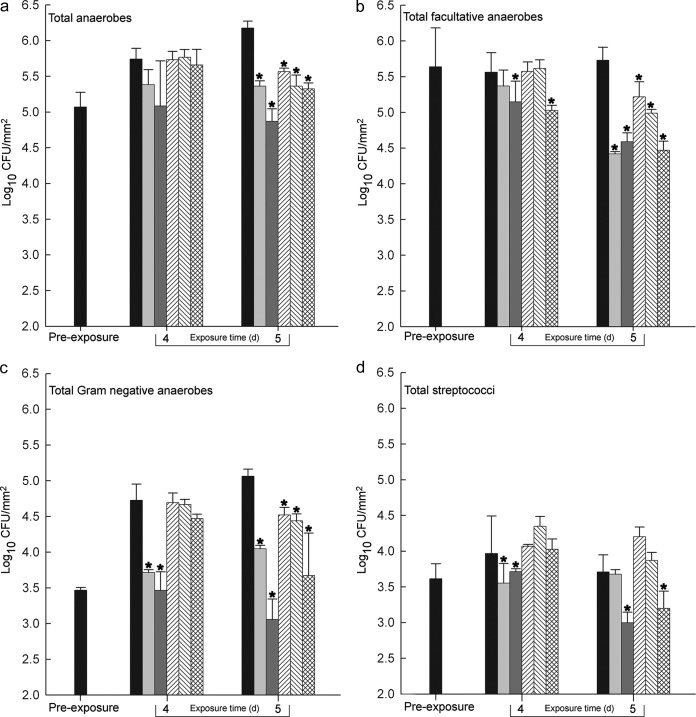
Fig. 3 shows the effects on plaques of daily dosing, as determined by differential culture. Following 4 days of dosing, none of the test (herbal) dentifrices had significantly reduced the numbers of total anaerobes. However, by day 5, all of the dentifrices had caused significant (P < 0.05) reductions in this group. KM significantly reduced the numbers of facultative anaerobes by day 4, with all the dentifrices causing significant reductions by day 5. All of the test dentifrices significantly reduced numbers of Gram-negative anaerobes, but only after 5 days of exposure. KM was the only test dentifrice causing reductions in streptococci. Of the herbal dentifrices, KM caused the most marked viability reductions in all groups of test bacteria and was the only test paste to cause significantly greater reductions than the CCP control (facultative anaerobes at 4 days and streptococci at 5 days). CT caused the greatest overall reductions. Effects against the streptococci and the Gram-negative anaerobes were significant compared to those of all test and control treatments.
FIG 3.
Effects of CCP (light-gray bars), CT (dark-gray bars), PF (left-to-right-diagonal-stripe bars), CD (right-to-left-diagonal-stripe bars), and KM (crosshatched bars) on anaerobic bacteria (a), viable facultative bacteria (b), Gram-negative anaerobes (c), and streptococci (d) in oral microcosms maintained in hydroxyapatite disc biofilm reactors after daily 5-min exposures. Values are means from three separate experiments. Statistical significance (compared to the untreated control; black bars) is indicated by single (P < 0.05) and double (P < 0.01) asterisks.
Effects of multiple exposures on dense plaques.
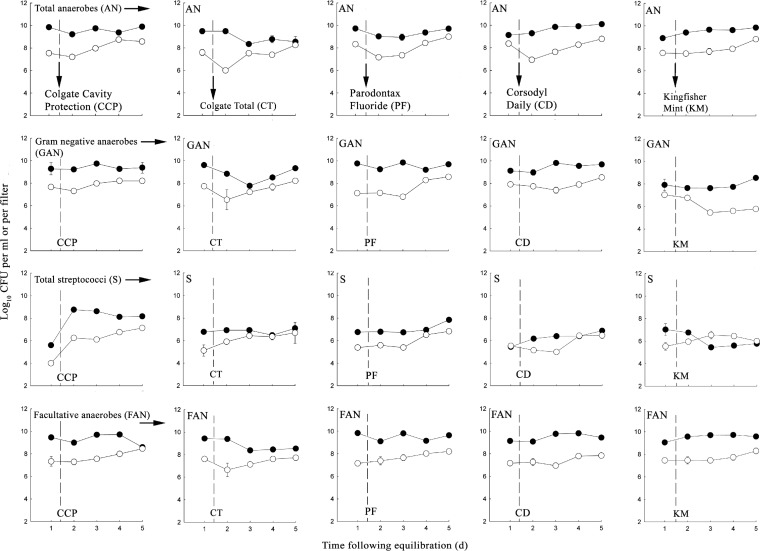
Figure 4 shows the effects of dosing of dense plaques daily with subinhibitory concentrations of dentifrices for 5 days. Data show viable counts from both the plaques and the perfusates. Analysis of the biofilm and planktonic phases in combination gives an indication of sloughing and/or dispersal of biofilm cells into the liquid phase. Overall, numbers of viable cells recovered from biofilms were broadly stable, while viable counts from the perfusates gradually increased following multiple treatments with herbal dentifrices. Two notable exceptions were apparent during treatment with KM. Numbers of Gram-negative anaerobes in the biofilms gradually increased over time, with a concurrent decrease in counts from the perfusates. Conversely, numbers of streptococci recovered from the biofilm decreased, corresponding to an increase in the numbers from the perfusates.
