ABSTRACT
The international effort to prevent HIV-1 infection by vaccination has failed to develop an effective vaccine. The aim of this vaccine trial in women was to administer by the vaginal mucosal route a vaccine consisting of HIV-1 gp140 linked to the chaperone 70-kDa heat shock protein (HSP70). The primary objective was to determine the safety of the vaccine. The secondary objective was to examine HIV-1 infectivity ex vivo and innate and adaptive immunity to HIV-1. Protocol-defined female volunteers were recruited. HIV-1 CN54gp140 linked to HSP70 was administered by the vaginal route. Significant adverse reactions were not detected. HIV-1 was significantly inhibited ex vivo in postimmunization CD4+ T cells compared with preimmunization CD4+ T cells. The innate antiviral restrictive factor APOBEC3G was significantly upregulated, as were CC chemokines which induce downregulation of CCR5 in CD4+ T cells. Indeed, a significant inverse correlation between the proportion of CCR5+ T cells and the concentration of CCL-3 or CCL-5 was found. Importantly, the upregulation of APOBEC3G showed a significant inverse correlation, whereas CCR5 exhibited a trend to correlate with inhibition of HIV-1 infection (r = 0.51). Furthermore, specific CD4+ and CD8+ T cell proliferative responses were significantly increased and CD4+ T cells showed a trend to have an inverse correlation with the viral load (r = −0.60). However, HIVgp140-specific IgG or IgA antibodies were not detected. The results provide proof of concept that an innate mechanism consisting of CC chemokines, APOBEC3G, and adaptive immunity by CD4 and CD8 T cells might be involved in controlling HIV-1 infectivity following vaginal mucosal immunization in women. (This study has been registered at ClinicalTrials.gov under registration no. NCT01285141.)
IMPORTANCE Vaginal immunization of women with a vaccine consisting of HIVgp140 linked to the 70-kDa heat shock protein (HSP70) elicited ex vivo significant inhibition of HIV-1 replication in postimmunization CD4+ T cells compared with that in preimmunization peripheral blood mononuclear cells. There were no significant adverse events. The vaccine induced the significant upregulation of CC chemokines and the downmodulation of CCR5 expression in CD4+ T cells, as well as an inverse correlation between them. Furthermore, the level of CCR5 expression was directly correlated with the viral load, consistent with the protective mechanism in which a decrease in CCR5 molecules on CD4+ T cells decreases HIV-1 envelope binding. Expression of the antiviral restriction factor APOBEC3G was inversely correlated with the viral load, suggesting that it may inhibit intracellular HIV-1 replication. Both CD4+ and CD8+ T cells showed HIVgp140- and HSP70-specific proliferation. A strong inverse correlation between the proportion of CC chemokine-modulated CCR5-expressing CD4+ T cells and the stimulation of CD4+ or CD8+ T cell proliferation by HIVgp140 was found, demonstrating a significant interaction between innate and adaptive immunity. This is the first clinical trial of vaginal immunization in women using only HIVgp140 and HSP70 administered by the mucosal route (3 times) in which a dual innate protective mechanism was induced and enhanced by significant adaptive CD4+ and CD8+ T cell proliferative responses.
INTRODUCTION
The global human immunodeficiency virus (HIV) pandemic continues, and an effective vaccine has so far not been produced. In a recent assessment in Nature Medicine of the latest of 5 well-conducted large-scale clinical trials of HIV type 1 (HIV-1) vaccines, 4 invited experts discussed the failure of the vaccines to prevent HIV infection or decrease the viral load set point (1). Two trials (STEP and Phambili) showed that the HIV infection rates after vaccination were higher than those achieved with placebo, and in both trials the higher HIV infection rates were attributed to the recombinant adenovirus type 5 vector (2). The exception was the RV144 clinical trial, which suggested that subcutaneous administration of an envelope-based vaccine may offer limited protection against HIV (3). Nonetheless, valuable lessons have been learned and cautious optimism was expressed. The overall strategy of these trials was the induction of the classical antibody and/or cellular immune response and the use of prime-boost strategies and more effective vectors. A great deal of attention has been paid to neutralizing antibodies targeting the V1 and V2 loops and specific sites within the structure of the HIV-1 trimer. While some of these approaches are proven strategies in vaccination that must be pursued, innate immunity, though often discussed, does not feature greatly in these trials, despite its importance in the most successful smallpox and yellow fever vaccines (4, 5).
We have pursued a strategy which attempts to induce first an early platform of innate immunity on the basis of two mechanisms: (i) inhibition of HIV-1 by downmodulation or blocking of the CCR5 coreceptor induced by an increase in the CC chemokines CCL-3, CCL-4, and CCL-5 (35, 36, 38) and (ii) inhibition of HIV-1 which may have escaped the CCR5-mediated mechanism by upregulation of the antiviral factor apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) and inactivation of Vif (6–9). Both mechanisms have been demonstrated in nonhuman primates and parts of the mechanism have also been demonstrated in humans, but not following immunization against HIV-1. Critically, early innate immunity is thought to boost subsequent HIV-1-specific adaptive cellular and antibody responses and may offer initial resistance to infection.
In order to establish a platform of innate immunity, the HIV CN54gp140 glycoprotein (10) was linked to the 70-kDa mycobacterial heat shock protein (HSP70). The former uses the outer envelope protein of a C-clade HIV isolate as the protective antigen. Although the rationale for using HIVgp140 has long been established (3), linking it to HSP70 is not well-known, and such an approach has not been used in clinical trials. HSP70 plays an important role in both innate and adaptive immunity (11), and it may facilitate loading and processing of antigenic epitopes into major histocompatibility complex (MHC) class II (12), as well as into MHC class I by cross-reactive priming (13). HSP70 is a potent stimulator of CC chemokines (14), cytokines (15), and APOBEC3G (16), which inhibits HIV-1 or simian immunodeficiency virus (SIV). HSP70 also functions as a coadjuvant by virtue of binding among other receptors, CD40 and the CCR5 molecules, and Toll-like receptor 4 (TLR4) in dendritic cells (DCs) and macrophages (14, 17–19). Furthermore, microbial HSP70 inhibits in vitro HIV-1 infection of human CD4+ T cells (20). Thus, HSP70 may function as a coadjuvant, enhancing innate immunity, and when linked to antigen it may induce adaptive immune responses.
In a preclinical toxicology, immunogenicity, and protective study in macaques, we used a vaccine preparation consisting of HIV envelope protein gp120 and SIVp27 mixed with three extracellular peptides from the human CCR5 molecule, all of which were linked to HSP70 (21). The vaccine was administered by the vaginal mucosal route to female rhesus macaques. Local or systemic adverse reactions were not recorded. CC chemokines and downmodulation of cell surface CCR5 expression were found in CD4+ T cells, which also showed proliferative responses, but low levels of serum and vaginal fluid IgG and IgA antibodies were detected. Vaginal challenge with simian-human immunodeficiency virus 89.6P (SHIV89.6P) infected all macaques, but sequential analysis showed that whereas the virus persisted in the four unimmunized animals, in two of the four vaginally immunized macaques, the virus was cleared and the CD4 T cell counts returned to normal levels. This experiment suggested that vaginal delivery of an HIV/SIV vaccine linked to HSP70 may elicit some protection in a nonhuman primate model.
