Abstract
The interferon (IFN)-inducible viperin protein restricts a broad range of viruses. However, whether viperin plays a role during herpes simplex virus 1 (HSV-1) infection is poorly understood. In the present study, it was shown for the first time that wild-type (WT) HSV-1 infection couldn't induce viperin production, and ectopically expressed viperin inhibited the replication of UL41-null HSV-1 but not WT viruses. The underlying molecular mechanism is that UL41 counteracts viperin's antiviral activity by reducing its mRNA accumulation.
TEXT
Viperin is a highly conserved, 361-amino-acid protein. It was first identified as a gamma interferon (IFN-γ)-inducible protein which is directly induced by human cytomegalovirus (HCMV), and its constitutive expression is low (1). The viperin gene (also known as cig5 or RASD2) can also be categorized as an antiviral interferon-stimulated gene (ISG) which limits the replication of many DNA and RNA viruses (1–14). However, whether viperin plays a role during herpes simplex virus 1 (HSV-1) infection is unknown.
To investigate whether HSV-1 could induce the expression of viperin, HEK293T cells were infected with wild-type (WT) HSV-1 at different multiplicities of infection (MOI) or with Sendai virus (SeV) (15). Infection with SeV induced a significant amount of viperin; however, infection with a low MOI (0.2) of HSV-1 induced only a trace amount of viperin, and infection with a moderate MOI (2) abrogated the expression of viperin (Fig. 1A).
FIG 1.
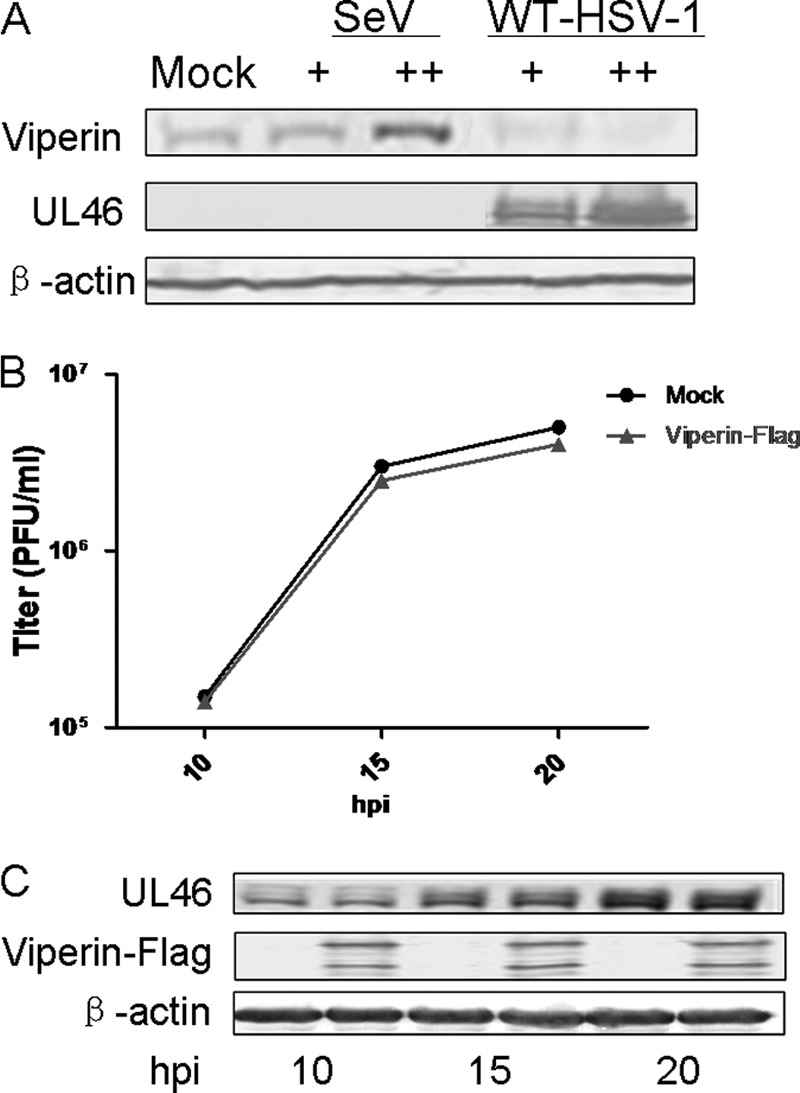
Ectopically expressed viperin did not inhibit the replication of WT HSV-1. HEK293T cells were infected with WT HSV-1 at an MOI of 0.2 or 2.0 or with SeV. (A) Twenty hours after infection, cells were harvested and subjected to WB analysis with antibodies against UL46, β-actin, or viperin. HEK293T cells were transfected with vector or with viperin-Flag plasmid. Twenty-four hours after transfection, the cells were infected with HSV-1 at an MOI of 0.2, and then cells were harvested at the indicated time points after infection and subjected to viral plaque assay (B) or WB analysis with antibodies against UL46, Flag, or β-actin (C). The results are from triplicate samples with standard deviations.
