ABSTRACT
Dengue virus (DENV) is the causative agent of dengue fever (DF). This disease can be caused by any of four DENV serotypes (DENV1 to -4) which share 67 to 75% sequence homology with one another. The effect of subsequent infections with different serotypes on the T cell repertoire is not fully understood. We utilized mice transgenic for human leukocyte antigens (HLA) lacking the alpha/beta interferon (IFN-α/β) receptor to study responses to heterologous DENV infection. First, we defined the primary T cell response to DENV3 in the context of a wide range of HLA molecules. The primary DENV3 immune response recognized epitopes derived from all 10 DENV proteins, with a significant fraction of the response specific for structural proteins. This is in contrast to primary DENV2 infection, in which structural proteins are a minor component of the response, suggesting differential antigen immunodominance as a function of the infecting serotype. We next investigated the effect of secondary heterologous DENV infection on the T cell repertoire. In the case of both DENV2/3 and DENV3/2 heterologous infections, recognition of conserved/cross-reactive epitopes was either constant or expanded compared to that in homologous infection. Furthermore, in heterologous infection, previous infection with a different serotype impaired the development of responses directed to serotype-specific but not conserved epitopes. Thus, a detrimental effect of previous heterotypic responses might not be due to dysfunctional and weakly cross-reactive epitopes dominating the response. Rather, responses to the original serotype might limit the magnitude of responses directed against epitopes that are either cross-reactive to or specific for the most recently infecting serotype.
IMPORTANCE DENV transmission occurs in more than 100 countries and is an increasing public health problem in tropical and subtropical regions. At present, no effective antiviral therapy or licensed vaccine exists, and treatment is largely supportive in nature. Disease can be caused by any of the four DENV serotypes (DENV1 to -4), which share a high degree of sequence homology with one another. In this study, we have addressed the question of how the T cell repertoire changes as a function of infections with different serotypes and of subsequent heterologous secondary infections. This is of particular interest in the field of dengue viruses, in which secondary infections with different DENV serotypes increase the risk of severe disease. Our results on the evolution of the immune response after primary and secondary infections provide new insights into HLA-restricted T cell responses against DENV relevant for the design of a vaccine against DENV.
INTRODUCTION
Dengue virus (DENV) is primarily transmitted by the mosquitoes Aedes aegypti and Aedes albopictus and is now endemic in more than 100 countries worldwide. It was recently reported that as many as 400 million dengue virus infections occur worldwide each year, including outbreaks in Europe and the United States (1, 2), thus making this infection potentially more prevalent than malaria (3). The severity of DENV-associated disease can range from asymptomatic to an acute self-limiting febrile illness (dengue fever [DF]) or to the severe forms of the disease, dengue hemorrhagic fever (DHF) and/or dengue shock syndrome (DSS). Disease can be caused by any of the four DENV serotypes (DENV1 to -4), which share 67 to 75% sequence homology with one another (4). No licensed vaccine or effective antiviral therapy is currently available. Treatment is largely supportive in nature, increasing the burden on the public health capacity of many tropical and subtropical principalities (5). One challenge in the development of a vaccine against DENV is the high degree of sequence variation characteristically associated with RNA viruses. This is of particular relevance in the case of DENV, since infection with one DENV serotype (primary infection) presumably affords lifelong, serotype-specific immunity but affords only partial and temporary protection to other serotypes in secondary-infection settings (6, 7). In fact, more severe infections resulting in DHF and DSS are associated with heterologous secondary infections (7).
One hypothesis to explain this phenomenon is termed the theory of original antigenic sin (8). According to this hypothesis, T cells induced by a primary infection dominate the secondary heterologous infection but are of lower efficacy in clearing the infection (9, 10). Peptide variants derived from the secondary infection serotype can induce a response that is qualitatively different from the response induced by the original antigen, such as inducing a different pattern of cytokine production, and thus contribute to immunopathogenesis of severe disease (11, 12). However, this hypothesis is in conflict with the observation that heterologous T cell responses are not always needed to produce DHF in infants. Indeed, the same severe clinical vascular permeability syndrome, as well as similar levels of cytokines in the blood, is seen during primary dengue immune responses in infants and children as is seen following secondary dengue virus infections (13), suggesting a role for maternal antibodies. Furthermore, a recent study has shown a temporal mismatch between the CD8+ T cell response and commencement of capillary leakage, suggesting that CD8+ T cells are not responsible for early triggering of capillary leakage in children with DHF (14).
We recently reported that a large fraction of responses in secondary infections in donors from an endemic Sri Lankan population (15), which were classified as secondary infections based on broadly neutralizing titers for more than one serotype, are directed to conserved/cross-reactive epitopes. This cross-reactive response was not associated with lower magnitude or functionality, thus calling into question epitope cross-reactivity as an explanation for the immunopathology associated with DENV infection. Limitations of that study, however, were that the predominant order of infection was inferred on the basis of epidemiological reports but was not experimentally determined or verified and that the time point of infection was unknown for these samples. Hence, the human leukocyte antigen (HLA)-transgenic model utilized in this study offered an ideal opportunity to address these issues in a well-defined experimental system.
Mice transgenic for HLA are widely used to study T cell responses restricted by human major histocompatibility complex (MHC) molecules (16–18), and it has been shown that mice lacking the alpha/beta interferon receptor (IFN-α/βR) support a productive DENV infection (19–22). To cover a wide range of HLA phenotypes, we backcrossed IFN-α/βR−/− mice with HLA A*0201, A*0101, B*0701, B*4001, and DRB1*0101 single-allele-transgenic mice. Previous studies applied bioinformatics-based HLA binding predictions to the DENV proteome and tested the recognition of predicted peptides after infection with DENV2 in these HLA-transgenic mouse strains. In the present study, we have further utilized these mice to similarly define the epitopes and corresponding antigens recognized following infection with DENV3. These studies enabled investigation, in a controlled model system, as to whether a different hierarchy of immundominance associated with the different DENV proteins was detectable as a function of the infecting serotype. Furthermore, we were able to determine the pattern of immunodominance of T cell responses following heterologous and/or homologous secondary infection.
MATERIALS AND METHODS
Ethics statement.
All murine experiments in this study were performed according to the National Research Council's Guide for the Care and Use of Laboratory Animals (23) and following Institutional Animal Care and Use Committee-approved animal protocols (protocol number AP071-AS4-0312).
Viral stocks.
