ABSTRACT
During HIV infection, increased CD57 expression among CD8+ T cells has been associated with immune senescence and defective immune responses. Interestingly, CD57-expressing CD8+ T cells exhibit a dual profile, being simultaneously highly cytotoxic (terminally differentiated effectors) and poorly proliferative (replicative senescent). Recent publications point toward a positive role of CD57-expressing CD8+ T cell subsets, presumably due to their high cytolytic activity. We further investigated the phenotype of CD57-expressing CD8+ T cells in healthy donors and during HIV infection combining CD57 expression to Eomesodermin (EOMES), a T box transcription factor which determines, coordinately with T-bet, effector and memory CD8+ T cell differentiation. We defined in healthy donors two functionally distinct CD57-expressing CD8+ T cell subsets exhibiting different levels of EOMES expression: EOMEShi CD57+ and EOMESint CD57+ CD8+ T cells. EOMEShi CD57+ cells exhibited low cytotoxic activity but preserved proliferative capacity and interleukin 7 (IL-7) receptor expression, whereas EOMESint CD57+ cells exhibited obvious cytotoxic functions and a more terminally differentiated phenotype. We next performed a similar analysis in different contexts of HIV infection: primary infected patients, long-term viremic patients, aviremic patients treated with antiretroviral therapy, and HIV controllers; we demonstrated a higher percentage of CD57-expressing cells in all HIV-infected patients regardless of virological status. When heterogeneity in EOMES expression among CD57 cells was taken into account, we detected significantly higher proportions of EOMEShi CD57+ cells among HIV-specific and nonspecific CD8+ T cells from HIV controllers than in aviremic antiretroviral-treated patients and viremic patients. Importantly, such a peculiar non-terminally differentiated EOMEShi CD57+ phenotypic profile was associated with viral control.
IMPORTANCE This study demonstrates that functional heterogeneity exists among CD57-expressing CD8 T cells, which include both terminally differentiated, highly cytotoxic EOMESint CD57+ CD8+ T cells and less differentiated EOMEShi CD57+ CD8 T cells, which do not exhibit immediate cytotoxic functions but present high proliferative capacity. Interestingly, HIV controllers present a high proportion of EOMEShi CD57 cells among CD57-expressing HIV-specific CD8 T cells compared to both long-term viremic and aviremic antiretroviral therapy (ART)-treated patients, suggesting a beneficial role for this cell subset in viral control.
INTRODUCTION
During chronic HIV infection, virus-specific CD8+ T cells functionally decline, progressively losing their proliferative capacity and cytotoxic potential and progressing to exhaustion and/or senescence (1, 2) except in rare individuals: the HIV controllers (HIC). These patients exhibit persistently undetectable HIV RNA in the absence of antiretroviral therapy (ART) (3) and maintain polyfunctional HIV-specific CD8+ T cells which retain proliferative potential (4–6) as well as the ability to produce effector cytokines and cytotoxic molecules (5–8). Such a peculiar, nonexhausted profile has been related to the presence of longer telomeres and higher levels of constitutive telomerase activity in HIV-specific CD8+ T cells from HIC (2). CD57 expression identifies senescent human T cells displaying a terminally differentiated phenotype (1, 10–12) and increases during HIV infection, probably as a result of chronic immune activation (11, 13). Interestingly, CD57-expressing CD8+ T cells exhibit a dual profile, being simultaneously highly efficient cytotoxic cells (terminally differentiated effectors) (14) and poor proliferative (replicative senescence) subsets (1).
However, recent publications provided new insights on the role of CD57-expressing cells during HIV infection. Lee et al. demonstrated that HIV and cytomegalovirus (CMV) differently regulate CD57 expression on CD8+ T cells, inducing terminal differentiation in CMV infection but accumulation of less differentiated cells in HIV infection, as assessed by a decreased proportion of CD57-expressing cells among CD28− CD8+ T cells (15). The same group also demonstrated that proportions of CD57-expressing CD28− CD8+ T cells were increased following ART treatment (16). Additionally, low proportions of CD28− CD8+ T cells expressing CD57 were a predictive marker of mortality among ART-treated HIV-infected patients with advanced disease (16). These recent data point toward a positive role for CD57-expressing CD8+ T cell subsets, presumably due to their high cytolytic activity, in contrast to the deleterious impact of immune senescence, usually associated with the CD57-expressing subsets. We further investigated the phenotype of CD57-expressing CD8+ T cells combining CD57 expression to Eomesodermin (EOMES), a T box transcription factor which determines, coordinately with T-bet, effector CD8+ T cell differentiation, regulating interferon gamma (IFN-γ), perforin, and granzyme B expression (17–19), as well as memory CD8+ T cell transition and maintenance (20–22). EOMES expression has been reported to be upregulated in early effectors and to further increase during memory differentiation (20). During murine chronic viral infections, maintained high T-bet expression has been associated with terminal effector differentiation (23, 24), whereas high EOMES expression correlates with the long-term memory fraction (25) and characterizes cells exhibiting increased proliferative potential, granzyme B production, and cytotoxicity (26). At present, the precise role performed by EOMES during HIV infection remains unclear: a recent report showed that EOMES expression was increased in viremic HIV patients (27), whereas Eomes mRNA has been shown to significantly decrease in HIV-specific CD8+ T cells from primary HIV infection to chronic phase (28), suggesting that loss of EOMES expression could be associated with CD8+ T cell functional decline.
In this report, we demonstrate functional heterogeneity among CD57-expressing CD8+ T cells. By combining CD57 with EOMES expression, we were able to identify in healthy donors (HD) two functionally distinct subsets: EOMEShi CD57+ CD8+ T cells displaying a memory phenotype and EOMESint CD57+ CD8+ T cells exhibiting high cytotoxic functions and a terminally differentiated phenotype. We performed a cross-sectional analysis of EOMES and CD57 expression in CD8+ T cells isolated from different groups of HIV-infected patients: primary infected patients, ART-treated aviremic patients, chronically viremic patients, and HIV controllers. We showed that CD57 expression is increased in all HIV-infected patient groups studied, including HIV controllers. Taking into account heterogeneity in EOMES expression among CD57-expressing CD8+ T cells, we showed that HIV controllers maintained significantly lower proportions of EOMESint CD57+ cells and higher fractions of EOMEShi CD57+ cells among both HIV-specific and nonspecific CD8+ T cells than both untreated viremic and ART-treated aviremic patients and that such a peculiar phenotype was associated with viral control.
These data suggest that CD57 expression per se is not a reliable marker of terminal differentiation, whereas the combination of EOMES and CD57 provided a more accurate insight on the tight balance between proliferating memory and cytotoxic terminally differentiated cells during HIV infection.
MATERIALS AND METHODS
Study participants.
