Summary
The application of high intense focused ultrasound (HIFU) is currently the subject of many experimental and clinical trials. The combination of HIFU with MRI guidance known as MR-guided focused ultrasound (MRgFUS) appears to be particularly promising to ablate tissues located deep in the brain. The method can be the beginning of interventional neurology and an important alternative to neurosurgery. Studies conducted to date show the effectiveness of the method both in chronic diseases and in emergency cases. The safety and effectiveness of this method have been observed in parkinsonian and essential tremor as well as in neuropathic pain. The procedure does not require anaesthesia. Ionizing radiation is not used and there is no risk of cumulative dose. Such advantages may result in low complication rates and medical justification for further development of MRgFUS.
Keywords: MR-guided focused ultrasound, essential tremor, parkinsonian tremor, neuropathic pain
Introduction
The prospects of non-invasive surgery for the diseases of the nervous system are based on two technologies, that is high intense focused ultrasound (HIFU), which heats and non-invasively destroys the targeted tissue, and magnetic resonance imaging (MRI) used for visualization of anatomical structures and treatment control. Magnetic resonance-guided focused ultrasound (MRgFUS) ablation is a combination of these two methods.
Ultrasound is widely used in medicine, both in diagnostic and therapeutic methods. The device generating and recording ultrasound waves provides images of internal organs. In urology lithotripsy is used to disintegrate kidney stones by a focused ultrasound beam. In ophthalmology ultrasound is used for cataract surgery by means of phacoemulsification. Ultrasound is also used in medical rehabilitation in physical therapy.
The characteristic feature of ultrasound is mechanical power which can be differently modified. Intensity, frequency, duration, or the shape of the ultrasonic pulse are subject to modification. Three mechanisms (thermal, stress and cavitation) must be considered in ultrasound therapy. Each of these non-linear physical processes is significant in sonication treatment that is the use of ultrasonic pulses to destroy the targeted tissue.
Non-invasiveness constitutes the main advantage of the method which does not require anaesthesia and offers an incisionless treatment through the intact skull. The procedure is characterized by high precision imaging and excellent tissue differentiation (intraoperative MR guidance allows targeted location with an accuracy of 1 mm) and safety (real-time thermal and procedural monitoring). The procedure causes only minor discomfort and can be performed without the need for hospitalization. It provides immediate therapeutic effect and a quick return to normal activities. The procedure carries a minimal risk of complications (no risk of infection, blood loss, shift of brain structures or allergic reactions). It is free of ionizing radiation therefore can be repeated as needed. The effectiveness of the method has been clinically proven.
Non-invasive neurosurgery
Neurological disorders such as essential tremor (ET), Parkinson’s disease (PD) and neuropathic pain (NP) affect the lives of millions of people. They may result in disability, suffering and drug dependence by lowering the quality of life of both patients and their caregivers.
Medicine is currently supported by technological development to find forms of treatment suitable for the patient. Such technological progress has allowed the use of surgical treatment in patients with ET and PD who do not respond to pharmacological treatment.
Damage to the thalamus was one of the surgical methods previously used in patients with unilateral tremor resistant to pharmacological treatment. It has been replaced by newer procedures. Deep brain stimulation (DBS), as opposed to previously used methods, is reversible and does not permanently result in brain damage. The implanted electrode changes brain activity in a controlled manner. However, DBS and other methods used to treat PD and ET such as radiofrequency ablation and radiosurgery are invasive or cause exposure to ionizing radiation. Furthermore, such procedures are associated with a recognized risk of complications and side-effects, including infection, haemorrhage and damage to the surrounding brain tissue.