The mouse-adapted DENV3 strain D3S5CX was derived from the clinical isolate UNC3001, obtained from Aravinda de Silva (UNC School of Medicine). IFN-α/βR−/− IFN-γR−/− mice were infected intravenously (i.v.) via the tail vein, and serum of these mice was harvested 3 days postinfection. Virus in the serum was amplified in C6/36 cells and then injected back into mice. After 5 cycles of passaging, the virus was injected into Cardif−/− mice via the tail vein, and spleens were harvested 3 days postinfection. Similar to this, the virus in the spleen homogenate supernatant was amplified in C6/36 cells and then injected back into mice for a total of 10 cycles. This resulted in strain D3S5CX, which replicates more efficiently in IFN-α/βR−/− mice than the parental strain. S221 is a plaque-purified DENV2 strain which was derived from the clinical isolate PL046 (24) by passaging through IFN-α/βR−/− IFN-γR−/− and mosquito cells, as previously described (20, 25). Viral stocks were amplified in C6/36 mosquito cells, also as previously described (26). Infectious doses were determined based on genomic equivalents (GE), which were quantified by reverse transcription-PCR (RT-PCR). There are ∼5 × 104 GE/PFU for S221, based on a plaque assay on baby hamster kidney cells as described previously (27).
Mice and infections.
Mice transgenic for HLA A*0101, A*0201/Kb, B*0702, B*4001, and DRB1*0101 were bred and backcrossed with IFN-α/βR−/− mice on the C57BL/6 background at the La Jolla Institute for Allergy and Immunology facility (La Jolla, CA) as previously described (22). Mice were used between 6 and 10 weeks of age. For all experiments, mice were infected i.v. (retro-orbitally) in groups of 3, with 1010 GE of DENV in 100 μl of phosphate-buffered saline (PBS) unless described otherwise. On day 7 postinfection, the mice were sacrificed and splenic CD8+ or CD4+ T cells from each experimental group were pooled and used in mouse IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assays. For secondary-infection experiments, B*0702 IFN-α/βR−/− mice were infected 28 days after primary of DENV3 (1010 GE) infection with either DENV3 (homologous) or DENV2 (heterologous). Similarly, B*0702 IFN-α/βR−/− mice were infected 28 days after primary DENV2 infection (1008 GE) with either DENV2 (homologous) or DENV3 (heterologous). Seven days after secondary infection, mice were sacrificed and splenic CD8+ T cells were used in mouse IFN-γ ELISPOT assays. To study the effects of heterologous effects on the T cell repertoire, we selected all 9- and 10-mer epitopes which had been detected after primary infection with DENV3 (n = 14) or DENV2 (n = 11) in the B*0702-transgenic IFN-α/βR−/− mice. A serotype-specific epitope has been defined as being an exact match to the serotype and sharing less than 80% homology with the other serotypes. Two of them were discovered after primary DENV2 and DENV3 infection and thus were only included once in the peptide set (n = 23). These 23 peptides were then grouped according sequence homology to the DENV3 and/or DENV2 strain. The majority of epitopes detected after primary infection with DENV2 shared >80% sequence homology with DENV3 and have thus been put in the conserved group.
Bioinformatic analyses and peptide synthesis.
The HLA A*0201, A*0101, B*0702, and B*4001 binding capacities of all 9- and 10-mer peptides encoded in the D3S5CX proteome were predicted using the command-line version of the MHC class I consensus prediction tool available on the Immune Epitope Database (IEDB) website (www.iedb.org) (28). Peptides were selected if they scored in the top 1% of all peptides for any of the 4 alleles. For the MHC class II DRB1*0101 allele, binding predictions were performed for all 15-mer peptides from the same proteome using the consensus approach, as previously described (29). The top 2% of predicted DRB1*0101 binders was selected for synthesis. Specifically, 83 A*0101, 92 A*0201, 83 B*0702, 77 B*4001, and 29 DRB1*0101 peptides were synthesized by Mimotopes (Victoria, Australia) as crude material on a 1-mg scale. For screening studies, the class I peptides were combined into pools of approximately 10 individual peptides, according to their predicted HLA restriction. This resulted in 8 A*0101-, 9 A*0201-, 8 B*0702-, and 8 B*4001-specific pools. MHC class II peptides were tested individually. DENV2 peptides specific for the S211 stain were predicted by following the same strategy, resulting in 99 A*0101, 118 A*0201, 110 A*1101, 104 B*0702, and 12 DRB1*0101 peptides.
For the peptides used in the threshold determination experiments, 9- and 10-mer peptides were picked based on their predicted binding affinity to the HLA B*0702 allele. Predicted peptides have been grouped depending on the number of amino acid substitutions compared to the D3S5CX strain used as the infecting agent in this study. This resulted in the synthesis of 63, 21, 24, 12, and 17 peptides with no, 1, 2, 3, or more than 4 amino acid substitutions, respectively.
MHC peptide-binding and restriction assays.
Purification of HLA A*0201, A*0101, B*0702, B*4001, and DRB1*0101 MHC molecules and the performance of quantitative competition assays to measure the binding affinities of all peptides used in this study to purified MHC were performed as described elsewhere (30). MHC peptide binding was measured only for the particular HLA allele expressed by the particular transgenic mice. It is thus possible that additional MHC molecules further restrict some of the epitopes. Of the five HLA transgenic mouse strains tested, the strains transgenic for A*0201 and B*4001 did coexpress murine MHC molecules together with the respective HLA molecule. In order to ensure that the observed responses were restricted solely by the human HLA class I molecule and not by murine class I, we performed restriction assays for A*0201 and B*4001 epitopes in addition to MHC peptide assays. To determine restriction for A*0201 and B*4001 epitopes, CD8+ T cells from DENV3-infected HLA A*A0201- and HLA B*4001-transgenic IFN-α/βR−/− mice were incubated with antigen-presenting cells (APC) pulsed with ascending concentrations of peptides and tested for IFN-γ production in an ELISPOT assay. The tumor cell line 721.221 (31), which lacks expression of HLA A, B, and C class I genes, was transfected with the HLA A*0201/Kb chimeric genes and used as APC in the A*0201 restriction assays. The nontransfected cell line was used as a negative control. An Epstein-Barr virus (EBV)-transformed B cell line expressing the B*4001 molecule (SVEIG) was utilized as APC in the B*4001 restriction assay. The LG2 cell line was used as a negative control.
IFN-γ ELISPOT assay.
For all murine experiments, splenic CD4+ or CD8+ T cells were isolated by magnetic bead positive selection (Miltenyi Biotec, Bergisch Gladbach, Germany) 7 days after infection with DENV. A total of 2 ×105 T cells were stimulated with 1 × 105 uninfected splenocytes as APC and pools of 10 individual DENV peptides in 96-well flat-bottom plates (Immobilon-P; Millipore, Bedford, MA) coated with anti-IFN-γ monoclonal antibody (MAb) (clone AN18; Mabtech, Stockholm, Sweden) as previously described (22). Positive pools were deconvoluted and the individual peptides responsible for the reactivity were determined. Responses are expressed as number of IFN-γ spot-forming cells (SFC) per 106 CD8+ T cells and considered positive if the magnitudes of response were ≥20 SFC and had a stimulation index (SI; ratio of test SFC to control SFC) of ≥2 and a P value of <0.05 in a t test comparing replicates with those from the negative control.
RESULTS
DENV3 peptide-specific responses in an HLA-transgenic mouse model.
