Abstract
Sorbic acid and acetic acid are among the weak organic acid preservatives most commonly used to improve the microbiological stability of foods. They have similar pKa values, but sorbic acid is a far more potent preservative. Weak organic acids are most effective at low pH. Under these circumstances, they are assumed to diffuse across the membrane as neutral undissociated acids. We show here that the level of initial intracellular acidification depends on the concentration of undissociated acid and less on the nature of the acid. Recovery of the internal pH depends on the presence of an energy source, but acidification of the cytosol causes a decrease in glucose flux. Furthermore, sorbic acid is a more potent uncoupler of the membrane potential than acetic acid. Together these effects may also slow the rate of ATP synthesis significantly and may thus (partially) explain sorbic acid's effectiveness.
INTRODUCTION
Various small weak organic acids (WOAs) have been used as food preservatives for a very long time. These weak acids slow the growth of various spoilage bacteria, yeasts, and molds without having overt undesired effects on taste or being toxic to the consumer. The undissociated states of the WOA preservatives are more effective in slowing growth than the dissociated form, although the latter may have some level of toxicity. As such, WOAs are most effective when applied at low pH values below their pKa value (1, 2). Under these conditions, the neutral acid is assumed to diffuse across the plasma membrane and dissociate in the cytosol, which generally has a higher pH. In this way, the proton gradient over the membrane is depleted and the anion may accumulate to potentially toxic levels inside the cell. This is known as the classical weak-acid preservative theory (3). Commonly used WOA preservatives include sorbic acid and acetic acid, which have a similar pKa value of 4.76 but dissimilar octanol/water partition coefficients (log Kow values) of 1.33 and −0.17, respectively (International Programme on Chemical Safety [http://www.inchem.org/]). This means that at a particular pH and the same total concentration, the concentrations of both undissociated acids are the same, but sorbic acid has a higher affinity for a hydrophobic (membrane) environment. Sorbic acid is clearly the more potent preservative of the two, but the exact reason why is still not fully clear (4, 5).
It is important to distinguish the different modes of action that WOAs may have on cell physiology. The classical weak-acid preservative theory (3) assumes entry only of the undissociated acid, dissociation in the cytosol, and cytosolic acidification. While this is sometimes described as uncoupling, we use the latter phrase for compounds that shuttle protons across the membrane and are thus protonophoric uncouplers. Others have also pointed to the potential toxicity of accumulated anions (1, 6, 7). If we assume that only the undissociated acid diffuses across the membrane, it follows from the Henderson-Hasselbalch equation (8) that the pH difference over the membrane (ΔpH) is equal to log([A−]in/[A−]out), where [A−]in is the concentration of the anion inside the cell and [A−]out is the concentration of the anion outside the cell, and thus, the internal concentration of the anion may become very high when a high ΔpH remains present. Also, specific binding of sorbate to cysteine (9) has been shown and is proposed to be an explanation for the higher toxicity of sorbic acid.
Several mechanisms of resistance against WOAs have been reported for yeasts, like Saccharomyces cerevisiae, Zygosaccharomyces bailii, and Zygosaccharomyces rouxii. These organisms induce the expression of H+ ATPases to regulate their cytosolic pH. S. cerevisiae uses a dedicated ATP binding cassette (ABC) transporter (Pdr12) to prevent accumulation of the anion (5). Also, S. cerevisiae plasma membrane components likely play an important role in the modulation of the influx of lipophilic weak organic acids (10, 11). Furthermore, Z. rouxii and Aspergillus niger have been shown to degrade sorbic acid to 1,3-pentadiene (12, 13).
The responses and potential resistance mechanisms against weak acids have not been as well described for bacteria as they have been for yeasts (14, 15). The level of growth reduction was modeled some 30 years ago (1), and the effect of sorbic acid on the membrane potential in Escherichia coli membrane vesicles has been described (16). More recently, Ter Beek et al. (2) performed a microarray study on Bacillus subtilis cells exposed to sorbic acid. Their results showed a broad transcriptomic response resembling a pattern typical of that for cells responding to nutrient limitation. The authors observed an upregulation of genes encoding potential efflux pumps as well as genes involved in remodeling of the plasma membrane.
Bacillus subtilis has been the model Gram-positive organism for decades, because it is generally recognized as safe (GRAS), it is genetically accessible, and it has a fully sequenced genome. It also forms heat-resistant spores and as such is a recognized spoilage organism (17). Spores of several related bacterial species are of great concern to the food industry because they are highly resistant to most preservation techniques and, once germinated, can cause food spoilage through the growth of vegetative cells that may produce toxins (15).
To comprehensively elucidate the physiological effects of WOAs on B. subtilis, we measured the effects of sorbic acid and acetic acid on the chemical (ΔpH) and electrical potential (Δψ) components of the proton motive force (PMF). With a depleted PMF, we speculated that the cell might alter its energy needs in terms of glucose consumption and the availability of its terminal electron acceptor (i.e., O2), which were hence determined. We assessed the rate and extent of the change in the internal pH (pHin) caused by these two weak acids using B. subtilis cells that either were directly exposed to both WOAs or had been preexposed to sorbic acid and reexposed to WOAs. The latter experiment was done because we inferred from the previously collected microarray data that cells elicit an adaptive response to these compounds (2).
MATERIALS AND METHODS
General growth conditions and strains.
For general purposes, B. subtilis PB2 strains were grown in lysogeny broth (LB). For weak-acid stress experiments and fluorescence measurements, B. subtilis strains were grown in defined liquid medium (M3G) (18) set at pH 5.5, 6.4 (buffered with 80 mM MES [morpholineethanesulfonic acid]), as well as 7.0 and 7.4 (buffered with 80 mM MOPS [morpholinepropanesulfonic acid]). The medium contained 5 mM glucose, 10 mM glutamate, and 10 mM NH4Cl as the carbon and nitrogen sources. All cultures were grown at 37°C under continuous agitation at 200 rpm. The wild-type strain used (B. subtilis PB2) was obtained from C. W. Price and A. Ter Beek (2). The strain expressing the improved version of the pH-sensitive fluorescent protein IpHluorin (B. subtilis PB2 PptsG-IpHluorin) was constructed as described previously (19). When required, 50 μg/ml spectinomycin was added to the medium.
Calibration of pHluorin and internal pH measurements.
The internal pH was measured as described previously (19). All strains were grown in M3G at pH 6.4. For rapid-exposure WOA stress experiments, potassium sorbate (K-S) and potassium acetate (K-Ac) were used at 250 mM and dissolved in M3G medium without glucose. Of these solutions, 2 to 10 μl was injected into the cell suspensions at 310 μl/s using the injector of the FluoStar Optima microplate reader (BMG Labtech, Germany). As a control experiment, the same concentrations of either KCl or NaCl were injected into the microtiter wells. For 50% and 80% growth inhibition experiments, 3 and 11 mM K-S, respectively, or 25 and 80 mM K-Ac, respectively, were used.
