Abstract
Some members of the family Enterobacteriaceae ferment sugars via the mixed-acid fermentation pathway. This yields large amounts of acids, causing strong and sometimes even lethal acidification of the environment. Other family members employ the 2,3-butanediol fermentation pathway, which generates comparatively less acidic and more neutral end products, such as acetoin and 2,3-butanediol. In this work, we equipped Escherichia coli MG1655 with the budAB operon, encoding the acetoin pathway, from Serratia plymuthica RVH1 and investigated how this affected the ability of E. coli to cope with acid stress during growth. Acetoin fermentation prevented lethal medium acidification by E. coli in lysogeny broth (LB) supplemented with glucose. It also supported growth and higher stationary-phase cell densities in acidified LB broth with glucose (pH 4.10 to 4.50) and in tomato juice (pH 4.40 to 5.00) and reduced the minimal pH at which growth could be initiated. On the other hand, the acetoin-producing strain was outcompeted by the nonproducer in a mixed-culture experiment at low pH, suggesting a fitness cost associated with acetoin production. Finally, we showed that acetoin production profoundly changes the appearance of E. coli on several diagnostic culture media. Natural E. coli strains that have laterally acquired budAB genes may therefore have escaped detection thus far. This study demonstrates the potential importance of acetoin fermentation in the ecology of E. coli in the food chain and contributes to a better understanding of the microbiological stability and safety of acidic foods.
INTRODUCTION
Two main sugar fermentation pathways have been described for the family Enterobacteriaceae. The mixed-acid fermentation pathway, which is used by genera such as Escherichia, Shigella, or Salmonella, generates mainly acidic end products, including acetic, lactic, succinic, and formic acids, and also some ethanol. Formic acid can be further converted to H2 and CO2 by the formate hydrogen lyase (FHL) complex (1). On the other hand, enterobacteria that make use of the 2,3-butanediol fermentation pathway, such as Serratia, Klebsiella, or Enterobacter species, ferment glucose primarily to nonacidic end products, such as ethanol, acetoin, and 2,3-butanediol, and produce only minor amounts of the organic acids mentioned above (2). In fact, the latter bacteria follow the mixed-acid fermentation route during the early stages of growth and switch to the 2,3-butanediol route in the late-exponential phase (3, 4). This switch is generally viewed as a strategy of these bacteria to prevent excessive medium acidification, and it has been demonstrated that knockout of the 2,3-butanediol pathway indeed results in more-pronounced acidification, early growth arrest, and cell death (5, 6).
The production of 2,3-butanediol from pyruvate, derived from glycolysis, consists of a three-step conversion (7). First, 2 molecules of pyruvate are converted to α-acetolactate and carbon dioxide by α-acetolactate synthase (α-ALS), after which α-acetolactate is decarboxylated to acetoin by α-acetolactate decarboxylase (α-ALD). Finally, 2,3-butanediol dehydrogenase (BDH) catalyzes the reversible reduction of acetoin to 2,3-butanediol. The switch from mixed-acid production to acetoin production reduces the fermentative energy yield from glucose, because it draws pyruvate from the route to acetate in which the last step—the conversion of acetyl phosphate to acetate by acetate kinase—generates ATP (8). Thus, 2,3-butanediol fermenters must coordinate the activity of the energy-conserving but acidifying acetate formation pathway with the activity of the non-energy-conserving but nonacidifying 2,3-butanediol pathway during sugar fermentation (9).
We have previously studied the role and regulation of 2,3-butanediol fermentation in Serratia plymuthica RVH1, an isolate from a food-processing environment (3, 6, 10). In this organism, the genes encoding the first two steps of the pathway, budB and budA, respectively, are clustered in the budAB operon, while the third gene, budC, is at a distant site in the chromosome (6, 11). This organization is similar to that found in Serratia marcescens (4), although the three genes can also form a single operon in some other members of the Enterobacteriaceae (12, 13). Most recently, we demonstrated that loss of the budAB operon not only results in stronger acidification and thus earlier growth arrest in (neutral) glucose-containing media but also impairs the ability of S. plymuthica RVH1 to initiate and sustain growth at low pH (14). In the present study, we transferred the budAB genes from S. plymuthica to Escherichia coli in order to investigate the impact of the potential acquisition of these genes on the fitness of E. coli in terms of survival of medium acidification and growth at low pH. This work provides novel insights into possible mechanisms of evolution and adaptation of food-borne bacteria to acid stress in the food chain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. The construction of the pTrc99A-Ptrc-budAB plasmid has been described previously (6). In addition to the pTrc99A backbone (15), pTrc99A-Ptrc-budAB contains an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible budAB operon from S. plymuthica RVH1. Both plasmids were introduced into E. coli MG1655 by electroporation and selection for ampicillin resistance. The standard growth medium for the preparation of bacterial inocula was lysogeny broth (LB) (10 g/liter tryptone, 5 g/liter yeast extract, 5 g/liter NaCl).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s) | Source or reference |
|---|---|---|
| E. coli strains | ||
| MG1655 | F− rph-1 | 43 |
| MG1655 lac::Cm | Chloramphenicol resistance cassette in lacY | Laboratory collection |
| MG1655 lac::Tc | Tetracycline resistance cassette in lacZ | 44 |
| Plasmids | ||
| pTrc99A | Cloning vector carrying IPTG-inducible trc promoter (Ptrc); Apr | 15 |
| pTrc99A-Ptrc-budAB | pTrc99A carrying the budAB operon downstream of Ptrc; Apr | 6 |
The growth of E. coli MG1655 derivatives was also evaluated on different diagnostic plating media, including LB agar (15 g/liter agar) with 0.5% glucose and 20 mg/liter phenol red, Violet Red Bile Glucose (VRBG) agar (Oxoid, Basingstoke, United Kingdom), MacConkey agar (Becton Dickinson, Sparks, MD, USA), and eosin-methylene blue (EMB) agar (Becton Dickinson, Sparks, MD, USA). In the latter two media, 0.5% glucose was used as the carbon source. The following concentrations of antibiotics (AppliChem, Darmstadt, Germany) were used when appropriate: 100 μg/ml ampicillin, 30 μg/ml chloramphenicol, and 10 μg/ml tetracycline.
Growth, acetoin, and pH measurements of E. coli MG1655 cultures in LB supplemented with glucose.
