ABSTRACT
Several studies have demonstrated that the delivery of type I, II, or III interferons (IFNs) by inoculation of a replication-defective human adenovirus 5 (Ad5) vector expressing IFNs can effectively control foot-and-mouth disease (FMD) in cattle and swine during experimental infections. However, relatively high doses are required to achieve protection. In this study, we identified the functional properties of a porcine fusion protein, poIRF7/3(5D), as a biotherapeutic and enhancer of IFN activity against FMD virus (FMDV). We showed that poIRF7/3(5D) is a potent inducer of type I IFNs, including alpha IFN (IFN-α), IFN-β, and IFN-ω but not type III IFN (interleukin-28B), without inducing cytotoxicity. Expression of poIRF7/3(5D) significantly and steadily reduced FMDV titers by up to 6 log10 units in swine and bovine cell lines. Treatment with an IFN receptor inhibitor (B18R) combined with an anti-IFN-α antibody neutralized the antiviral activity in the supernatants of cells transduced with an Ad5 vector expressing poIRF7/3(5D) [Ad5-poIRF7/3(5D)]. However, several transcripts with known antiviral function, including type I IFNs, were still highly upregulated (range of increase, 8-fold to over 500-fold) by poIRF7/3(5D) in the presence of B18R. Furthermore, the sera of mice treated with Ad5-poIRF7/3(5D) showed antiviral activity that was associated with the induction of high levels of IFN-α and resulted in complete protection against FMDV challenge at 6, 24, or 48 h posttreatment. This study highlights for the first time the antiviral potential of Ad5-poIRF7/3(5D) in vitro and in vivo against FMDV.
IMPORTANCE FMD remains one of the most devastating diseases that affect livestock worldwide. Effective vaccine formulations are available but are serotype specific and require approximately 7 days before they are able to elicit protective immunity. We have shown that vector-delivered IFN is an option to protect animals against many FMDV serotypes as soon as 24 h and for about 4 days postadministration. Here we demonstrate that delivery of a constitutively active transcription factor that induces the production of endogenous IFNs and potentially other antiviral genes is a viable strategy to protect against FMD.
INTRODUCTION
Foot-and-mouth disease (FMD) is one of the most contagious viral diseases that affect cloven-hoofed livestock worldwide. The disease is enzootic in many regions of Africa, South America, and Asia, causing enormous economic and social impacts (1, 2). The causative agent, FMD virus (FMDV), is a nonenveloped virus that belongs to the Picornaviridae family (1, 3, 4). FMDV is an antigenically variable virus comprising seven serotypes and multiple subtypes. Serotypes A, O, and C were first isolated in Europe and occur worldwide, while serotypes SAT-1 to SAT-3 and Asia-1 have traditionally been restricted to Africa and Asia, respectively (1, 4, 5).
Infection of animals with FMDV results in rapid replication, spread, and shedding of large amounts of virus, resulting in high morbidity. Therefore, in case of an outbreak, FMD is controlled by restriction of animal movement, slaughter of in-contact susceptible animals, and in some instances, vaccination with an inactivated vaccine followed by slaughter. Although in some countries where the disease is enzootic preventive vaccination is commonly used (1), FMD-free countries tend to avoid vaccination due to the more restrictive trading policies imposed by the World Organization for Animal Health (OIE) (1). The current inactivated whole-virus vaccine is effective, but a number of limitations, such as difficulty in distinguishing infected from vaccinated animals (DIVA) and the requirement for an expensive high-containment facility for vaccine production, have led investigators to develop alternative vaccine approaches (2, 6, 7). Although vaccination is largely utilized worldwide to protect against FMD in countries where it is enzootic, current vaccines do not always prevent infection but rather limit or block clinical signs and require at least 5 to 7 days to elicit a protective immune response, which results in some animals becoming long-term carriers. Therefore, in case of FMD outbreaks in disease-free countries, it is necessary to limit disease spread and thus potentially reduce the number of animals that have to be slaughtered by inducing rapid protection prior to the development of vaccine-induced adaptive immunity.
Biotherapeutics represent an option to induce very early protection against FMDV infection (8). The interferon (IFN) response is one of the first antiviral mechanisms naturally induced in an infected host cell (9–11). IFNs are produced upon viral infection and play a crucial role in early innate immunity as well as in subsequent adaptive immunity (12). The expression of type I IFN is regulated by the activation of transcription factors, such as members of the nuclear factor kappa B (NF-κB) family (13), activating transcription factor 2 (ATF-2)/c-Jun complex (14), interferon regulatory factor 7 (IRF7) (15), and IRF3 (11), that bind to specific sequences present at IFN promoter regions. IFN transcription, followed by translation, secretion, and binding to specific receptors, triggers the induction of IFN-stimulated genes (ISGs), which code for antiviral products that affect viruses at different stages of their replication cycle, and different viruses are susceptible to different ISG products (10, 16).