To determine the DENV3-specific T cell response, we utilized the mouse-adapted DENV3 strain D3S5CX to infect HLA A*0101-, A*0201-, B*0702-, B*4001-, and DRB1*0101-transgenic IFN-α/βR−/− mice. In the case of class I, the HLA A and B alleles studied were chosen as representative of the HLA A1, A2, B7, and B44 class I supertypes, respectively. Together, these class I supertypes are estimated to provide coverage of more than 90% of the general population (32). The population coverage values are calculated considering prevalence in (in alphabetical order) Australia, Europe, North Africa, North America, Northeast Asia, Oceania, South America, Southeast Asia, Southwest Asia, and sub-Saharan Africa. In the present study, the panel of alleles considered was taken as representative of the most common HLA class I supertypes (32, 33). DRB1*0101 was chosen as representative of the main HLA DR class II supertype based on studies in which DR1 was found to have the broadest repertoire and to also, in general, have the highest degree of cross-reactivity with other HLA class II specificities (33, 34). Given the high degree of repertoire overlap between HLA class II molecules, the main DR supertype is represented in more than 90% of individuals. Using bioinformatics-based algorithms, we generated panels of D3S5CX-derived peptides predicted to bind HLA A*0101, A*0201, B*0702, B*4001, or DRB1*0101 molecules.
For each allele, the corresponding predicted peptides were combined into pools of 10 peptides each and tested in IFN-γ ELISPOT assays using splenic T cells from HLA-transgenic IFN-α/βR−/− mice 7 days postinfection. Positive pools were deconvoluted, and the individual peptides responsible for the reactivity were determined. Using this approach, we identified a total of 59 responses, considering all HLA-transgenic IFN-α/βR−/− mouse strains tested. More specifically, these experiments revealed 3 A*0101-, 26 A*0201-, 19 B*0702-, 4 B*4001-, and 7 DRB1*0101-restricted epitopes (Fig. 1).
FIG 1.
DENV3-specific epitope identification in an HLA-transgenic mouse model. DENV-specific epitope identification was performed in five different mouse strains transgenic for HLA A*0101 (A), A*0201 (B), B*0702 (C), B*4001 (D), and DRB1*0101 (E). For all strains tested, IFN-γ ELISPOT assay was performed using splenic T cells isolated from HLA-transgenic IFN-α/βR−/− mice (black bars). Mice were infected retro-orbitally with 1 × 1010 GE of DENV3. For all MHC class I mouse strains, peptides were tested in pools of 10 peptides and subsequently deconvoluted if the pool was positive in two independent experiments. Shown are peptides from pools which have been identified as positive (5 A*0101 pools [A], 7 A*0201 pools [B], 7 B*0702 pools [C], and 4 B*4001 pools [D]). MHC class II peptides were tested individually (E). Seven days postinfection, CD8+ (A to D) or CD4+ (E) T cells were purified and tested against a panel of DENV3 predicted peptides. The data are expressed as mean numbers of SFC/106 T cells from two independent experiments. Error bars represent SEM. Responses against peptides were considered positive if the stimulation index (SI) exceeded double that of the mean negative-control wells (T cells plus APC without peptide) and net spots were above the threshold of 20 SFC/106 T cells in two independent experiments. Asterisks indicate peptides which were able to elicit a significant IFN-γ response in each individual experiment, according to the criteria described above.
Further characterization of the DENV3 epitopes.
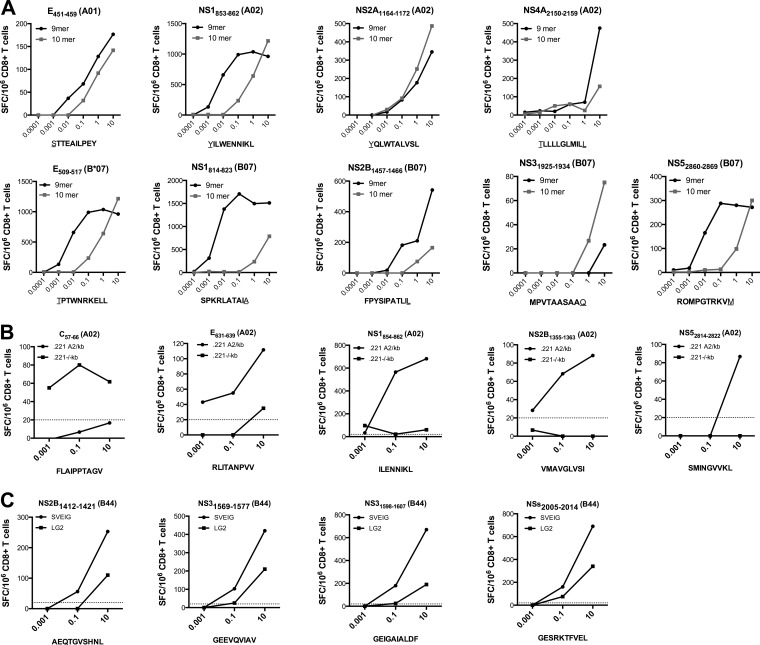
The A*0101, A*0201, and B*0702 epitopes identified included 9 pairs of nested epitopes, where a 10-mer and a nested 9-mer peptide were able to elicit immune responses (Fig. 1A to C). To determine which peptide was the optimal epitope, each nested peptide pair was further titrated, as shown in Fig. 2A. In eight out of nine cases, the optimal epitope could be unequivocally identified and therefore was utilized in all further studies. In the case of one A*0201 epitope (NS2A1164–1172) (Fig. 2), the 9-mer and the 10-mer showed equivalent dose-response curves. In this case, the 10-mer was selected for use in further experiments, since it also fully contains the 9-mer sequence.
FIG 2.
Further characterization of DENV3 epitopes. (A) To determine the optimal epitopes, CD8+ T cells were purified 7 days postinfection and incubated for 24 h with ascending concentrations of nested peptides. The peptides which were able to elicit stronger IFN-γ responses at various concentrations were considered the optimal epitopes. Cell lines expressing either A*0201 (B) or B*4001 (C) but no murine MHC molecules were used as APC to establish HLA restriction as described in Materials and Methods. Purified CD8+ T cells from DENV3-infected mice were incubated with ascending concentrations of peptides and tested for IFN-γ production in an ELISPOT assay. Representative graphs of CD8+ T cell responses for incubation with HLA-expressing cell lines (B and C; black circles) and control cell lines (B and C, black squares) are shown. Twenty SFC/106 CD8+ T cells was set as a threshold for positivity, as indicated by the dashed line.