FIG 4.
Effects of CCP, CT, PF, CD, and KM on viable anaerobes, Gram-negative anaerobes, streptococci, and facultative bacteria in oral microcosms maintained in multiple Sorbarod devices and treated for 5 days following a 24-hour equilibration to achieve a dynamic steady state. Closed circles, biofilms; open circles, perfusates. The dotted vertical line indicates the commencement of dentifrice dosing (dentifrice slurry [2 ml] added dropwise twice daily, 8 h apart).
Eubacterial DNA profiling of the biofilm communities was carried out using PCR-denaturing gradient gel electrophoresis (DGGE). Based on principal component analysis, all microcosm samples clustered together with the exception of those exposed to CT at day 5 (CTB5), which clustered separately (Fig. 5); however, individually, none of the five dentifrice formulations caused a significant reduction in bacterial diversity during the study period. Shannon-Wiener index (H′) analysis of resolved PCR-DGGE bands suggested that overall bacterial diversity remained comparable between control biofilm communities (day 1, mean H′ of 3.41, range of 3.30 to 3.71) and those exposed to toothpaste formulations for up to 5 days (mean H′ of 3.46, range of 3.32 to 3.61; P ≥ 0.05).
FIG 5.
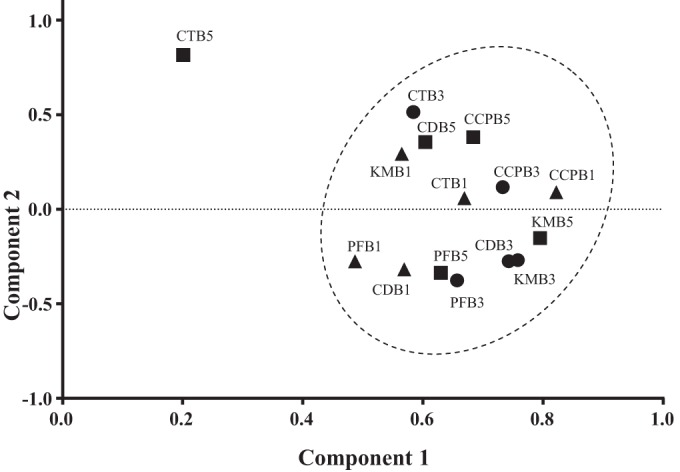
Two-factor principal components analysis of PCR-DGGE banding profiles representative of oral biofilms exposed to toothpaste formulations following 1-day (triangles), 3-day (circles), and 5-day (squares) periods. Formulations included the following: Colgate Cavity Protection (CCP), Colgate Total (CT), Kingfisher Mint (KM), Corsodyl Daily (CD), Parodontax fluoride (PF).
DISCUSSION
In the current investigation, four distinct approaches were used to evaluate the antibacterial efficacy of three herbal dentifrices. A dentifrice containing fluoride and another containing 0.3% triclosan and fluoride were included as nonherbal comparators. MIC and MBC values of formulations against oral bacteria in pure culture were determined by exposing planktonic suspensions to 2-fold serial dilutions of dentifrice slurries. Viable staining, which combines LIVE/DEAD fluorescent staining of plaques with epifluorescence or confocal microscopy, was used to assess immediate lethal effects of single exposures. Differential culture was used to determine effects on the viability of major functional groups of oral bacteria following single or multiple exposures to dentifrices in simulated supragingival plaques developed from human saliva.