FMDV has developed several mechanisms to evade the host immune response, including inhibition of cap-dependent host translation; inhibition of IFN expression and/or IFN signaling, presumably by virus-dependent degradation of NF-κB; suppression of IRF3 and IRF7 activation; and deubiquitination of retinoic acid-inducible gene I (RIG-I), TANK-binding kinase 1 (TBK1), tumor necrosis factor (TNF) receptor-associated factor 3 (TRAF3), and TRAF6 (17–23). However, IFN proteins are still detected in the serum and tissues of animals infected with FMDV, suggesting that inhibition of translation induced by the virus might be temporal and tissue or even cell specific (24). Animals that overexpress IFN, delivered by inoculation of a replication-defective human adenovirus type 5-based vector (Ad5) expressing IFNs, are protected against the clinical manifestations of disease and in some cases are protected from primary infection in a dose-dependent manner (25–28), suggesting that the strength, timing, and location of virus-host interactions are determinants critical to the outcome of the disease. In any case, high doses of Ad5 expressing IFNs (Ad5-IFNs) are required to achieve protection, resulting in an expensive approach to control FMD; therefore, there is a need to enhance the potency of this approach.
A construct, IRF7/3(5D), prepared using human sequences was previously described (29) and contains 246 amino acids from IRF7 (the DNA binding and constitutive activation domains) and 295 amino acids from IRF3(5D) (the transactivation and signal response domains). Expression of this construct in cultured human cells induced the activation of IFN promoters in vitro (29). Adjuvant properties of plasmids expressing IRF3(5D) or IRF7/3(5D) have also been described in mice, but IFN expression was not detected after intramuscular injection, presumably as a consequence of the low efficacy of plasmid-derived gene transfer in muscle tissue (30). Here, we describe the functional characterization of a constitutively active porcine IRF7/3(5D) [poIRF7/3(5D)] synthetic construct as an antiviral against FMDV. We found that this fusion protein is a potent inducer of several type I IFNs (but not type III IFNs) in cells from several species. Expression of poIRF7/3(5D) enhances the antiviral activity of an Ad5 vector expressing porcine beta IFN (Ad5–poIFN-β) against FMDV. Furthermore, mice inoculated with an Ad5 vector expressing poIRF7/3(5D) [Ad5-poIRF7/3(5D)] developed no viremia after FMDV serotype A24 challenge, and their sera had high levels of antiviral activity correlating with increased systemic levels of murine IFN-α/β (muIFN-α/β). This antiviral strategy can contribute to the development of improved biotherapeutics to control FMDV infection in animals.
MATERIALS AND METHODS
Cell and reagents.
Swine kidney (SK6 and IBRS-2) cells were obtained from the Foreign Animal Disease Diagnostic Laboratory (APHIS) at Plum Island Animal Disease Center (PIADC), Greenport, NY. Madin-Darby bovine kidney (MDBK; ATCC CCL-22), baby hamster kidney (BHK-21, clone 13, ATCC CCL-10), and mouse L929 (ATCC CCL-1) cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and were used for plasmid transfection or Ad5 vector transduction. Human 293 cells (ATCC CRL-1573) were also purchased from ATCC and were used to propagate recombinant Ad5 vectors (31). Mouse embryonic fibroblasts (MEFs) were propagated from original clones kindly provided by David E. Levy (New York University) (26). Cells were cultured under standard tissue culture conditions and maintained in minimal essential medium (MEM) containing 10% fetal bovine serum (FBS) supplemented with 1% antibiotics and nonessential amino acids. Ten percent tryptose phosphate broth was included in the medium for BHK-21 cells.
The inhibitor B18R (eBioscience, San Jose, CA) was used to block IFN type I receptor signaling. Prior to transfection or infection, cells were incubated for 1 h at room temperature in complete medium containing the inhibitor B18R at a concentration of 200 ng/ml. The inhibitor B18R was maintained in the medium during transfection and replenished after viral infection. Anti-pig IFN-α (clone K9) antibody (Ab; PBL Interferon Source, Piscataway, NJ) was used to neutralize the IFN-α activity present in the supernatants (3 μg of antibody/ml of supernatant) of treated cells. In some transfections, poly(I·C) (InvivoGen, San Diego, CA) was used as an inducer of IFN expression at the concentrations specified below.
Viral infections.
A laboratory-adapted vesicular stomatitis virus (VSV) serotype Indiana isolate was kindly provided by Judith Ball (Texas A&M University). A VSV serotype New Jersey field strain (95COB) and FMDV serotype A12 were generated from full-length-virus infectious clones (32). FMDV A24, which was isolated from the field and passed once in BHK-21 cells, was used for mouse experiments. All experimental infections using VSV serotype New Jersey or FMDV were conducted at the USDA-ARS Plum Island Animal Disease Center under biosafety level 3 agricultural hazard (BSL-3Ag) conditions. Infections with VSV serotype Indiana were performed at Texas A&M University under BSL-2 conditions.
Cells were infected at the times posttransfection specified below or transduced with Ad5 at the multiplicities of infection (MOIs) indicated below. In all cases, FMDV or VSV was adsorbed for 1 h at 37°C. For FMDV, unabsorbed virus was removed by washing the cells with 150 mM NaCl–20 mM morpholineethanesulfonic acid (MES; pH 6.0). Incubation continued for 24 h, unless otherwise specified. Virus was released by one freeze-thaw cycle. Viral titers were determined by a standard 50% tissue culture infective dose (TCID50) method using IBRS-2 cells, and results were expressed as the log10 number of TCID50/ml. Viral titers in FMDV-infected mouse serum were determined by plaque assay, using standard procedures (33), and expressed as the number of PFU/ml of serum.
Cell toxicity assay.
Cell toxicity after transfection or transduction of plasmids or Ad5 vectors expressing poIRF7/3(5D) was determined by using a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT)-based in vitro toxicology assay kit (Sigma, St. Louis, MO) following the manufacturer's recommendations. The optical density at 450 nm was read, and the optical density was filtered at 650 nm after 4 h of incubation. Microscopic examination of cell morphology in the monolayer after transfection/transduction was also used as an indicator of cell toxicity.
