Abstract
Legionnaires' disease is a severe form of pneumonia caused by Legionella spp., organisms often isolated from environmental sources, including soil and water. Legionella spp. are capable of replicating intracellularly within free-living protozoa, and once this has occurred, Legionella is particularly resistant to disinfectants. Citrus essential oil (EO) vapors are effective antimicrobials against a range of microorganisms, with reductions of 5 log cells ml−1 on a variety of surfaces. The aim of this investigation was to assess the efficacy of a citrus EO vapor against Legionella spp. in water and in soil systems. Reductions of viable cells of Legionella pneumophila, Legionella longbeachae, Legionella bozemanii, and an intra-amoebal culture of Legionella pneumophila (water system only) were assessed in soil and in water after exposure to a citrus EO vapor at concentrations ranging from 3.75 mg/liter air to 15g/liter air. Antimicrobial efficacy via different delivery systems (passive and active sintering of the vapor) was determined in water, and gas chromatography-mass spectrometry (GC-MS) analysis of the antimicrobial components (linalool, citral, and β-pinene) was conducted. There was up to a 5-log cells ml−1 reduction in Legionella spp. in soil after exposure to the citrus EO vapors (15 mg/liter air). The most susceptible strain in water was L. pneumophila, with a 4-log cells ml−1 reduction after 24 h via sintering (15 g/liter air). Sintering the vapor through water increased the presence of the antimicrobial components, with a 61% increase of linalool. Therefore, the appropriate method of delivery of an antimicrobial citrus EO vapor may go some way in controlling Legionella spp. from environmental sources.
INTRODUCTION
In 2011, 4,897 cases of Legionnaires' disease were reported by European Union member states, Norway, and Iceland, with six countries (France, Italy, Spain, Germany, Netherlands, and the United Kingdom) contributing to 83% of all the cases (1). In the same year, 239 cases were reported by the National Surveillance Scheme in England and Wales, with the number of cases steadily increasing since the mid-1990s, when on average, between 110 and 160 cases per annum were recorded (2).
Legionnaires' disease is a severe form of pneumonia (2, 3) caused by the Gram-negative, aerobic rod Legionella spp. It affects mainly the elderly and immunocompromised people and is more generally reported in men (3, 4). Legionella pneumophila is the predominant human-pathogenic strain and is responsible for about 90% of all human infections by Legionella spp. (5, 6). Sixteen serotypes of L. pneumophila exist, but serotype 1 is the most important clinically (5). An international collaborative survey showed that 84.2% of all isolates in patients with community-acquired Legionnaires' disease were serotype 1 (7). However, Legionella longbeachae and Legionella bozemanii were also isolated at rates of 3.9% and 2.4%, respectively (7). However, incidence rates vary from country to country and also from source to source. For example, L. longbeachae is the most commonly isolated species from patients in Australia (8), accounting for about 30% of Legionella isolates from Australia and New Zealand. A recent study by Currie et al. (9) in the United Kingdom has shown that 15 of 24 compost samples were positive for Legionella spp., with L. longbeachae being the most commonly isolated. In one study, L. longbeachae was found in the sputum of a patient who had been in contact with potting soil (6), and it was suggested that aerosol-aided spread and evaporation of water in the potting soil were the possible routes of transmission (6, 10). Legionella has also been isolated from waste management facilities dealing with unwashed solid articles, probably via exposure to soil (11).
The natural habitat of Legionella is freshwater, such as lakes and rivers (3), where it grows planktonically or in biofilms, with an optimum temperature range for growth and survival between 30°C and 40°C. However, it can enter man-made water systems and survive, thus creating a potential source for infection. Previous studies have isolated the bacterium from drinking water systems, cooling towers of air conditioning units, whirlpools, spas, fountains, ice machines, vegetable misters, dental devices, and shower heads (12). Infection in humans occurs via inhalation of an aerosolized form of Legionella spp. from a contaminated source or via aspiration of contaminated water, which can occur within milliseconds (5, 6, 10). There are no specific standards in the United Kingdom for acceptable levels of Legionella spp. in water; however, there is a statutory requirement that the owners of buildings that have equipment predisposed to harboring Legionella spp. must ensure that the equipment is maintained to prevent the growth and spread of the organism (13).
Legionella spp. are also capable of invading and replicating intracellularly within free-living protozoa (3, 12). Acanthamoeba polyphaga is the most common host of Legionella spp. in natural environments (3). Free-living amoebae are capable of forming cysts, which confer resistance to extreme temperatures, desiccation, and disinfection (14) and also provide protection to the intracellular Legionella cells, hence making them more able to survive similarly unfavorable conditions. Furthermore, several studies have shown that L. pneumophila exhibits a higher stress resistance and is more invasive and virulent after it has replicated within a protozoan cell than under other conditions (3, 12, 15). It has been suggested that Legionella cells invade and grow within human macrophages in a way similar to invasion and growth within protozoan cells (16).