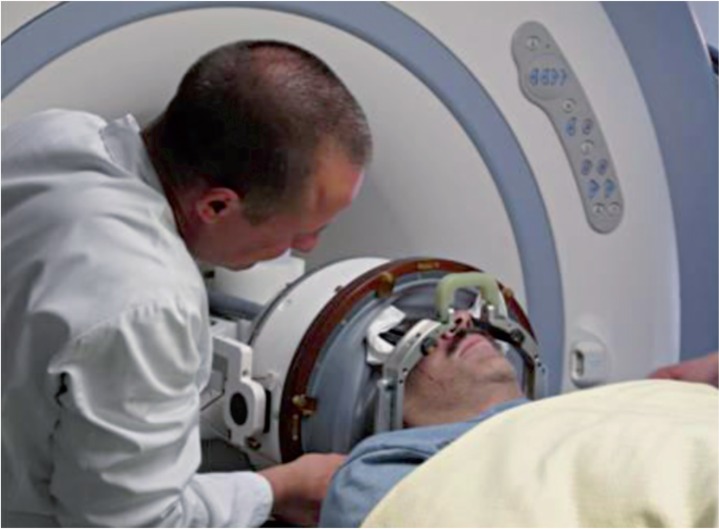
Magnetic resonance-guided focused ultrasound (MRgFUS) is a new generation treatment which minimizes the occurrence of the above complications. It appears to be a promising method for non-invasive treatment of ET, NP and PD. InSightec developed ExAblate which pioneered MrgFUS (Figures 1 and 2).
Figure 1.
Figure 2.
The ExAblate Neuro system has been awarded the European CE mark for targeted procedures in the thalamus, subthalamus and globus pallidus regions in tremor-dominant PD, ET and NP. The method may be a completely new approach and offer a potential alternative to neurosurgery, considering current preclinical and clinical studies focused on safety, efficacy and reproducibility of MRgFUS in the treatment of uterine fibroids, breast cancer, adenomyosis or palliative management of pain related to bone metastases.
Poor permeability and high ultrasound absorption by the skull were a technological barrier, complicating the easy transfer of HIFU methods (used so far in the soft tissue region) to intracranial applications. The problem was solved using a special phased array system operating at frequencies of 0.5 - 1.5 MHz and the helmet-like apparatus to prevent heating of the skull bones. Clinical studies are currently being conducted in Switzerland, the United States and South Korea. Preliminary results demonstrate the possibility of effective and precise thermal ablation of the areas located deep in the brain 1.
Theory
The ultrasound beam can be focused geometrically by special lenses, spherically curved transducers or electronically by a phased array, which is currently more frequent. A characteristic feature of HIFU is the use of the multi-element ultrasound phased array with a multichannel electronic system. It allows independent activation of the selected elements from the specified parameters of delay to control (aberration and focusing of) the ultrasound beam. Multiphased array differs from standard ultrasound heads in that it consists of multiple individual transducers (up to 1000), each of which can independently emit and receive ultrasonic waves.
Hariharan et al. 2 analysed the impact of the focused ultrasound of intensity below cavitation threshold and nonlinear boiling. This energy was large enough to raise tissue temperature by a few degrees per second. The extent of tissue damage can be predicted by the cumulative equivalent minutes. For most studies conducted on the basis of HIFU therapy, calculations are based on the model proposed by Dewey and Sapareto 3.
For this technology to be safely used for therapeutic purposes, it was necessary to develop methods allowing accurate imaging of the structure subjected to sonication and real-time precise three-dimensional thermal monitoring.
MRI met these requirements. In the 1980s, Bottomley et al. created the first 1.5 T MRI scanner, which allowed the first brain imaging. The studies of Corbett et al. from the 1990s proved that it is possible to measure temperature accurately in vivo in any brain region using magnetic resonance spectroscopy 4,5.
History
The study of Wood and Loomis conducted in 1927 was the earliest report on the effects of ultrasound on living tissues 6. The first studies on the use of HIFU for non-invasive ablation were reported by Lynn et al. at the beginning of the 1940s. They managed to perform controlled ablation of the liver of an ox without damaging the surrounding tissue 7. Soon afterwards, the Fry brothers started trials to use HIFU for brain tissue ablation. During their studies on cats they confirmed the non-invasiveness of HIFU. There was no need to cut brain tissue, interrupt blood vessels or open the dura mater 8,9. The first attempts to use focused ultrasound in humans for controlled brain tissue ablation were made in 1959. Patients with PD underwent therapy. During the procedure, part of the globus pallidus was damaged to reduce tremor. It was necessary to remove bone fragments of the skull. X-ray imaging was used. Despite the promising results, the development of the method was abandoned due to the difficulties related to the exact localization of brain structures for ablation and the introduction of levodopa, which brought good results with fewer treatment complications 10. The following years resulted in theoretical studies, indicating the potential benefits of non-invasive neurosurgery using focused ultrasound. The breakthrough was made as late as the 1990s when HIFU was combined with MRI. At the beginning of the 21st century the first precise procedures on rabbit brains were performed using MRI spectroscopy 11.
