Abstract
The structures of polysaccharides (PS) isolated from Lactobacillus rhamnosus LOCK 0900 and results from stimulation of mouse bone marrow-derived dendritic cells (BM-DC) and human embryonal kidney (HEK293) cells stably transfected with Toll-like receptors (TLR) upon exposure to these antigens were studied. L. rhamnosus LOCK 0900 produces PS that differ greatly in their structure. The polymer L900/2, with a high average molecular mass of 830 kDa, is a branched heteropolysaccharide with a unique repeating unit consisting of seven sugar residues and pyruvic acid, whereas L900/3 has a low average molecular mass of 18 kDa and contains a pentasaccharide repeating unit and phosphorus. Furthermore, we found that both described PS neither induce cytokine production and maturation of mouse BM-DC nor induce signaling through TLR2/TLR4 receptors. However, they differ profoundly in their abilities to modulate the BM-DC immune response to the well-characterized human isolate Lactobacillus plantarum WCFS1. Exposure to L900/2 enhanced interleukin-10 (IL-10) production induced by L. plantarum WCFS1, while in contrast, L900/3 enhanced the production of IL-12p70. We conclude that PS, probably due to their chemical features, are able to modulate the immune responses to third-party antigens. The ability to induce regulatory IL-10 by L900/2 opens up the possibility to use this PS in therapy of inflammatory conditions, such as inflammatory bowel disease, whereas L900/3 might be useful in reverting the antigen-dependent Th2-skewed immune responses in allergies.
INTRODUCTION
Human bodies provide a habitat for 10 trillion to 100 trillion microorganisms, such as bacteria, viruses, and eukaryotes, and collectively this complex community constitutes our microbiota. Accumulating evidence indicates that especially the intestinal microbiota plays a major role in health and disease in humans; it has been shown to regulate our physiology and metabolism and, perhaps more importantly, the gut microbiota provides signals for proper development of intestinal as well as systemic immune compartments (1, 2).
Probiotics, mainly lactobacilli and bifidobacteria, are live microorganisms which are able to exert beneficial effects on the host (3). These bacterial strains as well as their secreted products and surface antigens, i.e., bacteriocins, short-chain fatty acids, polysaccharides (PS), and surface proteins are recognized as immunomodulators (4). At the intestinal epithelial level, bacteria and antigens are sampled by dendritic cells (DC) and subsequently presented to naive T cells in Peyer's patches and mesenteric lymph nodes. Recognition of microbe-associated molecular patterns is known to be mediated by pattern recognition receptors (PRR), including the membrane-bound Toll-like receptor family (TLR) and the intracellular nucleotide-binding oligomerization domain proteins (NOD-like receptors [NLR]), that signal the presence of specific microorganisms to the host (5). However, the molecular mechanisms involved in this cross talk remain poorly understood.
The cell envelope of Lactobacillus species contains several effector molecules, including lipoteichoic acid, peptidoglycan, and (glyco)proteins, that are pivotal in the direct signaling capacity of these bacteria and that underlie their immunomodulatory effects. Moreover, the cell envelope contains several compounds, such as cell wall teichoic acid and PS, that may not be involved in direct signaling to the host cell but still affect signaling through shielding of other bacterial effector molecules (6). Bacterial PS consist of repeating mono- or oligosaccharide subunits connected by various glycosidic linkages, thereby generating homo- or heteropolymers, respectively, that are structurally very diverse (7). They can be replaced by nonsugar compounds and attain linear or ramified final conformations. They could be covalently linked to bacterial surface molecules, forming a capsule or loosely attached structures, or they can be secreted into the environment. As PS-producing bacterial strains have been traditionally used for the manufacture of fermented dairy products, most of the research concerning the lactobacilli polysaccharides has focused on their rheological properties. Although very little is known about the structure and biological functions of lactobacilli PS, it has been suggested that these antigens play an essential role in the adhesion phenomenon, e.g., they can reduce the adherence of probiotic strains and/or increase the adherence of enteropathogens (8). Recently, PS have been considered critical in host-microbe interactions (9) and function in immunomodulation (10), and they were reported to counteract the toxic effects of bacterial toxins and enteropathogens (11, 12).
The aim of this work was to isolate and characterize the structure and immunomodulatory properties of PS produced by the recently described probiotic strain Lactobacillus rhamnosus LOCK 0900 (13).
MATERIALS AND METHODS
Microorganisms and growth conditions.
Lactobacillus rhamnosus LOCK 0900 (formerly Lactobacillus casei LOCK 0900 [14]; U.S. patent application 209988) was isolated from feces of a healthy 26-year-old woman and was obtained from the Pure Culture Collection of the Technical University, Lodz, Poland (deposited under LOCK 0900). The species of the Lactobacillus genus was identified based on the sequences of genomic markers, such as 16S rRNA, rpoA, and pheS genes (15). The genome analysis of this bacterium confirmed the presence of a typical polysaccharide biosynthesis cluster that has been described for L. rhamnosus strains (16). Lactobacillus plantarum WCFS1 is a single-colony isolate from L. plantarum NCIMB8826, originally isolated from human saliva (National Collection of Industrial and Marine Bacteria, Aberdeen, United Kingdom) (17). It has been shown to have potent pro-Th1 immunomodulatory properties (18, 19). Strains were stored at −75°C in MRS broth (Difco) supplemented with 20% glycerol, and they were subcultured twice in MRS broth (Biocorp) under anaerobic conditions at 37°C for 48 h before use. For in vitro experiments, bacteria were inactivated in 1% formaldehyde–phosphate-buffered saline (PBS) as described previously (20).
Isolation of polysaccharide antigens.
Isolation of polysaccharide antigens from L. rhamnosus LOCK 0900 was performed as described before (21), with small modifications. Briefly, cells were harvested by centrifugation at 5,000 × g (4°C, 20 min) and washed twice with PBS and once with Milli-Q water and resuspended in 50 ml of PBS. Suspensions were transferred into plastic containers and frozen at −70°C. The frozen bacterial mass was desiccated under vacuum for 24 h (−55°C, 20 Pa). The freeze-dried bacterial mass (10 g) was extracted with 50 ml of 10% trichloroacetic acid (25°C, 2.5 h) and centrifuged at 13,000 × g for 20 min. The pellet was discarded and the supernatant was collected. Polysaccharide antigens were precipitated with 5 volumes of cold 96% ethanol (4°C, overnight), and after centrifugation the pellet was suspended in water (20 ml), dialyzed for 48 h against water, and then lyophilized. Polysaccharide antigens isolated from culture supernatants were precipitated by gradually adding cold ethanol to 80% (vol/vol). The material was dissolved in water and dialyzed for 48 h against water and then lyophilized.
Purification of exopolysaccharides.
The freeze-dried preparations of crude polysaccharide antigens (20 mg) were dissolved in 1 ml of buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2), treated with DNase (210 μg; Sigma) and RNase (210 μg; Sigma) at 37°C for 6 h and with protease from Streptomyces griseus (447 μg; 37°C, overnight; Sigma), and finally dialyzed against water at 4°C for 24 h. The polysaccharides were purified by ion-exchange chromatography on a DEAE–Sephadex A-25 column (1.6 by 20 cm; Pharmacia). The neutral fractions were eluted with 20 mM Tris buffer (pH 8.2), whereas the charged fractions were obtained by eluting with an NaCl gradient (0 to 2 M) in 20 mM Tris buffer (pH 8.2) at a flow rate of 0.4 ml/min, monitored at 220 nm with a UV-Vis absorbance detector (contaminant detection) and differential refractometer (Knauer); carbohydrate content was determined by using the phenol-sulfuric acid method (22). The amount of phosphate was determined as described before (23). The fractions containing polysaccharide were pooled, desalted by dialysis against water at 4°C for 24 h, lyophilized, and further purified by gel permeation chromatography on a TSK HW-55S column (1.6 by 100 cm; Amersham Pharmacia Biotech) fitted to a fast-performance liquid chromatography system (Amersham Pharmacia Biotech) and eluted with 0.1 M ammonium acetate buffer. The column effluents were monitored with an absorbance detector at a λ of 280 nm (protein contamination detection) and with a differential refractometer (Knauer), and also for the carbohydrate content as described above. Purified PS were tested in the Limulus amoebocyte lysate (LAL) assay (PyroGene recombinant factor C endotoxin detection assay; Lonza). The levels of endotoxin were below 0.1 endotoxin units per 1 μg of pure polysaccharide.