Commonly, chemical disinfectants or biocides are used to prevent microbial contamination and growth of prospective pathogenic microorganisms in man-made aquatic sites (14). However, they are effective only in high concentrations, which tend to be harmful to humans, and thus the use of natural alternatives to these chemicals may reduce the risk of toxicity. Citrus essential oils (EOs) were first noted for their antimicrobial effect in 1949 by Piacentini (17). In contrast to chemical disinfectants, citrus EOs are generally recognized as safe (GRAS) and therefore are acceptable for use in food and water systems.
In recent studies, a range of pathogenic bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and both vancomycin-susceptible and vancomycin-resistant strains of Enterococcus faecium and Enterococcus faecalis, have been shown to be susceptible to the vaporized form of the unique blend of the citrus essential oils at a concentration of 15 mg/liter air (18, 19). Furthermore, the citrus EO vapor used in this study has been shown to be effective against the food-borne pathogens Listeria monocytogenes, Bacillus cereus, Escherichia coli O157, and Campylobacter jejuni (20, 21). However, to date, the studies on the antimicrobial nature of the citrus EO vapor have tested its effectiveness only on surfaces such as stainless steel and other food contact surfaces and not against microorganisms in liquid systems because of the hydrophobic nature of its components. In addition, the use of EOs in water is not very effective because their vapors consist mainly of phenolic compounds, which have poor solubility, resulting in reduced antimicrobial activity. Water also reduces volatility, as compounds with hydroxyl groups may be more solvated and remain in the water phase (22). Previous studies using a bioautography method followed by atmospheric pressure chemical ionization mass spectrometry(APCI-MS) and solid-phase microextraction gas chromatography (SPME GC)-MS have shown that there is a favorable release of the active compounds (linalool, citral, and β-pinene) from the citrus EO vapor, which facilitates the antimicrobial activity (23).
The aim of this study was to investigate the effectiveness of the antimicrobial citrus EO vapor against Legionella spp. in soil and to establish if sintering is effective as an active delivery system against Legionella spp. and intra-amoebal L. pneumophila in water.
MATERIALS AND METHODS
All investigations were carried out in duplicate on at least three separate occasions.
Microorganisms and culturing methods.
Legionella pneumophila (ATCC 33152), Legionella longbeachae (ATCC-33462), and Legionella bozemanii (ATCC 33217) were grown on a Legionella charcoal-yeast extract (CYE) agar base (CM0655) supplemented with Legionella buffered CYE (BCYE) growth supplement (SR0110C) at 37°C for 48 h. Acanthamoeba polyphaga (CCAP 1501/14) was cultured using peptone yeast glucose (PYG) medium (10 g proteose-peptone, 0.5 g yeast extract, 0.1 M glucose, 25 ml Page's amoebal saline solution 1 [PAS 1], 25 ml PAS 2, 450 ml water) adjusted to pH 6.5 with KOH PAS solutions (24). Aliquots of 1 ml of A. polyphaga cultures were suspended in 5 ml PYG medium in tissue culture flasks. The protozoan cultures were incubated for 3 days at 35°C.
Preparation of Acanthamoeba polyphaga for coculture experiments.
PYG broth (22 ml) was inoculated with 2 ml of a 3-day A. polyphaga culture. The culture flasks were incubated horizontally for 3 days at room temperature.
After incubation, the flasks were shaken to remove the protozoa from their surface, and the sample was centrifuged at 400 × g (Hettich Rotanta 460 S; Hettich, Tuttlingen, Germany) for 6 min at room temperature. The pellet was then washed twice in 20 ml of PAS and resuspended in 15 ml of amoebal saline. Cell counts were obtained using a hemocytometer (Thoma, 0.1 mm, 1/400 mm2; Hawksley, London, United Kingdom). Coculturing required a final concentration of 105 cells ml−1.
Intra-amoebal culture of Legionella pneumophila.
A suspension of 10 ml A. polyphaga (105 cells ml−1) was mixed with a suspension of 10 ml L. pneumophila (102 cells ml−1) in a tissue culture flask, and the mixture was incubated at 35°C for 10 days. The sample was then centrifuged at 400 × g for 6 min at room temperature to remove the protozoa. The supernatant was subsequently centrifuged at 2,080 × g for 15 min at room temperature and discarded. The pellet was washed twice with 20 ml PAS. The resulting suspension (105 cells ml−1) was then mixed with 20 ml of a fresh 3-day-old culture of A. polyphaga (105 cells ml−1) and incubated again at 35°C for 3 days. The wash steps were then repeated. The final pellet was resuspended in 20 ml PAS, with a final concentration of Legionella of 108 cells ml−1.
Citrus EO vapor and vapor components.
The citrus EO blend consisted of orange (Citrus sinensis) and bergamot (Citrus bergamia) essential oils (Belmay, Northampton, United Kingdom) in a 1:1 (vol/vol) ratio. Limonene (97%, catalog no. 183164), linalool (97%, number W26, 350-8), citral (95%, number C8, 300-7), and β-pinene (99%, number 402753) were purchased from Sigma-Aldrich Co. Ltd. (Dorset, United Kingdom).