We collected samples from 147 HIV-infected individuals with their informed consent. Thirty-two primary infected untreated patients were enrolled in the French ANRS multicenter PRIMO cohort (Agence Nationale de Recherche sur le SIDA, CO06). Primary infection was defined by HIV RNA positivity and by a negative or emerging antibody response. We also studied 30 untreated chronically infected patients who are referred to as viremic patients. Thirty-one ART-treated aviremic individuals presenting plasma HIV RNA levels of <50 copies/ml and treated for at least 12 months were included in the study. Fifty-four patients were enrolled in the French HIV controller cohort (ANRS, CO21 CODEX) (inclusion criteria: no ART, HIV infection for >5 years, five last consecutive plasma HIV RNA values of <400 copies/ml). HIV RNA detection assay with a detection limit reaching <40 copies/ml was performed for all samples from HIV controllers. Clinical and biologic characteristics of participants are shown in Table 1. All HIV-infected patient groups were age matched with the exception of HIV controllers, which by definition are long-term HIV-infected patients and are older than others. Peripheral blood samples from 21 non-HIV-infected blood donors were obtained from the Etablissement Français du Sang (Saint Louis Hospital, Paris, France). These donors were selected to be age matched with HIV controllers.
TABLE 1.
Characteristics of HIV-infected patients and healthy controlsa
| Group (no. of individuals) | Age (yrs) | Viral load (log10 copies/ml) | CD4 count (cells/μl) |
|---|---|---|---|
| Healthy donors (21) | 49 (31–58) | ||
| Primary infected patients (32) | 35 (30–40) | 5.2 (4.7–5.8) | 469 (363–614) |
| ART untreated patients (30) | 37 (29–42) | 4.4 (3.9–4.8) | 488 (361–732) |
| ART treated patients (31) | 40 (33–48) | <1.7 | 771 (544–893) |
| HIV controllers (54) | 47 (40–53) | <2.6 | 771 (596–931) |
A total of 168 subjects were included in this study, in the 5 groups listed: healthy donors, HIV-infected patients studied during primary HIV infection, non-ART-treated viremic patients, aviremic (viral load < 50 copies/ml) ART-treated patients, and HIV controllers, defined as showing spontaneous viral control for more than 5 years (viral load < 400 copies/ml). Median values and 25th and 75th percentiles are presented (in parentheses) for age, viral load, and CD4 count for each group.
Laboratory studies. (i) Cell preparation.
Peripheral blood mononuclear cells (PBMCs) were isolated from anticoagulated blood by Ficoll density gradient centrifugation. Human leukocyte antigen (HLA) typing was done with the complement-dependent microlymphocytotoxic technique (One Lambda, Montpellier, France). Cells were cryopreserved in liquid nitrogen for subsequent analysis.
(ii) Flow cytometry.
PBMCs were analyzed by 10-color flow cytometry. Cryopreserved cells were thawed, and after incubation with purified Fc receptor binding inhibitor (e-Bioscience), samples were stained with labeled antibodies against surface markers for 15 min at 4°C. Conjugated antibodies against the following were used: CD4 (fluorescein isothiocyanate [FITC]), CD57 (phycoerythrin [PE]-CF594), CD27 (Alexa Fluor 700), CD45RA (allophycocyanin [APC]-H7), CD8 (V450), and CD3 (V500) from BD Biosciences (San Jose, CA) and CD57 (FITC or PE) from Miltenyi. Intracellular staining for cytotoxic molecules was performed using anti-granzyme B (FITC or Alexa Fluor 700; clone GB11 [BD Biosciences]) and antiperforin (FITC; clone B-D48 [Diaclone]). Intranuclear detection of EOMES (anti-EOMES Alexa Fluor 647; clone WD1928 [e-Bioscience]) and T-bet (anti-T-bet peridinin chlorophyll protein [PerCP]-Cy5.5; clone 4B10 [e-Bioscience]) was performed on fixed and permeabilized cells by following the manufacturer's instructions (e-Bioscience). Detection of perforin and granzyme B was performed ex vivo without any stimulation or brefeldin/monensin incubation. Samples were acquired on an LSRFortessa cell analyzer (BD Biosciences), and data files were analyzed using FlowJo software (Tree Star Inc.).
Peptide-HLA class 1 pentamers.
HIV-specific CD8+ T cells were identified by employing soluble PE-labeled peptide-HLA class 1 pentamers (Proimmune, Oxford, United Kingdom) derived from the HIV Gag, Nef, Pol, and Env proteins. The following epitopes were used: the HLA-A*0201-restricted peptide ligands SLYNTVATL (Gag residues 77 to 85) and ILKEPVHGV (Pol residues 476 to 484), the A*0301-restricted peptide ligands RLRPGGKKK (Gag residues 20 to 28) and QVPLRPMTYK (Nef residues 73 to 82), the A*1101-restricted ligand AVDLSHFLK (Nef residues 84 to 92), the A*2402-restricted peptide ligand RYPLTFGWCY (Nef residues 134 to 143), the B*0702-restricted peptide ligand IPRRIRQGL (Env residues 848 to 856), the B*0801-restricted peptide ligands GEIYKRWII (Gag residues 259 to 267) and FLKEKGGL (Nef residues 90 to 97), and the B*2705-restricted peptide ligand KRWIILGLNK (Gag residues 263 to 272).
Statistical methods.
Statistical analysis was performed using GraphPad prism software. Nonparametric Mann-Whitney test was employed to compare cell subsets or patient groups. Spearman's rank test was used to determine correlations. P values above 0.05 were considered not statistically significant.
RESULTS
CD57 expression is increased on both total and HIV-specific CD8+ T cells in chronically HIV-infected patients independently of viral load.
We first compared degrees of CD57 surface expression in CD8+ T cells isolated from HIV-negative subjects and from different HIV-infected patient groups, namely, subjects with primary HIV infection (PHI), long-term viremic (VIR) patients, ART-treated aviremic patients, and HIV controllers (HIC). For HIV-infected patients, analysis was performed on both total and HIV-specific CD8+ T cells identified using HIV epitope-HLA-I pentamers (Fig. 1A). In healthy donors, 11.5% (median; interquartile range [IQR], 3.5% to 38.0%) of CD8+ T cells expressed CD57 (Fig. 1B). Analysis of CD57 expression in HIV-infected individuals revealed a significant increase in CD57-expressing CD8+ T cell proportions in primary HIV-infected patients (24.5%; IQR, 9.0% to 64.0%; P = 0.0036), untreated viremic chronically infected patients (30.5%; IQR, 10.0% to 58.6%; P = 0.0004), and aviremic ART-treated patients (32.7%; IQR, 12.0% to 58.9%; P < 0.0001) compared with healthy donors (Fig. 1B). Interestingly, CD8+ T cells isolated from HIV controllers also expressed increased levels of CD57 (34.6%; IQR, 3.7% to 66.4%; P < 0.0001) compared to those expressed by healthy donors (Fig. 1B). These results indicate that CD57 expression in CD8+ T cells is increased during early and more advanced phases of HIV infection, independently of active viral replication.
FIG 1.