Plasmid construction.
Partial DNA sequences of porcine IRF7 (GenBank accession number AB287430, nucleotides 212 to 964) and IRF3 (GenBank accession number AB116563.1, nucleotides 400 to 1260) were used to synthesize the poIRF7/3(5D) fusion construct. This fusion construct was then cloned at the XbaI/EcoRV sites of the pcDNA 3.1 zeo+ vector (Invitrogen, Carlsbad, CA). A plasmid expressing the green fluorescent protein (GFP) in the pcDNA 3.1 zeo+ background (pGFP) was kindly provided by Michael Golding (Texas A&M University) and was used as a control.
Ad5 vector construction.
The pcDNA 3.1 zeo+ poIRF7/3(5D) construct was digested with ClaI and XbaI, and the resulting DNA fragment was ligated into a pAd5-Blue vector (34) that had been digested with the same enzymes to create recombinant Ad5-poIRF7/3(5D). Replication-defective human Ad5 expressing poIRF7/3(5D) was produced by transfection of 293 cells with the PacI-linearized recombinant pAd5-poIRF7/3(5D). Viruses were isolated, propagated, and purified by CsCl gradient centrifugation (34). The Ad5-Blue and Ad5–poIFN-β vectors were constructed previously (26, 35).
Analysis of mRNA expression by RT-qPCR.
Total RNA was isolated from cell lysates using a commercially available extraction kit (Qiagen, Valencia, CA). Two hundred to 1,000 ng of RNA was used to synthesize cDNA using random hexamers with qScript kit mix (Quanta Biosciences, Gaithersburg, MD) according to the manufacturer's instructions. Copied DNA was diluted 10-fold and used as the template for quantitative (qPCR) with PerfeCTa SYBR green FastMix and carboxy-X-rhodamine (Quanta Biosciences, Gaithersburg, MD). Samples were run in an Applied Biosystems 7500 or StepOne real-time PCR system (Applied Biosystems, Carlsbad, CA).
The expression of the genes of interest was normalized using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and β-actin. Relative quantification was performed for IRF7 and a panel of previously described ISGs (10, 27). Standard curves were run to standardize a SYBR-green based PCR array of the subtypes of porcine type I IFN. Sequences for detecting subtypes of porcine type I IFNs (IFN-α, -β, -κ, -ε, -ω, and -δ) were published previously (36–39).
Real-time qPCR (RT-qPCR) analysis was performed following the guidelines for the minimum information for publication of quantitative real-time PCR experiments (40). Data were analyzed using the comparative threshold cycle (ΔΔCT) method (41).
IFN bioassay.
The antiviral activity induced by IFN expression was tested with a VSV infection inhibition assay as previously described (20). Supernatants from cell cultures that had previously been transduced with Ad5 vectors were filtered through Centricon 100 columns (Millipore, Billerica, MA) to remove adenovirus particles.
Samples were diluted 2-fold and incubated on IBRS-2 cells for approximately 24 h. Supernatants were then removed and cells were infected with VSV serotype New Jersey (MOI = 2). Twenty-four or 48 h later, the cytopathic effect (CPE) was determined by microscopic examination, followed by staining with 1% crystal violet. Antiviral activity (the IFN concentration in units/ml) was expressed as the reciprocal of the highest dilution of supernatant able to suppress a VSV-induced cytopathic effect in 50% of the assayed wells.
The antiviral activity in serum samples from mice inoculated with the Ad5 vectors was tested on L929 cells as previously described (42). Briefly, 2-fold dilutions of serum samples were applied to confluent monolayers of L929 cells. At 24 h, the cells were infected with VSV (MOI = 20), followed by 48 to 72 h of incubation at 37°C. CPEs were scored by microscopic examination, followed by staining with 1% crystal violet.
Murine IFN ELISA.
Serum samples from mice infected with Ad5-poIRF7/3(5D) or the Ad5-Blue control vector were tested for the presence of muIFN-α and muIFN-β using VeriKine mouse enzyme-linked immunosorbent assays (ELISAs; PBL Interferon Source, Piscataway, NJ) per the manufacturer's directions. The absorbance at 450 nm was measured in an ELISA plate reader (VersaMax; Molecular Devices, Sunnyvale, CA). Cytokine concentrations were calculated on the basis of the optical densities obtained with standard curves.
Mouse challenge studies.
All animal work was conducted in compliance with the Animal Welfare Act (AWA), the 2011 Guide for the Care and Use of Laboratory Animals (43), the 2002 PHS Policy for the Humane Care and Use of Laboratory Animals, and U.S. Government Principles for Utilization and Care of Vertebrates Animal Used in Testing, Research and Training (44), as well as a specific animal protocol reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of PIADC.
Six- to 7-week-old female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and acclimated for 1 week. In the first experiment, two groups of five mice each were inoculated with Ad5-poIRF7/3(5D) (3 × 107 or 3 × 108 PFU/mouse) or Ad5-Blue (3 × 108 PFU/mouse) subcutaneously (s.c.) in the dorsal flank. One day after Ad5 treatment, serum samples were collected for testing by the IFN ELISAs and determination of total antiviral activity and the mice were euthanized.