E. coli MG1655 cultures containing pTrc99A or pTrc99A-Ptrc-budAB were grown overnight at 37°C in 4 ml LB with ampicillin (to ensure plasmid maintenance) and were subsequently diluted 1:1,000 in test tubes containing 10 ml of the same medium supplemented with 0.5% glucose as a carbon source for fermentation and 1 mM IPTG. During incubation at 37°C for 96 h, 300-μl samples were withdrawn at regular time points; the optical density at 600 nm (OD600) and the medium pH were measured, and the presence or absence of acetoin was determined. The latter was assessed using the Voges-Proskauer (VP) test as described previously (3). Additionally, plate counts were determined by plating a decimal dilution series in potassium phosphate buffer (10 mM; pH 7.00) on LB agar.
Growth experiments in LB supplemented with 0.5% glucose, 1 mM IPTG, and 100 μg/ml ampicillin, and acidified to different pH values with 37% HCl, were conducted similarly but used 300-μl culture volumes in 96-well microplates to allow higher throughput and an incubation temperature of 30°C, because 37°C is suboptimal at low pHs. The microplates were sealed with an oxygen-impermeable cover foil. Incubation and automatic measurement of the OD630 were carried out in a Multiskan Ascent plate reader (Thermo Labsystems, Helsinki, Finland). Plate counts and pHs were determined only at the start and end of this experiment. The growth data were fitted by the model of Baranyi and Roberts (16), using the Excel add-in package DMFit (Institute of Food Research, Norwich, United Kingdom).
Growth and pH measurements of E. coli MG1655 cultures in tomato juice.
E. coli MG1655 cultures containing pTrc99A or pTrc99A-Ptrc-budAB, grown overnight at 37°C in 4 ml LB with ampicillin, were diluted 1:1,000 in 0.9% NaCl. One hundred microliters of this suspension was inoculated into test tubes with 10 ml commercial appertized tomato juice (containing 6 g/liter NaCl; pH 4.20) that had first been adjusted to pH 4.30, 4.40, 4.50, 4.60, 4.80, or 5.00 with 1 M NaOH and supplemented with 1 mM IPTG. The inoculated tomato juice was covered with 2 ml paraffin oil to simulate the low oxygen availability in a commercial packaged juice and to stimulate fermentative metabolism and was then incubated at 20 or 30°C without shaking. At regular time points, and after careful penetration of the paraffin oil layer and pipetting up and down a few times to ensure good mixing, 300-μl samples were taken for plate counting and pH measurement.
Mixed-culture experiment in liquid medium and on solid medium.
Fifty microliters each of an overnight culture of E. coli MG1655 lac::Tc/pTrc99A and an overnight culture of E. coli MG1655 lac::Cm/pTrc99A-Ptrc-budAB were mixed together into 900 μl of potassium phosphate buffer (10 mM; pH 7.00). Forty microliters of this suspension was added to test tubes with 4 ml LB containing 0.5% glucose, 1 mM IPTG, and 100 μg/ml ampicillin, and the pH was adjusted to 5.00, while a dilution was plated onto the same medium (but solidified with 15 g/liter agar) in order to obtain 500 to 5,000 CFU per plate. The test tubes were incubated without shaking, while the plates were anaerobically incubated, both at 37°C. Every day, the liquid cultures were diluted 1:1,000 into fresh medium and were further incubated, while colonies from the plates were collected in 1 ml of potassium phosphate buffer (10 mM; pH 7.00), decimally diluted, plated again on fresh medium, and further incubated. Every day, counts of E. coli MG1655 lac::Tc/pTrc99A and E. coli MG1655 lac::Cm/pTrc99A-Ptrc-budAB in the mixed populations (in liquid and on solid medium) were determined by plating on LB agar with tetracycline or chloramphenicol, respectively. Both experiments were also carried out with the two plasmids swapped between the two strains.
Statistical analysis of data.
All experiments were carried out in triplicate using independent cultures, and results are presented as means ± standard deviations. The statistical significance of differences between mean values for different strains at each sampling moment was determined by Student t test analysis using the Microsoft Excel statistical package. Results were reported as significant when a P value of ≤0.05 was obtained.
RESULTS
Acetoin synthesis prevents lethal acidification during fermentative growth of E. coli.
First, the influence of the acetoin pathway in E. coli MG1655 was evaluated in growth medium with a neutral initial pH. To that end, MG1655 equipped with pTrc99A-Ptrc-budAB or the corresponding empty control plasmid (pTrc99A) was grown in LB with 0.5% glucose at 37°C for 96 h. The presence of the control plasmid had no effect on the growth of MG1655 (data not shown). Cultures were not shaken to stimulate fermentative growth. During the first 10 h, the OD600, the culture pH, and the production of acetoin were monitored every hour (Fig. 1). Additionally, culture aliquots were plated at 0, 10, 24, 48, 72, and 96 h (Table 2).
FIG 1.
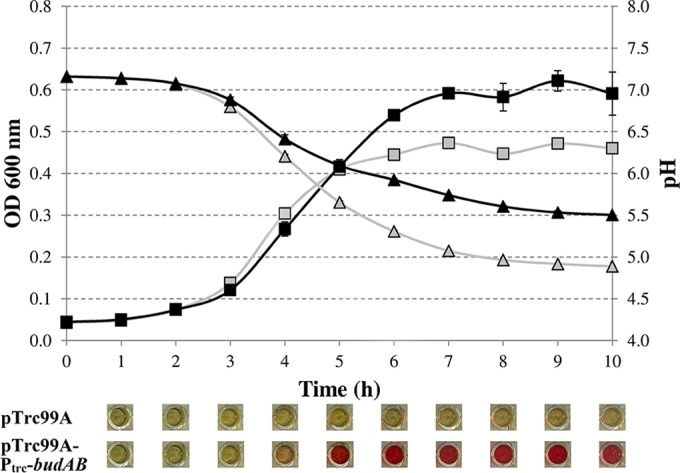
Growth (expressed as OD600) of E. coli MG1655 harboring pTrc99A (shaded squares) or pTrc99A-Ptrc-budAB (filled squares) in LB containing 0.5% glucose, 1 mM IPTG, and 100 μg/ml ampicillin at 37°C for 10 h. The pH of the medium is indicated by shaded triangles (pTrc99A) or filled triangles (pTrc99A-Ptrc-budAB). Error bars represent standard deviations. Pictures below the graph show the results of a qualitative Voges-Proskauer test demonstrating acetoin production (red color).
TABLE 2.