We report here the first phase 1 human trial of vaginal immunization with HIV CN54gp140 glycoprotein (HIVgp140) linked to an HSP70 vaccine (ClinicalTrials.gov NCT01285141 [http://clinicaltrials.gov/ct2/show/NCT01285141]). The vaccine did not induce adverse reactions, induced significant inhibition of HIV-1 ex vivo in peripheral blood mononuclear cells (PBMCs) postimmunization, and elicited innate and T cell immune responses.
MATERIALS AND METHODS
The protocol for this trial and supporting CONSORT checklist are available in Table S1A in the supplemental material.
Ethics statement.
Ethical approval for this study was obtained from the United Kingdom National Research Ethics Service, Wandsworth Research Ethics Committee reference 11/LO/0459. Written informed consent was obtained from all participants after the nature and possible consequences of the study were explained. This study protocol was registered on ClinicalTrials.gov (NCT01285141) and the European Clinical Trials Database (2010-022740-20) prior to subject recruitment. Clinical trial approval was obtained from the United Kingdom Medicines and Healthcare Products Regulatory Agency (reference 16745/0216/001-0001).
Objectives.
The primary objective was to determine the local and systemic safety and tolerability of vaginal immunization with CN54gp140 linked to the HSP70 vaccine administered 3 times over a 12-week period. The primary variables were the frequency of subject reporting of adverse events (AEs) during the entire study period from the first immunization. Exploratory variables included ex vivo inhibition of HIV-1 replication in PBMCs following immunization, the concentrations of three CC chemokines, the expression of cell surface CCR5 and CXCR4 and intracellular APOBEC3G in CD4+ T cells, the proliferative responses of PBMCs and CD4 and CD8 cells, and the concentrations of anti-HIVgp140- and anti-HSP70-specific IgG and IgA antibodies in serum and cervicovaginal secretions.
Participants.
As this was a hypothesis-generating pilot study, no placebo was included, and no formal power calculation for sample size was performed. The inclusion criteria for the 8 healthy female volunteers aged 18 to 45 years were that they provide written informed consent of good health, which was determined from their medical history and by physical examination; that they have a negative hematologic pregnancy test; that they be practicing appropriate contraception; and that they have a normal cervical smear within a calendar year of the date of screening. Exclusion criteria included hypersensitivity to any component of the vaccine; a positive test result for HIV antibody or HIV proviral DNA at the time of initial screening; positive results for hepatitis B or C virus, Chlamydia trachomatis, Neisseria gonorrhoeae, or Treponema pallidum infection; and the receipt of any medication via the vaginal route.
Description of procedures or investigations undertaken. (i) Vaccine formulation and production.
HIV-1 CN54gp140 is a subunit envelope protein of a C-clade HIV-1 isolate comprising a sequence of 634 amino acids (10). This HIV subtype is believed to cause more than 50% of cases of HIV-1 infection worldwide and is predominant in southern and eastern Africa and India (10). Mycobacterium tuberculosis HSP70 is a chaperone protein which is produced in Escherichia coli cells and purified by ultrafiltration, and the pH is adjusted to 8 (22). In macaques, HSP70 functions as a coadjuvant enhancing mostly the innate and cellular responses (14–21). Recombinant CN54gp140 was produced in a eukaryotic expression system under good manufacturing practice (GMP) guidelines by Polymun (Vienna, Austria), and HSP70 was expressed under the control of cGMP in E. coli by Biomeva (Heidelberg, Germany). The antigen must be conjugated with HSP70 (22), and conjugation was carried out by Biomeva under the control of cGMP using the method with N-succinimide 3-(2-pyridyldithio)-propionate (SPDP), a heterobifunctional membrane-permeant cross-linker acting through the reduction of —SH groups and linking through lysine within each protein molecule to form a stable covalent bond between the two proteins. Clinical batches of CN54gp140 glycoprotein-HSP70 conjugate vaccine were prepared under conditions of good manufacturing practice and presented as a solution with a total protein concentration of 400 μg/ml in phosphate-buffered saline (PBS)–EDTA buffer, pH 7.4. Each immunization comprised a 0.25-ml volume of vaccine containing a single 100-μg dose, selected on the basis of our previous preclinical and clinical programs (23, 24).
(ii) Immunization of human female volunteers.
Recumbent subjects received three immunizations with 0.25 ml (one standard dose) of vaccine at weeks 0, 4, and 12. The vaccine was administered directly into the vagina using a 1-ml syringe without an attached needle. Subjects remained recumbent for 15 min before mobilizing, and they were kept under direct observation for 1 h.
(iii) Sample collection.
A blood sample was taken before the first immunization, at the time of each immunization (0, 4, and 12 weeks), and then at weeks 16 and 20 after the first immunization. Cervical and vaginal secretions were sampled at the same times using a Weck-Cel surgical spear (Medtronic, Watford, United Kingdom) placed either in the cervical os or against the vaginal wall for 2 min. The secretions were then eluted (2 times) as described previously (24). Briefly, the spearheads were snipped into the top chamber of a Spin-X tube (Corning Inc., Corning, NY) containing 300 μl sterile-filtered extraction buffer (250 mM NaCl, 16 protease inhibitor cocktail set 1 [Calbiochem, United Kingdom]) in PBS and centrifuged, and the procedure was repeated prior to separation of the secretions in buffer into 200-μl aliquots, which were frozen at −80°C before antibody analysis.
(iii) Preclinical toxicology assessment in rabbits.
As part of the preclinical toxicology evaluation to support the clinical trial reported here, study 24953 was conducted under good laboratory practice (GLP) guidelines approved by the German Tierschutzgesetz (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit file reference 33.2-42502-05-LG-01-49/2009) in New Zealand White rabbits (n = 4) immunized intravaginally (days 1, 8, and 15) with 1× the human clinical dose of the HIV CN54gp140 glycoprotein linked to the HSP70 vaccine (100 μg total protein). A control group (n = 4) received a sham immunization with normal saline placebo. No significant toxicological abnormalities were observed.
(iv) HIV-1 (BaL) infectivity of PBMCs.
HIV-1 infection was tested as described before (25). PBMCs were activated with 10 μg/ml of phytohemagglutinin (PHA; Sigma) in supplemented RPMI for 5 days and then washed with medium and cultured in supplemented RPMI with 20 IU interleukin 2 (IL-2) overnight (Schiaparelli Biosystems BV, Woerden, Netherlands). Serial dilutions of primary HIV-1 strain BaL (a CCR5-binding strain) were prepared with a starting concentration of 20 ng/100 μl of p24. Aliquots of 2 × 105 cells were infected with serial dilutions with a 1:10 to 1:10−3 multiplicity of HIV-1 strains for 3 h. The cells were washed three times with medium and cultured in triplicate at 1 × 105 cells per well in 96-well culture plates. Every 2 days, 100 μl of culture supernatant was replaced with 100 μl of medium supplemented with 20 IU IL-2. On day 8, the cell-free culture supernatant was assayed with an in-house Aalto Bio Reagents Ltd. HIV-1 p24 antigen enzyme-linked immunosorbent assay (ELISA). HIV-1 (BaL) virions were lysed with Empigen BB detergent (catalog no. 45165; Sigma), and p24 antigen was bound to polyclonal antibody and detected with an alkaline phosphatase conjugate. The procedure was carried out as described by Aalto Bio Reagents Ltd. ELISA plates were read in a luminometer (Fluostar Omega; BMG Labtach), and the results are expressed as the mean ± standard error of the mean (SEM) for each concentration of p24 in the pre- and postimmunization PBMCs.
(v) Assay of RANTES, MIP-1α, and MIP-1β in plasma and culture supernatants.