In the second experiment, groups of five mice were inoculated s.c. with 3 × 108 PFU/mouse of Ad5-poIRF7/3(5D) or Ad5-Blue. A control group was inoculated with phosphate-buffered saline (PBS). At 6, 24, or 48 h after Ad5 treatment, mice were infected s.c. in the right rear footpad with 105 PFU of FMDV A24 in 50 μl of PBS as previously described (45). Animals were monitored for 7 days, and blood was collected at 1, 3, 5, and 7 days postchallenge. Viremia was determined by plaque assay on BHK-21 cells (33).
Similarly, in a third experiment, groups of five mice were inoculated s.c. with PBS, Ad5-poIRF7/3(5D) (3 × 107 PFU/mouse), Ad5-Blue (3 × 107 PFU/mouse), or combinations of these treatments with 100 IU of muIFN-α [(Ad5-poIRF7/3(5D) at 3 × 107 PFU/mouse plus 100 IU muIFN-α, Ad5-poIRF7/3(5D) at 3 × 106 PFU/mouse plus 100 IU muIFN-α, Ad5-Blue at 3 × 107 PFU/mouse plus 100 IU muIFN-α, Ad5-Blue at 3 × 106 PFU/mouse plus 100 IU muIFN-α, or 100 IU muIFN-α]. Ad5 inoculations were performed 48 h prior to FMDV challenge, while recombinant muIFN-α was applied 24 h before FMDV challenge. Two days after Ad5 treatment (or 24 h after IFN treatment), mice were infected s.c. in the right rear footpad with 105 PFU of FMDV A24. Animals were monitored for 7 days, and blood was collected at 1, 3, 5, and 7 days postchallenge. Viremia was determined by plaque assay on BHK-21 cells (33).
Statistics and data analysis.
Treatment differences were determined using Student's t test, Dunnett's method, or the Wilcoxon rank sum test, as indicated in the figure legends. Representative results of three independent replicates are shown for all experiments except the mouse experiments. Statistical analyses were performed using JMP software, version 8.0.2. Values are expressed as mean ± standard error or the mean (SEM), and statistical significance is indicated in the figure legends.
RESULTS
poIRF7/3(5D) induces high levels of ISG expression.
The IRF7 transcription factor shares structural features with IRF3 and includes a conserved DNA binding domain (DBD) and a serine-rich C-terminal region that is the target of virus-inducible phosphorylation (46). Here, using domains analogous to human IRF7/3A, we generated a chimeric construct of porcine IRF7 and IRF3, poIRF7/3(5D). The construct contains the DBD and constitutive activation domain (CAD) from porcine IRF7 but lacks the inhibitory domain (ID) (Fig. 1A). It also has the proline-rich domain (Pro), transactivation domain (TAD), and signal response domain (SRD) from porcine IRF3. Analogous to the human construct described previously (29), poIRF7/3(5D) contains mutations at 5 amino acids in the C-terminal IRF3 domain that mimic phosphorylation and therefore result in constitutive activation (Fig. 1A).
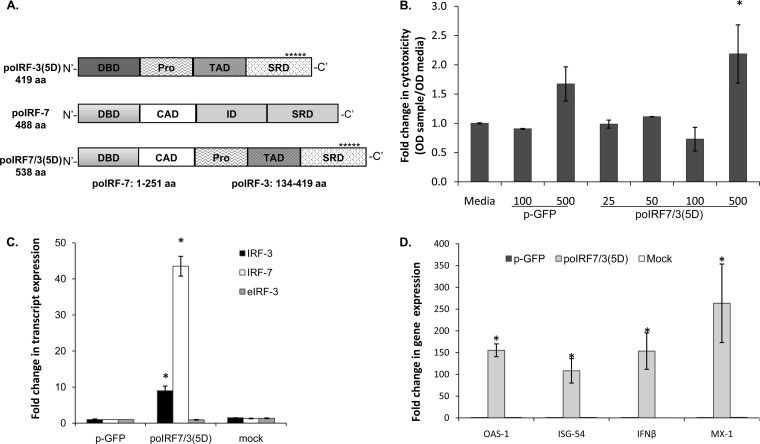
FIG 1.
Expression of poIRF3/7(5D) induces the expression of IFN and ISG mRNAs. (A) Schematics of porcine fusion [poIRF7/3(5D)] and parental [poIRF7 and poIRF3(5D)] proteins used in this work. Asterisks represent 5 phosphomimetic amino acid substitutions (5D) at the C terminus of the IRF3 DNA binding domain (DBD), proline-rich domain (Pro), transactivation domain (TAD), signal response domain (SRD), constitutive activation domain (CAD), and inhibitory domain (ID). aa, amino acids. (B) Cytotoxicity assays (XTT) on IBRS-2 cells 24 h after transfection with 25 to 500 ng of plasmid poIRF7/3(5D) or pGFP. OD, optical density; Media, transfection of medium alone. (C) Expression of plasmid-derived IRF3/IRF7 or endogenous IRF3 (eIRF3) mRNA was measured by RT-qPCR in SK6 cells at 24 h posttransfection with plasmids poIRF7/3(5D) and pGFP. Mock transfection was used as a control. (D) Expression of OAS1, ISG54, Mx1, or IFN-β mRNA was measured by RT-qPCR in SK6 cells at 24 h after plasmid or mock transfection. Statistical analyses were performed using Dunnett's method (*, P < 0.05) for panel B and the Wilcoxon rank sum test (*, P < 0.05) for panels C and D.
