Summary
Arterial ischaemic stroke is an important cause of morbidity in children. Timely diagnosis is necessary for acute stroke treatment but can be challenging in clinical practice. Due to a paucity of data there are no specific recommendations regarding the use of mechanical thrombectomy devices in current paediatric stroke guidelines. A 14-year-old boy presented with a severe acute left hemisphere stroke due to a proximal middle cerebral artery occlusion caused by emboli from an atrial myxoma. No clinical improvement was seen after administration of intravenous thrombolysis. Subsequent mechanical thrombectomy with a second-generation stent-based thrombectomy device resulted in successful recanalization and clinical improvement. To our knowledge, this is the first report of mechanical thrombectomy in a child with acute embolic stroke caused by atrial myxoma.
Keywords: paediatric stroke, myxoma, thrombolysis, CT angiography, thrombectomy
Introduction
The estimated incidence rate of arterial ischaemic stroke in children ranges from 1.2 to 7.9/100000 per year 1,2. Paediatric stroke is an increasingly recognized cause of long-term morbidity in the developing child and has an estimated case death rate of 6%.
No randomized controlled trials of treatment in acute childhood stroke have been performed. Current consensus guidelines do not recommend the use of thrombolytics outside of clinical trials in children age 14 and younger, although clinical practice in large centres differs 4. There is also no consensus in the AHA/ASA guidelines regarding the use of thrombolysis for older adolescents (age 15 and older) 5.
Paediatric stroke can be caused by several conditions, such as cardio-embolism, vasculopathy and prothrombotic conditions. A rare cause of embolism is atrial myxoma, which can spread emboli to the central nervous system. In adults with myxoma-related embolic ischaemic stroke, there is a concern of a higher risk of haemorrhage after intravenous thrombolysis (IVT) due to concomitant intracranial micro-aneurysms and occult tumour emboli 6,7. It has been argued that known myxoma is a relative contraindication to intravenous thrombolytic therapy, but often ischaemic stroke is the first presenting symptom of a myxoma 7. Symptomatic intracranial haemorrhage after IVT has been reported in one of seven previously reported cases, although no micro-aneurysms could be demonstrated on cerebral angiography 7-13. Successful and safe IVT has been described in younger adults with myxoma-related stroke 9-13. Uncomplicated intra-arterial thrombolysis (IAT) in an adolescent, as well as endovascular thrombectomy after IVT in four adults with myxoma-related ischaemic stroke have been reported, although with varying radiological