To further explore whether viperin could inhibit the replication of WT HSV-1, HEK293T cells with ectopic expression of viperin-Flag were infected with HSV-1 at an MOI of 0.2. Then cells were harvested at the time points indicated in the figures, and viral plaque assay was performed to determine viral replication (16). As a result, ectopically expressed viperin did not affect the replication of WT HSV-1 (Fig. 1B). The data from Western blot (WB) analysis also showed that viperin did not affect viral protein expression (Fig. 1C). These results demonstrated that ectopic expression of viperin failed to inhibit the replication of WT HSV-1.
The aforementioned data led us to hypothesize that at least one of the HSV-1 proteins could counteract the expression of viperin. As a member of the ISGs, viperin was effectively induced by SeV (Fig. 1) (15). With a high-throughput screen assay of all 84 proteins carried by HSV-1, dual-luciferase reporter gene assays were performed in HEK293T cells cotransfected with viperin-luciferase reporter plasmid and individual HSV-1 protein expression plasmid for 20 h and infected with SeV (17). As a result, ectopically expressed UL41 abrogated the expression of viperin; however, other HSV-1 proteins did not (data not shown). UL41 has been reported to degrade both viral and cellular mRNAs (18–26). Recently, mRNA of tetherin has been reported to be degraded by UL41 (27). Meanwhile, ICP0, an E3 ubiquitin ligase, promotes degradation of many cellular antiviral proteins, such as IRF3, IRF7, IFI16, and ATRX (28–32). To confirm whether ICP0 was involved in degradation of viperin at the protein level, HEK293T cells were cotransfected with UL41 or ICP0 and viperin-Flag plasmids, and the cells were harvested and subjected to WB analysis. UL41 abolished viperin-Flag expression in a dose-dependent manner, but ICP0 did not (Fig. 2).
FIG 2.
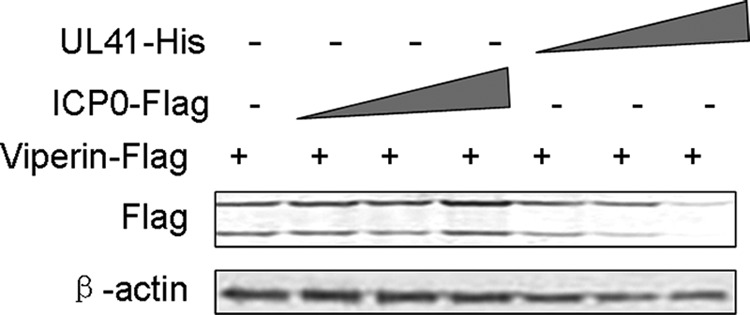
UL41, but not ICP0, decreased the expression of viperin. HEK293T cells were cotransfected with viperin-Flag and UL41-His or ICP0-Flag plasmids. Twenty-four hours after transfection, cells were harvested and subjected to WB analysis with antibodies against Flag or β-actin. The data represent results from one of the triplicate experiments.
It was reported that viperin was a target of human RNase endoribonuclease (33) and that UL41 was an endoribonuclease with a substrate specificity similar to that of RNase A (26). Therefore, it is very likely that UL41 abolishes viperin expression via its RNase activity to degrade viperin mRNA. To confirm this hypothesis, HEK293T cells were infected with WT HSV-1, R2621 (UL41-null) HSV-1, or SeV. Then cells were harvested at 8 h postinfection and subjected to reverse transcription (RT)-PCR to analyze the viperin mRNA (Fig. 3A). For normalization, 18S rRNA, which could not be degraded by UL41, was used as an internal control (27). WT HSV-1, but not R2621 HSV-1, significantly reduced the accumulation of viperin mRNA. Similarly, HEK293T cells were infected with WT or R2621 HSV-1 or SeV; 20 h after infection, the cells were harvested and subjected to WB analysis (Fig. 3B). The data showed that, compared with R2621 HSV-1, WT HSV-1 markedly abrogated viperin expression. Collectively, the data demonstrated that UL41 dampens the antiviral activity of viperin by reducing its mRNA accumulation.