Swine cells were transfected with the plasmid expressing the fusion protein. Since the proapoptotic and cytotoxic effects of a human IRF7/3(5D) construct have been previously described (29, 47), we evaluated the possible effects resulting from the overexpression poIRF7/3(5D) in swine cells. No cytotoxic effects of poIRF7/3(5D) were detected when 25 to 100 ng of plasmid was transfected in 2.5 × 105 cultured porcine cells (Fig. 1B). At a higher concentration, i.e., 500 ng, a significant increase in cytotoxicity was observed (P < 0.05).
Expression of poIRF7/3(5D) was analyzed by RT-qPCR using primers and probes that detected IRF7 and IRF3 transcribed regions. An IRF7 primer set detected both poIRF7/3(5D)-derived and cellular IRF7 mRNAs. However, two sets of primers were required to detect either plasmid-derived IRF3 (IRF3) or cellular IRF3 (endogenous IRF3 [eIRF3]) transcripts. Total IRF7 and specific IRF3 transcribed from the poIRF7/3(5D) plasmid were significantly upregulated (∼40-fold and 10-fold, respectively) in cells transfected with the poIRF7/3(5D) plasmid compared to the levels of regulation for the control groups of mock- or pGFP-transfected cells (P < 0.05) (Fig. 1C). Basal levels of IRF7 were detected in mock- or pGFP-transfected cells, similar to the findings for endogenous IRF3, whose levels also remained unchanged in the poIRF7/3(5D)-transfected cells.
To determine if the overexpression of poIRF7/3(5D) was able to induce changes in host gene profiles, three known ISGs, 2′,5′-oligoadenylate synthetase (OAS1), IFN-stimulated gene 54 (ISG54), and myxovirus resistance 1 (Mx1), as well as IFN-β, were analyzed by RT-qPCR. While little or no induction was detected in SK6 cells mock treated or transfected with pGFP, significant upregulation was detected in cells transfected with 25 ng of the poIRF7/3(5D) fusion protein (Fig. 1D). Levels of induction varying from 100- to 250-fold were detected for all analyzed genes, indicating that the fusion poIRF7/3(5D) protein was active.
poIRF7/3(5D) expressed in porcine cells has antiviral properties against FMDV and VSV.
To test the biological functions of the fusion protein poIRF7/3(5D) in the context of a viral infection, SK6 cells were mock transfected or transfected with 25 ng of poIRF7/3(5D) or pGFP and later infected with either FMDV or VSV. A striking reduction (5 to 6 log10 units) in the yield of FMDV (Fig. 2A) or VSV (Fig. 2B) (P < 0.0001 in all cases) was observed in cells transfected with poIRF7/3(5D), while no effect was detected in cells transfected with the control pGFP. Antiviral activity was consistently detected only in supernatants from poIRF7/3(5D)-transfected cells, and this activity was greatly decreased, but not completely neutralized, when cells were treated with an anti-poIFN-α antibody (Table 1).
FIG 2.
poIRF7/3(5D) has significant antiviral activity against FMDV and VSV in porcine cell lines. SK6 (A and B) or IBRS-2 (C and D) cells were transfected with 25 ng of plasmid poIRF7/3(5D) or pGFP or mock transfected with medium alone. At 24 h posttransfection, the cells were infected with FMDV A12 or VSV Indiana at an MOI of 0.1. Viral titers were determined by the TCID50 method at 24 h postinfection. Statistical analysis was performed using Student's t test. ***, P < 0.001.
TABLE 1.
Antiviral activity in the supernatants of porcine SK6 and IBRS-2 cells after transfection with poIRF7/3(5D)a
| Treatment | Neutralizationb | Mean ± SEM IFN concn (U/ml) |
|
|---|---|---|---|
| SK6 cells | IBRS-2 cells | ||
| poIRF7/3(5D) | 135.9 ± 39.8 | 22.8 ± 10.8 | |
| poIRF7/3(5D) | Anti-IFN-α | 10.5 ± 4.4 | 1.6 ± 0.6 |
| pGFP | <1 ± 0.0 | <1 ± 0.0 | |
| pGFP | Anti-IFN-α | <1 ± 0.0 | <1 ± 0.0 |
| Mock | <1 ± 0.0 | <1 ± 0.0 | |
| Mock | Anti-IFN-α | <1 ± 0.0 | <1 ± 0.0 |
An antiviral activity bioassay was performed in supernatants of SK6 or IBRS-2 cells collected at 24 h posttransfection of 25 ng of plasmid DNA.
In some supernatants, a neutralizing mouse anti-porcine IFN-α antibody was added to the cell supernatants prior to testing of VSV antiviral activity.
Antiviral activity against FMDV and VSV was also evaluated in the supernatants of IBRS-2 cells transfected with plasmids expressing the poIRF7/3(5D) construct (Fig. 2C and D). Similar to the findings for SK6 cells, there was a substantial reduction of viral titers (FMDV and VSV) varying from 4 to 6 log10 units after transfection with plasmids expressing poIRF7/3(5D), with no inhibition being detected in the pGFP-transfected cells. However, significantly less antiviral activity (6 times less) was detected in the supernatants of transfected IBRS-2 cells than in those of SK6 cells (Table 1). Most of the detected antiviral activity was neutralized by addition of an anti-porcine IFN-α antibody, suggesting that poIRF7/3(5D) mainly induces this type of IFN in swine cells (Table 1).
poIRF7/3(5D) steadily reduces viral yield and enhances the activity of Ad5–poIFN-β.