Cell counts and protein measurements.
In order to compare results per cell from the different experiments, cell counts were performed with a CASY counter (Roche, Germany) equipped with a 60-μm tube. The number of cells counted was 4 × 104 to 10 × 104 cells per ml. Protein concentrations were determined using a bicinchoninic acid assay kit (Thermo Scientific) according to the manufacturer's instructions. Finally, the optical density at 600 nm (OD600) of the cultures was measured with a FluoStar Optima microplate reader.
Membrane potential measurements.
B. subtilis PB2 and B. subtilis PptsG-IpHluorin were grown as described above in M3G, pH 6.4, to an OD600 of 0.4. Cells were harvested by centrifugation and resuspended in 1/10 volume M3G without glucose. To inhibit growth and protein synthesis between experiments, 10 μg/ml chloramphenicol was added. Cells were stored at 37°C. Δψ was measured using a tetraphenylphosphonium ion (TPP+) electrode (World Precision Instruments, Inc.) filled with 1 mM TPP+. All measurements were performed in a warmed (37°C), 2-ml measuring cell containing a TPP+ electrode, a reference electrode, and an oxygen sensor (see below). The cell suspension was stirred magnetically and aerated by a continuous flow of compressed air, so that at least 120 μM oxygen was present when the transmembrane electrical potential (Δψ) was measured. One milliliter of cell suspension was added to 1 ml medium with glucose; subsequently, weak organic acids and a TPP+ calibration series (1, 1, 2, 4, and 8 μl of 1 mM TPP+) were added. The addition of weak organic acid did affect the offset but not the slope of the calibration response. The stress conditions tested were exposure to 3 and 11 mM K-S and 25 and 80 mM K-Ac. These conditions were compared to nonexposure. The membrane potential was calculated as described previously (20–23) using deenergized cells (incubated for 10 min at 70°C) as a reference for nonspecific binding of the probe.
The electrical potential was calculated using the following equation (20, 23):
| (1) |
where Δψ is the transmembrane electrical potential (in mV; which is equal to ψin − ψout), Z (a conversion factor) is equal to 2.303 · (RT/nF) (where R is the universal gas constant [8.31 J K−1 mol−1], T is the absolute temperature [in K], n is the charge of the translocated ion, and F is Faraday's constant [96.6 J mV−1 mol−1]), C0 is the probe concentration in the medium without the addition of cells, Ce is the extracellular probe concentration, fcm is the ratio of the fractional cytoplasmic membrane and intracellular volume, Kcm is the cytoplasmic membrane partition coefficient, and x is the fractional internal volume.
The factor fcmKcm was determined by a probe binding assay (20) to be 14. In this simplified approach, one assumes that the amount of TPP+ bound to extracytoplasmic components of the cell (like parts of the cell wall) is equal to the amount of probe that binds to intracellular components, like nucleic acids. At the end of the experiment, 1.5-ml samples were collected for protein quantification as described above. Three biological replicates were measured for each condition.
Oxygen consumption measurements.
Oxygen consumption rates were measured simultaneously with measurement of the Δψ in the same measuring cell using a Neofox fiber optic oxygen sensor (Ocean Optics Inc.). Oxygen concentrations were measured every second from the maximally aerated to the fully oxygen depleted state. The slope of the straight part of the plot (at least 15 s) was used to derive the oxygen consumption rate. At the end of the experiment, 1.5-ml samples were collected for protein quantification as described above. Rates were normalized to the protein content.
Glucose consumption and metabolite measurements.
B. subtilis PB2 and B. subtilis PptsG-IpHluorin were grown as described above in M3G, pH 6.4, to an OD600 of 0.8. The cultures were split and exposed to different stress conditions (3 and 11 mM K-S or 25 and 80 mM K-Ac), and 10 mM glucose was added to each culture. A high OD600 was required to observe a significant decrease in glucose concentrations within the time frame of the experiment.
Samples, taken every 30 min, were snap-frozen in liquid nitrogen. The protein concentration of each sample was measured as described above. Samples were further processed for high-pressure liquid chromatography (HPLC) analysis; a 1-ml sample was mixed with 100 μl 35% perchloric acid, and subsequently, 55 μl 7 M KOH was added. Filtered supernatants were analyzed for the levels of glucose consumption by the cells as well as the presence of fermentation end products. Glucose, succinate, lactate, acetate, 2,3-butanediol, and ethanol contents were determined by HPLC (LKB) with a Rezex organic acid analysis column (Phenomenex) at 45°C with 7.2 mM H2SO4 as the eluent. An RI 1530 refractive index detector (Jasco) was used, and AZUR chromatography software was used for data integration. All measurements were performed with two biological replicates. From the obtained metabolite fluxes, a carbon balance was calculated with the assumption that similar molar amounts of CO2 were produced per amount of O2 consumed, that similar molar amounts of CO2 were produced per mole of acetate produced, and that 2 moles of CO2 was produced per mole of 2,3-butanediol. Oxygen consumption was corrected for directly related acetate and 2,3-butanediol production as follows:
| (2) |
where Cb is the carbon balance (in percent) and q indicates the flux of the indicated compound.
RESULTS
Internal pH during growth under WOA stress conditions remains low.
Weak organic acids have been shown to lower the internal pH of microorganisms (24, 25). Thus, we measured pHin during growth with a number of WOA stresses. To reduce the growth rate by approximately 50%, 3 mM K-S or 25 mM K-Ac was used. To reduce the growth rate by 85%, 10 and 80 mM, respectively, were used.
Growth and pHin were monitored for 6 h (Fig. 1A and B, respectively). The internal pH of nonstressed cultures dropped from 7.5 to 7.2 during this time. The internal pH of sorbic acid-stressed cultures continued to drop from time zero until the end of the experiment. With 80 mM K-Ac, this was not seen, and pHin remained stable at about 7.3. These results show that there is considerable acidification of the cell during growth and that there is no recovery of the pHin during extended exposure to WOAs, not even when growth resumes.
FIG 1.