Quantitation of RANTES, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β (CCL-5, CCL-3, and CCL-4, respectively) was carried out by a Luminex bead assay using Fluorokine multianalyte profiling (MAP) kits (R&D, Oxford, United Kingdom), as described previously (15).
(vi) Flow cytometry analysis of cell surface expression of CCR5 and CXCR4 on CD4+ T cells.
CCR5 and CXCR4 expression on CD4+ T cells was identified by incubating 1 × 106 PBMCs with antibodies specific to CCR5 or CXCR4 (BD Biosciences, United Kingdom). After 20 min of incubation, the cells were washed and analyzed by flow cytometry, and live cells were gated and expressed as the proportion of CCR5 or CXCR4 on CD4+ T cells.
(vii) Intracellular APOBEC3G and cytokine studies by flow cytometry.
Intracellular APOBEC3G protein expression on CD4+ T cells was assayed by intracellular staining with anti-APOBEC3G monoclonal antibodies (MAbs; kindly supplied by NIBSC, United Kingdom), as described elsewhere (8). APOBEC3G MAb was conjugated with fluorescein isothiocyanate (FITC) using a LYNX rapid fluorescein antibody conjugation kit (ABD Serotec, Oxford, United Kingdom). After cell surface staining with anti-CD4 MAb (Biolegend, United Kingdom), the cells were washed and fixed with a fixation buffer for 3 min (eBioscience, Insight Biotechnology, London, United Kingdom), followed by treatment with the permeabilization buffer (eBioscience). Ten microliters of FITC-conjugated APOBEC3G antibody (10 μg/ml) was added to the cell pellets, and following 30 min of incubation, the cells were washed and analyzed by flow cytometry on a FACSCanto II flow cytometer (BD Biosciences) using FACSDiva software.
For intracellular cytokine staining, cells were surface stained with CD4 antibody, fixed for 10 min, and permeabilized. The cell pellets were incubated with prediluted FITC-conjugated antibodies to IL-12, gamma interferon (IFN-γ), or IL-6 (all from Biolegend, United Kingdom). After 30 min of incubation, the cells were washed and analyzed by flow cytometry.
(viii) Lymphocyte proliferation assay.
Lymphocyte proliferation was determined by use of a Cell Trace carboxyfluorescein succinimidyl ester (CFSE) cell proliferation kit (Molecular Probes, Invitrogen, United Kingdom). PBMCs were isolated from blood by Ficoll-Hypaque separation and stored in liquid nitrogen. Unstimulated cells and HSP70-stimulated (10 μg/ml), HIVgp140-stimulated (10 μg/ml), or tetanus toxoid (TT)-stimulated (10 μg/ml) cells were cultured, cell proliferation was determined by dilution of the CFSE-labeled CD4+ and CD8+ T lymphocyte populations after 7 days of incubation by staining with the corresponding monoclonal antibodies (Biolegend, Cambridge Biosciences, United Kingdom), and the cells were gated for analysis. The results are expressed as a proportion of the total number of PBMCs and the total level of CD4 or CD8 T cell proliferation in the population.
(ix) Quantification of HIV CN54gp140- and HSP70-specific IgG and IgA antibodies in serum and cervical and vaginal secretions by ELISA.
The titer of HIVgp140 and HSP70-specific antibodies was measured by an indirect ELISA based on that described previously (23), using HIV-CN54gp140 (GMP product from Polymun, Vienna, Austria) and purified HSP70 (Lionex, Germany). Serum samples and samples of cervical and vaginal secretions were titrated at a starting dilution of 1/200 or 1/4 with 0.05% PBS-Tween 20, respectively. Goat anti-human IgG or IgA peroxidase conjugates were used for specific IgG and IgA antibodies, respectively; the latter recognize both monomeric and dimeric (mostly secretory) IgA antibodies.
Statistical methods.
In this pilot phase 1 clinical trial, no randomization was performed and the subjects were allocated to a single cohort. One subject missed the third immunization due to an unrelated adverse event but completed further safety assessments. One subject had a missing cervical secretion sample due to a laboratory processing error. There were no other protocol deviations. All data are expressed as means ± SEMs. The Wilcoxon rank sum test was used for analysis of the significance between the pre- and postimmunization values. Spearman correlation analysis was applied to examine the relation between the assay variables.
RESULTS
Subjects enrolled.
Nine female subjects aged 20 to 30 years (median age, 21 years) were enrolled. One subject was excluded before the first immunization due to a medical event, so the cohort consisted of 8 subjects (see Table S1A in the supplemental material).
AEs.
Generally, the vaccine was well tolerated, with only transient and mild-moderate AEs being experienced (see Table S1B in the supplemental material). A total of 3 AEs were thought to be possibly associated with vaccine administration on the basis of the temporal association: one subject reported moderate-intensity vulvitis and vulval erythema and increased vaginal discharge over a 3- to 4-day period commencing on the day of the second immunization. However, she had reported vulvitis on the day before this immunization, and similar symptoms with no obvious relationship to immunization occurred at three other times during the study period. There were no vaccine-related serious adverse events (SAEs).
Rabbit serum and vaginal IgG and IgA responses to CH54gp140 and HSP70.
A preclinical toxicology assessment was carried out in 4 New Zealand rabbits by vaginal immunization of the vaccine (3 times) to test for any toxicity of the vaccine, though an HIVgp120-HSP70 vaccine used in vaginal immunization in macaques was free of adverse affects (21). Four control rabbits received saline. Serum samples obtained before immunization and at day 16 after immunization were analyzed for antibodies by ELISA. At necropsy, mucosal secretions were collected from the vagina and vaginal vestibule (one sample from each site) using a Weck-Cel sponge. As the primary purpose of this study was toxicology, the accelerated intravaginal immunization schedule (days 1, 8, and 15) and sampling points (days 0 and 16) were not optimized to detect immune responses. Nevertheless, vaginal immunization induced an increase in the levels of serum IgG and IgA against CN54gp140 and HSP70 compared with those in sham-immunized animals, and the levels of mucosal IgA antibodies to CN54gp140 were higher in immunized animals than in sham-immunized animals (see Fig. S1 in the supplemental material).
CC chemokines in human plasma and culture supernatants from human PBMCs.
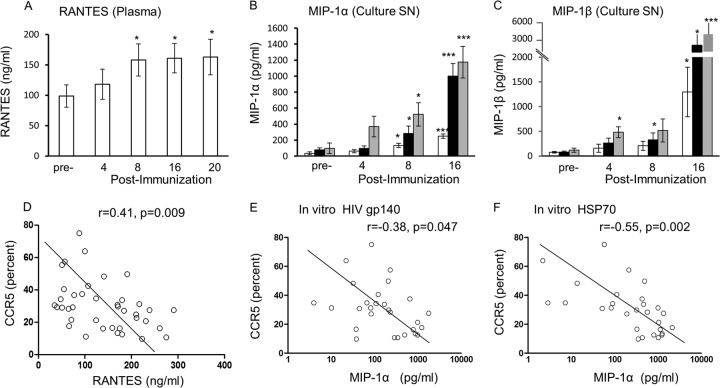
CC chemokines MIP-1α (CCL-3), MIP-1β (CCL-4), and RANTES (CCL-5) in plasma and culture supernatant were studied by the Luminex-bead assay, as these may bind CCR5 and thereby block and/or downmodulate the coreceptor. A significant increase in RANTES was found in plasma after the 2nd immunization (week 8; P < 0.05; Fig. 1A), and this increase was maintained after the 3rd immunization (weeks 16 and 20; from 98.8 ± 18.2 ng/ml before immunization to 163 ± 29.3 ng/ml after immunization). The MIP-1β level in plasma showed a significant increase from 132 ± 46.8 to 165 ± 52.9 pg/ml (P < 0.05) after the 1st immunization and a further increase after the 2nd immunization, but the variation (SEM) between the 8 subjects was large (see Fig. S1 in the supplemental material). MIP-1α, however, was not detected. The individual data are presented in Table S2 in the supplemental material.
