Abstract
This report describes a viral epidemiological study of wild fish around the Gulf of Cadiz (southwestern Iberian Peninsula) and is focused on infectious pancreatic necrosis virus (IPNV), viral hemorrhagic septicemia virus (VHSV), and viral nervous necrosis virus (VNNV). One fish species (Chelon labrosus) was sampled inside the gulf, at the mouth of the San Pedro River. Another 29 were sampled, in three oceanographic campaigns, at sites around the Bay of Cadiz. The fish were processed individually and subjected to isolation in cell culture and molecular diagnosis. VHSV was not isolated from any species. Thirteen IPNV-type isolates were obtained from barracuda (Sphyraena sphyraena), axillary seabream (Pagellus acarne), common two-banded seabream (Diplodus vulgaris), common pandora (P. erythrinus), Senegal seabream (D. bellottii), and surmullet (Mullus surmuletus). Six VNNV isolates were obtained from axillary seabream, common pandora, black seabream (Spondyliosoma cantharus), red mullet (Mullet barbatus), Lusitanian toadfish (Halobatrachus didactylus), and tub gurnard (Chelidonichtys lucerna). In the river mouth, viruses were detected only after reamplification, obtaining prevalence percentages of IPNV and VNNV (44.4 and 63.0%, respectively) much higher than those observed in the oceanographic campaigns (25.7 and 19.6%, respectively). The opposite results were obtained in the case of VHSV after reamplification: 11.1% in the river mouth and 43.6% in the oceanic locations. Analyzing the results with respect to the proximity of the sampling sites to the coast, an anthropogenic influence on wild fish is suggested and discussed. The type of viruses and the presence of natural reassortants are also discussed.
INTRODUCTION
Microbial pathogens and potential hosts have coexisted in the marine environment for a long time. This coexistence allows the achievement of a balance between the pathogen and its host: The pathogen self-limits its virulence to reduce the negative effect on specific hosts, and the host uses defense strategies to minimize the negative consequence of the infection. The stressful conditions of intensive culture, however, usually break that equilibrium, and consequently, disease episodes probably occur more frequently than in natural environments.
Among pathogens, the relevance of viral fish diseases relies on the high morbidity and mortality rates of infections and on the lack of effective treatments and prophylactic measures. In addition, several factors such as the vertical transmission of some fish viruses and the ability to establish chronic infections or carrier states among survivors must also be considered (1).
The Ninth Report of the International Committee on Taxonomy of Viruses (2) formally recognizes 40 species of fish viruses in 11 taxonomic families. However, only a few studies of wild fish viruses have been performed, in contrast to those done with cultured or ornamental fish. This is probably due to the important losses that viral diseases cause in cultured fish. Moreover, since diseased or moribund animals are rapidly captured and eliminated by predators or necrophages, the establishment of a relationship between the decrease of a wild population and its infection by a specific microbial pathogen is quite complex. In addition, those animals overcoming the disease, mainly in the case of viral infections, become asymptomatic carriers, exhibiting light viral loads and excreting viruses constantly (3). Therefore, research on the viral epidemiology of wild fish populations in the marine environment must be considered of special relevance. However, these kinds of studies are challenging because of the high diversity of fish species and viruses with different host ranges, the size of the ecosystems and fish populations under study, the complexity of the physical and chemical factors involved in the marine environment, and even the influence of climate change (4).
Knowledge of the sanitary status of specific wild fish populations is also important for aquaculture. In fact, several authors have pointed out the possibility of virus transmission between wild and cultured fish through different mechanisms that may facilitate or enhance that potential viral transmission. According to Kurath and Winton (5), the most important of those mechanisms might be the following: (i) the use of larvae, juveniles, or broodstocks from wild sources for rearing; (ii) the use of water supplies harboring infected wild fish; (iii) rearing fish in close proximity to wild virus reservoirs; (iv) the use of nonsterilized fish or fish products as feed for cultured animals; (v) stress or immunosuppressive conditions in cultured fish populations; (vi) continuous high-density culture; (vii) the culture of nonnative fish in areas where a virus is endemic; and (viii) the culture of multiple fish species in close proximity.
In the present study, we carried out a preliminary epidemiological investigation in the Gulf of Cadiz, a strategic area located between two continents, Europe and Africa, that is under the influence of the Atlantic Ocean and the Mediterranean Sea. In addition, this zone is of interest because it represents the main zone of commercial fishing of southern Spain and is the location of many fish farms. A virological survey of wild fish populations in these fishing zones was performed, and they were compared with the fish population near the mouth of the San Pedro River; the aim was to detect viruses in general but focus specially on the infectious pancreatic necrosis virus (IPNV) or IPNV-type viruses (family Birnaviridae), the viral nervous necrosis virus (VNNV; family Nodaviridae), and the viral hemorrhagic septicemia virus (VHSV; family Rhabdoviridae) since these are viruses of relevance for aquaculture worldwide, as well as for the southern Iberian Peninsula and northern Africa.
MATERIALS AND METHODS
Marine fish sampling.
Demersal trawling with research vessels was implemented to sample wild marine fish at 17 locations in the Gulf of Cadiz (near the Bay of Cadiz; Fig. 1A) in three oceanographic campaigns carried out by the Spanish Oceanographic Institute between 2010 (campaign I, in November) and 2011 (campaigns II, in March, and III, between October and December). In the three oceanographic campaigns, a total of 179 fish (28, 49 and 102, in campaigns I, II, and III, respectively) belonging to 29 species (the most representative in this zone; Table 1) were sampled. Most of the fish belonged to the family Sparidae or to other members of the order Perciformes and were captured at depths between 20 and 40 m, corresponding to water temperatures of around 18°C (except the Atlantic mackerel, Scomber scombrus, which was captured at more than 100 m, at 15°C). In most cases, non-Perciformes fish were captured in deeper waters, at 91 m (15°C) in the case of twaite shad (Alosa fallax) and tub gurnard (Chelidonichthys lucerna), or even at more than 660 m (13°C) in the case of blackbelly rosefish (Helicolenus dactylopterus), hollowsnout grenadier (Caelorinchus caelorinchus), rabbit fish (Chimaera monstrosa), blackmouth catshark (Galeus melastomus), and velvet belly (Etmopterus spinax). In addition, to monitor wild marine fish in areas with a putative influence on fish farms, 27 specimens of thicklip gray mullet (C. labrosus) were caught and sampled in October 2010 and May 2011 at two sites (close to each other) near the mouth of the San Pedro River (Fig. 1B). The San Pedro River is the main water source for several fish farms located in this zone, and it receives all of the wastewater from these aquaculture facilities.
FIG 1.
Sampling locations in the Gulf of Cadiz. (A) Oceanographic campaigns were performed during November 2010 (campaign I, at locations I.1 [36.4250N, 6.3510W], I.2 [36.4750N, 6.3990W], I.3 [36.5640N, 6.5230W], I.4 [36.4310N, 6.4400W], I.5 [36.4270N, 6.4760W], I.6 [36.3800N, 6.4710W], I.7 [36.5600N, 6.4160W], I.8 [36.1960N, 6.0400W], and I.9 [36.1820N, 6.2000W]), March 2011 (campaign II, at locations II.1 [36.2320N, 6.2380W], II.2 [36.2020N, 6.5090W], II.3 [36.4750N, 6.4030W], and II.4 [36.2760N, 6.2850W]), and October-November 2011 (campaign III, at locations III.1 [36.2260N, 6.3280W], III.2 [36.1820N, 6.2000W], III.3 [36.5780N, 6.4600W], and III.4 [36.4250N, 6.3510W]). (B) Samplings at the mouth of the San Pedro River were carried out during October 2010 and May 2011. Template map source, Google Earth.
TABLE 1.
Fish species sampled in three oceanographic campaigns
| Order, family, and species | Common name |
|---|---|
| Perciformes | |
| Sparidae | |
| Boops boops | Bogue |
| Pagellus bellottii | Red pandora |
| Pagellus erythrinus | Common pandora |
| Pagellus acarne | Axillary seabream |
| Diplodus vulgaris | Common two-banded seabream |
| Diplodus bellotii | Senegal seabream |
| Sparus aurata | Gilthead seabream |
| Lithognathus mormyrus | Sand steenbras |
| Spondyliosoma cantharus | Black seabream |
| Mullidae | |
| Mullus barbatus | Red mullet |
| Mullus surmuletus | Surmullet |
| Carangidae, Trachurus mediterraneus | Mediterranean horse mackerel |
| Haemulidae | |
| Pomadasys incisus | Bastard grunt |
| Plectorhinchus mediterraneus | Rubberlip grunt |
| Scianidae, Arginosomus regius | Meagre |
| Scombridae, Scomber scombrus | Atlantic mackerel |
| Sphiraenidae, Sphyraena sphyraena | Barracuda |
| Gadiformes, Gadidae, Micromesistius poutasso | Blue whiting |
| Gadiformes, Macrouridae, Caelorinchus caelorinchus | Hollowsnout grenadier |
| Clupeidormes, Engraulidae, Engraulis encrasicolus | Anchovy |
| Clupeiformes, Clupeidae, Alosa fallax | Twaite shad |
| Mugiliformes, Mugilidae, Liza ramada | Thinlip Gray mullet |
| Batrachoidiformes, Batrachoididae, Halobatrachus didactylus | Lusitanian toadfish |
| Pleuronectiformes, Soleidae, Microchirus azevia | Sole |
| Scorpaeniformes | |
| Triglidae, Chelidonichthys lucerna | Tub gurnard |
| Sebastidae, Helicolenus dactylopterus | Blackbelly rosefish |
| Chimaeriformes, Chimaeridae Chimaera monstrosa | Rabbit fish |
| Carcharhiniformes, Scyliorhinidae, Galeus melastomus | Blackmouth catshark |
| Squaliformes, Etmopteridae, Etmopterus spinax | Velvet belly |
Tissue sampling.
