Abstract
A study of prevalence, diversity, and antimicrobial resistance of Salmonella enterica in surface water in the southeastern United States was conducted. A new scheme was developed for recovery of Salmonella from irrigation pond water and compared with the FDA's Bacteriological Analytical Manual (8th ed., 2014) (BAM) method. Fifty-one isolates were recovered from 10 irrigation ponds in produce farms over a 2-year period; nine Salmonella serovars were identified by pulsed-field gel electrophoresis analysis, and the major serovar was Salmonella enterica serovar Newport (S. Newport, n = 29), followed by S. enterica serovar Enteritidis (n = 6), S. enterica serovar Muenchen (n = 4), S. enterica serovar Javiana (n = 3), S. enterica serovar Thompson (n = 2), and other serovars. It is noteworthy that the PulseNet patterns of some of the isolates were identical to those of the strains that were associated with the S. Thompson outbreaks in 2010, 2012, and 2013, S. Enteritidis outbreaks in 2011 and 2013, and an S. Javiana outbreak in 2012. Antimicrobial susceptibility testing confirmed 16 S. Newport isolates of the multidrug resistant-AmpC (MDR-AmpC) phenotype, which exhibited resistance to ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline (ACSSuT), and to the 1st, 2nd, and 3rd generations of cephalosporins (cephalothin, amoxicillin-clavulanic acid, and ceftriaxone). Moreover, the S. Newport MDR-AmpC isolates had a PFGE pattern indistinguishable from the patterns of the isolates from clinical settings. These findings suggest that the irrigation water may be a potential source of contamination of Salmonella in fresh produce. The new Salmonella isolation scheme significantly increased recovery efficiency from 21.2 (36/170) to 29.4% (50/170) (P = 0.0002) and streamlined the turnaround time from 5 to 9 days with the BAM method to 4 days and thus may facilitate microbiological analysis of environmental water.
INTRODUCTION
Salmonella enterica is a major food-borne pathogen that causes diseases in humans worldwide and also in animals, such as poultry (1, 2). According to the U.S. Centers for Disease Control and Prevention (CDC), in the United States from 1998 to 2008, Salmonella was the most common bacteriological etiological agent for food-borne diseases, responsible for 1,449 (18%) of the 7,998 outbreaks with a confirmed or suspected single etiology, 44% of the hospitalizations, and the most deaths (60 [30%] deaths) (3). It is estimated that approximately 1 million cases of salmonellosis occur in the United States each year (2) and that they cost more than 1 billion dollars (4). Moreover, despite substantial efforts by public health scientists to enhance the detection and monitoring systems of food-borne pathogens over the years, Salmonella infections have not declined significantly in more than a decade (5).
Salmonellosis in humans generally is a self-limiting disease, and patients frequently recover on their own without the need for medical attention. However, invasive salmonellosis is estimated to occur in 5% of cases, leading to life-threatening systemic infections that require effective chemotherapy (6). In the last 20 years, of increasing concern is the worldwide emergence of multidrug-resistant (MDR) phenotypes among Salmonella serotypes, in particular, Salmonella enterica serovar Typhimurium (7) and, more recently, S. enterica serovar Newport (8, 9). S. Typhimurium DT104, which is resistant to at least five antimicrobials including ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline, has caused severe infections and deaths in animals and humans worldwide (7). MDR S. Newport has recently been undergoing epidemic spread in both animals and humans throughout the United States (10). In addition to the pentadrug resistance found in S. Typhimurium DT104, S. Newport is also resistant to amoxicillin-clavulanic acid, cephalothin, cefoxitin, ceftiofur, and ceftriaxone; this serovar has accordingly been named S. enterica serovar Newport MDR-AmpC. A multistate outbreak of S. Newport MDR-AmpC was reported by the Centers for Disease Control and Prevention; raw or undercooked ground beef was implicated as the vehicle of transmission (11). The resistance of S. Newport MDR-AmpC to ceftiofur and ceftriaxone has significant clinical implications both in humans and for veterinary medicine. Ceftriaxone is the drug of choice for treatment of severe salmonellosis in humans, especially in children.
Over 2,600 serovars of Salmonella enterica have been identified (12), and they vary in their host ranges and abilities to cause disease in humans. Salmonella enterica is most commonly spread via contaminated foods, such as poultry, beef, pork, eggs, milk, seafood, and fresh produce (13), but it is also frequently isolated from fresh and marine waters (14, 15). Salmonella can be disseminated into environmental waters via human sewage, urban and agricultural runoff, and fecal matter from wildlife and domestic animals (16). Studies have demonstrated that food-borne pathogens such as Salmonella can survive for extended periods of time in the soil and water and can readily adapt to an aquatic lifestyle (17, 18). Furthermore, natural waters can serve as a vehicle for dissemination of Salmonella in the environment and as a route of transmission among hosts (16). Although Salmonella traditionally has been regarded as a host-associated food-borne pathogen, irrigation water has increasingly been recognized as a route of transmission of Salmonella (19), with subsequent introduction of Salmonella enterica to leafy vegetables (20, 21). Fruits and vegetables have been increasingly recognized as an important source of Salmonella enterica in food-borne outbreaks (22, 23). Irrigation with contaminated water not only can increase the level of bacteria such as Salmonella enterica on leafy green produce (24–26) but also can augment the internalization of Salmonella enterica into edible parts of leafy green vegetables through openings such as plant stomata and/or scars associated with plant health injuries (27, 28).
A rise in outbreaks caused by Salmonella enterica has been recognized in many parts of the world (19). In spite of the increased evidence of contaminated irrigation water as a suspected source of enteric pathogens during numerous produce-related outbreaks, little is known about the prevalence, distribution, diversity, and antimicrobial resistance of Salmonella enterica in surface water used for crop irrigation. A better understanding of the pathogen dynamics associated with surface waters would be indispensable for the development of effective strategies to mitigate the risk of produce contamination by Salmonella. To achieve this objective, rapid and efficient methods for the detection, isolation, and subtyping are desirable. Therefore, the aim of this study was to investigate the prevalence, diversity, and antimicrobial resistance of Salmonella enterica in irrigation pond water in produce farms in the southeastern United States. During the course of the study, we also developed an improved and efficient Salmonella isolation method and compared it to the traditional method in the U.S. FDA's Bacteriological Analytical Manual (29).
MATERIALS AND METHODS
Sampling locations and schedules.
