Abstract
Burkholderia sp. strain SJ98 (DSM 23195) utilizes 2-chloro-4-nitrophenol (2C4NP) or para-nitrophenol (PNP) as a sole source of carbon and energy. Here, by genetic and biochemical analyses, a 2C4NP catabolic pathway different from those of all other 2C4NP utilizers was identified with chloro-1,4-benzoquinone (CBQ) as an intermediate. Reverse transcription-PCR analysis showed that all of the pnp genes in the pnpABA1CDEF cluster were located in a single operon, which is significantly different from the genetic organization of all other previously reported PNP degradation gene clusters, in which the structural genes were located in three different operons. All of the Pnp proteins were purified to homogeneity as His-tagged proteins. PnpA, a PNP 4-monooxygenase, was found to be able to catalyze the monooxygenation of 2C4NP to CBQ. PnpB, a 1,4-benzoquinone reductase, has the ability to catalyze the reduction of CBQ to chlorohydroquinone. Moreover, PnpB is also able to enhance PnpA activity in vitro in the conversion of 2C4NP to CBQ. Genetic analyses indicated that pnpA plays an essential role in the degradation of both 2C4NP and PNP by gene knockout and complementation. In addition to being responsible for the lower pathway of PNP catabolism, PnpCD, PnpE, and PnpF were also found to be likely involved in that of 2C4NP catabolism. These results indicated that the catabolism of 2C4NP and that of PNP share the same gene cluster in strain SJ98. These findings fill a gap in our understanding of the microbial degradation of 2C4NP at the molecular and biochemical levels.
INTRODUCTION
As a typical representative of the chloronitrophenols, 2-chloro-4-nitrophenol (2C4NP), with high toxicity to humans, is widely utilized in the chemical syntheses of the fungicide nitrofungin and the pesticide dicapthon (1, 2). Structurally, 2C4NP is a chemical analogue of para-nitrophenol (PNP) that was listed as a priority environmental pollutant by the U.S. Environmental Protection Agency. The microbial degradation of PNP has been extensively investigated, in which either the hydroquinone (HQ) pathway (3–6) or the hydroxyquinol (BT) pathway (7–11) has been elucidated at both the genetic and biochemical levels. In contrast, the study of the degradation of its chlorinated derivatives is limited, with no genetic or biochemical investigation reported. This is because they are more resistant to microbial degradation due to the simultaneous existence of chlorine and nitro groups.
To date, four pure bacterial cultures have been isolated on the basis of the ability to utilize 2C4NP as a sole carbon and energy source (1, 12–14), and three different pathways based on different intermediates present during its degradation have been proposed. Rhodococcus imtechensis RKJ 300 (14) and Burkholderia sp. strain RKJ 800 (12) were reported to degrade 2C4NP via the HQ pathway, whereas Burkholderia sp. strain SJ98 degraded 2C4NP with the formation of PNP, which was further degraded via the BT pathway (13, 15). On the other hand, Arthrobacter sp. strain SJCon was thought to degrade 2C4NP with chlorohydroquinone (CHQ) as the ring cleavage substrate (1). However, none of these pathways has been characterized at the genetic and biochemical levels.
In this study, a 2C4NP catabolic pathway in Burkholderia sp. strain SJ98 that is different from those of all other 2C4NP utilizers was characterized at the genetic and biochemical levels. To our surprise, chloro-1,4-benzoquinone (CBQ) and CHQ, rather than PNP, 4-nitrocatechol (4-NC), and BT, as previously proposed (13), were identified during 2C4NP degradation. On the other hand, the pnpABA1CDEF cluster in a signal operon was proved to be also responsible for 2C4NP degradation by this strain, in addition to PNP degradation. This study fills a gap in our understanding of the mechanism of microbial 2C4NP degradation.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, chemicals, media, and culture conditions.
The bacterial strains and plasmids used in this study are described in Table 1, and the primers used are described in Table 2. Burkholderia strains and Pseudomonas sp. strain WBC-3 were grown at 30°C in minimal medium (MM) (16) with various carbon sources, and the ability to utilize a nitrophenol substrate (2C4NP or PNP) was determined by monitoring the growth of cells together with the consumption of the corresponding substrates. Escherichia coli strains were grown in lysogeny broth (LB) at 37°C. When necessary, 100 μg/ml of ampicillin, 50 μg/ml of kanamycin, 34 μg/ml of chloramphenicol, or 20 μg/ml of tetracycline was added to the medium. All reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) or Fluka Chemical Co. (Buchs, Switzerland).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or characteristic(s) | Reference or source |
|---|---|---|
| Burkholderia sp. strains | ||
| SJ98 | PNP and 2C4NP utilizer, wild type | 13 |
| SJ98ΔpnpA | SJ98 mutant with pnpA gene deleted | This study |
| SJ98ΔpnpA1 | SJ98 mutant with pnpA1 gene deleted | This study |
| SJ98ΔpnpA(pRK415-pnpA) | pnpA gene complemented by pRK415-pnpA in SJ98ΔpnpA | This study |
| Pseudomonas sp. strain WBC-3 | PNP degrader, wild type | 6 |
| E. coli strains | ||
| DH5α | supE44 lacU169 (φ80lacZΔM15) recA1 endA1 hsdR17 thi-1 gyrA96 relA1 | Novagen |
| Rosetta(DE3)pLysS | F− ompT hsdS (rB− mB+) gal dcm lacY1(DE3)/pLysSRARE (Cmr) | Novagen |
| WM3064 | Donor strain for conjugation, 2,6-diaminopimelic acid auxotroph, thrB1004 pro thi rpsL hsdS lacZΔM15 RP4-1360 Δ(araBAD)567 ΔdapA1341::(erm pir [wild type]) | 30 |
| Plasmids | ||
| pET-28a | Expression vector, Kanr | Novagen |
| pTnMod-Okm | Source of kanamycin resistance gene | 28 |
| pEX18Tc | Gene knockout vector, oriT+ sacB+ Tcr | 29 |
| pRK415 | Broad host range vector, Tcr | 32 |
| pET-pnpA | NdeI-XhoI fragment containing pnpA inserted into pET-28a | This study |
| pZWJJ009 | NdeI-XhoI fragment containing pnpA from WBC-3 inserted into pET-28a | 6 |
| pET-pnpA1 | NdeI-XhoI fragment containing pnpA1 inserted into pET-28a | This study |
| pET-pnpB | NdeI-XhoI fragment containing pnpB inserted into pET-28a | This study |
| pET-pnpCD | NdeI-XhoI fragment containing pnpCD inserted into pET-28a | This study |
| pET-pnpE | EcoRI-XhoI fragment containing pnpE inserted into pET-28a | This study |
| pET-pnpF | NdeI-XhoI fragment containing pnpF inserted into pET-28a | This study |
| pEX18Tc-pnpA | pnpA gene knockout vector containing two DNA fragments homologous to upstream and downstream regions of pnpA and kanamycin resistance gene | This study |
| pEX18Tc-pnpA1 | pnpA1 gene knockout vector containing two DNA fragments homologous to upstream and downstream regions of pnpA1 and kanamycin resistance gene | This study |
| pRK415-pnpA | Vector for pnpA gene complementation made by fusion of pnpA into pRK415 at KpnI/EcoRI restriction sites | This study |
TABLE 2.