Of the five HLA-transgenic mouse strains tested, the A*0201- and B*4001-transgenic mice coexpress murine MHC molecules. To confirm that the observed responses were restricted by the transfected human class I molecule and not the coexpressed murine class I, we tested purified T cells for the capacity to recognize the specific epitopes when pulsed on antigen-presenting cells expressing only human class I. Accordingly, for the A*0201 epitopes, we utilized HLA A*0201-transfected 721.221 cells, which are negative for expression of murine class I molecules. All 23 of the HLA*A0201-restricted epitopes stimulated a CD8+ T cell response when presented exclusively on HLA*0201 molecules (Fig. 2B). Similarly, all four B*4001-restricted epitopes were recognized when presented by corresponding cell lines expressing HLA B*4001 molecules (Fig. 2C) but no murine class I. It should be noted that there was some reactivity noted in the negative-control APC. The responses were seen only at the highest dose and were roughly 10-fold less than in the positive APC. At the same time, while binding predictions for HLA A and B, and comparison with known motifs for HLA C, would suggest otherwise, we cannot rule out that some of the response may have been elicited due to presentation by HLA class I on the negative APC. As an additional control, all A*0201 and B*4001 epitopes were tested for their reactivity in non-HLA-transgenic IFN-α/βR−/− mice and were found to not elicit CD8+ T cell responses in IFN-γ ELISPOT assays (data not shown).
To further characterize the MHC restriction of the identified epitopes, we measured their binding capacities for their putative restricting HLA allelic molecule in in vitro binding assays using purified MHC molecules (Table 1). Forty of the 50 peptides (80%) bound the corresponding predicted allele with high affinity, as indicated by a 50% inhibitory concentration (IC50) of <50 nM, including 26 peptides that bound with an affinity of 10 nM or better. Of the remaining 10 peptides, 9 (18%) bound the corresponding allele with intermediate affinity, with IC50s in the 50 to 500 nM range, and one (2%) bound with low affinity (IC50 > 1,000 nM).
TABLE 1.
DENV3-specific epitopes identified in this study
| Restriction class | Epitope | Sequence | T cell response (no. of SFC/106 T cells) | HLA binding (IC50, nM) | Conservation between virus strains (%) |
Reference(s) | |
|---|---|---|---|---|---|---|---|
| S221 | D3S5CX | ||||||
| A*0101 | E451–459 | TTEAILPEY | 429 | 31 | 56 | 100 | |
| NS11090–1099 | RSCTLPPLRY | 305 | 5.9 | 100 | 100 | 22 | |
| A*0201 | C57–66 | FLAIPPTAGV | 868 | 3.6 | 80 | 100 | 22 |
| C103–112 | SLCLMMILPA | 244 | 5.7 | 50 | 100 | 15 | |
| C106–114 | LMMILPAAL | 1,679 | 6.6 | 56 | 100 | 15 | |
| M250–259 | ILALFLAHYI | 1,595 | 6.3 | 50 | 100 | 15 | |
| M254–263 | FLAHYIGTSL | 1,363 | 4.8 | 50 | 100 | ||
| M268–276 | VIFILLMLV | 607 | 375 | 67 | 100 | ||
| E580–589 | YAMCTNTFVL | 658 | 33 | 50 | 100 | ||
| E631–639 | RLITANPVV | 418 | 20 | 78 | 100 | ||
| E727–735 | ALFSGVSWV | 1,086 | 20 | 78 | 100 | 15 | |
| NS1854–862 | ILWENNIKL | 2,238 | 2.7 | 67 | 100 | ||
| NS1987–996 | KLEKASLIEV | 488 | 77 | 80 | 100 | 39 | |
| NS2A1164–1172 | VLFTFVLLL | 1,363 | 10 | 45 | 100 | ||
| NS2A1202–1211 | YLALIATFKI | 1,089 | 10 | 70 | 100 | 15 | |
| NS2A1271–1280 | YQLWTALVSL | 1,122 | 1.7 | 40 | 100 | 15 | |
| NS2B1355–1363 | VMAVGLVSI | 442 | 21 | 78 | 100 | 15 | |
| NS2B1444–1453 | VLLKTALLIV | 203 | 23 | 50 | 100 | 47 | |
| NS31832–1840 | FAGKTVWFV | 415 | 11 | 89 | 100 | 39, 48 | |
| NS31876–1884 | KLNDWDFVV | 333 | 1.8 | 78 | 100 | 40 | |
| NS32013–2022 | ELMRRGDLPV | 757 | 22 | 90 | 100 | 15, 39 | |
| NS4A2150–2159 | TLLLLGLMIL | 990 | 52 | 60 | 100 | 11 | |
| NS4A2205–2213 | IVLEFFMMV | 469 | 11 | 67 | 100 | ||
| NS4B2311–2320 | SLAAIANQAV | 858 | 6.7 | 80 | 100 | ||
| NS52814–2822 | SMINGVVKL | 1,016 | 14 | 78 | 100 | 15 | |
| B*0702 | E509–517 | TPTWNRKEL | 1,193 | 2.8 | 56 | 100 | |
| NS1814–822 | SPKRLATAI | 1,186 | 1.5 | 67 | 100 | 15 | |
| NS11071–1079 | GPSLRTTTV | 115 | 2.2 | 89 | 100 | 15 | |
| NS2A1290–1298 | TVAWRTATL | 486 | 3.6 | 56 | 100 | ||
| NS2B1373–1382 | VPMAGPLVAG | 28 | 115 | 80 | 100 | 15 | |
| NS2B1457–1465 | FPYSIPATL | 802 | 1.0 | 67 | 100 | ||
| NS31648–1656 | EPDGPTPEL | 512 | 299 | 44 | 100 | ||
| NS31682–1690 | LPAIVREAI | 494 | 6.5 | 100 | 100 | 11, 15, 22, 39–41 | |
| NS31700–1709 | APTRVVAAEM | 291 | 4.6 | 100 | 100 | 15, 22, 39–41, 44, 48 | |
| NS31899–1907 | RVIDPRRCL | 260 | 146 | 89 | 100 | 15, 36 | |
| NS31925–1934 | MPVTAASAAQ | 690 | 1072 | 80 | 100 | 15 | |
| NS32070–2078 | RPRWLDART | 40 | 2.1 | 78 | 100 | 15, 40, 48 | |
| NS4A2113–2121 | LAHRTRNAL | 95 | 3.3 | 56 | 100 | ||
| NS52860–2868 | RPMPGTRKV | 461 | 2.7 | 44 | 100 | 15 | |
| B*4001 | NS2B1412–1421 | AEQTGVSHNL | 362 | 67 | 50 | 100 | |
| NS31569–1577 | GEEVQVIAV | 293 | 12 | 78 | 100 | 39 | |
| NS31598–1607 | GEIGAIALDF | 289 | 40 | 70 | 100 | 15 | |
| NS32005–2014 | GESRKTFVEL | 708 | 3.5 | 80 | 100 | 15, 40 | |
| DRB1*0101 | C101–115 | KTSLCLMMILPAALA | 82 | 12 | 40 | 100 | 15 |
| M268–282 | VIFILLMLVTPSMTM | 53 | 53 | 73 | 100 | 15 | |
| NS1984–998 | GSWKLEKASLIEVKT | 236 | 56 | 67 | 100 | 40 | |
| NS2B1359–1373 | GLVSILASSLLRNDV | 241 | 2.1 | 80 | 100 | ||
| NS31692–1706 | RRLRTLILAPTRVVA | 113 | 5.3 | 93 | 100 | 15, 39–41, 44, 48 | |
| NS31742–1756 | TFTMRLLSPVRVPNY | 70 | 1.5 | 100 | 100 | 15, 22, 40 | |
| NS52967–2981 | RAIWYMWLGARYLEF | 586 | 48 | 93 | 100 | 15 | |
Taken together, the data in this and the preceding section have defined a total of 50 different individual DENV3-derived, HLA-restricted T cell epitopes. As summarized in Table 1, 2 are restricted by A*0101, 23 by A*0201, 14 by B*0702, 4 by B*4001, and 7 by DRB1*0101.