All of the specimens were first examined for gross clinical signs and then aseptically dissected for virological analysis. From each fish, two pools of tissues were prepared, (i) eye and brain and (ii) spleen, head kidney, and heart, and each pool was split into two aliquots to be processed in duplicate in both of the laboratories involved in this study (Instituto de Acuicultura, University of Santiago de Compostela [IA-USC] and Facultad de Ciencias, University of Malaga [UMA]), employing their routine techniques of diagnosis described below. The tissues were resuspended 1/10 (wt/vol) in Leibovitz (L-15; Gibco) culture medium supplemented with 2% fetal bovine serum (FBS), 2% l-glutamine (Sigma), penicillin (1,000 IU/ml), and streptomycin (1,000 μg/ml) (Gibco). The mixture was homogenized with an MH400 homogenizer (Tetsch), and the homogenates were snap-frozen at −80°C.
Virus isolation.
The processed tissues were individually inoculated into cell cultures. For this purpose, monolayers of the BF-2 (bluegill fry) and E-11 (a clone of the striped snakehead SSN-1 cell line) cell lines were grown at 25°C in L-15 medium supplemented with 10 and 5% FBS (respectively), 2 mM glutamine, and a penicillin-streptomycin mixture (100 IU/ml and 100 μg/ml, respectively). When the cell monolayers reached 90% confluence, the medium was replaced with L-15 with 2% FBS. The BF-2 cell line, incubated at 15°C, was used for isolation of IPNV and VHSV, and E-11, incubated at 20°C, was employed for VNNV isolation. Inoculated cells were examined daily for the development of cytopathic effects (CPE) for up to 20 days postinoculation. Samples not causing CPE after a second passage were considered negative for the presence of infective viral particles.
Viral detection by amplification methods.
The EZNA Total RNA kit (Omega Bio-Tek, Inc.) was used by both laboratories for the extraction of total RNA from each individual fish homogenate (200 μl) in accordance with the manufacturer's instructions. The RNA concentration was determined at 260 nm with a NanoDrop ND-1000 (NanoDrop Instruments, Thermo Scientific), and the extracted RNA was stored at −80°C until use.
Two methods of IPNV amplification were used, one used by the IA-USC laboratory as reported by Heppell et al. (6), based on primers designed according to the sequence of the Jasper strain (genotype I), and a second one by the UMA laboratory as reported by López-Jimena et al. (7), based on the sequence of an Sp strain (genotype III). Primers Heppell F and Heppell R amplify a 359-bp fragment of the VP2-NS intergenic region of the polyprotein (Table 2). For seminested reamplification, an internal primer—Heppell F intro—was employed, resulting in the expected fragment of 323 bp. Primers designed by López-Jimena et al. (7) (Soleseg A F2 and Soleseg A R2) amplify a 599-bp fragment within the pVP2 region inside the gene encoding the polyprotein. A set of internal primers (R23 and R24) was designed for the reamplification by nested PCR of a 191-bp fragment. In addition, a dot blot hybridization (DBH) protocol was used in combination with the viral genome amplification procedure described by López-Jimena et al. (7).
TABLE 2.
Primers used in this study
| Virus, technique, and primer | Sequence | Gene product | Segment | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| IPNV | |||||
| RT-PCR | |||||
| SolesegA-F2 | 5′-GTGCTGGCCACAAACGACAAC-3′ | VP2 | A | 599 | 7 |
| SolesegA-R2 | 5′-ATTTGGTCTGCCGTTCCTA-3′ | VP2 | A | ||
| Seminested RT-PCR | |||||
| R23 | 5′-TCAACCAGCAGACAGCAATCGACAATG-3′ | VP2 | A | 191 | 7 |
| R24 | 5′-GTTTGGGATCAGCTCGTAGTTGGACAC-3′ | VP2 | A | ||
| RT-PCR | |||||
| Heppell F | 5′-AGAGATCACTGACTTCACAAGTGAC-3′ | VP2 | A | 359 | 6 |
| Heppell R | 5′-TGTGCACCACAGGAAAGATGACTC-3′ | VP2 | A | ||
| Seminested RT-PCR, Heppell F intro | 5′-AAAGGCATGGGGCTGGAGAG-3′ | VP2 | A | 323 | Dopazo et al., unpublished data |
| RT-PCR | |||||
| D543F | 5′-GAATCCMAACAAGACTCC-3′ | VP1 | B | 462 | Dopazo et al., unpublished data |
| D1005R | 5′-GTAGGGTAGGCCGGCTGAGGACTT-3′ | VP1 | B | ||
| Seminested RT-PCR, N753R | 5′-CACCATTGATAGYARTAGG-3′ | VP1 | B | 210 | Dopazo et al., unpublished data |
| VHSV | |||||
| RT-PCR | |||||
| cm3a | 5′-CAGGCGTTGTCCGTGCTTCT-3′ | N | 358 | 12 | |
| cm3b | 5′-ACCCTGCGGAGTTTCCTGATGG-3′ | N | |||
| Nested RT-PCR | |||||
| cm3a intro | 5′-CTATGTACTCCAAGGGAAC-3′ | N | 310 | 12 | |
| cm3b intro | 5′-CGGTGAAGTGCTGCAGTTC-3′ | N | |||
| VNNV | |||||
| RT-PCR | |||||
| Noda-Fwr1 | 5′-CCTGARGASACCACCGCTCCMAT-3′ | CP | RNA2 | 300 | 11 |
| Noda-Rev2 | 5′-CSCCAWCTGTGAAYGTMTTGT-3′ | CP | RNA2 | ||
| RT-PCR | |||||
| Noda F2 | 5′-CGTGTCAGTCATGTGTCGCT-3′ | CP | RNA2 | 427 | 8 |
| Noda R3 | 5′-AGTGTCTCCAGCTTTCTTCT-3′ | CP | RNA2 | ||
| Noda F2 ATL | 5′-CGTGTCGGTGTTATGTCGCT-3′ | CP | RNA2 | 427 | 47 |
| Noda R3 ATL | 5′-CGCATCGACCCTGGTGAAGG-3′ | CP | RNA2 | ||
| Nested PCR | |||||
| Noda F2.2 | 5′-CRTCYCTYGAGACACCTGA-3′ | CP | RNA2 | 179 | Dopazo et al., unpublished data |
| Noda R3.2 | 5′-TGTARTCAATGGRCARCGG-3′ | CP | RNA2 | ||
| RT-PCR | |||||
| Noda F7 | 5′-ATATCACGATGAGTTCACTA-3′ | Protein A | RNA1 | 641 | 50 |
| Noda R7 | 5′-CGATTCACTATTTTCAAGTC-3′ | Protein A | RNA1 |
For the detection of betanodavirus genomes, two methodologies were also employed. In the first one, an equimolar multiplex mixture of primers NodaF2, NodaR3 (8) NodaF2ATL, and NodaR3ATL (9) (Table 2), amplifying a 427-bp fragment within the T4 region in RNA2, was employed by IA-USC in accordance with the protocol described by Olveira et al. (10). For nested PCR reamplification of that fragment, internal primers NodaF2.2 and NodaR3.2 were employed to produce a fragment of 179 bp. The second procedure, performed by UMA, was designed by López-Jimena et al. (11) and used degenerate primers (Noda-Fwrl and Noda-Rev2) for the detection of the SJNNV and RGNNV genotypes. These primers amplify a fragment of 300 bp within the T4 region located in the RNA2 segment. Moreover, a hybridization assay was used in combination with this procedure as described.
Primers Cm3a and Cm3b were used for the detection of VHSV (12); these primers amplify a 358-bp fragment corresponding to the nucleoprotein gen. A set of internal primers (cm3a intro and cm3b intro) was used to reamplify a 310-bp fragment by nested PCR.
The identities of the amplicons were further confirmed by DBH as described by López-Jimena et al. (11). The use, in this methodology, of two sets of probes, specific for types SJNNV and RGNNV, additionally allowed the typing of some of the viruses detected.
Sequencing and phylogenetic analysis.