and clinical outcomes 14-17. We describe a case of safe and successful recanalization with subsequent clinical improvement using mechanical thrombectomy after intravenous thrombolysis in a 14-year-old patient with a cardio-embolic stroke due to atrial myxoma. This case indicates that mechanical thrombectomy may be considered in the treatment of acute large vessel paediatric ischaemic stroke due to atrial myxoma.
Case Report
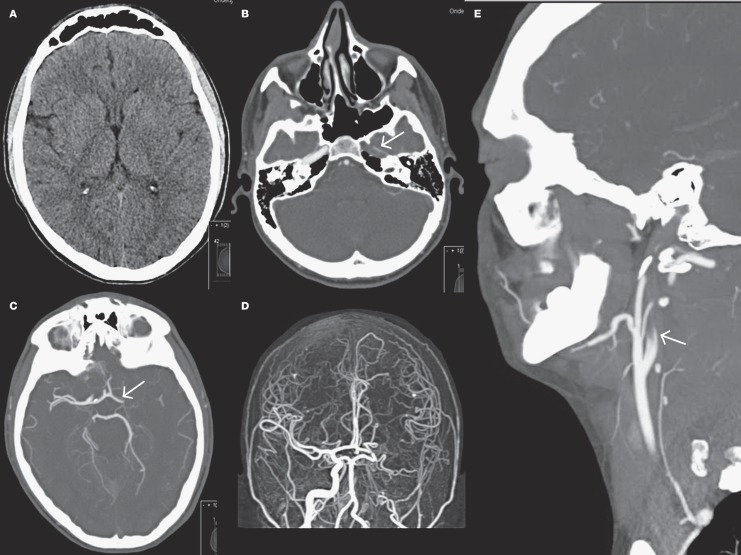
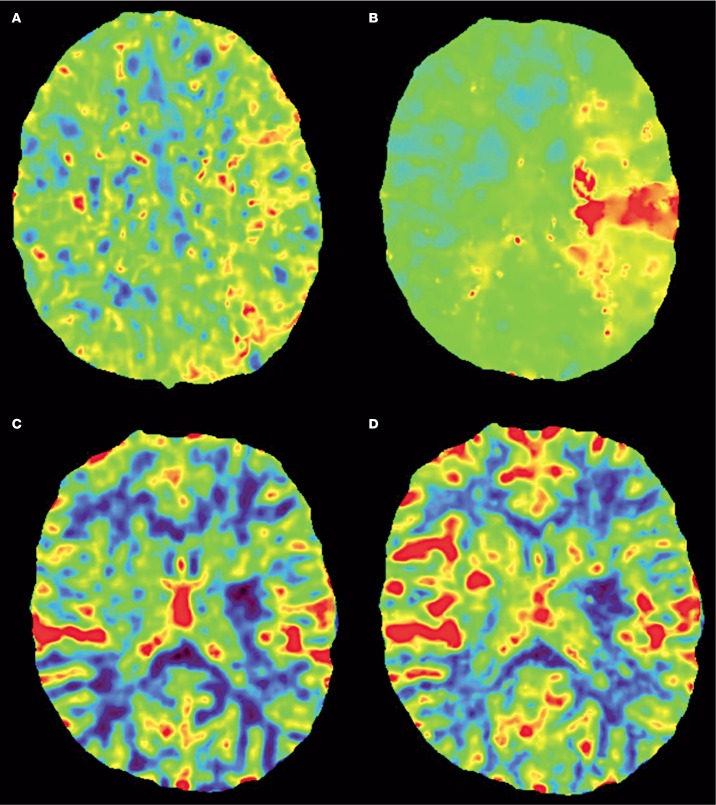
A 14-year-old, right-handed boy presented to our hospital after sudden onset severe aphasia, left gaze deviation and right-sided hemiplegia, resulting in a Paediatric National Institute of Health Stroke Scale (PedNIHSS) score of 21 suggesting a severe middle cerebral artery (MCA) stroke. His family history of stroke was negative and he did not use recreational drugs. Prior to his admission he had noticed typical skin lesions. His symptoms started a year before when he noticed purpuric macules on the palms of his hands, the soles of his feet, and calves. The macules seemed to appear after exercise and exposure to cold. Non-contrast computed tomography (Figure 1A) showed no early ischaemic changes except some hypo-attenuation and effacement in the striatum on the left side. CT angiography (CTA, Figure 1B-E) showed an occlusion of the left MCA as well as non-opacification of the left internal carotid artery (ICA) above the carotid bulb, which was interpreted as probable pseudo-occlusion 18. Good collateral supply of the occluded MCA territory was demonstrated by pial retrograde backfilling almost to the level of the occlusion. CT perfusion (CTP, Figure 2) showed an extensive area of the left MCA territory with increased mean transit time and time-to-peak (Figure 2A,B), while only a limited area showed a decreased cerebral blood volume (CBV) and flow (Figure 2C,D), indicating a favourable penumbral pattern 19.
Figure 1.
Axial non-contrast CT scan without early ischaemic changes except some effacement in the striatum on the left side. B) Axial CT angiography at the level of the cavernous segment shows absence of contrast at the left internal carotid artery (arrow). C,D) CT angiography with maximum intensity projection images of the intracranial arteries shows an occlusion of the left middle cerebral artery. E) Sagittal CT angiography image shows non-opacification of the left internal carotid artery above the carotid bulbus.
Figure 2.
Axial CT perfusion shows an extensive area of the left middle cerebral artery territory with increased mean transit time (A) and time-to-peak (B), while only a limited area shows a decreased cerebral blood volume (C) and flow (D), indicating a favourable penumbral pattern.