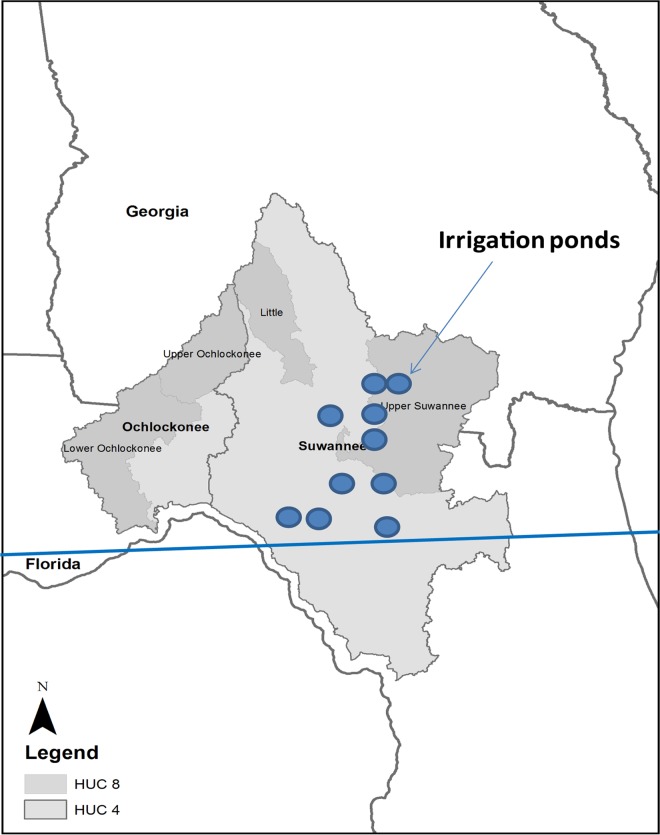
This research was conducted from July 2011 to September 2013 within the Suwannee River watershed. The studied area is located in a principal agricultural production area in southern Georgia, where vegetable production uses supplemental irrigation, as shown in Fig. 1. Ten ponds that serve as irrigation water sources for vegetable and crops throughout this area were chosen and coded as BB, VH, RT1, RT, CC1, SC, NP, LV, CC2, and MD1. Two sampling schedules were used in this study. Specifically, all 10 ponds were sampled from July 2011 to February 2012, while sampling of SC, NP, LV, CC2, and MD1 was extended from March 2012 to September 2013. The sizes and depths of these 10 selected irrigation ponds ranged from 12,000 to 93,000 m2 and from 3 to >10 m deep. The distances between ponds are listed in Table 1. In this study, a single 10-liter water sample close to the pumps was collected monthly from each pond. Collected water samples were stored on ice in the field and transported to a laboratory for analysis. In total, 170 samples were collected from these irrigation ponds during the 27-month study period.
FIG 1.
Map of the Suwannee River basin. HUC 4 refers to a watershed that is very large in area, typically representing a large river basin; HUC 8 refers to a smaller watershed, typically ranging from around 500 to 2,000 square miles in area. The locations and sizes of surveyed irrigation ponds in the map are not to scale.
TABLE 1.
Geographic distances (km) between irrigation ponds used in this study
| Pond | BB | VH | LV | RT2 | RT1 | SC | NP | MD1 | CC1 | CC2 |
|---|---|---|---|---|---|---|---|---|---|---|
| BB | 79.49 | 105.86 | 108.43 | 109.48 | 111.49 | 122.88 | 123.87 | 173.42 | 181.34 | |
| VH | 79.49 | 95.43 | 141.84 | 140.58 | 87.45 | 97.31 | 82.37 | 108.25 | 116.56 | |
| LV | 105.86 | 95.43 | 48.17 | 47.18 | 9.55 | 18.04 | 33.82 | 95.43 | 100.94 | |
| RT2 | 108.43 | 141.84 | 48.17 | 1.71 | 57.03 | 59.49 | 81.33 | 141.86 | 146.60 | |
| RT1 | 109.48 | 140.58 | 47.18 | 1.71 | 55.94 | 58.16 | 80.19 | 140.58 | 145.26 | |
| SC | 111.49 | 87.45 | 9.55 | 57.03 | 55.94 | 11.66 | 24.44 | 85.92 | 91.39 | |
| NP | 122.88 | 97.31 | 18.04 | 59.49 | 58.16 | 11.66 | 24.43 | 82.47 | 87.11 | |
| MD1 | 123.87 | 82.37 | 33.82 | 81.33 | 80.19 | 24.44 | 24.43 | 61.75 | 67.59 | |
| CC1 | 173.42 | 108.25 | 95.43 | 141.86 | 140.58 | 85.92 | 82.47 | 61.75 | 8.31 | |
| CC2 | 181.34 | 116.56 | 100.94 | 146.60 | 145.26 | 91.39 | 87.11 | 67.59 | 8.31 |
Water sample concentration and enrichment.
Each 1-liter water sample was vacuum filtered through a 1,000-ml corning filter system (Corning, Inc., Corning, NY) unit with a 0.45-μm-pore-size sterile nitrocellulose membrane. The membrane was broken into pieces by sterile tips and transferred to a 50-ml conical tube containing 10 ml of tryptic soy broth (TSB) (Difco/Becton, Dickinson, and Co., Franklin Lakes, NJ). The tube was vortexed vigorously for 30 s and subsequently incubated at 37°C with continuous shaking at 180 rpm for 18 h (Fig. 2).
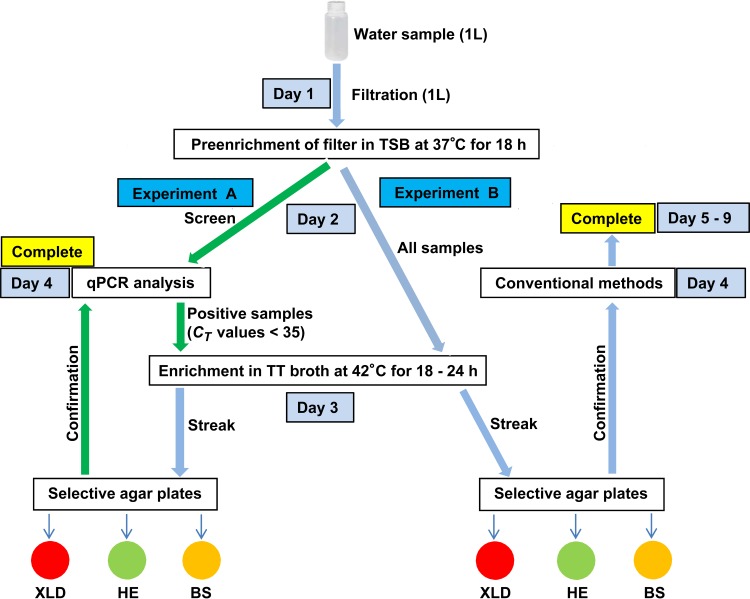
FIG 2.
Schemes of the Salmonella enterica isolation. Experiment A is a new protocol developed in this study; experiment B is a protocol based on the FDA's BAM isolation method forSalmonella. A green arrow indicates a change in procedure.
Development scheme for Salmonella detection and/or isolation from environmental water.
To improve the Salmonella isolation from environmental water, we combined our newly developed Salmonella detection quantitative PCR (qPCR) assay with sample concentration and enrichment and the BAM Salmonella isolation method (29) and compared the efficiencies between the two protocols. Two parallel sets of experiments (A and B) were performed. For experiment A, a qPCR assay was applied to preenriched water samples to determine the presence of Salmonella before proceeding further with the BAM procedures. Initially, 0.5 ml of the preenriched culture was centrifuged at 10,000 × g for 5 min; the supernatant was then removed, and the cell pellet was washed with 1 ml of phosphate-buffered saline (PBS), centrifuged, and suspended in 150 μl of PrepMan solution (Life Technologies, Grand Island, NY) for DNA extraction. The extracted DNA was subjected to a qPCR assay (30) to screen for the presence of Salmonella. Briefly, reaction mixtures consisted of 12.5 μl of 2× Universal Master Mix (Life Technologies), 200 nM forward and reverse primers targeting the invA gene in Salmonella, and 100 nM probe. Water sample DNA and an appropriate volume of nuclease-free water were added to reach a final reaction volume of 25 μl. The qPCR conditions were set as follows: activation of TaqMan (Life Technologies) at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 s and annealing/extension at 60°C for 1 min.