To determine if poIRF7/3(5D) could induce a sustained reduction of viral titers, IBRS-2 cells were transfected with poIRF7/3(5D) and infected with FMDV A12 at different times posttransfection. No differences in viral yields were detected earlier than 6 h posttransfection (hpt) with poIRF7/3(5D) or pGFP or after mock transfection (Fig. 3A). However, by 24 hpt, a 3-log10-unit reduction in virus yield was detected and was sustained for up to 120 h only in the cells transfected with poIRF7/3(5D).
FIG 3.
poIRF7/3(5D) induces sustained antiviral activity and potentiates the effects of Ad5–IFN-β. (A) IBRS-2 cells were transfected with 25 ng of plasmid poIRF7/3(5D) or pGFP or mock transfected. At the specified times posttransfection, cells were infected with FMDV A12 at an MOI of 1. At 24 h postinfection, supernatants were collected for viral titration by the TCID50 method. (B) SK6 cells were coinfected with Ad5–poIFN-β (MOI = 10e−4) and Ad5-poIRF7/3(5D) at three different MOIs (0.2, 0.1, or 0.02), with Ad5–poIFN-β (MOI = 10e−4) plus Ad5-Blue (MOI = 0.2) or medium alone, or with Ad5-poIRF7/3(5D) (MOI = 0.2). At 24 h, cells were challenged with FMDV at an MOI of 0.1 for 24 h, followed by determination of viral titers by the TCID50 method. Statistical analysis was performed using Student's t test. **, P < 0.01.
In order to deliver poIRF7/3(5D) more efficiently, we cloned its coding sequence into a replication-defective human Ad5 vector (Ad5-Blue) previously developed at PIADC (34). Infection of IBRS-2 cells with Ad5-poIRF7/3(5D) at an MOI of 2 resulted in an approximately 5-log10-unit reduction in the number of FMDV TCID50/ml (Table 2). Addition of IFN-neutralizing reagents (the inhibitor B18R and anti-IFN-α) neutralized most of the antiviral activity, although some residual activity (∼1 log10 unit) was still detected (Table 2).
TABLE 2.
Antiviral activity induced by Ad5-poIRF7/3(5D)a
| Treatment | Mean ± SEM log no. of TCID50/ml |
|
|---|---|---|
| B18R and anti-IFN-α negative | B18R and anti-IFN-α positive | |
| Ad5-poIRF7/3(5D) | <0.1 ± 0.0 | 4.1 ± 0.4 |
| Medium | 5.5 ± 0.0 | 6.3 ± 0.0 |
| Ad5-Blue | 5.1 ± 0.4 | 5.4 ± 0.4 |
FMDV titers (log TCID50/ml) recovered from the supernatants of IBRS-2 cells 24 h after infection with Ad5-poIRF7/3(5D) in the presence or absence of IFN-neutralizing agents (B18R and anti-porcine IFN-α antibody).
Next, we evaluated whether Ad5-poIRF7/3(5D) could enhance Ad5–poIFN-β antiviral activity (28). SK6 cells were infected with Ad5-poIRF7/3(5D) or combinations of Ad5–poIFN-β and Ad5-poIRF7/3(5D) or Ad5-Blue (empty vector) at 24 h prior to FMDV challenge. A reduction of approximately 3 log10 units was detected when cells were infected with Ad5-poIRF7/3(5D) at an MOI of 0.2. Interestingly, a dose-dependent decrease in viral yield of 2 to 3.5 log10 units compared to the viral yield with mock treatment was observed when cells were coinfected with Ad5-poIRF7/3(5D) at MOIs of 0.02, 0.1, and 0.2 combined with very small amounts of Ad5–poIFN-β (MOI = 10e−4) (Fig. 3B). Combinations of Ad5-poIRF7/3(5D) (MOI = 0.2) and Ad5–poIFN-β (MOI = 10e−4) resulted in a significant reduction in virus titer of ∼2 log10 units (P < 0.01) relative to that for cells treated with our control vector (Ad5-Blue) combined with Ad5–poIFN-β at similar MOIs. At the highest MOI used (0.2), Ad5-Blue did not significantly contribute to the reduction in virus titer induced by Ad5–poIFN-β (P > 0.1). These results suggest that treatment with Ad5-poIRF7/3(5D) enhances the antiviral activity of Ad5–poIFN-β against FMDV.
poIRF7/3(5D) induces antiviral responses in vitro in species other than swine.
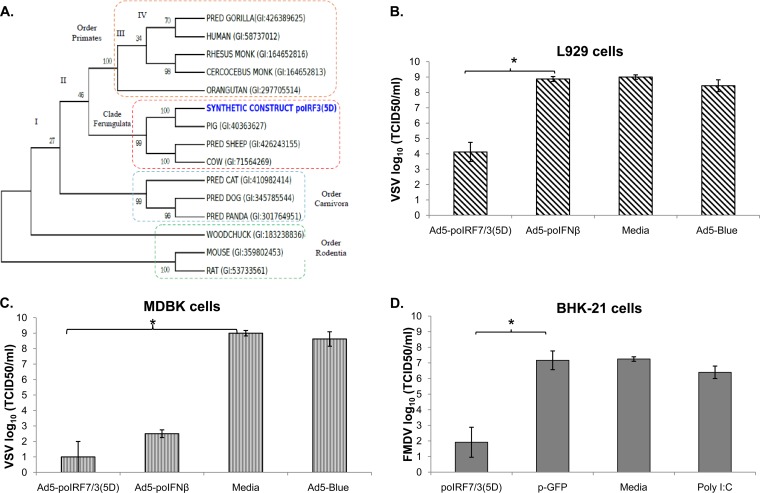
Since IRF family members share some homology, we studied the effect of poIRF7/3(5D) expression in vitro across several species. The phylogenetic relationships among several IRF3 protein sequences from some species available in public databases were deduced by maximum likelihood (ML) analysis (Fig. 4A) and verified by Bayesian inference. We confirmed that the IRF3 sequences of species from more closely related taxonomic groups, such as bovine, swine, and sheep, form a monophyletic group, while those of primates, carnivores, and rodents are more distantly related.