Assessment of a citrus EO vapor and its components against Legionella spp. in soil.
Potting soil (Miracle Gro potting mix; Scotts, United Kingdom) was sterilized, and 1.5 g was placed in a petri dish in a 1,000-ml beaker. The soil was inoculated with 400-μl suspensions of either L. pneumophila, L. longbeachae, or L. bozemanii. Filter papers (Whatman disks, 2 cm) were impregnated with the EO mix to give final concentrations of either 3.75 mg/liter air, 7.5 mg/liter air, or 15 mg/liter air and sealed with parafilm (FIL1026; Scientific Laboratory Supplies, United Kingdom). The beakers were incubated for 24 h at 37°C. The soil was then placed in 30 ml of maximum recovery diluent (MRD), vortexed for 2 min, spread plated onto CYE agar, and incubated for 48 h at 37°C, and counts were obtained. Controls were inoculated soil samples not exposed to the citrus EO vapor or components.
Survival of Legionella spp. in water after exposure to the citrus EO vapor. (i) Passive exposure.
Cells of either L. pneumophila, L. longbeachae, or L. bozemanii or of L. pneumophila that had been passaged through A. polyphaga were inoculated into sterile water in 1-liter beakers to give a final concentration of 107 cells ml−1. Filter papers impregnated with the citrus oil to give final concentrations of 3.75 mg/liter air, 7.5 mg/liter air, 15 mg/liter air, 150 mg/liter air, or 15 g/liter air in the atmosphere were placed in the beaker, which was sealed and incubated at room temperature for 24 h. After exposure, 100-μl samples were spread plated on CYE agar and incubated at 37°C for 48 h, and colonies were counted. Controls were water samples not exposed to the citrus EO vapor.
(ii) Active exposure.
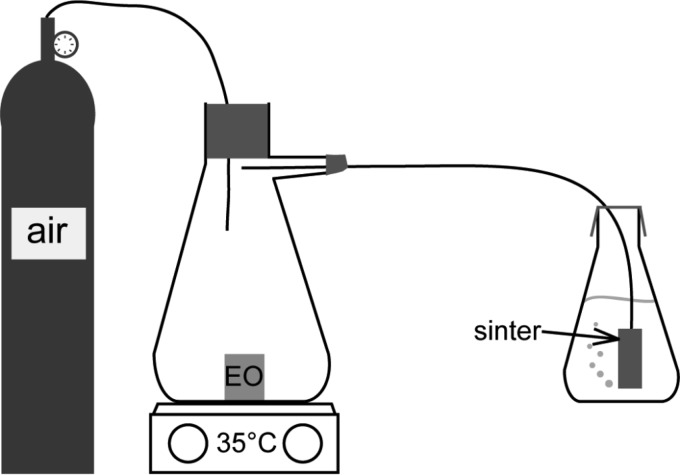
A cylinder filled with compressed air was connected to a 500-ml vacuum flask (headspace, 590 ml), containing either 150 mg/liter air, 820.5 mg/liter air, or 1,500 mg/liter air of citrus EO vapor. A sinter (10-μm pores; Sigma-Aldrich, United Kingdom) running from the vacuum flask was then placed into a 100-ml conical flask containing 100 ml water and L. pneumophila at a final concentration of 106 cells ml−1 (Fig. 1). The sinter forces the air containing the EO through micrometer-sized pores into the water, creating small bubbles that continuously move through the water sample. The citrus EO vapor was left to equilibrate in the vacuum flask for 15 min before the airflow (0.225 liter/min) and the heating plate (30°C) were switched on. Samples were removed at 0, 1, 2, 4, 6, and 24 h after starting the airflow, spread plated onto a CYE agar plate in triplicate, and incubated at 37°C for 48 h, and colonies were counted.
FIG 1.
Schematic diagram of the setup of active exposure of Legionella spp. to the vapor of a citrus EO.
The investigations were repeated using either L. longbeachae, L. bozemanii, or an intra-amoebal culture of L. pneumophila and the concentration of citrus EO vapor shown to be the most effective against L. pneumophila. Controls were cells exposed to pure airflow.
GC-MS analysis.
To quantify the active antimicrobial components (linalool, citral, and β-pinene) in the passive and active exposures, the same apparatus as the one described above was used, but without microorganisms added to the water. Additionally, the initial amount of citrus EO was increased 10-fold (15 g/liter) to enable detection. GC-MS analysis was undertaken on the citrus EO vapor in water or in ethanol without any further sample preparation. Experiments were performed for 24 h in the case of water and for 4 h in the case of ethanol due to volatility limitations (Table 1). All experiments were performed in duplicate.
TABLE 1.