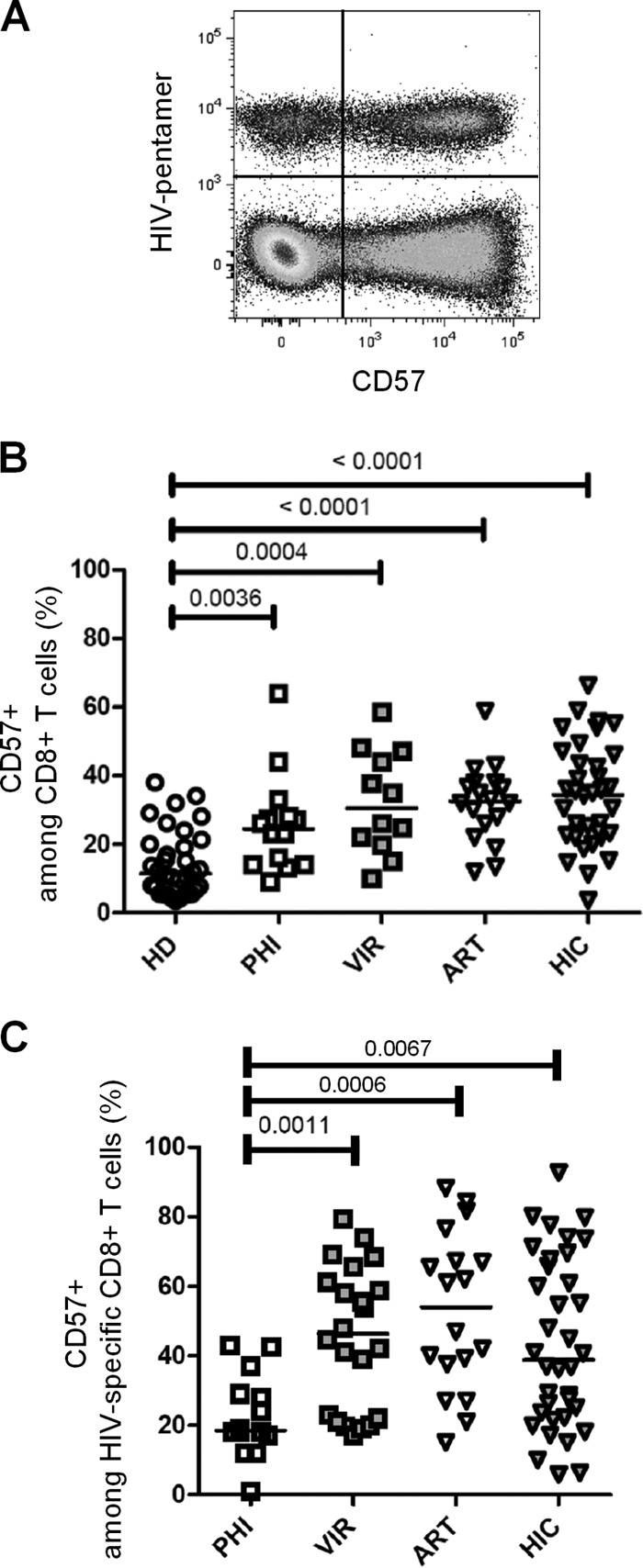
CD57 expression in CD8+ T cells during HIV infection. (A) Representative dot plot of CD57 expression on CD8+ T cells costained with pentamers folded with HIV-derived peptides recovered from one HIV-infected patient. (B) CD57-expressing cells among CD8+ T cells in peripheral blood mononuclear cells (PBMCs) from healthy donors (HD; open circles), primary HIV-infected patients (PHI; open squares), untreated viremic patients (VIR; filled gray squares), aviremic patients treated with ART (ART; open triangles), and HIV controllers (HIC; filled gray triangles). (C) CD57-expressing cells among HIV-specific CD8+ T cells, identified by pentamer staining as shown in panel A. Median percentages are represented for each group, and P values are indicated when significant.
We next assessed CD57 expression at the surface of HIV-specific CD8+ T cells. HIV-specific CD8+ T cells from PHI patients displayed only low proportions of CD57-expressing cells (18.50%; IQR, 1.0% to 43.0%) (Fig. 1C). Conversely, untreated viremic chronically infected patients displayed significantly higher proportions of CD57-positive HIV-specific CD8+ T cells (46.3%; IQR, 17.0% to 79.4%) than PHI patients (P = 0.0011) (Fig. 1C). Among aviremic patients, HIV-specific CD8+ T cells from ART-treated patients and from HIC contained high proportions of CD57-positive cells (ART-treated patients, 54.0% [IQR, 15.0% to 88.3%]; HIC, 38.8% [IQR, 5.9% to 92.7%]) (Fig. 1C). All chronically infected groups exhibited significantly higher proportions of CD57-expressing HIV-specific CD8+ T cells than did PHI patients (P = 0.0011, P = 0.0006, and P = 0.0067 for VIR and ART-treated patients and HIC, respectively). These results indicate that chronically HIV-infected patients, including HIV controllers, display high proportions of CD57-expressing HIV-specific and nonspecific CD8+ T cells.
Identification of two distinct CD57-expressing CD8+ T cell subsets based on EOMES expression.
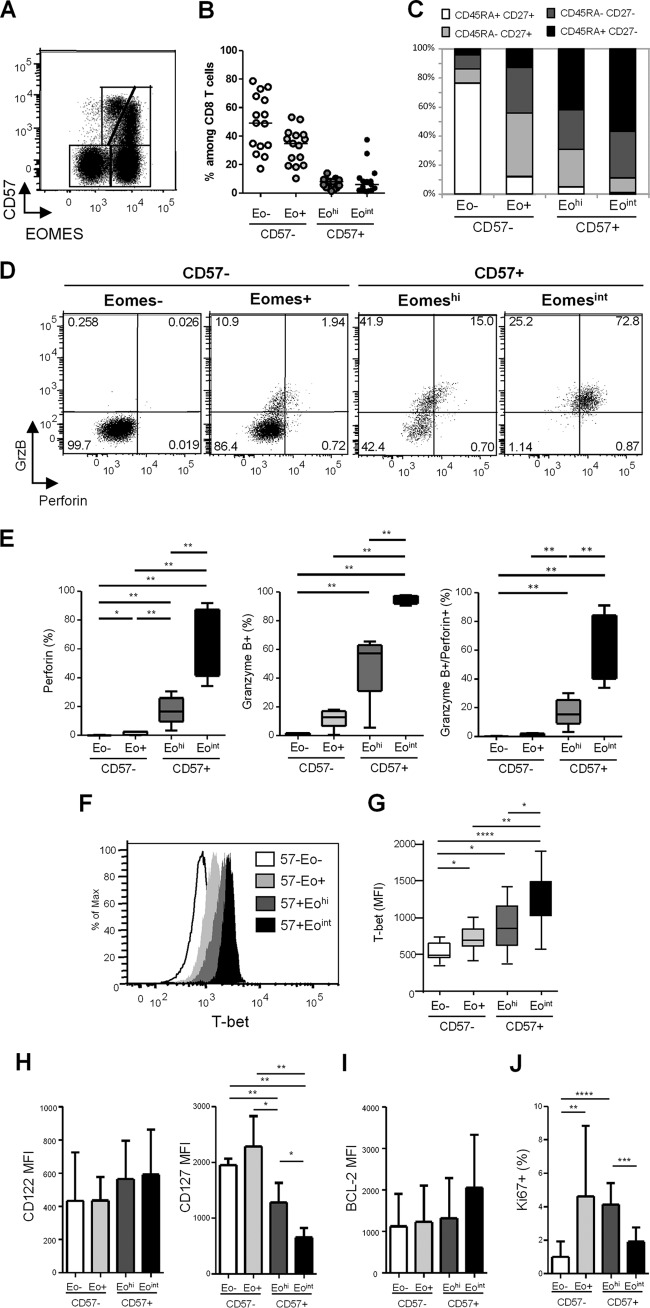
Expression of the T box transcription factor EOMES has been recently associated with terminal differentiation, CD57 being coexpressed with EOMES (29). In an analysis of EOMES and CD57 coexpression in CD8+ T cells, four cell subsets were identified (Fig. 2A and B): (i) a double negative subset, EOMES− CD57−, represented approximately half of CD8+ T cells in healthy donors (median, 49.8% [IQR, 34.8% to 66.1%]); (ii) an EOMES+ CD57− population represented the second most represented subset (median, 34.7% [22.7% to 42.3%]). Finally, EOMES+ CD57+ cells showed a heterogeneous level of EOMES expression, allowing us to identify two smaller subsets: (iii) an EOMEShigh CD57+ (EOMEShi CD57+) and (iv) an EOMESintermediate CD57+ subset (EOMESint CD57+), representing in healthy donors 7.6% (3.7% to 11.4%) and 4.0% (1.4% to 8.7%) of total CD8+ T cells, respectively. When surface expression of CD45RA and CD27 was analyzed to characterize cellular differentiation of the four CD8+ T cell subsets identified on the basis of EOMES and CD57 expression, we found that among CD57− CD8+ T cells, EOMES− cells were mainly composed of CD45RA+ CD27+ naive T cells (Fig. 2C). Conversely, EOMES+ CD57− cells contained the highest proportions of CD45RA− CD27+ memory CD8+ T cells and an important proportion of CD45RA− CD27− effector/memory CD8+ T cells (Fig. 2C). CD57+ cells, either EOMEShi or EOMESint, were mainly represented by CD45RA− CD27− effector/memory and CD45RA+ CD27− terminally differentiated CD8+ T cells (TEMRA) (Fig. 2C). The EOMESint CD57+ CD8+ T cell subset contained the highest proportions of CD45RA+ CD27− TEMRA CD8+ T cells, suggesting higher differentiation of this cell subset. However, we could not associate clear phenotypic discrimination between EOMEShi and EOMESint CD57-expressing CD8+ T cell fractions.