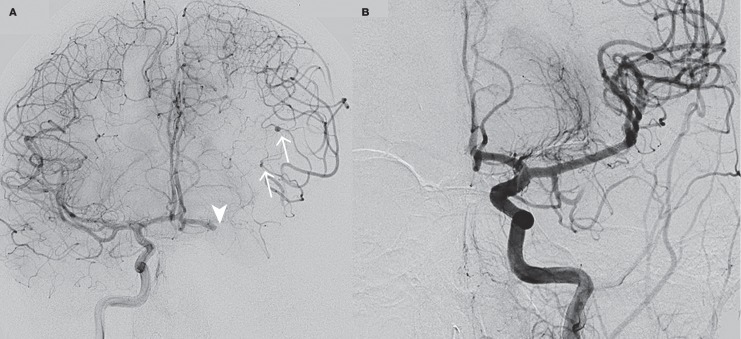
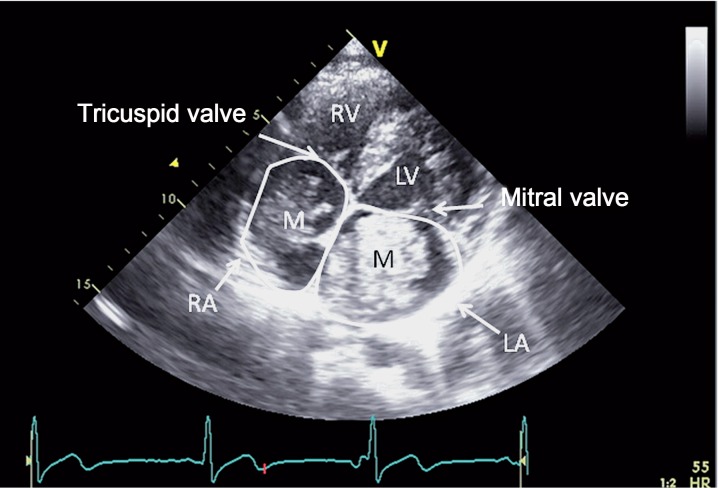
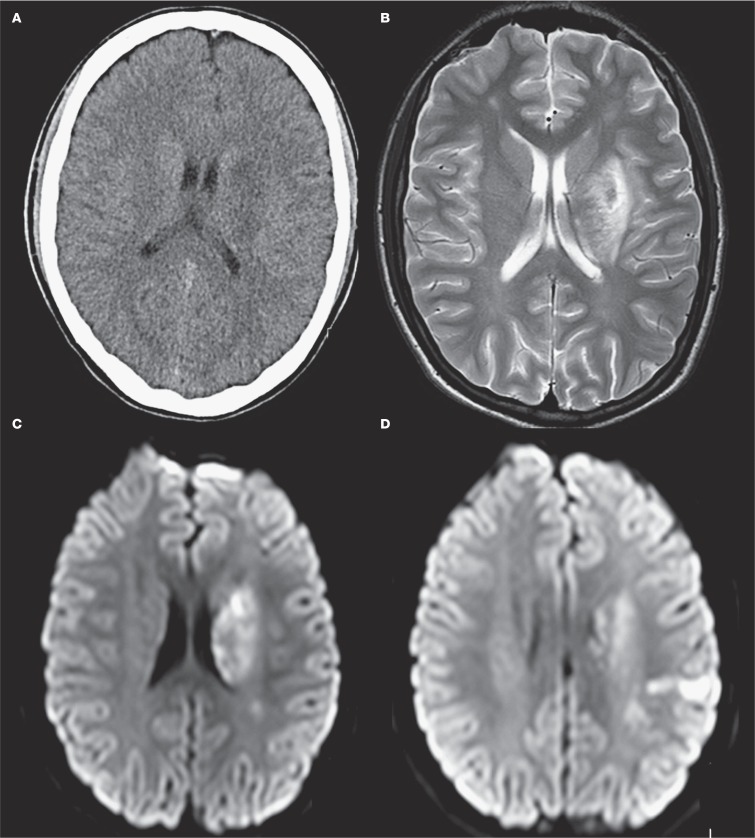
After the patient and his family were informed in detail about the risks, it was decided to treat the patient with IVT four hours after onset of symptoms (0.9 mg/kg recombinant tissue plasminogen activator). Because this treatment did not result in a rapid improvement of symptoms and based on careful analysis of imaging features showing only a small area of decreased CBV with good collateral supply, our patient underwent subsequent endovascular treatment five hours after stroke onset. After puncture of the right common femoral artery, catheterization of the left common carotid artery was performed using a 5F Guider Softip Guide Catheter (Boston Scientific, Natik, MA, USA). Digital subtraction angiography (DSA) confirmed the left MCA occlusion and also showed three small fusiform M3 aneurysms (Figure 3A). The left ICA was catheterized without any signs of occlusion or dissection, which confirmed our diagnosis of an ICA pseudo-occlusion 18. Mechanical thrombectomy was performed with the Solitaire Flow Restoration device (Covidien/EV3, Dublin, Ireland) which required only one pass and resulted in successful recanalization, TICI (Thrombolysis in Cerebral Infarction) grade 2b (Figure 3B). The puncture site was treated with ten minutes of manual compression. The next day a central retinal artery occlusion was noted on the left side. Echocardiography showed a mass in the right as well as left atrium, suspected for atrial myxoma (Figure 4). Follow-up brain imaging showed a limited area of ischaemia in the region of the basal ganglia, parietal cortex and mesencephalon on the left side, without evidence of haemorrhage (Figure 5). Twenty-four hours after presentation our patient underwent open-heart surgery and the mass was successfully removed without further complications. Pathological examination confirmed the diagnosis of myxoma. Since the patient had patchy blue pigmented lesions on his foot, Carney syndrome was suspected. Genetic analysis showed a (de novo) causative mutation of protein kinase A regulatory subunit-1-alpha gene (PRKAR1A) on chromosome 17, which resulted in the final diagnosis of Carney complex type 1, characterized by occurrence of atrial myxomas, skin abnormalities and endocrine tumours.
Figure 3.
