Abstract
Although multiple genotypes of Campylobacter jejuni may be isolated from the same commercial broiler flock, little is known about the infection dynamics of different genotypes within individuals or their colonization sites within the gut. Single experimental infections with C. jejuni M1 (sequence type 137, clonal complex 45) and C. jejuni 13126 (sequence type 21, clonal complex 21) revealed that 13126 colonized the ceca at significantly higher levels. The dissemination and colonization sites of the two C. jejuni strains then were examined in an experimental broiler flock. Two 33-day-old broiler chickens were infected with M1 and two with 13126, and 15 birds were left unchallenged. Cloacal swabs were taken postinfection to determine the colonization and shedding of each strain. By 2 days postinfection (dpi), 8/19 birds were shedding M1 whereas none were shedding 13126. At 8 dpi, all birds were shedding both strains. At 18 dpi, liver and cecal levels of each isolate were quantified, while in 10 birds they also were quantified at nine sites throughout the gastrointestinal (GI) tract. 13126 was found throughout the GI tract, while M1 was largely restricted to the ceca and colon. The livers of 7/19 birds were culture positive for 13126 only. These data show that 13126 has a distinctly different infection biology than strain M1. It showed slower colonization of the lower GI tract but was more invasive and able to colonize at a high level throughout the GI tract. The finding that C. jejuni strains have markedly different infection ecologies within the chicken has implications for control in the poultry industry and suggests that the contamination risk of edible tissues is dependent on the isolate involved.
INTRODUCTION
Campylobacter jejuni is the most frequent cause of food-borne bacterial gastroenteritis in the world and is estimated to infect 1% of the EU population each year. Chicken is the single largest source of infection, with up to 80% of retail poultry carcasses contaminated in the EU (1). Transmission from environmental sources is considered the primary route of flock colonization with Campylobacter (2).
C. jejuni colonizes the chicken gut and usually is found at the greatest levels in the mucosal crypts of the ceca (3). However, it also may be recovered at lower levels from the small intestines, crop, gizzard, and liver (4, 5). After infection, C. jejuni rapidly colonizes the two blind ceca to a high level and leads to fecal shedding (6). This high-level fecal shedding combined with the coprophagic behavior of the chickens means that once the first bird in a broiler flock becomes colonized, the bacterium then is able to pass rapidly through the entire flock in just a few days.
Although many broiler flocks are dominated by a single C. jejuni genotype (7), two or more genotypes can be isolated from the same flock (8–11). In most cases, where more than one genotype has colonized a flock, individual genotypes were found to coexist over time rather than exclude each other (12). However, replacement of one C. jejuni strain by another also has been observed in both field and experimental studies in chickens, indicating that some strains are more dominant than others (8, 13–15). Konkel and colleagues found that one C. jejuni strain could competitively inhibit a second C. jejuni strain from binding to epithelial cells, and when using the same two strains in infection experiments, the first C. jejuni strain also could prevent the second C. jejuni strain from colonizing chicks (15). A number of studies also have shown that different C. jejuni strains have various colonization abilities in chickens (6, 16–20).
While the colonization of chickens by C. jejuni has been well documented, very little is known about the dynamics of different genotypes in individual birds and whether different strains are able to colonize different sites in the gastrointestinal (GI) tract. We aimed to examine how two C. jejuni isolates, representing common genotypes found in broilers, disseminated and colonized birds in a small experimental broiler flock and to determine whether different strains are able to colonize different sites in the GI tract.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
C. jejuni M1 was selected for use in this study, as it is a well-characterized isolate (21–23) used in our laboratories and also is naturally resistant to tetracycline. C. jejuni 13126 also was selected, as it is naturally resistant to ciprofloxacin. Both strains also represent common genotypes found in UK retail poultry (24). C. jejuni M1 is a sequence type (ST) 137 (clonal complex [CC] 45) human isolate kindly provided by Lisa Williams (University of Bristol). C. jejuni 13126 is an ST 21 (CC-21) isolate from a UK poultry survey which was kindly provided by the UK Food Standards Agency (FSA). Bacteria were grown from stocks maintained at −80°C on Columbia blood agar (Lab M Ltd., Heywood, Lancashire, United Kingdom) supplemented with 5% defibrinated horse blood (Oxoid, Basingstoke, Hampshire, United Kingdom) for 48 h under microaerobic conditions (80% N2, 12% CO2, 5% O2 and 3% H2) at 41.5°C. Liquid cultures were grown for 24 h in 10 ml of Mueller-Hinton broth (MHB) under microaerobic conditions at 41.5°C and adjusted by dilution in fresh MHB to a final concentration of 106 CFU/ml for the initial infection and 108 CFU/ml for the subsequent infection. All microbiological media were purchased from Lab M Ltd. (Heywood, Lancashire, United Kingdom).
Experimental animals.