When the effects of antibacterials on oral microbial communities are assessed, specific groups of bacteria may be differentially inactivated, and this can alter the bacteriological composition of the plaques, particularly following repeated or long-term exposure. Therefore, in the current study, as well as being dosed singly, plaques were exposed daily for 5 days and monitored for the viability of major functional groups of oral bacteria. Furthermore, the use of a previously validated (15) filter-based continuous culture model facilitated the enumeration of viable cells both within the biofilm and among the cells released from it. This system, which has previously been used to grow plaque microcosms (30), establishes relatively high bacterial population densities and thus affords a considerable degree of recalcitrance to antibacterial treatments. Rather than focusing on gross reductions in viability in this system, the filter-based system allows more subtle changes in species composition to be measured and, where it occurs, to detect viable cells released from extant biofilms, such as would occur if a treatment caused dispersion or sloughing of viable bacterial cells (18). To provide an additional level of microbial characterization, the culture-independent profiling method PCR-DGGE was used. Resulting reproducible fingerprints thus generated (31) are amenable to objective analytical methods to reveal representative patterns of bacterial diversity, including the presence of nonculturable bacteria and loss or expansion of particular bacterial groups within the plaque or perfusate.
The inclusion of two previously well-studied nonherbal comparators allowed the potential antibacterial activities of the herbal pastes to be placed into context with those of well-studied formulations. Data obtained in the current study showed good consistency with previous reports, which also demonstrated specificity of the triclosan-containing formulation against Gram-negative anaerobes and streptococci (18, 32).
A range of dentifrices, including Parodontax, were previously assessed for antimicrobial potency against Candida albicans using a simple well diffusion assay (33). Antifungal effects were reported for certain herbal dentifrices that were approximately equivalent to those containing fluoride, although comparators formulated with antimicrobial active ingredients were not included (33). A similar assessment of 14 herbal formulations reported modest and variable effects against four bacterial species. However, the most effective herbal product in this investigation also contained zinc citrate, and it is not clear how much of the observed effect can be attributed to this active ingredient as opposed to the herbal ingredients (34). Compared to fluoride products and those containing triclosan, six herbal dentifrices were recently found to have only modest inhibitory effects against oral streptococci (35).
The current study highlights the utility of complex plaque models supporting multispecies, surface-associated biofilms derived from fresh human saliva. Confocal microscopy coupled with LIVE/DEAD staining (viability profiling), originally described by Netuschil et al. (27) and further developed by Hope and colleagues (28), is a powerful means of imaging the distribution of viability changes in plaque biofilms. The current investigation revealed such biofilms to be complex, containing three-dimensional structural features interspersed with channels. They therefore represent the complexity of dental plaque more closely than a planktonic monoculture with respect to species composition, physicochemical heterogeneity, and diversity of functional physiology. This distinction explains why data derived from complex plaque models (Fig. 2 and 3) and those from planktonic monoculture (Table 2) do not, at first glance, appear to be consistent. While the latter may yield useful data on a given number of species of interest, they do not necessarily take into account the numerical or physiological contribution of that species in the community as a whole and may therefore give an unrepresentative picture.
We are aware of only one other study which has assessed the effects of a herbal dentifrice (PF) on multispecies plaques (36). This study reported a 37% decrease in the viability of multispecies plaques following a single exposure to PF, as determined by viable staining, although this difference was considered to be statistically nonsignificant. Since in the Dutch study data were compared against a mouth rinse formulation rather than toothpaste, it is difficult to draw comparisons with the current study, in which a single exposure to PF caused no detectable reduction in the viability of simulated supragingival plaque. Viable staining in the current study indicated that KM was the only herbal dentifrice that caused reductions in the viability of plaques that were at least equivalent to those of the negative-control paste.
The current data indicate that all of the test dentifrices exhibit a variable degree of antibacterial activity, which generally increased after multiple doses. PF and CD showed modest antiplaque effects that were less than or equal to those of the fluoride paste. The antiplaque effects of KM were greater than or equal to those of CCP, particularly with respect to streptococci, although KM was less efficacious than CT in all cases. While multiple dosing of simulated supragingival plaques with KM revealed particular activity against the streptococci, exposure of dense plaques revealed that cell removal might be partially responsible for such effects. This observation highlights the fact that lethality is not necessarily needed to reduce bioburdens in sessile cells, but that other factors, for example, inhibition of coaggregation or interactions with extracellular polymeric matrix material, may play a part.