Chemical properties of antimicrobial components in the orange and bergamot EO blenda
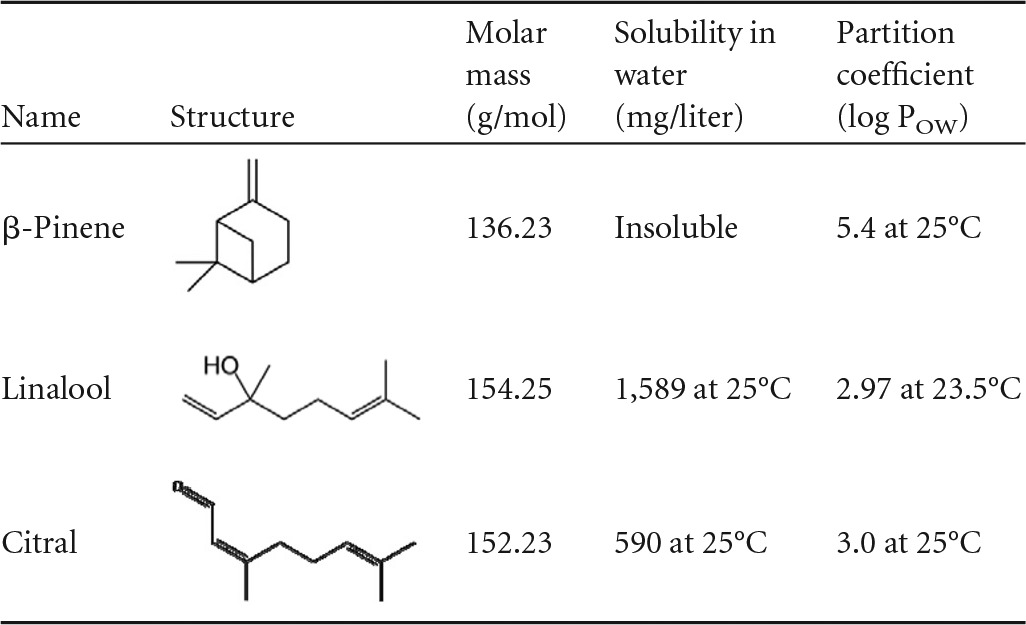
Data from reference 22. POW, octanol-water partition coefficient.
The GC-MS analyses were performed using a Bruker 450GC and 300-MS SQ mass spectrometer operated in electron ionization (EI) mode at 70 eV. A sample volume of 1 μl with a split ratio of 10:1 was injected at an inlet temperature of 250°C. The carrier gas was helium and maintained at a constant flow rate of 1.0 ml min−1. The gas chromatograph was equipped with a FactorFour VF-5MS capillary column (30-m long, 0.25-mm inner diameter [ID]) with 0.25-μm film thickness. The temperature of the column was held at 40°C for 2 min, ramped to 70°C at 10°C min−1, held for 5 min, ramped to 150°C at 5°C min−1, held for 1 min, and then ramped to 200°C at 10°C min−1.
The MS ion source temperature was 180°C. Quantitative analysis was carried out using the selected-ion monitoring (SIM) mode at 70 eV. For each compound, the most abundant ions were selected from its spectrum. The chosen ions for SIM were 69, 84, and 152 for citral, 93, 69, 79, and 136 for β-pinene, and 71, 93, and 154 for linalool. The limits of quantification for all three antimicrobial agents were 1.0 mg/liter.
RESULTS
The citrus EO vapor at concentrations as low as 3.75 mg/liter air reduced Legionella spp. by 0.5 to 1.5 log cells ml−1 in soil; however, when this concentration was increased to that of 15 mg/liter air, which had previously been shown to be effective against a range of different microorganisms on surfaces, up to an 8-log cells ml−1 reduction was observed against L. bozemanii compared to 1.53 log cells ml−1 and 0.7 log cells ml−1 log10 for L. longbeachae and L. pneumophila, respectively (Table 2).
TABLE 2.
Reduction of Legionella spp. in soil when exposed to a citrus EO vapor for 24 ha
| Citrus EO vapor concn (mg/liter) | Mean log reduction ± SE |
||
|---|---|---|---|
| L. longbeachae | L. bozemanii | L. pneumophila | |
| 0 (control) | 1.2 ± 0.2 | 1.05 ± 0.28 | 0.56 ± 0.22 |
| 3.75 | 1.47 ± 0.18 | 1.42 ± 0.32 | 0.55 ± 0.23 |
| 7.5 | 1.65 ± 0.16 | 1.71 ± 0.25 | 0.64 ± 0.24 |
| 15 | 1.53 ± 0.22 | 7.88 ± 0 | 0.7 ± 0.41 |
n = 3.
When water inoculated with Legionella cells was passively subjected to the citrus EO vapor, no reductions in counts were observed at 3.75 mg/liter air, 7.5 mg/liter air, 15 mg/liter air, or 150 mg/liter air. However, when subjected to 15 g/liter air, a 2-log cells ml−1 reduction occurred for L. longbeachae, although the other strains were unaffected (results not shown).
