Abstract
The contributions of human herpesvirus 8 (HHV-8) viral interleukin-6 (vIL-6) to virus biology remain unclear. Here we examined the role of vIL-6/gp130 signaling in HHV-8 productive replication in primary effusion lymphoma and endothelial cells. Depletion and depletion-complementation experiments revealed that endoplasmic reticulum-localized vIL-6 activity via gp130 and gp130-activated signal transducer and activator of transcription (STAT) signaling, but not extracellular signal-regulated kinase (ERK) activation, was critical for vIL-6 proreplication activity. Our data significantly extend current understanding of vIL-6 function and associated mechanisms in HHV-8 biology.
TEXT
Human herpesvirus 8 (HHV-8) is associated with multicentric Castleman's disease, primary effusion lymphoma (PEL), and Kaposi's sarcoma (1–3). HHV-8-encoded viral interleukin-6 (vIL-6) promotes cell growth and survival, inflammation, and angiogenesis and is implicated in pathogenesis (4–9). However, its role in normal virus biology remains unclear. Viral IL-6 and its human IL-6 (hIL-6) homolog share ∼25% sequence identity, fold into similar three-dimensional structures, and initiate signaling through similar complexes (10–15). Human IL-6 forms hexameric signaling complexes comprised of two gp80 receptor subunits, two gp130 signal transducers, and two hIL-6 molecules (gp802-gp1302-IL-62) (10). Viral IL-6 can also form signaling hexamers at the cell surface, but its unique signaling from the endoplasmic reticulum (ER) occurs via tetrameric complexes (gp1302-vIL-62) exclusively (8, 12, 15, 16). Viral IL-6 can be expressed during latent stages, in addition to lytic stages, of the viral life cycle (8, 17). Latently infected PEL cell viability is maintained in part via vIL-6/gp130 signaling from the ER and also through its interaction with vitamin K epoxide reductase complex subunit 1 variant 2 (VKORC1v2) (7, 18); the latter contributes to productive replication as well (19). Upon vIL-6-induced gp130 dimerization, phosphorylation of specific gp130 tyrosine residues initiates either signal transducer and activator of transcription 1 and 3 (STAT1/3) (Y767, Y804, Y905, and Y915) or mitogen-activated protein kinase (MAPK) (Y759) signaling (20–23). Activated STAT3 and extracellular signal-regulated kinase 1 and 2 (ERK1/2) have been shown to promote growth and survival of PEL cells, and vIL-6/gp130 signaling contributes (7, 24). The goal of this study was to determine the role of vIL-6/gp130 signaling in HHV-8 lytic replication in PEL and endothelial cells.
gp130 and vIL-6 promote HHV-8 replication in PEL and endothelial cells.
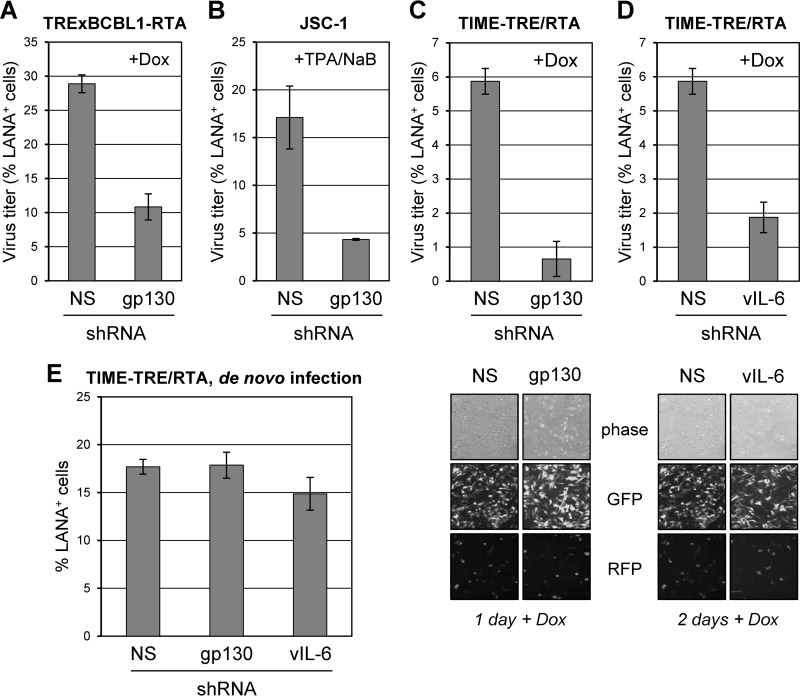
The gp130 signal transducer was depleted in TRExBCBL1-replication and transcription activator (RTA) and JSC-1 PEL cells (26, 27) and in TIME-TRE/RTA endothelial cells (28) latently infected with HHV-8 r219 (29) using previously established techniques (7, 8). Following depletion, lytic replication was induced with doxycycline (Dox) or 12-O-tetradecanoylphorbol-13-acetate (TPA) and sodium butyrate (NaB). HHV-8 was collected from PEL and endothelial cell culture media, and infectious viral titers were determined as undertaken previously (19). Depletion of gp130 led to decreased HHV-8 titers in PEL (∼3-fold) and endothelial cell (>8-fold) cultures (Fig. 1A, B, and C). Similarly, vIL-6 depletion in endothelial cells reduced viral titers by ∼3-fold (Fig. 1D). Rates of reactivation, measured by ORF59 antigen immunofluorescence (PEL cells; data not shown) and red fluorescent protein (RFP) imaging (Fig. 1C and D, bottom), were equivalent in depleted and control cultures. Combined, these data identify the importance of gp130 for productive replication in PEL and endothelial cells and the involvement of vIL-6 in support of productive replication in endothelial cells in addition to BCBL-1 and JSC-1 cells, which we previously reported (19). In contrast to effects on reactivated productive replication, gp130 or vIL-6 depletion had no substantial effect on latency titers (measured 3 days postinfection) following de novo infection of endothelial cells (Fig. 1E), a process known to involve a burst of expression of a subset of lytic genes, including vIL-6, prior to rapid latency establishment (30, 31).