Primers used in this study
| Primer | Sequence (5′–3′)a | Purpose |
|---|---|---|
| pnpA-F | GACTGACATATGGAAACGCTTGAAGGAG | Amplification of pnpA gene for expression |
| pnpA-R | GGTCTCGAGTTACGCTGCAAGCTTAAGAGG | |
| pnpA1-F | AGGAGACATATGATGGAGACGCTAGAAGG | Amplification of pnpA1 gene for expression |
| pnpA1-R | GGCCTCGAGTCACTTCAGGACGACTTGCG | |
| pnpB-F | AGAAACCATATGGCAACTAAGATTCAGATTGTG | Amplification of pnpB gene for expression |
| pnpB-R | GGTCTCGAGTTACTTGGACTGCGCGACCAGC | |
| pnpCD-F | AGGCACCATATGCAAGAAACGGTGTTCG | Amplification of pnpCD gene for expression |
| pnpCD-R | GGACTCGAGGAACTGGATCGGATTGGC | |
| pnpE-F | AGGCACCATATGCAAACCCAACTCTTCATTG | Amplification of pnpE gene for expression |
| pnpE-R | GGAGAATTCTCAGCGGGGGAAGTACG | |
| pnpF-F | AGGCACCATATGGAACCTTTCGTATATCAAAGC | Amplification of pnpF gene for expression |
| pnpF-R | GGACTCGAGCTACCTCGGTGCCCGCC | |
| RTA-F | GAAGGAGTGGTCGTTGTTGGTGGAG | Amplification of 725 bp of pnpA by RT-PCR |
| RTA-R | TCGGGCATACGACGACGCACTTC | |
| RTB-F | AACTCGTGCCCGAAGAAGTCCTG | Amplification of 445 bp of pnpB by RT-PCR |
| RTB-R | CGAGCGATTGCCAGTTCGTTTTC | |
| RTA1-F | GCCTATGTGACGATTGAAACCCG | Amplification of 555 bp of pnpA1 by RT-PCR |
| RTA1-R | TGCCGACTCTTTGATGACTGCG | |
| RTAB-F | TGCTTACACACGCAGGCACGGAC | Amplification of 743 bp of pnpA-pnpB-spanning region |
| RTAB-R | CGATAGCCCTTCATTCCGCTCTTAACC | |
| RTBA1-F | TGGTCATCGTTGGTGTGCCGTATT | Amplification of 665 bp of pnpB-pnpA1-spanning region |
| RTBA1-R | TCCGCCAACTACGACCACGCCTT | |
| RTA1C-F | CGGAAAACATTCAGAGCAGAG | Amplification of 440 bp of pnpA1-pnpC-spanning region |
| RTA1C-R | CCTTGCCTGTGATGATTTCG | |
| RTCD-F | CCGAAGGGAAAGCCGATG | Amplification of 421 bp of pnpC-pnpD-spanning region |
| RTCD-R | ACCGTAAAAGAAGCCCCAAGC | |
| RTDE-F | ACCAAATCATCTGGGACATCGC | Amplification of 459 bp of pnpD-pnpE-spanning region |
| RTDE-R | ACGGCACGGTCGATGTCG | |
| RTEF-F | AGCCCTGGTTCGCCTTTTG | Amplification of 514 bp of pnpE-pnpF-spanning region |
| RTEF-R | GGTCGGGATCGCAATGATAG | |
| RTFG-F | ATGGTCCGCAGGCTGTTTTC | Amplification of 679 bp of pnpF-pnpG-spanning region |
| RTFG-R | ACTTCTTCCCAGTTGCGGTCAG | |
| RTq16S-F | CGTGTAGCAGTGAAATGCGTAGAG | Amplification of 142-bp fragment of 16S rRNA genes for real-time qPCR |
| RTq16S-R | GACATCGTTTAGGGCGTGGAC | |
| RTq-pnpA-F | CGTCGCAACGAATGTCTTCTATG | Amplification of 172-bp fragment of pnpA for real-time qPCR |
| RTq-pnpA-R | CATACGACGACGCACTTCCTC | |
| RTq-pnpA1-F | CTGCCTATGTGACGATTGAAACC | Amplification of 134-bp fragment of pnpA1 for real-time qPCR |
| RTq-pnpA1-R | CCAGGTGGTGCCATCAAAAG | |
| W-pnpB-F | TACAAGATGGCCGAAGC | Amplification of fragment of pnpB for genomic walking |
| W-pnpB-R | AGGAAGTTGCGCATTTG | |
| GC-pnpA-F | GACGGTACCGATGGAAACGCTTGAAGGAGTGG | Amplification of pnpA for gene complementation |
| GC-pnpA-R | GGTGAATTCTTACGCTGCAAGCTTAAGAGGC | |
| KO-pnpAu-F | CCATGATTACGAATTGAGGGTTTCTAATCGGTTTCGC | Amplification of upstream fragment of pnpA for gene knockout |
| KO-pnpAu-R | AGAGATTTTGAGACATTTTCAGTCTCCTGTCACAACGC | |
| KO-pnpAd-F | GATGAGTTTTTCTAAATTTTGACCGCAATTTGGGAC | Amplification of downstream fragment of pnpA for gene knockout |
| KO-pnpAd-R | GGCCAGTGCCAAGCTTTGACGCGAACCATCTGCAC | |
| KO-pnpA1u-F | CCATGATTACGAATTGATGGCCGAAGCTATCGCG | Amplification of upstream fragment of pnpA1 for gene knockout |
| KO-pnpA1u-R | AGAGATTTTGAGACAGTGTCTCCTTTCAACTCGCGTTTC | |
| KO-pnpA1d-F | GATGAGTTTTTCTAAGCGTTTGCCTTCGGAACG | Amplification of downstream fragment of pnpA1 for gene knockout |
| KO-pnpA1d-R | GGCCAGTGCCAAGCTTGGTGCGTCCGAATTTGTCC | |
| KO-kan-F | TGTCTCAAAATCTCTGATGTTAC | Amplification of kanamycin resistance gene for gene knockout |
| KO-kan-R | TTAGAAAAACTCATCGAGCATC |
Specified restriction sites are underlined.
Biotransformation and intermediate identification.
Biotransformation was performed as described previously (17), with minor modifications. Strains SJ98 and WBC-3 were grown with 2 mM glucose to an optical density at 600 nm (OD600) of 0.3 and then induced with 0.3 mM 2C4NP or PNP for 5 h. Cells were harvested, washed twice, and diluted to an OD600 of 1.0 with phosphate buffer (20 mM, pH 7.4) and 1 mM 2,2′-dipyridyl. The cell suspension was incubated for 10 min before 2C4NP or PNP was added. Then 0.5-ml samples were withdrawn at regular intervals, mixed with an equal volume of methanol, and vortexed vigorously for 5 min. Each sample was then centrifuged at 15,000 × g at 4°C for 10 min before the supernatant was collected for high-performance liquid chromatography (HPLC) analysis. For gas chromatography-mass spectrometry (GC-MS) analysis, the supernatant was extracted with ether after acidification and the extract was dried over sodium sulfate. Quantitative HPLC analysis was done by using a standard curve prepared with authentic standards. One unit of activity was defined as the amount of cells (milligrams of cell dry weight) required to transform 1 μmol of substrate per min at 30°C.
Analytical methods.
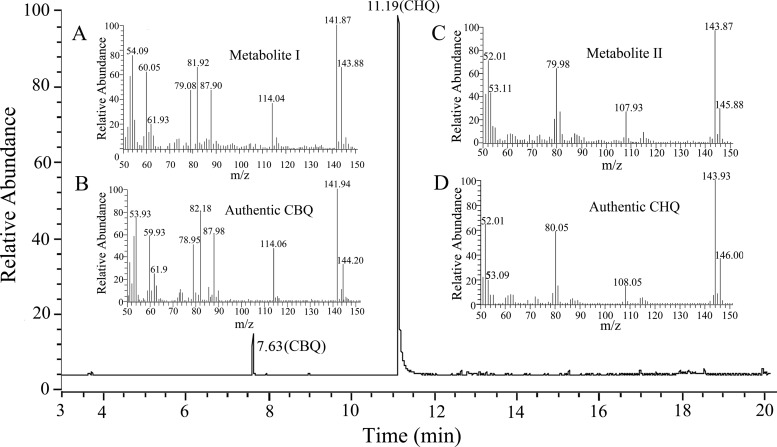
HPLC was performed with a Dionex UltiMate 3000 RS HPLC system with a diode array detector (DAD) and an Agilent ZORBAX Eclipse XDB-C18 column (250 by 4.6 mm, 5-μm particle size). The mobile phase consisted of solvents A (0.1% acetic acid in water) and B (methanol) with a gradient program started with 30% of B, followed by an increase to 80% B from 0 to 10 min, holding at 80% B from 10 to 15 min, and then back to 30% B in 0.1 min and equilibration for 2.9 min. The flow rate was 1.0 ml/min. The column temperature was 30°C. The injection volume was 20 μl, and the wavelength range used to monitor UV absorption with the DAD was 220 to 400 nm. Under these conditions, authentic PNP, 2C4NP, 1.4-benzoquinone (BQ), CBQ, HQ, and CHQ had retention times of 8.5, 11.1, 4.5, 6.8, 3.4, and 5.2 min, respectively. The conditions used for GC-MS analysis were the same as those described previously (6), except that the mass spectrometer recorded in the range of m/z 50 to m/z 200. Under these conditions, authentic CBQ and CHQ had GC retention times of 7.6 and 11.2 min, respectively. The intermediates were identified with an NIST98 MS data library, based on comparisons of the GC retention times and mass spectra with those of authentic compounds.