Possibilities of MRgFUS application in neurological conditions
Essential tremor
Essential tremor (ET) is one of the most common movement disorders. The incidence is estimated at 0.3-5.6% in the general population and it increases with age. Two prevalence peaks are observed, the first in early adulthood and the second the age of 60. On average, the first symptoms appear between the ages of 35 and 45. The cause of the disease and its pathomechanism are still unknown. The main symptoms include tremor of the hands, arms and head.
The clinical course is frequently benign, however disability due to ET is common. Eighty-five percent of ET patients report significant changes in their lives and 15% report significant disability related to the disease. In a study on hereditary ET, 60% of patients could not find employment, 25% changed jobs or took early retirement, 30% withdrew from social life and 20% stopped driving.
Pharmacotherapy is usually the first-line treatment in ET and most patients maintain a good quality of life when treated with propranolol and primidone. However, approximately 30% of patients do not respond to medications. In these “treatment-resistant patients” surgical treatment may be considered. Most frequently the ventral intermediate nucleus of the thalamus (VIM) is damaged using one of the following methods:
− RFID (radio-frequency identification): the tip of the electrode is placed in a given spot and heat from the high frequency alternating current is generated.
− Stereotactic radiosurgery as a form of radiotherapy focuses high-power x-rays on a small surface.
− Gamma knife thalamotomy is an approach in which the tissue is damaged by gamma radiation.
Another alternative is DBS. It is a surgical procedure in which a neurostimulator is implanted and produces an electrical stimulation of a given brain area blocking abnormal nerve signals which cause tremor. One of the advantages of this method is the possibility of adjusting the stimulation after implantation of the neurostimulator 12,13.
The results of the first clinical trial of MRgFUS application in ET treatment were presented by Elias et al. in 2012 14. The study was conducted on 15 ET patients at the University of Virginia who had significantly exacerbated symptoms of ET which complicated their normal activities and who did not show significant improvement after long-term pharmacotherapy. The therapeutic procedure consisted of unilateral thermal ablation of the ventral intermediate nucleus of the thalamus to reduce tremor on the dominant side. After stereotactic targeting controlled by MR, heating of a selected spot in the thalamus to 43°C was generated. MR spectroscopy was used to confirm that the targeted area was heated. Then a full-power beam was emitted resulting in a local temperature increase to about 60°C. The patient was conscious throughout the procedure. The assumption of the trial was to assess the usefulness and safety of MRgFUS in ET. It was determined that in each case ablation of the selected fragment in the thalamus was obtained without damage to other brain areas. An eighty percent tremor reduction was achieved on average with the symptoms maintained on the side which did not undergo the procedure. Imaging examinations conducted a week after the procedure revealed a small oedema surrounding the ablation site, which disappeared completely after one month. The results indicate an efficiency comparable to RFID or DBS without the adverse effects characteristic of these two methods.
Effectiveness of tremor suppression was measured using the Clinical Rating Scale for Tremor to calculate the total score (ranging from 0 to 160), hand subscore (primary outcome, ranging from 0 to 32), and disability subscore (ranging from 0 to 32), with higher scores indicating worse tremor. The Quality of Life in Essential Tremor Questionnaire was used to evaluate patients' perception related to treatment efficacy (ranging from 0 to 100%, with higher scores indicating greater perceived disability).
The recent study from Toronto 15 confirms the decrease by 89% and 81% in ET in patients treated with the same method measured after one and three months, respectively, following the procedure.
The clinical trial on 72 patients with ET has been in progress since May 2013. It is a randomized, double-blind study comparing the real intervention with the “fake” procedure. The estimated time of the study is 12 months.