FIG 1.
Involvement of gp130 and vIL-6 in promotion of HHV-8 productive replication. (A) HHV-8 titers following gp130 depletion in TRExBCBL1-RTA cells. The gp130 signal transducer was depleted in TRExBCBL1-RTA cells using lentivirus-encoded shRNAs (nonsilencing [NS] control or gp130-specific) described previously (7). Two days postransduction of lentiviral vectors, cell numbers were normalized and cultures were treated with doxycycline (1 μg/μl) to induce RTA expression and lytic reactivation. Media were collected following 4 days of induction, and virus was pelleted by ultracentrifugation. Aliquots of harvested virus were applied to naive TIME-TRE/RTA cells to determine infectious virus titers by staining for latency-associated nuclear antigen (LANA) as described previously (19). Multiple random fields were assessed to quantify the number of LANA-positive (LANA+) cells in the total [4′,6′-diamidine-2-phenylindole (DAPI)-positive] population. Densities of viable (trypan blue-excluding) TRExBCBL1-RTA cells at the time of medium harvest were not negatively affected by gp130 transduction (1.31 × 106 and 1.41 × 106 cells per ml for NS shRNA-transduced duplicate cultures versus 1.75 × 106 and 1.83 × 106 cells per ml for gp130 shRNA-transduced cells). Frequencies of reactivation, as determined by immunostaining for ORF59-encoded early antigen, were unaffected (data not shown). (B) Importance of gp130 in HHV-8 replication in JSC-1 cells. JSC-1 cells were depleted of gp130 (as described for panel A) and induced with sodium butyrate (NaB; 0.5 μM) and 12-O-tetradecanoylphorbol-13-acetate (TPA; 20 ng/ml) for 12 h and then continuously treated with TPA for 3 days. Released virus was pelleted from culture media and quantified as before. (C and D) Influence of gp130 and vIL-6 on HHV-8 replication in endothelial cells. TIME-TRE/RTA cells were first infected with HHV-8 r219 virus (29) at a multiplicity of infection yielding an infection rate (determined by the proportion of cells positive for green fluorescent protein [GFP]) of ∼90%; these cells were negative for RFP, confirming that they were latently infected. Cells were then transduced with shRNA-encoding lentiviral vectors (NS [control], gp130-specific, or vIL-6-specific [8]) 2 days post-HHV-8 infection and induced with doxycycline (1 μg/ml) 1 day post-lentiviral transduction. Lentiviral vector-encoded GFP, expressed above levels arising from latent r219 HHV-8 genomes, was used to monitor lentiviral infection, achieved in >90% of cells. HHV-8 reactivation was monitored by visualizing RTA-induced RFP expression from r219 viral genomes via fluorescence after doxycycline treatment (examples shown). Media containing virus were collected for 6 days and pooled prior to virus concentration and titration. TIME-TRE/RTA cells were visibly unaffected by gp130 or vIL-6 depletion, and rates of apoptosis (percent annexin V-Cy3+ cells) were equivalent in gp130- and vIL-6-depleted and NS shRNA-transduced cultures (data not shown). (E) NS, gp130, and vIL-6 shRNA-transduced TIME-TRE/RTA cells were infected with a subsaturating infectious dose (∼50% GFP+) of HHV-8 r219 2 days post-lentiviral transduction to determine possible effects of gp130 and vIL-6 depletion on de novo infection and establishment of latency, as determined by LANA staining 3 days post-HHV-8 r219 infection. For all panels, data were derived by counting LANA+ cells in multiple (>6) fields, together comprising 200 to 300 cells, to obtain the percentage of LANA+/DAPI+ cells. Average values from duplicate cultures are shown; error bars represent deviations of individual values from the means.