Sugar and methylation analysis, determination of molecular mass, and absolute configuration of monosaccharides.
For sugar analysis, the polysaccharide sample (0.5 mg) was hydrolyzed with 10 M HCl at 80°C for 25 min, followed by evaporation under a stream of N2. The resulting monosaccharides were converted into alditol acetates as described previously (24). For methylation analysis, the polysaccharide samples were first peracetylated with a mixture of trifluoroacetic anhydride and acetic acid (2:1 [vol/vol]; 25°C, 10 min) and then permethylated according to a method described before (25). The product was purified by water-chloroform extraction. The methylated polysaccharide was hydrolyzed with 2 M trifluoroacetic acid at 120°C for 2 h and evaporated under N2. Finally, methylated monosaccharides were reduced with NaBD4 and acetylated for gas-liquid chromatography–mass spectrometry (GLC-MS) analysis using the same conditions as for the sugar analysis and butyl glycosides as described below. The absolute configuration of the sugars present in the PS were determined as described in reference 26, using GLC-MS of their acetylated glycosides and (S)-2-butanol. Alditol acetates and acetylated 2-butyl glycosides were analyzed by GLC-MS using an ITQ 700 Thermo Scientific system equipped with a ZB-5HT capillary column (Phenomenex) with a temperature gradient from 150°C to 270°C at 8°C/min. The average molecular masses of the PS were determined by gel permeation chromatography (GPC; Dionex Ultimate 3000) on an OHpak SB-806M HQ column (8 by 300 mm; Shodex), calibrated with dextran standards (mass, 12, 25, 50, 80, 150, and 270 kDa) with 0.1 M ammonium acetate buffer as the eluent. The flow rate was 0.5 ml/min, and the column eluate was monitored with a refractive index detector (Shodex RI 102). The working detector temperature and sensitivity were adjusted to 35°C and 512×, respectively. GPC separation was performed using a OHpak SB-806M HQ apparatus (pore size maximum of 15,000 Å; Shodex, Munich, Germany). System control, data acquisition, and treatments were performed using Chromeleon software (Dionex).
NMR spectroscopy.
The nuclear magnetic resonance (NMR) spectra were obtained on a Bruker 600 MHz Avance III spectrometer with a 5-mm QCI 1H/13C/15N/31P probe equipped with a z-gradient. The NMR spectra were obtained for 2H2O solutions of the polysaccharides at 25°C by using acetone (δH 2.225, δC 31.05 ppm) as an internal reference. The polysaccharide (10 mg) was repeatedly exchanged with 2H2O with intermediate lyophilization. The data were acquired and processed using Bruker Topspin software (version 3.1) and SPARKY (27). The signals were assigned using one- and two-dimensional experiments, correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), nuclear Overhauser effect spectroscopy (NOESY), 1H-detected heteronuclear single-quantum coherence spectroscopy (HSQC) with and without carbon decoupling, HSQC-TOCSY, and 1H-detected heteronuclear multiple-bond correlation spectroscopy (HMBC). The TOCSY experiments were carried out with mixing times of 30, 60, and 100 ms, NOESY was with mixing times of 100 ms and 300 ms, and HMBC was performed with a 60-ms mixing time.
Preparation and activation of bone marrow-derived dendritic cells.
BM-DC were prepared from BALB/c mice. The mouse bone marrow precursors were isolated from femurs and tibias of mice. Cells were cultured at 4 × 105/ml in bacteriological petri dishes in 10 ml culture medium with granulocyte-macrophage colony-stimulating factor (20 ng/ml; eBioscience). Fresh medium was added at days 3 and 6, and BM-DC were used on day 8 of culture. The purity of the obtained BM-DC was assessed by CD11c staining and flow cytometry, and it ranged between 69% and 75% (data not shown). BM-DC (106 cells/ml) were stimulated with 10 μg/ml of polysaccharide antigens, 107 CFU/ml of formalin-inactivated L. rhamnosus LOCK 0900, 107 CFU/ml of formalin-inactivated L. plantarum WCFS1, and a mixture of polysaccharide and formalin-inactivated L. plantarum WCFS1 (107 CFU/ml) for 20 h. As controls, BM-DC were incubated with PAM3 (Pam3CSK4; 1 μg/ml; InvivoGen) or ultrapure lipopolysaccharide (LPS) from Escherichia coli (LPS-EB; 1 μg/ml; InvivoGen). Levels of IL-10 and IL-12p70 in culture supernatants were determined by using enzyme-linked immunosorbent assay (ELISA) Ready-Set-Go! kits (eBioscience) according to the manufacturer's instructions. The experiments were approved by the 1st Local Committee for Experiments with the Use of Laboratory Animals, Wroclaw, Poland (number 38/2012).
Flow cytometry analysis.
DC were labeled with monoclonal antibodies for CD11c (conjugated to fluorescein isothiocyanate), major histocompatibility complex II (MHC-II; conjugated to allophycocyanin), or CD40, CD80, or CD86 (each conjugated to phycoerythrin; eBioscience). Appropriate isotype antibodies were used as controls to determine nonspecific binding. Cells were analyzed using a FACSCalibur flow cytometer (Becton-Dickinson), and obtained data were analyzed with FlowJo 7.6.2 software (TreeStar).
Stimulation of HEK293 cells stably transfected with TLRs.
HEK293 cells stably transfected with plasmids carrying the human hTLR2/CD14 gene were kindly provided by M. Yazdanbakhsh (Leiden, Netherlands). Cells transfected with hTLR4/MD2/CD14 were a gift of B. Bohle (Vienna, Austria). Cells were stimulated with PS antigens (10 μg/ml), 107 CFU/ml of formalin-inactivated L. plantarum WCFS1, or a mixture of PS and formalin-inactivated L. plantarum WCFS1 (107 CFU/ml) for 20 h. TLR2 ligand PAM3 (Pam3CSK4; 1 μg/ml; InvivoGen) and TLR4 ligand ultrapure LPS from E. coli (LPS-EB; 1 μg/ml; InvivoGen) were used as positive controls. After the 20-h incubation period, culture supernatants were harvested and the concentration of human IL-8 was analyzed by ELISA (Thermo Scientific) according to the manufacturer's instructions.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test using Prism 5.04 software (GraphPad, San Diego, CA). P values of <0.05 were considered significant.
RESULTS
Polysaccharides were obtained from the bacterial masses and from the culture supernatants. Samples were treated with DNase/RNase and protease in order to eliminate contaminating nucleic acids and proteins. Antigens were purified by ion-exchange chromatography on DEAE–Sephadex A-25 columns. The typical chromatographic pattern of the ion-exchange chromatography consisted of a saccharide-positive fraction of neutral polysaccharide and a fraction typical for negatively charged polysaccharide. Further, both neutral and charged fractions were purified by gel chromatography on a TSK HW-55S column. Finally, two fractions from the culture supernatant (L900/1 and L900/2) and two fractions from the bacterial cell mass (L900/3 and L900/4), containing polysaccharides differing in molecular mass and charge, were obtained.
Chemical analysis.
The four fractions were investigated for monosaccharide composition and molecular mass. GLC-MS analysis of obtained alditol acetates (sugar and methylation analysis) and acetylated 2-butyl glycosides (determination of the absolute configuration) revealed the following.
(i) L900/1.
L900/1 is composed of d-Man, d-Glc, d-Gal, d-GlcNAc, and d-GalNAc in a molar ratio of 0.2:0.5:1:1:0.2. Methylation analysis revealed the presence of 6-substituted hexose, 3-substituted hexose, 4- or 5-substituted hexose, 3-substituted hexosamine, 3,6-disubstituted hexose, and t-hexosamine, in a molar ratio of 1.2:1:0.9:2:1:0.8. The average molecular mass was approximately 20 kDa.
(ii) L900/2.
L900/2 is composed of d-Fuc, d-Man, d-Glc, and d-Gal in a molar ratio of 3.5:1:1:1. Methylation analysis revealed the presence of t-Fuc, 3-substituted Fuc and hexose, 2,3-disubstituted Fuc, 3,4,6-trisubstituted hexose, and 2-substituted hexose in a molar ratio of 0.5:3:1:0.5:0.5:1. The average molecular mass was approximately 830 kDa.
(iii) L900/3.
L900/3 is composed of d-Glc, d-Gal, d-ManNAc, and d-GalNAc in a molar ratio of 1:0.5:0.5: 0.8. Methylation analysis revealed the presence of 6-substituted hexose, 3,4-disubstituted or 3,5-disubstituted hexose, 3-substituted GalNAc, 4- or 5-substituted hexose, and t-hexose in a molar ratio of 0.5:0.4:0.8:0.5:1. The average molecular mass was 18 kDa.