IPNV and VNNV isolates were subjected to viral typing by genome sequencing. For IPNV, both the polyprotein (segment A) and the RNA polymerase (segment B) genes were amplified with primers Heppell F, Heppell R, and Heppel F intro (for segment A) and D543F, D1005R, and N753R (for segment B) by using the last primer in each set for seminested reamplification (Table 2). In the case of VNNV, the RNA polymerase gene located in RNA1 was amplified by means of primers F7 and R7, and primers F2 and R3 were employed for the T4 gene, encoding the capsid protein (Table 2).
For sequencing, the amplicons were purified with the High Pure PCR Product Purification kit (Roche) with an automated DNA sequencer (ABI PRISM 3100 genetic analyzer; Applied Biosystems; UMA laboratory) or the CEQTM 8000 Genetic Analysis System (Beckman Coulter; IA-USC laboratory) in accordance with the technical instructions included.
Forward and reverse nucleotide sequences of all of the strains tested (13 IPNV and 6 VNNV strains) were edited with Lasergene v.8.1 SeqMan and EditSeq (DNASTAR) and subjected to multiple-sequence alignment with MegAlign software. The following reference strains were included in the phylogenetic analysis to ensure correct typing of the isolates: for IPNV, West Buxton (WB; accession no. AF078668 and AF078669, for segments A and B, respectively), Sp (AF342728 and M58757), Ab (AF342729 and AM114033), He (AF342730 and JF734351), Te (AF342731 and JF734352), C1 (AF342732 and JF734350), C2 (AF342733 and JF734354), C3 (AF342734 and JF734355), and Ja (AF342735 and M58756); for VNNV: types SJNNV (AB056571 and AB056572, for RNA1 and RNA2, respectively), RGNNV (NC_008040 and NC_008041), BFNNV (NC_011063 and EU826138), and TPNNV (NC_013460 and NC_013461). Alignment trees were constructed by Bayesian inference of phylogeny with BEAST 1.8 (Bayesian Markov Chain Monte Carlo analysis of molecular sequences) (13). Two Markov chains were run for 10,000,000 generations, and Bayesian probabilities were obtained from the 50% majority rule consensus of trees sampled every 100 generations after removal of the first 50,000 generations.
Nucleotide sequence accession numbers.
Sequences of RNA1 and RNA2 segments (respectively) of VNNV strains isolated are as follows: KM001709 and KM001708 (from Chelidonichthys lucerna), KM001711 and KM001710 (from Diplodus vulgaris), KM001713 and KM001712 (from Halobatrachus didactylus), KM001714 and KM001715 (from Mullus barbatus), KM001717 and KM001716 (from Pagellus acarne), and KM001719 and KM001718 (from Spondyliosoma cantharus). Novel IPNV sequences can be found in the supplemental material.
RESULTS
No VHSV isolates were obtained in any of the cell lines employed, and detection of this virus was achieved only by molecular techniques. Regarding IPNV, 13 isolates were obtained (Table 3), i.e., 1 from barracuda (Sphyraena sphyraena) in the first oceanographic campaign; 4 from axillary seabream (Pagellus acarne), 3 from common two-banded seabream (Diplodus vulgaris), 2 from common pandora (P. erythrinus), 1 from Senegal seabream (D. bellottii), and 1 from surmullet (Mullus surmulletus), in the second campaign; and 1 from common two-banded seabream in the third campaign. In the case of VNNV, six isolates were obtained only from fish in the first campaign: axillary seabream, common pandora, black seabream (Spondyliosoma cantharus), red mullet (Mullus barbatus), Lusitanian toadfish (Halobatrachus didactylus), and tub gurnard (Chelidonichthys lucerna).
TABLE 3.
Viral strains isolated from cell cultures
| Virus, campaign (date), and name of isolate | Fish species | Common name |
|---|---|---|
| Aquabirnavirus (IPNV type) | ||
| I (November 2010), SpSps-IAusc1538.10 | Sphyraena sphyraena | Barracuda |
| II (March 2011) | ||
| SpDv-IAusc518.11 | Diplodus vulgaris | Common two-banded seabream |
| SpDv-IAusc519.11 | Diplodus vulgaris | Common two-banded seabream |
| SpDv-IAusc520.11 | Diplodus vulgaris | Common two-banded seabream |
| SpDb-IAusc525.11 | Diplodus bellottii | Senegal seabream |
| SpPa-IAusc526.11 | Pagellus acarne | Axillary seabream |
| SpPa-IAusc527.11 | Pagellus acarne | Axillary seabream |
| SpPa-IAusc528.11 | Pagellus acarne | Axillary seabream |
| SpPa-IAusc530.11 | Pagellus acarne | Axillary seabream |
| SpPe-IAusc531.11 | Pagellus erythrinus | Common pandora |
| SpPe-IAusc535.11 | Pagellus erythrinus | Common pandora |
| SpMs-IAusc541.11 | Mullus surmulletus | Surmullet |
| III (November 2011), SpDv-IAusc221.12 | Diplodus vulgaris | Common two-banded seabream |
| Betanodavirus (VNNV) | ||
| I (November 2010) | ||
| SpMb-IAusc1544.10 | Mullus barbatus | Red mullet |
| SpHd-IAusc1547.10 | Halobatrachus didactylus | Lusitanian toadfish |
| SpDv-IAusc1549.10 | Diplodus vulgaris | Common two-banded seabream |
| SpPa-IAusc1551.10 | Pagellus acarne | Axillary seabream |
| SpChl-IAusc1554.10 | Chelidonichthys lucerna | Tub gurnard |
| SpSpc-IAusc1556.10 | Spondyliosoma cantharus | Black seabream |
No isolations were obtained, and no viruses were detected by direct reverse transcription (RT)-PCR in thicklip gray mullet (C. labrosus) in the San Pedro river mouth samplings (Table 4). After reamplification, the prevalence in this area and fish species rose, becoming high for IPNV in the first sampling period (100%; from 10 individuals) and for VNNV in both samplings (60% and 64.7%, respectively). The percentage of fish infected by any of the three viruses at this sampling site was 77.8%.
TABLE 4.
Results of detection of viruses by PCR confirmed by nested PCR and/or DBH
| Sample sourcea or parameter | nb | % of fish positive by PCR/reamplification and confirmation by nested PCR and/or DBH/total |
|||
|---|---|---|---|---|---|
| IPNV | VHSV | VNNV | Total | ||
| Oceanographic campaigns | |||||
| I | 28 | 7.1/35.7 | 7.1/7.1 | 17.9/32.1 | 28.6/53.6 |
| II | 49 | 22.5/49.0 | 2.0/59.2 | 2.0/12.2 | 24.5/71.4 |
| III | 102 | 0.0/11.8 | 0.0/46.1 | 3.9/19.6 | 3.9/39.2 |
| Total | 179 | 7.3/25.7 | 1.7/43.6 | 5.6/19.6 | 13.4/50.3 |
| River mouth samplingsc in: | |||||
| Zone 1 | 10 | 0.0/100 | 0.0/10.0 | 0.0/60.0 | 0/100 |
| Zone 2 | 17 | 0.0/11.8 | 0.0/11.8 | 0.0/64.7 | 0/64.7 |
| Total | 27 | 0.0/44.4 | 0.0/11.1 | 0.0/63.0 | 0/77.8 |
Samplings of the species shown in Tables 1 and 5, corresponding to oceanographic campaigns I, II, and III were performed in November 2010, March 2011, and October to December 2011, respectively.
Number of fish sampled.
Samplings in river mouth zones were carried out in October 2010 and May 2011, respectively, and samples were exclusively from thicklip gray mullet (C. labrosus) fish.
Table 4 also shows that the prevalence percentages of viruses detected by molecular techniques in fish from the oceanographic campaigns and, as observed, the percentage of fish infected by any of the three viruses were relatively low by direct RT-PCR (13.4%) but quite high after reamplification (50.3%). Regarding each virus, the prevalence percentages determined by single RT-PCR were also relatively low (below 10%) for the three viruses in most cases, except for VNNV (17.9%) and IPNV (22.5%) in the first and second campaigns, respectively. Reamplification by nested PCR and/or DBH increased the prevalence values, which averaged 25.7, 43.6, and 19.6% for IPNV, VHSV, and VNNV, respectively.
Table 5 shows the prevalence percentages of viruses distributed per family and species. Among the best-represented species (in terms of the number of specimens analyzed), only fish belonging to the genera Diplodus and Pagellus were positive for virus after a single RT-PCR: specimens of the genus Diplodus were positive for IPNV in the second campaign, and fish of the genus Pagellus were positive for VNNV in the third one, showing prevalence percentages (as determined by single RT-PCR) of 60 to 80% (3 or 4 positive fish out of 5 sampled per species) and 20% (2 out of 10 positive), respectively. Regarding the results obtained after reamplification, the three viruses were highly represented in the Perciformes families, mainly in the case of VHSV, which showed high prevalence values in the three best-represented families of the order Perciformes, i.e., 30, 40, and 50% in the families Mullidae, Sparidae, and Carangidae, respectively. In the case of IPNV, most of the carrier fish belonged to the family Sparidae (average prevalences over 30%). On the other hand, VNNV carrier fish were better represented in the families Mullidae and Carangidae (over 30 and 40%, respectively). In the case of non-Perciformes fish, only VHSV prevalence values exceeded 30%.