FIG 2.
Phenotypic and functional characterization of EOMES+ CD8+ T cells. (A) Representative dot plot of EOMES and CD57 expression in CD8+ T cells from one healthy donor (HD). (B) Proportions of CD8+ T cell subsets defined by EOMES and CD57 expression in HD: EOMES− CD57− (Eo− CD57−), EOMES+ CD57− (Eo+ CD57−), EOMEShi CD57+ (Eohi CD57+), and EOMESint CD57+ (Eoint CD57+). (C) Phenotypic characterization of EOMES− CD57−, EOMES+ CD57−, EOMEShi CD57+, and EOMESint CD57+ cells based on CD45RA and CD27 expression. Graphs represent proportions of CD45RA+ CD27+ (white bars), CD45RA− CD27+ (light gray bars), CD45RA− CD27− (dark gray bars), and CD45RA+ CD27− (black bars) CD8+ T cells. (D) Representative dot plots of granzyme B and perforin expression in CD57− and CD57+ CD8+ T cell subsets defined based on EOMES expression from one healthy donor. (E) Percentages of perforin-positive (left graph), granzyme B-positive (middle graph), and granzyme B- and perforin-coexpressing (right graph) cells among EOMES− CD57− (white bars), EOMES+ CD57− (light gray bars), EOMEShi CD57+ (dark gray bars), and EOMESint CD57+ (black bars) CD8+ T cells from HD (n = 10). (F) T-bet expression in CD8+ T cell subsets. Flow cytometry histograms show representative results from one individual. (G) Histograms represent the median T-bet median fluorescence intensity (MFI) ± SD in EOMES− CD57− (white bars), EOMES+ CD57− (light gray bars), EOMEShi CD57+ (dark gray bars), and EOMESint CD57+ (black bars) CD8+ T cells from 10 HD. (H to J) Mean MFI of CD122 and CD127 (H) and BCL-2 (I) and mean percentages of Ki-67+ cells (J) in EOMES-CD57− (white bars), EOMES+ CD57− (light gray bars), EOMEShi CD57+ (dark gray bars), and EOMESint CD57+ (black bars) CD8 T cells from 10 HD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
The EOMESint CD57+ phenotype is associated with higher cytotoxic potential and T-bet coexpression in CD8+ T cells.
We next studied the cytotoxic potential of the four CD8+ T cell subsets defined by EOMES and CD57 expression based on granzyme B and perforin expression. CD57− CD8+ T cells, either EOMES negative or positive, were almost completely deprived in cytotoxic molecule-producing cells (Fig. 2D and E). EOMEShi CD57+ cells contained significantly higher proportions of cells expressing perforin (16.6% [9.5% to 25.9%]), granzyme B (57.4% [31.1% to 63.0%]), or both (15.3% [9.0% to 25.4%]) than did EOMES− CD57− or EOMES+ CD57− cell subsets (Fig. 2D and E). Finally, EOMESint CD57+ cells presented the highest proportions of perforin-expressing (73.1% [41.4% to 87.2%]) and granzyme B-expressing (93.8% [91.9% to 97.5%]) cells, the great majority of EOMESint CD57+ CD8+ T cells (72.3% [40.1% to 84.5%]) coexpressing both granzyme B and perforin (Fig. 2D and E). We thus demonstrated that CD57-expressing CD8+ T cells include two fractions exhibiting highly different cytotoxic potentials that can be segregated using EOMES expression.
As the T box transcription factor T-bet also modulates CD8+ T-cell differentiation and granzyme B and perforin production in mice, we assessed T-bet expression in CD8+ T cell subsets identified based on EOMES and CD57 expression. The lowest levels of T-bet were expressed by EOMES-negative cells (median fluorescence intensity [MFI], 489 [440 to 651]), in accordance with their less differentiated resting state (Fig. 2F and G). A progressive increase in T-bet expression was identified from EOMES+ CD57− cells (MFI, 696 [597 to 846]) to EOMEShi CD57+ cells (MFI, 859 [613 to 1,160]) and EOMESint CD57+ cells (MFI, 1,328 [1,013 to 1,490]) (Fig. 2F and G). Interestingly, the EOMESint CD57+ subset, which presented the most terminally differentiated effector phenotype and the highest granzyme B and perforin production, expressed the highest levels of T-bet. Collectively, these data indicate that EOMESint CD57+ CD8+ T cells represent a terminally differentiated effector cell subset characterized by the highest cytotoxic potential and highest levels of T-bet expression.
The EOMEShi CD57+ phenotype is associated with higher CD127 expression and higher proliferation ability in CD57+ CD8+ T cells.
We next characterized CD8+ T cell subsets for surface expression of CD122 and CD127, which are necessary for transduction of interleukin 15 (IL-15) and IL-7 signaling, respectively, and therefore essential for CD8+ T cell homeostasis. As shown in Fig. 2H, we failed to detect any significant difference in CD122 expression at the surface of the four CD8+ T cell subsets identified based on CD57 and EOMES expression. Conversely, we found that EOMEShi and EOMESint CD57+ cells displayed significantly lower levels of CD127 than CD57-negative subsets (Fig. 2H). However, EOMEShi CD57+ cells retained higher levels of CD127 expression (mean MFI, 1,282 ± 351) than did the highly cytotoxic EOMESint CD57+ cells (MFI, 657 ± 166) (Fig. 2H).