FIG 1.
Vaginal immunization at weeks 0, 4, and 12 induces increases in RANTES concentrations (ng/ml) in plasma (A) and MIP-1α concentrations (pg/ml) (B) and MIP-1β (pg/ml) concentrations (C) in the culture supernatants (SN) of PBMCs alone (□) or PBMCs stimulated with HIVgp140 (■) or HSP70 ( ) (n = 8). The correlations between CCR5 and RANTES (D) and between CCR5 and MIP-1α after in vitro stimulation with HIVgp140 (E) and HSP70 (F) were assayed preimmunization and at 4, 8, 16, and 20 weeks postimmunization (all readings before and after each immunization), but only samples from 7 subjects were available because of the limited number of cells available at 4, 8, 16, and 20 weeks postimmunization. Statistical tests used the Wilcoxon rank sum test for panels A to C and Spearman correlation analyses for panels D to F. *, P < 0.05; ***, P < 0.005.
) (n = 8). The correlations between CCR5 and RANTES (D) and between CCR5 and MIP-1α after in vitro stimulation with HIVgp140 (E) and HSP70 (F) were assayed preimmunization and at 4, 8, 16, and 20 weeks postimmunization (all readings before and after each immunization), but only samples from 7 subjects were available because of the limited number of cells available at 4, 8, 16, and 20 weeks postimmunization. Statistical tests used the Wilcoxon rank sum test for panels A to C and Spearman correlation analyses for panels D to F. *, P < 0.05; ***, P < 0.005.
The three CC chemokines were also assayed in culture supernatants generated over the 7 days of culture of PBMCs pre- and postimmunization, as well as those stimulated in vitro with HIVgp140 or HSP70. The results demonstrated that while MIP1α was significantly upregulated after the 2nd (P < 0.05) and 3rd (P < 0.005) immunizations (Fig. 1B), MIP-1β showed a significant increase only after the 3rd immunization (Fig. 1C). Furthermore, MIP-1α and MIP-1β were significantly upregulated after the 2nd immunization when PBMCs were stimulated in vitro with either HIVgp140 or HSP70 (Fig. 1B and C). After the 3rd immunization, very significant upregulation of MIP-1α (P < 0.005) and MIP-1β (P < 0.05 and P < 0.005) was found in culture supernatants from HIVgp140- and HSP70-stimulated PBMCs, respectively (Fig. 1B and C). The individual data are presented in Table S3 in the supplemental material. While RANTES showed almost a 4-fold increase after the 2nd and 3rd immunizations, when HIVgp140- and HSP70-stimulated cell culture supernatants were tested, the 5% level of significance was reached only with HIVgp140 (P < 0.05; data not shown).
CCR5 and CXCR4 expression of human CD4+ T cells.
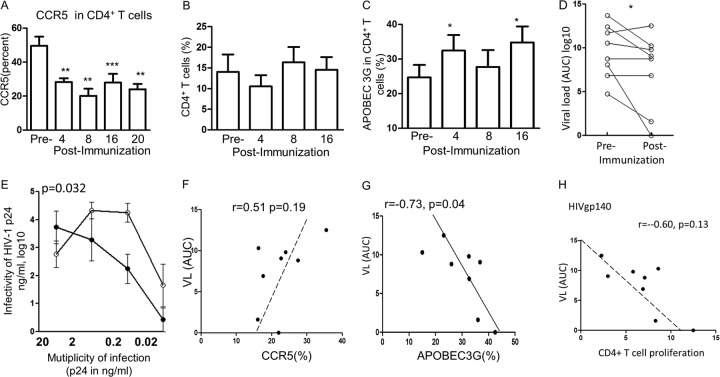
CCR5 is a major coreceptor for HIV-1 and is downmodulated by CC chemokines or HSP70, resulting in decreased HIV-1 infectivity. This was studied by multicolor flow cytometry, using anti-CCR5 or anti-CXCR4 and anti-CD4 antibodies. A significant decrease in CCR5 expression was found in the gated live cells after the 1st immunization (P = 0.01), and this decrease was well maintained throughout the period of investigation (Fig. 2A). The individual data are presented in Table S4 in the supplemental material. The CXCR4 coreceptor is bound only by the X4 strain of HIV-1; the level of this coreceptor was also decreased, but only after the second immunization, and the P value was lower (data not presented). It is noteworthy that the significant decrease in the CCR5+ subset of CD4+ T cells occurred without a corresponding change in the total CD4+ T cell population (Fig. 2B).
FIG 2.
(A) Downregulation of CCR5+ CD4+ T cells after each immunization at 0, 4, and 12 weeks; (B) regulation of the corresponding CD4+ T cells showing no change; (C) APOBEC3G upregulation in CD4+ T cells following each immunization; (D) viral load (expressed as the area under the curve [AUC]) pre- and postimmunization in the PBMCs of the 8 female volunteers; (E) multiplicity of infection of HIV-1 (BaL) (expressed as the mean ± SEM) preimmunization (○) and postimmunization (●) with the HIVgp140-HSP70 vaccine by the mucosal route (3 times). (F to H) Correlation between the viral load (VL) and CCR5 expression (F), APOBEC3G expression (G), and CD4+ T cell proliferation (H) after the 3rd immunization (week 16). Statistical analyses were performed as described in the legend to Fig. 1. *, P < 0.05; **, P < 0.01, ***, P < 0.001.
Correlation between CC chemokines and CCR5 expression.
Having found the upregulation of 3 CC chemokines and the downregulation of CCR5 expression, we studied the potential correlation between them. Indeed, the plasma RANTES (CCL-5) concentration was inversely correlated with the proportion of CCR5 molecules expressed by CD4+ T cells (P < 0.01; Fig. 1D), but the MIP-1β concentration was not, even though the concentration was significantly raised (data not presented). The MIP-1α concentration in the culture supernatant was also inversely correlated with the proportion of CCR5 molecules with stimulation in vitro with HIVgp140 (P < 0.05) or HSP70 (P < 0.01) (Fig. 1E and F), as well as without further stimulation (P < 0.01; data not presented). Although the MIP-1β concentration in the culture supernatants showed inverse correlations with CCR5 levels, it failed to reach the 5% level of significance (data not presented). The intriguing differences between the results for plasma and those for culture supernatants are presently not clear.
APOBEC3G in CD4+ T cells.
The purpose of studying the innate restriction factor APOBEC3G is based on its effect on the inhibition of intracellular HIV-1, once Vif has been inactivated by HSP70. Intracellular flow cytometry with anti-APOBEC3G monoclonal antibody showed significant upregulation after the 1st (P < 0.05) and 3rd (P < 0.05) immunizations (Fig. 2C). An early appearance of APOBEC3G might be critical in inhibiting any HIV which may have gained access into the CD4+ T cells. The individual data are presented in Table S5 in the supplemental material. Furthermore, APOBEC3G was positively correlated with MIP-1β (P < 0.05; see Fig. S2A in the supplemental material), and this is consistent with the latter binding CCR5 and upregulating APOBEC3G.