A) Digital subtraction angiography of the right internal carotid artery shows a left middle cerebral artery occlusion (arrowhead) as well as distal middle cerebral artery micro-aneurysms (arrows). B) Digital subtraction angiography after mechanical thrombectomy, TICI grade 2b.
Figure 4.
Cardiac ultrasound, apical 4 chamber view, shows a mass in the right as well as the left atrium (M), which turned out to be a myxoma (RV=right ventricle LV=left ventricle).
Figure 5.
A) Axial CT scan at day 1 shows a limited area of infarction in the left basal ganglia. T2 weighted (B) and diffusion-weighted (C,D) MRI at day 7: images show a limited area of infarction in the left basal ganglia and parietal cortex. A limited area of infarction was also noticed in the mesencephalon, not shown in this figure.
Neurological improvement was noted after mechanical thrombectomy, in which the aphasia disappeared and there was only a mild remaining weakness of the right arm and leg. Visual loss of the left eye, however, persisted. The patient was referred to an ambulant rehabilitation program and later returned to school. At three-month follow-up the Paediatric NIHSS score was 5 and the modified Rankin scale was 2, corresponding to slight disability.
Discussion
The current treatment for children with acute ischaemic stroke is difficult because of age-related differences in the coagulation and fibrinolytic systems, different stroke pathophysiology compared to adults and lack of randomized controlled trials in the young population 4,20. Based on a review of the scarce literature covering endovascular acute ischaemic stroke therapy in children, mechanical approaches seem safer than treatment with intra-arterial tissue plasminogen activator because of a lower haemorrhage rate 21. Recently published cases of paediatric acute ischaemic stroke treated with mechanical endovascular therapies suggested that the use of endovascular treatment can be beneficial 22,23. Successful and safe bridging therapy with IV tPA followed by mechanical thrombectomy in paediatric stroke has also been reported previously 24. Until data from randomised trials are available to support the use of endovascular thrombolytic therapies in children with ischaemic stroke, the management of these challenging cases must be individualized 21, taking into consideration the stroke aetiology, the natural history of the disease, the clinical status, imaging features and the experience of the neurointerventional team.