DENV epitopes identified in the transgenic mouse model reflect the T cell repertoire in humans following natural exposure.
To investigate if the epitopes identified in the HLA-transgenic mouse model are also recognized in context of natural infection in humans, we performed a search of the Immune Epitope Database (IEDB) (35). We found that 34 (68%) of the 50 DENV3 epitopes detected in this study were independently described to elicit a T cell response in humans exposed to dengue virus. The corresponding references are listed in Table 1. At the same time, we performed a similar analysis of the DENV2 epitopes previously identified by us in the HLA-transgenic mouse system (22) and found that 32 (76%) of the 42 epitopes have also been described to occur in humans (see Table S1 in the supplemental material). The overall high concordance, 72% (66 out of 92 epitopes), confirms that the HLA-transgenic IFN-α/βR−/− mice are a reliable model of T cell responses relevant to human infection with DENV.
DENV2 and DENV 3 responses are serotype specific and largely nonoverlapping.
We have previously identified DENV2-specific epitopes recognized in the HLA-transgenic IFN-α/βR−/− mouse system, following the exact same methodology as described herein (22). We were interested to analyze the degree of overlap between the repertoires of HLA-transgenic mice infected with the two different DENVs. Table 1 indicates for each of the DENV3-specific epitopes whether they are also conserved in the DENV2 strain S221, which was used in the previous study (22). Only 4 of the 50 DENV3 epitopes share 100% sequence identity with the DENV2 S221 strain (NS11090–1099 A*0101, NS31682–1690 and NS31700 + 1709 B*0702, and NS31742–1756 DRB1*0101). Not surprisingly, all 4 of these epitopes were also independently identified after infection with DENV2 (22). None of the remaining 46 DENV3-specific epitopes, or variants thereof, were identified after infection with DENV2 (see Table S1). In one case (E451–459), two corresponding sequences with only 60% homology were identified in DENV2 and DENV3. Some fully conserved epitopes identified in DENV2 were not detected (e.g., NS31681–1689). This maybe reflects sensitivity issues or differences in the way these peptide regions are processed in the context of the two different (DENV2 versus DENV3) polyproteins. These results demonstrate that the T cell repertoires for DENV2 and DENV3 are largely nonoverlapping and suggest that the primary T cell response is serotype specific.
Differential pattern of immunogenicity after infection with DENV3 compared to DENV2.
Next, we investigated the specificity of the DENV3 immune response at the antigen level. To this end, we analyzed the relative strengths of recognition of the 10 DENV proteins, namely, the three structural proteins (capsid [C], membrane [M], and envelope [E]) and the seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). As shown in Fig. 3A, the immune response against DENV3 was broad, and epitopes originating from all 10 proteins were recognized. The majority (37 out of 50 [74%]) of the epitopes were derived from the seven nonstructural proteins and accounted for two-thirds (67%) of the total IFN-γ response observed. Within the seven nonstructural proteins, NS3 and NS1 were the most dominantly targeted, accounting for 17% and 15% of the total IFN-γ response, respectively. Thirteen of the 50 (26%) epitopes were derived from the three structural proteins and accounted for one-third (33%) of the total IFN-γ response observed. This is in contrast to infection with DENV2, with which only 3% of the responses were directed against structural proteins as previously reported (22) and also shown in Fig. 3B for comparison purposes. The remaining 97% of the total response was derived from the nonstructural proteins and accounted for 39 out of 42 (93%) of the epitopes detected. No significant differences have been detected for viremia titers 4 days after infection with either of the strains (mean logs of relative DENV copies, 6.52 ± 0.55 for DENV2 and 6.67 ± 0.36 for DENV3). This unexpected difference between DENV2 and DENV3 in the recognition of structural versus nonstructural proteins was statistically highly significant (post hoc analysis, P < 0.0001 in Fisher's exact test for distribution of SFC and P = 0.025 for distribution of epitopes). These results suggest different hierarchies of immunodominance associated with the different DENV proteins as a function of the infecting serotype.
FIG 3.

Differential patterns of immunogenicity after infection with DENV3 compared to DENV2. All identified DENV3-specific (A) and DENV2-specific (B) (22) epitopes were grouped according to the protein of provenance. IFN-γ responses of individual epitopes derived from the three structural (capsid [C], premembrane [prM/M], and envelope [E]) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins are shown. Numbers below the proteins indicate the relative (percentage of total) response against the corresponding protein. Numbers in the upper right corners of the boxes represent the relative responses either targeted against structural proteins (left boxes) or nonstructural proteins (right boxes).
Operational threshold for cross-reactive epitope recognition.
Of the five HLA-transgenic mouse strains tested, only the A*0101- and B*0702-transgenic strains did not coexpress murine MHC molecules together with the respective HLA molecule. In order to ensure that the observed responses were restricted solely by the human HLA class I molecule and not by murine class I and because low responses have been observed in the mice transgenic for HLA A*0101, we have chosen B*0702-transgenic IFN-α/βR−/− mice as a representative strain for additional studies. We further analyzed the observed immunodominance differences associated with DENV2 and DENV3 primary infection and the effects of preexisting immunity and heterologous infection on T cell recognition. To enable these studies, we first sought to experimentally define a homology threshold associated with cross-reactivity at the T cell level. The phenomenon of T cell-mediated cross-reactivity between various serotypes has been the subject of much discussion in the context of dengue virus infection (11, 12, 36, 37), but a definition of the threshold for cross-reactivity broadly applicable to large number of epitopes has not been experimentally addressed.
For these analyses, we synthesized a panel of 137 peptides corresponding to naturally occurring DENV3 variants with various degrees of sequence homology to the D3S5CX strain. This panel was then tested for T cell reactivity following infection of B*0702-transgenic IFN-α/βR−/− mice with this strain. As shown in Fig. 4, the majority of responses, in terms of both frequency (15 of 63 peptides [24%]) and magnitude, was detected in peptides with 100% sequence identity to the infecting strain. Another roughly 20% of the total response (5 out of 45 peptides) observed was elicited by variants with just one or two amino acid variations, corresponding to ≥78% and ≥80% sequence identities in the cases of 9-mer and 10-mer peptides, respectively. No response was observed in variants with 3 or more amino acid differences compared to the corresponding D3S5CX strain. These results thus identify a sequence homology threshold that discriminates conserved or cross-reactive recognition and non-cross-reactive (and hence serotype-specific) immune responses.