(iv) L900/4.
L900/4 is composed of d-Fuc, d-Man, d-Glc, and d-Gal in a molar ratio of 3:1:1:1. Methylation analysis revealed the presence of t-Fuc, 3-substituted Fuc and hexose, 2,3-disubstituted Fuc, 3,4,6-trisubstituted hexose, and 2-substituted hexose in a molar ratio of 0.6:4:1:0.5:0.8:1. The average molecular mass was approximately 920 kDa.
Structural studies of polysaccharides L900/2 (L900/4) and L900/3.
The NMR analysis showed that L900/2 and L900/4 have the same structure whereas L900/1 and L900/3 are different. The detailed NMR analysis was performed for L900/2 (L900/4) and L900/3 due to their interesting biological properties, i.e., their ability to modulate the immune response of BM-DC induced by L. plantarum WCFS1 (see “Polysaccharides modulate cytokine production of mouse BM-DC exposed to L. plantarum WCFS1 in an opposite manner,” below). Due to the fact that PS L900/1 had no effect on the immune response induced by L. plantarum WCFS1 (data not shown), a comprehensive analysis of its structure was not performed.
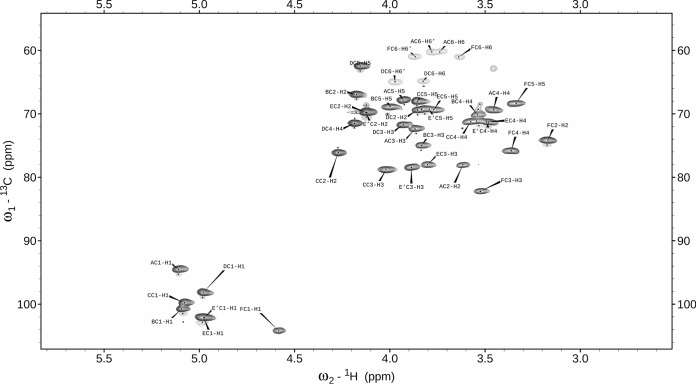
The one-dimensional 1H NMR spectrum at 600 MHz of L900/2 contained seven anomeric proton signals (residues A, B, C, D, E, E′, and F) at 5.11, 5.09, 5.08, 4.98, 4.978, 4.969, and 4.58 ppm. They were correlated with carbon resonances at 94.5, 100.8, 99.7, 98.1, 102.1 (for both residues E and E′), and 104.2 ppm, respectively, as shown in the 1H-13C HSQC spectrum for L900/2 in Fig. 1.
FIG 1.
Selected part of the 1H-13C HSQC NMR spectrum for the L900/2 polysaccharide.
The 1H NMR spectrum revealed also the presence of the pyruvyl groups (δ 1.37 ppm), as well as the four CH3-C groups of the deoxysugars (H-6 at δ 1.106, 1.225, 1.224, and 1.236 ppm). In the COSY experiment, the cross-peaks between the anomeric positions (H-1) and the neighboring H-2 followed by H-3, H-4, and H-5 were observed. Comparison of the TOCSY spectra for increasing mixing times allowed for the assignment of the sequential order of the chemical shifts belonging to a single spin system. The spin systems for all sugar moieties were determined to have a pyranose form. The 1H and 13C NMR resonances were obtained using different two-dimensional (2D) NMR experiments as well as by comparison with previously published 1H and 13C NMR data (28–31); the chemical shifts are reported in Table 1.
TABLE 1.
1H and 13C NMR chemical shifts and selected interresidue connectivities from the anomeric protons of L900/2 (L900/4) from Lactobacillus rhamnosus strain LOCK 0900a
| Sugar residue |
1H, 13C chemical shifts (ppm) |
Pyruvic acid residue 1H, 13C shift (ppm) |
Selected connectivity(ies)b (ppm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-1, C-1 | H-2, C-2 | H-3, C-3 | H-4, C-4 | H-5, C-5 | H-6, C-6 | H-6′ | CH3 | C | CO2H | |||
| A | →2)-α-d-Glcp-(1→ | 5.11, 94.5 | 3.62, 77.9 | 3.87, 72.8 | 3.45, 67.7 | 3.93, 69.4 | 3.72, 60.2 | 3.77 | A1-D3 (71.6) | |||
| B | α-d-Fucp-(1→ | 5.09, 100.8 | 4.18, 66.9 | 3.88, 75.0 | 3.54, 70.1 | 3.93, 68.8 | 1.106, 16.5 | B1-B3 and B5 (intra), B1-F3 (82.2) | ||||
| C | →2,3)-α-d-Fucp-(1→ | 5.08, 99.7 | 4.27, 76.0 | 4.02, 78.7 | 3.58, 71.1 | 3.85, 69.3 | 1.225, 16.5 | C1-C3 (intra), C1-A2 (77.9), C1-E2 (69.7) | ||||
| D | →3,4,6)-α-d-Galp-(1→ | 4.98, 98.1 | 3.85, 68.0 | 3.93, 71.6 | 4.18, 71.5 | 4.15, 62.4 | 3.81, 64.8 | 3.96 | D1-E′3 (78.4) | |||
| E | →3)-α-d-Fucp-(1→ | 4.978, 102.1 | 4.12, 69.7 | 3.79, 77.9 | 3.48, 71.1 | 3.78, 69.3 | 1.236, 16.5 | E1-C2 (76.0) | ||||
| E′ | →3)-α-d-Fucp-(1→ | 4.969, 102.1 | 4.12, 69.7 | 3.88, 78.4 | 3.48, 71.3 | 3.79, 69.2 | 1.224, 16.5 | E′1-E3 (77.9) | ||||
| F | →3)-α-d-Manp-(1→ | 4.58, 104.2 | 3.17, 74.2 | 3.53, 82.2 | 3.36, 75.8 | 3.34, 68.3 | 3.64, 60.9 | 3.86 | F1-C3 (78.7) | |||
| Pyruvate | 1.37, 25.0 | —,c 101.6 | —, 175.8 | Pyr-D4 (71.5), D6 (64.8) | ||||||||
Spectra were obtained for 2H2O solutions at 25°C via HMBC. Acetone (δH 2.225, δC 31.05 ppm) was used as an internal reference.
Results in boldface denote interresidual cross peaks, indicating glycosidic linkages between sugar residues in the main chain; “intra” indicates the cross peaks of the protons and carbons ascribed to the intraresidual cross peaks.
—, no 1H shift data.
Residue A was recognized as glucose based on the 1H and 13C chemical shift and strong vicinal coupling constants between all protons in the sugar ring. Residues B, C, E, and E′ were determined to be fucose on the basis of the characteristic proton spin system and 1H and 13C chemical shift of the H-6/C-6 signal. Residue D was recognized as galactose due to the large vicinal coupling constant between H-2 and H-3 and the small vicinal coupling constants between H-3, H-4, and H-5, whereas residue F was assigned as mannose on the basis of the 1H and 13C chemical shift. C-2 and C-3 of pyruvic acid were assigned in the HMBC spectrum. The 1H and 13C NMR chemical shifts for C-5, at δ 69.4 68.8, 69.3, 62.4, 69.3, 69.2, and 68.3 ppm for residues A to F, respectively, indicated that all residues are α-linked (32). Downfield displacements of the signals for C-3 of residues C, D, E, E′, and F (δ 78.7, 71.6, 77.9, 78.4, and 82.2 ppm, respectively), C-2 of residue C (δ 76.0 ppm), and C-2 of residue A (δ 77.9 ppm), compared with their respective chemical shifts in the spectra of the corresponding nonsubstituted monosaccharides, revealed the substitution pattern in PS L900/2 (33).
The HMBC spectra showed cross-peaks between the anomeric proton and the carbon at the linkage positions of sugars in L900/2 (Table 1). A strong HMBC cross-peak was found for the C-2 of the pyruvyl between 13C (δ 101.6 ppm) and 1H (δ 4.18, 3.81, and 3.96 ppm), corresponding to H-4 and two H-6s of the D residue, respectively. Attribution of the chemical shifts corresponding to the quaternary carbons (C and COOH) of the substituents was performed using HMBC data. Only one signal was observed for the pyruvylated hexose, indicating that pyruvylation is complete.