Plate counts and pH values in cultures of E. coli MG1655 harboring pTrc99A or pTrc99A-Ptrc-budABa
| Time (h) | pTrc99A |
pTrc99A-Ptrc-budAB |
||
|---|---|---|---|---|
| Plate count (log[CFU/ml]) | pH | Plate count (log[CFU/ml]) | pH | |
| 0 | 5.4 ± 0.5 A | 7.16 ± 0.01 A | 5.4 ± 0.1 A | 7.16 ± 0.01 A |
| 10 | 8.3 ± 0.1 A | 4.89 ± 0.01 B | 8.6 ± 0.2 A | 5.50 ± 0.01 A |
| 24 | 7.9 ± 0.2 B | 4.63 ± 0.02 B | 8.4 ± 0.1 A | 5.61 ± 0.33 A |
| 48 | 6.2 ± 0.5 B | 4.63 ± 0.02 B | 8.4 ± 0.1 A | 6.33 ± 0.18 A |
| 72 | 5.2 ± 0.4 B | 4.62 ± 0.02 B | 8.4 ± 0.3 A | 6.78 ± 0.13 A |
| 96 | 3.0 ± 0.3 B | 4.59 ± 0.01 B | 8.8 ± 0.1 A | 7.14 ± 0.06 A |
Strains were grown in LB containing 0.5% glucose, 1 mM IPTG, and 100 μg/ml ampicillin at 37°C for 96 h. Values are means ± standard deviations. Plate counts or pH values followed by different capital letters in the same row are significantly different (P < 0.05).
The OD600 curves show comparable lag phases and exponential growth rates for the two strains but suggest that entry into stationary phase was delayed in the strain containing budAB. The plate counts in early-stationary phase (10 h) tend to confirm that this strain grew to a slightly higher stationary-phase level, although the difference from the strain without budAB was marginally nonsignificant (P = 0.07). As expected, the growth of the control strain with the empty plasmid was accompanied by a rapid decrease in the medium pH due to the production of acidic end products generated by the mixed-acid fermentation of glucose. In contrast, the pH of the medium for MG1655 carrying the pTrc99A-Ptrc-budAB plasmid decreased more slowly and to a lesser extent (Fig. 1). This can be attributed to the production of acetoin and to a reduction in the amount of acidic fermentation products. Acetoin production was indeed confirmed by the VP test (Fig. 1) and was detectable from 4 h of incubation onward. Interestingly, during further incubation up to 96 h, the pH of the medium returned to neutral for MG1655 carrying the budAB operon, while it decreased further, to 4.59, for the empty-vector control strain. This acidification of the medium by the control strain was lethal, as illustrated by the strong decline in the plate count to 3.0 log(CFU/ml) after 96 h. On the other hand, the strain containing budAB was not subject to this lethal acidification, and its plate count remained almost constant or even increased slightly, to 8.8 log(CFU/ml), after 96 h (Table 2).
Acetoin synthesis changes the appearance of E. coli colonies on diagnostic plating media.
Given the profound impact of the acetoin pathway on the pH evolution of MG1655 cultures in the experiment described above, we investigated whether the pathway would also influence the appearance of E. coli colonies grown on the solid media frequently used for the identification and enumeration of Enterobacteriaceae (Fig. 2). These media often rely on the color change of a pH indicator as a result of acid production from glucose or another sugar to distinguish Enterobacteriaceae from nonfermenting bacteria. On VRBG agar, which contains glucose and the pH indicator neutral red, MG1655 with pTrc99A formed purple colonies surrounded by opaque halos of precipitated bile salts, with a purple discoloration of the medium, characteristic of Enterobacteriaceae. In contrast, the colonies formed by MG1655 with budAB genes and the surrounding medium were gray, and there were no halos. Likewise, on MacConkey agar with glucose as a carbon source, which is very similar in composition to VRBG agar, the colonies and surrounding medium of the strain without budAB turned deep red after incubation, while those for the strain containing budAB turned yellow-orange. On EMB agar, containing eosin Y and methylene blue dyes in addition to glucose, strain MG1655 without budAB formed blue-black colonies with a green metallic sheen, typical for E. coli, while colonies of the strain containing budAB were colorless. Finally, on LB agar containing glucose and phenol red as a pH indicator, the strain without budAB caused a color shift to yellow, while the strain containing budAB caused a color shift to red, after incubation. The distinct appearance of MG1655 containing budAB genes can be explained by the less-pronounced acidification caused by this strain, as observed above in liquid LB containing glucose (Fig. 1 and Table 2).
FIG 2.
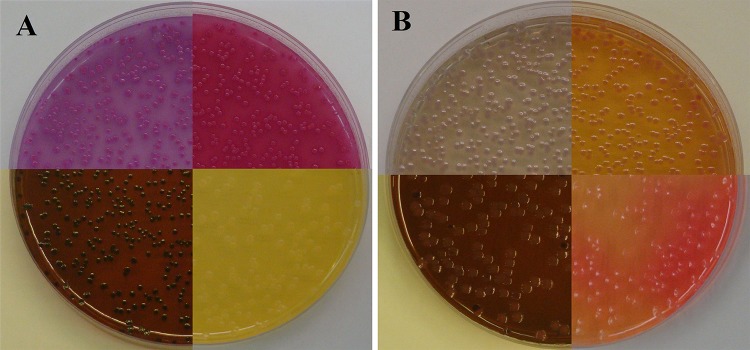
E. coli MG1655 harboring pTrc99A (A) or pTrc99A-Ptrc-budAB (B) grown on VRBG agar (top left), MacConkey agar containing 0.5% glucose (top right), EMB agar containing 0.5% glucose (bottom left), or LB agar containing 0.5% glucose and 20 mg/liter phenol red (bottom right) at 37°C for 24 h. All media contained 1 mM IPTG.
Acetoin synthesis supports the growth of E. coli at low pH in LB with glucose.
Since we showed previously that knockout of the budAB operon impairs growth at a low initial pH in the 2,3-butanediol-fermenting organism S. plymuthica RVH1 (14), we now addressed the question of whether the acquisition of this operon would improve the ability of E. coli to grow at low pH in LB with glucose (initial plate counts, around 5.7 log[CFU/ml]). Based on the OD630 data, there was no growth of MG1655 harboring pTrc99A or pTrc99A-Ptrc-budAB at pH 4.00. On the contrary, the final plate counts indicated that cell death occurred at this pH (Table 3). At an initial pH of 4.10, the counts of the strain without budAB decreased to 2.6 log(CFU/ml) after 72 h, whereas those of the strain containing budAB increased to 8.3 log(CFU/ml). This difference in growth was also reflected by a difference in pH evolution. Whereas the pH increased only marginally, to 4.29, for the strain without budAB, the strain containing budAB increased the pH to 5.23 after 72 h (Table 3).
TABLE 3.