Human T cell proliferative responses.
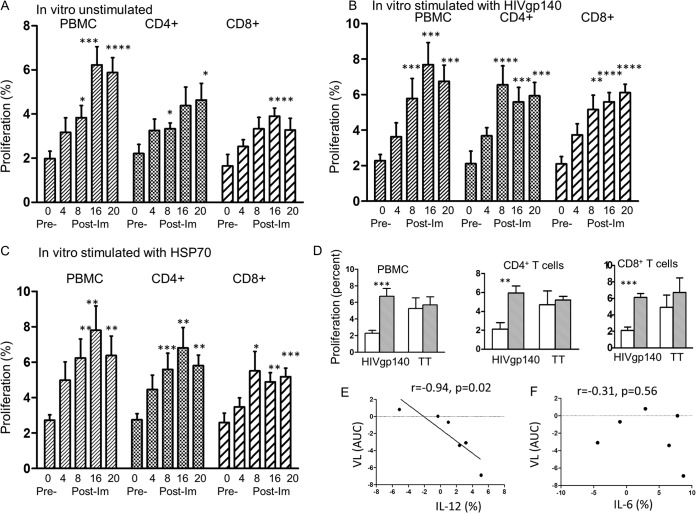
PBMC and CD4+ and CD8+ T cell proliferative responses were tested with samples of blood taken before (week 0) and after each of the 3 immunizations to study any cellular responses induced by immunization. These were tested by the CFSE method, which showed a significantly increased proliferation of PBMCs after the 2nd immunization (P < 0.05) and greatly enhanced proliferation after the 3rd immunization compared with that at the baseline, before immunization (P < 0.001) (Fig. 3A). In vitro stimulation with HIVgp140 or HSP70 also resulted in a significant increase in proliferation after the 2nd immunization with HIVgp140 (P < 0.001; Fig. 3B) or HSP70 (P < 0.01; Fig. 3C) compared with that at the baseline. Furthermore, CD4+ T cells showed a progressive increase in proliferation following immunization, and the increase reached a significant level after the 2nd immunization (P < 0.05), but the increase for CD8+ T cells was significant only after the 3rd immunization (P < 0.001) (Fig. 3A). In vitro stimulation with HIVgp140 or HSP70 elicited very significant specific proliferation by the CD4+ and CD8+ T cells after the 2nd stimulation, but the P values for HIVgp140 were higher than those for HSP70 (Fig. 3B and C). The individual data are presented in Tables S6A to C in the supplemental material. The nonspecific TT, to which most people have been vaccinated, stimulated pre- and postimmunization PBMC, CD4+, and CD8+ proliferative responses higher than those stimulated by HIVgp140 (Fig. 3D) or HSP70 (not presented), but the responses to HIVgp140 and HSP70 were significantly increased (P < 0.01 to P < 0.001) and maintained for PBMCs and CD4+ and CD8+ T cells up to 8 weeks after the 3rd immunization, when the experiment was terminated. Altogether, in vivo vaginal immunization elicited significant CD4+ and CD8+ T cell proliferation, and the CD4+ and CD8+ T cells showed specific HIVgp140 and HSP70 responses when challenged in vitro with these immunogens.
FIG 3.
Proliferative responses of PBMCs and CD4+ and CD8+ T cells of 8 women immunized at 0, 4, and 12 weeks. Blood samples were taken before and 4 weeks after each vaginal immunization, and the last reading was 8 weeks after the final (3rd) immunization. (A) Proliferative response of PBMCs and CD4+ and CD8+ T cells before and after each immunization with no stimulation in vitro. (B and C) Proliferative responses with in vitro stimulation with HIVgp140 (B) and HSP70 (C). (D) Proliferative responses before immunization □ and after the 3rd immunization ( ) with stimulation in vitro with TT compared with that with HIVgp140. Pre- versus postimmunization statisticsl analyses were carried out by the Wilcoxon rank sum test. (E) The net difference between the pre- and postimmunization values for cytokines IL-12 and IL-6 (see Table S7 in the supplemental material) was plotted against the viral load (VL) as the area under the curve. *, P < 0.05; **, P ≤ 0.01; ***, P < 0.001; ****, P < 0.005. Im, immunization.
) with stimulation in vitro with TT compared with that with HIVgp140. Pre- versus postimmunization statisticsl analyses were carried out by the Wilcoxon rank sum test. (E) The net difference between the pre- and postimmunization values for cytokines IL-12 and IL-6 (see Table S7 in the supplemental material) was plotted against the viral load (VL) as the area under the curve. *, P < 0.05; **, P ≤ 0.01; ***, P < 0.001; ****, P < 0.005. Im, immunization.
Cytokines in CD4+ T cells.
The cytokine effector response in CD4+ T cells was assayed by intracellular staining with monoclonal antibodies to IL-12, IFN-γ, and IL-6. Adequate numbers of PBMCs were found in 6 of the 8 cases pre- and postimmunization (see Table S7 in the supplemental material). The levels of IL-12 and IL-6 were increased in 4 out of 6 samples, and the net difference between the pre- and postimmunization levels was plotted against the viral load (Fig. 3E and F). The levels of both cytokines showed an inverse correlation, but only that for IL-12 was significant (P = 0.02). IFN-γ showed no changes, and the data for IFN-γ are not presented. IL-12 is a potent Th-1 cytokine and may contribute to the protection against HIV. IL-6 plays an important role in the mechanism of transition from innate to acquired immunity (26).
Correlation between CCR5 or APOBEC3G and T cell proliferative responses.
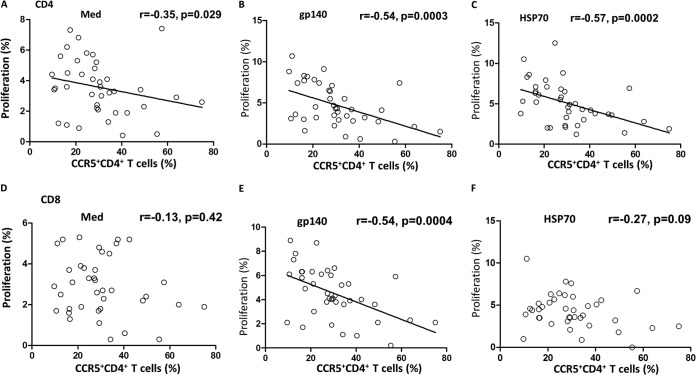
We next examined any correlation between the innate CCR5 or APOBEC3G response and the T cell adaptive proliferative response. Surprisingly, very significant inverse correlations were found between CCR5 and CD4+ T cell or PBMC proliferation when the cells were stimulated with HIVgp140 or HSP70 (P < 0.0005; Fig. 4B and C) and to a limited extent when the cells were unstimulated (P < 0.05; Fig. 4A). However, the corresponding PBMCs showed very significant correlations (P = 0.002 to P < 0.0001; see Fig. S2B to D in the supplemental material). CD8+ T cells showed a significant correlation only when stimulated with HIVgp140 (P = 0.0004; Fig. 4E). APOBEC3G, however, showed no significant correlations with any of the proliferative responses (data not presented). Thus, a novel interaction between the innate and adaptive cellular responses was recorded.
FIG 4.