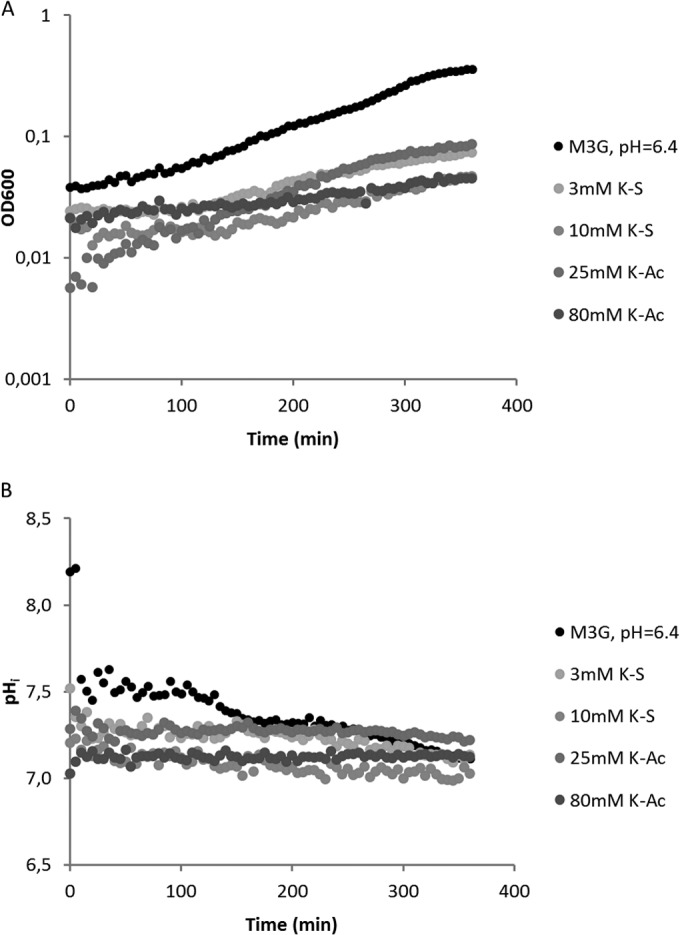
Growth curve (A) and internal pH (B) of B. subtilis PB2 pDG-IpHluorin monitored for 6 h under various stress conditions. The medium pH was 6.4. In M3G, the growth rate is the highest, but a continuous decrease in pHin is seen. Sorbic acid stress causes the growth rate to be reduced, as well as a continuous decrease in internal pH. Acetic acid stress has a similar effect on the growth rate, but pHin remains constant.
Weak organic acids cause a rapid drop in internal pH.
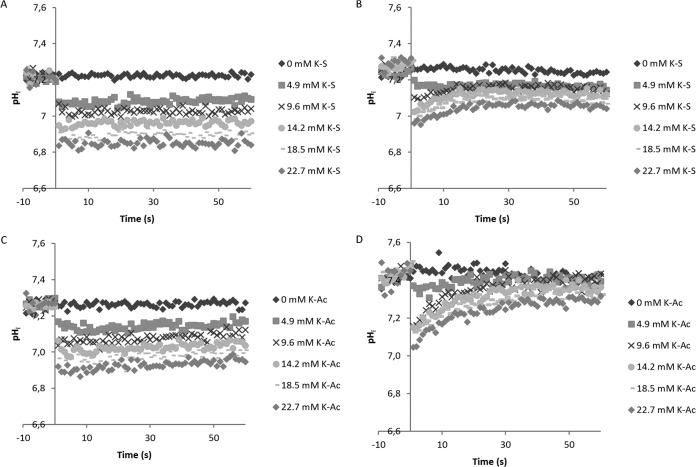
Long-term (6-h) exposure to WOAs, as described above, showed no long-term recovery of the pHin of the cells. The largest drop in pHin upon weak-acid exposure occurred within the first minutes after addition. To investigate the rate of influx of weak organic acid preservatives and their dependence on the metabolic activity of the cell, we investigated the short-term effects of WOA injections into the medium.
Before acid injection, the pHin of starved wild-type B. subtilis cells was 7.30 ± 0.05. Injection of either KCl or NaCl at concentrations identical to those of the weak acids had no effect on pHin within 1 min after injection (not shown). Upon glucose injection, the pHin rapidly increased to 7.5 within 1 min (not shown). When WOAs were injected, the pH dropped to its lowest point within 1 to 4 s. In starved cells, this pH remained stable, but in the presence of glucose, pHin recovered quickly to a new equilibrium (Fig. 2A to D). The acidification of starved cells was fitted with a first-order kinetic equation,
| (3) |
where pHin(t) is the pHin at time t, pHin(0) is the offset (pHin at time zero, before injection), ki is the rate constant (s−1), Bi is the amplitude factor (ΔpH/mM) indicating the intracellular buffering capacity, and [HA] is the concentration of undissociated weak acid (in mM). Using this equation, values for Bi and ki were determined for sorbic acid and acetic acid. The value for ΔpHin or the amplitude of acidification has a linear relation with the concentration of undissociated acid in the medium (Fig. 3). These data (with starved cells) show that sorbic acid causes a change in pHin similar to that caused by acetic acid. The rate of acidification is high and similar for both acids, 1.29 ± 0.09 s−1 for sorbic acid and 1.27 ± 0.33 s−1 for acetic acid. However, with a measuring frequency of once per second, this rate is most likely set by our detection system and the actual rate is likely higher. Cells preexposed to sorbic acid had Bi and ki values similar to those for nonstressed cells for either WOA, showing no sign of adaptation at this level (see Table S1 in the supplemental material; our unpublished data).
FIG 2.
Acidification and recovery upon weak organic acid addition to B. subtilis PB2 PptsG-IpHluorin. Sorbic acid (A and B) or acetic acid (C and D) was injected at time zero. The medium pH was 6.4. The internal pH drops to its lowest point within seconds (A and C) and recovers fast when glucose is available (B and D). The pH recovers to higher values with acetic acid stress. Data from typical examples are shown.
FIG 3.
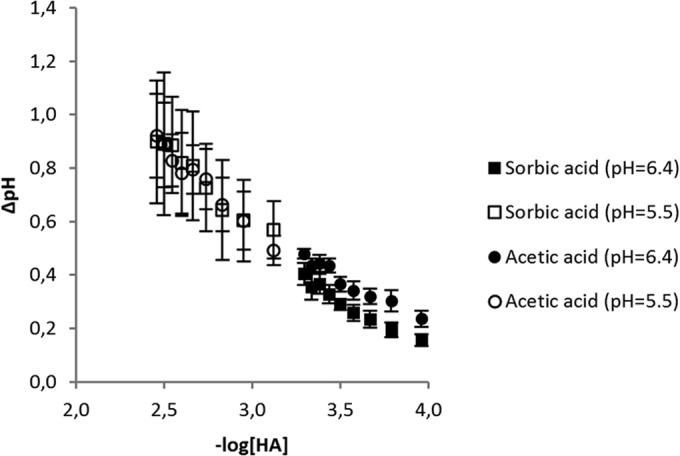
Amplitude of acidification by addition of sorbic or acetic acid. A linear trend can be observed between ΔpH and −log[HA] ([HA] is in M). Data from experiments at extracellular pHs of 5.5 and 6.4 are combined. Error bars indicate standard deviations.
With glucose added to the cultures, recovery of the pHin started immediately after the injection of weak acid. This curve, too, was fitted, but with an additional factor describing recovery:
| (4) |
where Br is the amplitude of recovery and kr is the rate constant of the pH recovery. Recovery kinetics did not seem to change between unexposed and preexposed cultures. There was, however, a clear difference between sorbic acid and acetic acid. The pHin of acetic acid-stressed cultures recovered to a higher value than that of sorbic acid-stressed cultures (Fig. 4; see Table S1 in the supplemental material). The amplitude of recovery also appeared to have a linear relation with the acid concentration. The acquired constants allowed predictions for both acidification and subsequent recovery (see Fig. S1 in the supplemental material).