All work was conducted in accordance with United Kingdom legislation governing experimental animals under project license PPL 40/3652 and was approved by the University of Liverpool ethical review process prior to the award of the license. All animals were checked a minimum of twice daily to ensure their health and welfare. One-day-old Ross 308 chicks, the most commonly reared broiler breed in the United Kingdom, were obtained from a commercial hatchery. Chicks were maintained in floor pens at Home Office-recommended stocking levels, and were given ad libitum access to water and a pelleted vegetable protein-based diet (SDS, Witham, Essex, United Kingdom). Chicks were housed at a temperature of 30°C, which was reduced to 20°C when the birds were 3 weeks of age. Prior to experimental infection, all birds were confirmed as Campylobacter free by taking cloacal swabs, which were streaked onto Campylobacter-selective blood-free agar (mCCDA) supplemented with Campylobacter enrichment supplement (SV59; Mast Group Ltd., Bootle, Merseyside, United Kingdom) and grown for 48 h in microaerobic conditions at 41.5°C.
Effect of strain on C. jejuni infection.
One-day-old Ross 308 chicks, the most commonly reared broiler breed in the United Kingdom, were obtained from a commercial hatchery and housed as described above. Birds (n = 22) were divided into two groups, each containing 11 birds. At 21 days of age, birds in one group were infected orally with 105 cells of C. jejuni M1 in 0.2 ml of MHB, while those in the other group were infected orally with 105 cells of C. jejuni 13126 in 0.2 ml MHB. Cloacal swabs were performed at 4 days postinfection (dpi) to confirm colonization with Campylobacter. All birds were killed at 11 dpi (32 days of age) for postmortem examination, and liver tissue and gastrointestinal contents from the ileum and ceca were taken for Campylobacter enumeration using the method described below.
Assessment of C. jejuni load.
Enumeration of C. jejuni on the cloacal swabs was carried out using a semiquantitative approach (25, 26). Briefly, swabs were eluted in 2 ml modified Exeter broth consisting of 1,100 ml nutrient broth, 11 ml lysed defibrinated horse blood (Oxoid, Basingstoke, Hampshire, United Kingdom), Campylobacter enrichment supplement SV59 (Mast Diagnostics), and Campylobacter growth supplement SV61 (Mast Diagnostics). Swabs then were plated onto mCCDA agar supplemented with SV59 and 30 μg/ml tetracycline and mCCDA agar supplemented with SV59 and 12 μg/ml ciprofloxacin. The enriched swabs also were incubated at 41.5°C for 48 h before the swab was replated onto mCCDA agar supplemented with antibiotics as described above. Plates were incubated for 48 h at 41.5°C before being scored for the level of bacterial growth as heavy (H; >50 colonies after initial direct plating), moderate (D; between 1 and 50 colonies after initial direct plating), and total amount of C. jejuni isolated by plating after enrichment in modified Exeter broth for 48 h (T). All antibiotics were purchased from Sigma-Aldrich (Dorset, United Kingdom).
At 11 dpi, all birds were killed, and at postmortem examination, samples of liver and gastrointestinal contents from the ileum and ceca were taken aseptically for Campylobacter enumeration. Serial 10-fold dilutions were made of each sample in maximum recovery diluent (MRD), and using the method of Miles and Misra, triplicate 20-μl spots were plated in duplicate onto mCCDA agar supplemented with antibiotics as described above. Plates were incubated under microaerobic conditions at 41.5°C for 48 h and enumerated to give CFU/g of sample. Liver samples also were collected from all birds, homogenized, diluted in 9 volumes of MRD, and plated onto mCCDA agar supplemented with antibiotics as described above. To enrich liver samples, 100 μl of each homogenized liver sample was enriched in 2 ml of modified Exeter broth and incubated under microaerobic conditions at 41.5°C for 48 h, followed by plating onto mCCDA agar supplemented with antibiotics as described above.
Dual infection with Campylobacter jejuni strains.
One-day-old Ross 308 chicks, the most commonly reared broiler breed in the United Kingdom, were obtained from a commercial hatchery and housed as described above. When the birds were 21 days old, all birds (n = 19) were released from the floor pen into the room (total area, 60 ft2). Two “seeder” birds then were orally infected with 105 cells of C. jejuni M1 in 0.2 ml of MHB and two different seeder birds with 105 cells of C. jejuni 13126 in 0.2 ml of MHB. The remaining birds were not infected. Following infection, the litter in the room was not changed. Cloacal swabbing of the birds at 2, 5, and 8 days postinfection (dpi) revealed that all, including the seeder birds, were Campylobacter negative. Therefore, when the birds were 33 days old, the same two seeder birds were rechallenged orally with 107 cells of C. jejuni M1 in 0.2 ml of MHB and the same two seeder birds with 107 cells of C. jejuni 13126 in 0.2 ml of MHB. The remaining birds were not challenged, and the litter in the room was not changed.
Assessment of C. jejuni load.
Cloacal swabs were taken at 2, 5, 8, 12, and 15 dpi following the rechallenge to determine the course of each Campylobacter strain during infection. Bacterial enumeration of the cloacal swabs was carried out using the semiquantitative approach described above.