In experiment A, the approach was to perform the isolation procedures only on samples with positive qPCR results (threshold cycle [CT] of <35), while in experiment B, the BAM protocol was followed to process all preenriched samples (Fig. 2). The samples with positive qPCR results in experiment A and all preenriched samples in experiment B were further enriched in tetrathionate (TT) broth. Specifically, 0.5 ml of preenriched sample was inoculated into 4.5 ml of TT broth and incubated at 42°C with shaking at 180 rpm for 18 h. A loopful of enriched sample (about 10 μl) was streaked onto three selective agar plates, xylose lysine desoxycholate (XLD), Hektoen enteric (HE), and bismuth sulfite (BS), and incubated at 35°C for 24 ± 2 h. After incubation, four to eight well-separated colonies that exhibited typical colony morphology of Salmonella on XLD (black), HE (dark greenish), or BS (brownish) agar were picked and inoculated into 100 μl of LB medium in a 96-well culture plate. The inoculated plate was incubated at 37°C for 4 h. The culture on the plate was split into two plates with a multichannel pipette. A part of the culture was saved as seeds for the picked colonies, and the other part of the culture was used for DNA extraction, which was performed using PrepMan Ultra sample preparation reagent (Life Technologies) according to the manufacturer's instructions. In experiment A, a qPCR assay was performed to confirm the presumptive Salmonella-positive colonies as described above (30), whereas in experiment B, conventional methods were used as described in the BAM (29) (Fig. 2). The confirmed Salmonella isolates were frozen at −80°C in LB medium supplemented with 15% glycerol for subsequent analyses and long-term storage.
PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed according to a protocol developed by the CDC in 2002 using Salmonella Braenderup H9812 as the control strain. Agarose-embedded DNA was digested with 50 U of XbaI or BlnI (Boehringer-Mannheim, Indianapolis, IN) for least 4 h in a water bath at 37°C. The restriction fragments were separated by electrophoresis in 0.5× TBE (Tris-borate-EDTA) buffer (Invitrogen, Carlsbad, CA) at 14°C for 18 h using a Chef Mapper electrophoresis system (Bio-Rad, Hercules, CA) with pulse times of 2.16 to 63.8 s. Isolates showing DNA smears were retested using plugs digested with XbaI or BlnI and electrophoresis buffer containing 50 μM thiourea in 0.5× TBE buffer. The gels were stained with ethidium bromide, and DNA bands were visualized with UV transillumination (Bio-Rad). PFGE results were analyzed using BioNumerics software (Applied-Maths, Kortrijk, Belgium), and banding pattern similarity was compared using the average of two enzyme analyses with a 1.5% band position tolerance. The generated unique PFGE patterns were compared with those in the Center for Veterinary Medicine (CVM) PFGE database and queried against the CDC PulseNet as well.
Antimicrobial susceptibility.
Fifty-one Salmonella isolates were assessed for antimicrobial susceptibility by the Kirby-Bauer method using Sensi-Disc susceptibility test disks (Becton, Dickinson, and Co., Sparks, MD, USA) according to the manufacturer's instructions. Briefly, disks with antimicrobials were loaded onto a bacterial lawn made by swabbing 0.1 ml of bacterial culture (optical density at 260 nm [OD260] of 0.5) in LB broth onto Mueller-Hinton agar plates (Difco). Plates were incubated at 37°C for 16 to 18 h. Antimicrobial susceptibility of Salmonella isolates was evaluated by measuring the diameters of inhibition zones and referring to the zone diameter interpretive chart from the Sensi-Disc kit (31). Our susceptibility test panel covered 16 antimicrobials, including ampicillin-sulbactam (test disc code SAM-20), amikacin (AN-30), amoxicillin-clavulanic acid (AMC-30), cefotaxime (CTX-30), ceftazidime (CAZ-30), ceftriaxone (CRO-30), cephalothin (CF-30), gentamicin (GM-120), chloramphenicol (CHL-30), ciprofloxacin (CIP-5), kanamycin (KAN-30), nalidixic acid (NAL-30), streptomycin (STR-10), tetracycline (TET-30), trimethoprim-sulfamethoxazole (SXT), and trimethoprim (TMP-5). Escherichia coli (ATCC 25922) was used as the control strain for antibiotic susceptibility testing.
Statistical analyses.
Data were analyzed using SAS, version 9.3 (Cary, NC). The comparative analysis of recovery of Salmonella from the irrigation pond water by the new and old schemes was conducted with McNemar's test (or paired chi-square test). The seasonal factors on the recovery of Salmonella from the five irrigation ponds and the prevalence of the individual irrigation ponds were analyzed with Friedman's x2 test, followed by a Kruskal-Wallis test. For all measures of association, P values of ≤0.05 were considered significant.
RESULTS
Detection and isolation of Salmonella enterica from water samples.
In an effort to improve the efficiency of detection and isolation of Salmonella, in this study, we developed a new Salmonella isolation scheme and compared it with the original BAM method (29). To assess the efficiency of the two isolation schemes for recovery of Salmonella from environmental water samples, we tested a total of 170 water samples from the 10 selected irrigation ponds between July 2011 and September 2013, including water samples from five ponds that were sampled from July 2011 to February 2012. After water samples were concentrated and preenriched, a qPCR assay was performed to detect the presence of Salmonella in the water samples. Two parallel sets of experiments were made: in experiment A, isolation procedures were performed only on samples with positive qPCR results (CT of <35), while in experiment B, the BAM protocol for Salmonella isolation was performed on all preenriched samples (Fig. 2). A summary of the results of Salmonella recovery from pond water samples is shown in Table 2. As a result, a total of 14 more isolates were recovered with the new scheme than with the original BAM scheme.
TABLE 2.