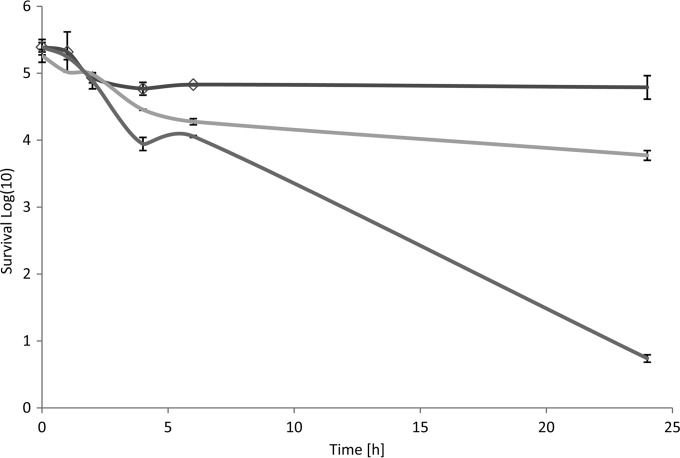
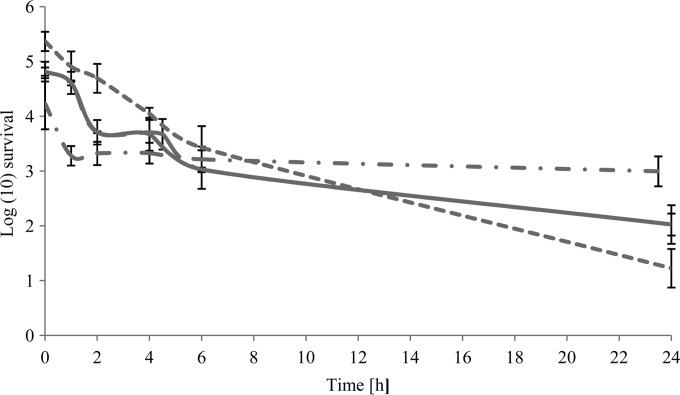
Actively sintering the citrus EO vapor into water inoculated with L. pneumophila resulted in reductions over 24 h of 1.5 log cells ml−1 and 4.5 log cells ml−1 (P ≤ 0.05) for 150 mg/liter air and 1,500 mg/liter air, respectively (Fig. 2). These concentrations are 10- to 100-fold higher than that previously shown (15 mg/liter air) to reduce microorganisms on surfaces such as stainless steel (19).
FIG 2.
Mean survival of L. pneumophila when exposed to an antimicrobial citrus EO vapor in water via a sintering system. Dark grey line, control (exposed to air only); light gray line, 150 mg EO vapor/liter air; medium grey line, 15 g EO vapor/liter air.
A reduction in cell numbers of Legionella spp. in water treated with a citrus EO vapor (15 g/liter) through a sintering system was observed at 2 h of exposure with reductions of between 1 and 2 log cells ml−1. L. pneumophila was the most susceptible species, with a 4-log cells ml−1 (P ≤ 0.05) reduction in cell numbers at 24 h, while L. bozemanii and L. longbeachae were reduced by 2.8 log cells ml−1 and 2.2 log cells ml−1, respectively. However, the vapor had only a minimal effect on the cocultured L. pneumophila, with a 1.24-log cells ml−1 reduction in cell numbers over 24 h (Fig. 3). There was no significant difference (P ≤ 0.05) between the active and passive systems of delivery of the citrus EO vapor against L. longbeachae, for which a 2-log cells ml−1 reduction was observed in both systems.
FIG 3.
Mean survival of Legionella spp. when exposed to an antimicrobial citrus EO vapor (15 g/liter air) in water via a sintering system. Dashed line, L. pneumophila; continuous line, L. longbeachae; long-dashed line, L. bozemanii (mostly overlapping the L. longbeachae line); dash-and-dot line, cocultured L. pneumophila.
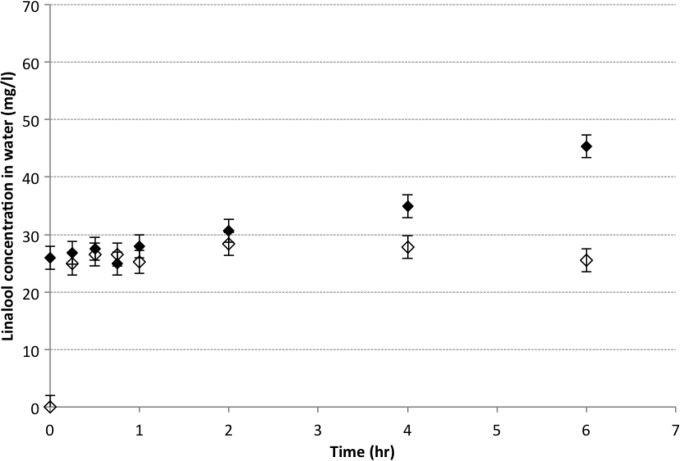
Only linalool was detected in water when 15 g/liter citrus EO vapor was passed through either passively or via the sintering system (active diffusion), which can be attributed to the relatively high water solubility of linalool (Table 1). As shown in Fig. 4, there was no significant difference in the linalool content of water between the passive and active systems, with concentrations of 24.9 mg/liter and 26.5 mg/liter linalool, respectively, after 45 min of exposure. From 1 h onwards, a significant difference (P ≤ 0.05) in the concentration of linalool in the water was noted. After 24 h of exposure, the linalool concentrations were 35.43 mg/liter and 57.17 mg/liter in the passive and active systems, respectively, making the linalool content in the active system 61% greater than that in the passive system.