TABLE 5.
Prevalence of fish viruses in wild populations in the Gulf of Cadiz as determined by RT-PCR and confirmed by nested PCR and/or DBH
| Order, family, and species or parameter | Common name | Results of oceanographic campaign(s)a: |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I |
II |
III |
All |
||||||||||||||||||||
| nb | Depth (m)c | Locationd | IPNV | VHSV | VNNV | n | Depth (m) | Location | IPNV | VHSV | VNNV | n | Depth (m) | Location | IPNV | VHSV | VNNV | n | IPNV | VHSV | VNNV | ||
| Perciformes | |||||||||||||||||||||||
| Sparidae | |||||||||||||||||||||||
| Boops boops | Bogue | 1 | 21 | I.7 | 0/1e | 0/0 | 0/0 | 5 | 51 | II.1 | 0/1 | 0/5 | 0/0 | 10 | 46 | III.2 | 0/2 | 0/6 | 0/0 | 16 | 0/4 | 0/11 | 0/0 |
| Pagellus bellottii | Red pandora | 0 | 5 | 28 | II.3 | 0/0 | 0/2 | 0/1 | 10 | 46 | III.2 | 0/4 | 0/5 | 2/2 | 15 | 0/4 | 0/7 | 2/3 | |||||
| Pagellus erythrinus | Common pandora | 2 | 30, 21 | I.2, I.7 | 0/1 | 0/0 | 0/0 | 5 | 51 | II.1 | 0/5†† | 0/4 | 0/0 | 10 | 46 | III.2 | 0/1 | 0/9 | 2/3 | 17 | 0/7 | 0/13 | 2/3 |
| Pagellus acarne | Axillary seabream | 1 | 21 | I.7 | 0/0 | 0/0 | 1/1* | 5 | 51 | II.1 | 4/4‡‡ | 0/5 | 1/2 | 10 | 46 | III.2 | 0/1 | 0/0 | 0/0 | 16 | 4/5 | 0/5 | 2/3 |
| Diplodus vulgaris | Common two-banded seabream | 2 | 30, 21 | I.2, I.7 | 1/2 | 0/0 | 1/1† | 5 | 51 | II.1 | 4/4§§ | 1/3 | 0/0 | 10 | 93 | III.2 | 0/1††† | 0/0 | 0/0 | 17 | 5/7 | 1/3 | 1/1 |
| Diplodus bellootii | Senegal seabream | 0 | 5 | 56 | II.4 | 3/5∥∥ | 0/4 | 0/0 | 12 | 26 | III.4 | 0/1 | 0/1 | 0/3 | 17 | 3/6 | 0/5 | 0/3 | |||||
| Sparus aurata | Gilthead seabream | 1 | 21 | I.7 | 0/1 | 0/0 | 0/1 | 0 | 0 | 1 | 0/1 | 0/0 | 0/1 | ||||||||||
| Lithognathus mormyrus | Sand steenbras | 1 | 21 | I.7 | 0/1 | 0/0 | 0/0 | 0 | 0 | 1 | 0/1 | 0/0 | 0/0 | ||||||||||
| Spondyliosoma cantharus | Black seabream | 1 | 61 | I.4 | 1/1 | 0/0 | 1/1‡ | 0 | 0 | 1 | 1/1 | 0/0 | 1/1 | ||||||||||
| Summary for family Sparidae | 9 | 2/6 (22.2/66.7)f | 0/0 (0/0) | 3/4 (33.3/44.4) | 30 | 11/19 (36.7/63.3) | 1/23 (3.3/76.7) | 1/3 (3.3/10) | 62 | 0/10 (0/16.1) | 0/21 (0/33.9) | 4/8 (6.5/12.9) | 101 | 13/35 (12.9/34.7) | 1/44 (1.0/43.6) | 8/15 (7.9/15.0) | |||||||
| Mullidae | |||||||||||||||||||||||
| Mullus barbatus | Red mullet | 1 | 30 | I.2 | 0/0 | 0/0 | 1/1§ | 0 | 0 | 1 | 0/0 | 0/0 | 1/1 | ||||||||||
| Mullus surmuletus | Surmullet | 1 | 30 | I.2 | 0/0 | 0/0 | 0/0 | 4 | 51 | II.1 | 0/1*** | 0/1 | 0/1 | 10 | 46 | III.2 | 0/0 | 0/4 | 0/3 | 15 | 0/1 | 0/5 | 0/4 |
| Summary for family Mullidae | 2 | 0/0 (0/0) | 0/0 (0/0) | 1/1 (50.0/50.0) | 4 | 0/1 (0/25.0) | 0/1 (0/25.0) | 0/1 (0/25.0) | 10 | 0/0 (0/0) | 0/4 (0/40.0) | 0/3 (0/30.0) | 16 | 0/1 (0/6.3) | 0/5 (0/31.3) | 1/5 (6.3/31.3) | |||||||
| Carangidae, Trachurus mediterraneus | Mediterranean horse mackerel | 1 | 28 | I.1 | 0/0 | 0/0 | 0/0 | 5 | 51 | II.1 | 0/1 | 0/2 | 0/2 | 10 | 93.5 | III.1 | 0/2 | 0/7 | 0/5 | 16 | 0/3 | 0/9 | 0/7 |
| Other Perciformes | |||||||||||||||||||||||
| Haemulidae | |||||||||||||||||||||||
| Pomadasys incisus | Bastard grunt | 1 | 28 | I.1 | 0/1 | 0/0 | 0/0 | 0 | 0 | 1 | 0/1 | 0/0 | 0/0 | ||||||||||
| Plectorhinchus mediterraneus | Rubberlip grunt | 1 | 44 | I.9 | 0/1 | 1/1 | 0/0 | 0 | 0 | 1 | 0/1 | 1/1 | 0/0 | ||||||||||
| Scianidae, Arginosomus regius | Meagre | 1 | 28 | I.1 | 0/1 | 0/0 | 0/1 | 0 | 0 | 1 | 0/1 | 0/0 | 0/1 | ||||||||||
| Scombridae, Scomber scombrus | Atlantic mackerel | 1 | 107 | I.6 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Sphiraenidae, Sphyraena sphyraena | Barracuda | 1 | 28 | I.1 | 0/1** | 0/0 | 0/1 | 0 | 0 | 1 | 0/1 | 0/0 | 0/1 | ||||||||||
| Summary for other Perciformes | 5 | 0/4 (0/80.0) | 1/1 (20.0/20.0) | 0/2 (0/40.0) | 0 | 0 | 5 | 0/4 (0/80.0) | 1/1 (20.0/20.0) | 0/2 (0/40.0) | |||||||||||||
| Summary for order Perciformes | 17 | 2/10 (11.8/58.8) | 1/1 (5.9/5.9) | 4/7 (23.5/41.2) | 39 | 11/21 (28.2/53.9) | 1/26 (2.6/66.7) | 1/6 2.6/15.4 | 82 | 0/12 (0.0/14.6) | 0/37 (0/45.1) | 4/16 (4.9/19.5) | 138 | 13/43 (9.4/31.2) | 2/64 (1.5/46.4) | 9/29 (6.5/21.0) | |||||||
| Families belonging to other orders | |||||||||||||||||||||||
| Gadiformes, Gadidae, Micromesistius poutasso | Blue whiting | 1 | 665 | I.8 | 0/0 | 0/0 | 0/0 | 5 | 48 | II.2 | 0/1 | 0/2 | 0/0 | 0 | 6 | 0/1 | 0/2 | 0/0 | |||||
| Gadiformes, Macrouridae, Caelorinchus caelorinchus | Hollowsnout grenadier | 1 | 665 | I.8 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Clupeidormes, Engraulidae, Engraulis encrasicolus | Anchovy | 0 | 5 | 56 | II.4 | 0/2 | 0/1 | 0/0 | 20 | 27 | III.3 | 0/0 | 0/10 | 0/4 | 25 | 0/2 | 0/11 | 0/4 | |||||
| Clupeiformes, Clupeidae, Alosa fallax | Twaite shad | 1 | 90 | I.5 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Mugiliformes, Mugilidae, Liza ramada | Thinlip Gray mullet | 1 | 28 | I.1 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Batrachoidiformes, Batrachoididae, Halobatrachus didactylus | Lusitanian toadfish | 1 | 21 | I.7 | 0/0 | 0/0 | 1/1¶ | 0 | 0 | 1 | 0/0 | 0/0 | 1/1 | ||||||||||
| Pleuronectiformes, Soleidae, Microchirus azevia | Sole | 1 | 48 | I.3 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Scorpaeniformes | |||||||||||||||||||||||
| Triglidae, Chelidonichthys lucerna | Tub gurnard | 1 | 90 | I.5 | 0/0 | 1/1 | 0/1∥ | 0 | 0 | 1 | 0/0 | 1/1 | 0/1 | ||||||||||
| Sebastidae, Helicolenus dactylopterus | Blackbelly rosefish | 1 | 665 | I.8 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Chimaeriformes, Chimaeridae, Chimaera monstrosa | Rabbit fish | 1 | 665 | I.8 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Carcharhiniformes, Scyliorhinidae, Galeus melastomus | Blackmouth catshark | 1 | 665 | I.8 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Squaliformes, Etmopteridae, Etmopterus spinax | Velvet belly | 1 | 665 | I.8 | 0/0 | 0/0 | 0/0 | 0 | 0 | 1 | 0/0 | 0/0 | 0/0 | ||||||||||
| Summary for other families | 11 | 0/0 (0/0) | 1/1 (9.1/9.1) | 1/2 (9.1/18.2) | 10 | 0/3 (0/30.0) | 0/3 (0/30.0) | 0/0 (0/0) | 20 | 0/0 (0/0) | 0/10 (0/50.0) | 0/4 (0/20.0) | 41 | 0/3 (0/7.3) | 1/14 (2.4/34.1) | 1/6 (2.4/14.6) | |||||||
| Total | 28 | 2/10 (7.1/35.7) | 2/2 (7.1/7.1) | 5/9 (17.9/32.1) | 49 | 11/24 (22.5/49.0) | 1/29 (2.0/59.2) | 1/6 (2.0/12.2) | 102 | 0/12 (0/11.8) | 0/47 (0/46.1) | 4/20 (3.9/19.6) | 179 | 13/46 (7.3/25.7) | 3/78 (1.7/43.6) | 10/35 (5.6/19.6) | |||||||
Oceanographic campaigns were performed during November 2010 (campaign I), March 2011 (campaign II), and October to December 2011 (campaign III).