ER-localized vIL-6/gp130 signaling contributes to productive replication.
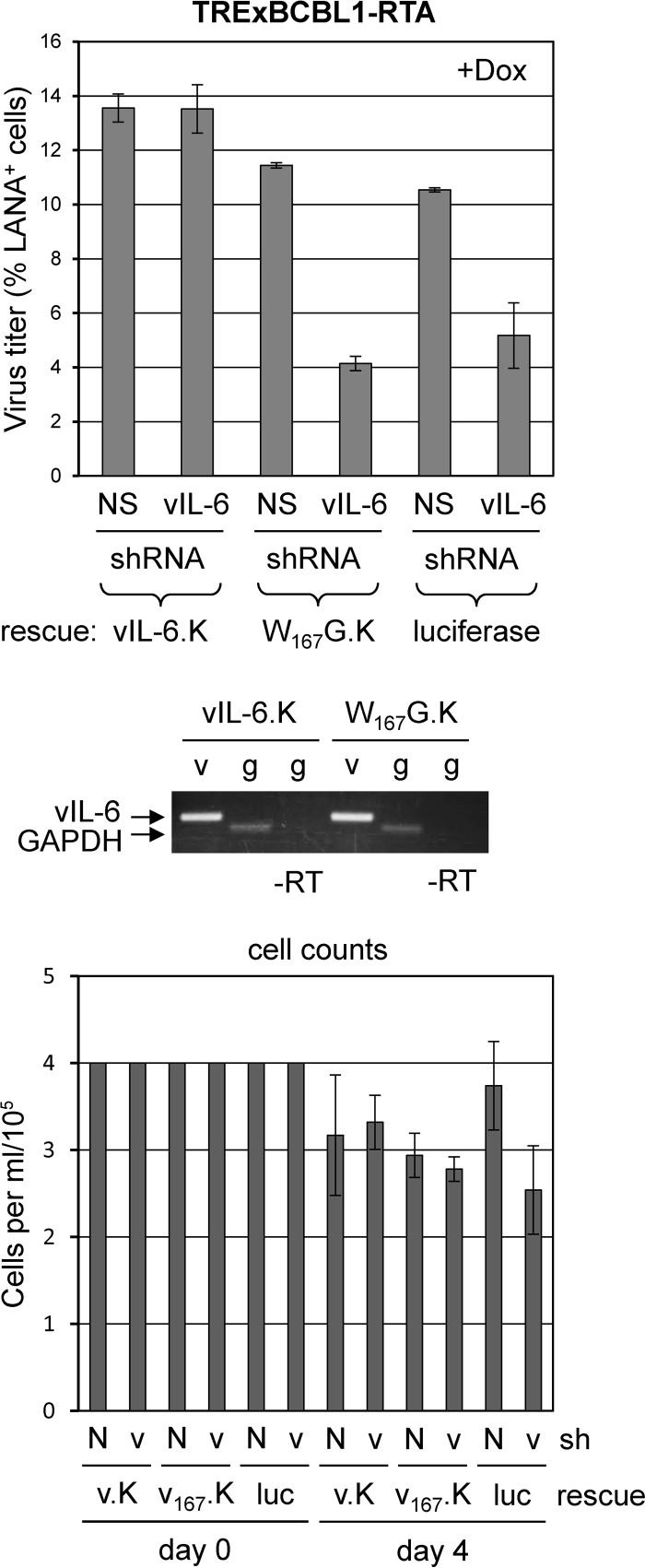
Dox-inducible TRExBCBL1-RTA PEL cells were used to test the ability of gp130 signaling-competent and ER-retained vIL-6 to rescue the vIL-6 depletion phenotype. As before, lentiviral vectors encoding either control (NS) or vIL-6 mRNA-directed short hairpin RNAs (shRNAs) were used for transduction. Depletion of vIL-6 led to reduced virus production (luciferase control), and this phenotype could be rescued with lentiviral vector-transduced vIL-6.KDEL (ER-retained [8]) but not with vIL-6.W167G.KDEL (ER-retained and gp130 dimerization-incompetent in the ER [7, 32]) (Fig. 2). These data identify the involvement of ER-localized vIL-6/gp130 signaling in support of HHV-8 productive replication.