FIG 4.
Linalool content in 100 ml water exposed to 15 g/liter antimicrobial citrus oil vapor in passive and active modes. Symbols: ◇, linalool passive; ◆, linalool active.
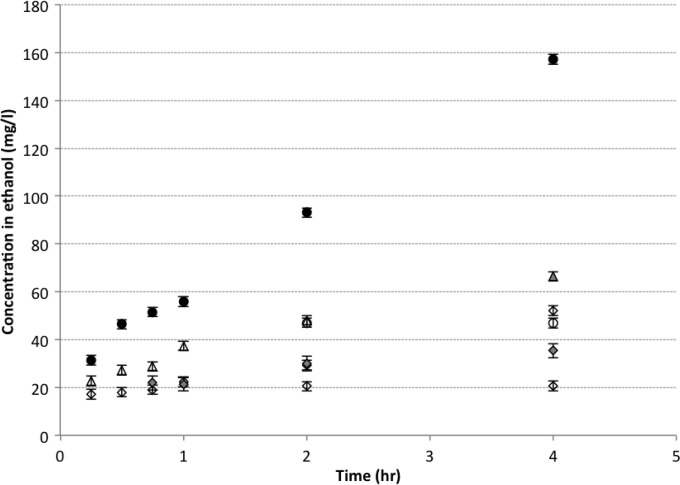
When ethanol was the solute, no linalool was detected within the first 30 min in solution when the citrus EO vapor was diffused either passively or by active sintering. After 4 h, the linalool content was 46% higher in the active system (Fig. 5). This trend of a higher linalool concentration in solution in the active system is similar to that observed when water was used as the solute, and this is also the case for both citral and β-pinene. However, citral showed the highest difference with up to 2.35-fold greater concentration in the active system after 4 h.
FIG 5.
Antimicrobial agents in 100 ml ethanol exposed to 15 g/liter antimicrobial citrus oil vapor in passive and active modes. Symbols: △, β-pinene passive; ▲, β-pinene active; ◇, linalool passive; ◆, linalool active; ○, citral passive; ●, citral active.
DISCUSSION
Overall, the citrus antimicrobial vapor was active against Legionella spp. However, the extent of its efficacy was dependent on strain and substrate. In soil, the vapor was most effective against L. bozemanii at 15 mg/liter air (Table 2) with a 7.88-log cells ml−1 reduction, and there was no significant difference in reductions between the controls and 15 mg/liter air of citrus EO vapor against L. longbeachae and L. pneumophila, demonstrating the vapor to have strain-specific activity. L. longbeachae is the most isolated Legionella sp. from potting soil in Australia (58%), with the rates of isolation of L. pneumophila being 13.3% (10). Against both L. longbeachae and L. bozemanii, the most effective concentration of the citrus EO vapor was 15 mg/liter air. Similar results have been previously reported for Enterococcus survival on a range of surfaces, including lettuce, cucumber, and stainless steel, with reductions of up to 5 log cells ml−1 (19, 25).
The use of essential oils (EOs) as antimicrobials in water-based environments has not been explored in depth, which is probably due to the lipophilic nature of the EOs and their relative insolubility in water. Traditionally, when assessing EO MICs, an agar dilution method is usually chosen over a broth dilution method for this very reason (6). However, improvements in methodologies for the determination of the antimicrobial efficacy of EOs in broth cultures with the use of emulsifiers have been made, making the assessment of EOs in aqueous solutions more effective (26). The use of a sintering system to force the vapors of EOs through water eliminates the need for other emulsifying agents such as ethanol and Tween 80, thus increasing its potential use within equipment predisposed to harboring Legionella spp., such as air conditioning units.
The use of the vapors of EOs rather than EOs per se allows for single components to be targeted and analyzed for their solubility and antimicrobial efficacy in water. Previous studies have shown that linalool, citral, and β-pinene are the main antimicrobial components of the citrus EO vapor as determined by a bioautography method (23).
Figures 1 to 4 demonstrate that the use of a sintering system, forcing the vapors components through the water, gives a greater reduction in Legionella spp. than does natural diffusion of the components. The consequent reduction in cell numbers increased from zero in the passive system to 4.5 log10 (Fig. 2) in the active system against L. pneumophila at a citrus EO vapor concentration of 15 g/liter air. However, the concentration of the vapor needed to reduce the Legionella counts in water had to increase 100-fold, from the 15-mg/liter air concentration observed to be active in a soil system and other surfaces to 15 g/liter air when being sintered into water. There is limited published research on the effect of EOs in water environments. The use of buffered yeast extract broth with Tween as an emulsifier has been shown to be a suitable medium to assess tea tree EO activity against Legionella spp. (26), and Chang et al. (27) assessed the use of cinnamon oil in hot-spring water at a range of pHs with ethanol being used as an emulsifying agent. Minimum bacterial concentrations (MBCs) against L. pneumophila ranged from 400 to 1,200 mg μl−1 with a contact time of 10 min and from 400 to 750 μg ml−1 with a contact time of 60 min.