Cloning of PNP and 2C4NP degradation genes and sequence analyses.
To amplify a potential BQ reductase gene from strain SJ98, primers (Table 2) were designed on the basis of a conserved region of BQ reductases (accession no. C1I202 and ACZ51379) and the codon preferences of Burkholderia spp. The flanking regions of the fragment obtained were cloned by the genome walking strategy (18). The nucleotide sequence was determined by Tsingke BioTech Co. (Beijing, China). Analyses of open reading frames (ORFs) and amino acid identities were performed with ORF finder programs and BLASTX at the NCBI website (19).
RT-PCR and real-time PCR.
Total RNA was isolated from strain SJ98 with an RNAprep pure kit for bacteria (Tiangen Biotech, Beijing, China) and reverse transcribed into cDNA with a PrimeScript RT Reagent kit (TaKaRa, Dalian, China). Reverse transcription (RT)-PCR was carried out with the primers described in Table 2. Real-time quantitative PCR (qPCR) was performed with a CFX Connect real-time PCR detection system (Bio-Rad, Hercules, CA) with iQ SYBR green Supermix (Bio-Rad, Hercules, CA) and the primers described in Table 2. A 142-bp fragment of the 16S rRNA gene of strain SJ98 was used as the reference to evaluate the relative difference in integrity between individual RNA samples. The 2−ΔΔCT method was used to calculate relative changes in gene expression (20).
Protein expression and purification.
pnpA, pnpA1, pnpB, pnpCD, pnpE, and pnpF were amplified from genomic DNA of strain SJ98 by PCR with the primers in Table 2 and cloned into pET-28a to obtain the expression constructs listed in Table 1. The resultant plasmids were transformed into E. coli Rosetta(DE3)pLysS for protein expression and purification as described previously (21).
Enzyme assays.
The activities of the 2C4NP and PNP 4-monooxygenase (PnpA) and CBQ and BQ reductase (PnpB) enzymes were assayed as previously described for PNP 4-monooxygenase and BQ reductase, respectively (6). The molar extinction coefficients for NAD(P)H, PNP, and 2C4NP were 6,220 M−1 cm−1 at 340 nm (22), 7,000 M−1 cm−1 at 420 nm (23), and 14,580 M−1 cm−1 at 405 nm (24), respectively. The products of H6-PnpA- or H6-PnpB-catalyzed reactions were identified by HPLC and GC-MS as described previously (6). The Michaelis-Menten kinetics of 2C4NP monooxygenation catalyzed by PnpA were determined by plotting reaction rates against seven concentrations of PNP (5 to 150 μM) or 2C4NP (2 to 50 μM) from three independent sets of experiments when the NADPH concentration was fixed at 400 μM. Data were fitted with the Michaelis-Menten equation by OriginPro 8 software (OriginLab, Northampton, MA). Protein concentrations were determined by the Bradford method (25) with bovine serum albumin as the standard. One unit of enzyme activity was defined as the amount of enzyme required to catalyze the disappearance of 1 μmol of substrate per min at 30°C. Specific activities are expressed in units per milligram of protein.
Detection of the enzymes involved in the sequential conversions of CHQ and HQ were performed as follows. The ring cleavage activity of CHQ and HQ dioxygenase (PnpCD) was determined by monitoring the spectral changes from 290 to 320 nm (26). For PnpCD inhibition, the reaction mixtures were incubated for 10 min with 1 mM 2,2′-dipyridyl before the addition of the substrates. The products of PnpCD-catalyzed reactions were used to detect (chloro-)4-hydroxymuconic semialdehyde dehydrogenase (PnpE) activity, which was performed by monitoring the decrease in absorption at 320 nm (3), and the increase in NADH was monitored by measuring absorption at 340 nm. In the (chloro)maleylacetate reductase (PnpF) assay, the decrease in absorption at 340 nm was used to monitor the conversion of NADH to NAD+ as an indication of its activity (27).
Gene knockout and complementation.
pEX18Tc-pnpA and pEX18Tc-pnpA1 for gene knockout were constructed by fusing the upstream and downstream fragments of the target gene (pnpA or pnpA1) and the kanamycin resistance gene amplified from plasposon pTnMod-Okm (28) to EcoRI/HindIII-digested pEX18Tc (29) with the In-Fusion HD Cloning kit (TaKaRa, Dalian, China) (the primers used are described in Table 2). The plasmids were then transformed into E. coli WM3064 before conjugation with strain SJ98 as described previously (30, 31). The SJ98ΔpnpA and SJ98ΔpnpA1 double-crossover recombinants were screened on LB plates containing 10% (wt/vol) sucrose and 50 μg/ml kanamycin. pRK415-pnpA for gene complementation was constructed by cloning PCR products of pnpA into KpnI/EcoRI-digested pRK415 (32). It was transformed into E. coli WM3064 and then mated with pnpA deletion-containing strain SJ98 by conjugation to obtain SJ98ΔpnpA(pRK415-pnpA). Strain SJ98 and its derivatives were grown on 0.3 mM substrate (2C4NP or PNP), and their ability to utilize the substrate was determined by monitoring the growth of cells together with the consumption of the substrate. The growth curves were fitted by the modified Gompertz equation (33) with OriginPro 8 software, and their maximum specific growth rates (μm, per hour) were calculated.
RESULTS
CBQ and CHQ are metabolites of 2C4NP degradation.
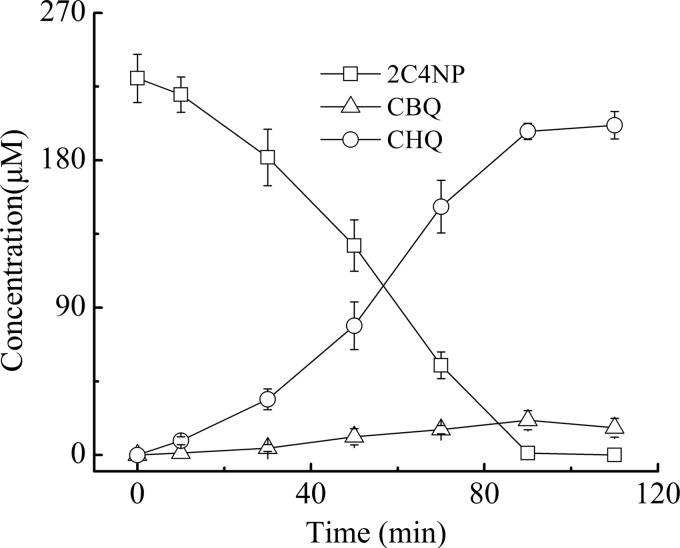
Strain SJ98 is able to utilize 2C4NP and PNP as a sole source of carbon and energy; however, no intermediate was initially detected by HPLC during the degradation of these two compounds. Therefore, 2,2′-dipyridyl, an iron(II) chelator used to inhibit a number of heme-dependent aromatic ring dioxygenases (26, 34), was added to the biotransformation mixtures in order to capture the intermediates. For 2C4NP degradation, two metabolites with HPLC retention times of 5.2 and 6.8 min, respectively, were accumulated with 2C4NP consumption (see Fig. S1 in the supplemental material). These two retention times precisely matched those obtained with standard CHQ and CBQ. Furthermore, the identification of CBQ and CHQ was also confirmed by GC-MS by comparison with the GC retention times and mass spectra of the authentic compounds (Fig. 1). In a biotransformation time course, 2C4NP consumption (230 μM) was approximately equivalent to the total accumulation of both CHQ (201 μM) and CBQ (12 μM) (Fig. 2), indicating nearly stoichiometric formation of CBQ and CHQ from 2C4NP. Similarly, BQ (with an HPLC retention time of 4.5 min) and HQ (retention time of 3.4 min) were detected during PNP degradation and more than 90% of the PNP (244 μM) was converted to BQ (8 μM) and HQ (217 μM) (see Fig. S2 in the supplemental material). Therefore, the identification of intermediates clearly demonstrated that strain SJ98 degraded 2C4NP with CBQ and CHQ as the intermediates before ring cleavage and degraded PNP via the typical HQ pathway (Fig. 3A).