While it may be assumed that the herbal components, rather than the excipients in these dentifrices, exert the reported antibacterial activities, it remains to be determined which herbal components, if any, have significant bactericidal effects over and above the excipients and whether or not specific combinations of active ingredients are associated with potentiation. Sodium lauryl sulfate (SLS) is known to exert antibacterial activity against simulated supragingival plaques (32), and the SLS content of KM might partially explain its efficacy in comparison to an SLS-containing formulation (CCP). However, its particular selectivity against streptococci suggests additional specific activity.
The focus of the current study was antibacterial activity, which represents an important outcome of oral hygiene. Antibacterial potency in oral hygiene formulations may be particularly advantageous when mechanical oral hygiene measures are not fully effective. Herbal dentifrices may display beneficial characteristics that are independent of effects on bacterial viability. Identification of such activities could be achieved by clinical evaluation of patients in controlled trials. However, to date, clinical studies of herbal toothpastes have been inconclusive, showing varied effects of treatment with herbal toothpastes on plaque and gingival scores. The viability of volunteer plaque bacteria reportedly decreased following exposure to PF, although effects were moderate compared to those of the nonherbal, triclosan formulation (37). No significant differences in plaque and gingival inflammation scores were reported in a previous investigation when PF was compared to a fluoride-containing toothpaste in a 21-day clinical trial (38). Compared to treatment with a fluoride toothpaste, treatment with PF reportedly reduced plaque accumulation and gingival inflammation scores among patients with chronic gingivitis (39).
ACKNOWLEDGMENT
This work was funded by Colgate-Palmolive.
Footnotes
Published ahead of print 8 August 2014
REFERENCES
- 1.Morris AJ, Steele J, White DA. 2001. The oral cleanliness and periodontal health of UK adults in 1998. Br. Dent. J. 191:186–192. 10.1038/sj.bdj.4801135 [DOI] [PubMed] [Google Scholar]
- 2.Autio-Gold J. 2008. The role of chlorhexidine in caries prevention. Oper. Dent. 33:710–716. 10.2341/08-3 [DOI] [PubMed] [Google Scholar]
- 3.Jenkins S, Addy M, Newcombe RG. 1994. A comparison of cetylpyridinium chloride, triclosan and chlorhexidine mouthrinse formulations for effects on plaque regrowth. J. Clin. Periodontol. 21:441–444. 10.1111/j.1600-051X.1994.tb00743.x [DOI] [PubMed] [Google Scholar]
- 4.Svatun B, Attramadal A. 1978. The effect of stannous fluoride on human plaque acidogenicity in situ (Stephan curve). Acta Odontol. Scand. 36:211–218. 10.3109/00016357809004670 [DOI] [PubMed] [Google Scholar]
- 5.Kjaerheim V, Skaare A, Barkvoll P, Rolla G. 1996. Antiplaque, antibacterial, and anti-inflammatory properties of triclosan mouthrinses in combination with zinc citrate or polyvinylmethylether maleic acid (PVM-MA) copolymer. Eur. J. Oral Sci. 104:529–534. 10.1111/j.1600-0722.1996.tb00137.x [DOI] [PubMed] [Google Scholar]
- 6.Fine DH, Sreenivasan PK, McKiernan M, Tischio-Bereski D, Furgang D. 2012. Whole mouth antimicrobial effects after oral hygiene: comparison of three dentifrice formulations. J. Clin. Periodontol. 39:1056–1064. 10.1111/j.1600-051X.2012.01938.x [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Chaknis P, DeVizio W, Petrone M, Panagakos FS, Proskin HM. 2010. A clinical investigation of the efficacy of three commercially available dentifrices for controlling established gingivitis and supragingival plaque. J. Clin. Dent. 21:105–110 [PubMed] [Google Scholar]