FIG 2.
Importance of ER-localized vIL-6/gp130 interaction for HHV-8 replication. TRExBCBL1-RTA cells were transduced with lentiviral expression vectors encoding KDEL motif-tagged (ER-retained) and shRNA-resistant forms of vIL-6 (vIL-6.K) or vIL-6.W167G (W167G.K) (7, 8) or with lentivirus encoding luciferase (negative control). Two days posttransduction, cells were transduced with control (NS) shRNA-encoding lentivirus (N) or lentivirus expressing vIL-6-specific shRNA (v). Dually transduced cells were then normalized for cell density and induced the following day with doxycycline. Virus was collected and titers were determined as before (see Fig. 1 legend). RNA was harvested from a subset of cells, and cDNA was amplified for vIL-6.KDEL (7) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; used for normalization and positive control) mRNA to verify expression of the introduced vIL-6 genes (v, vIL-6 reverse transcription [RT]-PCR; g, GAPDH RT-PCR; −RT, no reverse transcriptase). Densities of viable cells at the time of Dox treatment (day 0, normalized) and at virus harvest (day 4) are shown (bottom panel). v.K, vIL-6-KDEL; v167.K, vIL-6.W167G-KDEL; luc, luciferase.
gp130-mediated STAT signaling, but not ERK signaling, supports HHV-8 replication.
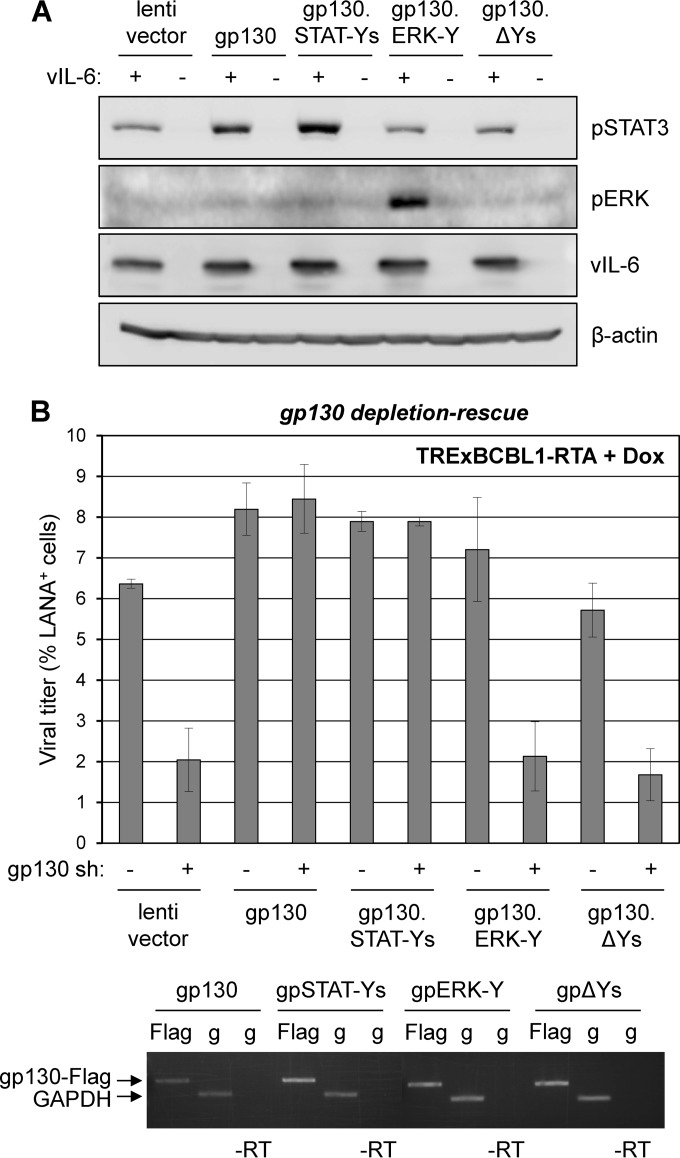
To determine the significance of gp130-activated STAT versus ERK signaling in HHV-8 replication, we employed signaling-altered gp130 variants in gp130 depletion-rescue experiments. Four C-tail tyrosines of gp130 initiate STAT1/3 signaling, and one induces MAPK signaling (20–23). Lentiviral vector-cloned gp130 open reading frames (ORFs) for complementation encoded wild-type gp130 (WT), gp130.STAT-Ys (Y759F mutated; ERK1/2 signaling-null), gp130.ERK-Y (Y759 only; STAT1/3 signaling-null), and gp130.ΔYs (inactive). Functional testing in transfected cells verified that in the presence of vIL-6, introduced WT and gp130.STAT-Ys induced STAT3 phosphorylation above levels supported by endogenous gp130, and gp130.ERK-Y exclusively induced phospho-ERK levels (Fig. 3A). Unexpectedly, WT gp130 did not support detectable ERK signaling in response to vIL-6, but the same result was seen for hIL-6 in the presence of overexpressed gp80 (data not shown). In TRExBCBL1-RTA-based depletion-complementation experiments, WT and gp130.STAT-Ys, but not gp130.ERK-Y or negative controls gp130.ΔY and empty vector, fully rescued replication (Fig. 3B), indicating that STAT signaling alone is sufficient for vIL-6/gp130-mediated support of HHV-8 replication.