FIG 1.
GC-MS analysis of the intermediates captured during 2C4NP degradation by Burkholderia sp. strain SJ98. Shown is the ion current chromatogram at m/z 142.00 ± 0.50 and 144.00 ± 0.50 extracted from the total ion current chromatogram. The mass spectra of metabolite I (A), authentic CBQ (B), metabolite II (C), and authentic CHQ (D) are shown within the gas chromatogram.
FIG 2.
Time course of 2C4NP degradation by Burkholderia sp. strain SJ98 in whole-cell biotransformation. In order to capture the intermediates before ring cleavage, 1 mM 2,2′-dipyridyl was added to inhibit the ring cleavage enzyme. Samples were withdrawn at the time points indicated and treated immediately as described in the text. The disappearance of 2C4NP and the appearance of the products (CBQ and CHQ) were quantified by HPLC. The experiments were performed in triplicate, the results shown are average values of three independent experiments, and error bars indicate standard deviations.
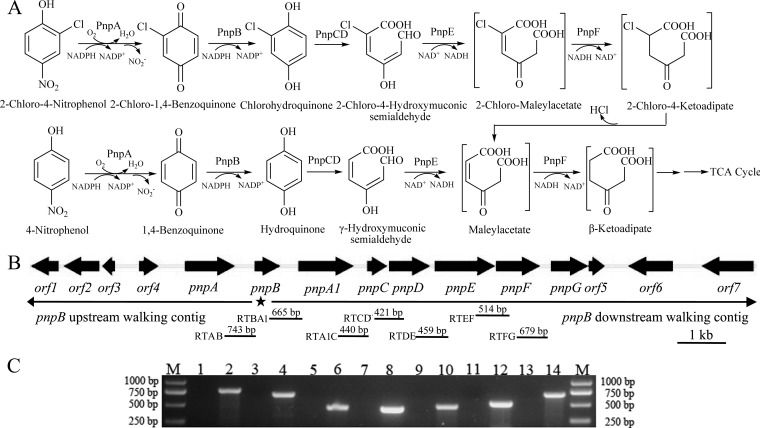
FIG 3.
(A) Proposed pathways of 2C4NP and PNP catabolism in Burkholderia sp. strain SJ98, together with the catabolic reactions catalyzed by pnp gene products. The reactions catalyzed by PnpA and PnpB in PNP catabolism were suggested previously (36). TCA, tricarboxylic acid. (B) Organization of the pnp gene cluster of strain SJ98. The large black arrows indicate the sizes and directions of transcription of the genes and ORFs. The star below pnpB indicates the region where genome walking in both directions started. The locations of primer sets RTAB, RTBA1, RTA1C, RTCD, RTDE, RTEF, and RTFG and the DNA fragments amplified for RT-PCR are indicated below. (C) Analysis of pnpABA1CDEFG transcription by RT-PCR. Total RNAs of strain SJ98 with or without induction by 2C4NP were prepared for RT-PCR, and reactions performed without RT were used as negative controls. Lanes: M, molecular size markers; 2, 4, 6, 8, 10, 12, and 14 (template from glucose-grown strain SJ98 induced by 2C4NP), products amplified with the RTAB, RTBA1, RTA1C, RTCD, RTDE, RTEF, and RTFG primer sets, respectively, with products of RT; 1, 3, 5, 7, 9, 11, and 13, corresponding negative controls. Transcription with the template from glucose-grown strain SJ98 is not shown.
2C4NP degradation is induced by either 2C4NP or PNP.
The initial steps of 2C4NP degradation are analogous to those of PNP degradation by strain SJ98. Therefore, whole-cell biotransformation was carried out in order to investigate whether the catabolism of 2C4NP and that of PNP share the same enzymes in this strain. Negligible activity for these two nitrophenols was observed in uninduced cells of strain SJ98. However, 2C4NP-induced cells exhibited a specific activity of 2.16 ± 0.52 U mg−1 for 2C4NP, and PNP-induced cells exhibited a specific activity of 4.86 ± 0.97 U mg−1 for PNP. These findings indicate that the genes encoding the enzymes involved in the catabolism of PNP and 2C4NP by strain SJ98 are inducible. Moreover, PNP-induced cells degrade 2C4NP (2.28 ± 0.18 U mg−1) while strain SJ98 cells induced by 2C4NP also have the ability to degrade PNP (4.37 ± 0.34 U mg−1). This indicates that the enzymes responsible for PNP catabolism are also likely to be involved in 2C4NP degradation. Interestingly, Pseudomonas sp. strain WBC-3, a PNP rather than 2C4NP utilizer, was also found to be able to degrade 2C4NP after PNP induction (see Fig. S3 in the supplemental material) in an experiment following the above observations in stain SJ98.
Cloning and sequence analyses of the 2C4NP catabolic gene cluster.
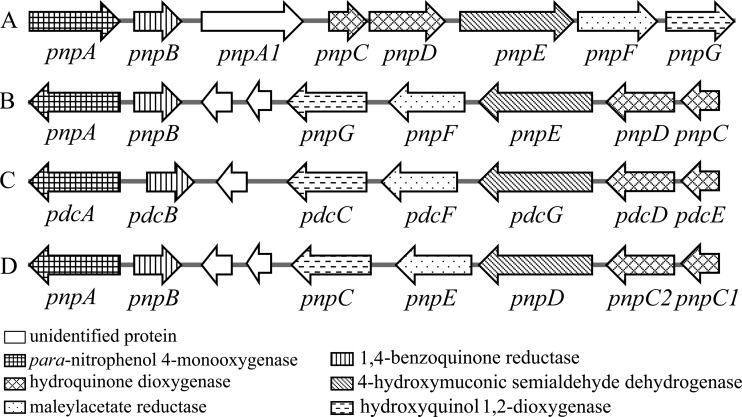
A pair of primers based on a conserved region of the BQ reductase genes was used to amplify a PCR product with an anticipated size of 242 bp from strain SJ98. Subsequently, a 17,429-bp DNA fragment extending from this 242-bp region was obtained by genome walking as outlined and annotated in the legend to Fig. 3B. The genes in this fragment have been designated the pnp genes, and the nucleotide sequence of this fragment is identical to that in a subsequently available draft genomic sequence of strain SJ98 (35), equivalent to nucleotides 1036909 to 1054337 of contig 12 (GenBank accession no. AJHK02000012). Among the products encoded by these genes, PnpA was reported to catalyze the monooxygenation of PNP to BQ, which was then reduced by PnpB (36), during the preparation of this report. While PnpA1 (80% identical to PnpA), PnpC, PnpD, PnpE, and PnpF exhibit high degrees of identity with the enzymes involved in PNP degradation from several PNP utilizers (3–6), PnpG has a high level of identity with the BT 1,2-dioxygenase of Pseudomonas sp. strain WBC-3 (37).
pnp genes are in a single operon that is upregulated in 2C4NP-induced cells of strain SJ98.
RT-PCR was performed with RNA derived from glucose-grown cultures of strain SJ98, with or without the addition of substrates. This showed that transcription of the pnp cluster was detected only under inducing conditions, and pnpABA1CDEFG was suggested to be in a single transcriptional operon, as shown in Fig. 3C. In addition, real-time qPCR analysis showed a 34-fold increase in gene transcription of the pnp cluster from the 2C4NP-induced samples, in comparison with the data from the noninduced samples. It also showed a 44-fold increase in gene transcription of the pnp cluster from PNP-induced samples.
pnpA is essential for strain SJ98 to utilize 2C4NP.