To determine whether such a difference in CD127 was associated with any homeostatic advantage, we studied the expression of the antiapoptotic molecule BCL-2 as a marker of survival and the expression of Ki-67 as a proliferation marker. As shown in Fig. 2I, we failed to detect any significant difference in BCL-2 expression among the four subsets. Conversely, we found lower proportions of Ki-67-expressing cells in EOMES− CD57− cells (1.0% ± 0.9%) compared to both EOMES+ CD57− (4.6% ± 4.2%) and EOMEShi CD57+ CD8+ (4.1% ± 1.3%) T cells (Fig. 2J). Interestingly, EOMEShi CD57+ cell subsets presented significantly higher proportions of Ki-67+ cells than did EOMESint CD57+ CD8+ T cells (1.9% ± 0.8%) (Fig. 2J). Collectively, these data suggest a higher homeostatic potential for EOMEShi CD57+ CD8+ T cells, which exhibited higher expression of IL-7 receptor and proliferative capacity compared with the high immediate cytotoxicity provided by the EOMESint CD57+ subset.
Higher levels of EOMEShi CD57+ CD8+ T cells in HIV controllers.
We then investigated CD57 expression during HIV infection introducing EOMES expression. Primary HIV-infected patients displayed significantly reduced proportions of EOMEShi CD57+ cells (3.4% [1.1% to 36.5%]) compared to those displayed by healthy donors (7.6% [1.3% to 26.2]; P = 0.0440) and viremic individuals (8.4% [1.1% to 20.0%]; P = 0.0209) (Fig. 3A, upper graph). Conversely, proportions of EOMEShi CD57+ CD8+ T cells in chronically HIV-infected patients, either viremic or aviremic ART treated (8.8% [2.8% to 29.4%]), were not different from those observed in healthy individuals (Fig. 3A, upper graph). Interestingly, CD8+ T cells from HIV controllers displayed significantly higher proportions of EOMEShi CD57+ cells (13.9% [3.3% to 37.9%]) than did healthy donors (P = 0.0025) and primary HIV-infected (P < 0.0001), chronically viremic (P = 0.0139), and aviremic ART-treated (P = 0.0321) patients (Fig. 3A, upper graph). When we focused on the EOMESint CD57+ CD8+ T cell subset, i.e., the fraction exhibiting high cytolytic content ex vivo, we observed higher proportions in HIV-infected patient groups, including primary infected individuals (14.1% [2.4% to 29.9%]; P = 0.0004), chronically infected patients (18.8% [4.6% to 35.2%]; P < 0.0001), aviremic ART-treated patients (23.9% [2.7% to 40.2%]; P < 0.0001), and HIV controllers (16.4% [0.2% to 42.3%]; P = 0.0018) than in healthy donors (4.0% [0.5% to 24.4%]), while we failed to detect any significant difference among HIV-infected patient groups (Fig. 3A, lower graph). Because such increases may be directly driven by the higher percentage of CD57-expressing cells among CD8+ T cells observed in HIV-infected patients (as shown in Fig. 1B), we analyzed the proportions of EOMEShi CD57+ and EOMESint CD57+ fractions among CD57-expressing CD8+ T cells. We observed that primary HIV-infected, chronically viremic, and aviremic ART-treated patients presented a significant reduction in the proportions of EOMEShi cells among CD57+ CD8+ T cells associated with an increase on the EOMESint fraction (Fig. 3B). Interestingly, HIV controllers represented the only patient group retaining a balance of these two subsets similar to that observed in healthy donors (Fig. 3C). Collectively, these results show that while displaying proportions of CD57+ CD8+ T cells similar to those of other groups (Fig. 1B), HIV controllers retained higher proportions of EOMEShi cells among CD57+ cells.
FIG 3.
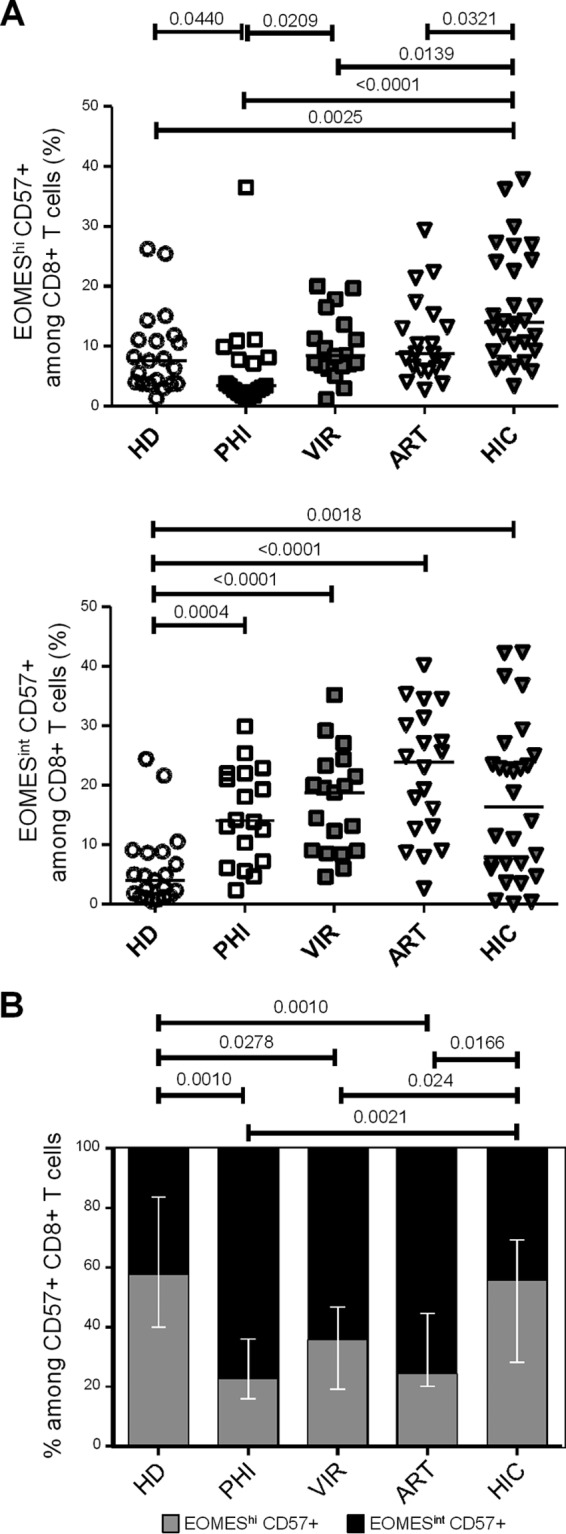
Effects of different phases of HIV infection on CD57- and EOMES-expressing CD8+ T cell subsets. (A and B) Graphs present EOMEShi CD57+ (A) and EOMESint CD57+ (B) percentages among CD8+ T cells in PBMCs from HD, primary HIV-infected patients (PHI; open squares), untreated viremic patients (VIR; filled gray squares), ART-treated aviremic patients (ART; open triangles), and HIV controllers (HIC; filled gray triangles). (C) Proportions of EOMEShi CD57+ (gray bars) and EOMESint CD57+ (black bars) among CD57+ CD8+ T cells from HD and PHI, VIR, ART, and HIC patients. P values are indicated.
HIV-specific CD8+ T cells from HIV controllers present an EOMEShi CD57+ phenotype.