FIG 3.
Contributions of gp130-mediated STAT and ERK signaling to HHV-8 productive replication. (A) Functional assessment of gp130 signaling-tyrosine variants. HEK293T cells were cotransfected with empty vector (−) or vIL-6 plasmid (+) together with empty lentiviral vector or lentiviral vectors expressing wild-type gp130, gp130.STAT-Ys (Y759F-mutated), gp130.ERK-Y (Y759 only; other C-tail tyrosines mutated to F), or gp130.ΔYs (pan-Y-to-F-mutated). Two days postransduction, cells were lysed for Western blot analysis to detect phospho-STAT3 (pSTAT3) and pERK as indicators of gp130-activated STAT and MAPK pathways. (B) Role of STAT and ERK signaling in vIL-6/gp130 promotion of HHV-8 replication. TRExBCBL1-RTA PEL cells were transduced with empty lentiviral vector (pDUET001 [39]) or vectors expressing Flag-tagged wild-type gp130, gp130.STAT-Ys, gp130.ERK-Y, or gp130.ΔYs. Two days postransduction, cells were transduced with either NS (control) or gp130-specific shRNA-encoding lentiviral vectors. Following doxycycline-induced HHV-8 reactivation for 4 days, virus was collected and titers were determined as described previously. To verify appropriate expression of wild-type gp130 and the tyrosine-mutated gp130 variants, we lysed a subset of cells in TRIzol for preparation of RNA samples. RNA was reverse transcribed and amplified using 3′-Flag- and 5′-gp130-directed primers (TAGATGGCGGTGATGGTATTT and CTTGTCATCGTCGTCCTTGTA) to assess expression of transduced gp130-Flag mRNAs. GAPDH-specific primers were used for normalization and to provide a positive control. Flag, gp130-Flag RT-PCR; g, GADPH RT-PCR; −RT, no reverse transcriptase.
Contribution of STAT3 to HHV-8 replication.
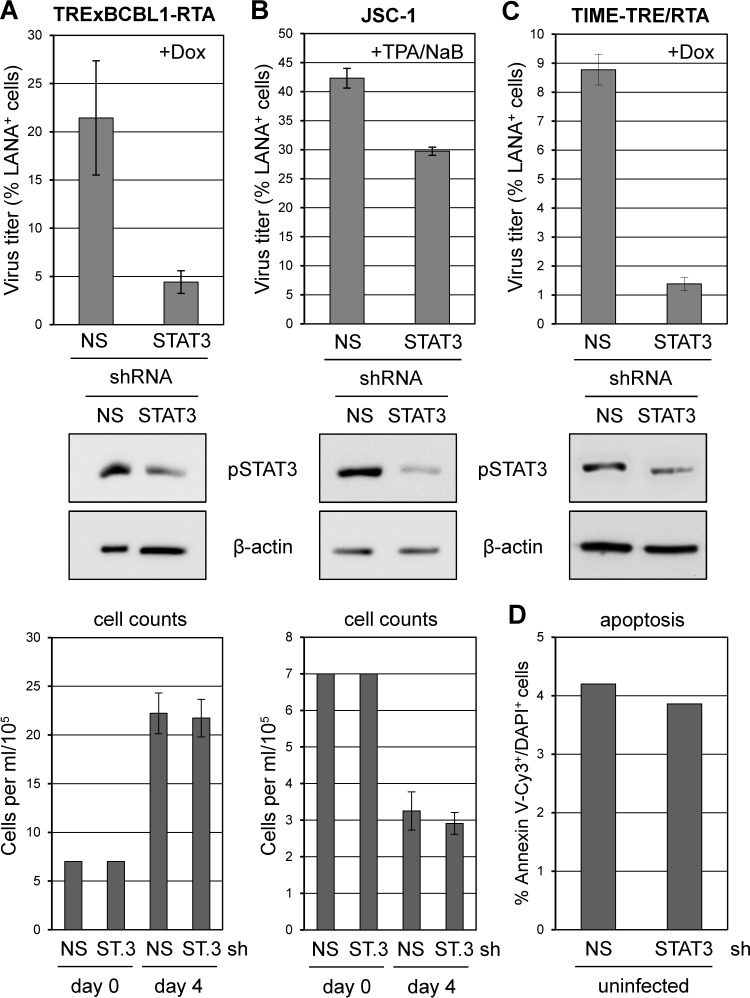
As previous reports have demonstrated the importance of STAT3 for latent PEL cell proliferation and viability (7, 24), we examined its role in productive replication. STAT3 depletion led to reduced HHV-8 titers induced from both PEL and endothelial cell cultures, although effects in TPA/NaB-induced JSC-1 cultures were modest despite robust STAT3 depletion (Fig. 4). The latter may reflect different PEL cell-specific thresholds of STAT3 functionality or different dependencies on STAT3 for support of HHV-8 replication. It is important to note that viable cell densities at the end of the experiment were equivalent in NS and STAT3-directed shRNA-transduced cultures (Fig. 4A and B, bottom), indicating that proproliferative and antiapoptotic activities of STAT3 operating in latently infected PEL cells (7, 24) do not account directly for diminished replication resulting from STAT3 depletion in reactivated cultures. Also, STAT3 depletion in TIME-TRE/RTA cells had no detectable effect on cell viability (Fig. 4D). Our data show that STAT3 supports HHV-8 replication, and together with the vIL-6 and gp130 depletion-rescue data (Fig. 2 and 3) indicate that vIL-6/gp130 signaling via STAT3 contributes significantly to the proreplication activities of vIL-6 and gp130.
FIG 4.