FIG 4.
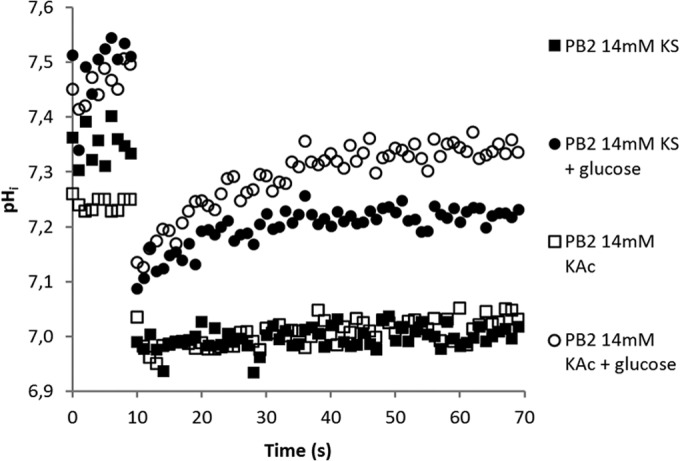
Acidification and recovery upon weak organic acid addition to B. subtilis PB2 PptsG-IpHluorin. Acetic or sorbic acid was injected at 10 s. The medium pH was 6.4. The internal pH drops to its lowest point within seconds and recovers fast when glucose is available. With glucose available, the pHin at time zero is also higher. The pH recovers to higher values with acetic acid stress. Data from a typical example are shown.
Sorbic acid affects both ΔpH and Δψ.
Proton translocation by the electron transport chain through the cell membrane results in both a gradient in the chemical potential of protons (i.e., ΔpH) and an electrical potential (Δψ). This electrochemical proton gradient exerts an inward-directed proton motive force (PMF) (26, 27). The PMF can drive protons back into the cell via the F1Fo ATPase for ATP synthesis and is also required for various membrane transport processes and for flagellar rotation. The PMF is defined as follows (e.g., see reference 28):
| (5) |
where Δp is the PMF (in mV), ΔμH+ is the transmembrane electrochemical proton potential (in J/mol), F is Faraday's constant [96.6 J mV−1 mol−1], Δψ is the transmembrane electrical potential difference (in mV; ψin − ψout), Z (a conversion factor) is equal to 2.303 · (RT/nF) (where R is the universal gas constant [8.31 J K−1 mol−1], T is the absolute temperature [in K], and n is the charge of the translocated ion), and ΔpH is the transmembrane pH gradient (pHin − pHout, where pHin is the pH inside the cell and pHout is the pH outside the cell).
In our experiments, which were performed at 37°C, Z was equal to 61.4 mV. Our measurements with TPP+ show that under nonstressed conditions with an extracellular of pH 6.4, Δψ is equal to 103 ± 9 mV and pHin is equal to 7.78 ± 0.05. This thus gives a Z · ΔpH value of 84 ± 3 mV and a total PMF of 188 ± 9 mV (Fig. 5). This value for Δψ is similar to values reported earlier (22, 29) and the pHin value is slightly higher (29). Sorbic acid has a severe effect on Δψ, reducing it by 64% even at 3 mM K-S. Acetic acid, however, does not deplete Δψ as strongly. With 25 mM K-Ac, the Δψ is 17% lower, and with 80 mM K-Ac only a 45% reduction is seen. It appears that sorbic acid acts as an uncoupler more than acetic acid does.
FIG 5.
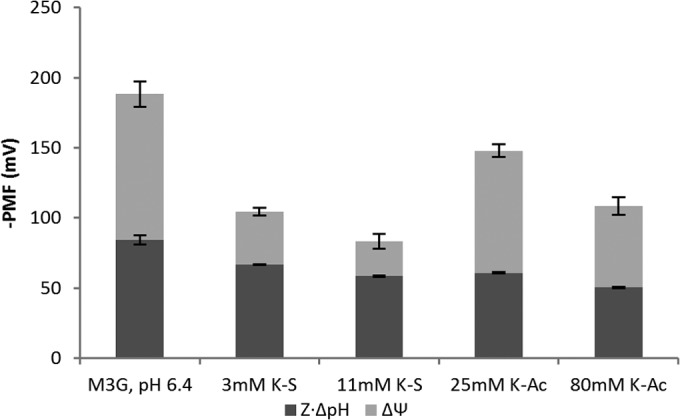
Differential effects of sorbic acid and acetic acid on the proton motive force. Sorbic acid stress affects both ΔpH and Δψ. Acetic acid affects ΔpH but has little effect on Δψ. Data are from cultures exposed to weak acids for approximately 5 min. Results are based on 3 biological replicates. Error bars indicate standard deviations.
Together, Δψ − (Z · ΔpH) comprises the proton motive force. In total, WOA stress causes a dissipation from 188 ± 9 mV in nonstressed cells to 83 ± 5 mV with 11 mM K-S and 108 ± 7 mV with 80 mM K-Ac. This, too, shows that sorbic acid has a stronger effect on the electrochemical gradient for protons than acetic acid does at concentrations that lead to similar levels of growth inhibition (see Table S2 in the supplemental material).
Oxygen consumption is reduced upon addition of high WOA concentrations.
Because of the depleted PMF, the cell may experience energy depletion stress, since the F1Fo ATPase depends on the proton gradient for its activity. It is possible that the cell tries to compensate for the decrease in PMF by increasing the flux through the electron transfer chain and increasing proton pumping activity. This also requires a terminal electron acceptor (e.g., O2). We therefore measured the oxygen levels during our PMF experiments and made sure that at least 120 μM O2 was measured when Δψ was measured. Nonstressed cells consumed oxygen at a rate of 7.4 mmol s−1 (mg protein)−1. Sorbic acid stress reduced the rate of oxygen consumption to 3.9 and 1.5 mmol s−1 (mg protein)−1 with 3 and 11 mM K-S, respectively. K-Ac at 25 mM had a nonsignificant effect on the oxygen consumption rate, but 80 mM reduced it to 1.1 mmol s−1 (mg protein)−1 (Fig. 6). These effects took place immediately after addition of the acid. These observations show that the cell consumes less O2 when faced with weak organic acid stress.
FIG 6.