FIG 3.
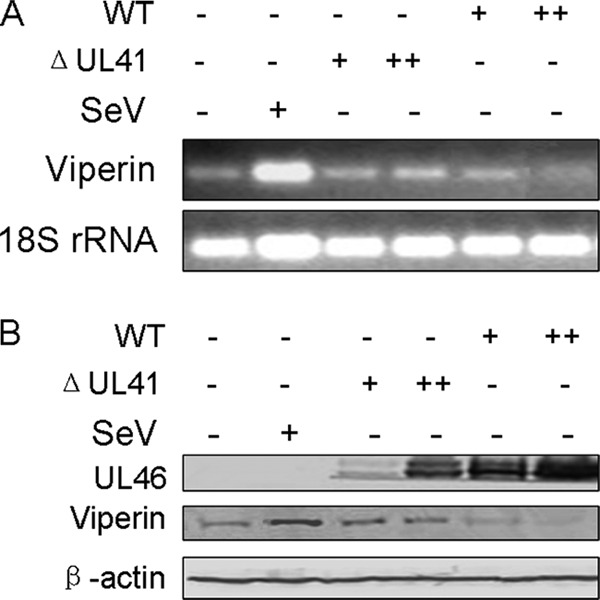
HSV-1 UL41 protein reduced the accumulation of viperin mRNA. (A) HEK293T cells were infected with WT or R2621 HSV-1 at an MOI of 0.5 (+) or 5.0 (++) or with SeV. Eight hours postinfection, cells were harvested and then subjected to RT-PCR (Roche). (B) HEK293T cells were infected as described for panel A with an MOI of 0.2 (+) or 2.0 (++). Twenty hours postinfection, cells were harvested and subjected to WB analysis with antibodies against viperin and β-actin.
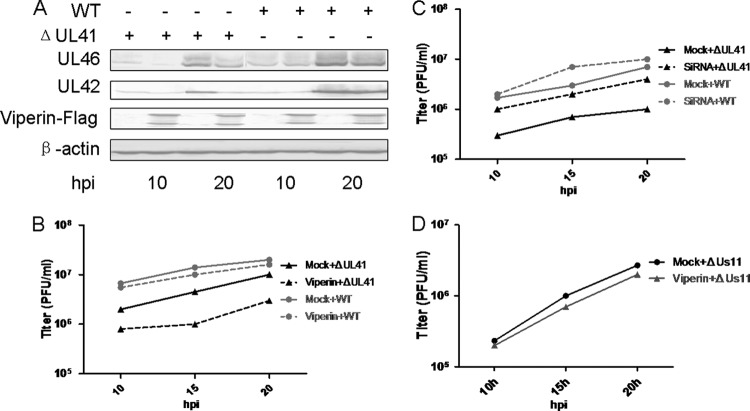
The above-described data led us to hypothesize that viperin could inhibit replication of UL41-null virus. To test this assumption, HEK293T cells with ectopic expression of viperin-Flag were infected with WT or R2621 HSV-1 and harvested at the indicated time points for WB analysis and viral plaque assay (16). As a result, ectopically expressed viperin significantly reduced the expression of UL46 and UL42 from R2621 but not WT HSV-1 (Fig. 4A). The viral plaque assay showed that ectopic expression of viperin significantly inhibited the replication of R2621 but not WT HSV-1 (Fig. 4B).
FIG 4.
Viperin counteracted the replication of UL41-null HSV-1. HEK293T cells were transfected with vector or with viperin-Flag plasmid. Twenty-four hours after transfection, the cells were infected with the indicated viruses at an MOI of 0.2, and then cells were harvested at the indicated time points postinfection and subjected to WB analysis with antibodies against UL46, UL42, Flag, and β-actin (A) or viral plaque assay on Vero cells (B). (C) HEK293T cells were transfected with control or with siRNA specific to viperin. Twenty-four hours after transfection, the cells were infected and subjected to viral plaque assay as described for panel A. (D) HEK293T cells were transfected and infected with ΔUs11 HSV-1 at an MOI of 0.2 and subjected to viral plaque assay as described for panel A. The data represent results from one of the triplicate experiments.