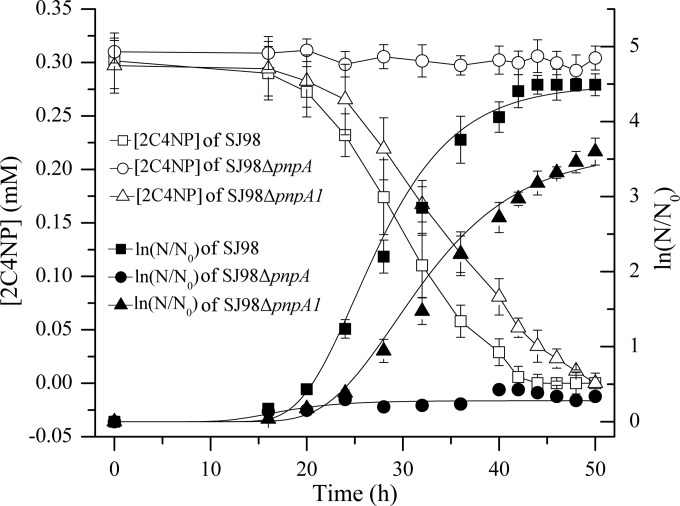
To investigate the physiological roles of pnpA and pnpA1 in 2C4NP degradation in vivo, derivatives of strain SJ98 with deletion of pnpA or pnpA1 were individually constructed. Strain SJ98ΔpnpA (with pnpA deleted) completely lost its ability to grow on 2C4NP, as well as PNP, and pnpA-complemented mutant SJ98ΔpnpA(pRK415-pnpA) regained its ability to grow on these two substrates (Fig. 4). On the other hand, in addition to a lower rate of 2C4NP or PNP removal, strain SJ98ΔpnpA1 (with pnpA1 deleted) also exhibited a lower growth rate than the wild type on these two substrates (Fig. 4), with its maximum specific growth rates (μm, per hour) being approximately 25% lower than those of wild-type SJ98 (see Table S1 in the supplemental material). In biotransformation assays, SJ98ΔpnpA1 exhibited specific activities for 2C4NP (1.73 ± 0.45 U mg−1) and PNP (3.74 ± 0.56 U mg−1) approximately 20% lower than those of wild-type SJ98.
FIG 4.
Time course of 2C4NP degradation and cell growth in cultures of Burkholderia sp. strains SJ98, SJ98ΔpnpA (with pnpA deleted), and SJ98ΔpnpA1 (with pnpA1 deleted). The data for pnpA-complemented mutant SJ98ΔpnpA(pRK415-pnpA) are not shown. The results obtained with PNP were similar to those obtained with 2C4NP (data not shown). N, number of cells; N0, initial number of cells. The growth curves were fitted by the modified Gompertz equation (33) with OriginPro (version 8) software. All of the experiments were performed in triplicate, the results shown are average values of three independent experiments, and error bars indicate standard deviations.
Expression and purification of enzymes involved in 2C4NP degradation.
Recombinant PnpA, PnpA1, PnpB, PnpCD, PnpE, and PnpF were individually overexpressed in E. coli Rosetta(DE3)pLysS as N-terminally His6-tagged fusion proteins for easy purification. H6-PnpB, H6-PnpCD, H6-PnpE, and H6-PnpF were largely soluble and readily purified, whereas only small amounts of H6-PnpA and H6-PnpA1 were soluble even when E. coli cells were induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 16°C. Therefore, the small amounts of H6-PnpA and H6-PnpA1 that were obtained from large quantities of cells were concentrated by ultrafiltration through an Ultra-15 filter unit with a molecular mass cutoff of 10 kDa.
In this way, a total of 0.78 mg of H6-PnpA with a specific activity of 7.2 U mg−1 for 2C4NP (5.6 U mg−1 for PNP) was purified from 6,000 ml of culture. Even less H6-PnpA1 was purified from 6,000 ml of culture, which hampered its further analysis in vitro. For H6-PnpB purification, 28.2 mg of H6-PnpB with a specific activity of 23.8 U mg−1 for CBQ (31.4 U mg−1 for BQ) was obtained from 1,000 ml of culture. On the other hand, 21.4 mg of H6-PnpCD, 7.9 mg of H6-PnpE, and 8.7 mg of H6-PnpF were each purified from 1,000 ml of culture. SDS-PAGE analysis of all of the above purified proteins showed that the molecular masses of H6-PnpA, H6-PnpB, H6-PnpC, PnpD, H6-PnpE, and H6-PnpF are about 45, 25, 20, 38, 55, and 40 kDa (see Fig. S4 in the supplemental material), respectively, corresponding to the molecular masses deduced from their amino acid sequences.
PnpA catalyzes the monooxygenation of 2C4NP to CBQ.
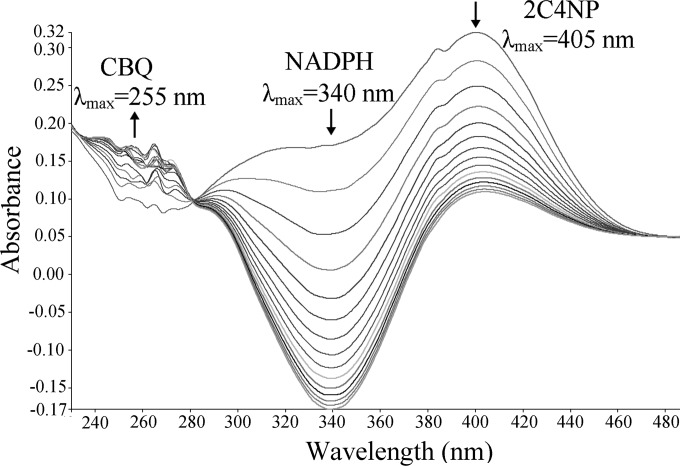
In parallel to our study, it was recently reported that PnpA catalyzed the NADPH-dependent monooxygenation of PNP to BQ in PNP catabolism in this strain (36), but its role in 2C4NP degradation was not characterized. In our investigation, after the confirmation of PnpA as a PNP monooxygenase, the activity of PnpA against 2C4NP was assayed by measuring the consumption of both 2C4NP and NADPH. Rapid degradation of 2C4NP (λmax, 405 nm) by H6-PnpA occurred, as shown in Fig. 5, together with consumption of NADPH (λmax, 340 nm). An isobestic point at 280 nm was observed, indicating the transformation of 2C4NP to a new product with maximum absorption at around 255 nm. By HPLC and GC-MS analyses, both CBQ (λmax, 255 nm) and CHQ (λmax, 291 nm) were identified as products of 2C4NP monooxygenation in the system containing purified H6-PnpA. The detection of CHQ may be due to the nonenzymatic reduction of CBQ in the presence of NADPH, and a similar explanation was proposed previously for the production of HQ when PNP was catalyzed by PNP monooxygenases in Pseudomonas sp. strain WBC-3 (6) and Arthrobacter sp. strain JS443 (38).
FIG 5.
Spectral changes during the transformation of 2C4NP by purified H6-PnpA. Sample and reference cuvettes contained 0.1 mM NADPH, 0.03 mM flavin adenine dinucleotide, 50 mM phosphate buffer (pH 7.4), and 10 μg H6-PnpA in a 0.5-ml volume. The reaction was initiated by the addition of 25 μM 2C4NP, and the spectra were recorded every minute after the addition of 2C4NP. The arrows indicate the directions of spectral changes.
Kinetic assays revealed that the Km value of H6-PnpA for 2C4NP (6.2 ± 0.76 μM) was lower than the value previously reported (35.4 ± 5.95 μM) (36) or that determined in this study (25.4 ± 3.63 μM) for PNP. Ironically, the PNP 4-monooxygenase (PnpA) from the PNP utilizer Pseudomonas sp. strain WBC-3 was also found in this study to exhibit a higher affinity and catalytic efficiency for 2C4NP (Km = 8.1 ± 1.13 μM, kcat/Km = 1.13 ± 0.080 μM−1 min−1) than PNP (Km = 20.3 ± 2.70 μM, kcat/Km = 0.665 ± 0.043 μM−1 min−1), despite its inability to grow on 2C4NP observed in this study.
PnpB catalyzes the reduction of CBQ to CHQ.
Recently, PnpB was reported to catalyze the reduction of BQ in PNP catabolism (36), but its product was not identified. During this study, in addition to the HPLC identification of HQ as the product of BQ catalysis by PnpB, the activity of PnpB against CBQ was performed in order to prove the formation of CBQ during 2C4NP catabolism by strain SJ98. The product of the reaction catalyzed by H6-PnpB was identified as CHQ by HPLC analysis. Moreover, when H6-PnpB was added to the reaction mixture containing H6-PnpA with 2C4NP or PNP, the activity of H6-PnpA was enhanced significantly (Fig. 6). This is similar to the phenomenon described by Zhang et al. (6) during PNP monooxygenation in the presence of BQ reductase from strain WBC-3. A probable explanation for this enhanced activity is that the formed quinone (CBQ or BQ) is reduced by H6-PnpB, possibly avoiding product inhibition of PnpA. In particular, the enhanced 2C4NP monooxygenase activity with H6-PnpB in this study further demonstrates the involvement of CBQ during 2C4NP degradation by strain SJ98, apart from the GC-MS identification of CBQ as an intermediate.