Determination of growth parameters in acidified LB mediuma
| Initial pH | Final plate count (log[CFU/ml]) |
Final pH |
Lag phase (h) |
Growth rate (1/h) |
Final OD |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Without budAB | With budAB | Without budAB | With budAB | Without budAB | With budAB | Without budAB | With budAB | Without budAB | With budAB | |
| 4.00 | 4.0 ± 0.2 A | 3.9 ± 0.3 A | 4.08 ± 0.01 A | 4.08 ± 0.01 A | ||||||
| 4.10 | 2.6 ± 0.3 B | 8.3 ± 0.1 A | 4.29 ± 0.01 B | 5.23 ± 0.33 A | 29.7 ± 3.0 | 0.040 ± 0.007 | 0.813 ± 0.118 | |||
| 4.20 | 6.5 ± 0.2 B | 8.4 ± 0.1 A | 4.39 ± 0.04 B | 5.56 ± 0.03 A | 9.3 ± 0.3 A | 9.2 ± 0.4 A | 0.011 ± 0.002 B | 0.063 ± 0.001 A | 0.378 ± 0.023 B | 0.976 ± 0.006 A |
| 4.30 | 7.2 ± 0.1 B | 8.3 ± 0.1 A | 4.36 ± 0.02 B | 5.73 ± 0.03 A | 6.0 ± 0.3 A | 6.1 ± 0.2 A | 0.018 ± 0.002 B | 0.071 ± 0.001 A | 0.418 ± 0.032 B | 1.008 ± 0.022 A |
| 4.40 | 7.2 ± 0.2 B | 8.4 ± 0.1 A | 4.40 ± 0.01 B | 5.78 ± 0.04 A | 5.3 ± 0.2 A | 5.3 ± 0.3 A | 0.026 ± 0.001 B | 0.077 ± 0.001 A | 0.461 ± 0.022 B | 1.026 ± 0.025 A |
| 4.50 | 7.5 ± 0.1 B | 8.3 ± 0.1 A | 4.42 ± 0.01 B | 5.88 ± 0.05 A | 2.0 ± 0.2 A | 2.1 ± 0.3 A | 0.026 ± 0.004 B | 0.076 ± 0.003 A | 0.483 ± 0.033 B | 1.046 ± 0.016 A |
E. coli MG1655 harboring pTrc99A (without budAB) or pTrc99A-Ptrc-budAB (with budAB) was grown in LB (acidified to different initial pH values) containing 0.5% glucose, 1 mM IPTG, and 100 μg/ml ampicillin at 30°C for 72 h. Lag phases, growth rates, and final ODs were determined after fitting of the growth curves using DMFit. Values are means ± standard deviations. Values for each parameter followed by different capital letters in the same row are significantly different (P < 0.05).
The lowest pH value at which the strain without budAB could initiate growth (pHmin) was 4.20. After 72 h at this pH, its plate count increased to 6.5 log(CFU/ml), which was significantly less than the final count of the strain containing budAB (8.4 log[CFU/ml]) (P = 3.7 × 10−4). In parallel, there was only a small increase in the medium pH for the strain without budAB, and a much stronger increase for the strain containing budAB. Also at initial pH values of 4.30, 4.40, and 4.50, MG1655 clearly benefited from the presence of the budAB operon during growth in acidified LB with regard to growth rates and final plate counts (Table 3). At these three pH values, there was a statistically significant difference (P < 0.007) of around 1 log(CFU/ml) in the final plate count between the two strains. Again, under these three conditions, the final pH was much lower for the strain without budAB than for the strain containing budAB. The empty pTrc99A plasmid had no effect on the growth of MG1655 in this experiment (data not shown).
Acetoin synthesis supports the growth of E. coli in tomato juice.
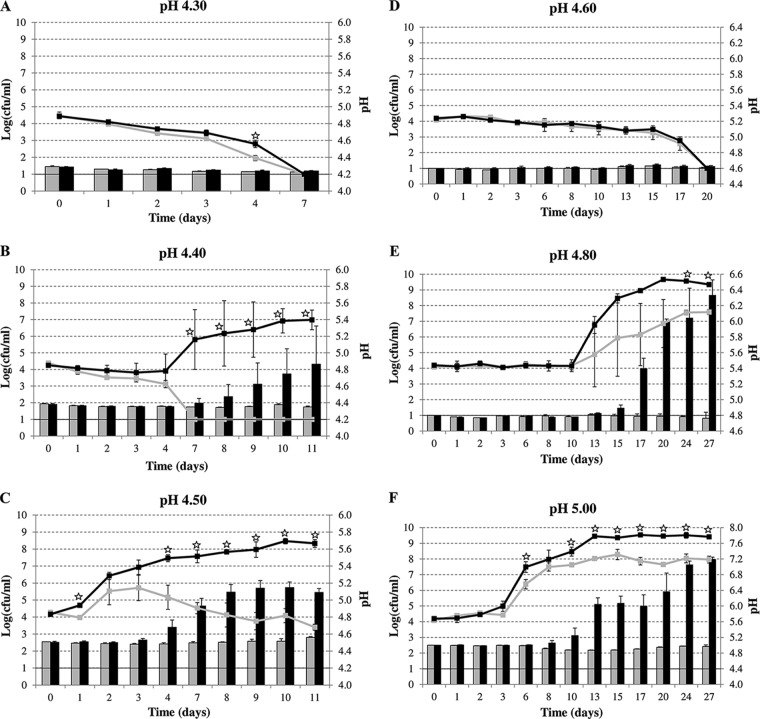
To assess the potential relevance of the acetoin pathway to the safety and stability of acidic foods, MG1655, without or with the budAB operon, was inoculated at about 4.0 log(CFU/ml) into tomato juices adjusted to a pH of 4.30 to 5.00. The inoculated juices were stored at a temperature close to the optimum for growth (30°C) or at a more moderate temperature (20°C). As such, the experiment is representative of bacterial growth in mildly acidic foods that are stored without refrigeration, such as unprocessed fresh products (tomatoes and several fruits) or processed products that have been subject to contamination, or in refrigerated products that are subjected to severe temperature abuse.
At an incubation temperature of 30°C, the plate counts of both strains, without and with budAB, declined steadily at an initial pH of 4.30 (Fig. 3A), to fall below the detection limit within 7 days. At an initial pH of 4.40 (Fig. 3B), there was a period of 4 days during which the counts remained roughly constant (for the strain containing budAB) or decreased slightly (for the strain without budAB). After that, the counts of the strain without budAB rapidly dropped below the detection limit (day 7). In contrast, the strain containing budAB started growing and reached 7.0 log(CFU/ml) after 11 days. This was accompanied by an increase in the pH of the tomato juice to 4.86. In tomato juice with an initial pH of 4.50 (Fig. 3C), both strains were able to initiate growth, but the strain without budAB stopped growing after 3 days, and its counts then declined until day 11. Since the pH of the juice remained constant, the reason for this decline is probably the accumulation of acids during the growth phase. In contrast, the strain containing budAB grew to a final cell density of 8.3 log(CFU/ml) over the entire 11-day period.