FIG 4.

Threshold for cross-reactive epitope recognition. A panel of 137 peptides corresponding to naturally occurring DENV variants of a set of B*0702 epitopes, with various degrees of sequence homology to DENV3 strain D3S5CX, was synthesized and tested for T cell reactivity after infection of B*0702-transgenic IFN-α/βR−/− mice with D3S5CX. The data are expressed as mean numbers of SFC/106 CD8+ T cells from two independent experiments. Responses against peptides were considered positive if the stimulation index (SI) exceeded double the mean of negative-control wells (T cells plus APC without peptide) and net spots were above the threshold of 20 SFC/106 CD8+ T cells in two independent experiments. aa, amino acid.
Effect of heterologous DENV3 followed by DENV2 infection on the T cell repertoire.
The results shown above demonstrate remarkable differences in the immunodominance patterns of response to DENV3 and DENV2. This observation prompted us to test whether preimmunization with the DENV3 serotype would interfere with development of immunity to the DENV2 serotype, because the pattern of response imprinted by the original infection would be preserved in the heterologous infection. Accordingly, we infected B*0702-transgenic IFN-α/βR−/− mice with DENV3 and subsequently with DENV2 (DENV3/2 heterologous secondary infection). According to the threshold established as described above, the B*0702 epitopes were divided into DENV3-specific (sequence identities as follows: D3S5CX, ≥80%, and S221, <80%), conserved (D3S5CX, ≥80%, and S221, ≥80%), and DENV2-specific (D3S5CX, <80%, and S221, ≥80%) subsets.
Figure 5A shows the patterns of response elicited by the individual epitopes after either primary DENV3 infection (gray bars), homologous DENV3/3 immunization (black bars), or heterologous secondary infection with DENV2 (white bars). Both the primary and the homologous secondary infections resulted in a B*0702-restricted immune response targeting 7 out of the 10 DENV proteins. While primary infection or homologous DENV3/3 immunization elicited responses prevalently directed toward DENV3-specific epitopes (72% to 62% [Fig. 5C, dark gray]), these responses were reduced in the heterologous DENV3/2 infection (19% [Fig. 5C, light gray]). Conversely, the response directed against DENV2-specific epitopes increased to 13%, while the conserved epitopes accounted for the remaining 68% (Fig. 5C, black).
FIG 5.
Effect of heterologous infection on the T cell repertoire. (A) Groups of B*0702-transgenic IFN-α/βR−/− mice were infected with DENV3 as described in Materials and Methods. For primary-infection experiments, the mice were sacrificed 7 days postinfection and splenic CD8+ were tested against a panel of previous identified epitopes in IFN-γ ELISPOT assays (white bars). For secondary-infection experiments, mice were infected with DENV2 28 days after primary DENV3 infection. Seven days after secondary infection, mice were sacrificed and splenic CD8+ T cells were used in mouse IFN-γ ELISPOT assays (black bars). The data are expressed as mean numbers of SFC/106 CD8+ T cells from two independent experiments. (B) Groups of B*0702-transgenic IFN-α/βR−/− mice were infected with DENV2 as described in Materials and Methods. Twenty-eight days after primary DENV2 infection, mice were infected with either DENV2 (white bars) or DENV3 (black bars). Seven days after secondary infection, mice were sacrificed and splenic CD8+ T cells were used in mouse IFN-γ ELISPOT assays. The data are expressed as mean numbers of SFC/106 CD8+ T cells from two independent experiments. (C) All responses measured after primary or secondary infection (average SFC/106 CD8+ T cells in two independent experiments) were added, and the relative responses against DENV3-specific (dark gray), DENV2-specific (light gray), or conserved (black) epitopes after primary (left chart) or secondary (right charts) infection are shown.
In conclusion, the controlled experimental conditions enabled by the HLA-transgenic mouse system demonstrated that the pattern of dominance following heterologous secondary infection is associated with contraction of the serotype-specific responses directed against the first serotype and expansion of the serotype-specific response directed against the second serotype.
Effect of heterologous DENV2 followed by DENV3 infection on the T cell repertoire.
The results presented above demonstrate that responses following secondary DENV3/2 heterologous infection are associated with recognition of both serotype-specific and conserved/cross-reactive epitopes. We next performed further experiments, in which the order of heterologous immunization was reversed (DENV2/3).
We infected B*0702-transgenic IFN-α/βR−/− mice with either DENV2 followed by DENV3 (DENV2/3 heterologous secondary infection) or DENV2/2 (DENV2 homologous secondary infection). Figure 5B shows the patterns of response elicited by the individual epitopes after either homologous DENV2/2 immunization (white bars) or heterologous secondary DENV2/3 infection (black bars). Homologous secondary infection elicited responses prevalently directed toward DENV2 specific epitopes (61% [Fig. 5C, light gray]). These responses were reduced in the heterologous DENV2/3 infection (42% [Fig. 5C]). Conversely, the response directed against DENV3-specific epitopes increased to 21% (Fig. 5C, dark gray), while the conserved epitopes accounted for the remaining 37%.
Thus, in the case of both DENV2/3 and DENV3/2 heterologous infections, recognition of conserved/cross-reactive epitopes is either constant or expanded compared to that in homologous infection. It is also apparent that in the setting of heterologous infection, previous infection with a different serotype impairs the development of responses directed to serotype-specific (19 versus 62% for DENV3 and 42 versus 61% for DENV2) but not conserved epitopes.
DISCUSSION
The study of DENV infection in humans and immune correlates associated with protection on one hand and immunopathology on the other is fraught with considerable complexities. One of the issues contributing to this complexity is that individuals in areas where the infection is endemic, in general, and those affected by the more severe forms of disease, in particular, are typically afflicted by multiple heterologous infections. As a result, the patterns of reactivity associated with primary DENV3 infections are relatively less well defined. We have previously shown that the virus load is significantly reduced in IFN-α/βR−/− mice if T cells are primed by peptide immunization before homologous challenge (20). It has been suggested that heterologous infection leads to the preferential recognition of sequences cross-reactive between the two (or more) infecting viruses but that this cross-reactive response is of lower efficacy in controlling viral disease. This possibility has been described as the original antigenic sin hypothesis (9). In most cases, however, the exact infecting serotypes and the corresponding order and times of infection are unknown, highlighting the need for a more controlled experimental model system to study the evolution of HLA-restricted T cell responses to dengue virus.