FIG 6.
H6-PnpB enhances 2C4NP and PNP degradation by H6-PnpA. The PnpA specific activity of 7.2 U mg−1 against 2C4NP and 5.6 U mg−1 against PNP were defined as 100% when no H6-PnpB was added.
Enzymatic assays of PnpCD, PnpE, and PnpF by sequential catalyses.
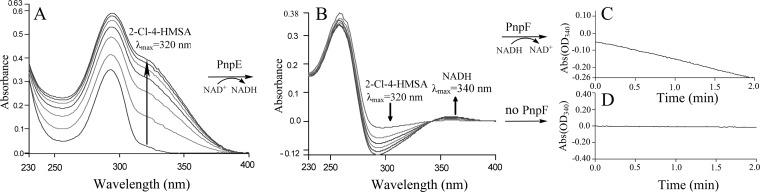
E. coli cells carrying pET-pnpCD were found, by HPLC analysis, to degrade HQ and CHQ, while cells harboring only the vector (pET-28a) were unable to do so. When purified His6-PnpCD was added to the reaction mixture containing CHQ, a spectral change from 290 to 320 nm occurred to form a new peak with a λmax of 320 nm (Fig. 7A), in line with the spectral property of 2-chloro-4-hydroxymuconic semialdehyde (26). On the other hand, the dioxygenase activity of PnpCD was Fe2+ dependent and the activity was completely abolished in the presence of the ferrous ion chelator 2,2′-dipyridyl. Sequential catalytic reactions were carried out in order to investigate whether (chloro)4-hydroxymuconic semialdehyde dehydrogenase (PnpE) and (chloro)maleylacetate reductase (PnpF) are involved in 2C4NP degradation. For the PnpE assay, the substrate contained in the reaction mixture was the ring cleavage product of CHQ from the above PnpCD-catalyzed dioxygenation, presumably, 2-chloro-4-hydroxymuconic semialdehyde. As shown in Fig. 7B, the absorbance of 2-chloro-4-hydroxymuconic semialdehyde became progressively lower after the addition of purified His6-PnpE, together with the production of NADH, presumably forming 2-chloromaleylacetate. Following the complete oxidation of 2-chloro-4-hydroxymuconic semialdehyde by PnpE, the reaction mixture was used to assay the activity of PnpF, according to the method for assaying maleylacetate reductase from Pseudomonas sp. strain B13 (monitoring the decrease in the cofactor NADH by measuring absorption at 340 nm) (27). A rapid oxidation of NADH was observed (Fig. 7C) upon the addition of purified His6-PnpF to the assay mixture, an indication of the presence of active 2-chloromaleylacetate reductase. The product of this reaction is presumably maleylacetate resulting from the dechlorination of 2-chloromaleylacetate by PnpF, similar to the reports in which maleylacetate reductases were involved in the dechlorination of 2-chloromaleylacetate to form β-ketoadipate via maleylacetate (27, 39, 40). However, no oxidation of NADH was observed when His6-PnpF was omitted from the reaction mixture (Fig. 7D). When nonchlorinated HQ was the starting substrate, PnpCD, PnpE, and PnpF worked in the same way, in which the spectral changes are similar to those shown in Fig. 7 and the products are suggested to be 4-hydroxymuconic semialdehyde (5, 26), maleylacetate, and β-ketoadipate, respectively (41).
FIG 7.
Assays of PnpCD, PnpE, and PnpF enzyme activities by sequential catalytic reactions with CHQ as the starting substrate. (A) Spectral changes during rapid oxidation of CHQ by purified His6-PnpCD. Sample and reference cuvettes contained 20 mM phosphate buffer (pH 7.4), 0.04 mM Fe2+, and 5 μg purified His6-PnpCD in a 0.5-ml volume. The reaction was initiated by the addition of 0.1 mM CHQ. Spectra were recorded every minute after the addition of CHQ. (B) Enzyme activity assay of PnpE. Following the complete oxidation of CHQ to 2-chloro-4-hydroxymuconic semialdehyde by PnpCD, 50 μM NAD+ was added to the sample cuvette from the reaction in panel A and then the contents were divided between two cuvettes (sample and reference) for assay of PnpE activity. Spectra were recorded every minute after the addition of 20 μg purified H6-PnpE. (C, D) His6-PnpF enzyme activity assays. Following the complete conversion of 2-chloro-4-hydroxymuconic semialdehyde to 2-chloromaleylacetate, 50 μM NADH was added to the sample cuvette from the reaction in panel B and then the contents were divided between two cuvettes (sample and reference) for assay of PnpF activity. To initiate the assay, 20 μg purified H6-PnpF (C) or buffer without H6-PnpF (D) was added to the sample cuvette.
DISCUSSION
It was generally accepted that the BT pathway is the predominant pathway of PNP degradation by Gram-positive strains, which was initiated by a two-component PNP monooxygenase (7–11), while the HQ pathway is preferentially found in Gram-negative bacteria and is initiated by a single-component PNP monooxygenase (3–6). Previously, 2C4NP degradation by strain SJ98 was reported to be initiated by reductive dechlorination with the formation of PNP, which was further degraded via a BT pathway (Fig. 8B) (13). In this study, an initial bioinformatic analysis did not identify genes encoding the potential reductive dehalogenase or two-component PNP monooxygenase in its draft genome. Subsequent biochemical and genetic analyses demonstrated that 2C4NP metabolism in strain SJ98 occurs via the CHQ pathway rather than the BT pathway, revealing the catabolic mechanism of 2C4NP degradation at the molecular and enzymatic levels in a bacterial strain.
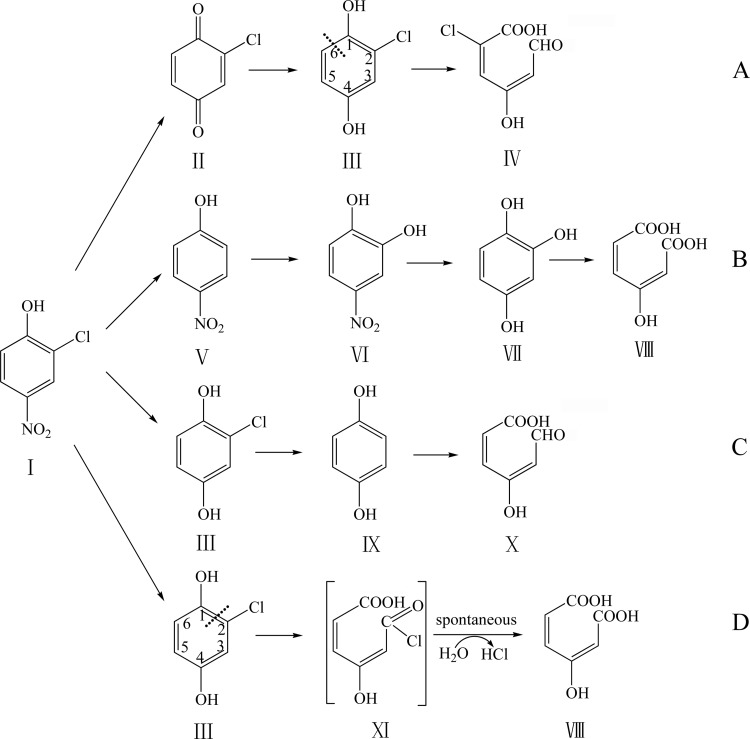
FIG 8.
Proposed pathways of 2C4NP degradation by different 2C4NP utilizers. Panels: A, Burkholderia sp. strain SJ98 (present study); B, Burkholderia sp. strain SJ98 (13); C, R. imtechensis RKJ 300 (14) and Burkholderia sp. strain RKJ 800 (12); D, Arthrobacter sp. strain SJCon (1). The dash lines in panels A and D indicate the ring cleavage positions. Structures: I, 2C4NP; II, CBQ; III, CHQ; IV, 2-chloro-4-hydroxymuconic semialdehyde; V, PNP; VI, 4-NC; VII, BT; VIII, maleylacetate; IX, HQ; X, 4-hydroxymuconic semialdehyde; XI, acyl chloride.