At 18 dpi, all birds were killed by neck dislocation, and at postmortem examination, samples of tissue and gut contents were taken aseptically for Campylobacter enumeration. In order to determine the levels of C. jejuni colonization in each of the gastrointestinal sites, in 10 of the birds gastrointestinal contents were collected from the crop, gizzard, duodenum, jejunum, proximal ileum, distal ileum, ceca, and colon. Samples of liver tissue also were collected. Samples were processed and quantitative bacteriology performed as described previously. The presence/absence of hock marks and/or pododermatitis also was recorded for every bird at postmortem examination.
Statistical analyses.
Statistical analyses were performed using SPSS v20 (IBM). For comparison of the levels of each of the C. jejuni strains in the different gastrointestinal sites, the Mann-Whitney U (nonparametric) test was applied, as the data were not normally distributed. Differences were considered significant when P < 0.05.
RESULTS
Effect of strain on C. jejuni infection.
At 4 dpi, 10/11 birds infected with C. jejuni M1 were shedding this strain, whereas none of the birds infected with C. jejuni 13126 were shedding this strain. However, by 11 dpi, all of the birds infected with C. jejuni M1 and all of the birds infected with C. jejuni 13126 were shedding their respective strains. Differences were found in the level of colonization of the two strains in the gastrointestinal tract, although both colonized the ceca and the ileum (Fig. 1).
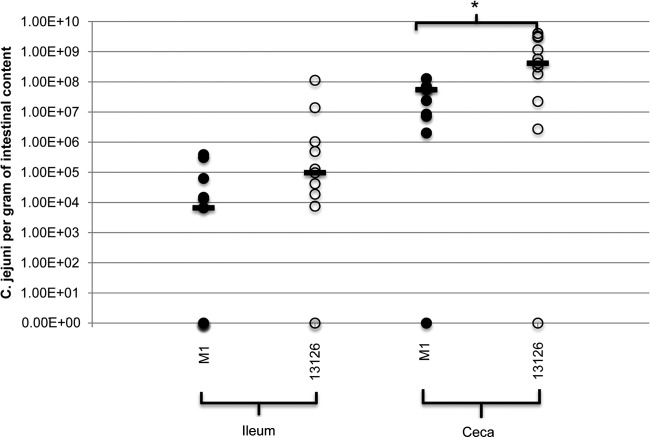
FIG 1.
Gastrointestinal colonization of the C. jejuni strains M1 (closed circles) and 13126 (open circles) at 11 dpi. Colonization of each strain was examined in the ileum and ceca and is presented as the CFU of C. jejuni per gram of gastrointestinal content. Bars represent the median values for each site. Significant differences between the strains were examined using a Mann-Whitney U test.
C. jejuni 13126 colonized the ceca to a significantly higher level than M1 (P = 0.023). However, no significant differences (P = 0.065) were found between the isolates in terms of their ability to colonize the ileum.
Extraintestinal spread of C. jejuni strains M1 and 13126.
At postmortem examination, one liver from the group of birds infected with C. jejuni M1 contained C. jejuni M1, whereas in the group of birds infected with C. jejuni 13126, eight livers were positive for this bacterium. Infection with C. jejuni 13126 was associated with significantly higher levels of liver positivity than M1 (P = 0.010). These results suggest C. jejuni 13126 is more capable of extraintestinal spread.
Dual infection with Campylobacter jejuni strains. (i) Determination of the dynamics of C. jejuni colonization.
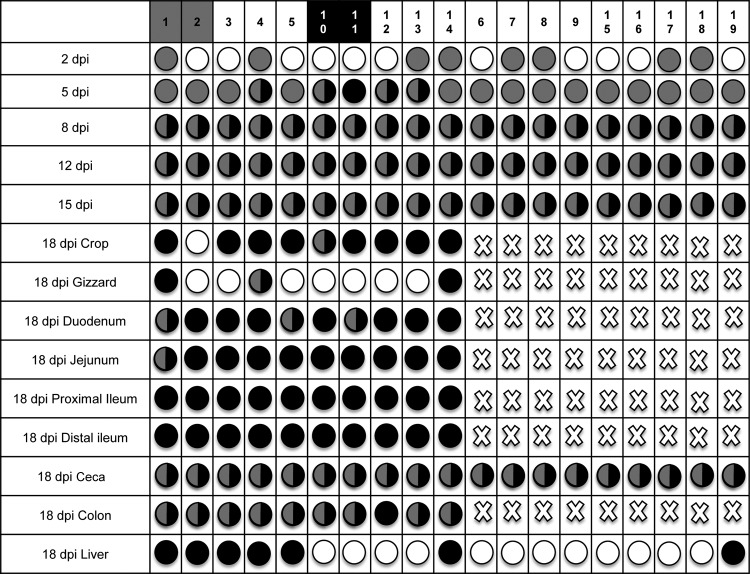
Between 2 dpi and 15 dpi (following reinfection), cloacal swabs were used to determine the dynamics of the two strains in the population of birds. A semiquantitative method was used to allow determination of the levels of each of the strains. At 2 dpi, 8/19 birds were shedding C. jejuni M1, whereas none were shedding C. jejuni 13126 (Table 1 and Fig. 2). At 5 dpi, nearly all of the birds (18/19) were shedding C. jejuni M1, whereas only 5 were shedding C. jejuni 13126 (Table 1 and Fig. 2). However, by 8 dpi, all birds were positive for both strains. All birds shed C. jejuni 13126 at a high level, with 16/19 also showing high-level shedding of C. jejuni M1 (Table 1 and Fig. 2). All birds continued to shed both strains at the 12- and 15-dpi time points (Table 1 and Fig. 2). However, by 15 dpi, although most (18/19) birds continued to shed C. jejuni 13126 at a high level, only 10/19 showed a pattern similar to that of M1.