Recovery and antimicrobial susceptibility of Salmonella isolates from irrigation pond water samplesa
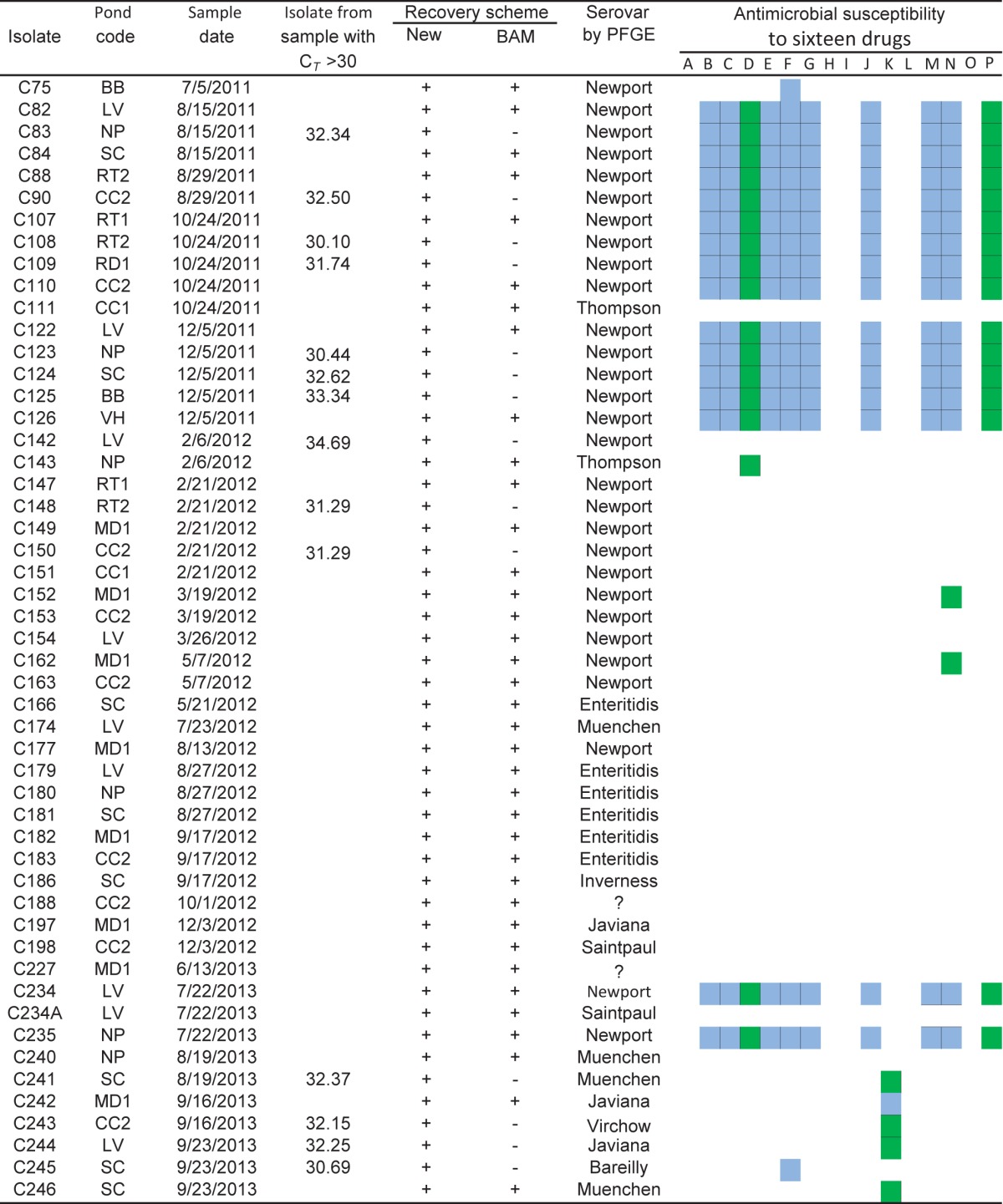
The drugs used in susceptibility testing are as follows (Sensi-Disc code): A, amikacin (AN-30); B, amoxicillin-clavulanic acid (AmC-30); C, ampicillin-sulbactam (SAM-20); D, cefotaxime (CTX-30); E, ceftazidime (CAZ-30); F, ceftriaxone (CRO-30); G, cephalothin (CF-30); H, gentamicin (GM-120); I, ciprofloxacin (CIP-5); J, chloramphenicol (CHL-30); K, kanamycin (KAN-30); L, nalidixic acid (NAL-30); M, streptomycin (STR-10); N, tetracycline (TET-30); O, trimethoprim (TMP-5); P, trimethoprim-sulfamethoxazole (SXT). Results are coded as follows: blank, sensitive; blue, resistant; green, intermediate.
A comparison of the results obtained from the two methods demonstrated that the Salmonella recovery rate in experiment A with the new method (29.4%, 50/170) was significantly higher (P = 0.0002) than that in experiment B with the original BAM method (21.2%, 36/170) (Table 3).
TABLE 3.
Prevalence of Salmonella enterica in irrigation pond water by serovar
| S. enterica isolate | No. of PFGE patterns | No. of isolates (%) |
|---|---|---|
| S. Newport | 2 | 29 (56.9) |
| S. Enteritidis | 1 | 6 (11.8) |
| S. Muenchen | 4 | 4 (7.8) |
| S. Javiana | 2 | 3 (5.9) |
| S. Thompson | 1 | 2 (3.9) |
| S. Saintpaul | 2 | 2 (3.9) |
| S. Bareilly | 1 | 1 (2.0) |
| S. Inverness | 1 | 1 (2.0) |
| S. Virchow | 1 | 1 (2.0) |
| Nontypeable | 2 | 2 (3.9) |
Subtyping Salmonella enterica by PFGE analysis.
In order to increase the discriminatory power of the PFGE analysis, two restriction enzymes, XbaI and BlnI, were used for PFGE analysis. Among the 51 isolates analyzed by PFGE, a total of 17 different PFGE patterns, representing nine different serovars, were obtained (Fig. 3). Of the 17 PFGE patterns, two patterns represented two different lineages of the same serovar, S. Newport. These two patterns covered 13 (cluster C) and 16 (cluster D) isolates, respectively, and represented the most commonly found serovar (29/51, 56.9%). The third largest cluster was comprised of six isolates (cluster B) that were identified as S. enterica serovar Enteritidis. Four S. enterica serovar Muenchen isolates were identified, and each isolate had a unique PFGE pattern. Three S. enterica serovar Javiana isolates were confirmed, and two isolates shared a PFGE pattern while the third one had a unique PFGE pattern. Two S. enterica serovar Thompson isolates shared a PFGE pattern in cluster A, while each of two S. enterica serovar Saintpaul isolates had its own specific PFGE pattern. The remainder recovered Salmonella serovars included single isolates of S. enterica serovar Inverness, S. enterica serovar Bareilly, and S. enterica serovar Virchow and two Salmonella isolates (C188 and C227) which were not typeable by PFGE; each serovar had a specific PFGE pattern (Fig. 3).
FIG 3.
XbaI and BlnI PFGE-based dendrogram of Salmonella enterica isolates from irrigation ponds.
Spatial-temporal factors of Salmonella prevalence in the irrigation water ponds.
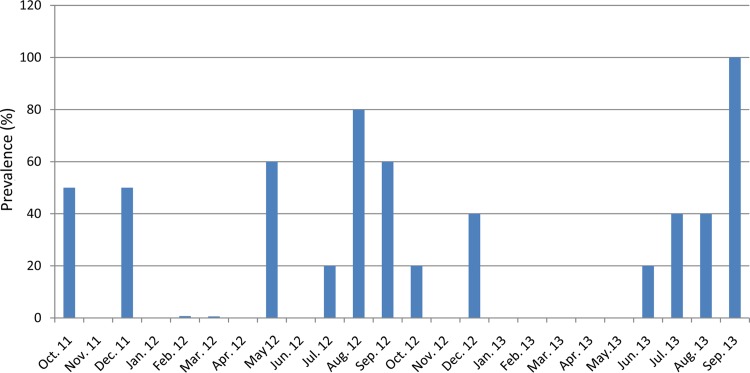
Of the 170 water samples from the 10 selected irrigation ponds, 50 were positive for Salmonella by the qPCR assay, and at least one Salmonella strain was isolated from each PCR-positive water sample. The overall Salmonella prevalence was 29.4% (50/170) for the 10 irrigation ponds for the 27 months of the survey period. During the survey period, multiple projects shared water samples, and some projects had overlaps in the sample scheduling. This resulted in some inconsistencies in sample scheduling; i.e., five ponds were surveyed for 27 months while the five other ponds were surveyed for 8 months. Hence, to remedy this for the purpose of analyzing prevalence, we used five ponds for 2 years and left out the other five ponds (Table 4). But for Salmonella isolation, we used all 10 ponds for the entire 27 months. The overall Salmonella prevalence was 30% (36/120) for the five irrigation ponds for the 24 months of the survey period. The prevalence for each irrigation water pond ranged from 25.0% (6/24) positive at NP and SC to 37.5% (3/8) positive at MD1 (Table 4). Although the prevalences of some ponds such as MD and CC2 were higher than those of the others, there were no statistically significant differences observed among the five ponds (x2 = 1.61, df = 4, P = 0.7416). However, there was an obvious seasonality in Salmonella prevalence, as shown in Fig. 4. The recovery of Salmonella in warmer months (July, August, and September) from five ponds, including MD1, CC2, LV, NP, and SC, was significantly higher than that in the other months (x2 = 11.82, df = 3, P = 0.008) over the 2-year study period.