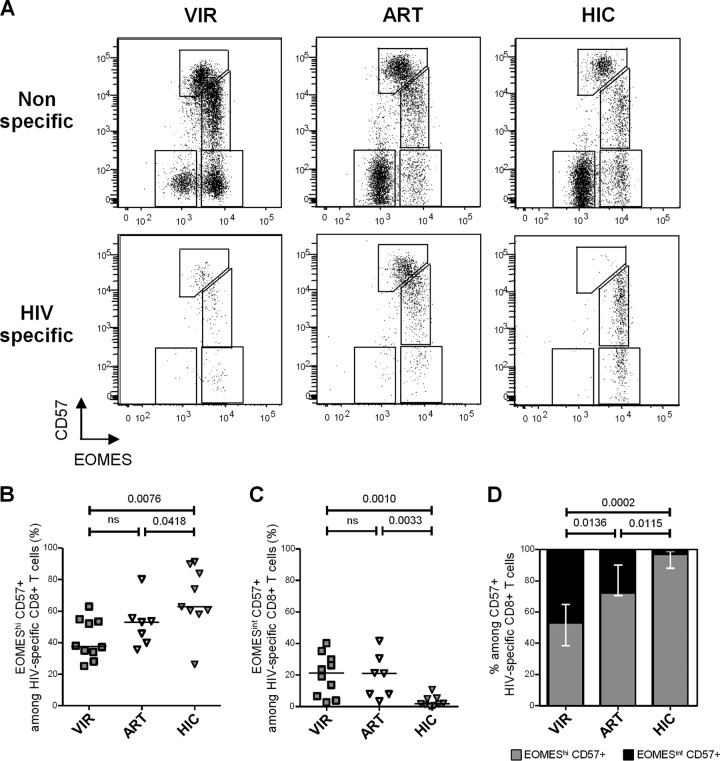
We next assessed EOMES and CD57 expression in HIV-specific CD8+ T cells from chronically viremic patients (n = 10), aviremic ART-treated individuals (n = 7), and HIV controllers (n = 9) identified using HIV epitope-HLA-I pentamers (Fig. 4A). We detected significantly higher proportions of EOMEShi CD57+ cells (i.e., the fraction exhibiting higher survival and proliferation profiles) among HIV-specific CD8+ T cells from HIV controllers (62.8% [26.3% to 91.2%]) than in cells from both chronic viremic (37.7% [25.2% to 62.9%]; P = 0.0076) and ART-treated (53.1% [35.7% to 80.2%]; P = 0.0418) patients (Fig. 4B). Conversely, lower proportions of EOMESint CD57+ cells (i.e., the fraction exhibiting higher cytolytic molecule contents ex vivo) among HIV-specific CD8+ T cells were observed in HIV controllers (1.9% [0.2% to 10.7%]) than in both chronic viremic (21.4% [2.7% to 40.1%]; P = 0.0010) and ART-treated (21.1% [3.5% to 41.7%]; P = 0.0418) patients (Fig. 4C). Restricting the analysis to CD57+ HIV-specific CD8+ T cells, we observed the lowest proportions of EOMEShi cells among CD57-expressing cells in chronical viremic HIV-infected individuals (53.1% [30.9% to 90.3%]) (Fig. 4D). In contrast, CD57+ HIV-specific CD8+ T cells from HIV controllers were almost completely constituted by EOMEShi CD57+ cells (96.7% [85.5% to 99.7%]), EOMESint CD57+ cells being barely detectable (Fig. 4D). These data confirm in HIV-specific CD8+ T cells results obtained in analyses performed on global CD8+ T cells and indicate that CD57-expressing HIV-specific CD8+ T cells from HIV controllers presented a skewed phenotype toward an EOMEShi CD57+ phenotype compared with both those from viremic and aviremic ART-treated patients.
FIG 4.
HIV-specific CD8+ T cells from HIV controllers present a peculiar EOMEShi CD57+ phenotype. (A) Representative dot plots of EOMES and CD57 expression in total (upper graphs) and HIV-specific (lower graphs) CD8+ T cells from VIR, ART, and HIC patients. (B and C) Proportions of EOMEShi CD57+ (B) and EOMESint CD57+ (C) cells among HIV-specific CD8+ T cells from VIR (gray filled squares), ART (open triangles), and HIC (gray filled triangles) patients. (D) Proportions of EOMEShi CD57+ (gray bars) and EOMESint CD57+ (black bars) among HIV-specific CD57+ CD8+ T cells from VIR, ART, and HIC patients. P values are indicated.
Higher EOMEShi CD57+ proportions among both global and HIV-specific CD8+ T cells are associated with lower viral loads.
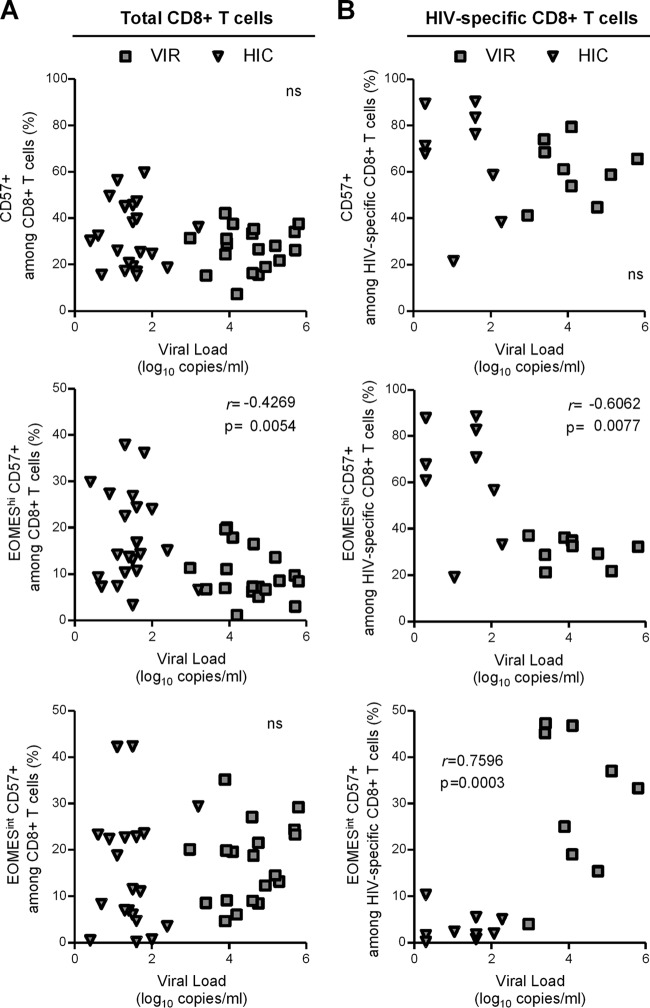
Finally, we studied whether CD57-expressing CD8+ T cells subsets correlated with viral load in chronically HIV-infected untreated patients. HIV load did not correlate with CD57+ proportions of either total (Fig. 5A, upper graph) or HIV-specific (Fig. 5B, upper graph) CD8+ T cells. However, when we took into account CD57+ CD8+ T cell heterogeneity in terms of EOMES expression, we detected a significant inverse correlation between viral load and EOMEShi CD57+ proportions among both total (r = −0.4269; P = 0.0054) and HIV-specific (r = −0.6062; P = 0.0077) CD8+ T cells (Fig. 5, middle graphs). In contrast, while no association with viral load was observed when considering the immediately cytotoxic EOMESint CD57+ subset among total CD8 T cells (Fig. 5A, lower graph), we found a significant, positive correlation between viral levels and the proportion of EOMESint CD57+ cells among HIV-specific CD8+ T cells (r = −0.7596, P = 0.0003) (Fig. 5B, lower graph). Collectively, these results revealed differential impact of CD57-expressing subsets on viral load and supported the notion that the EOMEShi CD57+ subset, which exhibits a higher proliferation capacity and homeostatic potential but lower immediate cytotoxic potential, could play a role in viral control.