Parkinson's disease
Parkinson's disease (PD) is a progressive, multifactorial neurodegenerative disorder. The incidence is estimated at 0.15% in the general population and there is a tenfold increase to 1.5% over the age of 70. In Poland 80 000 patients are affected by the disease and 4000-8000 new cases are diagnosed annually. The average age at onset is about 56, but early-onset PD (between 21-40) and juvenile-onset PD (before the age of 21) also exist.
The type and severity of symptoms vary depending on the stage of the disease. The most common symptoms include tremor, rigidity, bradykinesia, gait disturbances and unstable posture. The main PD symptoms are the consequence of a significantly reduced activity of dopamine-generating cells caused by neuronal death in the substantia nigra of the midbrain. As the disease progresses, dopamine concentration decreases, impairing the patient's mobility.
The cause of the progressive atrophy of dopamine-generating nerve cells is unknown. Studies on PD aetiology are primarily based on hypotheses. Presumably, various environmental and genetic factors are related to the origin of the disease. So far, no treatment has been able to stop the natural progression of the disease. Current methods of treatment provide only symptomatic improvement. The treatment method depends on the stage of the disease.
As the disease progresses, the administration of levodopa is necessary. However, within a few years after the beginning of the therapy, levodopa-resistant symptoms develop together with movement fluctuations (ON-OFF phases) and dyskinesias. In spite of the optimal intervals and doses of levodopa, in the late PD stage a decrease in proper motor control is observed. Typically, at this time many patients consider surgical intervention to improve the quality of their lives. Currently, DBS of the subthalamic nucleus is used. Stereotactic procedures to reduce tremor by irreversible damage to the thalamus or the globus pallidus are less frequently adopted 16-19.
The first report on the application of sonication in PD came from Jeanmonod et al. 20 who performed ablation of the fibres that join the thalamus with the globus pallidus, which resulted in the spot increase in temperature up to 59°C. Half of the patients were given one impulse, whereas in the other patients sonication was repeated five to six times during the procedure. After three months both groups were assessed on the Unified Parkinson's Disease Rating Scale (UPDRS) by independent neurologists. Patients from the first group obtained improvement by 7.6% on average, while the other group improved by 57.1%. No complications or other undesirable effects were noted. This may result from mean absolute global targeting accuracies between 0.54 and 0.72 mm (SDs between 0.34 and 0.42 mm), which were obtained by employing measurement methods proposed by Moser et al. 1. Magnetic resonance images performed three months after the procedure show the foci of the lesion which are visible only in patients with multiple sonication. The pallido-thalamic tractotomy target placed in a dense fibre bundle is more resistant to heating than nuclear (thalamic) targets.
Since the beginning of 2013 there has been enrolment for another clinical trial by Elias et al. Observation is scheduled for 12 months. Thirty patients with tremor-dominant PD will be studied.
Neuropathic pain
Neuropathic pain (NP) is frequently the result of damage to or dysfunction of the nerve fibres which send incorrect signals to pain centres. Pain significantly lowers patients' quality of life and complicates normal functioning. Unfortunately, as the study results show, the efficiency of NP management is not satisfactory despite regular introduction of new methods and medications and the continuous search for more effective therapeutic options 21-23. It appears that only 50% of NP patients achieve pain relief by 30-50%. However, no improvement is observed in the remaining patients.
Studies on low-threshold calcium spike bursts became the basis for performing central lateral thalamotomy 24,25. Studies of neurosurgeons from Zurich University Hospital proved the procedure to be effective in NP management 26.
In the conducted clinical study, 11 patients with chronic therapy-resistant NP underwent treatment with MRgFUS. Thermal ablation (3-4 mm) was performed in the part of the central lateral thalamic nucleus at peak temperatures between 51 and 64° C with the aid of real-time patient monitoring and MR imaging and MR thermometry guidance. The treated NP syndromes had peripheral (five patients) or central (six patients) origins and covered various parts of the body (face, arm, leg, trunk).