FIG 3.
Survival or growth of E. coli MG1655 harboring pTrc99A (gray line) or pTrc99A-Ptrc-budAB (black line), in tomato juice containing 1 mM IPTG at 30°C (A, B, and C) or 20°C (D, E, and F) at different initial pHs. The evolution of the juice pH during the experiment is indicated by shaded bars (pTrc99A) or filled bars (pTrc99A-Ptrc-budAB). Error bars represent standard deviations. The horizontal lines at 1 log(CFU/ml) represent the detection limit. Stars indicate statistically significant differences (P < 0.05) between the plate counts of the two strains.
At an incubation temperature of 20°C, both strains generally grew more slowly, and the lowest pH value at which growth could be initiated (pH 4.80) was higher than at 30°C. At pH 4.60 (Fig. 3D), both strains survived well for 15 days, but upon longer incubation, counts decreased, to sink below the detection limit after 20 days. There was no difference between the two strains at this pH. At an initial pH of 4.80 (Fig. 3E), there was a long lag phase during which plate counts remained constant. Thereafter, the plate counts of both strains started to increase, but more slowly for the strain without budAB than for the strain containing budAB. The lag phase at an initial pH of 5.00 (Fig. 3F) was much shorter than that at an initial pH of 4.80, and both strains started growing after 2 to 3 days, reaching plateaus at approximately 8.0 and 9.4 log(CFU/ml) for the strain without budAB and the strain containing budAB, respectively, after about 13 days. Both at pH 4.80 and at pH 5.00, a strong pH increase was observed for the strain containing budAB, while the pH remained almost constant for the strain without budAB.
Acetoin synthesis provides no fitness advantage to E. coli in LB at low pH.
In the next experiment, we investigated whether the capacity of an E. coli strain to produce acetoin can confer a growth advantage at low pH in a mixed culture with an E. coli strain lacking this capacity. To allow counting of both the strain without budAB and the strain containing budAB in the mixed culture, we introduced the pTrc99A-Ptrc-budAB plasmid and the pTrc99A plasmid into two MG1655 strains with different antibiotic resistance cassettes inserted into the lac operon. Equal amounts of MG1655 lac::Tc/pTrc99A and MG1655 lac::Cm/pTrc99A-Ptrc-budAB were mixed together, and the culture was grown at 37°C in LB with glucose at pH 5.00 for as long as 10 consecutive days, with daily dilution at 1:1,000 in fresh medium. Colony counts of the strains lacking and containing budAB were determined daily by plating on LB agar containing tetracycline or chloramphenicol, respectively. The results of these experiments are shown in Fig. 4A as the difference in log(CFU/ml) between the strain lacking budAB and the strain containing budAB. The results indicate that the strain containing the acetoin synthesis operon was gradually outcompeted by the strain lacking the operon. The same observation was made when the experiment was repeated at pH 4.6 (data not shown). This result contrasts with the finding described above for pure cultures, that the acetoin pathway improves growth at low pH.
FIG 4.
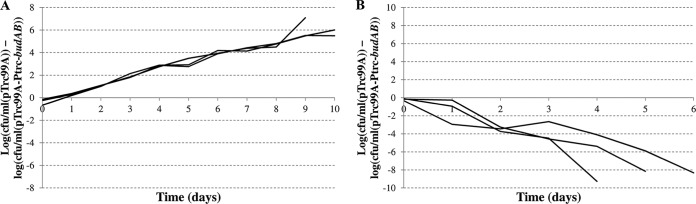
Difference in plate counts (expressed in log[CFU/ml]) between E. coli MG1655 lac::Tc/pTrc99A and E. coli MG1655 lac::Cm/pTrc99A-Ptrc-budAB during a 10-day cocultivation experiment with daily transfer to fresh medium. The medium was liquid LB (A) or LB agar (B) at an initial pH of 5.00 with 0.5% glucose, 1 mM IPTG, and 100 μg/ml ampicillin, and the growth temperature was 37°C. The lines shown represent the results of three independent replicate experiments. The plate counts of the two strains were significantly different from day 2 onward in both experiments.
A repetitive mixed-culture experiment was also carried out on an acidic solid medium, under conditions where discrete colonies are formed that are sufficiently separated to allow the creation of a local pH environment. In these experiments (Fig. 4B), MG1655 possessing the budAB operon quickly got the upper hand and completely outcompeted the other strain after 4 to 6 days, depending on the experiment. A typical example of colony formation in the mixed culture on LB agar at pH 5.00 is shown in Fig. 5. Similar results were obtained, both in liquid and on solid medium, when the plasmids were swapped between the two strains in order to exclude potential fitness effects of the antibiotic resistance markers (data not shown).
FIG 5.
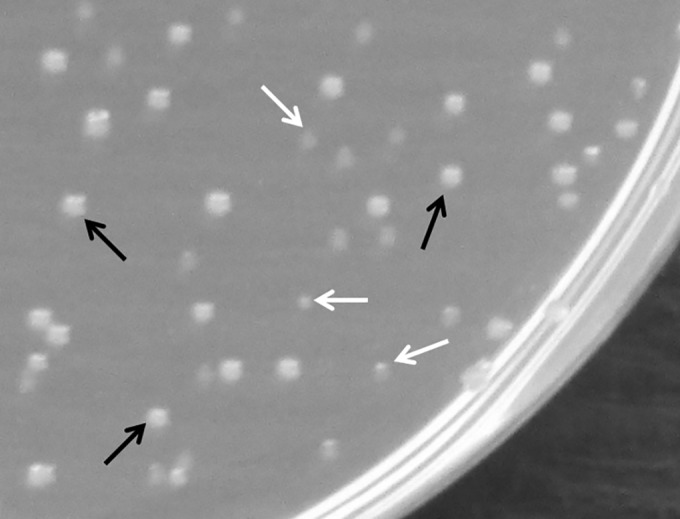
Mixed culture of E. coli MG1655 lac::Tc/pTrc99A (white arrows) and E. coli MG1655 lac::Cm/pTrc99A-Ptrc-budAB (black arrows), grown anaerobically on LB agar at pH 5.00 with 0.5% glucose, 1 mM IPTG, and 100 μg/ml ampicillin at 37°C for 24 h.