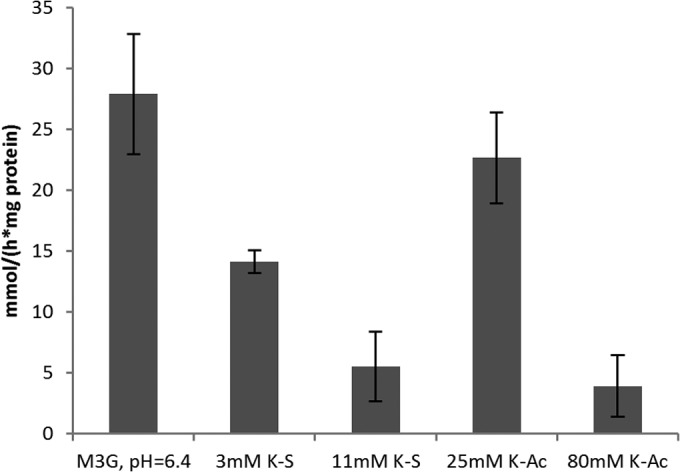
Weak organic acid stress reduces respiration. Respiration was monitored for 1 min or the amount of time that it took to reduce O2 levels to 0 μmol. Results are based on 3 biological replicates. Error bars indicate standard deviations.
Glucose metabolism is affected by weak organic acid stress.
Because weak organic acids partially dissipate the PMF, we speculated that B. subtilis might alter its metabolism.
Glucose consumption was the highest for nonstressed cells, reaching 7.8 mmol s−1 (mg protein)−1. Addition of weak acids lowered glucose consumption rates, with 80 mM K-Ac resulting in 1.45 mmol s−1 (mg protein)−1 (Fig. 7; see also Table S3 in the supplemental material).
FIG 7.
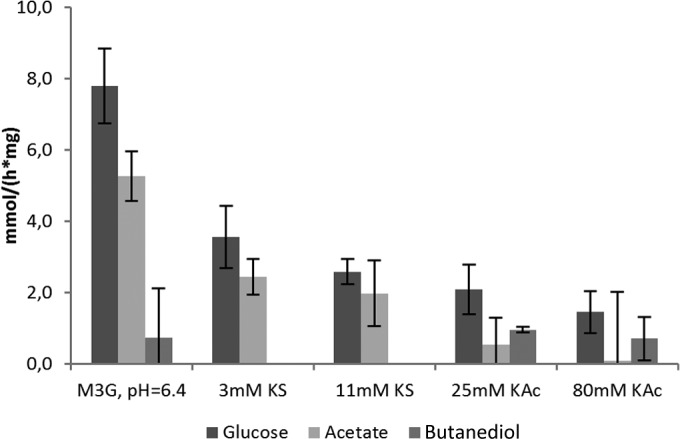
Increased weak organic acid stress lowers the glucose consumption rate. The rate of glucose consumption was compared to the rate of acetate and 2,3-butanediol production. Results are based on two biological replicates. Error bars indicate standard deviations.
Acetate was produced under all conditions tested and was already present in small amounts at the start of the experiment. Nonstressed cultures produced acetate at the highest rate, together with the K-S-exposed cells. Addition of K-S lowered the rate of acetate synthesis. Acetic acid-stressed cells had the lowest rate of acetate production. These cultures redirected fermentation routes by switching to 2,3-butanediol fermentation. Some 2,3-butanediol was also found in the final sample of nonstressed cells but not with sorbic acid-stressed cells.
Carbon flux.
When confronted with a lack of an electron acceptor, B. subtilis employs a mixed acid fermentation and has been shown to produce lactate, acetate, acetoin, ethanol, 2,3-butanediol, and succinate as fermentation products when grown on glucose (30), but it also produces a lot of acetate as a result of overflow metabolism (31). In our experiments, only acetate and 2,3-butanediol were found.
Although batch cultures are not ideally suited for accurate carbon flux determinations, our data do seem to be in line with reported values for wild-type B. subtilis (32). With the assumptions made previously regarding O2 utilization, our data (see Table S3 in the supplemental material) for WOA-stressed cells show reasonably close C balances for all stresses, apart from 25 mM K-Ac, with which cells appeared to use about 3.5 times as much O2 as the amount estimated on the basis of carbon fluxes based solely on glycolysis and fermentation.
The calculated qATP, based on qacetate and q2,3-butanediol [qATP = (2 · qacetate) + (2 · q2,3-butanediol)] through glycolysis and fermentation, displayed a similar WOA concentration-dependent behavior as qglucose. That is, increasing concentrations of weak acid caused a reduction in fluxes. This behavior seems to be analogous to the effect on pHin, which depends more on the concentration than the nature of the acid (as shown above). Indeed, there appears to be a linear relation between pHin and qglucose (Fig. 8), as well as between pHin and the calculated amounts of ATP generated via production of qacetate and q2,3-butanediol (our unpublished calculations). When looking at correlations between Δψ and qglucose, such a correlation does not exist for either WOA. Only for sorbic acid might a linear correlation be observed, but more experiments will be needed to confirm this relationship. The relation between qO2 and the decrease in pHin seems to be rather constant, while a decrease in qO2 seems to correlate with a decrease in Δψ. However, more experiments will be needed for an accurate description.
FIG 8.
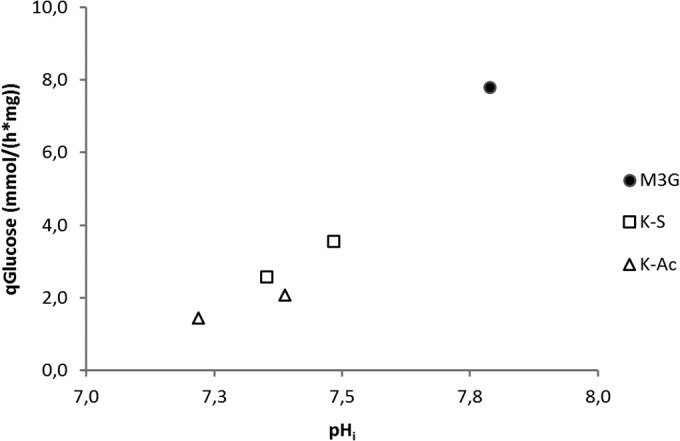
Glucose flux versus internal pH due to weak organic acid stress (no stress, 3 and 11 mM K-S, 25 and 80 mM K-Ac). A decrease in pHin due to WOA stress is accompanied by a decrease in glucose flux.
DISCUSSION
To reduce the growth rate to a similar level, more acetic acid than sorbic acid at an identical external pH value is required. Both sorbic acid and acetic acid lower pHin and reduce the growth rate of B. subtilis. When cells are growing with WOA stress, the internal pH is lower than that of cells not exposed to WOAs during the early exponential phase. Even though in the first minute after WOA exposure some recovery takes place, the internal pH does not seem to recover fully from this acidification, even after 6 h of growth. Given that the pKa for both weak acids is almost equal, this makes sorbic acid a more effective preservative.
Homeostasis of the pHin is crucial for cell physiology. Optimal proton extrusion clearly depends on the presence of an energy source (i.e., glucose) in the medium, because recovery of the pHin does not reach the original values. It appears that a new ΔpH equilibrium is established within a few minutes, i.e., before most transcriptomic changes take effect. The level of cytosolic acidification after injection of weak acid into the medium is very similar for both acids without glucose present. This suggests that in starved cells, an equilibrium for acid dissociation is established. Since the only factor measured in this regard is pH or [H+], the anion accumulation level is unknown. It can, however, be estimated if it is assumed that ΔpH is equal to log([A−]in/[A−]out). The cell has to establish a new equilibrium with a ΔpH low enough to reduce anion concentrations but high enough to allow metabolism. As is clear from the data, the ability to restore pHin differs between sorbic acid-treated cells and acetic acid-treated cells.