Following the procedure, patients experienced significant pain relief at the three-month follow-up and at the 12-month follow-up (nine and eight patients, respectively). At the same time, according to the visual analogue scale, mean improvement amounted to 42% and 41%.
Six patients experienced immediate post-surgical improvement. Quantitative electroencephalography (EEG) showed a significant reduction in EEG spectral overactivities. MRI revealed thermal ablation sites which showed sharply delineated ellipsoidal thermolesions surrounded by transient oedema. There was one complication noted (bleeding), which resulted in the introduction of the following safety measures, that is using the detection of cavitation and the maintenance of sonication temperature below 60°C 27.
Other potential applications of MRgFUS
A decade ago, in preclinical studies McDannold et al. found that the blood-brain barrier (BBB) may be temporarily disrupted without causing damage. This may be achieved using low-intensity ultrasonic pulses in combination with microbubble contrast agents for ultrasound imaging 19. One of the possible applications of this method is breast cancer. Many female patients respond well to chemotherapy before brain metastases develop. Because of the disruption of BBB, the efficiency of chemotherapeutic drugs in the CNS region is increased and thus an increased penetration of Herceptin and NK-92 cells in primates was demonstrated 29,30. Studies on humans have not been conducted yet.
MRgFUS may also be beneficial in the treatment of haemorrhagic stroke where clotted blood can be dissolved using focused ultrasound, which decreases intracranial pressure. In vivo animal studies and human cadaver studies showed that due to ultrasound blood clots may be nearly completely dissolved without additional damage to the brain tissue. This, in turn, facilitates a minimally invasive clot evacuation through a keyhole craniotomy and aspiration tubes 31. The application of MRgFUS for the ablation of glioblastomas in three patients did not bring therapeutic results. It was possible to reach the pathological tissue and increase its temperature but the power of the device was insufficient. Further attempts after the modification of the equipment will be necessary 32.
Patients with epilepsy who due to insufficient seizure control experience a significant decrease in quality of life despite optimal pharmacotherapy consider invasive methods. Neurosurgery offers implantation of vagus nerve stimulators, removal of the epileptogenic focus or severing of the corpus callosum. The first experiments concerning the application of HIFU in seizure disorders were performed on rats. Generalized seizures in rats were induced by pentylenetetrazole. Afterwards, low-powered pulses of short duration targeted at the thalamus were used. On average, a 30% decrease in the number of spikes on EEG was observed 33. The application of HIFU to remove epileptogenic foci may be a promising therapy. Cerebrovascular accidents in Poland, similarly to other European countries, constitute the second most common cause of death. Since the introduction of recombinant tissue plasminogen activator (rtPA) as the standard treatment for acute ischaemic strokes, methods improving the efficacy of this treatment have been searched for. Sonothrombolysis appears to be the most promising in this respect. It is based on directing the energy of ultrasound frequency of 2 MHz to the occluded vessel site. Ultrasound provided in this manner for at least 60 min increases the chances of vessel recanalization with rtPA. The method is safe and does not increase the risk of haemorrhage 34. However, the significant limitation of the method is a substantial loss of energy passing through the temporal bone (65-90%), which also produces an adverse thermal effect inside the bone. However, MRgFUS lacks such limitations. It is capable of simultaneously emitting multiple independent beams, each of which is of lower intensity compared to the ones emitted by diagnostic heads. However, at the beam intersection a 2×3 mm sharp ellipsoidal area is formed with very high signal intensity. Focused beams may be manoeuvred within a radius of 3 cm without losing the beam intensity. Many in vitro trials have been performed using skulls and vascular models with human blood clots. Each time clot dissolution was achieved after 30 seconds of sonication without rtPA 35. The first in vivo experiments on rabbits indicate the need for the cavitation effect to occur for good vascular recanalization. There are also reports on haemorrhagic complications, which may possibly be eliminated using shorter ultrasound pulses 36,37.
Acknowledgments
The authors thank Arkadiusz Badziński MA for translating the article.