Here we show that in the cases of both DENV2/3 and DENV3/2 heterologous infections, recognition of conserved/cross-reactive epitopes was either constant or expanded compared to that in homologous infection, which is in agreement with previously published studies (15). Thus, a detrimental effect of previous heterotypic responses (original antigenic sin), if existing, might not be due to dysfunctional and weakly cross-reactive epitopes dominating the response. Rather, responses to the original serotype might limit the magnitude of responses directed against epitopes that are either cross-reactive or specific for the most recently infecting serotype. This situation might be exacerbated in the case of alleles associated with lower intrinsic magnitudes of CD8+ T cell responses, which have been shown to be associated with increased predisposition to severe disease (15).
In our study, only one allele and one viral isolate per serotype have been investigated, and it is possible that the foci of the immune response differ between alleles and/or in other DENV strains. It should be noted that B*0702 responses were dominant after infection with DENV2, while A*0201-restricted responses were dominant after infection with DENV3. It might be of interest to verify in future studies whether similar conclusions would be reached with A*0201-transgenic mice.
The primary immune response against DENV3 was broad and targeted all 10 DENV proteins. One-third of the responses identified were elicited by T cell epitopes derived from the three structural proteins (C, M, and E). This is in contrast to the T cell targets observed after infection with DENV2, where the vast majority of responses (97%) were targeted toward epitopes derived from the nonstructural proteins and not the structural proteins. It is possible that differences in viral replication and adaptation between the two viruses might contribute to some of the differences observed. However, a study of viral titers, pathogenicity, and disease protection and susceptibility was beyond the scope of the present study and will be addressed in future experiments.
The stark difference in the immune responses depending on the serotype of infection (DENV3 versus DENV2) has implications for vaccine design and development. The most advanced dengue vaccine to date consists of live-attenuated tetravalent chimeric dengue-yellow fever vaccine strains, and it incorporates DENV serotype-specific membrane (M) and envelope (E) proteins in a yellow fever virus17D backbone. Results from a recent clinical trial demonstrated partial (60 to 80%) protection against 3 of the 4 DENV serotypes but no protection against DENV2 infection (38). We and others have previously shown that in humans, the majority of the DENV2-specific T cell responses are directed against the nonstructural proteins (15, 22, 39–42), which are absent in the tetravalent vaccine. This deficiency may contribute to the lack of protective immunity against DENV2. Similarly, our data for HLA-transgenic mice demonstrate that while only 3% of the DENV2-specific immune response is focused on the structural proteins, about one-third of the DENV3-specific response is directed against the premembrane (prM) and E structural proteins, both of which are present in the vaccine. These substantial serotype-specific differences could provide an explanation as to why the live-attenuated tetravalent chimeric vaccine was able to partially protect against 3 of the 4 dengue virus serotypes but not DENV2.
Another protein dominantly targeted after infection with DENV3 but not following DENV2 infection is the NS1 protein. Unlike other nonstructural proteins, NS1 can also be secreted, and detection of early concentrations of NS1 in blood is positively associated with disease severity (43). It has been suggested that NS1 from dengue virus-infected cells contributes to dengue shock syndrome by forming complexes with prothrombin. Formation of such complexes may result in a prolongation of activated partial thromboplastin times, values that have been shown to be the strongest correlate of vascular permeability in patients with dengue virus infection (44, 45). Fifteen percent of all DENV3-specific reactivity was targeted against the NS1 protein, whereas NS1 reactivity was basically absent after primary DENV2 infection. Furthermore, DENV3-specific NS1 reactivity was not restricted to certain alleles, since we have identified at least one NS1 epitope in 4 out of 5 HLA class I alleles tested. Thus, the DENV3-specific response against NS1 could contribute to protection, while this immune response is absent in infection with DENV2, which has in fact been reported as a risk factor for severe disease (46). It has to be noted that other epidemiological studies have shown that DENV3 causes massive epidemics of DHF and that people infected with DENV3 (the majority having a different HLA type than the mice used in this study) do get severe disease. It is possible that HLA alleles other than the ones tested in this study induce a T cell response different than the one described herein.
In conclusion, our results highlight the fact that different DENV strains are associated with different and unique hierarchies in terms of the specific antigens that are immunodominant for cellular immunity. These findings have potential relevance for both vaccine design and DENV immunopathogenesis. Furthermore, the results clearly demonstrate how heterologous infection might be associated with an impairment of responses against serotype-specific CD8+ T cell responses. As our recent data (15) suggest that weak CD8+ T cells responses are associated with increased risk with disease susceptibility, this impairment might contribute to the increased risk of severe disease associated with heterologous infection in humans.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health contract no. HHSN272200900042C (to A.S.) and National Institutes of Health grant no. U54AI057517 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (to S.S.) (47, 48).
Footnotes
Published ahead of print 23 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01108-14.
REFERENCES
- 1.Bouri N, Sell TK, Franco C, Adalja AA, Henderson DA, Hynes NA. 2012. Return of epidemic dengue in the United States: implications for the public health practitioner. Public Health Rep. 127:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank C, Hohle M, Stark K, Lawrence J. 2013. More reasons to dread rain on vacation? Dengue fever in 42 German and United Kingdom Madeira tourists during autumn 2012. Euro Surveill. 18:20446. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. 1998. Phylogeny of the genus Flavivirus. J. Virol. 72:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LC, Tan LH, Kongsin S, Jiamton S, Garrido F, Montoya R, Armien B, Huy R, Castillo L, Caram M, Sah BK, Sughayyar R, Tyo KR, Halstead SB. 2009. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am. J. Trop. Med. Hyg. 80:846–855 [PubMed] [Google Scholar]
- 6.Kurane I. 2007. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp. Immunol. Microbiol. Infect. Dis. 30:329–340. 10.1016/j.cimid.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 7.Halstead SB. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476–481. 10.1126/science.3277268 [DOI] [PubMed] [Google Scholar]
- 8.Rothman AL. 2011. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 11:532–543. 10.1038/nri3014 [DOI] [PubMed] [Google Scholar]
- 9.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9:921–927. 10.1038/nm887 [DOI] [PubMed] [Google Scholar]
- 10.Halstead SB, Rojanasuphot S, Sangkawibha N. 1983. Original antigenic sin in dengue. Am. J. Trop. Med. Hyg. 32:154–156 [DOI] [PubMed] [Google Scholar]
- 11.Bashyam HS, Green S, Rothman AL. 2006. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J. Immunol. 176:2817–2824. 10.4049/jimmunol.176.5.2817 [DOI] [PubMed] [Google Scholar]