- 8.Jeon JG, Rosalen PL, Falsetta ML, Koo H. 2011. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 45:243–263. 10.1159/000327250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins MA, Charles HP. 1987. Antimicrobial activity of carnosol and ursolic acid. Two anti-oxidant constituents of Rosmarinus officinalis. Food Microbiol. 4:311–315 [Google Scholar]
- 10.McKay DL, Blumberg JB. 2006. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 20:619–633. 10.1002/ptr.1936 [DOI] [PubMed] [Google Scholar]
- 11.Srivastava JK, Pandey M, Gupta S. 2009. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 85:663–669. 10.1016/j.lfs.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson JB. 2012. Applications of the phytomedicine Echinacea purpurea (purple coneflower) in infectious diseases. J. Biomed. Biotechnol. 2012:769896. 10.1155/2012/769896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhari LK, Jawale BA, Sharma S, Sharma H, Kumar CD, Kulkarni PA. 2012. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J. Contemp. Dent. Pract. 13:71–74 [DOI] [PubMed] [Google Scholar]
- 14.Fowler ZL, Shah K, Panepinto JC, Jacobs A, Koffas MA. 2011. Development of non-natural flavanones as antimicrobial agents. PLoS One 6:e25681. 10.1371/journal.pone.0025681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan MM. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimica-Dukic N, Kujundzic S, Sokovic M, Couladis M. 2003. Essential oil composition and antifungal activity of Foeniculum vulgare Mill obtained by different distillation conditions. Phytother. Res. 17:368–371. 10.1002/ptr.1159 [DOI] [PubMed] [Google Scholar]
- 17.Kaur GJ, Arora DS. 2009. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 9:30. 10.1186/1472-6882-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledder RG, McBain AJ. 2012. An in vitro comparison of dentifrice formulations in three distinct oral microbiotas. Arch. Oral Biol. 57:139–147. 10.1016/j.archoralbio.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 19.Saporito RA, Boneta AR, Feldman CA, Cinotti W, Sintes JL, Stewart B, Volpe AR, Proskin HM. 2000. Comparative anticaries efficacy of sodium fluoride and sodium monofluorophosphate dentifrices. A two-year caries clinical trial on children in New Jersey and Puerto Rico. Am. J. Dent. 13:221–226 [PubMed] [Google Scholar]
- 20.Sullivan RJ, Fletcher R, Bachiman R, Penugonda B, LeGeros RZ. 1995. Intra-oral comparison and evaluation of the ability of fluoride dentifrices to promote the remineralization of caries-like lesions in dentin and enamel. J. Clin. Dent. 6:135–138 [PubMed] [Google Scholar]
- 21.Boneta AE, Aguilar MM, Romeu FL, Stewart B, DeVizio W, Proskin HM. 2010. Comparative investigation of the efficacy of triclosan/copolymer/sodium fluoride and stannous fluoride/sodium hexametaphosphate/zinc lactate dentifrices for the control of established supragingival plaque and gingivitis in a six-month clinical study. J. Clin. Dent. 21:117–123 [PubMed] [Google Scholar]
- 22.Mateu FA, Boneta AE, DeVizio W, Stewart B, Proskin HM. 2008. A clinical investigation of the efficacy of two dentifrices for controlling established supragingival plaque and gingivitis. J. Clin. Dent. 19:85–94 [PubMed] [Google Scholar]
- 23.McBain AJ, Ledder RG, Sreenivasan P, Gilbert P. 2004. Selection for high-level resistance by chronic triclosan exposure is not universal. J. Antimicrob. Chemother. 53:772–777. 10.1093/jac/dkh168 [DOI] [PubMed] [Google Scholar]
- 24.Marsh PD, Hunter JR, Bowden GH, Hamilton IR, McKee AS, Hardie JM, Ellwood DC. 1983. The influence of growth rate and nutrient limitation on the microbial composition and biochemical properties of a mixed culture of oral bacteria grown in a chemostat. J. Gen. Microbiol. 129:755–770 [DOI] [PubMed] [Google Scholar]