References
- 1.Moser D, Zadicario E, Schiff G, et al. Measurement of targeting accuracy in focused ultrasound functional neurosurgery. Neurosurg Focus. 2012;32(1):E2. doi: 10.3171/2011.10.FOCUS11246. doi: 10.3171/2011.10.FOCUS11246. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan P, Myers MR, Banerjee RK. HIFU procedures at moderate intensities-effect of large blood vessel. Phys. Med. Biol. 2007;52:3493–3513. doi: 10.1088/0031-9155/52/12/011. doi: 10.1088/0031-9155/52/12/011. [DOI] [PubMed] [Google Scholar]
- 3.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800. doi: 10.1016/0360-3016(84)90379-1. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 4.Corbett RJ, aptook AR, Tollefsbol G, et al. Validation of a noninvasive method to measure brain temperature in vivo using 1H NMR spectroscopy. J Neurochem. 1995;64(3):1224–1230. doi: 10.1046/j.1471-4159.1995.64031224.x. doi: 10.1046/j.1471-4159.1995.64031224.x. [DOI] [PubMed] [Google Scholar]
- 5.Corbett R, Laptook AR, Weatherall P. Noninvasive measurements of human brain temperature using volume-localized proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1997;17(4):363–369. doi: 10.1097/00004647-199704000-00001. doi: 10.1097/00004647-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Wood EW, Loomis AL. The physical and biological effects of high-frequency sound-waves of great intensity. Philos Mag. 1927;Series 7(4):417–436. [Google Scholar]
- 7.Lynn J, Zwemer R, Chick A, et al. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26(6):179–193. doi: 10.1085/jgp.26.2.179. doi: 10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry W, Barnard J, Fry F, et al. Ultrasonic lesions in the mammalian central nervous system. Science. 1942;122:517–518. doi: 10.1126/science.122.3168.517. [PubMed] [Google Scholar]
- 9.Fry WJ, Mosberg WH, Barnard JW, et al. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg. 1954;11(5):471–478. doi: 10.3171/jns.1954.11.5.0471. doi: 10.3171/jns.1954.11.5.0471. [DOI] [PubMed] [Google Scholar]
- 10.Meyers R, Fry WJ, Fry FJ, et al. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J Neurosurg. 1959;16(1):32–54. doi: 10.3171/jns.1959.16.1.0032. doi: 10.3171/jns.1959.16.1.0032. [DOI] [PubMed] [Google Scholar]
- 11.Vykhodtseva N, McDannold N, Martin H, et al. Apoptosis in ultrasound-produced threshold lesions in the rabbit brain. Ultrasound Med Biol. 2001;27(1):111–117. doi: 10.1016/s0301-5629(00)00275-1. doi: 10.1016/S0301-5629(00)00275-1. [DOI] [PubMed] [Google Scholar]
- 12.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(suppl 3):2–23. doi: 10.1002/mds.870131303. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 13.Zesiewicz TA, Elble RJ, Louis ED, et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology. 2011;77(19):1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369(7):640–648. doi: 10.1056/NEJMoa1300962. doi: 10.1056/NEJMoa1300962. [DOI] [PubMed] [Google Scholar]
- 15.Lipsman N, Schwartz ML, Huang Y, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12(5):462–468. doi: 10.1016/S1474-4422(13)70048-6. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 16.Horstink M, Tolosa E, Bonuccelli U, et al. European Federation of Neurological Societies; Movement Disorder Society-European Section. Review of the therapeutic management of Parkinson's disease. Report of a joint task force of the European Federation of Neurological Societies and the Movement Disorder Society-European Section. Part I: early (uncomplicated) Parkinson's disease. Eur J Neurol. . 2006;13(11):1170–1185. doi: 10.1111/j.1468-1331.2006.01547.x. doi: 10.1111/j.1468-1331.2006.01547.x. [DOI] [PubMed] [Google Scholar]
- 17.Horstink M, Tolosa E, Bonuccelli U, et al. European Federation of Neurological Societies; Movement Disorder Society-European Section. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson's disease. Eur J Neurol. 2006;13(11):1186–1202. doi: 10.1111/j.1468-1331.2006.01548.x. doi: 10.1111/j.1468-1331.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson's disease. Eur J Neurol. 2013;20(1):5–15. doi: 10.1111/j.1468-1331.2012.03866.x. doi: 10.1111/j.1468-1331.2012.03866.x. [DOI] [PubMed] [Google Scholar]