TABLE 4.
Prevalence and distribution of Salmonella enterica in irrigation ponds in produce farms
| Pond code | Recovery of Salmonella in: |
Prevalence (%) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 |
2012 |
2013 |
|||||||||||||||||||||||
| Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | June | July | Aug | Sept | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | June | July | Aug | Sept | ||
| MD1 | + | + | + | + | + | + | + | + | + | 37.5 | |||||||||||||||
| CC2 | + | + | + | + | + | + | + | + | 33.3 | ||||||||||||||||
| LV | + | + | + | + | + | + | + | 29.2 | |||||||||||||||||
| NP | + | + | + | + | + | + | 25.0 | ||||||||||||||||||
| SC | + | + | + | + | + | + | 25.0 | ||||||||||||||||||
FIG 4.
Prevalence of Salmonella enterica in the five irrigation ponds from October 2011 to September 2013.
Antimicrobial susceptibility.
Fifty-one Salmonella isolates were tested for antimicrobial susceptibility. Of the 16 antimicrobial agents tested, the most common resistance phenotypes observed were to ceftriaxone (35.3%, 18/51), while all isolates were susceptible to gentamicin, ciprofloxacin, nalidixic acid, and trimethoprim. Among the 51 isolates, 22 isolates, including 6 S. Enteritidis isolates, were pan-susceptible to all 16 antibiotics tested (Table 2). It is noteworthy that S. Newport isolates in cluster D (n = 16) were resistant to nine antibiotics: amoxicillin-clavulanic acid, ampicillin-sulbactam, ceftazidime, cephalothin, ceftriaxone, chloramphenicol, streptomycin, tetracycline, and trimethoprim-sulfamethoxazole. These S. Newport strains also exhibited reduced susceptibility to cefotaxime (Table 2). On the other hand, among the S. Newport isolates in cluster C (n = 13), only one isolate (C75) was resistant to ceftriaxone, and two isolates (C152 and C162) showed reduced susceptibility to tetracycline. Besides the antimicrobial susceptibility demonstrated by these S. Newport isolates, a few non-Newport isolates also demonstrated moderate antibiotic resistance. An S. Bareilly (C245) isolate was resistant to ceftriaxone, and one of the three S. Javiana isolates (C242) was resistant to kanamycin. In addition, two of the four S. Muenchen isolates (C241 and C246), the S. Virchow isolate (C243), and one of the three S. Javiana isolates (C244) showed intermediate resistance to kanamycin (Table 2).
DISCUSSION
At the present time, food safety professionals and farmers are facing a Janus-like dilemma, i.e., a growing demand for fresh produce accompanied by an increasing trend of food-borne outbreaks associated with consuming fresh produce (32). The increase in food-borne outbreaks may be attributed to two factors: the improved surveillance of Salmonella contaminations of fresh produce over the years and the growing consumption of fresh produce due to a shift in people's eating habits toward healthier lifestyles where more vegetables and fruits instead of meat are consumed. It is predicted that consumption and production of fresh produce will continue to rise (33). Salmonella transmission and routes of entry into the food chain are complex and diverse; contamination can occur in multiple steps along the “farm-to-fork” continuum. Outbreaks of Salmonella caused by contaminated fresh produce have shown that the contamination can occur locally or regionally or be more widely distributed. To constrain and/or reverse these increasing trends, we need to expand our knowledge of the prevalence, distribution, and diversity of Salmonella enterica in irrigation pond waters used by produce farms.
Given that irrigation water can be a source of Salmonella contamination of vegetables, the prevention of outbreaks could be accomplished by consistent monitoring of the presence of Salmonella enterica in water supplies. However, detection of Salmonella in water samples can be complicated by factors such as fecal inhibitors in nucleic acid-based detection assays (34), inhibitors from soil suspension in water samples (35), and a low number of cells requiring a large volume of sample. Thus, a key aspect of obtaining accurate assessments of the prevalence of Salmonella in irrigation water relies greatly on the availability of sensitive and specific detection methodologies. Traditional methods for isolating and identifying Salmonella in food rely on its growth on selective and differential media and serological identification. The major limitation of these methods is that they typically take 5 to 9 days to produce results, and the sensitivity is relatively low. Detection methodologies for Salmonella have undergone dramatic improvements over the past 2 decades; DNA-based molecular detection methods such as qPCR have been widely used for bacterial diagnostics. To streamline the process for detection of Salmonella enterica from surface water, in this study we screened the preenriched water samples with a qPCR assay prior to the isolation process and used the qPCR result as an indicator for the presence of Salmonella enterica in the samples. In our experience, if preenriched samples showed CT values of <35 in qPCR, it was very likely that Salmonella isolates would be recovered by a subsequent culture isolation procedure (Table 2); on the other hand, if the CT values were >35, no isolates would be recovered in the isolation procedure. For instance, for all preenriched water samples screened by qPCR with CT values of >30 (n = 14), isolates were successfully recovered in experiment A, owing to the incorporated qPCR screen step in the new protocol (Fig. 2). In these cases, the qPCR results showed CT values of >30 but <35 (Table 2), which gave a hint that Salmonella was present in the samples, albeit the numbers of Salmonella cells were quiet low. Hence, special attention was paid to these samples in the isolation process; for example, additional streaking steps were taken by picking some material from the incubated plates which showed growth of a possible mixture of Salmonella and non-Salmonella bacteria (dark blue to blackish appearance). In this way, Salmonella strains were eventually isolated from samples with higher CT values (>30 but <35) (n = 14) in experiment A. In contrast, in experiment B, these 14 samples were disregarded and deemed negative samples in the initial isolation step because typical Salmonella colonies did not appear on the three selective medium plates. Empirically, with this new protocol, we were able to improve the Salmonella recovery rate from 21.1% (36/170) to 29.4% (50/170) from irrigation pond water, substantially cut down the workload involved in the current isolation procedures, and shorten the turnaround time from 5 to 9 days to 3 to 4 days (Fig. 2).