DISCUSSION
In this study, we investigated the impact of the potential acquisition of the genes encoding the acetoin pathway from pyruvate by E. coli. This pathway exists in a number of Gram-positive and Gram-negative bacteria, including some members of the Enterobacteriaceae, and is believed to be important for their lifestyle, because it reduces acid production during fermentative growth and has also been shown to promote growth at low pH (14, 17). In line with those previous findings, we observed that medium acidification by E. coli in LB at an initially neutral pH and with glucose as a fermentable carbon source was reduced after introduction of the budAB genes from S. plymuthica. As a result, the strain containing budAB grew to a higher stationary-phase cell density and showed no decline in plate counts during the entire observation period of 96 h, while the plate counts of the strain without budAB declined more than 5 log units. Our results confirm earlier findings on the effect of expression of Bacillus subtilis α-ALS in E. coli (18). However, while those authors were interested in enhancing cell yields and yields of recombinant proteins produced in E. coli by reducing fermentative acetate accumulation, our interest is in the potential impact of the acquisition of the acetoin pathway on the behavior of E. coli in the food production chain and, more specifically, on growth in acidic foods.
In a second experiment, we found that acetoin production supported better growth at a low initial pH, both in acidified LB with glucose and in tomato juice. E. coli with the budAB operon generally reached stationary-phase levels about 1 log(CFU/ml) higher than those of the strain without this operon in acidified LB. Moreover, the budAB operon decreased the minimal pH allowing MG1655 growth by 0.10 pH unit. This may have important consequences, because fresh fruits and other acidic, slightly acidic, or acidified foods, such as dressings and sauces, salads, yoghurt, fermented sausages, and unpasteurized fruit juices, have been increasingly recognized as sources of food-borne outbreaks caused by enteric pathogens such as Salmonella and E. coli O157:H7 in many parts of the world (19–27). These pathogens have a low infective dose and hence can cause illness even in foods that do not support their growth. Evidently, the risk of illness would increase if they could acquire the ability to grow in such foods. A widely used practical guideline in food preservation is that enterobacterial pathogens do not grow in acidic foods (pH <4.5). However, an increasing number of observations suggest that pH 4.5 is not an absolute barrier, and even growth at pH values as low as 3.5 has been reported for E. coli O157:H7 on peach plugs stored at 25°C (28). Growth of E. coli O157:H7 has also been reported on fresh cut mangoes (pH 4.2; 23°C), and in apple cider (pH 3.6 and 23°C or pH 4.0 and 25°C) (29–31). Also in tomato products, as in processed whole tomatoes (pH 4.6; 25°C) and processed tomato juice (pH 4.4; 25°C), growth of E. coli O157:H7 has been observed (32). In this context, the question of the lower pH limit for the growth of these enteropathogens is very pertinent. In the current work, we showed that, at least under some conditions, the pHmin can be lowered by acquisition of the acetoin pathway.
Although the decrease in the pHmin in acidified LB and in tomato juice was rather small, the possible shift of the boundaries for some intrinsic or extrinsic factors allowing growth toward the more extreme values due to new genes or functions is an important issue for food safety. An interesting proof of principle that such a shift is possible was provided by a study that reported the reduction of the minimal growth temperature of an E. coli strain from 8°C to below 4°C upon the transfer of two chaperonin genes from a psychrophilic bacterium (33). If the genes responsible for this shift of growth range can be transferred to other hosts by horizontal gene transfer, the corresponding traits can move among bacterial species and change their niche specificities (34). Several genes encoding new metabolic functions have been found previously to be horizontally acquired by pathogens, providing a selective advantage in host tissues and access to new niches (35).
With regard to 2,3-butanediol fermentation, indications of the ecological significance of this pathway have emerged from a comparison between the two biotypes of Vibrio cholerae serogroup O1, the classical and El Tor biotypes (36). A unique difference in the carbohydrate metabolism between the two biotypes is that V. cholerae El Tor is able to produce 2,3-butanediol and consequently to suppress the accumulation of organic acids during growth in the presence of carbohydrates, whereas the classical biotype has no functional 2,3-butanediol fermentation pathway and thus strongly acidifies media with fermentable sugars, resulting in a loss of viability. Although the genes involved in the 2,3-butanediol pathway are present in both biotypes, their level of transcription in the classical biotype is very low compared to that in the El Tor biotype. It has been proposed that these differences might account for the improved evolutionary fitness of the El Tor biotype over that of the classical biotype, explaining the displacement of the classical biotype by the El Tor biotype since the 7th cholera pandemic in 1961 (36). Although mixed-acid fermenters, such as E. coli, Salmonella, or Shigella strains, do not normally perform acetoin fermentation, the acquisition of the acetoin pathway from other bacteria is possible in principle and could have important consequences for food safety, particularly the safety of (mildly) acidic foods. It is noteworthy in this context that the superior ability of E. coli and Shigella strains, relative to that of other Enterobacteriaceae, to survive extreme acid exposure (as opposed to the ability to grow under moderate acid stress, as investigated in this work) has been linked to the presence of a so-called acid fitness island containing several genes of the glutamate decarboxylase (GAD) system and a number of chaperones, which has probably been acquired by horizontal gene transfer (37, 38).
Since the acetoin pathway potentially provides a growth advantage in substrates with a fermentable carbon source at both neutral and low pHs, the question arises whether strains of, e.g., E. coli or Salmonella containing this fermentation pathway have emerged. In this context, it is relevant to observe that α-acetolactate is an intermediate in branched-chain amino acid synthesis. The first reaction in this anabolic pathway is catalyzed by acetohydroxyacid synthase (AHAS), which converts 2 molecules of pyruvate to α-acetolactate, the same reaction as that catalyzed by the catabolic α-ALS enzyme (39). E. coli in fact possesses three AHAS isozymes, and although their basal activity is insufficient to provide an adequate flux toward α-acetolactate for the production of acetoin or 2,3-butanediol (40), it is conceivable that one of these could acquire a catabolic function under appropriate selection conditions. The acquisition of a functional acetoin pathway would then require horizontal transfer of only an α-ALD gene. A search in the available sequence databases did not reveal putative α-ALD genes in E. coli, Shigella, or Salmonella. However, since isolation of these bacteria by selective plating methods usually relies on acid production by glucose and/or lactose fermentation, acetoin-fermenting strains of bacteria are likely to have escaped detection. Using some widely used diagnostic agar media, such as VRBG agar, MacConkey agar, and EMB agar, we showed that the presence of the acetoin pathway indeed renders the colony appearance atypical. Therefore, to find out whether acetoin-fermenting E. coli, Shigella, or Salmonella strains exist in nature, it will be necessary to screen a large collection of strains that have been isolated and identified without relying on acid production from sugar fermentation.