- 25.Shah HN, Williams RA, Bowden GH, Hardie JM. 1976. Comparison of the biochemical properties of Bacteroides melaninogenicus from human dental plaque and other sites. J. Appl. Bacteriol. 41:473–495. 10.1111/j.1365-2672.1976.tb00660.x [DOI] [PubMed] [Google Scholar]
- 26.McBain AJ, Bartolo RG, Catrenich CE, Charbonneau D, Ledder RG, Gilbert P. 2003. Effects of triclosan-containing rinse on the dynamics and antimicrobial susceptibility of in vitro plaque ecosystems. Antimicrob. Agents Chemother. 47:3531–3538. 10.1128/AAC.47.11.3531-3538.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netuschil L, Reich E, Unteregger G, Sculean A, Brecx M. 1998. A pilot study of confocal laser scanning microscopy for the assessment of undisturbed dental plaque vitality and topography. Arch. Oral Biol. 43:277–285. 10.1016/S0003-9969(97)00121-0 [DOI] [PubMed] [Google Scholar]
- 28.Hope CK, Clements D, Wilson M. 2002. Determining the spatial distribution of viable and nonviable bacteria in hydrated microcosm dental plaques by viability profiling. J. Appl. Microbiol. 93:448–455. 10.1046/j.1365-2672.2002.01703.x [DOI] [PubMed] [Google Scholar]
- 29.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. 2005. Development and characterization of a simple perfused oral microcosm. J. Appl. Microbiol. 98:624–634. 10.1111/j.1365-2672.2004.02483.x [DOI] [PubMed] [Google Scholar]
- 30.Ledder RG, Gilbert P, Pluen A, Sreenivasan PK, De Vizio W, McBain AJ. 2006. Individual microflora beget unique oral microcosms. J. Appl. Microbiol. 100:1123–1131. 10.1111/j.1365-2672.2006.02847.x [DOI] [PubMed] [Google Scholar]
- 31.Neilson JW, Jordan FL, Maier RM. 2013. Analysis of artifacts suggests DGGE should not be used for quantitative diversity analysis. J. Microbiol. Methods 92:256–263. 10.1016/j.mimet.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledder RG, Sreenivasan PK, DeVizio W, McBain AJ. 2010. Evaluation of the specificity and effectiveness of selected oral hygiene actives in salivary biofilm microcosms. J. Med. Microbiol. 59:1462–1468. 10.1099/jmm.0.024372-0 [DOI] [PubMed] [Google Scholar]
- 33.Ellepola AN, Khan ZU, Chandy R, Philip L. 2011. A comparison of the antifungal activity of herbal toothpastes against other brands of toothpastes on clinical isolates of Candida albicans and Candida dubliniensis. Med. Princ. Pract. 20:112–117. 10.1159/000321199 [DOI] [PubMed] [Google Scholar]
- 34.Lee SS, Zhang W, Li Y. 2004. The antimicrobial potential of 14 natural herbal dentifrices: results of an in vitro diffusion method study. J. Am. Dent. Assoc. 135:1133–1141. 10.14219/jada.archive.2004.0372 [DOI] [PubMed] [Google Scholar]
- 35.Sentila R, Gandhimathi A, Karthika S, Suryalakshmi R, Michael A. 2011. In-vitro evaluation and comparison of the anti-microbial potency of commercially available oral hygiene products against Streptococcus mutans. Indian J. Med. Sci. 65:250–259. 10.4103/0019-5359.107026 [DOI] [PubMed] [Google Scholar]
- 36.Verkaik MJ, Busscher HJ, Jager D, Slomp AM, Abbas F, van der Mei HC. 2011. Efficacy of natural antimicrobials in toothpaste formulations against oral biofilms in vitro. J. Dent. 39:218–224. 10.1016/j.jdent.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 37.Arweiler NB, Auschill TM, Reich E, Netuschil L. 2002. Substantivity of toothpaste slurries and their effect on reestablishment of the dental biofilm. J. Clin. Periodontol. 29:615–621. 10.1034/j.1600-051X.2002.290705.x [DOI] [PubMed] [Google Scholar]
- 38.Pannuti CM, Mattos JP, Ranoya PN, Jesus AM, Lotufo RF, Romito GA. 2003. Clinical effect of a herbal dentifrice on the control of plaque and gingivitis: a double-blind study. Pesqui. Odontol. Bras. 17:314–318. 10.1590/S1517-74912003000400004 [DOI] [PubMed] [Google Scholar]
- 39.Al-Kholani AI. 2011. Comparison between the efficacy of herbal and conventional dentifrices on established gingivitis. Dent. Res. J. 8:57–63 [PMC free article] [PubMed] [Google Scholar]