In this study, the presence and diversity of Salmonella enterica in irrigation pond waters of the southeastern United States were assessed for 27 months (July 2011 to September 2013). Of the 10 ponds, 5 ponds were surveyed for only 8 months, thus resulting in some inconsistency in sample scheduling. To remedy that, for the purpose of analyzing prevalence, we used five ponds for 2 years and left out the other five ponds. But we used all 10 ponds for the entire 27 months for isolation purposes. The overall prevalence in the five ponds was 29.4% for the 24 months. The prevalence of Salmonella for each pond ranged from 25.0% to 37.5% (Table 4). There was one multiserovar sample (C234) that yielded two different serovars. Interestingly, almost all 51 Salmonella isolates were found to be members of the most common food-borne pathogenic Salmonella serovars; S. Newport (n = 29) was the most commonly detected serovar, followed by S. Enteritidis (n = 6), S. Muenchen (n = 4), S. Javiana (n = 3), S. Thompson (n = 2), S. Saintpaul (n = 2) and single isolates of other serovars (Fig. 3). However, one of the most common serovars, S. Typhimurium, which is also one of the major Salmonella serovars associated with waterborne salmonellosis (36), was not present during this survey period. The reason for the absence of S. Typhimurium in the recovery list (Table 4) was not completely known; however, it may be due to the microecology of the irrigation ponds, which depends heavily on the microbial populations in the local environment and some specific chronological events, such as rainfall, outbreaks, and intrusions of wildlife. On the other hand, an S. Virchow isolate (C243), a serovar found in many European, Asian, and Oceanic countries (37, 38), was recovered. It is worth noting that this serovar is clinically significant due to the frequency at which it causes invasive infections and to its association with food-borne outbreaks (39); this isolate also demonstrated reduced susceptibility to kanamycin (Table 2). Overall, the prevalence (29.4%) of Salmonella in the surface waters in the sampled ponds was low but within the range described by previous studies. Specifically, this prevalence was higher than the 7.1% prevalence rate that was reported by Gorski et al. (18) from water samples from an agricultural region in California and the 7.7% prevalence rate reported by Micallef et al. (40) from water ponds associated with Mid-Atlantic tomato farms, but it is lower than the prevalence rate of 79.2% that was reported by Haley et al. for the watershed in the Suwannee River Basin of the southern Georgia (15). The variations in the prevalences may be due to differences in the microenvironment surrounding the ponds, geography, water sources, sampling schemes, and detection methodologies. Furthermore, irrigation water supplies are intrinsically different, with the West Coast using aqueducts and the East Coast using surface catchment.
Seasonality was shown with a higher Salmonella prevalence in the summer months (July, August, and September) than in other months (P = 0.0218) during the 2 years of surveyed period (Fig. 4). This elevated prevalence in the summer months may be influenced by temperature and precipitation, as evidenced by temperature and pond water level record in the irrigation pond field sheet (data not shown). This is in agreement with the previous report on the Salmonella seasonality in the Little River watershed (15), which is located in the State of Georgia and next to Suwannee River Basin. There are about 80,000 irrigation ponds in use by produce farms within the Suwannee River watershed. Half of them are man-made and represent relatively isolated water bodies for agricultural purposes or produce production. The southeastern coastal plain (SECP) is an ecoregion which spans portions of Louisiana, Mississippi, Tennessee, Alabama, Florida, Georgia, South Carolina, North Carolina, and Virginia. It is an important vegetable production area of the United States. The 10 selected irrigation ponds covered a relatively large area in southern Georgia, which has been identified by federal agencies and researchers as being representative of the agricultural practices, climate, and water resources of the SECP (41, 42). Thus, the information on the prevalence and distribution of Salmonella in these irrigation ponds can bridge the knowledge gap for this larger area and provide a reference for other areas in the United States. PFGE analyses separated the 29 S. Newport isolates into two clusters, i.e., clusters C and D. Previous studies have demonstrated that S. Newport is polyphylogenetic and is further grouped into three different genotypical clusters (43, 44). More importantly, with PFGE PulseNet pattern numbers, we were able to demonstrate that the water sample isolates (Fig. 3) were indistinguishable from the clinical isolates. Some of them showed identical PFGE PulseNet patterns to those of isolates from the recent outbreaks, such as Salmonella outbreaks of S. Thompson in 2010, 2012, and 2013, of S. Enteritidis in 2011 and 2013, and of S. Javiana in 2012. It is of interest that some of the serovars such as S. Newport had been present in the surveyed area sporadically over the 2-year-period. However, the presence of S. Newport in the irrigation ponds was not consistent because there was no positive detection of the same serovar (S. Newport) in the same pond for two or more consecutive months (Fig. 3). These results suggest that the irrigation water may be a potential source of contamination of Salmonella in fresh produce and underscore the necessity for close monitoring of microbial populations such as Salmonella in surface water used for irrigation; the information on prevalence, distribution, and diversity of Salmonella enterica in irrigation water will be valuable for the development of strategies to minimize the risk of Salmonella outbreaks associated with fresh produce farms.
Another key finding of the present study is the recovery of the MDR-AmpC-phenotype S. Newport strains from irrigation pond water in produce farms. The PFGE analysis on the isolates grouped the 29 S. Newport isolates into two different clusters, i.e., clusters C and D (Fig. 3). This prompted us to assess the antimicrobial susceptibility of all 51 isolates to 16 commonly used antibiotics. Surprisingly, the 16 S. Newport isolates in cluster D were confirmed to be resistant to 9 of the 16 tested antibiotics tested (amoxicillin-clavulanic acid, ampicillin-sulbactam, ceftazidime, ceftriaxone, cephalothin, chloramphenicol, streptomycin, and tetracycline, and trimethoprim-sulfamethoxazole) (Table 2), and some of the antibiotics, such as ceftriaxone and cephalothin, are the third generation of cephalosporins. Besides the S. Newport isolates, an S. Bareilly isolate (C245) also demonstrated resistance to ceftriaxone. The PFGE pattern of the 16 S. Newport isolates perfectly matched the PulseNet pattern number JJPX01.0085, which is one of most common of S. Newport MDR-AmpC patterns isolated from human clinical samples, beef, and poultry in the U.S. PulseNet database. Recovery of the MDR-AmpC-phenotype S. Newport isolates from irrigation ponds in produce farms is of importance in epidemiology and clinical settings in several respects.
First, salmonellosis caused by nontyphoidal Salmonella (NTS) serovars ranges from mild diarrheal disease to severe systemic infections; it is generally self-limiting, and antimicrobial therapy is not needed. However, antimicrobial therapy plays a critical role in treating salmonellosis in young children, the elderly, and immunocompromised patients (45). Currently, effective antimicrobial therapy for severe salmonellosis depends on extended-spectrum cephalosporins and fluoroquinolones (46). However, the recent emergence of MDR serotypes of Salmonella enterica has raised an issue in the management of salmonellosis (47, 48). Salmonella of the MDR-AmpC phenotype resistant to extended-spectrum cephalosporins, the third-generation cephalosporins, has become a global problem since the 1980s, with reports from Europe, North and South America, north and west Africa, and Asian countries. Resistance to extended-spectrum cephalosporins is associated with clinical failures, including death, in patients with systemic infections. Ceftriaxone is the drug of choice for treatment of severe salmonellosis in humans, especially in children. Therefore, identification of the S. Newport MDR-AmpC from irrigation pond water raises significant public health concerns. To our knowledge, this is the first to report the presence of the S. Newport MDR-AmpC phenotype in irrigation pond water in produce farms, and the investigation on the occurrence and distribution of the S. Newport MDR-AmpC phenotype in this study provided knowledge for the development of a strategy of safety and efficient irrigation with surface water. Second, the PulseNet pattern number of the isolates of the S. Newport MDR-AmpC phenotype (Fig. 3) matched that from clinical isolates, suggesting that the S. Newport MDR-AmpC phenotype isolated from the irrigation ponds might be associated with the Salmonella infections. Third, the prevalence of the S. Newport MDR-AmpC phenotype in the selected irrigation ponds was alarmingly high (9.4%, 16/170) in irrigation ponds in the southeastern United States, considering that this particular Salmonella phenotype is more commonly found in areas of high population density and relatively high milk cow density in the northern parts of the United States (9). This suggests that special attention should be given to the development of a strategy of safety and efficient irrigation with surface water. Last, the 10 surveyed irrigation ponds are in a relatively large area, and some ponds are as far as 181 km apart (Table 1). The presence of the S. Newport MDR-AmpC phenotype in the studied area was widespread (90%) during the period of survey (August to December 2011) (Table 4); the first occurrence of the S. Newport MDR-AmpC phenotype in irrigation ponds (LV and NP) was in August 2011, and the second and third times of recovery of this particular Salmonella phenotype from the same ponds were in December 2011 and July 2013, respectively (Fig. 3). This suggested that the presence of the S. Newport MDR-AmpC phenotype in the studied area (10 ponds) was not accidental or coincidental; rather, it must have underlying reasons or mechanisms. To identify and reveal the possible reasons or mechanisms for this high prevalence of the S. Newport MDR-AmpC phenotype in the irrigation ponds warrants future study. Our finding further strengthened the argument for close monitoring of microbial populations, such as the food-borne pathogen Salmonella, in surface water used for irrigation in produce farms as this surface water might involve the risk of contaminating produce with the S. Newport MDR-AmpC phenotype pathogen.