Cardiac disease, which constitutes a potentially modifiable risk factor, is identified in almost a third of children with acute ischaemic stroke 25. Often the diagnosis of cardiac embolism is not made before acute stroke treatment is started. Patients with cardiac myxoma usually present with one or more of the triad of embolism, both cerebral and peripheral, intracardiac obstruction, and constitutional symptoms like fever and weight loss. Recurrent multiple purpuric patches on the palms or soles can be an important clue to the diagnosis of a cardiac myxoma 26, which allows for early diagnosis if recognized. Neurologic manifestations of atrial myxomas are frequent and have been reported in 25-45% of cases, of which embolic stroke is the most common manifestation 27,28. Emboli from atrial myxoma are composed of thrombus, neoplasm, or a composite of both. Thrombotic emboli are rich in fibrin, similar to other cardiac sources and are likely to be amenable to thrombolysis. The response of a myxomatous embolism to thrombolysis is unpredictable 14 and tumour emboli from myxoma are unlikely to lyse with thrombolytic therapy 7. In general, prompt resection of a myxoma is required because of the risk of embolization or cardiovascular complications, including sudden death. The results of surgical resection are generally good, with low peri-operative mortality rates 29,30.
Our patient was almost 15 years old, had severe neurological deficits with an acute large vessel occlusion of undetermined cause at presentation, good collateral status and a suggestion of a large area of salvageable brain. Since there is no consensus in the guidelines 5 regarding the use of thrombolysis for older adolescents, we employed IVT in accordance with the eligibility criteria as used in adults. Because of failure of intravenous thrombolysis and the presence of a favourable imaging pattern, endovascular treatment with a second-generation stent-based thrombectomy device was initiated.
In the present case, the central retinal artery occlusion was likely not related to the endovascular treatment because of a more than four-hour time difference between procedure and event. Moreover follow-up MR angiography excluded carotid artery dissection. The visual loss could have been a result of subsequent cardiac embolization 12,31 suggesting a high recurrent stroke risk and in the absence of cerebral haemorrhage an indication for urgent cardiac surgery.
Finally, our report confirms the previously documented association between atrial myxoma in younger patients and the formation of multiple cerebral micro-aneurysms 6. The distal location, fusiform shape and multiplicity are typical for myxomatous aneurysms 32. To our knowledge, this is the first report in which safe IVT has been described in the presence of multiple micro-aneurysms associated with cardiac myxoma. The presence of myxoma-related micro-aneurysms and the composition of myxomatous emboli could argue against treatment with thrombolytics, while mechanical thrombectomy may carry less risk and have potentially more benefit in such cases. To further investigate the efficacy and safety of thrombolysis and thrombectomy in acute paediatric stroke, collaboration across institutes is needed.
Acknowledgments
The authors thank Arno AW Roest, MD PhD, Paediatric Cardiologist, Department of Paediatric Cardiology, Leiden University Medical Centre, for his assistance in the preparation of this manuscript.