(A to C) Proliferative responses of CD4+ T cells plotted against the proportion of CCR5+ CD4+ T cells not stimulated (use of medium [Med] alone) (A), stimulated in vitro with HIVgp140 (B), or stimulated in vitro with HSP70 (C). (D to F) Proliferative responses of CD8+ T cells plotted against the proportion of CCR5+ CD4+ T cells not stimulated (D), stimulated with HIVgp140 (E), and stimulated with HSP70 (F). The statistical analyses used Spearman correlation.
HIV-1 infectivity ex vivo.
To determine if immunization inhibits HIV-1, we determined the ex vivo infectivity of HIV-1 for the pre- and postimmunization PBMCs by testing the multiplicity of infection of the HIV-1 BaL strain for activated PBMCs for 8 days, and the culture supernatants were tested for HIV-1 p24 by ELISA. These assays showed a significant inhibition of infectivity of PBMCs with HIV-1 (BaL) postimmunization compared with that preimmunization, as assayed by ELISA for determination of the concentration of p24 from the 2 areas under the curves for activated CD4+ T cells (P = 0.032; Fig. 2E). Of the PBMCs from 8 subjects, PBMCs from 5 demonstrated a decrease in infectivity, PBMCs from 2 showed no change, and PBMCs from 1 showed an increase in infectivity of <1 log unit (P = 0.032; Fig. 2D).
The infectivity of PBMCs collected after immunization was then analyzed in comparison with the innate immune parameters of CD4+ T cells. CCR5 showed a positive correlation, as expected, with a correlation coefficient of 0.51, which failed, however, to reach the 5% level of significance (Fig. 2F). APOBEC3G was significantly inversely correlated (P < 0.05; Fig. 2G). The CD4+ T cell proliferative response with stimulation with HSP70 (r = −0.60; Fig. 2H) or HIVgp140 (r = −0.38; data not presented) also showed an inverse correlation but failed to reach significance, even though the former showed a high r value. Thus, the 2 innate and 1 adaptive immune responses studied showed either a significant or a strong trend toward protective correlations with HIV-1 infectivity.
IgG and IgA responses to HIV-1 CN54gp140 and HSP70 in human serum and cervical and vaginal secretions.
The levels of anti-HIV-1 and hsp70 IgG and IgA antibodies in serum and cervical and vaginal secretions were measured before and after each immunization with HIVgp140 and HSP70 using an antigen-specific antibody binding ELISA. No systemic or mucosal IgG and IgA response to HIVgp140 or HSP70 was detected after vaginal immunization (data not shown). The discrepancy in eliciting rabbit but not human antibodies is not surprising, as, for ill-defined reasons, antibodies can be readily induced in rabbits.
DISCUSSION
A number of studies have been carried out in macaques (27–29) and women (24, 30), in which the vaginal mucosal route of immunization of HIV-1 antigens was compared with the systemic, oral, or nasal route of immunization. The subjects were free of adverse effects, and the immunizations yielded variable immune responses, depending on the immunogen, adjuvant, the combined route of immunization, and the parameters investigated. The present phase 1 trial of vaginal immunization with HIV-1 CN54gp140 linked to HSP70 in women showed that it is a safe procedure. A significant inhibition of HIV-1 infectivity of PBMCs was found ex vivo postimmunization compared with that preimmunization. Although the infection was assayed ex vivo, there is evidence from studies in rhesus macaques that ex vivo viral inhibition is consistent with in vivo viral control following viral challenge of the vaccinated animals (31).
HIV tropism is largely generated by coreceptor selection, but initial transmission is commonly by the R5 strain of HIV-1, which utilizes the CCR5 coreceptor (32–34). The mature envelope glycoprotein spikes, comprised of a trimer of a gp120-gp41 heterodimer, first bind the CD4 receptor on CD4+ T cells, and the bound HIVgp140 undergoes conformational changes and then binds the CCR5 coreceptor. The lower that the level of expression of CCR5 is, the less binding of HIVgp140 that takes place, consistent with the present findings. Three CC chemokines, CCL-3, CCL-4, and CCL-5, bind to CCR5, which may block and/or downmodulate the coreceptors, thereby inhibiting HIV transmission in vitro (35) and in vivo (36). The most striking resistance to HIV infection occurs in individuals with the naturally occurring homozygous Δ32 CCR5 mutation, which is found in approximately 1% of Caucasians (37). These individuals lack cell surface expression of CCR5, have increased concentrations of the three CC chemokines, and do not suffer from ill health.
Inhibition of HIV-1 infectivity is likely to have been associated with the significant downregulation of CCR5 in CD4+ T cells, which was recorded after the 1st immunization and maintained throughout the investigation (P < 0.01 to 0.001). This was most likely due to the significant increase in plasma CCL-5 and/or CCL-3 and CCL-4 levels in the culture supernatants of PBMCs. Administration of HSP70 to naive nonhuman primates stimulates CC chemokines by T cells (38). HSP70 activates the CCR5 and CD40 receptors on CD4+ T cells and DCs, respectively, eliciting the upregulation of CC chemokines and the downregulation of CCR5. Indeed, the present data showed a significant inverse correlation between CCR5 expression in CD4+ T cells and the concentration of CCL-5 (RANTES) in plasma or MIP-1α (CCL-3) in the culture supernatants treated in vitro with either HIVgp140 or HSP70 (P < 0.05 to P < 0.01). These data are consistent with an inverse correlation between CCR5 and the CC chemokines in macaques, in which inhibition of SHIV89.6p infection was recorded in vivo (21).
The increase in the level of the innate anti-HIV-1 factor APOBEC3G after the 1st immunization was similar to that found after in vivo rectal mucosal alloimmunization in macaques (8) and was inversely correlated with that after rectal challenge with SHIVSF162.P4, suggesting a protective immune response. HSP70 upregulates APOBEC3G in human CD4+ T cells and DCs by ligating the CCR5 and CD40 molecules (7). It is noteworthy that both the innate antiviral restrictive factor APOBEC3G and the CCL-4 chemokine were upregulated early after the 1st immunization, and this is commensurate with the early downregulation of CCR5 expression of CD4+ T cells. Importantly, CCR5 showed a direct correlation with the viral load, whereas APOBEC3G showed an inverse correlation with the viral load, consistent with a decrease in CCR5 and an increase in APOBEC3G in CD4+ T cells inhibiting HIV-1 infectivity. These findings suggest a dual innate mechanism in which HIV-1 is inhibited by CC chemokines blocking and downmodulating CCR5 and any virus that may have entered the CD4+ T cells is likely inhibited by upregulated APOBEC3G. Vif, which counteracts APOBEC3G, is unlikely to cope with the increased amount of APOBEC3G, but quite apart from this, HSP70 inhibits HIV-1 Vif-mediated ubiquitination and the degradation of APOBEC3G (9).