Conclusions.
A novel Salmonella isolation scheme was developed for investigation of the prevalence, diversity, and antimicrobial resistance of Salmonella enterica in surface water in the southeastern United States. The new isolation scheme significantly increased Salmonella recovery (29.4%) from the irrigation pond water samples compared with the recovery (21.2%) using the BAM method and shortened the turnaround time to 4 days from 5 to 9 days (BAM method). The PFGE analyses demonstrated that the Salmonella isolates from the irrigation water in the produce farms were identical to those of the Salmonella outbreaks in 2010, 2012, and 2013 (S. Thompson) and in 2012 (S. Javiana). It is of note that some of the Salmonella isolates, including 16 S. Newport MDR-AmpC phenotype isolates, exhibited indistinguishable PFGE patterns with strains that were associated with some of the recent Salmonella outbreaks or strains isolated from clinical settings. These findings suggest that the irrigation water may be a potential source of contamination of Salmonella in fresh produce and underscore the necessity for close monitoring of microbial populations, such as the food-borne pathogen Salmonella, in farm surface water used for irrigation. This information may be useful for the development of strategies to minimize the risk of Salmonella outbreaks associated with fresh produce.
ACKNOWLEDGMENTS
We thank Jason Abbott for his help on PFGE analyses with Salmonella isolates and Keith Lampel and Ben Tall for reading the manuscript and providing useful suggestions.
Footnotes
Published ahead of print 8 August 2014
REFERENCES
- 1.Alali WQ, Thakur S, Berghaus RD, Martin MP, Gebreyes WA. 2010. Prevalence and distribution of Salmonella in organic and conventional broiler poultry farms. Foodborne Pathog. Dis. 7:1363–1371. 10.1089/fpd.2010.0566 [DOI] [PubMed] [Google Scholar]
- 2.Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. 2011. Foodborne illness acquired in the United States—unspecified agents. Emerg. Infect. Dis. 17:16–22. 10.3201/eid1701.P21101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. 2013. Surveillance for foodborne disease outbreaks—United States, 1998–2008. MMWR Surveill. Summ. 62:1–34 http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6202a1.htm [PubMed] [Google Scholar]
- 4.Todd EC. 1989. Costs of acute bacterial foodborne disease in Canada and the United States. Int. J. Food Microbiol. 9:313–326. 10.1016/0168-1605(89)90099-8 [DOI] [PubMed] [Google Scholar]
- 5.CDC. 2010. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2009. MMWR Morb. Mortal. Wkly. Rep. 59:418–422 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5914a2.htm [PubMed] [Google Scholar]
- 6.Helms M, Vastrup P, Gerner-Smidt P, Molbak K. 2003. Excess mortality associated with antibiotic resistant Salmonella typhimurium. Ugeskr. Laeger. 165:235–239 (In Danish.) http://www.ncbi.nlm.nih.gov/pubmed/12555707 [PubMed] [Google Scholar]
- 7.Threlfall EJ. 2000. Epidemic Salmonella typhimurium DT 104—a truly international multiresistant clone. J. Antimicrob. Chemother. 46:7–10. 10.1093/jac/46.1.7 [DOI] [PubMed] [Google Scholar]
- 8.Zansky S, Wallace B, Schoonmaker-Bopp D, Smith P, Ramsey F, Painter J, Gupta A, Kalluri P, Noviello S. 2002. From the Centers for Disease Control and Prevention. Outbreak of multi-drug resistant Salmonella Newport—United States, January-April 2002. JAMA 288:951–953. 10.1001/jama.288.8.951-JWR0828-2-1 [DOI] [PubMed] [Google Scholar]
- 9.Greene SK, Stuart AM, Medalla FM, Whichard JM, Hoekstra RM, Chiller TM. 2008. Distribution of multidrug-resistant human isolates of MDR-ACSSuT Salmonella Typhimurium and MDR-AmpC Salmonella Newport in the United States, 2003–2005. Foodborne Pathog. Dis. 5:669–680. 10.1089/fpd.2008.0111 [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Qaiyumi S, Friedman S, Singh R, Foley SL, White DG, McDermott PF, Donkar T, Bolin C, Munro S, Baron EJ, Walker RD. 2003. Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J. Clin. Microbiol. 41:5366–5371. 10.1128/JCM.41.12.5366-5371.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. 2002. Outbreak of multidrug-resistant Salmonella Newport—United States, January–April 2002. MMWR Morb. Mortal. Wkly. Rep. 51:545–548 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5125a1.htm [PubMed] [Google Scholar]
- 12.Guibourdenche M, Roggentin P, Mikoleit M, Fields PI, Bockemuhl J, Grimont PA, Weill FX. 2010. Supplement 2003–2007 (no. 47) to the White-Kauffmann-Le Minor scheme. Res. Microbiol. 161:26–29. 10.1016/j.resmic.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Gomez TM, Motarjemi Y, Miyagawa S, Kaferstein FK, Stohr K. 1997. Foodborne salmonellosis. World Health Stat. Q. 50:81–89 [PubMed] [Google Scholar]
- 14.Walters SP, Thebo AL, Boehm AB. 2011. Impact of urbanization and agriculture on the occurrence of bacterial pathogens and stx genes in coastal waterbodies of central California. Water Res. 45:1752–1762. 10.1016/j.watres.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 15.Haley BJ, Cole DJ, Lipp EK. 2009. Distribution, diversity, and seasonality of waterborne salmonellae in a rural watershed. Appl. Environ. Microbiol. 75:1248–1255. 10.1128/AEM.01648-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters SP, Gonzalez-Escalona N, Son I, Melka DC, Sassoubre LM, Boehm AB. 2013. Salmonella enterica diversity in central Californian coastal waterways. Appl. Environ. Microbiol. 79:4199–4209. 10.1128/AEM.00930-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winfield MD, Groisman EA. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69:3687–3694. 10.1128/AEM.69.7.3687-3694.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorski L, Parker CT, Liang A, Cooley MB, Jay-Russell MT, Gordus AG, Atwill ER, Mandrell RE. 2011. Prevalence, distribution, and diversity of Salmonella enterica in a major produce region of California. Appl. Environ. Microbiol. 77:2734–2748. 10.1128/AEM.02321-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen CS, Bech TB. 2012. Soil survival of Salmonella and transfer to freshwater and fresh produce. Food Res. Int. 45:557–566. 10.1016/j.foodres.2011.07.026 [DOI] [Google Scholar]
- 20.Shirron N, Yaron S. 2011. Active suppression of early immune response in tobacco by the human pathogen Salmonella Typhimurium. PLoS One 6:e18855. 10.1371/journal.pone.0018855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapidot A, Yaron S. 2009. Transfer of Salmonella enterica serovar Typhimurium from contaminated irrigation water to parsley is dependent on curli and cellulose, the biofilm matrix components. J. Food Prot. 72:618–623 [DOI] [PubMed] [Google Scholar]