References
- 1.Broderick J, Talbot GT, Prenger E, et al. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8(3):250–255. doi: 10.1177/088307389300800308. doi: 10.1177/088307389300800308. [DOI] [PubMed] [Google Scholar]
- 2.Giroud M, Lemesle M, Gouyon JB, et al. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48(11):1343–1348. doi: 10.1016/0895-4356(95)00039-9. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 3.Steinlin M, Pfister I, Pavlovic J, et al. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): a population-based study of incidence, symptoms and risk factors. Neuropediatrics. 2005;36(2):90–97. doi: 10.1055/s-2005-837658. doi: 10.1055/s-2005-837658. [DOI] [PubMed] [Google Scholar]
- 4.Simma B, Holiner I, Luetschg J. Therapy in pediatric stroke. Eur J Pediatr. 2013;172(7):867–875. doi: 10.1007/s00431-012-1863-9. doi: 10.1007/s00431-012-1863-9. [DOI] [PubMed] [Google Scholar]
- 5.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39(9):2644–2691. doi: 10.1161/STROKEAHA.108.189696. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 6.Sabolek M, Bachus-Banaschak K, Bachus R, et al. Multiple cerebral aneurysms as delayed complication of left cardiac myxoma: a case report and review. Acta Neurol Scand. 2005;111(6):345–350 2005; 111 (6): 345-350.. doi: 10.1111/j.1600-0404.2005.00413.x. doi: 10.1111/j.1600-0404.2005.00413.x. [DOI] [PubMed] [Google Scholar]
- 7.Chong JY, Vraniak P, Etienne M, et al. Intravenous thrombolytic treatment of acute ischemic stroke associated with left atrial myxoma: a case report. J Stroke Cerebrovasc Dis. 2005;14(1):39–41. doi: 10.1016/j.jstrokecerebrovasdis.2004.09.002. doi: 10.1016/j.jstrokecerebrovasdis.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Acampa M, Tassi R, Guideri F, et al. Safety of intravenous thrombolysis in ischemic stroke caused by left atrial myxoma. Curr Drug Saf. 2011;6(5):343–345. doi: 10.2174/157488611798918665. doi: 10.2174/157488611798918665. [DOI] [PubMed] [Google Scholar]
- 9.da Silva IR, de Freitas GR. Is it safe to proceed with thrombolytic therapy for acute ischemic stroke in a patient with cardiac myxoma? Case report and review of the literature. Eur Neurol. 2012;68(3):185–186. doi: 10.1159/000340019. doi: 10.1159/000340019. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim M, Iliescu C, Safi HJ, et al. Biatrial myxoma and cerebral ischemia successfully treated with intravenous thrombolytic therapy and surgical resection. Tex Heart Inst J. 2008;35(2):193–195. [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy CD, Levy M, Mulhearn TJ, et al. Safe and effective intravenous thrombolysis for acute ischemic stroke caused by left atrial myxoma. J Stroke Cerebrovasc Dis. 2009;18(5):398–402. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.012. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Ong CT, Chang RY. Intravenous thrombolysis of occlusion in the middle cerebral and retinal arteries from presumed ventricular myxoma. Stroke Res Treat. 2010;2011:735057. doi: 10.4061/2011/735057. doi: 10.4061/2011/735057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun MC, Tai HC, Lee CH. Intravenous thrombolysis for embolic stroke due to cardiac myxoma. Case Rep Neurol. 2011;3:21–26. doi: 10.1159/000324095. doi: 10.1159/000324095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bekavac I, Hanna JP, Wallace RC, et al. Intra-arterial thrombolysis of embolic proximal middle cerebral artery occlusion from presumed atrial myxoma. Neurology. 1997;49(2):618–620. doi: 10.1212/wnl.49.2.618. doi: 10.1212/WNL.49.2.618. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Ptacek S, Matias-Guiu JA, Valencia-Sánchez C, et al. Mechanical endovascular treatment of acute stroke due to cardiac myxoma. J Neurointerv Surg. 2014;6(1):e1. doi: 10.1136/neurintsurg-2012-010343. doi: 10.1136/neurintsurg-2012-010343. [DOI] [PubMed] [Google Scholar]