The 2C4NP catabolic pathway reported here is different from those of all other 2C4NP utilizers in terms of the initial reactions or the ring cleavage reaction. CHQ was identified as the ring cleavage compound in strain SJ98 (Fig. 8A), but HQ was identified as the ring cleavage compound in the previously reported degradation pathway of 2C4NP in Rhodococcus imtechensis RKJ 300 (14) and Burkholderia sp. strain RKJ 800 (Fig. 8C) (12). This clearly indicates that removal of the chloro group occurs after ring cleavage in strain SJ98, whereas the chloro group was removed before ring cleavage in other two strains. Although CHQ was also identified as the ring cleavage compound during 2C4NP degradation by Arthrobacter sp. strain SJCon (Fig. 8D) (1), CBQ was not detected in this case. In addition, the regioselectivity of dioxygenation of the ring cleavage substrate CHQ is remarkably different between the (chloro)hydroquinone dioxygenases of strains SJ98 and SJCon (Fig. 8A and D). The ring cleavage position of CHQ dioxygenase in cell extracts of strain SJCon was proposed in line with that of the (chloro)hydroquinone dioxygenase (LinE) from Sphingomonas paucimobilis UT26 (42) and the 2,6-dichlorohydroquinone dioxygenase (PcpA) from Sphingobium chlorophenolicum ATCC 39723 (43), splitting the ring of CHQ between C-1 and C-2 with the formation of MA (λmax, 243 nm) via a transient intermediate (1). However, in this study, the product of CHQ catalysis by PnpCD was suggested to be 2-chloro-4-hydroxymuconic semialdehyde (λmax, 320 nm), which indicated that the ring cleavage position of CHQ dioxygenase of strain SJ98 is the same as that of (chloro)hydroquinone dioxygenase (HapCD) from Pseudomonas fluorescens ACB (26), splitting the ring of CHQ between C-1 and C-6. Therefore, CHQ was possibly catalyzed by a LinE-like single-subunit dioxygenase in strain SJCon (although its protein sequence is unknown), whereas CHQ was split by a two-subunit dioxygenase (PnpCD) in strain SJ98.
Although three different pathways have been proposed for the microbial degradation of 2C4NP (1, 12–14), none of these pathways has been characterized at the genetic and biochemical levels. In this study, the high transcription of the pnp cluster in the 2C4NP-induced cell indicated that the enzymes encoded by the pnp cluster were likely involved in the catabolism of 2C4NP. Enzyme activity assay results and intermediate identification have shown that PnpA has the ability to catalyze the oxidation of 2C4NP to CBQ, and its encoding gene is necessary for strain SJ98 to utilize 2C4NP. The lower Km value of PnpA for 2C4NP than PNP indicates that 2C4NP is the probable physiological substrate for PnpA in strain SJ98. In addition, the enhancement of 2C4NP monooxygenase activity of PnpA by PnpB, a reductase catalyzing the reduction of CBQ to CHQ, clearly suggests the involvement of CBQ during 2C4NP degradation. Despite the significant identity between clusters pnpDE1E2F and pnpCDEF (between 68 and 85% identity of the gene products at the amino acid level), PnpE1E2 was found to be able catalyze the ring cleavage of HQ but not CHQ (44). In contrast, PnpCD, PnpE, and PnpF, reported here, were found to be able to catalyze the sequential reactions starting from both HQ and CHQ. Considering that pnpCDEF are located in the same operon as pnpAB, it is reasonable to conclude that pnpCDEF are the functional genes responsible for the lower pathway of the catabolism of both 2C4NP and PNP in strain SJ98.
Unlike all other utilizers of PNP via the HQ pathway, in which the pnp catabolic genes are located on three operons (3–6), the genetic organization of the pnp cluster (pnpABA1CDEF) involved in the degradation of both 2C4NP and PNP in strain SJ98 is a single operon (Fig. 9), indicating its unique evolutionary pattern in acquiring 2C4NP and PNP catabolic ability. On the other hand, despite the observations that PnpA from strain WBC-3 (a well-characterized representative of the PNP utilizers) is able to catalyze the monooxygenation of both PNP and 2C4NP and all other Pnp proteins share significant homology with their counterparts in strain SJ98, strain WBC-3 does not grow on 2C4NP. Nevertheless, it is able to degrade 2C4NP after PNP induction. These observations suggest that different regulatory mechanisms are involved in the initiation of pnp gene transcription in strains SJ98 and WBC-3.
FIG 9.
Comparison of the genetic organization of PNP catabolic clusters from different PNP utilizers. Panels: A, Burkholderia sp. strain SJ98 (accession no. AJHK02000012) (35); B, Pseudomonas sp. strain WBC-3 (accession no. EF577044) (6) and Pseudomonas sp. strain NyZ402 (accession no. GU123925) (4); C, Pseudomonas sp. strain 1-7 (accession no. FJ821777) (3); D, Pseudomonas putida DLL-E4 (accession no. FJ376608) (5).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant 31270103) and the National Key Basic Research Program of China (973 Program, grant 2012CB725202).
We are grateful to the Core Facility and Technical Support in the Wuhan Institute of Virology, Chinese Academy of Sciences.
Footnotes
Published ahead of print 1 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02093-14.
REFERENCES
- 1.Arora PK, Jain RK. 2011. Pathway for degradation of 2-chloro-4-nitrophenol in Arthrobacter sp. SJCon. Curr. Microbiol. 63:568–573. 10.1007/s00284-011-0022-2 [DOI] [PubMed] [Google Scholar]
- 2.Arora PK, Sasikala C, Ramana ChV. 2012. Degradation of chlorinated nitroaromatic compounds. Appl. Microbiol. Biotechnol. 93:2265–2277. 10.1007/s00253-012-3927-1 [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Sun W, Xu L, Zheng X, Chu X, Tian J, Wu N, Fan Y. 2012. Identification of the para-nitrophenol catabolic pathway, and characterization of three enzymes involved in the hydroquinone pathway, in Pseudomonas sp. 1-7. BMC Microbiol. 12:27. 10.1186/1471-2180-12-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Q, Liu H, Zhang JJ, Wang SH, Xiao Y, Zhou NY. 2010. Characterization of a para-nitrophenol catabolic cluster in Pseudomonas sp. strain NyZ402 and construction of an engineered strain capable of simultaneously mineralizing both para- and ortho-nitrophenols. Biodegradation 21:575–584. 10.1007/s10532-009-9325-4 [DOI] [PubMed] [Google Scholar]
- 5.Shen W, Liu W, Zhang J, Tao J, Deng H, Cao H, Cui Z. 2010. Cloning and characterization of a gene cluster involved in the catabolism of p-nitrophenol from Pseudomonas putida DLL-E4. Bioresour. Technol. 101:7516–7522. 10.1016/j.biortech.2010.04.052 [DOI] [PubMed] [Google Scholar]
- 6.Zhang JJ, Liu H, Xiao Y, Zhang XE, Zhou NY. 2009. Identification and characterization of catabolic para-nitrophenol 4-monooxygenase and para-benzoquinone reductase from Pseudomonas sp. strain WBC-3. J. Bacteriol. 191:2703–2710. 10.1128/JB.01566-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto K, Nishimura M, Kato DI, Takeo M, Negoro S. 2011. Identification and characterization of another 4-nitrophenol degradation gene cluster, nps, in Rhodococcus sp. strain PN1. J. Biosci. Bioeng. 111:687–694. 10.1016/j.jbiosc.2011.01.016 [DOI] [PubMed] [Google Scholar]
- 8.Liu PP, Zhang JJ, Zhou NY. 2010. Characterization and mutagenesis of a two-component monooxygenase involved in para-nitrophenol degradation by an Arthrobacter strain. Int. Biodeter. Biodegr. 64:293–299. 10.1016/j.ibiod.2010.03.001 [DOI] [Google Scholar]