For sorbic acid, the inability to recover pHin during acid stress reflects the ability of sorbate to act as a classic uncoupler, shuttling protons over the membrane, whereas the less lipophilic acetate is believed to do this to a lesser extent. The latter is corroborated by the greater effect that sorbic acid has on the membrane potential, while acetic acid carries only bulk volume protons across the membrane until a steady state is reached, allowing Δψ to remain relatively unaffected. Even though some authors have pointed to the similar effects of the uncoupler 2,4-dinitrophenol and sorbic acid on growth and metabolism (9, 33, 34), to our knowledge no quantitative measurements of the effect that sorbic acid has on Δψ have been reported thus far. While relatively little research on the effects that WOA preservatives have on the membrane potential of microorganisms has been published (6, 16, 29, 35, 36), the depletion of Δψ may have a plethora of effects. The consequences may range from reduced transporter activity (37) to destabilization of the bacterial cytoskeleton (38), although we have not seen evidence for the latter effect (our unpublished results). Anion efflux from the cytosol would be driven by the remaining Δψ. This effect was clearly shown for the permeant ion picrate, which mainly acts as an uncoupler in everted cell membranes when a high Δψ is established (39). It has also been observed that lipophilic compounds such as ethanol can stimulate the leakage of protons over the membrane of S. cerevisiae (40, 41). However, recent results show that this is not the case for sorbic acid or acetic acid (5).
Other commonly used preservatives, such as lactic acid, benzoic acid, and propionic acid, are also expected to diffuse and dissociate, in agreement with the Henderson-Hasselbalch equation (9). Future experiments should establish to what extent these and other preservatives have uncoupling properties and what the quantitative effect of Δψ depletion on the growth rate is. It is likely that their ability to cross the membrane barrier (log Kow) into the aqueous phase will play a role, as seems to be the case with sorbic acid and acetic acid (42, 43).
With a low pH outside the cell (pHout), the balance of the PMF is shifted toward ΔpH, so the effectiveness of weak organic acids at low pH is 2-fold: the concentration of undissociated acid is higher, and the WOAs act on the dominant component of the PMF. In the search for food preservatives that are also active near neutral pH, the food industry should preferably consider uncoupler-like molecules similar to or better than sorbate. At an even higher pH, permeant cations might be useful to deplete the membrane potential. In food with low glucose concentrations, the recovery of pHin might be less; hence, less growth is observed with lower preservative concentrations.
We observed a decrease in the O2 consumption rate with WOA-stressed cells. This suggests that there are fewer electrons to feed the electron transport chain and that metabolism may be affected by acidification of the cytosol. Because weak organic acids partially dissipate the PMF, the amount of ATP that the cells can generate through the F1Fo ATPase is considerably smaller. So, to produce a sufficient amount of ATP to restore pH homeostasis and proliferate, the cell might alter its glucose metabolism to generate ATP through substrate-level phosphorylation. Because increased energy needs have been observed in WOA-stressed yeast (44, 45) and a starvation-like response was reported for sorbic acid-stressed B. subtilis (2), we measured the glucose consumption rate as well as the production of fermentation products.
Glucose metabolism slows in the presence of WOAs, depending on the concentration of undissociated acid, as has also been described for E. coli and Saccharomyces cerevisiae (24, 46, 47). This results in a linear relation between pHin and qglucose. The observation that acetic acid continues to be produced when cells are exposed to sorbic acid [2 mmol h−1 (mg protein)−1 with 11 mM K-S] indicates that acetic acid may be an extra stress factor for cells growing under these conditions and may be part of the explanation for the observed continued decrease in pHin during sorbic acid stress. Addition of K-S lowered the rate of acetate synthesis, possibly due to growth reduction or a decrease in glycolytic activity.
Glycolytic flux can be controlled, among other factors, by pH (48), affecting phosphofructokinase (Pfk) activity (49) or, specifically in the case of Bacillus species, phosphoglycerate mutase activity (50), which is also very sensitive to pH changes. Glucose is generally assumed to be the preferred carbon and energy source for B. subtilis, which is taken up via the phosphoenolpyruvate-sugar phosphotransferase system (PTS). It is subsequently converted into fructose 1,6-bisphosphate (FBP) by Pfk. FBP is required for the phosphorylation of CcpA, one of the main repressors in carbon catabolite repression (CCR) in B. subtilis. In this way, FBP availability causes CCR (51). Also, CCR inhibits expression of citric acid cycle enzymes when glucose concentrations are high. When a decrease of the internal pH reduces the activity of Pfk, the concentration of FBP is reduced, CcpA (52) activity is reduced and CCR is released. This may explain how in earlier studies (2) a starvation-like response could be observed in B. subtilis upon sorbic acid stress.
With high concentrations of acetic acid, it is likely that fermentation routes toward acetate are diverted to 2,3-butanediol. Synthesis of 2,3-butanediol depends on bdhA, a gene that encodes acetoin reductase (30) and that has SigB-controlled expression. Acetic acid stress can trigger stressosome activity and, indeed, results in the upregulation of acetoin reductase (53, 54). Therefore, when acetate is present as a preservative, putative organoleptic properties of 2,3-butanediol should be considered when B. subtilis or related species may be present. Weak organic acid stress also causes an upregulation of citric acid cycle enzymes (2, 53). This route may also consume added acetic acid, thereby providing an extra carbon source and eliminating this weak organic acid. The high oxygen consumption rate that we observed in the presence of 25 mM and 80 mM K-Ac may be explained by the utilization of acetate. While growth rates are reduced by 80% with both 11 mM K-S and 80 mM K-Ac, the glucose consumption rate with 80 mM K-Ac is almost half of that with 11 mM K-S. Future experiments should be conducted in a turbidostat setup and include CO2 measurements to allow a direct measurement of the carbon balance under these conditions. Therefore, knowledge of the pHin can be used to predict glycolysis, and this knowledge might be useful for modeling purposes in food fermentation (48, 55).
In summary, oxidative phosphorylation is an important source of ATP for aerobic, nonstressed B. subtilis cells. The O2 consumption rates follow a trend similar to that for qglucose, qacetate, and q2,3-butanediol, apart from the 25 mM K-Ac stress. The level of WOA stress may leave too little PMF to allow F1Fo ATPase to produce ATP. Sorbic acid has an effect similar to that of acetic acid on pHin, but it is more effective in depleting Δψ. This may partially explain the observation that sorbic acid is a more potent preservative than acetic acid. In the quest for preservatives active at nearly neutral pH, industry should best focus on uncoupler-like molecules that act similarly to or better than sorbic acid.