Finally, we also conducted a competition experiment between an acetoin-producing E. coli strain and a non-acetoin-producing E. coli strain in a liquid medium at low pH. Although the acetoin producer had a growth advantage in pure culture, it was quickly outcompeted by the nonproducer in a mixed culture. The competition experiment does not allow us to identify the reason for the reduced competitiveness of the acetoin producer, but two possible explanations can be proposed. The first is that the cost for the maintenance and expression of the budAB operon leads to a reduced growth rate. The second and more likely explanation is that the partial rerouting of pyruvate from mixed-acid fermentation to acetoin fermentation leads to a lower energy yield. This is because the production of acetate is coupled to ATP production through the action of acetate kinase and provides a major portion of the ATP produced during anaerobic growth (41). E. coli expressing α-ALS from B. subtilis in batch cultures synthesized less acetate, and, as a result, had a lower ATP yield, than the corresponding wild-type strain (42).
In conclusion, we demonstrated in this work that the introduction of the acetoin pathway profoundly affects the ability of E. coli to cope with acid stress during growth. Not only does it prevent excessive lethal acidification during fermentative growth; it also reduces the minimal pH at which E. coli can initiate growth. Acquisition of the pathway by pathogenic E. coli, Shigella, or Salmonella strains, which would require only the lateral transfer of one or two genes, could potentially have important consequences for the safety of acidic and mildly acidic foods.
ACKNOWLEDGMENTS
This work was supported by a doctoral fellowship to B.V. from the Agency for Innovation by Science and Technology (IWT) and by research grants from the Research Foundation—Flanders (FWO) (G.A061.11), and from the KU Leuven Research Fund (METH/07/03).
Footnotes
Published ahead of print 25 July 2014
REFERENCES
- 1.Clark DP. 1989. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 63:223–234. 10.1111/j.1574-6968.1989.tb03398.x [DOI] [PubMed] [Google Scholar]
- 2.White D. 2000. The physiology and biochemistry of prokaryotes, 2nd ed. Oxford University Press, Inc, New York, NY [Google Scholar]
- 3.Van Houdt R, Moons P, Buj MH, Michiels CW. 2006. N-Acyl-l-homoserine lactone quorum sensing controls butanediol fermentation in Serratia plymuthica RVH1 and Serratia marcescens MG1. J. Bacteriol. 188:4570–4572. 10.1128/JB.00144-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao B, Zhang LY, Sun J, Su G, Wei D, Chu J, Zhu J, Shen Y. 2012. Characterization and regulation of the 2,3-butanediol pathway in Serratia marcescens. Appl. Microbiol. Biotechnol. 93:2147–2159. 10.1007/s00253-011-3608-5 [DOI] [PubMed] [Google Scholar]
- 5.Marquez-Villavicencio MDP, Weber B, Witherell RA, Willis DK, Charkowski AO. 2011. The 3-hydroxy-2-butanone pathway is required for Pectobacterium carotovorum pathogenesis. PLoS One 6:e22974. 10.1371/journal.pone.0022974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moons P, Van Houdt R, Vivijs B, Michiels CW, Aertsen A. 2011. Integrated regulation of acetoin fermentation by quorum sensing and pH in Serratia plymuthica RVH1. Appl. Environ. Microbiol. 77:3422–3427. 10.1128/AEM.02763-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celińska E, Grajek W. 2009. Biotechnological production of 2,3-butanediol—current state and prospects. Biotechnol. Adv. 27:715–725. 10.1016/j.biotechadv.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Neijssel OM, de Mattos MJT, Tempest DW. 1996. Growth yield and energy distribution, p 1683–1692 In Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 2 ASM Press, Washington, DC [Google Scholar]
- 9.Mayer D, Schlensog V, Böck A. 1995. Identification of the transcriptional activator controlling the butanediol fermentation pathway in Klebsiella terrigena. J. Bacteriol. 177:5261–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Houdt R, Moons P, Jansen A, Vanoirbeek K, Michiels CW. 2005. Genotypic and phenotypic characterization of a biofilm-forming Serratia plymuthica isolate from a raw vegetable processing line. FEMS Microbiol. Lett. 246:265–272. 10.1016/j.femsle.2005.04.016 [DOI] [PubMed] [Google Scholar]
- 11.Van Houdt R, Van der Lelie D, Izquierdo JA, Aertsen A, Masschelein J, Lavigne R, Michiels CW, Taghavi S. 2014. Genome sequence of Serratia plymuthica RVH1, isolated from a raw vegetable-processing line. Genome Announc. 2(1):e00021-14. 10.1128/genomeA.00021-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Z, Lu JR. 2014. Strategies for enhancing fermentative production of acetoin: a review. Biotechnol. Adv. 32:492–503. 10.1016/j.biotechadv.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 13.Yang TH, Rathnasingh C, Lee HJ, Seung D. 2014. Identification of acetoin reductases involved in 2,3-butanediol pathway in Klebsiella oxytoca. J. Biotechnol. 172:59–66. 10.1016/j.jbiotec.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 14.Vivijs B, Moons P, Geeraerd AH, Aertsen A, Michiels CW. 2014. 2,3-Butanediol fermentation promotes growth of Serratia plymuthica at low pH but not survival of extreme acid challenge. Int. J. Food Microbiol. 175:36–44. 10.1016/j.ijfoodmicro.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 15.Amann E, Ochs B, Abel KJ. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315. 10.1016/0378-1119(88)90440-4 [DOI] [PubMed] [Google Scholar]
- 16.Baranyi J, Roberts TA. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277–294. 10.1016/0168-1605(94)90157-0 [DOI] [PubMed] [Google Scholar]
- 17.Johansen L, Bryn K, Stormer FC. 1975. Physiological and biochemical role of the butanediol pathway in Aerobacter (Enterobacter) aerogenes. J. Bacteriol. 123:1124–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aristidou AA, San KY, Bennett GN. 1994. Modification of central metabolic pathway in Escherichia coli to reduce acetate accumulation by heterologous expression of the Bacillus subtilis acetolactate synthase gene. Biotechnol. Bioeng. 44:944–951. 10.1002/bit.260440810 [DOI] [PubMed] [Google Scholar]