Based on these data, the structure of the heptasaccharide repeating unit of the L. rhamnosus LOCK 0900 polysaccharide L900/2 (and L900/4) was determined and is shown in Fig. 2.
FIG 2.
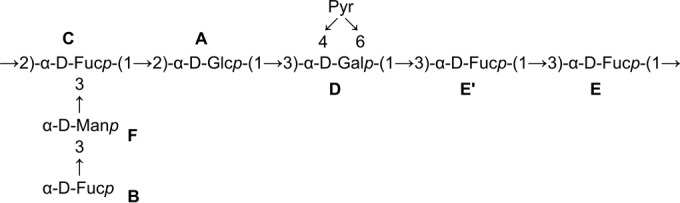
The structure of the heptasaccharide repeating unit of the L. rhamnosus LOCK 0900 polysaccharide L900/2 (L900/4).
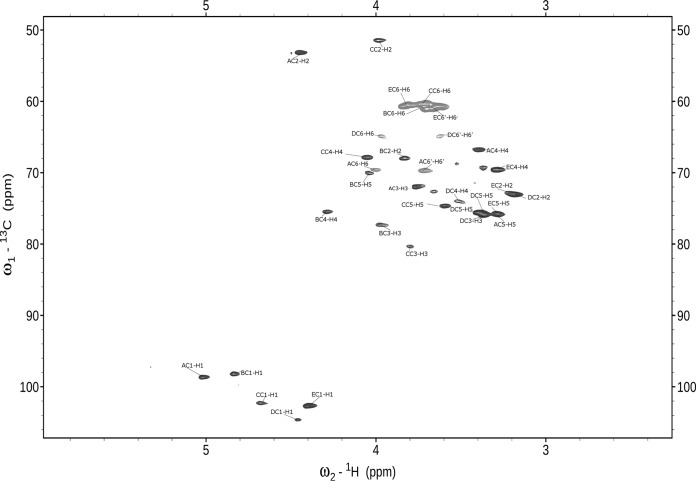
The one-dimensional 1H NMR spectrum at 600 MHz of L900/3 revealed five anomeric proton signals at 5.02, 4.81, 4.65, 4.46, and 4.37 ppm. They were correlated with five carbon resonances at 98.6, 98.2, 103.3, 104.6, and 102.5 ppm, respectively, as shown in the 1H-13C HSQC spectrum of L900/3 in Fig. 3.
FIG 3.
Selected part of the 1H-13C HSQC NMR spectrum for the L900/3 polysaccharide.
These chemical shifts are characteristic for anomeric protons and carbons of pyranoses (34). The 31P NMR spectrum contained one signal for a phosphomonoester group at 2.6 ppm. The 1H NMR spectrum also contained signals for acetyl groups at 1.93 to 1.95 ppm. The five monosaccharides of L900/3 were designated as residues A to E, according to their proton chemical shifts. The complete 1H and 13C chemical shifts for all components of L900/3 were assigned by two-dimensional COSY, TOCSY, and HSQC experiments, as well as by comparison with previously published 1H and 13C NMR data (28–33); the chemical shifts are reported in Table 2.
TABLE 2.
1H and 13C NMR chemical shifts and selected interresidue connectivities from the anomeric protons of L900/3 from Lactobacillus rhamnosus strain LOCK 0900a
| Sugar or phosphorus residue |
1H, 13C chemical shifts (ppm) |
Selected connectivity(ies)b (ppm) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H-1, C-1 | H-2, C-2 | H-3, C-3 | H-4, C-4 | H-5, C-5 | H-6, C-6 | H-6′ | CH3CO | NOESY | HMBC | ||
| A | →6)-β-d-ManpNAc-(1→ | 5.02, 98.6 | 4.43, 53.0 | 3.74, 71.8 | 3.46, 66.8 | 3.26, 75.6 | 4.01, 69.4 | 3.72 | 1.93, 22.1 | A1-B4 (4.26) | A1-A2 (intra), A1-B4 (75.5) |
| B | →3,4)-α-d-Galp-(1→ | 4.81, 98.2 | 3.80, 67.9 | 3.95, 77.4 | 4.26, 75.5 | 4.06, 69.9 | 3.68, 61.0 | B1-D4 (3.52), B1-B5 (4.06), B1-B6 (3.68) | B1-B5 (intra) | ||
| C | →3)-β-d-GalpNAc-(1→ | 4.65, 103.3 | 3.97, 51.5 | 3.77, 80.3 | 4.02, 67.9 | 3.56, 74.7 | 3.70, 60.3 | 1.95, 21.9 | C1-B3 (3.95) | C1-B3 (77.4) | |
| D | →4,6)-β-d-Glcp-(1→ | 4.46, 104.6 | 3.28, 72.8 | 3.36, 75.7 | 3.52, 73.8 | 3.39, 75.3 | 3.95, 64.8 | 3.65 | D1-C3 (3.77) | D1-C3 (80.3) | |
| E | β-d-Glcp-(1→ | 4.37, 102.5 | 3.26, 72.9 | 3.37, 75.5 | 3.27, 69.2 | 3.38, 75.9 | 3.63, 60.6 | 3.80 | E1-A6 (4.01 and 3.72) | E1-A6 (69.4) | |
| P | 2.6 | P-D6 (64.8) | |||||||||
Spectra were obtained for 2H2O solutions at 25°C. Acetone (δH 2.225, δC 31.05 ppm) was used as an internal reference.
Results in boldface denote interresidual cross peaks, indicating glycosidic linkages between sugar residues in the main chain; “intra” indicates cross peaks of the protons and carbons ascribed to the intraresidual cross peaks.
Spin systems of all five sugar residues were identified basing on typical 3JH,H coupling constant values (35). The TOCSY spectrum showed cross-peaks of H-1 with H-2 to H-5 of residues A, D, and E, as well as H-1 with H-2 to H-4 of residues B and C. Assignment was completed by the following correlations in the COSY spectrum: H-5/H-6 for residues A, D, and E and H-4/H-5 and H-5/H-6 for residues B and C. Identification of spin systems of the N-acetylamino sugars was confirmed by correlation of protons at carbons bearing nitrogen to the corresponding carbons at 53.0 ppm (C-2 of residue A) and 51.5 ppm (C-2 of residue C), as revealed by the 1H-13C HSQC experiment. From the analysis of TOCSY spectra and 1H and 13C chemical shifts, residue B was determined to have the galacto configuration (large vicinal coupling constant between H-2 and H-3 and the small vicinal coupling constants between H-3, H-4, and H-5), whereas residues D and E have the gluco configuration (small coupling constants). The four anomeric resonances of residues A, C, D, and E had large 3JH1,H2 coupling constants, indicating β-linked pyranosidic residues. Residue B was identified as an α-linked pyranosidic residue based on the 1H and 13C chemical shifts similar to those from α-d-Galp. The chemical shifts identified residues A, B, C, D, and E as N-acetylmannosamine, galactose, N-acetylgalactosamine, and two glucose residues, respectively. The sequence of the monosaccharide residues within the repeating unit of the L900/3 polysaccharide was obtained by assignment of the interresidue connectivities observed in the 2D NOESY and HMBC spectra, which showed cross-peaks between the anomeric protons and the carbons at the linkage positions (Table 2). The phosphate substitution position was revealed by the 1H,31P correlation experiment (HMQC and HSQC). The 31P resonance at δ 2.6 ppm showed connectivity to the H-6 signal at δ 3.95 ppm (H-6 of residue D) and the carbon signal at δ 64.8 ppm (C-6 of residue D). The structure of the pentasaccharide repeating unit of the L. rhamnosus LOCK 0900 polysaccharide L900/3 is depicted in Fig. 4.
FIG 4.
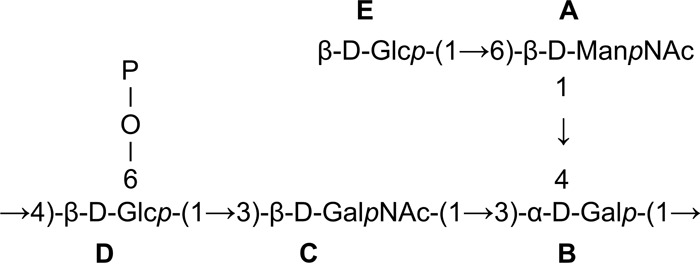
The structure of the pentasaccharide repeating unit of the L. rhamnosus LOCK 0900 polysaccharide L900/3.