TABLE 1.
Distribution of birds at each time point with heavy, directly detectable, and total cloacal colonizationa
| Day postinfection | No. (%) of birds colonized by: |
|||||
|---|---|---|---|---|---|---|
| M1 |
13126 |
|||||
| H | D | T | H | D | T | |
| 2 | 1 (5.3) | 2 (5.3) | 8 (42.1) | 0 | 0 | 0 |
| 5 | 13 (68.4) | 2 (10.5) | 18 (94.7) | 1 (5.3) | 1 (5.3) | 5 (26.3) |
| 8 | 16 (84.2) | 3 (15.8) | 19 (100) | 19 (100) | 0 | 19 (100) |
| 12 | 14 (73.7) | 5(26.3) | 19 (100) | 19 (100) | 0 | 19 (100) |
| 15 | 10 (52.6) | 9 (47.4) | 19 (100) | 18 (94.7) | 1 (5.3) | 19 (100) |
Cloacal colonization of birds after seeders were infected with C. jejuni M1 and C. jejuni 13126 at 33 days of age. Birds were monitored by cloacal swabbing at 2, 5, 8, 12, and 15 dpi. Results are expressed as both the number and percentage of birds falling into each of three levels of colonization (H, D, and T). The total (T) includes positives from H and D colonization along with those obtained from enrichment culture.
FIG 2.
Cloacal colonization of the C. jejuni strains M1 and 13126 between 2 dpi and 15 dpi and gastrointestinal colonization at the 18-dpi postmortem examination. Colonization of each strain was examined in the crop, gizzard, duodenum, jejunum, proximal ileum, distal ileum, ceca, and colon and is presented as C. jejuni strain per gram of gastrointestinal content. Birds 1 and 2 were the seeder birds for C. jejuni M1, while birds 10 and 11 were the seeder birds for C. jejuni 13126. Gray circles represent sites where only C. jejuni M1 was found, black circles represent sites were only C. jejuni 13126 was found, circles which are half gray and half black represent sites where both C. jejuni M1 and C. jejuni 13126 were found, white circles represent Campylobacter-negative sites, and white crosses are sites that were not sampled at that time point.
(ii) Gastrointestinal colonization of the C. jejuni strains M1 and 13126.
At postmortem examination, C. jejuni was isolated from the duodenum, jejunum, proximal ileum, distal ileum, ceca, and colon in all 10 birds (Fig. 2). It also was isolated from the crop in most birds (9/10) and, less frequently, the gizzard of some birds (3/10) (Fig. 2).
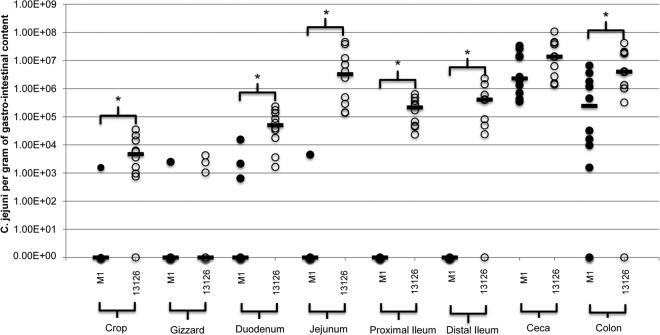
Differences were found in the colonization patterns of the two strains in the gastrointestinal tract. Both strains were present in the ceca of all of the birds sampled and in all but one of the colon samples (Fig. 2). C. jejuni M1 was not isolated from any of the proximal or distal ileum samples, and it was rarely isolated from any of the crop, gizzard, duodenum, or jejunum samples (Fig. 2). In contrast, C. jejuni 13126 was isolated from all of the duodenum, jejunum, and proximal and distal ileum samples (Fig. 2). C. jejuni 13126 also was isolated from 9/10 crop samples and 3/10 gizzard samples (Fig. 2). Significant differences were found in the levels of each of the C. jejuni strains in the crop (P ≤ 0.001), duodenum (P ≤ 0.001), jejunum (P ≤ 0.001), proximal and distal ileum (both P ≤ 0.001), and colon (P ≤ 0.001) (Fig. 3).
FIG 3.