- 19.Besser RE, Lett SM, Weber JT, Doyle MP, Barrett TJ, Wells JG, Griffin PM. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217–2220. 10.1001/jama.1993.03500170047032 [DOI] [PubMed] [Google Scholar]
- 20.Erickson JP, Stamer JW, Hayes M, McKenna DN, Van Alstine LA. 1995. An assessment of Escherichia coli O157:H7 contamination risks in commercial mayonnaise from pasteurized eggs and environmental sources, and behavior in low pH dressings. J. Food Prot. 58:1059–1064 [DOI] [PubMed] [Google Scholar]
- 21.Holck AL, Axelsson L, Rode TM, Høy M, Måge I, Alvseike O, L'Abée-Lund TM, Omer MK, Granum PE, Heir E. 2011. Reduction of verotoxigenic Escherichia coli in production of fermented sausages. Meat Sci. 89:286–295. 10.1016/j.meatsci.2011.04.031 [DOI] [PubMed] [Google Scholar]
- 22.Jain S, Bidol SA, Austin JL, Berl E, Elson F, LeMaile-Williams M, Deasy M, Moll ME, Rea V, Vojdani JD, Yu PA, Hoekstra RM, Braden CR, Lynch MF. 2009. Multistate outbreak of Salmonella Typhimurium and Saintpaul infections associated with unpasteurized orange juice—United States, 2005. Clin. Infect. Dis. 48:1065–1071. 10.1086/597397 [DOI] [PubMed] [Google Scholar]
- 23.Lynch MF, Tauxe RV, Hedberg CW. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137:307–315. 10.1017/S0950268808001969 [DOI] [PubMed] [Google Scholar]
- 24.Morgan D, Newman CP, Hutchinson DN, Walker AM, Rowe B, Majid F. 1993. Verotoxin producing Escherichia coli O157 infections associated with the consumption of yoghurt. Epidemiol. Infect. 111:181–187. 10.1017/S0950268800056880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilden J, Young W, McNamara AM, Custer C, Boesel B, Lambert-Fair MA, Majkowski J, Vugia D, Werner SB, Hollingsworth J, Morris JG. 1996. A new route of transmission for Escherichia coli: infection from dry fermented salami. Am. J. Public Health 86:1142–1145. 10.2105/AJPH.86.8_Pt_1.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tribst AAL, Sant'Ana AS, Massaguer PR. 2009. Microbiological quality and safety of fruit juices—past, present and future perspectives. Crit. Rev. Microbiol. 35:310–339. 10.3109/10408410903241428 [DOI] [PubMed] [Google Scholar]
- 27.Vojdani JD, Beuchat LR, Tauxe RV. 2008. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J. Food Prot. 71:356–364 [DOI] [PubMed] [Google Scholar]
- 28.Alegre I, Abadias M, Anguera M, Usall J, Viñas I. 2010. Fate of Escherichia coli O157:H7, Salmonella and Listeria innocua on minimally-processed peaches under different storage conditions. Food Microbiol. 27:862–868. 10.1016/j.fm.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 29.Ryu JH, Beuchat LR. 1998. Influence of acid tolerance responses on survival, growth, and thermal cross-protection of Escherichia coli O157:H7 in acidified media and fruit juices. Int. J. Food Microbiol. 45:185–193. 10.1016/S0168-1605(98)00165-2 [DOI] [PubMed] [Google Scholar]
- 30.Strawn LK, Danyluk MD. 2010. Fate of Escherichia coli O157:H7 and Salmonella spp. on fresh and frozen cut mangoes and papayas. Int. J. Food Microbiol. 138:78–84. 10.1016/j.ijfoodmicro.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 31.Ukuku DO, Zhang H, Huang L. 2009. Growth parameters of Escherichia coli O157:H7, Salmonella spp., Listeria monocytogenes, and aerobic mesophilic bacteria of apple cider amended with nisin–EDTA. Foodborne Pathog. Dis. 6:487–494. 10.1089/fpd.2008.0233 [DOI] [PubMed] [Google Scholar]
- 32.Eribo B, Ashenafi M. 2003. Behavior of Escherichia coli O157:H7 in tomato and processed tomato products. Food Res. Int. 36:823–830. 10.1016/S0963-9969(03)00077-2 [DOI] [Google Scholar]
- 33.Ferrer M, Chernikova TN, Yakimov MM, Golyshin PN, Timmis KN. 2003. Chaperonins govern growth of Escherichia coli at low temperatures. Nat. Biotechnol. 21:1266–1267. 10.1038/nbt1103-1266 [DOI] [PubMed] [Google Scholar]
- 34.de Lorenzo V. 2011. Genes that move the window of viability of life: lessons from bacteria thriving at the cold extreme. Bioessays 33:38–42. 10.1002/bies.201000101 [DOI] [PubMed] [Google Scholar]
- 35.Rohmer L, Hocquet D, Miller SI. 2011. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 19:341–348. 10.1016/j.tim.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon SS, Mekalanos JJ. 2006. 2,3-Butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect. Immun. 74:6547–6556. 10.1128/IAI.00695-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter MQ, Louie JW, Fagerquist CK, Sultan O, Miller WG, Mandrell RE. 2012. Evolutionary silence of the acid chaperone protein HdeB in enterohemorrhagic Escherichia coli O157:H7. Appl. Environ. Microbiol. 78:1004–1014. 10.1128/AEM.07033-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hommais F, Krin E, Coppée JY, Lacroix C, Yeramian E, Danchin A, Bertin P. 2004. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology 150:61–72. 10.1099/mic.0.26659-0 [DOI] [PubMed] [Google Scholar]
- 39.McCourt JA, Duggleby RG. 2006. Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids. Amino Acids 31:173–210. 10.1007/s00726-005-0297-3 [DOI] [PubMed] [Google Scholar]
- 40.Nielsen DR, Yoon SH, Yuan CJ, Prather KLJ. 2010. Metabolic engineering of acetoin and meso-2,3-butanediol biosynthesis in E. coli. Biotechnol. J. 5:274–284. 10.1002/biot.200900279 [DOI] [PubMed] [Google Scholar]
- 41.Moat AG, Foster JW, Spector MP. 2002. Microbial physiology, 4th ed. Wiley-Liss, Inc, New York, NY [Google Scholar]
- 42.Aristidou AA, San KY, Bennett GN. 1999. Metabolic flux analysis of Escherichia coli expressing the Bacillus subtilis acetolactate synthase in batch and continuous cultures. Biotechnol. Bioeng. 63:737–749. [DOI] [PubMed] [Google Scholar]
- 43.Guyer MS, Reed RR, Steitz JA, Low KB. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb. Symp. Quant. Biol. 45:135–140. 10.1101/SQB.1981.045.01.022 [DOI] [PubMed] [Google Scholar]
- 44.Moons P, Van Houdt R, Aertsen A, Vanoirbeek K, Engelborghs Y, Michiels CW. 2006. Role of quorum sensing and antimicrobial component production by Serratia plymuthica in formation of biofilms, including mixed biofilms with Escherichia coli. Appl. Environ. Microbiol. 72:7294–7300. 10.1128/AEM.01708-06 [DOI] [PMC free article] [PubMed] [Google Scholar]