Adaptive immunity was also elicited by HIVgp140 and HSP70, indicated by CD4+ and CD8+ T cell proliferation after the 2nd immunization, and was most significant with HIVgp140-stimulated PBMCs and CD4+ and CD8+ T cells (P < 0.01 to P < 0.001). The kinetics of the immunological responses observed are consistent with the early development of innate immunity, followed by cellular immunity. A surprising strong correlation was found, especially between the CD4+ T cells expressing CCR5 and CD4+ and CD8+ T cells and PBMCs stimulated with HIVgp140 (P = 0.0003, 0.0004, and 0.002, respectively). This potentially important finding suggests a link between the CCR5+ CD4+ T cells, downregulated by the innate CC chemokines and HSP70, and the corresponding CD4+ T cell adaptive response. This is enhanced by the strong trend toward an inverse correlation between CD4+ T cell proliferation and CCR5 expression that was exhibited (r = 0.60) or a significant correlation between IL-12 expression (r = 0.73, P = 0.04) and protection against HIV-1 infectivity. The mechanism of this novel finding will need to be further studied. However, the data suggest that the critical downregulation of CCR5+ CD4 T cells takes place without a decrease of the overall proportion of CD4+ T cells. Altogether, the data suggest the paradigm that the vaccine suppresses HIV-1 infectivity by significantly downmodulating CCR5 expression in CD4+ T cells without compromising the immune system by maintaining the total proportion of CD4+ T cells and proliferative responses. The critical issue is the early production of the dual innate immune responses, consisting of CC chemokines and APOBEC3G, allowing the adaptive immune responses to mature.
HSP70 functions as a coadjuvant stimulating innate and cellular immunity, but it has a limited effect on the Th2 type of immunity. This has been observed previously in macaques immunized vaginally or by targeted immunization of the iliac lymph node with a vaccine consisting of HIVgp120, SIVp27, and CCR5 peptides, all covalently linked to HSP70. The immunization elicited T cell proliferation and CC chemokines but poor serum and vaginal fluid IgG and IgA antibody titers to HIVgp120 yet caused a significant inhibition of SHIV89.6P (21). The failure to elicit specific antibodies to HIVgp140, in addition to the low dose of HIVgp140 used (100 μg in women compared with 150 μg in macaques), can be accounted for by HSP70 acting as a coadjuvant, enhancing predominantly innate immune responses (14–16) and DC and macrophage stimulation (14, 17–19) but inducing poor Th-2 cell activation. The mechanism whereby the vaginal mucosal immunization elicits CC chemokines, APOBEC3G, and T cell proliferative responses is based on the concept of the common mucosal immune system, in which antigenic challenge via the cervicovaginal mucosa elicits the inductive site of immunity by Langerhans cells and macrophages in the mucosa, which process antigens and migrate via the afferent lymphatics to the iliac lymph nodes. Vaginal mucosal immunization with HIV or SIV envelope antigen predominantly induces CD4+ and CD8+ T and B cells in the iliac lymph nodes; these cells then exit via the efferent lymphatics into the circulation, and some home into the genital-associated lymphoid tissue (27, 39, 40). Cells from these lymph nodes preferentially traffic to the regional mucosal site. Thus, the circulating T cells express what takes place in the lymph nodes and the mucosa, which was the site of vaccination. These cells are sensitized to generate CC chemokines and APOBEC3G, which elicit a dual innate response.
The present study provides proof of concept that anti-HIV-1 innate immune responses of APOBEC3G and CC chemokines can be generated by vaginal mucosal immunization in women. APOBEC3G in nonhuman primates has been maintained in vivo for over 20 weeks (8), and ex vivo in humans it is expressed in CD4+ CD45 RO+ memory T cells, so both findings are consistent with a memory-like function (16). Furthermore, it is highly likely that other IFN-stimulated gene products, apart from APOBEC3G, are stimulated. Repeated immunization with SIVgp120 and p27 in macaques enhanced CC chemokine expression, suggestive of secondary responses (41). The conventional view that innate immunity lacks memory has been challenged, as NK cells demonstrate a memory-like effect in the absence of T cells (42), and activation of TLR expressed on DCs stimulates antigen-specific T and B cells and modulates their memory (43). APOBEC3G is predominantly expressed in CD4 CD45 RO+ memory T cells in both nonhuman primates (8) and humans (16). These memory cells in macaques have been maintained for over 20 weeks. However, long-term observations, as well as the adaptive transfer of memory T cells, are needed to establish memory in these innate immune responses.
The sequential appearance of the innate viral restriction factor APOBEC3G, followed by the CCL-3, CCL-4, and CCL-5 chemokines, cellular immune responses, and finally, specific antibodies, is the desired response to a vaccine. Extensive investigations of vaccination against yellow fever virus and smallpox virus have underlined the critical significance of innate immunity to virus infection, in addition to eliciting adaptive immune responses for the long-term maintenance of protective immunity (4, 5). The present study has demonstrated that atraumatic vaginal immunization 3 times with a simple vaccine elicited ex vivo inhibition of HIV-1 replication and an early innate response and later CD4+ and CD8+ immune responses in women. This establishes a template for the development of a broadly based vaccine against HIV-1.
Supplementary Material
ACKNOWLEDGMENTS
The work presented here was funded under contracts with the European Commission's Sixth Framework Programme (EUROPRISE contract LSHP-CT-2006-037611 and MUVAPRED contract LSHP-CT-2003-503240) and Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 280873 ADITEC.
Footnotes
Published ahead of print 9 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01621-14.
REFERENCES
- 1. McMichael A, Picker LJ, Moore JP, Burton DR. 2013. Another HIV vaccine failure: where to next? Nat. Med. 19:1576–1577. 10.1038/nm.3413 [DOI] [PubMed] [Google Scholar]
- 2. Gray G, Buchbinder S, Duerr A. 2010. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr. Opin. HIV AIDS 5:357–361. 10.1097/COH.0b013e32833d2d2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haynes BF, McElrath MJ. 2013. Progress in HIV-1 vaccine development. Curr. Opin. HIV AIDS 8:326–332. 10.1097/COH.0b013e328361d178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131–1137. 10.1038/nm917 [DOI] [PubMed] [Google Scholar]
- 5. Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, III, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sekaly RP. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 205:3119–3131. 10.1084/jem.20082292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheehy AM, Gaddis NC, Choi JD, Malim MH. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646–650. 10.1038/nature00939 [DOI] [PubMed] [Google Scholar]
- 7. Pido-Lopez J, Whittall T, Wang Y, Bergmeier LA, Babaahmady K, Singh M, Lehner T. 2007. Stimulation of cell surface CCR5 and CD40 molecules by their ligands or by HSP70 up-regulates APOBEC3G expression in CD4(+) T cells and dendritic cells. J. Immunol. 178:1671–1679. 10.4049/jimmunol.178.3.1671 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Bergmeier LA, Stebbings R, Seidl T, Whittall T, Singh M, Berry N, Almond N, Lehner T. 2009. Mucosal immunization in macaques upregulates the innate APOBEC 3G anti-viral factor in CD4(+) memory T cells. Vaccine 27:870–881. 10.1016/j.vaccine.2008.11.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sugiyama R, Nishitsuji H, Furukawa A, Katahira M, Habu Y, Takeuchi H, Ryo A, Takaku H. 2011. Heat shock protein 70 inhibits HIV-1 Vif-mediated ubiquitination and degradation of APOBEC3G. J. Biol. Chem. 286:10051–10057. 10.1074/jbc.M110.166108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su L, Graf M, Zhang Y, von Briesen H, Xing H, Kostler J, Melzl H, Wolf H, Shao Y, Wagner R. 2000. Characterization of a virtually full-length human immunodeficiency virus type 1 genome of a prevalent intersubtype (C/B′) recombinant strain in China. J. Virol. 74:11367–11376. 10.1128/JVI.74.23.11367-11376.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srivastava P. 2002. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2:185–194. 10.1038/nri749 [DOI] [PubMed] [Google Scholar]