Supplementary Material
ACKNOWLEDGMENTS
Jos Arents is thanked for his assistance with the membrane potential measurements. Gertien Smits is acknowledged for critically reading initial versions of the manuscript.
Footnotes
Published ahead of print 18 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01391-14.
REFERENCES
- 1.Eklund T. 1983. The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J. Appl. Bacteriol. 54:383–389. 10.1111/j.1365-2672.1983.tb02632.x [DOI] [PubMed] [Google Scholar]
- 2.Ter Beek A, Keijser BJF, Boorsma A, Zakrzewska A, Orij R, Smits GJ, Brul S. 2008. Transcriptome analysis of sorbic acid-stressed Bacillus subtilis reveals a nutrient limitation response and indicates plasma membrane remodeling. J. Bacteriol. 190:1751–1761. 10.1128/JB.01516-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratford M, Anslow PA. 1998. Evidence that sorbic acid does not inhibit yeast as a classic “weak acid preservative.” Lett. Appl. Microbiol. 27:203–206. 10.1046/j.1472-765X.1998.00424.x [DOI] [PubMed] [Google Scholar]
- 4.Stratford M, Plumridge A, Nebe-von-Caron G, Archer DB. 2009. Inhibition of spoilage mould conidia by acetic acid and sorbic acid involves different modes of action, requiring modification of the classical weak-acid theory. Int. J. Food Microbiol. 136:37–43. 10.1016/j.ijfoodmicro.2009.09.025 [DOI] [PubMed] [Google Scholar]
- 5.Ullah A, Orij R, Brul S, Smits GJ. 2012. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 78:8377–8387. 10.1128/AEM.02126-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell JB. 1992. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J. Appl. Microbiol. 73:363–370 [Google Scholar]
- 7.Carpenter CE, Broadbent JR. 2008. External concentration of organic acid anions and pH: key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. J. Food Sci. 74:R12–R15. 10.1111/j.1750-3841.2008.00994.x [DOI] [PubMed] [Google Scholar]
- 8.Henderson L. 1908. Concerning the relationship between the strength of acids and their capacity to preserve neutrality. Am. J. Physiol. 21:173–179 [Google Scholar]
- 9.York GK, Vaughn RH. 1964. Mechanisms in the inhibition of microorganisms by sorbic acid. J. Bacteriol. 88:411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollapour M, Fong D, Balakrishnan K, Harris N, Thompson S, Schüller C, Kuchler K, Piper PW. 2004. Screening the yeast deletant mutant collection for hypersensitivity and hyper-resistance to sorbate, a weak organic acid food preservative. Yeast 21:927–946. 10.1002/yea.1141 [DOI] [PubMed] [Google Scholar]
- 11.Golden DA, Beuchat LR, Hitchcock HL. 1994. Changes in fatty acid composition of various lipid components of Zygosaccharomyces rouxii as influenced by solutes, potassium sorbate and incubation temperature. Int. J. Food Microbiol. 21:293–303. 10.1016/0168-1605(94)90059-0 [DOI] [PubMed] [Google Scholar]
- 12.Casas E, de Ancos B, Valderrama MJ, Cano P, Peinado JM. 2004. Pentadiene production from potassium sorbate by osmotolerant yeasts. Int. J. Food Microbiol. 94:93–96. 10.1016/j.ijfoodmicro.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Plumridge A, Stratford M, Lowe KC, Archer DB. 2008. The weak-acid preservative sorbic acid is decarboxylated and detoxified by a phenylacrylic acid decarboxylase, PadA1, in the spoilage mold Aspergillus niger. Appl. Environ. Microbiol. 74:550–552. 10.1128/AEM.02105-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mols M, Abee T. 2011. Bacillus cereus responses to acid stress. Environ. Microbiol. 13:2835–2843. 10.1111/j.1462-2920.2011.02490.x [DOI] [PubMed] [Google Scholar]
- 15.Brul S, Coote P. 1999. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 50:1–17 [DOI] [PubMed] [Google Scholar]
- 16.Eklund T. 1985. The effect of sorbic acid and esters of p-hydroxybenzoic acid on the protonmotive force in Escherichia coli membrane vesicles. J. Gen. Microbiol. 131:73–76 [DOI] [PubMed] [Google Scholar]
- 17.Oomes SJCM, van Zuijlen ACM, Hehenkamp JO, Witsenboer H, van der Vossen JMBM, Brul S. 2007. The characterisation of Bacillus spores occurring in the manufacturing of (low acid) canned products. Int. J. Food Microbiol. 120:85–94. 10.1016/j.ijfoodmicro.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 18.Keijser BJF, Ter Beek A, Rauwerda H, Schuren F, Montijn R, van der Spek H, Brul S. 2007. Analysis of temporal gene expression during Bacillus subtilis spore germination and outgrowth. J. Bacteriol. 189:3624–3634. 10.1128/JB.01736-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Beilen JWA, Brul S. 2013. Compartment-specific pH monitoring in Bacillus subtilis using fluorescent sensor proteins; a tool to analyse the antibacterial effect of weak organic acids. Front. Microbiol. 4:157. 10.3389/fmicb.2013.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lolkema J, Hellingwerf K, Konings W. 1982. The effect of “probe binding”on the quantitative determination of the proton-motive force in bacteria. Biochim. Biophys. Acta 150:1183–1191 [Google Scholar]
- 21.Lolkema JS, Abbing A, Hellingwerf KJ, Konings WN. 1983. The transmembrane electrical potential in Rhodopseudomonas sphaeroides determined from the distribution of tetraphenylphosphonium after correction for its binding to cell components. Eur. J. Biochem. 292:287–292 [DOI] [PubMed] [Google Scholar]
- 22.Zaritsky A, Kihara M, Macnab RM. 1981. Measurement of membrane potential in Bacillus subtilis: a comparison of lipophilic cations, rubidium ion, and a cyanine dye as probes. J. Membr. Biol. 63:215–231. 10.1007/BF01870983 [DOI] [PubMed] [Google Scholar]
- 23.De Vrij W, Driessen AJ, Hellingwerf KJ, Konings WN. 1986. Measurements of the proton motive force generated by cytochrome c oxidase from Bacillus subtilis in proteoliposomes and membrane vesicles. Eur. J. Biochem. 156:431–440. 10.1111/j.1432-1033.1986.tb09600.x [DOI] [PubMed] [Google Scholar]
- 24.Salmond CV, Kroll RG, Booth IR. 1984. The effect of food preservatives on pH homeostasis in Escherichia coli. J. Gen. Microbiol. 130:2845–2850 [DOI] [PubMed] [Google Scholar]
- 25.Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA. 2009. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 55:1–79, 317. 10.1016/S0065-2911(09)05501-5 [DOI] [PubMed] [Google Scholar]
- 26.Mitchell P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148. 10.1038/191144a0 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell P. 1977. Vectorial chemiosmotic processes. Annu. Rev. Biochem. 46:996–1005. 10.1146/annurev.bi.46.070177.005024 [DOI] [PubMed] [Google Scholar]