Gastrointestinal colonization of the C. jejuni strains M1 (closed circles) and 13126 (open circles) at 18 dpi. Colonization of each strain was examined in the crop, gizzard, duodenum, jejunum, proximal ileum, distal ileum, ceca, and colon and is presented as CFU of C. jejuni per gram of gastrointestinal content. Bars represent the median values for each site. Significant differences between the strains were examined using a Mann-Whitney U test.
(iii) Extraintestinal spread of C. jejuni strains M1 and 13126.
At postmortem examination, one liver from the group of birds contained C. jejuni 13126 by direct plating, and six livers were positive for this bacterium by enrichment culture (Fig. 2). In contrast, no C. jejuni M1 was detected by either direct plating or enrichment culture (Fig. 2). These results further suggest that C. jejuni 13126 is more invasive and be better able to show extraintestinal spread and infect the liver, as well as colonize the whole of the gastrointestinal tract.
Welfare indications.
Four out of the 19 birds had evidence of mild pododermatitis at postmortem examination (birds 3, 5, 6 and 10), but no hock marks were observed.
DISCUSSION
Here, we show that different C. jejuni genotypes display distinct differences in both their dynamics of infection and gut ecology in commercial broiler chickens. C. jejuni M1 shows classical infection biology of rapid colonization and shedding that is largely associated with the ceca. In contrast, C. jejuni 13126 is slower to colonize yet is more able to colonize the upper GI tract, and it is significantly more capable of extraintestinal spread. This has implications for the control of C. jejuni in poultry production and processing. Control in the United Kingdom recently has focused on reducing the cecal load of C. jejuni in an attempt to reduce its public health impact (http://www.food.gov.uk/policy-advice/microbiology/campylobacterevidenceprogramme/#Thecampylobactertarget). Our data show that the ST 21 isolate 13126 is capable of colonizing many sites in the GI tract to a high level, meaning that carcass contamination may originate from parts of the GI tract other than the ceca and colon, increasing the loading of this organism on the carcass; this is something that has clear implications at slaughter and for postslaughter processing. Furthermore, C. jejuni 13126 is more capable of extraintestinal spread than isolate M1, leading to a greater risk of contaminating the liver and potentially other edible tissues, such as breast meat, which would be largely unaffected by proposed postslaughter interventions.
To determine differences in the dynamics and ecology of C. jejuni infection, we utilized single experimental infections and a dual seeder infection approach in Ross 308 broiler chickens infected at 21 days of age. In the single experimental infections, both C. jejuni M1 and C. jejuni 13126 were able to colonize the ileum and ceca. Significant differences were found between the levels to which C. jejuni M1 and C. jejuni 13126 were able to colonize the ceca. C. jejuni 13126 was able to colonize to a much higher level. Furthermore, C. jejuni 13126 spreads more readily from the intestines than isolate M1, leading to a greater risk of contaminating the liver. The mechanisms that allow this are not yet described, but a greater ability to invade via the intestinal epithelium is likely. In the dual seeder infection model, following the infection of the seeder birds at 21 days of age, none of the birds within the flock, including the seeder birds, became Campylobacter positive. Therefore, when the seeders were rechallenged subsequently at 33 days of age, we used a relatively high challenge dose (107 bacteria) to enable the seeders to shed the bacteria effectively and allow the other birds in the flock to become infected. It is unclear why the seeders in the second experiment failed to become infected using the lower challenge dose (105 bacteria), especially given that, in the first experiment, this dose was sufficient to establish infection. One plausible reason is that we are using a low challenge dose in only a small number of birds; thus, if infection is not established in the seeder birds, it is unable to spread throughout the flock. This possibility can be examined in experiment 1, where all of the birds were infected, so even if infection does not become established in some of the birds it will still be able to spread throughout the flock due to other birds being colonized. There is also evidence that some well-characterized strains, such as C. jejuni 11168, have variable colonization (27). Once the seeders were given the higher challenge dose (107 bacteria), they were able to shed the bacteria effectively and allow the other birds in the flock to become infected.
One of the seeder birds had begun to shed C. jejuni M1 at levels high enough to be detected by cloacal swabbing at 2 dpi following rechallenge. However, the shedding of the respective C. jejuni isolates from the remaining three seeder birds was not detected by cloacal swabbing until somewhere between 3 and 5 dpi. Although both strains were given to the respective seeders at the same time and at a similar level, C. jejuni M1 appears to colonize birds in the flock and is detectable by cloacal swabbing much sooner than C. jejuni 13126. There may be a number of reasons why there is a delay in the shedding of C. jejuni 13126. A previous study has found that there may be an upper limit to the number of C. jejuni bacteria that can be present in the cecum (15). If this is indeed true, when there is a sequential infection one strain may need to be partially displaced for a second to establish colonization. However, in this experiment, as the strains were introduced to the respective seeder birds at the same time and at similar levels, the experiment is more like a coinfection scenario than sequential infection (where one strain fully colonizes the flock, followed by challenge with a second strain), so it is unlikely that this explains why we see a delay in the dissemination of C. jejuni 13126 through the flock. Strains with greater motility also may have an advantage in vivo, as C. jejuni bacteria preferentially colonize the mucus-filled cecal crypts (15). If C. jejuni M1 has greater motility than C. jejuni 13126, this may allow the strain to colonize the ceca sooner and begin to be shed at levels detectable by cloacal swabbing earlier.