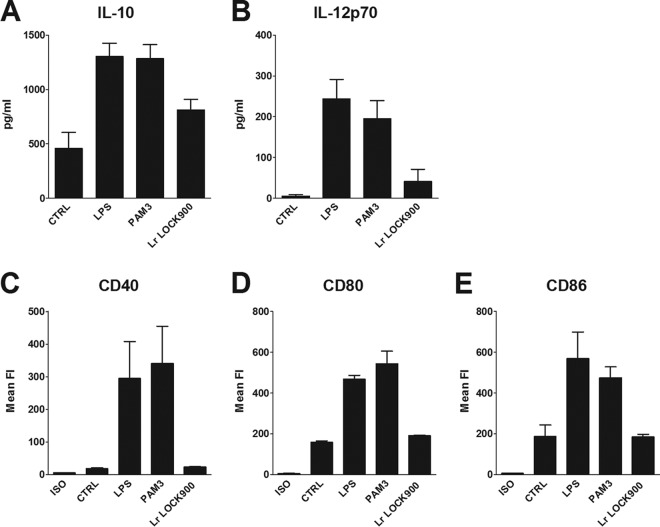
L. rhamnosus LOCK 0900 induces low cytokine production and maturation of bone marrow-derived DC.
Stimulation of BM-DC from BALB/c mice with L. rhamnosus LOCK 0900 induced very low induction of CD40, CD80, and CD86 costimulatory molecules (Fig. 5C to E). Expression levels of these molecules were similar to levels in untreated controls and substantially lower than levels induced by LPS or PAM3. Similarly, the levels of IL-10 and IL-12p70 induced by 107 CFU/ml of L. rhamnosus LOCK 0900 were low (Fig. 5A and B).
FIG 5.
Maturation of DC and cytokine production induced by L. rhamnosus LOCK 0900. BM-DC from naive BALB/c mice were cultured with medium alone (CTRL), ultrapure LPS from E. coli (1 μg/ml), PAM3 (1 μg/ml), or 107 CFU/ml formalin-inactivated L. rhamnosus LOCK 0900 for 20 h. Production of IL-10 (A) and IL-12p70 (B) in culture supernatant was determined by ELISA. Pooled results from three independent experiments are shown. Means ± SEM are shown. BM-DC were gated as MHC-II+ CD11c+ and analyzed by flow cytometry for CD40, CD80, and CD86 expression (C, D, and E). Numbers shown are mean fluorescence units. ISO, isotype controls. Pooled results from three independent experiments are shown.
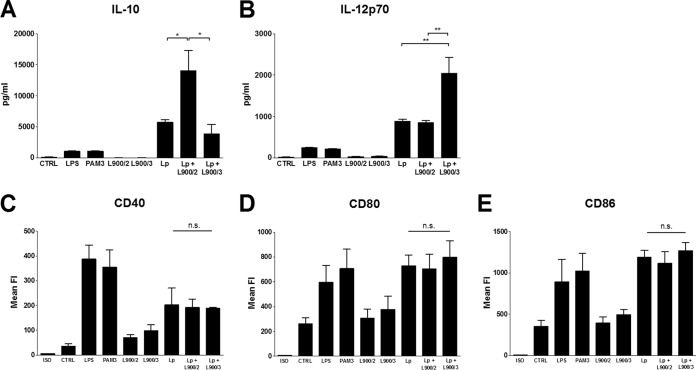
Polysaccharides modulate cytokine production of mouse BM-DC exposed to L. plantarum WCFS1 in an opposite manner.
Stimulation of BM-DC from BALB/c mice with PS L900/2 and L900/3 induced very low induction of CD40, CD80, and CD86 costimulatory molecules (Fig. 6C to E). Expression levels of these molecules were similar to those in untreated controls and substantially lower than levels induced by LPS or PAM3. Similarly, the levels of IL-10 and IL-12p70 induced by 10 μg/ml of L900/2, as well as by L900/3, were low/undetectable and didn't differ significantly from the untreated control groups (Fig. 6A and B). Cocultivation of BM-DC with L. plantarum WCFS1 led to their maturation, as seen from increased expression of CD40, CD80, and CD86 costimulatory molecules (Fig. 6C to E) and induction of both IL-10 and IL-12p70 cytokines (Fig. 5A and B). Interestingly, stimulation of BM-DC with a mixture of L. plantarum WCFS1 and PS led to significant modification of the induced cytokine levels. The mixture of L. plantarum WCFS1 and PS L900/2 induced a significant increase of IL-10 and no changes in IL12p70 production compared to L. plantarum WCFS1 alone (Fig. 5A). On the other hand, the mixture of L900/3 and L. plantarum WCFS1 induced significantly higher levels of IL12p70 while not changing the IL-10 levels (Fig. 6B). Admixing PS with L. plantarum WCFS1 didn't change the levels of costimulatory molecules on DC surfaces (Fig. 6C to E), suggesting that the maturation of BM-DC was not altered.
FIG 6.
Maturation of DC and cytokine production induced by PS L900/2, PS L900/3, L. plantarum WCFS1, and their mixtures. BM-DC from naive BALB/c mice were cultured with medium alone (CTRL), ultrapure LPS from E. coli (1 μg/ml), PAM3 (1 μg/ml), 10 μg/ml of polysaccharide L900/2 or L900/3, 107 CFU/ml of formalin-inactivated L. plantarum, or the mixture of L. plantarum and corresponding PS (L. plantarum plus L900/2 and L. plantarum plus L900/3) for 20 h. Production of IL-10 (A) and IL-12p70 (B) in culture supernatant was determined by ELISA. Pooled results from three independent experiments are shown. Data are means ± SEM. BM-DC were gated as MHC-II+ CD11c+ and analyzed by flow cytometry for CD40, CD80, and CD86 expression (C, D, and E). Numbers shown are mean fluorescence units. ISO, isotype controls. Pooled results from three independent experiments are shown. *, P < 0.05; **, P < 0.01; n.s., not significant.
Polysaccharides are not recognized by TLR2 and TLR4 and they do not affect the recognition of L. plantarum WCFS1.
HEK293 cells stably transfected with TLR2 or TLR4 were stimulated with 10 μg/ml of either L900/2 or L900/3, with L. plantarum WCFS1 (107 CFU/ml) or with a mixture of L. plantarum WCFS1 and the corresponding PS. PAM3 was used as a positive control for TLR2, and LPS was used as the positive control for TLR4. We have shown that polysaccharides do not induce the production of IL-8 in either TLR2- or TLR4-transfected HEK293 cells. Stimulation of HEK293/TLR2 with L. plantarum WCFS1 led to production of IL-8, whereas no induction was observed after stimulation of HEK293/TLR4. Admixing PS L900/2 or PS L900/3 with L. plantarum WCFS1 had no effect on the production of IL-8 compared to L. plantarum WCFS1 alone (Fig. 7A and B).
FIG 7.
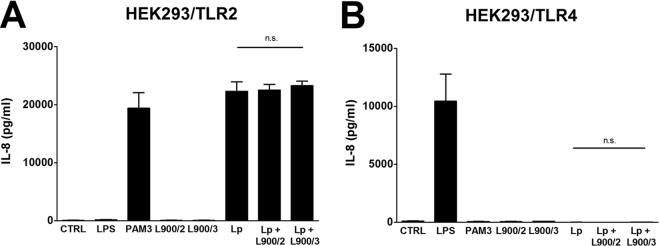
Activation of TLR receptors by PS L900/2, PS L900/3, L. plantarum WCFS1, and their mixture. HEK293 cells stably transfected with an expression vector for human TLR2 (293-hTLR2/CD14) (A) or TLR4 (293-hTLR4/MD2/CD14) (B) were cultured for 20 h with 10 μg/ml of polysaccharide L900/2, L900/3, 107 CFU/ml of formalin-inactivated L. plantarum, or the mixture of L. plantarum and the corresponding PS (L900/2 or L900/3). PAM3 (1 μg/ml) and ultrapure LPS from E. coli (1 μg/ml) were used as positive controls for TLR2 and TLR4, respectively. Unstimulated cells (CTRL) were used as controls. Stimulation was evaluated by measurement of IL-8 production; results are expressed as means ± SEM. One representative experiment out of three is shown. n.s., not significant.