- 12.Beaumier CM, Mathew A, Bashyam HS, Rothman AL. 2008. Cross-reactive memory CD8(+) T cells alter the immune response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. J. Infect. Dis. 197:608–617. 10.1086/526790 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, Chen LC, Huang JH, Lam TM, Liu CC, Halstead SB. 2004. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J. Infect. Dis. 189:221–232. 10.1086/380762 [DOI] [PubMed] [Google Scholar]
- 14.Dung NT, Duyen HT, Thuy NT, Ngoc TV, Chau NV, Hien TT, Rowland-Jones SL, Dong T, Farrar J, Wills B, Simmons CP. 2010. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J. Immunol. 184:7281–7287. 10.4049/jimmunol.0903262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, Mattia KA, Doranz BJ, Grey HM, Shresta S, Peters B, Sette A. 2013. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. U. S. A. 110:E2046–E2053. 10.1073/pnas.1305227110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotturi MF, Botten J, Maybeno M, Sidney J, Glenn J, Bui HH, Oseroff C, Crotty S, Peters B, Grey H, Altmann DM, Buchmeier MJ, Sette A. 2010. Polyfunctional CD4+ T cell responses to a set of pathogenic arenaviruses provide broad population coverage. Immunome Res. 6:4. 10.1186/1745-7580-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotturi MF, Assarsson E, Peters B, Grey H, Oseroff C, Pasquetto V, Sette A. 2009. Of mice and humans: how good are HLA transgenic mice as a model of human immune responses? Immunome Res. 5:3. 10.1186/1745-7580-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasquetto V, Bui HH, Giannino R, Banh C, Mirza F, Sidney J, Oseroff C, Tscharke DC, Irvine K, Bennink JR, Peters B, Southwood S, Cerundolo V, Grey H, Yewdell JW, Sette A. 2005. HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products. J. Immunol. 175:5504–5515. 10.4049/jimmunol.175.8.5504 [DOI] [PubMed] [Google Scholar]
- 19.Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S. 2010. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J. Immunol. 185:5405–5416. 10.4049/jimmunol.1001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S. 2009. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 182:4865–4873. 10.4049/jimmunol.0801974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 78:2701–2710. 10.1128/JVI.78.6.2701-2710.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiskopf D, Yauch LE, Angelo MA, John DV, Greenbaum JA, Sidney J, Kolla RV, De Silva AD, de Silva AM, Grey H, Peters B, Shresta S, Sette A. 2011. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J. Immunol. 187:4268–4279. 10.4049/jimmunol.1101970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 24.Lin YL, Liao CL, Chen LK, Yeh CT, Liu CI, Ma SH, Huang YY, Huang YL, Kao CL, King CC. 1998. Study of dengue virus infection in SCID mice engrafted with human K562 cells. J. Virol. 72:9729–9737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry ST, Prestwood TR, Lada SM, Benedict CA, Shresta S. 2009. Cardif-mediated signaling controls the initial innate response to dengue virus in vivo. J. Virol. 83:8276–8281. 10.1128/JVI.00365-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prestwood TR, Prigozhin DM, Sharar KL, Zellweger RM, Shresta S. 2008. A mouse-passaged dengue virus strain with reduced affinity for heparan sulfate causes severe disease in mice by establishing increased systemic viral loads. J. Virol. 82:8411–8421. 10.1128/JVI.00611-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond MS, Edgil D, Roberts TG, Lu B, Harris E. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814–7823. 10.1128/JVI.74.17.7814-7823.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Ponomarenko J, Zhu Z, Tamang D, Wang P, Greenbaum J, Lundegaard C, Sette A, Lund O, Bourne PE, Nielsen M, Peters B. 2012. Immune epitope database analysis resource. Nucleic Acids Res. 40:W525–W530. 10.1093/nar/gks438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. 2008. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput. Biol. 4:e1000048. 10.1371/journal.pcbi.1000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, Sette A. 2013. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Curr. Protoc. Immunol. 100:18.3.1–18.3.36. 10.1002/0471142735.im1803s100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu Y, DeMars R. 1989. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J. Immunol. 142:3320–3328 [PubMed] [Google Scholar]
- 32.Sette A, Sidney J. 1999. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50:201–212. 10.1007/s002510050594 [DOI] [PubMed] [Google Scholar]
- 33.Greenbaum J, Sidney J, Chung J, Brander C, Peters B, Sette A. 2011. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics 63:325–335. 10.1007/s00251-011-0513-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A. 1998. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immunol. 160:3363–3373 [PubMed] [Google Scholar]
- 35.Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, Damle R, Sette A, Peters B. 2010. The Immune Epitope Database 2.0. Nucleic Acids Res. 38:D854–D862. 10.1093/nar/gkp1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appanna R, Huat TL, See LL, Tan PL, Vadivelu J, Devi S. 2007. Cross-reactive T-cell responses to the nonstructural regions of dengue viruses among dengue fever and dengue hemorrhagic fever patients in Malaysia. Clin. Vaccine Immunol. 14:969–977. 10.1128/CVI.00069-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friberg H, Burns L, Woda M, Kalayanarooj S, Endy TP, Stephens HA, Green S, Rothman AL, Mathew A. 2011. Memory CD8+ T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunol. Cell Biol. 89:122–129. 10.1038/icb.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567. 10.1016/S0140-6736(12)61428-7 [DOI] [PubMed] [Google Scholar]
- 39.Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, Hanson BJ, Smith KG, Bertoletti A, Kemeny DM, MacAry PA. 2013. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J. Virol. 87:2693–2706. 10.1128/JVI.02675-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons CP, Dong T, Chau NV, Dung NTP, Chau TNB, Thao LTT, Dung NT, Hien TT, Rowland-Jones S, Farrar J. 2005. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 79:5665–5675. 10.1128/JVI.79.9.5665-5675.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathew A, Kurane I, Rothman AL, Zeng LL, Brinton MA, Ennis FA. 1996. Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a. J. Clin. Invest. 98:1684–1691. 10.1172/JCI118964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, Mongkolsapaya J, Screaton G. 2010. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. U. S. A. 107:16922–16927. 10.1073/pnas.1010867107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165–1168. 10.1086/343813 [DOI] [PubMed] [Google Scholar]
- 44.Wills B, Tran VN, Nguyen TH, Truong TT, Tran TN, Nguyen MD, Tran VD, Nguyen VV, Dinh TT, Farrar J. 2009. Hemostatic changes in Vietnamese children with mild dengue correlate with the severity of vascular leakage rather than bleeding. Am. J. Trop. Med. Hyg. 81:638–644. 10.4269/ajtmh.2009.08-0008 [DOI] [PubMed] [Google Scholar]
- 45.Halstead SB. 2013. Dengue vascular permeability syndrome: what, no T cells? Clin. Infect. Dis. 56:900–901. 10.1093/cid/cis1047 [DOI] [PubMed] [Google Scholar]
- 46.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2–9. 10.1086/315215 [DOI] [PubMed] [Google Scholar]
- 47.Malavige GN, McGowan S, Atukorale V, Salimi M, Peelawatta M, Fernando N, Jayaratne SD, Ogg G. 2012. Identification of serotype-specific T cell responses to highly conserved regions of the dengue viruses. Clin. Exp. Immunol. 168:215–223. 10.1111/j.1365-2249.2012.04566.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangada MM, Rothman AL. 2005. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J. Immunol. 175:2676–2683. 10.4049/jimmunol.175.4.2676 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.