Number of fish sampled for viral analysis.
Depth of the trawling transect. Depths and temperatures: 20 to 30 m, around 18°C; 40 to 50 m, around 16.5°C; 90 to 100 m, around 15°C, and ≥600 m, around 13°C.
Capture locations: I.1, 36.4250N, 6.3510W; I.2, 36.4750N, 6.3990W; I.3, 36.5640N, 6.5230W; I.4, 36.4310N, 6.4400W; I.5, 36.4270N, 6.4760W; I.6, 36.3800N, 6.4710W; I.7, 36.5600N, 6.4160W; I.8, 36.1960N, 6.0400W; I.9, 36.1820N, 6.2000W; II.1, 36.2320N, 6.2380W; II.2, 36.2020N, 6.5090W; II.3, 36.4750N, 6.4030W; II.4, 36.2760N, 6.2850W; III.1, 36.2260N, 6.3280W; III.2, 36.1820N, 6.2000W; III.3, 36.5780N, 6.4600W; III.4, 36.4250N, 6.3510W).
Number of positive fish, as determined by RT-PCR/nested PCR and/or DBH.
Prevalence percentage determined by RT-PCR/nested PCR and/or DBH. Code(s) of viral isolates obtained from certain fish species: *, SpPa-IAusc1551.10; †, SpDv-IAusc1549.10; ‡, SpSpc-IAusc1556.10; §, SpMb-IAusc1544.10; ¶, SpHd-IAusc1547.10; ∥, SpChl-IAusc1554.10; **, SpSps-IAusc1538.10; ††, SpPe-IAusc531.11 and SpPe-IAusc535.11; ‡‡, SpPa-IAusc526.11, SpPa-IAusc527.11, SpPa-IAusc528.11, and SpPa-IAusc530.11; §§, SpDv-IAusc518.11, SpDv-IAusc519.11, and SpDv-IAusc520.11; ∥∥, SpDb-IAusc525.11; ***, SpMs-IAusc541.11; †††, SpDv-IAusc221.12.
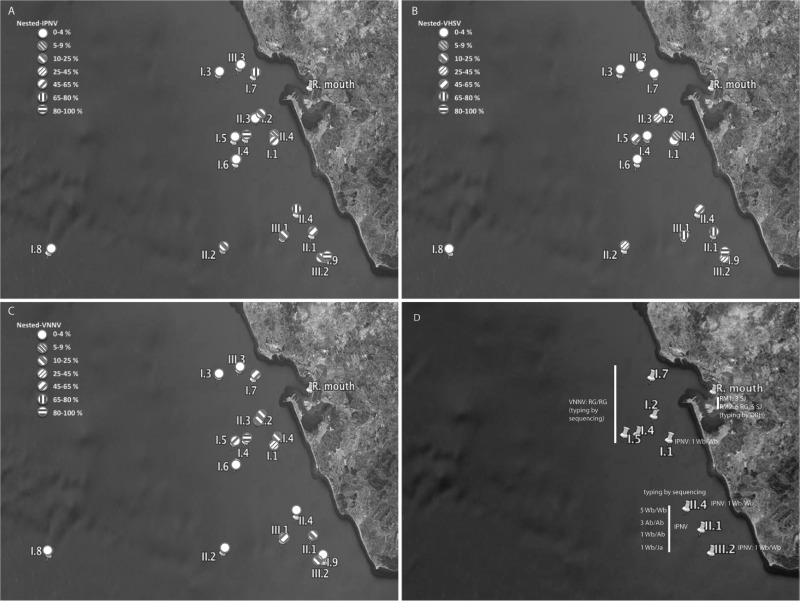
Figure 2 shows the distribution of sampling sites in the oceanographic campaigns with the presence of carrier fish for each specific virus and for viruses in general. We must remark that there were two main areas of sites in the oceanographic sampling zone (one close to the Bay of Cadiz and another one slightly to the southeast), in both cases next the coast, and two individual sites far away from the cost. The highest prevalence percentages of carrier fish in general corresponded to the sites located near the coast. However, the interesting results came from the analysis of data from specific viruses. In this sense, whereas the highest prevalences of IPNV carrier fish were represented in both main site areas (Fig. 2A), the area close to the Bay of Cadiz showed a higher prevalence of VNNV carriers (Fig. 2C), and the southeastern area showed a higher prevalence of VHSV carrier fish (Fig. 2B).
FIG 2.
Distribution of viral prevalences, as determined after reamplification, of IPN-type virus (A), VHSV (B), and VNNV (C) among the sampling site locations. Panel D shows the locations of the typed isolates. Template map source, Google Earth.
Typing of viral strains by genome sequencing was possible only with the 13 IPNV and 6 VNNV isolates obtained from fish from the oceanographic campaigns (Table 6 and Fig. 2D). Unfortunately, in the remaining cases (viruses detected only by direct amplification and reamplification) the template quantities were not enough for sequencing. Primer pairs R23/R24 and D543F/N753R were used to sequence small fragments (around and under 200 bp) of segments A and B (respectively) of IPNV. Primer pairs Noda-F7/R7 and Noda-F2/R3 were employed to sequence 641- and 427-bp segments RNA1 and RNA2 of VNNV, respectively. As shown in Table 6, most of the IPNV isolates were of the WB/WB (segments A/B) type; three were of the Ab/Ab type, and two turned out to be natural reassortants of types WB/Ab (strain SpDv-IAusc519.11) and WB/Ja (strain SpPa-IAusc527.11). The six VNNV isolates showed both segments of the RG type. The application of type-specific DBH for the confirmation of RT-PCR detection of VNNV allowed the typing of some nonisolated strains, all of them detected in thicklip gray mullet from the river mouth sampling area (Fig. 2D). In this respect, six individuals (from the second sampling time at this site) were carriers of an RG (corresponding to the type of segment RNA2) type strain and eight (three from the first sampling and five from the second) were carriers of the SJ type strain (data not shown).
TABLE 6.
Genotyping of IPNV and VNNV isolated from the oceanographic campaigns
| Isolate type, OC,a and strain name | Fish species | Typeb | GenBank accession no. |
|---|---|---|---|
| IPNV | |||
| I, SpSps-IAusc1538.10 | Sphyraena sphyraena | WB/WB | NPc |
| II | |||
| SpDv-IAusc518.11 | Diplodus vulgaris | Ab/Ab | NP |
| SpDv-IAusc519.11 | Diplodus vulgaris | WB/Ab | NP |
| SpDv-IAusc520.11 | Diplodus vulgaris | Ab/Ab | NP |
| SpDb-IAusc525.11 | Diplodus bellottii | WB/WB | NP |
| SpPa-IAusc526.11 | Pagellus acarne | WB/WB | NP |
| SpPa-IAusc527.11 | Pagellus acarne | WB/Ja | NP |
| SpPa-IAusc528.11 | Pagellus acarne | Ab/Ab | NP |
| SpPa-IAusc530.11 | Pagellus acarne | WB/WB | NP |
| SpPe-IAusc531.11 | Pagellus erythrinus | WB/WB | NP |
| SpPe-IAusc535.11 | Pagellus erythrinus | WB/WB | NP |
| SpMs-IAusc541.11 | Mullus surmulletus | WB/WB | NP |
| III, SpDv-IAusc221.12 | Diplodus vulgaris | WB/WB | NP |
| VNNV, I | |||
| SpMb-IAusc1544.10 | Mullus barbatus | RG/RG | KM001714, KM001715 |
| SpHd-IAusc1547.10 | Halobatrachus didactylus | RG/RG | KM001713, KM001712 |
| SpDv-IAusc1549.10 | Diplodus vulgaris | RG/RG | KM001711, KM001710 |
| SpPa-IAusc1551.10 | Pagellus acarne | RG/RG | KM001717, KM001716 |
| SpChl-IAusc1554.10 | Chelidonichthys lucerna | RG/RG | KM001709, KM001708 |
| SpSpc-IAusc1556.10 | Spondyliosoma cantharus | RG/RG | KM001719, KM001718 |
OC, oceanographic campaigns (I, November 2012; II, March 2011; III, October to December 2011).