- 19.Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES/ENS corrected recommendations for the diagnosis of Parkinson's disease. Eur J Neurol. 2013;20(1):16–34. doi: 10.1111/ene.12022. doi: 10.1111/ene.12022. [DOI] [PubMed] [Google Scholar]
- 20.Jeanmonod D, Moser D, Magara D, et al. Study on incisionless transcranial MR-guided focused ultrasound treatment of Parkinson's disease; safety accuracy and initial clinical outcomes. Current and future applications of focused ultrasound;; Oct 14-17; Washington. 2012. [Google Scholar]
- 21.Consensus guidelines: treatment planning and options. Mayo Clin. Proc. 2006;81(Suppl 4):12–25. doi: 10.1016/s0025-6196(11)61475-4. doi: 10.1016/S0025-6196(11)61475-4. [DOI] [PubMed] [Google Scholar]
- 22.Attal N, Gruccu G, Haanpaa M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13(11):1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 23.Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(Suppl):S3–14. doi: 10.4065/mcp.2009.0649. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996;119(Pt 2):363–375. doi: 10.1093/brain/119.2.363. doi: 10.1093/brain/119.2.363. [DOI] [PubMed] [Google Scholar]
- 25.Sarnthein J, Stern J, Aufenberg C, et al. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129(Pt 1):55–64. doi: 10.1093/brain/awh631. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 26.Jeanmonod D, Magnin M, Morel A, et al. Surgical control of the human thalamocortical dysrhythmia: I Central lateral thalamotomy in neurogenic pain. Thalamus& Related Systems. 2001;1:245–254. doi: 10.1016/S1472-9288(01)00026-7. [Google Scholar]
- 27.Jeanmonod D, Werner B, Morel A, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32(1):1–11. doi: 10.3171/2011.10.FOCUS11248. doi: 10.3171/2011.10.FOCUS11248. [DOI] [PubMed] [Google Scholar]
- 28.Hynynen K, McDannold N, Sheikov NA, et al. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 29.McDannold N, Arvanitis CD, Vykhodtseva N, et al. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14):3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73(6):1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteith SJ, Harnof S, Medel R, et al. Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance-guided focused ultrasound. J Neurosurg. 2013;118(5):1035–1045. doi: 10.3171/2012.12.JNS121095. doi: 10.3171/2012.12.JNS121095. [DOI] [PubMed] [Google Scholar]
- 32.McDannold N, Clement GT, Black P, et al. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66(2):323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boison D. The sound of noninvasive seizure control. Epilepsy Curr. 2011;11(6):196–197. doi: 10.5698/1535-7511-11.6.196. doi: 10.5698/1535-7511-11.6.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bor-Seng-Shu E, Nogueira Rde C, Figueiredo EG, et al. Sonothrombolysis for acute ischemic stroke: a systematic review of randomized controlled trials. Neurosurg Focus. 2012;32(1):E5. doi: 10.3171/2011.10.FOCUS11251. doi: 10.3171/2011.10.FOCUS11251. [DOI] [PubMed] [Google Scholar]
- 35.Hölscher T, Fisher DJ, Raman R, et al. Noninvasive transcranial clot lysis using high intensity focused ultrasound. J Neurol Neurophysiol. 2011;S1 doi: 10.4172/2155-9562.S1-002. [Google Scholar]
- 36.Wright C, Hynynen K, Goertz D. In vitro and in vivo high-intensity focused ultrasound thrombolysis. Invest Radiol. 2012;47(4):217–225. doi: 10.1097/RLI.0b013e31823cc75c. doi: 10.1097/RLI.0b013e31823cc75c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess A, Huang Y, Waspe AC, et al. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS One. 2012;7(8):e42311. doi: 10.1371/journal.pone.0042311. doi: 10.1371/journal.pone.0042311. [DOI] [PMC free article] [PubMed] [Google Scholar]