Another hypothesis is that C. jejuni 13126 colonizes the chicken initially further up the gastrointestinal tract than C. jejuni M1, so it is less readily detected by cloacal swabbing at the earlier time points. We have shown that birds mount a prolonged local inflammatory response to C. jejuni M1 (22), and it may be that some strains, such as C. jejuni 13126, are more susceptible to this prolonged inflammatory response, delaying cecal colonization of the birds with this strain. Alternatively, there may be differences in metabolic capacity that mean some isolates are more adaptable to higher-level colonization and to the range of differences in physical conditions that are found throughout the GI tract, including pH, oxygen tension, levels of bile, mucins, or differences in the resident microbiota. A number of studies have shown that different C. jejuni strains have various colonization abilities in chickens (6, 16–19).
When Friis and colleagues examined the genome of C. jejuni M1, they found a number of the genes, which have previously been described as being related to chicken colonization, to be present in this strain (21). These included genes such as cadF, which encodes the Campylobacter adhesion to fibronectin protein (28, 29), and flpA, which has been shown to be important in chicken colonization (30). However, other genes, such as capA, which also have been identified as being important for chicken colonization (31), were missing from C. jejuni M1. However, this is not unusual, as capA is known to be absent from many C. jejuni isolates (30). Without the genome sequence of C. jejuni 13126, it is difficult to make direct comparisons of the virulence factors which are present in the two strains, although we plan to do this in the future.
Here, we have confirmed the findings of previous studies that birds can be colonized by more than one strain (10, 12, 15). However, this study has revealed that different strains appear to have different niches within the gastrointestinal tract. C. jejuni M1 was rarely found in sites other than the ceca and colon, whereas C. jejuni 13126 was found throughout the gastrointestinal tract and at significantly higher levels in all sites except the gizzard and ceca.
One explanation for the difference in gastrointestinal niches is that the birds were starting to clear C. jejuni M1 at those sites, particularly as it appeared to colonize the birds earlier than C. jejuni 13126. Other studies have shown that birds are able to clear Campylobacter (32), and competition between strains may be an important mechanism leading to clearance of a strain (33). Further work would be needed to confirm whether the two strains do in fact have different niches within the gastrointestinal tract or whether, at earlier time points, C. jejuni M1 is found higher up the gastrointestinal tract. The fact that C. jejuni 13126 is able to colonize throughout the gastrointestinal tract is a finding which warrants further investigation. Of particular interest is the fact that C. jejuni 13126 appears to be a more invasive strain which is capable of extraintestinal spread to liver tissue. C. jejuni 13126 also appears to be a better colonizer of the crop, as it was isolated from the crop in all but one of the birds in our study. The crop is an acidic environment, so it may be that C. jejuni 13126 is more acid tolerant and able to colonize, while colonization by C. jejuni M1 may be inhibited by the acidic conditions. The crop is thought to be a possible source of cross-contamination of meat at slaughter, as it is more likely to rupture during the evisceration process than the intestines (34). The threat of this cross-contamination may be increased, as the process of feed withdrawal in broilers before slaughter has been found to significantly increases the isolation frequency of Campylobacter from the crop (5, 35, 36).
One of the limitations of this study is that we used media containing antibiotics to differentiate between the two C. jejuni strains. There is a small possibility that resistance determinants transferred between the strains during colonization. However, we feel this is unlikely, as although Campylobacter are naturally transformable, the experiments were carried out over a short time period and the birds were not under any selective pressure. Ciprofloxacin resistance arises as a result of mutations in the quinolone resistance-determining region (QRDR) of the gyrA gene (37, 38). In Campylobacter species, high-level tetracycline resistance usually is associated with the tet(O) gene carried on transmissible plasmids (39). In order to confirm that C. jejuni M1 had not acquired resistance to ciprofloxacin and C. jejuni 13126 had not acquired resistance to tetracycline during the colonization of the birds, a proportion of the isolates isolated at 18 dpi using the mCCDA agar supplemented 30 μg/ml tetracycline and a proportion of the of the isolates isolated at 18 dpi using mCCDA agar supplemented with 12 μg/ml ciprofloxacin were replated onto mCCDA agar supplemented with both 30 μg/ml tetracycline and 12 μg/ml ciprofloxacin. None of the 20 isolates grew on the plates containing both antibiotics, confirming that C. jejuni M1 had not acquired resistance to ciprofloxacin and C. jejuni 13126 had not acquired resistance to tetracycline during colonization of the birds.
Understanding how different C. jejuni strains colonize poultry is important for developing strategies to reduce or eliminate C. jejuni carriage. The ability of C. jejuni strains to colonize the digestive tract of the bird is clearly multifactorial. However, if certain strains are able to colonize the crop and are more likely to cross-contaminate meat during evisceration at slaughter, or they are strains which are capable of extraintestinal spread to edible tissues, such as liver or breast muscle, then these findings would have profound implications for public health. Further work is necessary to elucidate the mechanisms by which strains such as C. jejuni 13126 are able to leave the gut and infect the liver.