Role of STAT3 in HHV-8 replication in PEL and endothelial cells. (A) TRExBCBL1-RTA cells were transduced with control (NS) and STAT3 (ST.3)-specific shRNA-encoding lentiviral vectors. After 2 days, lytic reactivation was induced with doxycycline for 4 days, and virus was then collected and concentrated from culture media for titration. Experimental procedures and data collection and calculations were as outlined in the legend to Fig. 1. Subsets of transduced cells were harvested at the time of induction and lysed for Western blot analysis to verify STAT3 depletion. Densities of viable (trypan blue-excluding) cells were normalized at the time of lytic induction (day 0) and determined at the end of the experiment (day 4) (bottom panel). (B) The same analyses were undertaken for JSC-1 cultures induced with TPA/NaB and harvested after 4 days for virus titration. A subset of cells was lysed for Western blotting at the time of induction to verify STAT3 depletion. Densities of viable cells at the start and end of the experiment are shown (bottom). (C) Doxycycline-induced TIME-TRE/RTA cultures were analyzed in the same way except that virus was harvested following 6 days of doxycycline treatment; parallel cultures were harvested at the time of induction for Western blot analysis. Cultures were not visibly affected by STAT3 depletion during the course of infection (not shown). (D) Rates of apoptosis (% annexin V-Cy3+/DAPI+ cells) in similarly transduced TIME-TRE/RTA cultures were unaffected by STAT3 depletion.
The likely role of vIL-6 in pathogenesis has been described in numerous reports (4–9). However, investigation of the role of vIL-6 in HHV-8 replication is restricted to one study of vIL-6-null virus replication in (HHV-8-negative) BJAB cells, which noted no phenotype (33), and our own preliminary vIL-6 depletion experiments in PEL cells (19). The present study significantly extends these previous reports, demonstrating that vIL-6 promotes HHV-8 productive replication in endothelial cells in addition to PEL cells, that gp130 contributes significantly to HHV-8 replication in these cell types, that vIL-6 activity via gp130 can be mediated largely or exclusively through ER-localized signaling via tetrameric complexes, and that STAT3 signaling, but not gp130-activated MAPK signaling, is likely critical for vIL-6/gp130-enhanced replication. While it is possible that gp130-activated STAT1 signaling could also contribute, the lack of detectable effects of STAT1 depletion on PEL cell growth (7) coupled with the well-established role of STAT1 in promotion of antiviral interferon and apoptotic signaling (34) indicates that this is unlikely. It is noteworthy that previous studies have reported the importance of STAT3 signaling for the replication of viruses, including varicella-zoster virus (VZV) and hepatitis C virus (35, 36). For VZV, STAT3-activated survivin was found to be involved in proreplication activity, reflecting the prosurvival activities of STAT3 via survivin reported for PEL cells (24). However, human cytomegalovirus blocks STAT3 phosphorylation, though it requires it for optimal replication, and mouse cytomegalovirus induces phosphorylation of STAT3 but not STAT3-responsive cellular genes, suggesting novel, virus-redirected activities of the transcription factor (37, 38). Our findings of vIL-6/gp130 and STAT3 involvement in HHV-8 productive replication could facilitate the development of therapeutic interventions for the treatment of HHV-8-associated diseases, to which productive replication contributes significantly.