DISCUSSION
The present study was initiated in order to investigate the relationship between the biological role and chemical structure of polysaccharide antigens isolated from the probiotic bacterium Lactobacillus rhamnosus LOCK 0900. We have shown that L. rhamnosus LOCK 0900 produces polysaccharide antigens that differ in molecular mass: the small molecules L900/1 and L900/3 and large molecules L900/2 and L900/4. These antigens also differ in terms of sugar composition: no acetylated amino sugars (L900/2 and L900/4) versus acetylated amino sugars (L900/1 and L900/3). Polymers L900/2, L900/3, and L900/4 are negatively charged, whereas L900/1 is neutral. Moreover, fraction L900/2 (L900/4) is pyruvylated, while fraction L900/3 contains a phosphomonoester. The structure of L900/2 was characterized and found to be identical to the structure of L900/4, whereas L900/3 and L900/1 were different. We comprehensively characterized the structures of two polysaccharides, L900/2 and L900/3. The polymer L900/2, with a high average molecular mass of 830 kDa, is a branched heteropolysaccharide with a repeating unit consisting of seven monosaccharide residues (one Gal, four Fuc, one Man, and one Glc) and pyruvic acid. The presence of 4,6-0-(1-carboxyethylidene) α-galactose as a constituent of the backbone chain makes the structure of polysaccharide L900/2 uncommon. Previously, the pyruvic acid has been described in the exopolysaccharide of Lactobacillus rhamnosus strains RW-9595M and R (36), but mostly it has been reported to occur in extracellular acidic polysaccharides of pathogenic bacteria, including Streptococcus pneumoniae (37), Corynebacterium insidiosum (38), Escherichia coli (39), Klebsiella (40, 41), Rhizobium (42), and Rhodococcus (32). The polymer L900/3, with a low average molecular mass of 1.8 kDa, contains five monosaccharide residues (two Glc, one Gal, one ManNAc, and one GalNAc) and one phosphate group in the repeating unit. The monosaccharides occurring most frequently in the various forms of lactobacillus polysaccharides are glucose, galactose, rhamnose, mannose, fucose, arabinose, and xylose; sugar compounds such as N-acetylgalactosamine and N-acetylglucosamine are also found (7). To our knowledge, this is the first time the presence of N-acetylmannosamine has been described for L. rhamnosus.
In our study, we have shown that neither L900/2 nor L900/3 signal through the TLR4 and TLR2 receptors (whole-bacterium L. rhamnosus LOCK 0900 binds TLR2 [data not shown]). Studies with PS showed that the PS neither changed the BM-DC maturation level nor stimulated the production of IL-10 and IL-12. These results are similar to the results obtained after the stimulation of BM-DC with the whole-bacterium L. rhamnosus LOCK 0900. This is in line with a previous report showing that PS enables commensal bacteria to remain immunologically silent (11). Interestingly, we showed that purified PS can effectively modulate the immune response patterns elicited by another bacterium, L. plantarum WCFS1. We observed significant changes in IL-10 and IL-12p70 levels induced by L. plantarum WCFS1 admixed with polysaccharides compared to results obtained for this bacterium alone. The L900/2 (as a mixture with L. plantarum WCFS1) significantly increased the cytokine IL-10 level, suggesting the induction of regulatory immune responses. On the contrary, L. plantarum WCFS1 admixed with L900/3 significantly increased the IL-12 level, suggesting the potentiation of proinflammatory Th1 responses. Recently, Shan et al. (43) showed that hyperglycosylated mucin MUC2 constrains the immunogenicity of gut antigens by delivering tolerogenic signals to dendritic cells and that this action is MUC2 glycan dependent. In this context, our results demonstrated that the glycans produced by the bacteria also possess the ability to modulate the immune response of DC to antigens and open up a new direction in the way we perceive the bacterium-bacterium and bacterium-host interactions. Although the molecular mechanism of reported activities of L900/2 and L900/3 have yet to be determined, we can speculate that PS might bind to components of the L. plantarum WCFS1 surface and restrain them from interacting with host cell surface pattern recognition receptors, thus altering the recognition and signaling pathway activation. Alternatively, PS may bind directly to the receptors on the host cell surface, such as C-type lectin receptors, e.g., dectin-1, dectin-2, or mannan receptors, altering the signaling pathway induced by bacteria (44). Given the fact that we have seen no alteration in the BM-DC maturation status and TLR signaling for L. plantarum WCFS1 admixed with PS compared to L. plantarum WCFS1 alone, the latter possibility seems more probable.
It is not clear which polysaccharides are the most suitable molecules for the specific beneficial properties. However, it has been hypothesized that negatively charged polysaccharides with low molecular weights (MW) seem to be able to act as stimulators of immune cells, whereas neutral and high-MW polymers elicit a suppressing profile (45, 46). Shao et al. showed that a polysaccharide with a low MW was a stronger stimulator of the proliferation of splenocytes compared to PS with a high MW (47). However, the monosaccharide compositions were diametrically different, suggesting that the observed results may also have been dependent on structure rather than molecular mass. Interestingly, Surayot et al. (48) showed that glucan from L. confusus TISTR 1498, with the relatively high-MW ranges of 65 × 106 to 506 × 106, was not able to stimulate RAW264.7 cells. However, when partially hydrolyzed (MW of ≤70 × 103), the PS significantly activated the macrophages and induced considerable amounts of NO and TNF-α, IL-1, IL-6, and IL-10. This could be due to the fact that polysaccharides with low MW have better binding capacities to the cell receptors than those with higher MW (48). In this study, we showed that even if a PS did not induce the immune response by itself, it was able to elicit different effects when applied in conjunction with L. plantarum WCFS1. Our results are in accordance with the results reported above, because we also observed that L900/3 (a small, negatively charged polymer) was able to act as a strong stimulator of immune cells (increased IL-12p70 production). The large L900/2, although it is negatively charged, exhibited a suppressive/regulatory induction profile.
Most reports have shown that polysaccharides isolated from lactic acid bacteria are immunomodulators themselves. They have been shown to exhibit significant in vitro antioxidant activities and immunomodulatory properties (49), inhibit the proliferation potential of human gastric cancer BGG-823 cells (50), show strong antitumor activities against Caco-2, BCG-823, and HT-28 cells (51), increase the IgA-positive cells in the intestine and the serum levels of IL-4, IL-10, and IFN-γ (52, 53), and ameliorate arthritis in active models of collagen-induced arthritis (54). The fact that in most studies that described the biological functions of PS (45–59) there was only a partial chemical characterization of these molecules renders it difficult to make general statements about the properties that PS possess, since the natures of PS molecules are highly diverse. It seems that immunomodulatory properties are both physical and chemical property dependent (43, 48). However, to draw a general conclusion about the capability of a polysaccharide to induce a given immune response, it is necessary to conduct a comprehensive immunochemical characterization (sugar composition, molecular mass, presence of charged substituents, type of polysaccharide). Moreover, in most published studies the presence of lipo-contamination, e.g., lipoteichoic acid or LPS, is not reported, although the presence of these compounds might lead to false attribution of results to the studied PS. Furthermore, to gain insight into a molecular mechanism involved in polysaccharide-induced effects, high-purity antigens are required.
Conclusions.
The gastrointestinal tract provides a protective interface between the internal environment and the challenge from food-derived and microbial antigens of the external environment. A more complete understanding of how the microflora, the mucosal immune system, and the epithelium of the host communicate with each other and how the cumulative output of this conversation communicates with the host outside the gut represent both a potent challenge and a tremendous opportunity in critical care research. The interactions between bacteria and between a bacterium and its host are mediated mainly by cell surface components, which are able to interact with specific receptors. Bacterial polysaccharides are one of the surface molecules that could play a role as mediators of this cross talk. It has been recently shown that glycans associated with mucus are able to imprint dendritic cells with anti-inflammatory properties and thus actively change the immune response toward the bacterial/food antigens (43). Interestingly, we also found that L. rhamnosus-derived PS could actively change the immune response to another bacterium. Our study shows that one strain can produce PS that differ in immunomodulatory effects, and it suggests that the high-molecular-mass PS L900/2 acts as a regulatory molecule, whereas the small polymer L900/3 is able to elicit an increased proinflammatory response. It is tempting to speculate that the ability to induce regulatory IL-10 by L900/2 opens up the possibility to use this PS in therapy of inflammatory conditions, such as inflammatory bowel disease, whereas L900/3 might be useful in reverting the antigen-dependent Th2-skewed immune responses in allergies. However, further experiments to evaluate these concepts are needed.
ACKNOWLEDGMENT
This work was supported by a grant cofounded by the National Science Centre of Poland under grant decision number UMO-2012/05/D/NZ7/02494.