ACKNOWLEDGMENTS
We gratefully acknowledge funding from BBSRC (grant number BB/I024674/1), UK food retailers, poultry breeding companies, and Danisco.
We thank the staff in the infection facilities for their excellent work in caring for the chickens.
Footnotes
Published ahead of print 8 August 2014
REFERENCES
- 1.EFSA. 2010. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008. Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 8:1503–1602. 10.2903/j.efsa.2010.1503 [DOI] [Google Scholar]
- 2.Sahin O, Morishita TY, Zhang Q. 2002. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 3:95–105. 10.1079/AHRR200244 [DOI] [PubMed] [Google Scholar]
- 3.Beery JT, Hugdahl MB, Doyle MP. 1988. Colonisation of gastrointestinal tract of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young CR, Ziprin RL, Hume ME, Stanker LH. 1999. Dose response and organ invasion of day-of-hatch leghorn chicks by different isolates of Campylobacter jejuni. Avian Dis. 43:763–767 [PubMed] [Google Scholar]
- 5.Achen M, Morishita TY, Ley EC. 1998. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 42:732–737 [PubMed] [Google Scholar]
- 6.Shanker S, Lee A, Sorrell TC. 1990. Horizontal transmission of Campylobacter jejuni amongst broiler chicks—experimental studies. Epidemiol. Infect. 104:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ring M, Zychowska MA, Stephan R. 2005. Dynamics of Campylobacter spp. spread investigated in 14 broiler flocks in Switzerland. Avian Dis. 49:390–396. 10.1637/7319-010305R1.1 [DOI] [PubMed] [Google Scholar]
- 8.Jacobs-Reitsma WF, Van de Giessen AW, Bolder NM, Mulder RWA. 1995. Epidemiology of Campylobacter spp. at 2 Dutch broiler farms. Epidemiol. Infect. 114:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berndtson E, Emanuelson U, Engvall A, Dainelsson Tham ML. 1996. A 1-year epidemiological study of campylobacters in 18 Swedish chicken farms. Prev. Vet. Med. 26:167–185 [Google Scholar]
- 10.Thomas LM, Long KA, Good RT, Panaccio M, Widders PR. 1997. Genotypic diversity among Campylobacter jejuni isolates in a commercial broiler flock. Appl. Environ. Microbiol. 63:1874–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiett KL, Stern NJ, Fedorka-Cray P, Cox NA, Musgrove MT, Ladely S. 2002. Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl. Environ. Microbiol. 68:6220–6236. 10.1128/AEM.68.12.6220-6236.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hook H, Fattah MA, Ericsson H, Vagsholm I, Danielsson-Tham ML. 2005. Genotype dynamics of Campylobacter jejuni in a broiler flock. Vet. Microbiol. 106:109–117. 10.1016/j.vetmic.2004.12.017 [DOI] [PubMed] [Google Scholar]
- 13.Korolik V, Alderton MR, Smith SC, Chang J, Coloe PJ. 1998. Isolation and molecular analysis of colonising and non-colonising strains of Campylobacter jejuni and Campylobacter coli following experimental infection of young chickens. Vet. Microbiol. 60:239–249 [DOI] [PubMed] [Google Scholar]
- 14.Calderon-Gomez LI, Hartley LE, McCormack A, Ringoir DD, Korolik V. 2009. Potential use of characterised hyper-colonising strain(s) of Campylobacter jejuni to reduce circulation of environmental strains in commercial poultry. Vet. Microbiol. 134:353–361. 10.1016/j.vetmic.2008.09.055 [DOI] [PubMed] [Google Scholar]
- 15.Konkel ME, Christensen JE, Dhillon AS, Lane AB, Hare-Sanford R, Schaberg DM, Larson CL. 2007. Campylobacter jejuni strains compete for colonization in broiler chicks. Appl. Environ. Microbiol. 73:2297–2305. 10.1128/AEM.02193-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanker S, Lee A, Sorrell TC. 1988. Experimental colonization of broiler chicks with Campylobacter jejuni. Epidemiol. Infect. 100:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HC, Stern NJ. 2001. Competitive exclusion of heterologous Campylobacter spp. in chicks. Appl. Environ. Microbiol. 67:848–851. 10.1128/AEM.67.2.848-851.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern NJ, Bailey JS, Blankenship LC, Cox NA, McHan F. 1988. Colonization characteristics of Campylobacter jejuni in chick caeca. Avian Dis. 32:330–334 [PubMed] [Google Scholar]