Genotyping of segment A/segment B of IPNV and RNA1/RNA2 of VNNV.
NP, sequences not published in GenBank because they were under 200 bp (see the supplemental material).
DISCUSSION
The present report describes an epidemiological study of wild fish populations inhabiting different areas around the Gulf of Cadiz (South Atlantic coast of the Iberian Peninsula). This survey, performed between 2010 and 2011, was focused on three viruses of high relevance in aquaculture (aquabirnaviruses and IPNV-type viruses, VHSV, and betanodaviruses). Since all of the fish sampled were asymptomatic, light viral loads in the fish tissues were expected. For that reason, viral diagnosis was performed simultaneously in both laboratories (IA-USC and UMA), by their own routine procedures, which included, in addition to cell culture isolation, detection by RT-PCR, followed by reamplification (a confirmatory test) by nested PCR (IA-USC) and DBH (UMA). The first remarkable result obtained in this study was that the three viruses were detected in the wild fish populations screened both near and far from the coast, although the detection percentages varied according to the fish species sampled, the season, and the geographical locations of the sampling areas. In fact, these results are not surprising at all, considering that the ubiquity of these three viruses in wild fish has been previously established in several surveys (14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24). In most cases, the wild fish sampled in those surveys were asymptomatic, although several outbreaks with pathological signs of VNNV infection and detection of the virus in wild fish have been recorded in Europe, near the coasts of Portugal and Italy (11, 25, 26).
Most of the fish sampled in the oceanographic campaigns reported here belonged to the order Perciformes, mainly to the families Sparidae, Mullidae, and Carangidae, though a significant number of other non-Perciformes fish, such as those of the orders Gadiformes and Clupeiformes, were also sampled. On the other hand, previous surveys of these viruses focused on different fish species in wild populations have been aimed mostly at fish of the orders Pleuronectiformes (flatfish), Clupeiformes (mainly herring and sprat), and Gadiformes (15, 16, 18, 19, 21, 27, 28, 29, 30) and to a lesser extent Perciformes (17, 20, 23, 28, 29). Most of these surveys employed isolation in cell culture for detection of carrier fish and reported very low prevalence values, which agrees with our results obtained with that procedure. However, several studies (14, 16, 18, 30, 31) reported obtaining high prevalence percentages of VHSV and/or IPNV with this same method of detection. Curiously, a large percentage of the fish tested in those surveys were Pleuronectiformes, which may suggest a higher susceptibility of flatfish to these viruses. Nevertheless, in the present study, our results demonstrated a higher prevalence of carrier fish among of order Perciformes, not only in terms of isolated viruses but also considering detection by molecular techniques. As expected, with reamplification techniques, higher prevalence values were obtained, which is in agreement with the results of others (17, 32).
Considering the distribution of viruses among the fish species in our study, the aquabirnaviruses showed the highest prevalence in fish of the genera Diplodus and Pagellus (family Sparidae). Unfortunately, these genera have been poorly studied in other surveys and therefore a comparison is not possible; however, Kim et al. (23) analyzed (by isolation in cell culture) other species from the same family and did not detect any IPNV-type virus. In addition, other authors reported obtaining results similar to ours when working with other Perciformes families (21, 23). Regarding betanodavirus, in the present study, VNNV carriers were found mainly in the families Mullidae and Carangidae (considering results from reamplification) but also in the genera Pagellus and Diplodus (considering viral isolation and single RT-PCR). A high prevalence of this virus in fish of the family Carangidae was also reported by Gómez et at (17) in Japanese waters but not by Baeck et al. (20) in South Korea. No isolates of VHSV were obtained in the sampled areas around the Bay of Cadiz, which coincides with the low prevalences observed by other authors in larger surveys (in terms of the numbers of samples and fish species tested) aimed at this virus. However, the percentage of carrier fish determined after reamplification exceeded the values reported by other authors, even when screening exclusively certain species already considered natural carriers of the virus (32, 33).
The emergence of some viral fish diseases has occurred through the expansion of the known geographic ranges via migration and natural movements of infected hosts, vectors, or carriers, with subsequent exposure to naive and often highly susceptible species. In addition, anthropogenic and environmental factors unrelated to aquaculture, such as the movement of pathogens or hosts via ballast water in ships and movement of bait by anglers and environmental changes, must also be considered (34). The interaction between aquaculture and wild fish populations is a matter of permanent controversy. Whereas the transmission of viral diseases from wild to cultured fish seems to be clear according to many authors (35, 36, 37, 38), only a few have suggested the possibility of the opposite route of transmission (21, 26). In this sense, according to Kurath and Winton (5), the conditions present in aquaculture provide an increased potential for the transmission and spreading of viruses that enter farmed fish populations through interaction with wild fish. In addition, host switches occur when wild viruses encounter the characteristic conditions present in aquaculture (38), which enhances the spreading of diseases.
In the present study, we screened four areas with different levels of putative anthropogenic influence: three corresponding to oceanographic campaigns (one far away from the coast, a second one close to the coast, southeast of the Bay of Cadiz, and another one next to the bay), and one in a river mouth zone. The mouth of the San Pedro River is a strategic zone of interaction between fish farms and the marine environment. The thicklip mullet (C. labrosus), the fish species sampled at this location, migrates from the marine environment to zones with influence of aquaculture practices, where this wild fish interacts with cultured fish. Therefore, this fish species might be considered a model to determine the flow of microbial pathogens between wild and cultured hosts and vice versa. To our knowledge, this is the first report on the viral state of this species, although it has previously been reported that C. labrosus is highly susceptible to parasitic diseases (39). Our results demonstrate a high percentage of carriers of VNNV and IPNV-type viruses of this fish species, but only after reamplification, suggesting light viral loads in carrier fish. These values are much higher than those observed in the oceanographic populations, which could be indicative of an anthropogenic influence. On the other hand, the prevalence of VHSV in this fish population was significantly lower than both the prevalence of the other two viruses in this fish species and the prevalence of the same virus in the oceanographic fish populations. A deeper analysis of the geographical distribution of the three viruses at the oceanographic locations revealed interesting data. Thus, as shown in Fig. 2, whereas the IPNV carrier fish were present at all of the sampling sites, the highest prevalences of VNNV were detected at the sites next to the Bay of Cadiz, closer to the river mouth, and in the case of VHSV, the highest prevalence values were observed at sites southeast of the gulf, at least theoretically far from the influence of the aquaculture facilities. These results suggest an anthropogenic influence on the establishment of the carrier state in fish with this virus. In fact, the presence of these viruses as a result of aquaculture in the area near the San Pedro River has been well documented (7, 40). Unfortunately, phylogenetic analysis to compare strains from both geographical locations (oceanic and river mouth) was not possible since only genome sequencing of viral isolates was effective and no isolates could be obtained from the river mouth. On the other hand, the presence of viruses in these wild fish could be due to interactions between the two populations (oceanic sites and river mouth) and/or to the existence of marine currents—from north to south along the cost—with a suction effect on waters inside the Bay of Cadiz. In addition, the differences in the distribution of the three viruses between the two populations could be due to differences in water temperature (1 to 2 degrees higher in surface waters in the river mouth than in waters outside the bay) or to different susceptibilities of the fish species to these viruses, as a possible explanation for the absence of VHSV in C. labrosus. However, this hypothesis needs further challenge studies to be demonstrated.
Typing of aquabirnaviruses and betanodaviruses in this study provided interesting results. Regarding aquabirnavirus, in the present work, both genome segments of 13 IPNV isolates collected from the Gulf of Cadiz were typed. Although some strains were of the European type Ab/Ab (segments A and B of type Ab), most of them unexpectedly showed the American type WB/WB and their distribution was not related to the fish species (since this type was detected in all of them). IPNV types have been traditionally correlated to their geographical locations. The Sp, Ab, He, and Te type strains would constitute the European types, and WB, Jasper, and other related strains such as Dry Mills were reported mainly in North America (41, 42, 43). However, at present, this geographical distribution has been altered probably because of natural migrations of wild fish populations, the use of fish as feed in aquaculture, and/or commercial movements of fish stocks (3, 5). In this sense, a screening for IPNV performed by Cutrín et al. (44) in northwestern Spain showed that most of the isolates belonged to genotypes II (Ab) and III (Sp), but some were typed as American strains of genotype I (WB and Jasper type strains), a genotype that could have entered that region through the importation of trout eggs from North America, as the authors hypothesized. The same research team, studying the wild fish populations of the Flemish Cap (Newfoundland) observed that although most of the strains detected were typed as WB, Dry Mills, or Jasper, a few isolates were included in typically European genotype II (Ab strain) (45); those findings were explained by the authors as being due to the migration of fish from Europe to Newfoundland. They additionally reported, for the first time, the occurrence of natural reassortment in aquabirnaviruses. In the present study, two natural reassortants of the types WB/Ab and WB/Ja were also detected, and this is the first time a recombinant of two American types has been detected. These results include a new area of Europe where the American strains are also present and confirm the conclusions of other authors about the lack of a direct and clear relationship between the genotype and geographic distribution of these viruses.