Footnotes
Published ahead of print 8 August 2014
REFERENCES
- 1.Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. 2011. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 19:349–359. 10.1016/j.tim.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Srůtková D, Zídek Z, Schwarzer M, Drastich P, Funda DP. 2011. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 8:110–120. 10.1038/cmi.2010.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani BR, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11:506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 4.Ruas-Madiedo P, Medrano M, Salazar N, de los Reyes-Gavilán CG, Pérez P, Abraham AG. 2010. Exopolysaccharides produced by Lactobacillus and Bifidobacterium strains abrogate in vitro de cytotoxic effect of bacterial toxins on eukaryotic cells. J. Appl. Microbiol. 109:2079–2086. 10.1111/j.1365-2672.2010.04839.x [DOI] [PubMed] [Google Scholar]
- 5.Philpott DJ, Girardin SE. 2004. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol. Immunol. 41:1099–1108. 10.1016/j.molimm.2004.06.012 [DOI] [PubMed] [Google Scholar]
- 6.Lee I, Timita S, Kleerebezem M, Bron PA. 2013. The quest for probiotic effector molecules: unraveling strain specificity at the molecular level. Pharmacol. Res. 69:61–74. 10.1016/j.phrs.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 7.Górska S, Grycko P, Rybka J, Gamian A. 2007. Exopolysaccharide of lactic acid bacteria: structure and biosynthesis. Postępy Hig. Med. Dosw. 61:805–818 [PubMed] [Google Scholar]
- 8.Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilan CG, Salminen S. 2006. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J. Food Prot. 69:2011–2015 [DOI] [PubMed] [Google Scholar]
- 9.Conover MS, Sloan GP, Love CF, Sukumar N, Deora R. 2010. The Bps polysaccharide of Bordetella pertussis promotes colonization and biofilm formation in the nose by functioning as an adhesin. Mol. Microbiol. 77:1439–1455. 10.1111/j.1365-2958.2010.07297.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebeer S, Vanderleyden J, de Keersmaecker SCJ. 2010. Host interactions of probiotic bacterial surface molecule: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8:171–184. 10.1038/nrmicro2297 [DOI] [PubMed] [Google Scholar]
- 11.Fanning S, Hall LJ, van Sinderen D. 2012. Bifidobacterium breve UCC2003 surface exopolysaccharide production is a beneficial trait mediating commensal-host interaction through immune modulation and pathogen protection. Gut Microbes 3:420–425. 10.4161/gmic.20630 [DOI] [PubMed] [Google Scholar]
- 12.Medrano M, Hamet MF, Abraham AG, Pérez PF. 2009. Kefiran protects Caco-2 cells from cytopathic effects induced by Bacillus cereus infection. Antonie Van Leeuwenhoek 96:505–513. 10.1007/s10482-009-9366-z [DOI] [PubMed] [Google Scholar]
- 13.Cukrowska B, Rosiak I, Klewicka E, Motyl I, Schwarzer M, Libudzisz Z, Kozakova H. 2010. Impact of heat-inactivated Lactobacillus casei and Lactobacillus paracasei strains on cytokine responses in whole blood cell cultures of children with atopic dermatitis. Folia Microbiol. (Praha) 55:277–280. 10.1007/s12223-010-0041-6 [DOI] [PubMed] [Google Scholar]
- 14.Aleksandrzak-Piekarczyk T, Koryszewska-Bagińska A, Bardowski J. 2013. Genome sequence of the probiotic strain Lactobacillus rhamnosus (formerly Lactobacillus casei) LOCK900. Genome Announc. 1:e00640-13. 10.1128/genomeA.00640-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naser SM, Dawyndt P, Hoste B, Gevers D, Vandemeulebroecke K, Cleenwerck I, Vancanneyt M, Swings J. 2007. Identification of lactobacilli by pheS and rpoA gene sequence analyses. Int. J. Syst. Evol. Microbiol. 57:2777–2789. 10.1099/ijs.0.64711-0 [DOI] [PubMed] [Google Scholar]
- 16.Péant B, LaPointe G, Gilbert C, Atlan D, Ward P, Roy D. 2005. Comparative analysis of the exopolysaccharide biosynthesis gene clusters from four strains of Lactobacillus rhamnosus. Microbiology 151:1839–1851. 10.1099/mic.0.27852-0 [DOI] [PubMed] [Google Scholar]
- 17.Hayward AC, Davis GHG. 1956. The isolation and classification of Lactobacillus strains from Italian saliva. Br. Dent. J. 101:43–46 [Google Scholar]
- 18.Rigaux P, Daniel C, Hisbergues M, Muraille E, Hols P, Pot B, Pestel J, Jacquet A. 2009. Immunomodulatory properties of Lactobacillus plantarum and its use as a recombinant vaccine against mite allergy. Allergy 64:406–414. 10.1111/j.1398-9995.2008.01825.x [DOI] [PubMed] [Google Scholar]
- 19.Schwarzer M, Repa A, Daniel C, Schabussova I, Hrncir T, Pot B, Stepankova R, Hudcovic T, Pollak A, Tlaskalova-Hogenova H, Wiedermann U, Kozakova H. 2011. Neonatal colonization of mice with Lactobacillus plantarum producing the aeroallergen Bet v 1 biases towards Th1 and T-regulatory responses upon systemic sensitization. Allergy 66:368–375. 10.1111/j.1398-9995.2010.02488.x [DOI] [PubMed] [Google Scholar]
- 20.Repa A, Grangette C, Daniel C, Hochreiter R, Hoffmann-Sommergruber K, Thalhamer J, Kraft D, Breiteneder H, Mercenier A, Wiedermann U. 2003. Mucosal co-application of lactic acid bacteria and allergen induces counter-regulatory immune responses in a murine model of birch pollen allergy. Vaccine 22:87–95. 10.1016/S0264-410X(03)00528-0 [DOI] [PubMed] [Google Scholar]
- 21.Górska-Frączek S, Sandström C, Kenne L, Paściak M, Brzozowska E, Strus M, Heczko P, Gamian A. 2013. The structure and immunoreactivity of exopolysaccharide isolated from Lactobacillus johnsonii strain 151. Carbohydr. Res. 378:148–153. 10.1016/j.carres.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 22.Dubois M, Giller KA, Rebers PA, Smith FA. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356. 10.1021/ac60111a017 [DOI] [Google Scholar]
- 23.Chen PS, Toribara TV, Warner H. 1956. Microdetermination of phosphorus. Anal. Chem. 28:1756–1758. 10.1021/ac60119a033 [DOI] [Google Scholar]
- 24.Sawardeker JS, Sloneker JH, Jeanes A. 1956. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 37:1602–1603 [Google Scholar]
- 25.Ciukanu I, Kerek FA. 1984. simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209–217. 10.1016/0008-6215(84)85242-8 [DOI] [Google Scholar]
- 26.Gerwig GJ, Kamerling JP, Vliegenthart JFG. 1979. Determination of the absolute configuration of monosaccharides in complex carbohydrates by capillary G.L.C. Carbohydr. Res. 77:10–17 [DOI] [PubMed] [Google Scholar]
- 27.Goddard TD, Kneller DG. 2001. SPARKY, 3rd ed. University of California, San Francisco, San Francisco, CA [Google Scholar]
- 28.Bock K, Pedersen C. 1983. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv. Carbohydr. Chem. Biochem. 41:27–66. 10.1016/S0065-2318(08)60055-4 [DOI] [Google Scholar]
- 29.Lipkind GM, Shashkov AS, Knirel YA, Vinogradov EV, Kochetkov NK. 1988. A computer-assisted structural analysis of regular polysaccharides on the basis of carbon-13 NMR data. Carbohydr. Res. 175:59–75. 10.1016/0008-6215(88)80156-3 [DOI] [PubMed] [Google Scholar]
- 30.Gorin PAJ, Mazurek M. 1975. Further studies on the assignment of signals in 13C magnetic resonance spectra of aldoses and derived methyl glycosides. Can. J. Chem. 53:1212–1223. 10.1139/v75-168 [DOI] [Google Scholar]