- 19.Sahin O, Zhang Q, Meitzler J. 2001. Effects of anti-Campylobacter maternal antibody on the colonization of Campylobacter jejuni in poultry, p 742–743 Abstr. 101st Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 20.Hepworth PJ, Ashelford KE, Hinds J, Gould KA, Witney AA, Williams NJ, Leatherbarrow H, French NP, Birtles RJ, Mendonca C, Dorrell N, Wren BW, Wigley P, Hall N, Winstanley C. 2011. Genomic variations define divergence of water/wildlife-associated Campylobacter jejuni niche specialists from common clonal complexes. Environ. Microbiol. 13:1549–1560. 10.1111/j.1462-2920.2011.02461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friis C, Wassenaar TM, Javed MA, Snipen L, Lagesen K, Hallin PF, Newell DG, Toszeghy M, Ridley A, Manning G, Ussery DW. 2010. Genomic characterization of Campylobacter jejuni strain M1. PLoS One 5:e12253. 10.1371/journal.pone.0012253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey S, Chaloner G, Kemmett K, Davidson N, Williams N, Kipar A, Humphrey T, Wigley P. 2014. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio 5:e01364-14. 10.1128/mBio.01364-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aroori SV, Cogan TA, Humphrey TJ. 2013. The effect of growth temperature on the pathogenicity of Campylobacter. Curr. Microbiol. 67:333–340. 10.1007/s00284-013-0370-1 [DOI] [PubMed] [Google Scholar]
- 24.Sheppard SK, Colles F, Richardson J, Cody AJ, Elson R, Lawson A, Brick G, Meldrum R, Little CL, Owen RJ, Maiden MCJ, McCarthy ND. 2010. Host association of Campylobacter genotypes transcends geographic variation. Appl. Environ. Microbiol. 76:5269–5277. 10.1128/AEM.00124-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith HW, Tucker JF. 1975. Effect of antibiotic therapy on fecal excretion of Salmonella typhimurium by experimentally infected chickens. J. Hyg. 75:275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd Y, Herbert EG, Marston KL, Jones MA, Barrow PA. 2005. Host genes affect intestinal colonisation of newly hatched chickens by Campylobacter jejuni. Immunogenetics 57:248–253. 10.1007/s00251-005-0790-6 [DOI] [PubMed] [Google Scholar]
- 27.Jones MA, Marston KL, Woodall CA, Maskell DJ, Linton D, Karlyshev AV, Dorrell N, Wren BW, Barrow PA. 2004. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect. Immun. 72:3769–3776. 10.1128/IAI.72.7.3769-3776.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konkel ME, Garvis SG, Tipton SL, Anderson DE, Cieplak W. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24:953–963 [DOI] [PubMed] [Google Scholar]
- 29.Ziprin RL, Young CR, Stanker LH, Hume ME, Konkel ME. 1999. The absence of cecal colonization of chicks by a mutant of Campylobacter jejuni not expressing bacterial fibronectin-binding protein. Avian Dis. 43:586–589 [PubMed] [Google Scholar]
- 30.Flanagan RC, Neal-McKinney JM, Dhillon AS, Miller WG, Konkel ME. 2009. Examination of Campylobacter jejuni putative adhesins leads to the identification of a new protein, designated FlpA, required for chicken colonization. Infect. Immun. 77:2399–2407. 10.1128/IAI.01266-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashgar SSA, Oldfield NJ, Wooldridge KG, Jones MA, Irving GJ, Turner DPJ, Ala'Aldeen DAA. 2007. CapA, an autotransporter protein of Campylobacter jejuni, mediates association with human epithelial cells and colonization of the chicken gut. J. Bacteriol. 189:1856–1865. 10.1128/JB.01427-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin O, Luo ND, Huang SX, Zhang QJ. 2003. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl. Environ. Microbiol. 69:5372–5379. 10.1128/AEM.69.9.5372-5379.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conlan AJK, Coward C, Grant AJ, Maskell DJ, Gog JR. 2007. Campylobacter jejuni colonization and transmission in broiler chickens: a modelling perspective. J. R. Soc. Interface 4:819–829. 10.1098/rsif.2007.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrd JA, Hargis BM, Corrier DE, Brewer RL, Caldwell DJ, Stanker LH. 1999. Fluorescent marker for the detection of crop and upper gastrointestinal leakage in poultry processing plants. Poult. Sci. 78:124–124 [DOI] [PubMed] [Google Scholar]
- 35.Byrd JA, Corrier DE, Hume ME, Bailey RH, Stanker LH, Hargis BM. 1998. Incidence of Campylobacter in crops of preharvest market-age broiler chickens. Poult. Sci. 77:1303–1305 [DOI] [PubMed] [Google Scholar]
- 36.Byrd JA, Corrier DE, Hume ME, Bailey RH, Stanker LH, Hargis BM. 1998. Effect of feed withdrawal on Campylobacter in the crops of market-age broiler chickens. Avian Dis. 42:802–806 [PubMed] [Google Scholar]
- 37.Gootz TD, Martin BA. 1991. Characterization of high-level quinolone resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 35:840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Huang WM, Taylor DE. 1993. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob. Agents Chemother. 37:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor DE, Courvalin P. 1988. Mechanisms of antibiotic resistance in Campylobacter species. Antimicrob. Agents Chemother. 32:1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]