- 22.Schnetzinger F, Pan Y, Nocker A. 2013. Use of propidium monoazide and increased amplicon length reduce false-positive signals in quantitative PCR for bioburden analysis. Appl. Microbiol. Biotechnol. 97:2153–2162. 10.1007/s00253-013-4711-6 [DOI] [PubMed] [Google Scholar]
- 23.Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, Frankel G. 2010. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 12:2385–2397. 10.1111/j.1462-2920.2010.02297.x [DOI] [PubMed] [Google Scholar]
- 24.Benjamin L, Atwill ER, Jay-Russell M, Cooley M, Carychao D, Gorski L, Mandrell RE. 2013. Occurrence of generic Escherichia coli, E. coli O157 and Salmonella spp. in water and sediment from leafy green produce farms and streams on the Central California coast. Int. J. Food Microbiol. 165:65–76. 10.1016/j.ijfoodmicro.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Villanova Ruiz B, Cueto Espinar A, Bolaños Carmona MJ. 1987. A comparative study of strains of Salmonella isolated from irrigation waters, vegetables and human infections. Epidemiol. Infect. 98:271–276. 10.1017/S0950268800062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan TY, Holley RA. 2003. Pathogen survival in swine manure environments and transmission of human enteric illness—a review. J. Environ. Qual. 32:383–392 (Erratum, 32:1153.) [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Allard S, Reynolds S, Millner P, Arce G, Blodgett RJ, Brown EW. 2013. Colonization and internalization of Salmonella enterica in tomato plants. Appl. Environ. Microbiol. 79:2494–2502. 10.1128/AEM.03704-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D, Sela S. 2009. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl. Environ. Microbiol. 75:6076–6086. 10.1128/AEM.01084-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews WH, Jacobson A, Hammack T. 2014. Salmonella. In Bacteriological analytical manual, 8th ed. Center for Food Safety and Applied Nutrition, U.S. FDA, College Park, MD: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149.htm [Google Scholar]
- 30.Li B, Chen JQ. 2013. Development of a sensitive and specific qPCR assay in conjunction with propidium monoazide for enhanced detection of live Salmonella spp. in food. BMC Microbiol. 13:273. 10.1186/1471-2180-13-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becton, Dickinson, and Company. 2011. BBL Sensi-Disc antimicrobial susceptibility test discs kit package insert. Becton, Dickinson, and Company, Franklin Lakes, NJ [Google Scholar]
- 32.Doyle MP, Erickson MC. 2008. Summer meeting 2007—the problems with fresh produce: an overview. J. Appl. Microbiol. 105:317–330. 10.1111/j.1365-2672.2008.03746.x [DOI] [PubMed] [Google Scholar]
- 33.Beuchat LR. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413–423. 10.1016/S1286-4579(02)01555-1 [DOI] [PubMed] [Google Scholar]
- 34.Loge FJ, Thompson DE, Call DR. 2002. PCR detection of specific pathogens in water: a risk-based analysis. Environ. Sci. Technol. 36:2754–2759. 10.1021/es015777m [DOI] [PubMed] [Google Scholar]
- 35.Juen A, Traugott M. 2005. Detecting predation and scavenging by DNA gut-content analysis: a case study using a soil insect predator-prey system. Oecologia 142:344–352. 10.1007/s00442-004-1736-7 [DOI] [PubMed] [Google Scholar]
- 36.Covert TC. 1999. Salmonella, p 107–110 In American Water Works Association manual of water supply practices, waterborne pathogens. American Water Works Association, Denver, CO [Google Scholar]
- 37.Matheson N, Kingsley RA, Sturgess K, Aliyu SH, Wain J, Dougan G, Cooke FJ. 2010. Ten years experience of Salmonella infections in Cambridge, UK. J. Infect. 60:21–25. 10.1016/j.jinf.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 38.Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DM, Jensen AB, Wegener HC, Aarestrup FM. 2011. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 8:887–900. 10.1089/fpd.2010.0787 [DOI] [PubMed] [Google Scholar]
- 39.Bachmann NL, Petty NK, Ben Zakour NL, Szubert JM, Savill J, Beatson SA. 2014. Genome analysis and CRISPR typing of Salmonella enterica serovar Virchow. BMC Genomics 15:389. 10.1186/1471-2164-15-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Micallef SA, Rosenberg Goldstein RE, George A, Kleinfelter L, Boyer MS, McLaughlin CR, Estrin A, Ewing L, Jean-Gilles BJ, Hanes DE, Kothary MH, Tall BD, Razeq JH, Joseph SW, Sapkota AR. 2012. Occurrence and antibiotic resistance of multiple Salmonella serotypes recovered from water, sediment and soil on Mid-Atlantic tomato farms. Environ. Res. 114:31–39. 10.1016/j.envres.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 41.Jang T, Vellidis G, Hyman JB, Brooks E, Kurkalova LA, Boll J, Cho J. 2013. Model for prioritizing best management practice implementation: sediment load reduction. Environ. Manage. 51:209–224. 10.1007/s00267-012-9977-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho JG, Vellidis Bosch DD, Lowrance R, Strickland T. 2010. Water quality effects of simulated conservation practice scenarios in the Little River experimental watershed. J. Soil Water Conserv. 45:463–473. 10.2489/jswc.65.6.463 [DOI] [Google Scholar]
- 43.Harbottle H, White DG, McDermott PF, Walker RD, Zhao S. 2006. Comparison of multilocus sequence typing, pulsed-field gel electrophoresis, and antimicrobial susceptibility typing for characterization of Salmonella enterica serotype Newport isolates. J. Clin. Microbiol. 44:2449–2457. 10.1128/JCM.00019-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sangal V, Harbottle H, Mazzoni CJ, Helmuth R, Guerra B, Didelot X, Paglietti B, Rabsch W, Brisse S, Weill FX, Roumagnac P, Achtman M. 2010. Evolution and population structure of Salmonella enterica serovar Newport. J. Bacteriol. 192:6465–6476. 10.1128/JB.00969-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parry CM, Threlfall EJ. 2008. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 21:531–538. 10.1097/QCO.0b013e32830f453a [DOI] [PubMed] [Google Scholar]
- 46.Zhao S, McDermott PF, White DG, Qaiyumi S, Friedman SL, Abbott JW, Glenn A, Ayers SL, Post KW, Fales WH, Wilson RB, Reggiardo C, Walker RD. 2007. Characterization of multidrug resistant Salmonella recovered from diseased animals. Vet. Microbiol. 123:122–132. 10.1016/j.vetmic.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 47.Piddock LJ. 2002. Fluoroquinolone resistance in Salmonella serovars isolated from humans and food animals. FEMS Microbiol. Rev. 26:3–16. 10.1111/j.1574-6976.2002.tb00596.x [DOI] [PubMed] [Google Scholar]
- 48.Whichard JM, Gay K, Stevenson JE, Joyce KJ, Cooper KL, Omondi M, Medalla F, Jacoby GA, Barrett TJ. 2007. Human Salmonella and concurrent decreased susceptibility to quinolones and extended-spectrum cephalosporins. Emerg. Infect. Dis. 13:1681–1688. 10.3201/eid1311.061438 [DOI] [PMC free article] [PubMed] [Google Scholar]