- 31.Urai M, Aizawa T, Anzai H, Oihara J, Iwabuchi N, Neilan B, Couperwhite I, Nakajima M, Sunairi M. 2006. Structural analysis of an extracellular polysacchardie porduced by a benzene tolerant bacterium, Rhodococcus sp. 33. Carbohydr. Res. 341:616–623. 10.1016/j.carres.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 32.Katzenellenbogen E, Kocharova NA, Toukach PV, Górska S, Bogulska M, Gamian A, Knirel AY. 2012. Structures of a unique O-polysaccharide of Edwardsiella tarda PCM 1153 containing an amide of galacturonic acid with 2-aminopropane-1,3-diol and an abequose-containing O-polysaccharide shared by E. tarda PCM 1145, PCM 1151 and PCM 1158. Carbohydr. Res. 355:56–62. 10.1016/j.carres.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 33.Jansson PE, Kenne L, Widmalm G. 1989. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H- and 13C-NMR data. Carbohydr. Res. 188:169–191. 10.1016/0008-6215(89)84069-8 [DOI] [PubMed] [Google Scholar]
- 34.Gorin PA. 1981. Carbon-13 nuclear magnetic resonance spectroscopy of polysaccharides. Adv. Carbohydr. Chem. Biochem. 38:13–104. 10.1016/S0065-2318(08)60309-1 [DOI] [Google Scholar]
- 35.Altona C, Haasnoot CAG. 1980. Prediction of anti and gauche vicinal proton-proton coupling constants in carbohydrates: a simple additivity rule for pyranose rings. Org. Magn. Reson. 13:417–429. 10.1002/mrc.1270130606 [DOI] [Google Scholar]
- 36.Van Calsteren MR, Pau-Roblot C, Begin A, Roy D. 2002. Structure determination of the exopolysaccharide produced by Lactobacillus rhamnosus strains RW-9595M and R. Biochem. J. 253:7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansson PE, Lindberg B, Lindquist U. 1981. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 4. Carbohydr. Res. 95:73–80. 10.1016/S0008-6215(00)85296-9 [DOI] [PubMed] [Google Scholar]
- 38.Gorin PA, Spencer JF, Lindberg B, Lindh F. 1980. Structure of the extracellular polysaccharide from Corynebacterium insidiosum. Carbohydr. Res. 79:313–315. 10.1016/S0008-6215(00)83846-X [DOI] [PubMed] [Google Scholar]
- 39.Lawson CJ, McCleary CW, Nakada HI, Rees DA, Sutherland IW, Wilkinson JF. 1969. Structural analysis of colanic acid from Escherichia coli by using methylation and base-catalysed fragmentation. Comparison with polysaccharides from other bacterial sources. Biochem. J. 115:947–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabat EA, Liao J, Bretting H, Franklin EC, Geltner D, Frangione B, Koshland ME, Shyong J, Osserman EF. 1980. Human monoclonal macroglobulins with specificity for Klebsiella K polysaccharides that contain 3,4-pyruvylated-d-galactose and 4,6-pyruvylated-d-galactose. J. Exp. Med. 152:979–995. 10.1084/jem.152.4.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gormus BJ, Wheat RW, Porter JF. 1971. Occurrence of pyruvic acid in capsular polysaccharides from various Klebsiella species. J. Bacteriol. 107:150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansson PE, Kenne L, Lindberg B, Ljunggren H, Lonngren J, Ruden U, Svensson S. 1977. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of Rhizobium meliloti by sequential degradation. J. Am. Chem. Soc. 99:3812–3815. 10.1021/ja00453a049 [DOI] [PubMed] [Google Scholar]
- 43.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Kang Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A. 2013. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342:447–453. 10.1126/science.1237910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta R, Eric Altermann E, Anderson RC, McNabb WC, Moughan PJ, Roy NC. 2013. The role of cell surface architecture of Lactobacilli in hostmicrobe interactions in the gastrointestinal tract. Mediators Inflamm. 2013:237921. 10.1155/2013/237921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hidalgo-Cantabrana C, López P, Gueimonde M, de los Reyes-Gavilán CG, Suárez A, Margolles A, Ruas-Madiedo P. 2012. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob. Proteins 4:227–237. 10.1007/s12602-012-9110-2 [DOI] [PubMed] [Google Scholar]
- 46.López P, Monteserín DC, Gueimonde M, de los Reyes-Gavilán CG, Margolles A, Suárez A, Ruas-Madiedo P. 2012. Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro response upon human cells. Food Res. Int. 46:99–107. 10.1016/j.foodres.2011.11.020 [DOI] [Google Scholar]
- 47.Shao L, Wu Z, Zhang H, Chen W, Ai L, Guo B. 2014. Partial characterization and immunostimulatory activity ofexopolysaccharides from Lactobacillus rhamnosus KF. Carbohydr. Polym. 107:51–56. 10.1016/j.carbpol.2014.02.037 [DOI] [PubMed] [Google Scholar]
- 48.Surayot U, Wang J, Seesuriyachan P, Kuntiya A, Tabarsa M, Lee YJ, Kim JK, Park WJ, You SG. 2014. Exopolysaccharides from lactic acid bacteria: structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 68:233–240. 10.1016/j.ijbiomac.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 49.Liu CF, Tseng KC, Chiang SS, Lee BH, Hsu WH, Pan TM. 2011. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 91:2284–2291. 10.1002/jsfa.4456 [DOI] [PubMed] [Google Scholar]
- 50.Li W, Ji J, Tang W, Rui X, Chen X, Jiang M, Dong M. 2014. Characterization of an antiproliferative exopolysaccharide (LHEPS-2) from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 105:334–340. 10.1016/j.carbpol.2014.01.093 [DOI] [PubMed] [Google Scholar]
- 51.Wang K, Li W, Riu X, Chen X, Jiang M, Dong M. 2014. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 67:71–78. 10.1016/j.ijbiomac.2014.02.056 [DOI] [PubMed] [Google Scholar]
- 52.Uchida M, Ishii I, Inoue C, Akisato Y, Watanabe K, Hosoyama S, Toida T, Ariyoshi N, Kitada M. 2010. Kefiran reduces atherosclerosis in rabbits fed a high cholesterol diet. J. Atheroscler. Thromb. 17:980–988. 10.5551/jat.4812 [DOI] [PubMed] [Google Scholar]
- 53.Vinderola G, Perdigon G, Duarte J, Farnworth E, Matar C. 2006. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 36:254–260. 10.1016/j.cyto.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 54.Nowak B, Ciszek-Lenda M, Sróttek M, Gamian A, Kontny E, Górska-Frączek S, Marcinkiewicz J. 2012. Lactobacillus rhamnosus exopolysaccharide ameliorates arthritis induced by the systemic injection of collagen and lipopolysaccharide in DBA/1 mice. Arch. Immunol. Ther. Exp. 60:211–220. 10.1007/s00005-012-0170-5 [DOI] [PubMed] [Google Scholar]
- 55.Chabot S, Yu HL, de Léséleuc L, Cloutier D, van Calsteren MR, Lessard M, Roy D, Lacroix M, Oth D. 2001. Exopolysaccharides from Lactobacillus rhamnosus RW-9595M stimulate TNF, IL-6 and IL-12 in human and mouse cultured immunocompetent cells, and IFN-c in mouse splenocytes. Lait 81:683–687. 10.1051/lait:2001157 [DOI] [Google Scholar]
- 56.Medina M, Izquierdo E, Ennahar S, Sanz Y. 2007. Differential immunomodulatory properties of Bifidobacterium longum strains: relevance to probiotic selection and clinical applications. Clin. Exp. Immunol. 150:531–538. 10.1111/j.1365-2249.2007.03522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makino S, Ikegami S, Kano H, Sashihara T, Sugano H, Horiuchi H, Saito T, Oda M. 2006. Immunomodulatory effects of polysaccharides produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 89:2873–2881. 10.3168/jds.S0022-0302(06)72560-7 [DOI] [PubMed] [Google Scholar]
- 58.Bleau C, Monges A, Rashidan K, Laverdure JP, Lacroix M, van Calsteren MR, Millette M, Savard R, Lamontagne L. 2010. Intermediate chains of expolysaccharides form Lactobacillus rhamnosus RW-9595M increase IL-10 production by macrophages. J. Appl. Microbiol. 108:666–675. 10.1111/j.1365-2672.2009.04450.x [DOI] [PubMed] [Google Scholar]
- 59.Hidalgo-Cantabrana C, Nikolic M, López P, Suárez A, Miljkovic M, Kojic M, Margolles A, Golic N, Ruas-Madiedo P. 2014. Exopolysaccharide-producing Bifidobacterium animalis subsp. lactis strains and their polymers elicit different responses on immune cells from blood and gut associated lymphoid tissue. Anaerobe 26:24–30. 10.1016/j.anaerobe.2014.01.003 [DOI] [PubMed] [Google Scholar]