FIG 5.
Relationship between CD57-expressing CD8+ T cell subsets and viral load. Correlation between viral load and CD57+ (upper graphs), EOMEShi CD57+ (middle graphs), or EOMESint CD57+ (lower graphs) cells among total (A) and HIV-specific (B) CD8+ T cells from untreated chronically HIV-infected patients. Different symbols identify patients groups: viremic patients (gray filled squares) and HIV controllers (filled gray triangles). Correlations were evaluated using a Spearman rank correlation coefficient test. Spearman r index and P value are indicated.
DISCUSSION
The CD57 antigen, a member of the N-CAM family initially described as a natural killer cell marker, has been reported to identify human CD8+ T cells displaying high cytotoxic activity and poor proliferative activity leading to the coexistence of terminally differentiated phenotype and senescence. In vitro studies revealed functional impairment of HIV-specific CD57+ CD8+ T cells as indicated by their high susceptibility to apoptosis and activation-induced cell death associated with a reduced capacity to proliferate in response to appropriate stimulation (1, 10, 11). Interestingly, Lee et al. (16) recently demonstrated that the proportions of CD57-expressing cells among CD28− CD8+ T cells were increased following ART treatment and represented a favorable prognostic factor during HIV infection, pointing toward a positive role for the CD57-expressing fraction. We hypothesized that such a discrepancy relied on functional heterogeneity among CD57-expressing CD8+ T cells. We thus aimed to further characterize CD57-expressing CD8+ T cells during HIV infection by analyzing the expression of Eomesodermin (EOMES) and T-bet, two transcription factors determining coordinately memory (20, 21) and effector (30) CD8+ T cell fate.
In healthy subjects, we distinguished two functionally different subsets among CD57-expressing cells; the EOMESint CD57+ fraction but not the EOMEShi CD57+ fraction exhibited high granzyme B and/or perforin contents. Our results show that highly cytotoxic, terminally differentiated EOMESint CD57+ CD8 T cells presented the highest levels of T-bet expression, in accordance with data from Hersperger et al. (31). Conversely, EOMEShi CD57+ cell subsets presented lower levels of T-bet expression associated with a less differentiated memory phenotype and retain high homeostatic and proliferative potential, as revealed by high levels of CD127 expression and a high proportion of proliferating cells ex vivo, respectively. Such an EOMES/T-bet coexpression pattern supports in human CD8+ T cells a model in which the relative expression levels of T-bet and EOMES in CD8+ T cells reciprocally promote a terminal effector differentiation versus a memory fate, as previously suggested for mice (20–22). Such heterogeneity may provide a rationale for discrepancies concerning functional characteristics of CD57- or EOMES-expressing cells (1, 14, 26, 29).
CD57+ CD8+ T cell proportions increase physiologically during aging (32), presumably mostly driven by chronic infections such as with CMV. An increase in CD57 expression during chronic HIV infection has also been documented, occurring probably as a result of chronic immune activation (11, 13). Taking advantage of the study of several French cohorts of patients at different phases of HIV infection, we confirmed in this work that CD57 expression in both total and HIV-specific CD8+ T cells is increased during the chronic phase of infection. Interestingly, equally high percentages of CD57-expressing CD8+ T cells were observed at the surface of CD8+ T cells isolated from viremic and aviremic ART-treated patients and HIV controllers. Primary HIV-infected patients exhibited a significantly reduced proportion of CD57-expressing HIV-specific CD8+ T cells compared to those exhibited by all chronically infected groups. Such a low proportion of CD57-expressing cells, which include potent cytotoxic CD8+ T cells, is in accordance with an important role for chronic immune activation on CD57 expression and may participate in the insufficient viral control during primary infection. We thus further focused on the chronic stages of HIV infection. By integrating EOMES expression in the analysis, we demonstrated that HIV controllers exhibited a significantly higher proportion of EOMEShi CD57+ CD8+ T cells than did both viremic and aviremic ART-treated patients. Extending our analysis to HIV-specific responses, we observed that HIV-specific CD8+ T cells from HIV controllers retain an EOMEShi CD57+ phenotype, in contrast to cells from viremic and ART-treated aviremic patients, which mostly exhibited terminally differentiated EOMESint CD57+ cells. Our study suggests that maintenance of high EOMES expression in CD57+ cells could contribute to the higher efficiency of CD8+ T cell responses in HIC (5–8). Indeed, among untreated chronically infected patients, proportions of EOMEShi CD57+ cells among total and HIV-specific CD8+ T cells inversely correlate with viral loads, suggesting a beneficial role for this cell population in viral control. Conversely, the proportion of EOMESint CD57+ cells among HIV-specific CD8+ T cells was positively associated with viral load, suggesting that viral persistence contributed to terminal differentiation. However, viral load is probably not the only factor responsible for the expansion of the EOMESint CD57+ fraction, as HIV-specific CD8+ T cells from HIV controllers still exhibited significantly lower proportions of the EOMESint CD57+ fraction and higher proportions of the EOMEShi CD57+ fraction than did ART-treated aviremic patients (i.e., a patient group with an equivalent viral load).
Previous reports have suggested that HIV-specific CD8+ T cells from HIV controllers have a greater capacity to produce cytotoxic molecules (5, 8). Our results, demonstrating higher frequencies of non-terminally differentiated, actively proliferating EOMEShi CD57+ CD8+ T cells in HIV controllers, are only in apparent contrast with previous reports. Migueles and coworkers reported that high perforin production by CD8+ T cells from HIV long-term nonprogressors (LNTPs) was a consequence of their ability to more readily proliferate and undergo greater numbers of divisions upon antigen stimulation (5). Our results using EOMES/CD57 identification led to a similar observation: the EOMEShi CD57+ fraction, which exhibited a higher proliferative capacity, was associated with better viral control. It is tempting to speculate that the large and rapid expansion of HIV-specific CD8+ T cells from LNTPs partly reflects the higher fraction of EOMEShi CD57+ fraction among CD57-expressing HIV-specific CD8+ T cells. The use of the EOMES/CD57 combination requires intracellular staining and thus does not allow us to directly demonstrate the cytotoxic potential of the EOMEShi CD57+ fraction upon in vitro simulation. CD57 expression was higher in the EOMESint CD57+ fraction than in the EOMEShi CD57+ fraction, which leads us to question whether a higher level of CD57 expression may reflect higher terminal differentiation. We may therefore speculate that EOMEShi CD57+ CD8+ T cells could represent a progenitor subset ready to rapidly proliferate and further differentiate into fully functional, perforin-producing EOMESint CD57+ cells upon antigenic encounter. Accordingly, Buckheit et al. have previously demonstrated that HIV-specific CD8+ T cell subsets exhibit different in vitro suppressive activities depending on the time point considered: terminally differentiated cells were more suppressive at an early stage, whereas a less differentiated fraction exhibited an equally suppressive function at later time points (33). Polyfunctionality, a marker of efficient T cell responses, may thus also rely on the diversity of CD8+ T cell differentiation ensuring a large timescale of cytotoxic responses balancing from immediate to late induced cytotoxic activity.