ACKNOWLEDGMENTS
We thank Daming Chen for technical and scientific advice.
This work was supported by NIH grant R01-CA76445 to J.N. and NIH Ruth Kirschstein Fellowship F31-CA171933 to E.C.
Footnotes
Published ahead of print 30 July 2014
REFERENCES
- 1. Ensoli B, Sturzl M. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 9:63–83. 10.1016/S1359-6101(97)00037-3 [DOI] [PubMed] [Google Scholar]
- 2. Schulz TF. 2006. The pleiotropic effects of Kaposi's sarcoma herpesvirus. J. Pathol. 208:187–198. 10.1002/path.1904 [DOI] [PubMed] [Google Scholar]
- 3. Uldrick TS, Polizzotto MN, Yarchoan R. 2012. Recent advances in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Curr. Opin. Oncol. 24:495–505. 10.1097/CCO.0b013e328355e0f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholas J. 2007. Human herpesvirus 8-encoded proteins with potential roles in virus-associated neoplasia. Front. Biosci. 12:265–281. 10.2741/2063 [DOI] [PubMed] [Google Scholar]
- 5. Sakakibara S, Tosato G. 2011. Viral interleukin-6: role in Kaposi's sarcoma-associated herpesvirus: associated malignancies. J. Interferon Cytokine Res. 31:791–801. 10.1089/jir.2011.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. 1999. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 94:2871–2879 [PubMed] [Google Scholar]
- 7. Cousins E, Nicholas J. 2013. Role of human herpesvirus 8 interleukin-6-activated gp130 signal transducer in primary effusion lymphoma cell growth and viability. J. Virol. 87:10816–10827. 10.1128/JVI.02047-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen D, Sandford G, Nicholas J. 2009. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J. Virol. 83:722–733. 10.1128/JVI.01517-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034–4043 [PubMed] [Google Scholar]
- 10. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. 2003. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374:1–20. 10.1042/BJ20030407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272:19625–19631. 10.1074/jbc.272.31.19625 [DOI] [PubMed] [Google Scholar]
- 12. Chen D, Nicholas J. 2006. Structural requirements for gp80 independence of human herpesvirus 8 interleukin-6 (vIL-6) and evidence for gp80 stabilization of gp130 signaling complexes induced by vIL-6. J. Virol. 80:9811–9821. 10.1128/JVI.00872-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adam N, Rabe B, Suthaus J, Grotzinger J, Rose-John S, Scheller J. 2009. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J. Virol. 83:5117–5126. 10.1128/JVI.01601-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chow D, He X, Snow AL, Rose-John S, Garcia KC. 2001. Structure of an extracellular gp130 cytokine receptor signaling complex. Science 291:2150–2155. 10.1126/science.1058308 [DOI] [PubMed] [Google Scholar]
- 15. Boulanger MJ, Chow DC, Brevnova E, Martick M, Sandford G, Nicholas J, Garcia KC. 2004. Molecular mechanisms for viral mimicry of a human cytokine: activation of gp130 by HHV-8 interleukin-6. J. Mol. Biol. 335:641–654. 10.1016/j.jmb.2003.10.070 [DOI] [PubMed] [Google Scholar]
- 16. Meads MB, Medveczky PG. 2004. Kaposi's sarcoma-associated herpesvirus-encoded viral interleukin-6 is secreted and modified differently than human interleukin-6: evidence for a unique autocrine signaling mechanism. J. Biol. Chem. 279:51793–51803. 10.1074/jbc.M407382200 [DOI] [PubMed] [Google Scholar]
- 17. Chandriani S, Ganem D. 2010. Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 84:5565–5573. 10.1128/JVI.02723-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen D, Cousins E, Sandford G, Nicholas J. 2012. Human herpesvirus 8 viral interleukin-6 interacts with splice variant 2 of vitamin K epoxide reductase complex subunit 1. J. Virol. 86:1577–1588. 10.1128/JVI.05782-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen D, Gao Y, Nicholas J. 2014. Human herpesvirus 8 interleukin-6 contributes to primary effusion lymphoma cell viability via suppression of proapoptotic cathepsin D, a cointeraction partner of vitamin K epoxide reductase complex subunit 1 variant 2. J. Virol. 88:1025–1038. 10.1128/JVI.02830-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamanaka Y, Nakajima K, Fukada T, Hibi M, Hirano T. 1996. Differentiation and growth arrest signals are generated through the cytoplasmic region of gp130 that is essential for Stat3 activation. EMBO J. 15:1557–1565 [PMC free article] [PubMed] [Google Scholar]