The way in which the components are being passed through the water may also be crucial to the antimicrobial efficacy of the citrus EO vapor. Linalool, which has a higher solubility in water (1,589 mg/liter at 25°C) when sintered, is trapped within the water, thus continuing to have an antimicrobial effect on the Legionella cells after 2 h of exposure (Fig. 4), resulting in an accumulation effect; this in part may explain the 61% difference in the concentrations of linalool between the passive and active systems at 24 h. However, both citral and β-pinene have a lower solubility in water (590 mg/liter at 25°C and no solubility, respectively) and are not retained within the water when sintered, as shown by the amount of citral and β-pinene in the water at any given time as they pass through the water before they evaporate (Fig. 5). This suggests that the antimicrobial effect of citral and β-pinene may be based on a collision process between the compounds and the Legionella cells as they pass through the water. The increase in concentration of the antimicrobial compounds is noted from 2 h onwards (Fig. 3 and 4), corresponding to a reduction in the Legionella cells in water from the same time point (Fig. 3). The increase of the antimicrobial compounds after 2 h is also noted in the vapor release intensity of headspace with a 0.5-log cells ml−1 increase for linalool and β-pinene and 2-log cells ml−1 increase for citral (23).
Since there are no acceptable levels of Legionella spp. specified by the Health and Safety Executive (HSE) in the United Kingdom, the maintenance of equipment that is predisposed to harboring Legionella spp. is the responsibility of the manager of the site/equipment; therefore, a range of different disinfectants and physical treatments are used, including chlorine, monochloramine, UV, and heat, all of which have drawbacks, including the need for rinsing, expensive equipment, and high running costs and are often not effective against intercellular L. pneumophila. The use of citrus antimicrobial vapor that is sintered through an enclosed water system such as air conditioning units and cooling towers may be a natural alternative, which the FDA has deemed GRAS under the general provisions of essential oils, oleoresins (solvent free), and natural extractives (28). The components identified to be antimicrobial (linalool, citral, and β-pinene) are widely found in plants, including fruits and herbs, and are often used within the food and fragrance industries. Linalool has no recommended threshold limit value (TLV) or biological exposure index (BEI), and citral and β-pinene are listed by the International Fragrance Association (IFRA) as being commonly found in fragrances safe for use (29–31).
In conclusion, this citrus EO vapor may be a potential solution to controlling Legionella spp. from environmental sources such as soil and water. The novel delivery system using sinters to force hydrophobic compounds through water could allow for the use of EO-based products in new arenas.
Footnotes
Published ahead of print 25 July 2014
REFERENCES
- 1.European Centre for Disease Prevention and Control. 2013. Legionnaires' disease in Europe, 2011, surveillance report. http://ecdc.europa.eu/en/publications/publications/legionnaires-disease-in-europe-2011.pdf
- 2.Health Protection Agency. 2011. National increase in cases of legionnaires' disease. http://www.hpa.org.uk/cdr/archives/archive06/News/news3706.htm
- 3.Steinert M, Hentschel U, Hacker J. 2002. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 26:149–162. 10.1111/j.1574-6976.2002.tb00607.x [DOI] [PubMed] [Google Scholar]
- 4.Health Protection Agency. 2011. Legionnaires' disease. http://www.hpa.nhs.uk/Topics/InfectiousDiseases/InfectionsAZ/LegionnairesDisease
- 5.Robert Koch Institut. 2006. Legionellose. Robert Koch Institut, Berlin, Germany: http://www.rki.de/DE/Content/InfAZ/L/Legionellose/Legionellose.html [Google Scholar]
- 6.den Boer JW, Yzerman EPF, Jansen R, Bruin JP, Verhoef LPB, Neve G, Van der Zwaluw K. 2007. Legionnaires' disease and gardening. Clin. Microbiol. Infect. 13:88–91. 10.1111/j.1469-0691.2006.01562.x [DOI] [PubMed] [Google Scholar]
- 7.Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186:127–128. 10.1086/341087 [DOI] [PubMed] [Google Scholar]
- 8.Health Protection Agency. 2010. Compost and Legionella longbeachae. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1279889169500
- 9.Currie S, Beattie K, Knapp C, Lindsay D. 2014. Legionella spp. in UK composts—a potential public health issue? Clin. Microbiol. Infect. 20:O224–O229. 10.1111/1469-0691.12381 [DOI] [PubMed] [Google Scholar]