In conclusion, our results demonstrate functional heterogeneity among CD57-expressing CD8+ T cells. CD57-expressing CD8+ T cells include both terminally differentiated, highly cytotoxic CD8+ T cells and less differentiated cells that may act as progenitors capable of rapidly proliferating and further differentiating into fully functional, perforin-producing EOMESint CD57+ cells upon antigenic encounter. We identified a skewed balance between EOMEShi CD57+ and EOMESint CD57+ CD8+ T cells in HIV controllers, who exhibited a more preserved EOMEShi CD57+ fraction. Importantly, such a less differentiated profile was associated with viral control. Our study suggests that maintenance of high EOMES expression could contribute to the higher efficiency of cytotoxic responses by developing an adequate balance between less differentiated and terminally differentiated fractions.
ACKNOWLEDGMENTS
We thank Marc Tardieu and Jean-François Delfraissy for their support.
We declare no competitive financial interests.
This work was supported by the Agence Nationale de la recherche contre le SIDA et les hépatites virales (ANRS) and Fondation de France. Federico Simonetta was also supported by the Fondation pour la recherche médicale (FRM).
Footnotes
Published ahead of print 6 August 2014
REFERENCES
- 1. Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711–2720. 10.1182/blood-2002-07-2103 [DOI] [PubMed] [Google Scholar]
- 2. Lichterfeld M, Mou D, Cung TDH, Williams KL, Waring MT, Huang J, Pereyra F, Trocha A, Freeman GJ, Rosenberg ES, Walker BD, Yu XG. 2008. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood 112:3679–3687. 10.1182/blood-2008-01-135442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, Rouzioux C, Venet A, Delfraissy JF. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053–1056. 10.1086/433188 [DOI] [PubMed] [Google Scholar]
- 4. Card CM, Keynan Y, Lajoie J, Bell CP, Dawood M, Becker M, Kasper K, Fowke KR. 2012. HIV controllers are distinguished by chemokine expression profile and HIV-specific T-cell proliferative potential. J. Acquir. Immune Defic. Syndr. 59:427–437. 10.1097/QAI.0b013e3182454fcd [DOI] [PubMed] [Google Scholar]
- 5. Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068. 10.1038/ni845 [DOI] [PubMed] [Google Scholar]
- 6. Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 102:7239–7244. 10.1073/pnas.0502393102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. 10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD, Betts MR. 2010. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 6:e1000917. 10.1371/journal.ppat.1000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reference deleted.
- 10. Le Priol Y, Puthier D, Lecureuil C, Combadiere C, Debre P, Nguyen C, Combadiere B, Lécureuil C, Combadière C, Debré P, Combadière B. 2006. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J. Immunol. 177:5145–5154. 10.4049/jimmunol.177.8.5145 [DOI] [PubMed] [Google Scholar]
- 11. Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, Dunbar PR, Shepherd D, Cerundolo V, Emery V, Griffiths P, Conlon C, McMichael AJ, Richman DD, Rowland-Jones SL, Appay V. 2004. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2:E20. 10.1371/journal.pbio.0020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, Douek DC, Mueller YM, Jacobson JM, Kulkarni V, Felber BK, Pavlakis GN, Katsikis PD, Koup RA. 2009. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J. Immunol. 183:1120–1132. 10.4049/jimmunol.0900182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis DE, Puck JM, Babcock GF, Rich RR. 1985. Disproportionate expansion of a minor T cell subset in patients with lymphadenopathy syndrome and acquired immunodeficiency syndrome. J. Infect. Dis. 151:555–559. 10.1093/infdis/151.3.555 [DOI] [PubMed] [Google Scholar]
- 14. Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. 2009. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 85:88–97. 10.1189/jlb.0208107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SA, Sinclair E, Hatano H, Hsue PY, Epling L, Hecht FM, Bangsberg DR, Martin JN, McCune JM, Deeks SG, Hunt PW. 2014. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One 9:e89444. 10.1371/journal.pone.0089444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SA, Sinclair E, Jain V, Huang Y, Epling L, Van Natta M, Meinert CL, Martin JN, McCune JM, Deeks SG, Lederman MM, Hecht FM, Hunt PW. 2014Low proportions of CD28− CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J. Infect. Dis. 210:372–382. 10.1093/infdis/jiu109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. 2009. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206:51–59. 10.1084/jem.20081242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. 2008. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321:408–411. 10.1126/science.1159806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. 2003. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302:1041–1043. 10.1126/science.1090148 [DOI] [PubMed] [Google Scholar]
- 20. Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. 2010. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185:4988–4992. 10.4049/jimmunol.1002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6:1236–1244. 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- 22. Rao RR, Li Q, Odunsi K, Shrikant PA. 2010. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32:67–78. 10.1016/j.immuni.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. 2007. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 204:2015–2021. 10.1084/jem.20070841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McLane LM, Banerjee PP, Cosma GL, Makedonas G, Wherry EJ, Orange JS, Betts MR. 2013. Differential localization of T-bet and Eomes in CD8 T cell memory populations. J. Immunol. 190:3207–3215. 10.4049/jimmunol.1201556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. 2012. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338:1220–1225. 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasley RB, Hong C, Li W, Friesen T, Nakamura Y, Kim GY, Park J-H, Hixon JA, Durum S, Hu Z, Sneller MC, Oguariri R, Imamichi T, Lane HC, Catalfamo M. 2013. HIV immune activation drives increased Eomes expression in memory CD8 T cells in association with transcriptional downregulation of CD127. AIDS 27:1867–1877. 10.1097/QAD.0b013e3283618487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ribeiro-dos-Santos P, Turnbull EL, Monteiro M, Legrand A, Conrod K, Baalwa J, Pellegrino P, Shaw GM, Williams I, Borrow P, Rocha B. 2012. Chronic HIV infection affects the expression of the 2 transcription factors required for CD8 T-cell differentiation into cytolytic effectors. Blood 119:4928–4938. 10.1182/blood-2011-12-395186 [DOI] [PubMed] [Google Scholar]
- 29. Dolfi DV, Mansfield KD, Polley AM, Doyle SA, Freeman GJ, Pircher H, Schmader KE, Wherry EJ. 2013. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J. Leukoc. Biol. 93:825–836. 10.1189/jlb.0912438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. 2003. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl. Acad. Sci. U. S. A. 100:15818–15823. 10.1073/pnas.2636938100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hersperger AR, Martin JN, Shin LY, Sheth PM, Kovacs CM, Cosma GL, Makedonas G, Pereyra F, Walker BD, Kaul R, Deeks SG, Betts MR. 2011. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 117:3799–3808. 10.1182/blood-2010-12-322727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Peña J, Solana R. 2000. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech. Ageing Dev. 121:77–88. 10.1016/S0047-6374(00)00199-8 [DOI] [PubMed] [Google Scholar]
- 33. Buckheit RW, Salgado M, Silciano RF, Blankson JN. 2012. Inhibitory potential of subpopulations of CD8+ T cells in HIV-1-infected elite suppressors. J. Virol. 86:13679–13688. 10.1128/JVI.02439-12 [DOI] [PMC free article] [PubMed] [Google Scholar]