In the case of VNNV, several studies have established that this virus is globally distributed in the environment (46, 47, 48). In the fish populations analyzed in the present work, RGNNV was the only genotype detected in the oceanographic campaigns, whereas in the thicklip mullet, the genotype of VNNV detected varied with the sampling period: SJNNV in 2010 and both SJNNV and RGNNV in 2011. These results support previous data obtained by us and others. Thus, Vendramin et al. (26) detected only RGNNV in wild fish collected in southern Italy; however, López-Jimena et al. (11) in a study of fish from southern Portugal detected both genotypes, although RGNNV was more frequently detected. On the contrary, SJNNV was consistently detected in cultured fish from a farm located near the San Pedro River (40) and in other geographic farms of the Iberian Peninsula (49). Our analysis suggests a distribution of betanodaviruses according to their source (wild or farmed), and both genotypes were detected in C. labrosus only in 2011, which is indicative of a flow of SJNNV from cultured to wild fish. However, further investigations are required to verify if this viral flow is uni- or bidirectional.
Finally, further surveys must be conducted to study the evolution of the frequencies of carriers among these wild fish populations and the correlation with the presence of those viruses in cultured fish in the same area.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grant P09-RNM-4898 from Junta de Andalucía (Proyecto de Excelencia) and partially by grant CSD2007-00002 Aquagenomics (funded by the program Consolider-Ingenio 2010) from the Ministerio de Educación y Ciencia (MEC).
Footnotes
Published ahead of print 15 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02090-14.
REFERENCES
- 1.Kibenge FSB, Godoy MG, Fast M, Workenho S, Kibenge MJT. 2012. Countermeasures against viral diseases of farmed fish. Antiviral Res. 95:257–281. 10.1016/j.antiviral.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 2.King AMQ, Adams MJ, Carstends EB, Lefkowitz EJ. 2012. Virus taxonomy. Ninth report of the International Committee on Taxonomy of Viruses. Academic Press, Elsevier, London, United Kingdom [Google Scholar]
- 3.Gozlan RE, Peeler EJ, Longshaw M, St-Hilaire S, Feist SW. 2006. Effect of microbial pathogens on the diversity of aquatic populations, notably in Europe. Microbes Infect. 8:1358–1364. 10.1016/j.micinf.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 4.Marcogliese DJ. 2008. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Tech. 27:467–484 [PubMed] [Google Scholar]
- 5.Kurath G, Winton J. 2011. Complex dynamics at the interface between wild and domestic viruses of finfish. Curr. Opin. Virol. 1:73–80. 10.1016/j.coviro.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 6.Heppell J, Berthiaume L, Tarrab E, Lecomte J, Arella M. 1992. Evidence of genomic variations between infectious pancreatic necrosis virus strains determined by restriction fragment profiles. J. Gen. Virol. 73:2863–2870. 10.1099/0022-1317-73-11-2863 [DOI] [PubMed] [Google Scholar]
- 7.López-Jimena B, García-Rosado E, Infante C, Cano I, Manchado M, Castro D, Borrego JJ, Alonso MC. 2010. Detection of infectious pancreatic necrosis virus (IPNV) from asymptomatic redbanded seabream, Pagrus auriga Valenciennes, and common seabream, Pagrus pagrus (L.), using a non-destructive procedure. J. Fish Dis. 33:311–319. 10.1111/j.1365-2761.2009.01123.x [DOI] [PubMed] [Google Scholar]
- 8.Nishizawa T, Kinoshita S, Yoshimizu M. 2005. An approach for genogrouping of Japanese isolates of aquabirnaviruses in a new genogroup, VII, based on the VP2/NS junction region. J. Gen. Virol. 86:1973–1978. 10.1099/vir.0.80438-0 [DOI] [PubMed] [Google Scholar]
- 9.Thiéry R, Arnauld C, Delsert C. 1999. Two isolates of sea bass, Dicentrachus labrax L., nervous necrosis virus with distinct genomes. J. Fish Dis. 22:201–207. 10.1046/j.1365-2761.1999.00164.x [DOI] [Google Scholar]
- 10.Olveira JG, Soares F, Engrola S, Dopazo CP, Bandín I. 2008. Antemortem versus postmortem methods for detection of betanodavirus in Senegalese sole (Solea senegalensis). J. Vet. Diagn. Invest. 20:215–219. 10.1177/104063870802000212 [DOI] [PubMed] [Google Scholar]
- 11.López-Jimena B, Cherif N, García-Rosado E, Infante C, Cano I, Castro D, Hammami S, Borrego JJ, Alonso MC. 2010. A combined RT-PCR and dot-blot hybridization method reveals the coexistence of SJNNV and RGNNV betanodavirus genotypes in wild meagre (Argyrosomus regius). J. Appl. Microbiol. 109:1361–1369. 10.1111/j.1365-2672.2010.04759.x [DOI] [PubMed] [Google Scholar]
- 12.López-Vázquez C, Dopazo CP, Olveira JG, Barja JL, Bandín I. 2006. Development of a rapid, sensitive and non-lethal diagnostic assay for the detection of viral haemorrhagic septicaemia virus. J. Virol. Methods 133:167–174. 10.1016/j.jviromet.2005.10.033 [DOI] [PubMed] [Google Scholar]
- 13.Drummond AJ, Suchard MA, Dong X, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29:1969–1973. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takano R, Nishizawa T, Arimoto M, Muroga K. 2000. Isolation of viral haemorrhagic septicaemia virus (VHSV) from wild Japanese flounder, Paralichtys olivaceus. Bull. Eur. Assoc. Fish Pathol. 20:186–192 [Google Scholar]
- 15.King JA, Snow M, Smail DA, Raynard RS. 2001. Distribution of viral haemorrhagic septicaemia virus in wild fish species of the North Sea, north east Atlantic Ocean and Irish Sea. Dis. Aquat. Organ. 47:81–86. 10.3354/dao047081 [DOI] [PubMed] [Google Scholar]
- 16.Dopazo CP, Bandín I, López-Vázquez C, Lamas J, Noya M, Barja JL. 2002. Isolation of viral hemorrhagic septicemia virus from Greenland halibut Reinhardtius hippoglossoides caught at the Flemish Cap. Dis. Aquat. Organ. 50:171–179. 10.3354/dao050171 [DOI] [PubMed] [Google Scholar]
- 17.Gómez DK, Sato J, Mushiake K, Isshiki T, Okinaka Y, Nakai T. 2004. PCR-based detection of betenodaviruses from cultures and wild marine fish with no clinical signs. J. Fish Dis. 27:603–608. 10.1111/j.1365-2761.2004.00577.x [DOI] [PubMed] [Google Scholar]
- 18.Romero-Brey I, Batts WN, Bandín I, Winton JR, Dopazo CP. 2004. Molecular characterization of birnaviruses isolated from wild marine fishes at the Flemish Cap (Newfoundland). Dis. Aquat. Organ. 6:1–10. 10.3354/dao061001 [DOI] [PubMed] [Google Scholar]
- 19.Skall HF, Olesen NJ, Mellergaard S. 2005. Prevalence of viral haemorrhagic septicaemia virus in Danish marine fishes and its occurrence in new host species. Dis. Aquat. Organ. 66:146–151. 10.3354/dao066145 [DOI] [PubMed] [Google Scholar]
- 20.Baeck GW, Gomez DK, Oh KS, Kim JH, Choresca CH, Jr, Park SC. 2007. Detection of piscine nodavirus from apparently healthy wild marine fish in Korea. Bull. Eur. Assoc. Fish Pathol. 27:116–122 [Google Scholar]
- 21.Wallace IS, Gregory A, Murray AG, Munroe ES, Raynard RS. 2008. Distribution of infectious pancreatic necrosis virus (IPNV) in wild marine fish from Scottish waters with respect to clinically infected aquaculture sites producing Atlantic salmon, Salmo salar L. J. Fish Dis. 31:177–186. 10.1111/j.1365-2761.2007.00886.x [DOI] [PubMed] [Google Scholar]
- 22.Isidan H, Bolat Y. 2011. A survey of viral hemorrhagic septicemia (VHS) in Turkey. Turk. J. Fish. Aquat. Sci. 11:507–513. 10.4194/1303-2712-v11_4_01 [DOI] [Google Scholar]