The fact that ectopically expressed viperin inhibits the replication of the R2621 mutant does not mean that the lower constitutive expression of viperin would play an important role in inhibition of viral replication. To address this issue, HEK293T cells were transfected with a viperin-specific small interfering RNA (siRNA) prior to infection, and then the replications of WT and R2621 HSV-1 were compared with that in cells that had been transfected with a nontargeting siRNA (9). As presented, knockdown of viperin did not affect the replication of WT HSV-1 but did promote the replication of the R2621 HSV-1 (Fig. 4C).
To rule out the involvement of other late proteins in viperin regulation other than UL41, Us11 was chosen, as ΔUs11 HSV-1 had been constructed in our lab (34). Us11 is an RNA binding tegument protein that prevents the activation of protein kinase R (PKR) and oligoadenylate synthetases (OAS) and impairs type I IFN responses by antagonizing retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5) (34–36). HEK293T cells were transfected with viperin-Flag or vector plasmids prior to being infected with ΔUs11 HSV-1 at an MOI of 0.2, and the titers were tested. As shown, ectopic expression of viperin did not affect the replication of ΔUs11 HSV-1 (Fig. 4D). Taken together, these results indicated that UL41, but not other viral proteins, promotes HSV-1 replication by ablation of the antiviral activity of viperin.
Viperin restricts the replication of many RNA viruses, including HIV, hepatitis C virus (HCV), SeV, and influenza virus, and also DNA virus HCMV (1, 2, 9, 11, 14, 15, 37). Viperin effectively affects the replication of influenza virus by inhibiting its release from the plasma membrane of infected cells (10) and inhibits HCV replication by localizing and interacting with HCV nonstructural protein 5A at the lipid-droplet interface (6). Surprisingly, we found that HSV-1 infection abolished viperin expression and ectopic expression of viperin could not restrict the replication of HSV-1, and HSV-1 UL41 protein was demonstrated for the first time to dampen the antiviral activity of viperin.
To establish effective infection, HSV-1 has evolved multiple mechanisms to evade host innate immunity (34, 38–44). UL41 is an mRNA-specific RNase that triggers rapid degradation of host mRNAs to facilitate the sequential expression of viral proteins (19, 20, 22, 26, 45–48). Our data demonstrated that ectopic expression of UL41 or WT HSV-1 infection reduced the accumulation of viperin mRNA, suggesting that UL41 degraded viperin mRNA to promote the replication of HSV-1.
In brief, we have demonstrated for the first time that HSV-1 UL41 dampens expression of viperin to abrogate the antiviral activity of viperin by reducing its mRNA accumulation. These findings will lead us to better understand the mechanisms employed by HSV-1 to evade host antiviral activity and develop novel effective therapeutics to modulate HSV-1 pathogenesis.
ACKNOWLEDGMENTS
We thank Hui Zheng for viperin antibody, Yi-Ling Lin for viperin plasmid, Katherine A. Fitzgerald for viperin reporter plasmids, and Bernard Roizman for R2621 virus.
This work was supported by grants from the National Natural Science Foundation of China (81371795 and 81171584), Innovative Research Team in Soochow University (PCSIRT, IRT 1075), and Jiangsu Provincial Innovative Research Team.