FIG 4.
poIRF7/3(5D) induces an antiviral response in vitro in cells from several species. (A) Consensus tree generated using ML. Bootstrapping values are displayed in each branch. Boxes represent the four different taxonomic groups represented in the tree. PRED, predicted, MONK, monkey. L929 (B) or MDBK (C) cells were infected with Ad5-poIRF7/3(5D), Ad5–poIFN-β, or Ad5-Blue at an MOI of 20 or mock treated with medium. At 24 h posttreatment, cells were infected with VSV serotype New Jersey at an MOI of 1 for 24 h. Viral titers were determined by the TCID50 method. (D) BHK-21 cells were transfected with plasmid poIRF7/3(5D) or pGFP or mock transfected with medium or were treated with poly(I·C) (100 ng/ml). At 24 h posttreatment, cells were infected with FMDV A12 at an MOI of 0.1 for 24 h. Viral titers were determined by the TCID50 method. Statistical analysis was performed using Student's t test. *, P < 0.05.
Interestingly, poIRF7/3(5D) induced a functional antiviral response in vitro in cell lines from several species, including MDBK (bovine), BHK-21 (hamster), or L929 (mouse) (Fig. 4). After infection with Ad5 poIRF7/3(5D) or transfection with poIRF7/3(5D) and subsequent challenge with FMDV or VSV, these cell lines exhibited a drastic reduction in viral yield compared with that from mock-treated cells. The antiviral effect in mouse L929 cells (Fig. 4B) or MEFs (not shown) was less than that in porcine (Fig. 2A to D) and bovine (Fig. 4C) cells. Consistent with our previous data (42), transduction with Ad5–poIFN-β did not protect murine cell lines from FMDV infection (Fig. 4B). The reduction in the FMDV yield in BHK-21 cells treated with the fusion protein (Fig. 4D) was similar to the effect observed in SK6 cells (Fig. 3A), but BHK-21 cells did not develop an antiviral response after poly(I·C) stimulation.
Characterization of the antiviral response induced by poIRF7/3(5D) in swine cells.
The antiviral activity elicited by transfection of the plasmid expressing poIRF7/3(5D) in SK6 or IBRS-2 cells was not fully neutralized by addition of an anti-IFN-α antibody (Table 1). To determine whether the residual antiviral activity could be attributed to the expression of other subtypes of porcine type I IFN, we quantitated the relative transcript levels of the IFN type I subtypes (IFN-α, -β, -κ, -ε, -ω, -δ) in cells treated with the Ad5-poIRF7/3(5D). Infection with Ad5-poIRF7/3(5D) induced expression of IFN-α, -β, and -ω in IBRS-2 cells (Fig. 5) and SK6 cells (data not shown). However, IFN-κ, -ε, or -δ mRNA or interleukin-28B (IL-28B; IFN-λ3) mRNA was not upregulated in any of these two cell lines.
FIG 5.
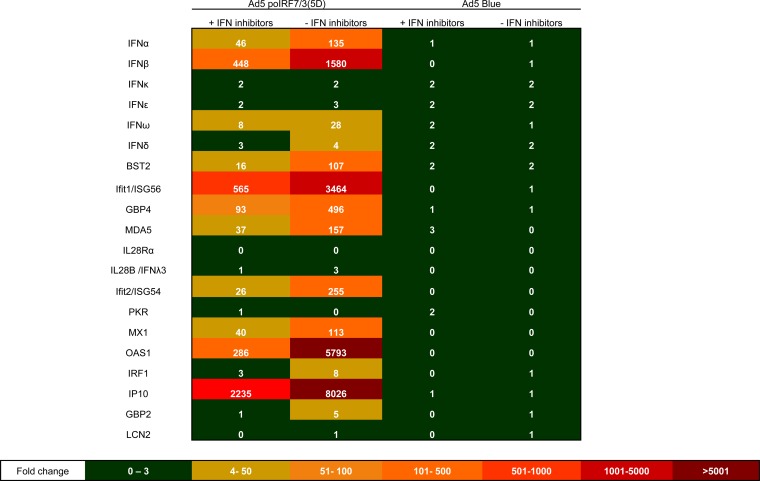
Characterization of several type I IFNs and other genes with antiviral functions induced by Ad5-poIRF7/3(5D) in porcine cells. Analysis of gene expression in IBRS-2 cells infected with Ad5-poIRF7/3(5D) or Ad5-Blue was performed by RT-qPCR. Relative gene expression was analyzed in cells infected with Ad5-poIRF7/3(5D) or Ad5-Blue in the presence (+) or absence (−) of the inhibitor B18R and an anti-IFN-α antibody. Mock-treated cells were used as a reference to calculate relative gene expression using the ΔΔCT method. IL-28Rα, interleukin-28α receptor.