- 16.Gassanov N, Nia AM, Dahlem KM, et al. Local thrombolysis for successful treatment of acute stroke in an adolescent with cardiac myxoma. Scientific World Journal. 2011;11:891–893. doi: 10.1100/tsw.2011.90. doi: 10.1100/tsw.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamanome T, Yoshida K, Miura K, et al. [Superselective fibrinolysis for a middle cerebral artery embolism caused by a left atrial myxoma: case report] No Shinkei Geka. 2000;28(7):653–658. [PubMed] [Google Scholar]
- 18.Marquering HA, Nederkoorn PJ, Beenen LF, et al. Carotid pseudo-occlusion on CTA in patients with acute ischemic stroke: A concerning observation. Clin Neurol Neurosurg. 2013;115(9):1591–1594. doi: 10.1016/j.clineuro.2013.02.008. doi: 10.1016/j.clineuro.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Kidwell CS, Wintermark M, De Silva DA, et al. Multiparametric MRI and CT models of infarct core and favorable penumbral imaging patterns in acute ischemic stroke. Stroke. 2013;44(1):73–79. doi: 10.1161/STROKEAHA.112.670034. doi: 10.1161/STROKEAHA.112.670034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amlie-Lefond C, deVeber G, Chan AK, et al. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8(6):530–536. doi: 10.1016/S1474-4422(09)70106-1. doi: 10.1016/S1474-4422(09)70106-1. [DOI] [PubMed] [Google Scholar]
- 21.Ellis MJ, Amlie-Lefond C, Orbach DB. Endovascular therapy in children with acute ischemic stroke: review and recommendations. Neurology. 2012;79(13) Suppl 1:S158–S164. doi: 10.1212/WNL.0b013e31826958bf. doi: 10.1212/WNL.0b013e31826958bf. [DOI] [PubMed] [Google Scholar]
- 22.Tatum J, Farid H, Cooke D, et al. Mechanical embolectomy for treatment of large vessel acute ischemic stroke in children. J Neurointerv Surg. 2013;5(2):128–134. doi: 10.1136/neurintsurg-2011-010100. doi: 10.1136/neurintsurg-2011-010100. [DOI] [PubMed] [Google Scholar]
- 23.Sainz de la Maza S, Felipe AD, Matute MC, et al. Acute ischemic stroke in a 12-year-old successfully treated with mechanical thrombectomy. J Child Neurol. 2014;29(2):269–273. doi: 10.1177/0883073813509889. doi: 10.1177/0883073813509889. [DOI] [PubMed] [Google Scholar]
- 24.Fink J, Sonnenborg L, Larsen LL, et al. Basilar artery thrombosis in a child treated with intravenous tissue plasminogen activator and endovascular mechanical thrombectomy. J Child Neurol. 2013;28(11):1521–1526. doi: 10.1177/0883073812460334. doi: 10.1177/0883073812460334. [DOI] [PubMed] [Google Scholar]
- 25.Mackay MT, Wiznitzer M, Benedict SL, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69(1):130–140. doi: 10.1002/ana.22224. doi: 10.1002/ana.22224. [DOI] [PubMed] [Google Scholar]
- 26.Lee HJ, Park JY, Kim YS, et al. Cardiac myxoma diagnosed by signs of purpuric macules on both palms and soles. Ann Dermatol. 2012;24(3):337–340. doi: 10.5021/ad.2012.24.3.337. doi: 10.5021/ad.2012.24.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekinci EI, Donnan GA. Neurological manifestations of cardiac myxoma: a review of the literature and report of cases. Intern Med J. 2004;34(5):243–249. doi: 10.1111/j.1444-0903.2004.00563.x. doi: 10.1111/j.1444-0903.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 28.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80(3):159–172. doi: 10.1097/00005792-200105000-00002. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Keeling IM, Oberwalder P, Anelli-Monti M, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg. 2002;22(6):971–977. doi: 10.1016/s1010-7940(02)00592-4. doi: 10.1016/S1010-7940(02)00592-4. [DOI] [PubMed] [Google Scholar]
- 30.Selkane C, Amahzoune B, Chavanis N, et al. Changing management of cardiac myxoma based on a series of 40 cases with long-term follow-up. Ann Thorac Surg. 2003;76(6):1935–1938. doi: 10.1016/s0003-4975(03)01245-1. doi: 10.1016/S0003-4975(03)01245-1. [DOI] [PubMed] [Google Scholar]
- 31.Rafuse PE, Nicolle DA, Hutnik CM, et al. Left atrial myxoma causing ophthalmic artery occlusion. Eye (Lond) 1997;11(Pt 1):25–29. doi: 10.1038/eye.1997.5. doi: 10.1038/eye.1997.5. [DOI] [PubMed] [Google Scholar]
- 32.Tamuleviciute E, Taeshineetanakul P, Terbrugge K, et al. Myxomatous aneurysms: a case report and literature review. Interv Neuroradiol. 2011;17(2):188–194. doi: 10.1177/159101991101700208. [DOI] [PMC free article] [PubMed] [Google Scholar]