Footnotes
Published ahead of print 30 July 2014
REFERENCES
- 1. Chin KC, Cresswell P. 2001. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 98:15125–15130. 10.1073/pnas.011593298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boudinot P, Riffault S, Salhi S, Carrat C, Sedlik C, Mahmoudi N, Charley B, Benmansour A. 2000. Vesicular stomatitis virus and pseudorabies virus induce a vig1/cig5 homologue in mouse dendritic cells via different pathways. J. Gen. Virol. 81:2675–2682 [DOI] [PubMed] [Google Scholar]
- 3. Carlton-Smith C, Elliott RM. 2012. Viperin, MTAP44, and protein kinase R contribute to the interferon-induced inhibition of Bunyamwera orthobunyavirus replication. J. Virol. 86:11548–11557. 10.1128/JVI.01773-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fredericksen BL, Smith M, Katze MG, Shi PY, Gale M., Jr 2004. The host response to West Nile virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 78:7737–7747. 10.1128/JVI.78.14.7737-7747.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helbig KJ, Carr JM, Calvert JK, Wati S, Clarke JN, Eyre NS, Narayana SK, Fiches GN, McCartney EM, Beard MR. 2013. Viperin is induced following dengue virus type-2 (DENV-2) infection and has anti-viral actions requiring the C-terminal end of viperin. PLoS Negl. Trop. Dis. 7:e2178. 10.1371/journal.pntd.0002178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helbig KJ, Eyre NS, Yip E, Narayana S, Li K, Fiches G, McCartney EM, Jangra RK, Lemon SM, Beard MR. 2011. The antiviral protein viperin inhibits hepatitis C virus replication via interaction with nonstructural protein 5A. Hepatology 54:1506–1517. 10.1002/hep.24542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hinson ER, Joshi NS, Chen JH, Rahner C, Jung YW, Wang XY, Kaech SM, Cresswell P. 2010. Viperin is highly induced in neutrophils and macrophages during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 184:5723–5731. 10.4049/jimmunol.0903752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGillivary G, Jordan ZB, Peeples ME, Bakaletz LO. 2013. Replication of respiratory syncytial virus is inhibited by the host defense molecule viperin. J. Innate Immun. 5:60–71. 10.1159/000342473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nasr N, Maddocks S, Turville SG, Harman AN, Woolger N, Helbig KJ, Wilkinson J, Bye CR, Wright TK, Rambukwelle D, Donaghy H, Beard MR, Cunningham AL. 2012. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood 120:778–788. 10.1182/blood-2012-01-407395 [DOI] [PubMed] [Google Scholar]
- 10. Zhang YG, Burke CW, Ryman KD, Klimstra WB. 2007. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 81:11246–11255. 10.1128/JVI.01282-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sen Tan K, Olfat F, Phoon MC, Hsu JP, Howe JLC, Seet JE, Chin KC, Chow VTK. 2012. In vivo and in vitro studies on the antiviral activities of viperin against influenza H1N1 virus infection. J. Gen. Virol. 93:1269–1277. 10.1099/vir.0.040824-0 [DOI] [PubMed] [Google Scholar]
- 12. Szretter KJ, Brien JD, Thackray LB, Virgin HW, Cresswell P, Diamond MS. 2011. The interferon-inducible gene viperin restricts West Nile virus pathogenesis. J. Virol. 85:11557–11566. 10.1128/JVI.05519-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teng TS, Foo SS, Simamarta D, Lum FM, Teo TH, Lulla A, Yeo NK, Koh EG, Chow A, Leo YS, Merits A, Chin KC, Ng LF. 2012. Viperin restricts Chikungunya virus replication and pathology. J. Clin. Invest. 122:4447–4460. 10.1172/JCI63120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang XY, Hinson ER, Cresswell P. 2007. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2:96–105. 10.1016/j.chom.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 15. DeFilippis VR, Sali T, Alvarado D, White L, Bresnahan W, Fruh KJ. 2010. Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B. J. Virol. 84:8913–8925. 10.1128/JVI.00169-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Zhao L, Wang S, Xing J, Zheng C. 2012. Identification of a novel NLS of herpes simplex virus type 1 (HSV-1) VP19C and its nuclear localization is required for efficient production of HSV-1. J. Gen. Virol. 93:1869–1875. 10.1099/vir.0.042697-0 [DOI] [PubMed] [Google Scholar]
- 17. Severa M, Coccia EM, Fitzgerald KA. 2006. Toll-like receptor-dependent and -independent viperin gene expression and counter-regulation by PRDI-binding factor-1/BLIMP1. J. Biol. Chem. 281:26188–26195. 10.1074/jbc.M604516200 [DOI] [PubMed] [Google Scholar]
- 18. Chee AV, Roizman B. 2004. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J. Virol. 78:4185–4196. 10.1128/JVI.78.8.4185-4196.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Everly DN, Feng PH, Mian IS, Read GS. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560–8571. 10.1128/JVI.76.17.8560-8571.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng P, Everly DN, Jr, Read GS. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272–10280. 10.1128/JVI.75.21.10272-10280.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Read GS. 2013. Virus-encoded endonucleases: expected and novel functions. Wiley Interdiscip. Rev. RNA 4:693–708. 10.1002/wrna.1188 [DOI] [PubMed] [Google Scholar]