- 9.Takeo M, Murakami M, Niihara S, Yamamoto K, Nishimura M, Kato D, Negoro S. 2008. Mechanism of 4-nitrophenol oxidation in Rhodococcus sp. strain PN1: characterization of the two-component 4-nitrophenol hydroxylase and regulation of its expression. J. Bacteriol. 190:7367–7374. 10.1128/JB.00742-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitagawa W, Kimura N, Kamagata Y. 2004. A novel p-nitrophenol degradation gene cluster from a Gram-positive bacterium, Rhodococcus opacus SAO101. J. Bacteriol. 186:4894–4902. 10.1128/JB.186.15.4894-4902.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadiyala V, Spain JC. 1998. A two-component monooxygenase catalyzes both the hydroxylation of p-nitrophenol and the oxidative release of nitrite from 4-nitrocatechol in Bacillus sphaericus JS905. Appl. Environ. Microbiol. 64:2479–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora PK, Jain RK. 2012. Metabolism of 2-chloro-4-nitrophenol in a gram negative bacterium, Burkholderia sp RKJ 800. PLoS One 7(6):e38676. 10.1371/journal.pone.0038676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey J, Heipieper HJ, Chauhan A, Arora PK, Prakash D, Takeo M, Jain RK. 2011. Reductive dehalogenation mediated initiation of aerobic degradation of 2-chloro-4-nitrophenol (2C4NP) by Burkholderia sp. strain SJ98. Appl. Microbiol. Biotechnol. 92:597–607. 10.1007/s00253-011-3254-y [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A, Khurana M, Chauhan A, Takeo M, Chakraborti AK, Jain RK. 2010. Degradation of 4-nitrophenol, 2-chloro-4-nitrophenol, and 2,4-dinitrophenol by Rhodococcus imtechensis strain RKJ300. Environ. Sci. Technol. 44:1069–1077. 10.1021/es9034123 [DOI] [PubMed] [Google Scholar]
- 15.Chauhan A, Pandey G, Sharma NK, Paul D, Pandey J, Jain RK. 2010. p-Nitrophenol degradation via 4-nitrocatechol in Burkholderia sp. SJ98 and cloning of some of the lower pathway genes. Environ. Sci. Technol. 44:3435–3441. 10.1021/es9024172 [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Wang SJ, Zhang JJ, Dai H, Tang H, Zhou NY. 2011. Patchwork assembly of nag-like nitroarene dioxygenase genes and the 3-chlorocatechol degradation cluster for evolution of the 2-chloronitrobenzene catabolism pathway in Pseudomonas stutzeri ZWLR2-1. Appl. Environ. Microbiol. 77:4547–4552. 10.1128/AEM.02543-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YF, Chao H, Zhou NY. 2014. The catabolism of 2,4-xylenol and p-cresol share the enzymes for the oxidation of para-methyl group in Pseudomonas putida NCIMB 9866. Appl. Microbiol. Biotechnol. 98:1349–1356. 10.1007/s00253-013-5001-z [DOI] [PubMed] [Google Scholar]
- 18.Siebert PD, Chenchik A, Kellogg DE, Lukyanov KA, Lukyanov SA. 1995. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 23:1087–1088. 10.1093/nar/23.6.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye J, McGinnis S, Madden TL. 2006. BLAST: improvements for better sequence analysis. Nucleic Acids Res. 34:W6–W9. 10.1093/nar/gkl164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 21.Liu TT, Zhou NY. 2012. Novel l-cysteine-dependent maleylpyruvate isomerase in the gentisate pathway of Paenibacillus sp. strain NyZ101. J. Bacteriol. 194:3987–3994. 10.1128/JB.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontecave M, Eliasson R, Reichard P. 1987. NAD(P)H:flavin oxidoreductase of Escherichia coli. A ferric iron reductase participating in the generation of the free radical of ribonucleotide reductase. J. Biol. Chem. 262:12325–12331 [PubMed] [Google Scholar]
- 23.Spain JC, Wyss O, Gibson DT. 1979. Enzymatic oxidation of p-nitrophenol. Biochem. Biophys. Res. Commun. 88:634–641. 10.1016/0006-291X(79)92095-3 [DOI] [PubMed] [Google Scholar]
- 24.Winn-Deen ES, David H, Sigler G, Chavez R. 1988. Development of a direct assay for α-amylase. Clin. Chem. 34:2005–2008 [PubMed] [Google Scholar]
- 25.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 26.Moonen MJ, Synowsky SA, van den Berg WA, Westphal AH, Heck AJ, van den Heuvel RH, Fraaije MW, van Berkel WJ. 2008. Hydroquinone dioxygenase from Pseudomonas fluorescens ACB: a novel member of the family of nonheme-iron(II)-dependent dioxygenases. J. Bacteriol. 190:5199–5209. 10.1128/JB.01945-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaschabek SR, Reineke W. 1995. Maleylacetate reductase of Pseudomonas sp. strain B13: specificity of substrate conversion and halide elimination. J. Bacteriol. 177:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis JJ, Zylstra GJ. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of Gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710–2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. 10.1016/S0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- 30.Dehio C, Meyer M. 1997. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 179:538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saltikov CW, Newman DK. 2003. Genetic identification of a respiratory arsenate reductase. Proc. Natl. Acad. Sci. U. S. A. 100:10983–10988. 10.1073/pnas.1834303100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keen NT, Tamaki S, Kobayashi D, Trollinger D. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191–197. 10.1016/0378-1119(88)90117-5 [DOI] [PubMed] [Google Scholar]
- 33.Zwietering MH, Jongenburger I, Rombouts FM, Vantriet K. 1990. Modeling of the bacterial-growth curve. Appl. Environ. Microbiol. 56:1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira MI, Marchesi JR, Janssen DB. 2008. Degradation of 4-fluorophenol by Arthrobacter sp. strain IF1. Appl. Microbiol. Biotechnol. 78:709–717. 10.1007/s00253-008-1343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Vikram S, Raghava GP. 2012. Genome sequence of the nitroaromatic compound-degrading bacterium Burkholderia sp. strain SJ98. J. Bacteriol. 194:3286. 10.1128/JB.00497-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vikram S, Pandey J, Kumar S, Raghava GP. 2013. Genes involved in degradation of para-nitrophenol are differentially arranged in form of non-contiguous gene clusters in Burkholderia sp. strain SJ98. PLoS One 8(12):e84766. 10.1371/journal.pone.0084766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei M, Zhang JJ, Liu H, Zhou NY. 2010. para-Nitrophenol 4-monooxygenase and hydroxyquinol 1,2-dioxygenase catalyze sequential transformation of 4-nitrocatechol in Pseudomonas sp. strain WBC-3. Biodegradation 21:915–921. 10.1007/s10532-010-9351-2 [DOI] [PubMed] [Google Scholar]
- 38.Perry LL, Zylstra GJ. 2007. Cloning of a gene cluster involved in the catabolism of p-nitrophenol by Arthrobacter sp. strain JS443 and characterization of the p-nitrophenol monooxygenase. J. Bacteriol. 189:7563–7572. 10.1128/JB.01849-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vollmer MD, Stadlerfritzsche K, Schlomann M. 1993. Conversion of 2-chloromaleylacetate in Alcaligenes eutrophus JMP134. Arch. Microbiol. 159:182–188. 10.1007/BF00250280 [DOI] [PubMed] [Google Scholar]
- 40.Kaschabek SR, Reineke W. 1992. Maleylacetate reductase of Pseudomonas sp. strain B13: dechlorination of chloromaleylacetates, metabolites in the degradation of chloroaromatic compounds. Arch. Microbiol. 158:412–417 [DOI] [PubMed] [Google Scholar]
- 41.Spain JC, Gibson DT. 1991. Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl. Environ. Microbiol. 57:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyauchi K, Adachi Y, Nagata Y, Takagi M. 1999. Cloning and sequencing of a novel meta cleavage dioxygenase gene whose product is involved in degradation of γ-hexachlorocyclohexane in Sphingomonas paucimobilis. J. Bacteriol. 181:6712–6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohtsubo Y, Miyauchi K, Kanda K, Hatta T, Kiyohara H, Senda T, Nagata Y, Mitsui Y, Takagi M. 1999. PcpA, which is involved in the degradation of pentachlorophenol in Sphingomonas chlorophenolica ATCC 39723, is a novel type of ring cleavage dioxygenase. FEBS Lett. 459:395–398. 10.1016/S0014-5793(99)01305-8 [DOI] [PubMed] [Google Scholar]
- 44.Vikram S, Pandey J, Kumar S, Raghava GP. 2013. Genes involved in degradation of para-nitrophenol are differentially arranged in form of non-contiguous gene clusters in Burkholderia sp. strain SJ98. PLoS One 8(12):e84766. 10.1371/journal.pone.0084766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.