- 21. Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. 1998. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334:297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schiemann WP, Bartoe JL, Nathanson NM. 1997. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. Evidence for participation of multiple signaling pathways which converge at Ras. J. Biol. Chem. 272:16631–16636 [DOI] [PubMed] [Google Scholar]
- 23. Schaper F, Gendo C, Eck M, Schmitz J, Grimm C, Anhuf D, Kerr IM, Heinrich PC. 1998. Activation of the protein tyrosine phosphatase SHP2 via the interleukin-6 signal transducing receptor protein gp130 requires tyrosine kinase Jak1 and limits acute-phase protein expression. Biochem. J. 335:557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aoki Y, Feldman GM, Tosato G. 2003. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101:1535–1542. 10.1182/blood-2002-07-2130 [DOI] [PubMed] [Google Scholar]
- 25. Reference deleted.
- 26. Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, Jung JU. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J. Virol. 77:4205–4220. 10.1128/JVI.77.7.4205-4220.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cannon JS, Ciufo D, Hawkins AL, Griffin CA, Borowitz MJ, Hayward GS, Ambinder RF. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187–10193. 10.1128/JVI.74.21.10187-10193.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choi YB, Sandford G, Nicholas J. 2012. Human herpesvirus 8 interferon regulatory factor-mediated BH3-only protein inhibition via Bid BH3-B mimicry. PLoS Pathog. 8:e1002748. 10.1371/journal.ppat.1002748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vieira J, O'Hearn PM. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325:225–240. 10.1016/j.virol.2004.03.049 [DOI] [PubMed] [Google Scholar]
- 30. Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601–3620. 10.1128/JVI.78.7.3601-3620.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toth Z, Brulois K, Lee HR, Izumiya Y, Tepper C, Kung HJ, Jung JU. 2013. Biphasic euchromatin-to-heterochromatin transition on the KSHV genome following de novo infection. PLoS Pathog. 9:e1003813. 10.1371/journal.ppat.1003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wan X, Wang H, Nicholas J. 1999. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J. Virol. 73:8268–8278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen L, Lagunoff M. 2007. The KSHV viral interleukin-6 is not essential for latency or lytic replication in BJAB cells. Virology 359:425–435. 10.1016/j.virol.2006.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Najjar I, Fagard R. 2010. STAT1 and pathogens, not a friendly relationship. Biochimie 92:425–444. 10.1016/j.biochi.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen N, Che X, Rajamani J, Zerboni L, Sung P, Ptacek J, Arvin AM. 2012. Signal transducer and activator of transcription 3 (STAT3) and survivin induction by varicella-zoster virus promote replication and skin pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 109:600–605. 10.1073/pnas.1114232109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCartney EM, Helbig KJ, Narayana SK, Eyre NS, Aloia AL, Beard MR. 2013. Signal transducer and activator of transcription 3 is a proviral host factor for hepatitis C virus. Hepatology 58:1558–1568. 10.1002/hep.26496 [DOI] [PubMed] [Google Scholar]
- 37. Reitsma JM, Sato H, Nevels M, Terhune SS, Paulus C. 2013. Human cytomegalovirus IE1 protein disrupts interleukin-6 signaling by sequestering STAT3 in the nucleus. J. Virol. 87:10763–10776. 10.1128/JVI.01197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trilling M, Le VT, Rashidi-Alavijeh J, Katschinski B, Scheller J, Rose-John S, Androsiac GE, Jonjic S, Poli V, Pfeffer K, Hengel H. 2014. “Activated” STAT proteins: a paradoxical consequence of inhibited JAK-STAT signaling in cytomegalovirus-infected cells. J. Immunol. 192:447–458. 10.4049/jimmunol.1203516 [DOI] [PubMed] [Google Scholar]
- 39. Zhou BY, Ye Z, Chen G, Gao ZP, Zhang YA, Cheng L. 2007. Inducible and reversible transgene expression in human stem cells after efficient and stable gene transfer. Stem Cells 25:779–789 [DOI] [PubMed] [Google Scholar]