- 22. Read GS, Frenkel N. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Read GS, Karr BM, Knight K. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schek N, Bachenheimer SL. 1985. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 55:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smiley JR. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063–1068. 10.1128/JVI.78.3.1063-1068.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taddeo B, Roizman B. 2006. The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A. J. Virol. 80:9341–9345. 10.1128/JVI.01008-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zenner HL, Mauricio R, Banting G, Crump CM. 2013. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J. Virol. 87:13115–13123. 10.1128/JVI.02167-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boutell C, Sadis S, Everett RD. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841–850. 10.1128/JVI.76.2.841-850.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hagglund R, Van Sant C, Lopez P, Roizman B. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. U. S. A. 99:631–636. 10.1073/pnas.022531599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson KE, Chikoti L, Chandran B. 2013. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J. Virol. 87:5005–5018. 10.1128/JVI.00082-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin R, Noyce RS, Collins SE, Everett RD, Mossman KL. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675–1684. 10.1128/JVI.78.4.1675-1684.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanni E, Gatherer D, Tong LL, Everett RD, Boutell C. 2012. Functional characterization of residues required for the herpes simplex virus 1 E3 ubiquitin ligase ICP0 to interact with the cellular E2 ubiquitin-conjugating enzyme UBE2D1 (UbcH5a). J. Virol. 86:6323–6333. 10.1128/JVI.07210-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mattijssen S, Hinson ER, Onnekink C, Hermanns P, Zabel B, Cresswell P, Pruijn GJM. 2011. Viperin mRNA is a novel target for the human RNase MRP/RNase P endoribonuclease. Cell. Mol. Life Sci. 68:2469–2480. 10.1007/s00018-010-0568-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xing J, Wang S, Lin R, Mossman KL, Zheng C. 2012. Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-I and MDA-5. J. Virol. 86:3528–3540. 10.1128/JVI.06713-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poppers J, Mulvey M, Khoo D, Mohr I. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74:11215–11221. 10.1128/JVI.74.23.11215-11221.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanchez R, Mohr I. 2007. Inhibition of cellular 2′-5′ oligoadenylate synthetase by the herpes simplex virus type 1 Us11 protein. J. Virol. 81:3455–3464. 10.1128/JVI.02520-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang YG, Burke CW, Ryman KD, Klimstra WB. 2007. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 81:11246–11255. 10.1128/JVI.01282-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang K, Ni L, Wang S, Zheng C. 7 May 2014. Herpes simplex virus type 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-kappaB activation. J. Virol. 10.1128/JVI.03394-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S, Wang K, Li J, Zheng C. 2013. Herpes simplex virus 1 ubiquitin-specific protease UL36 inhibits beta interferon production by deubiquitinating TRAF3. J. Virol. 87:11851–11860. 10.1128/JVI.01211-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J. Virol. 87:12814–12827. 10.1128/JVI.02355-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-kappaB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J. Virol. 87:9788–9801. 10.1128/JVI.01440-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang J, Wang K, Wang S, Zheng C. 2013. Herpes simplex virus 1 E3 ubiquitin ligase ICP0 protein inhibits tumor necrosis factor alpha-induced NF-kappaB activation by interacting with p65/RelA and p50/NF-kappaB1. J. Virol. 87:12935–12948. 10.1128/JVI.01952-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang J, Wang S, Wang K, Zheng C. 2013. Herpes simplex virus 1 DNA polymerase processivity factor UL42 inhibits TNF-alpha-induced NF-kappaB activation by interacting with p65/RelA and p50/NF-kappaB1. Med. Microbiol. Immunol. 202:313–325. 10.1007/s00430-013-0295-0 [DOI] [PubMed] [Google Scholar]
- 44. Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF3-mediated innate immune response through degradation of activated IRF3. J. Virol. 85:11079–11089. 10.1128/JVI.05098-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doepker RC, Hsu WL, Saffran HA, Smiley JR. 2004. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 78:4684–4699. 10.1128/JVI.78.9.4684-4699.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Esclatine A, Taddeo B, Roizman B. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. U. S. A. 101:18165–18170. 10.1073/pnas.0408272102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feng P, Everly DN, Jr, Read GS. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 79:9651–9664. 10.1128/JVI.79.15.9651-9664.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sarma N, Agarwal D, Shiflett LA, Read GS. 2008. Small interfering RNAs that deplete the cellular translation factor eIF4H impede mRNA degradation by the virion host shutoff protein of herpes simplex virus. J. Virol. 82:6600–6609. 10.1128/JVI.00137-08 [DOI] [PMC free article] [PubMed] [Google Scholar]