- 12. Panjwani N, Akbari O, Garcia S, Brazil M, Stockinger B. 1999. The HSC73 molecular chaperone: involvement in MHC class II antigen presentation. J. Immunol. 163:1936–1942 [PubMed] [Google Scholar]
- 13. Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. 2000. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J. Exp. Med. 191:1957–1964. 10.1084/jem.191.11.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Kelly CG, Karttunen JT, Whittall T, Lehner PJ, Duncan L, MacAry P, Younson JS, Singh M, Oehlmann W, Cheng G, Bergmeier L, Lehner T. 2001. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15:971–983. 10.1016/S1074-7613(01)00242-4 [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T. 2002. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J. Immunol. 169:2422–2429. 10.4049/jimmunol.169.5.2422 [DOI] [PubMed] [Google Scholar]
- 16. Pido-Lopez J, Wang Y, Seidl T, Babaahmady K, Vaughan R, Lehner T. 2009. The effect of allogeneic in vitro stimulation and in vivo immunization on memory CD4(+) T-cell APOBEC3G expression and HIV-1 infectivity. Eur. J. Immunol. 39:1956–1965. 10.1002/eji.200939228 [DOI] [PubMed] [Google Scholar]
- 17. Becker T, Hartl FU, Wieland F. 2002. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J. Cell Biol. 158:1277–1285. 10.1083/jcb.200208083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Floto RA, MacAry PA, Boname JM, Mien TS, Kampmann B, Hair JR, Huey OS, Houben EN, Pieters J, Day C, Oehlmann W, Singh M, Smith KG, Lehner PJ. 2006. Dendritic cell stimulation by mycobacterial Hsp70 is mediated through CCR5. Science 314:454–458. 10.1126/science.1133515 [DOI] [PubMed] [Google Scholar]
- 19. Whittall T, Wang Y, Younson J, Kelly C, Bergmeier L, Peters B, Singh M, Lehner T. 2006. Interaction between the CCR5 chemokine receptors and microbial HSP70. Eur. J. Immunol. 36:2304–2314. 10.1002/eji.200635953 [DOI] [PubMed] [Google Scholar]
- 20. Babaahmady K, Oehlmann W, Singh M, Lehner T. 2007. Inhibition of human immunodeficiency virus type 1 infection of human CD4+ T cells by microbial HSP70 and the peptide epitope 407-426. J. Virol. 81:3354–3360. 10.1128/JVI.02320-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bogers WM, Bergmeier LA, Ma J, Oostermeijer H, Wang Y, Kelly CG, Ten Haaft P, Singh M, Heeney JL, Lehner T. 2004. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. AIDS 18:25–36. 10.1097/00002030-200401020-00003 [DOI] [PubMed] [Google Scholar]
- 22. Barrios C, Georgopoulos C, Lambert PH, Del Giudice G. 1994. Heat shock proteins as carrier molecules: in vivo helper effect mediated by Escherichia coli GroEL and DnaK proteins requires cross-linking with antigen. Clin. Exp. Immunol. 98:229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curran RM, Donnelly L, Morrow RJ, Fraser C, Andrews G, Cranage M, Malcolm RK, Shattock RJ, Woolfson AD. 2009. Vaginal delivery of the recombinant HIV-1 clade-C trimeric gp140 envelope protein CN54gp140 within novel rheologically structured vehicles elicits specific immune responses. Vaccine 27:6791–6798. 10.1016/j.vaccine.2009.08.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis DJ, Fraser CA, Mahmoud AN, Wiggins RC, Woodrow M, Cope A, Cai C, Giemza R, Jeffs SA, Manoussaka M, Cole T, Cranage MP, Shattock RJ, Lacey CJ. 2011. Phase I randomised clinical trial of an HIV-1(CN54), clade C, trimeric envelope vaccine candidate delivered vaginally. PLoS One 6:e25165. 10.1371/journal.pone.0025165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kingsley C, Peters B, Babaahmady K, Pomeroy L, Rahman D, Vaughan R, Lehner T. 2009. Heterosexual and homosexual partners practising unprotected sex may develop allogeneic immunity and to a lesser extent tolerance. PLoS One 4:e7938. 10.1371/journal.pone.0007938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoebe K, Janssen E, Beutler B. 2004. The interface between innate and adaptive immunity. Nat. Immunol. 5:971–974. 10.1038/ni1004-971 [DOI] [PubMed] [Google Scholar]
- 27. Lehner T, Bergmeier LA, Panagiotidi C, Tao L, Brookes R, Klavinskis LS, Walker P, Walker J, Ward RG, Hussain L. 1992. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science 258:1365–1369. 10.1126/science.1360702 [DOI] [PubMed] [Google Scholar]
- 28. Klavinskis LS, Bergmeier LA, Gao L, Mitchell E, Ward RG, Layton G, Brookes R, Meyers NJ, Lehner T. 1996. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J. Immunol. 157:2521–2527 [PubMed] [Google Scholar]
- 29. Wang Y, Abel K, Lantz K, Krieg AM, McChesney MB, Miller CJ. 2005. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J. Virol. 79:14355–14370. 10.1128/JVI.79.22.14355-14370.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566–574. 10.4049/jimmunol.169.1.566 [DOI] [PubMed] [Google Scholar]
- 31. Stephenson KE, Li H, Walker BD, Michael NL, Barouch DH. 2012. Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys. J. Virol. 86:9583–9589. 10.1128/JVI.00996-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. 10.1126/science.272.5270.1955 [DOI] [PubMed] [Google Scholar]
- 33. Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. 10.1038/381661a0 [DOI] [PubMed] [Google Scholar]
- 34. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. 10.1038/381667a0 [DOI] [PubMed] [Google Scholar]
- 35. Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811–1815. 10.1126/science.270.5243.1811 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Tao L, Mitchell E, Bravery C, Berlingieri P, Armstrong P, Vaughan R, Underwood J, Lehner T. 1999. Allo-immunization elicits CD8+ T cell-derived chemokines, HIV suppressor factors and resistance to HIV infection in women. Nat. Med. 5:1004–1009. 10.1038/12440 [DOI] [PubMed] [Google Scholar]
- 37. Paxton WA, Martin SR, Tse D, O'Brien TR, Skurnick J, VanDevanter NL, Padian N, Braun JF, Kotler DP, Wolinsky SM, Koup RA. 1996. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat. Med. 2:412–417. 10.1038/nm0496-412 [DOI] [PubMed] [Google Scholar]
- 38. Lehner T, Bergmeier LA, Wang Y, Tao L, Sing M, Spallek R, van der Zee R. 2000. Heat shock proteins generate beta-chemokines which function as innate adjuvants enhancing adaptive immunity. Eur. J. Immunol. 30:594–603 http://dx.doi.org/10.1002/1521-4141(200002)30:2<594::AID-IMMU594>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- 39. Lehner T, Tao L, Panagiotidi C, Klavinskis LS, Brookes R, Hussain L, Meyers N, Adams SE, Gearing AJ, Bergmeier LA. 1994. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J. Virol. 68:1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN, Step Study Protocol Team. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Tao L, Mitchell E, Bergmeier L, Doyle C, Lehner T. 1999. The effect of immunization on chemokines and CCR5 and CXCR4 coreceptor functions in SIV binding and chemotaxis. Vaccine 17:1826–1836. 10.1016/S0264-410X(98)00482-4 [DOI] [PubMed] [Google Scholar]
- 42. O'Leary JGM, Goofdarzi M, Drayton DL, von Andrian UH. 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 5:507–516. 10.1038/ni1332 [DOI] [PubMed] [Google Scholar]
- 43. Pulendran B, Ahmed R. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849–863. 10.1016/j.cell.2006.02.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.