- 10.Casati S, Gioria-Martinoni A, Gaia V. 2009. Commercial potting soils as an alternative infection source of Legionella pneumophila and other Legionella species in Switzerland. Clin. Microbiol. Infect. 15:571–575. 10.1111/j.1469-0691.2009.02742.x [DOI] [PubMed] [Google Scholar]
- 11.Ali S, Phillips CA, Phillips PS, Bates M. 2010. Isolation and genetic diversity of Legionella pneumophila from material reclamation facilities. Int. J. Environ. Health Res. 20:367–377. 10.1080/09603123.2010.484859 [DOI] [PubMed] [Google Scholar]
- 12.Atlas RM. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283–293. 10.1046/j.1462-2920.1999.00046.x [DOI] [PubMed] [Google Scholar]
- 13.Health and Safety Executive. 2013. HS(G)70: the control of legionellosis including Legionnaires disease. http://www.hse.gov.uk/legionnaires/
- 14.Cooper IR, Hanlon GW. 2010. Resistance of Legionella pneumophila serotype 1 biofilms to chlorine-based disinfection. J. Hosp. Infect. 74:152–159. 10.1016/j.jhin.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Buse HY, Ashbolt NJ. 2011. Differential growth of Legionella pneumophila strains within a range of amoebae at various temperatures associated with in-premise plumbing. Lett. Appl. Microbiol. 53:217–224. 10.1111/j.1472-765X.2011.03094.x [DOI] [PubMed] [Google Scholar]
- 16.Katagiri N, Shobuike T, Chang B, Kukita A, Miyamoto H. 2012. The human apoptosis inhibitor NAIP induces pyroptosis in macrophages infected with Legionella pneumophila. Microbes Infect. 14:1123–1132. 10.1016/j.micinf.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 17.Subba MS, Soumithri TC, Rao RS. 2006. Antimicrobial action of citrus oils. J. Food Sci. 32:225–227. 10.1111/j.1365-2621.1967.tb01299.x [DOI] [Google Scholar]
- 18.Fisher K, Phillips C. 2009. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 106:1343–1349. 10.1111/j.1365-2672.2008.04102.x [DOI] [PubMed] [Google Scholar]
- 19.Fisher K, Phillips C. 2009. In vitro inhibition of vancomycin-susceptible and vancomycin-resistant Enterococcus faecium and E. faecalis in the presence of citrus essential oils. Br. J. Biomed. Sci. 66:180–185 [DOI] [PubMed] [Google Scholar]
- 20.Fisher K, Phillips CA. 2006. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 101:1232–1240. 10.1111/j.1365-2672.2006.03035.x [DOI] [PubMed] [Google Scholar]
- 21.Fisher K, Phillips C. 2008. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci. Technol. 19:156–164. 10.1016/j.tifs.2007.11.006 [DOI] [Google Scholar]
- 22.Sato K, Krist S, Buchbauer G. 2006. Antimicrobial effect of trans-cinnamaldehyde, (-)-perillaldehyde (-)-citronellal, citral, eugenol and carvacrol on airborne microbes using an airwasher. Biol. Pharm. Bull. 29:2292–2294. 10.1248/bpb.29.2292 [DOI] [PubMed] [Google Scholar]
- 23.Phillips Gkatzionis C, Laird K, Score J, Kant A, Fielder M. 2012. Identification and quantification of the antimicrobial components of a citrus essential oil vapour. Nat. Prod. Commun. 7:103–107 [PubMed] [Google Scholar]
- 24.Culture Collection of Algae and Protzoa. 2007. PAS (Page's Amoeba Saline Solution). http://www.ccap.ac.uk/media/documents/PAS_000.pdf
- 25.Fisher K, Phillips C, McWatt L. 2009. The use of an antimicrobial citrus EO vapour to reduce Enterococcus spp. on salad products. Int. J. Food Sci. Technol. 44:1748–1754. 10.1111/j.1365-2621.2009.01992.x [DOI] [Google Scholar]
- 26.Mondello F, Girolamo A, Scaturro M, Ricci ML. 2009. Determination of Legionella pneumophila susceptibility to Melaleuca alternifolia Cheel (tea tree) oil by an improved broth micro-dilution method under vapour controlled conditions. J. Microbiol. Methods 77:243–248. 10.1016/j.mimet.2009.02.012 [DOI] [PubMed] [Google Scholar]
- 27.Chang C, Chang W, Chang S. 2008. Influence of pH on bioactivity of cinnamon oil against Legionella pneumophila and its disinfection efficacy in hot springs. Water Res. 42:5022–5030. 10.1016/j.watres.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 28.Food and Drug Administration. 2013. CFR—Code of Federal Regulations Title 21; substances generally recognized as safe, essential oils, oleoresins (solvent-free), and natural extractives (including distillates). http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=582.20
- 29.International Fragrance Association. 2007. Fragrance ingredients. http://www.ifraorg.org/en/ingredients#.U6Lv6rFwbcs
- 30.Technical Resources International. 1997. Summary of data for chemical selection; linalool, CAS No. 78-70-6. http://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/linalool_508.pdf
- 31.Tisserand R, Young R. 2013. Essential oil safety: a guide for health care professionals, 2nd ed. Elsevier, Amsterdam, Netherlands [Google Scholar]