- 23.Kim W-S, Choi S-Y, Kim D-H, Oh M-J. 2013. A survey of fish viruses isolated from wild marine fishes from the coastal waters of southern Korea. J. Vet. Diagn. Invest. 25:750–755. 10.1177/1040638713504755 [DOI] [PubMed] [Google Scholar]
- 24.Giacopello C, Foti M, Bottari T, Fisichella V, Barbera G. 2013. Detection of viral encephalopathy and retinopathy virus (VERV) in wild marine fish species of the South Tyrrhenian Sea (Central Mediterranean). J. Fish Dis. 36:819–821. 10.1111/jfd.12095 [DOI] [PubMed] [Google Scholar]
- 25.Ciulli S, Galletti E, Grodzki M, Alessi A, Battilani M, Prosperi S. 2007. Isolation and genetic characterization of betanodavirus from wild marine fish from the Adriatic Sea. Vet. Res. Commun. 31(Suppl 1):221–224. 10.1007/s11259-007-0010-y [DOI] [PubMed] [Google Scholar]
- 26.Vendramin N, Patarnello P, Toffan A, Panzarin V, Cappellozza E, Tedesco P, Terlizzi A, Terregino C, Cattoli G. 2013. Viral encephalopathy and retinopathy in groupers (Epinephelus spp.) in Southern Italy: a threat for wild endangered species? BMC Vet. Res. 9:20. 10.1186/1746-6148-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortensen HF, Heuer OE, Lorenzen N, Otte L, Olesen NJ. 1999. Isolation of viral haemorrhagic septicaemia virus (VHSV) from wild marine fish species in the Baltic Sea, Kattegat, Skagerrak and the North Sea. Virus Res. 63:95–106. 10.1016/S0168-1702(99)00062-3 [DOI] [PubMed] [Google Scholar]
- 28.Skall HF, Mellergaard S, Olesen NJ. 2000. Isolation of Birnavirus serogrouop B in wild and aquaculture fish species. Bull. Eur. Ass. Fish Pathol. 20:229–236 [Google Scholar]
- 29.Brudeseth BE, Evensen Ø. 2002. Occurrence of viral haemorrhagic septicaemia virus (VHSV) in wild marine fish species in the coastal regions of Norway. Dis. Aquat. Organ. 52:21–28. 10.3354/dao052021 [DOI] [PubMed] [Google Scholar]
- 30.Watanabe L, Pakingking R, Iida H, Nishizawa T, Iida Y, Arimoto M, Muroga K. 2002. Isolation of aquabirnavirus and viral hemorrhagic septicemia virus (VHSV) in wild marine fishes. Fish Pathol. 37:189–191. 10.3147/jsfp.37.189 [DOI] [Google Scholar]
- 31.Takano R, Mori K, Nishizawa T, Arimoto M, Muroga K. 2001. Isolation of viruses from wild Japanese flounder, Paralichtys olivaceus. Fish Pathol. 36:153–160. 10.3147/jsfp.36.153 [DOI] [Google Scholar]
- 32.Johansen R, Bergh Ø, Modahl I, Dahle G, Gjerset B, Holst JC, Sandlund N. 2013. High prevalence of viral haemorrhagic septicaemia virus (VHSV) in Norwegian spring spawning herring. Mar. Ecol. Prog. Ser. 478:223–230. 10.3354/meps10208 [DOI] [Google Scholar]
- 33.Skall HF, Olesen NJ, Mellergaard S. 2005. Viral haemorrhagic septicaemia virus in marine fish and its implications for fish farming—a review. J. Fish Dis. 28:509–529. 10.1111/j.1365-2761.2005.00654.x [DOI] [PubMed] [Google Scholar]
- 34.Walker PJ, Winton JR. 2010. Emerging viral diseases of fish and shrimp. Vet. Res. 41:51. 10.1051/vetres/2010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amos K, Thomas J. 2002. Disease interactions between wild and cultured fish: observations and lessons learned in the Pacific Northwest. Bull. Eur. Ass. Fish Pathol. 22:95–102 [Google Scholar]
- 36.Olivier G. 2001. Disease interactions between wild and cultured fish—perspectives from the American Northwest (Atlantic Provinces). Bull. Eur. Ass. Fish Pathol. 22:103–109 [Google Scholar]
- 37.Garver KA, Traxler GS, Hawley LM, Ruchard J, Ross JP, Lovy J. 2013. Molecular epidemiology of viral haemorrhagic septicaemia virus (VHSV) in British Columbia, Canada, reveals transmission from wild to farmed fish. Dis. Aquat. Organ. 104:93–104. 10.3354/dao02588 [DOI] [PubMed] [Google Scholar]
- 38.Johansen LH, Jensen I, Mikkelsen H, Bjorn PA, Jansen P, Bergh O. 2011. Disease interaction and pathogens exchange between wild and farmed fish populations with special reference to Norway. Aquaculture 315:167–186. 10.1016/j.aquaculture.2011.02.014 [DOI] [Google Scholar]
- 39.Fioravanti ML, Caffara M, Florio D, Gustinelli A, Marcer F, Quaglio F. 2006. Parasitic diseases of marine fish: epidemiological and sanitary considerations. Parasitologia 48:15–18 [PubMed] [Google Scholar]
- 40.García-Rosado E, Cano I, Martin-Antonio B, Labella A, Manchado M, Alonso MC, Castro D, Borrego JJ. 2007. Co-occurrence of viral and bacterial pathogens in disease outbreaks affecting newly cultured sparid fish. Int. Microbiol. 10:193–199. 10.2436/20.1501.01.27 [DOI] [PubMed] [Google Scholar]
- 41.Dobos P. 1995. The molecular biology of infectious pancreatic necrosis virus (IPNV). Annu. Rev. Fish Dis. 5:25–54. 10.1016/0959-8030(95)00003-8 [DOI] [Google Scholar]
- 42.Rodriguez Saint-Jean S, Borrego JJ, Perez-Prieto SI. 2003. Infectious pancreatic necrosis virus: biology, pathogenesis, and diagnostic methods. Adv. Virus Res. 62:113–165. 10.1016/S0065-3527(03)62003-8 [DOI] [PubMed] [Google Scholar]
- 43.Cutrín JM, Barja JL, Nicholson BL, Bandín I, Blake S, Dopazo CP. 2004. Restriction fragment length polymorphisms and sequence analysis: an approach for genotyping infectious pancreatic necrosis virus reference strains and other aquabirnaviruses isolated from northwestern Spain. Appl. Environ. Microbiol. 70:1059–1067. 10.1128/AEM.70.2.1059-1067.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cutrín JM, Olveira JG, Barja JL, Dopazo CP. 2000. Diversity of infectious pancreatic necrosis virus strains isolated from fish, shellfish, and other reservoirs in northwestern Spain. Appl. Environ. Microbiol. 66:839–843. 10.1128/AEM.66.2.839-843.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero-Brey I, Bandín I, Cutrín JM, Vakharia VN, Dopazo CP. 2009. Genetic analysis of aquabirnaviruses isolated from wild fish reveals occurrence of natural reassortment of infectious pancreatic necrosis virus. J. Fish Dis. 32:585–595. 10.1111/j.1365-2761.2009.01020.x [DOI] [PubMed] [Google Scholar]
- 46.Skliris GP, Krondiris JV, Sideris DC, Shinn AP, Starkey WG, Richards RH. 2001. Phylogenetic and antigenic characterization of new fish nodavirus isolates from Europe and Asia. Virus Res. 75:59–67. 10.1016/S0168-1702(01)00225-8 [DOI] [PubMed] [Google Scholar]
- 47.Thiéry R, Cozien J, de Boisseson C, Kerbart-Boscher S, Nevarez L. 2004. Genomic classification of new betanodavirus isolates by phylogenetic analysis of the coat protein gene suggests a low host-fish species specificity. J. Gen. Virol. 85:3079–3087. 10.1099/vir.0.80264-0 [DOI] [PubMed] [Google Scholar]
- 48.Toffolo V, Negrisolo E, Maltese C, Bov G, Belveder P, Colombo L, Valle LD. 2007. Phylogeny of betanodaviruses and molecular evolution of their RNA polymerase and coat proteins. Mol. Phylogenet. Evol. 43:298–308. 10.1016/j.ympev.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 49.Cutrín JM, Dopazo CP, Thiéry R, Leao P, Olveira JG, Barja JL, Bandin I. 2007. Emergence of pathogenic betanodaviruses belonging to the SJNNV genogroup in farmed fish species from the Iberian Peninsula. J. Fish Dis. 30:225–232. 10.1111/j.1365-2761.2007.00803.x [DOI] [PubMed] [Google Scholar]
- 50.Névarez L, Cozien J, Thiéry R. 2005. Analyse phylogénétique comparée des gènes de la protéine de capside et de l'ARN polymérase des nodavirus de poissons. A52. VIIèmes Journées francophones de Virologie, 28-29 Avril 2005 Paris. Virologie 9:115–166 (In French.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.