- 28.Bulthuis BA, Koningstein GM, Stouthamer AH, van Verseveld HW. 1993. The relation of proton motive force, adenylate energy charge and phosphorylation potential to the specific growth rate and efficiency of energy transduction in Bacillus licheniformis under aerobic growth conditions. Antonie Van Leeuwenhoek 63:1–16. 10.1007/BF00871725 [DOI] [PubMed] [Google Scholar]
- 29.Shioi JI, Matsuura S, Imae Y. 1980. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J. Bacteriol. 144:891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz Ramos H, Hoffmann T, Marino M, Nedjari H, Presecan-Siedel E, Dreesen O, Glaser P, Jahn D. 2000. Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J. Bacteriol. 182:3072–3080. 10.1128/JB.182.11.3072-3080.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frädrich C, March A, Fiege K, Hartmann A, Jahn D, Härtig E. 2012. The transcription factor AlsR binds and regulates the promoter of the alsSD operon responsible for acetoin formation in Bacillus subtilis. J. Bacteriol. 194:1100–1112. 10.1128/JB.06425-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tännler S, Decasper S, Sauer U. 2008. Maintenance metabolism and carbon fluxes in Bacillus species. Microb. Cell Fact. 7:19. 10.1186/1475-2859-7-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickett MJ, Clifton CE. 1943. The effect of selective poisons on the utilization of glucose and intermediate compounds by microorganisms. J. Cell. Comp. Physiol. 22:147–165. 10.1002/jcp.1030220206 [DOI] [Google Scholar]
- 34.Stratford M, Anslow PA. 1996. Comparison of the inhibitory action on Saccharomyces cerevisiae of weak-acid preservatives, uncouplers, and medium-chain fatty acids. FEMS Microbiol. Lett. 142:53–58. 10.1111/j.1574-6968.1996.tb08407.x [DOI] [PubMed] [Google Scholar]
- 35.Kinderlerer JL, Hatton PV. 1990. Fungal metabolites of sorbic acid. Food Addit. Contam. 7:657–669. 10.1080/02652039009373931 [DOI] [PubMed] [Google Scholar]
- 36.Russell JB, Diez-Gonzalez F. 1998. The effects of fermentation acids on bacterial growth. Adv. Microb. Physiol. 39:205–234 [DOI] [PubMed] [Google Scholar]
- 37.Culham DE, Romantsov T, Wood JM. 2008. Roles of K+, H+, H2O, and ΔΨ in solute transport mediated by major facilitator superfamily members ProP and LacY. Biochemistry 47:8176–8185. 10.1021/bi800794z [DOI] [PubMed] [Google Scholar]
- 38.Strahl H, Hamoen LW. 2010. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. U. S. A. 107:12281–12286. 10.1073/pnas.1005485107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michels M, Bakker EP. 1981. The mechanism of uncoupling by picrate in Escherichia coli K-12 membrane systems. Eur. J. Biochem. 116:513–519. 10.1111/j.1432-1033.1981.tb05366.x [DOI] [PubMed] [Google Scholar]
- 40.Sikkema J, de Bont JA, Poolman B. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leão C, Van Uden N. 1984. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 774:43–48. 10.1016/0005-2736(84)90272-4 [DOI] [PubMed] [Google Scholar]
- 42.Spycher S, Smejtek P, Netzeva TI, Escher BI. 2008. Toward a class-independent quantitative structure-activity relationship model for uncouplers of oxidative phosphorylation. Chem. Res. Toxicol. 21:911–927. 10.1021/tx700391f [DOI] [PubMed] [Google Scholar]
- 43.Chu S, Hawes JW, Lorigan GA. 2009. Solid-state NMR spectroscopic studies on the interaction of sorbic acid with phospholipid membranes at different pH levels. Magn. Reson. Chem. 47:651–657. 10.1002/mrc.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holyoak CD, Stratford M, McMullin Z, Cole MB, Crimmins K, Brown AJ, Coote PJ. 1996. Activity of the plasma membrane H+-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl. Environ. Microbiol. 62:3158–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pampulha ME, Loureiro-Dias MC. 2000. Energetics of the effect of acetic acid on growth of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 184:69–72. 10.1111/j.1574-6968.2000.tb08992.x [DOI] [PubMed] [Google Scholar]
- 46.Ugurbil K, Rottenberg H, Glynn P, Shulman RG. 1978. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc. Natl. Acad. Sci. U. S. A. 75:2244–2248. 10.1073/pnas.75.5.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs HA, Wiggins D, Stubbs M, Sols A, Bedoya F. 1983. Studies on the mechanism of the antifungal action of benzoate. Biochem. J. 214:657–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vojinović V, von Stockar U. 2009. Influence of uncertainties in pH, pMg, activity coefficients, metabolite concentrations, and other factors on the analysis of the thermodynamic feasibility of metabolic pathways. Biotechnol. Bioeng. 103:780–795. 10.1002/bit.22309 [DOI] [PubMed] [Google Scholar]
- 49.Wu TF, Davis EJ. 1981. Regulation of glycolytic flux in an energetically controlled cell-free system: the effects of adenine nucleotide ratios, inorganic phosphate, pH, and citrate. Arch. Biochem. Biophys. 209:85–99. 10.1016/0003-9861(81)90260-5 [DOI] [PubMed] [Google Scholar]
- 50.Kuhn N, Setlow B, Setlow P. 1995. Cooperative manganese(II) activation of 3-phosphoglycerate mutase of Bacillus megaterium: a biological pH-sensing mechanism in bacterial spore formation and germination. Arch. Biochem. Biophys. 320:35–42. 10.1006/abbi.1995.1339 [DOI] [PubMed] [Google Scholar]
- 51.Fujita Y. 2009. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci. Biotechnol. Biochem. 73:245–259. 10.1271/bbb.80479 [DOI] [PubMed] [Google Scholar]
- 52.Sonenshein AL. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 5:917–927. 10.1038/nrmicro1772 [DOI] [PubMed] [Google Scholar]
- 53.Ter Beek AS. 2009. Weak organic acid stress in Bacillus subtilis. Ph.D. thesis University of Amsterdam, Amsterdam, The Netherlands [Google Scholar]
- 54.Ter Beek A, Wijman JGE, Zakrzewska A, Orij R, Smits GJ, Brul S. 2014. Comparative physiological and transcriptional analysis of weak organic acid stress in Bacillus subtilis. Food Microbiol. 10.1016/j.fm.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 55.Ter Beek A, Hornstra LM, Pandey R, Kallemeijn WW, Smelt JP, Manders EM, Brul S. 2011. Models of the behaviour of (thermally stressed) microbial spores in foods: tools to study mechanisms of damage and repair. Food Microbiol. 28:678–684. 10.1016/j.fm.2010.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.