We also questioned if an antiviral effect independent of type I IFNs might be involved in the strong antiviral response of IBRS-2 cells even when these cells showed less of an ability to induce antiviral responses than SK6 cells (Table 1). To neutralize the IFN-induced response, we used the inhibitor B18R, a product of vaccinia virus that competes with IFN for binding to the type I IFN receptor. Based on the production of antiviral activity induced by poIRF7/3(5D) in SK6 or IBRS-2 cells (Table 1), we used a dose of B18R that was sufficient to neutralize up to 500 units of IFN-α without causing toxicity. Transduction of IBRS-2 cells with Ad5-poIRF7/3(5D) completely blocked FMDV replication, and this effect was only partially reversed by the addition of the inhibitor B18R (Table 2). Although the antiviral activity in the supernatants of the cells was fully neutralized by B18R (Table 3), inhibition of FMDV replication was partially reversed (Table 2) and 46-, 448-, and 8-fold upregulation of the IFN-α, -β, and -ω transcripts, respectively (Fig. 5), was observed even in the presence of the inhibitor B18R combined with an anti-IFN-α antibody. Even though treatment with the inhibitor B18R in combination with anti-IFN-α markedly reduced the expression of all the ISGs tested, several ISGs, including BST2, IFN-γ-induced protein 10 (IP-10), ISG56, ISG54, GBP4, MDA5, and OAS1, were upregulated to relatively high levels in cells maintained with the IFN-neutralizing treatment (Fig. 5). The fold change in transcript levels of genes such as those for IP-10, OAS1, and ISG56 dropped from 8,026 to 2,235, 5,793 to 286, and 3,464 to 565, respectively. These results suggest that poIRF7/3(5D) may stimulate genes with antiviral function even when type I IFNs are neutralized. However, the identity of the antiviral genes induced by poIRF7/3(5D) fully independently of IFN stimuli in a porcine system remains to be determined.
TABLE 3.
Antiviral activity of filtered supernatants of IBRS-2 cells after treatment with Ad5-poIRF7/3(5D) in the presence or absence of IFN-neutralizing treatmenta
| Treatment | IFN concn (U/ml) |
|
|---|---|---|
| B18R and anti-IFN-α negative | B18R and anti-IFN-α positive | |
| Ad5-poIRF7/3(5D) | 11.3 | 0.0 |
| Medium | 0.0 | 0.0 |
| Ad5-Blue | 0.0 | 0.0 |
The bioassay for antiviral activity was performed in supernatants of IBRS-2 cells collected 24 h after infection with Ad5-poIRF7/3(5D) in the presence or absence of IFN-neutralizing agents (B18R and anti-porcine IFN-α antibody).
Ad5-poIRF7/3(5D) protects mice against FMDV challenge.
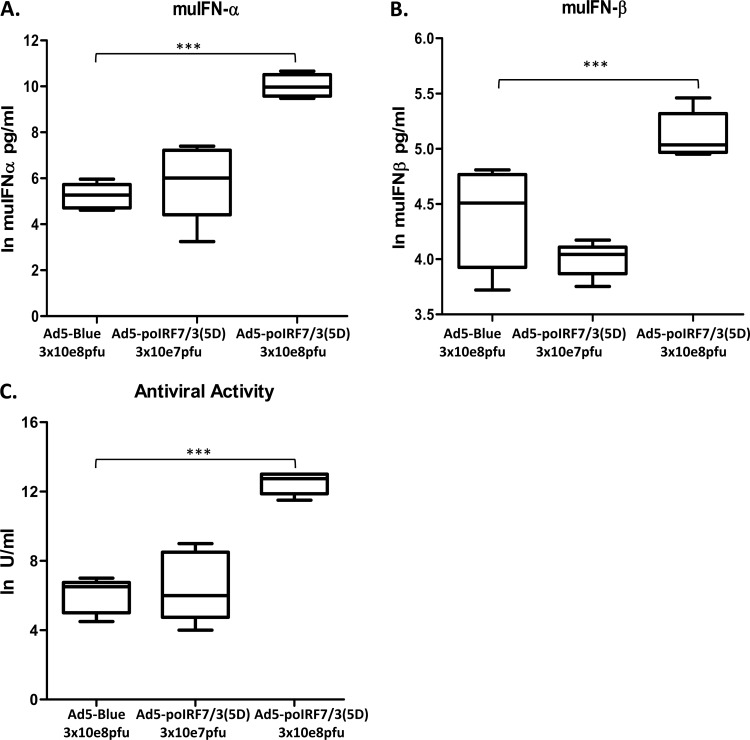
It has previously been shown that high doses of FMDV cause fatal disease in adult (6- to 7-week-old) C57BL/6 mice (45) that can be prevented by treatment with IFN or IFN-inducing agents (42). To determine if Ad5-poIRF7/3(5D) could induce the production of systemic IFN and protect against FMDV challenge, we inoculated mice with two doses (3 × 107 or 3 × 108 PFU/mouse) of Ad5-poIRF7/3(5D) or the Ad5-Blue control. We found that mice inoculated with the high dose (3 × 108 PFU/mouse) of Ad5-poIRF7/3(5D) had statistically significantly higher levels of IFN-α (P < 0.001), IFN-β (P < 0.05), and total induced antiviral activity (P < 0.001) than those inoculated with an equivalent dose of the Ad5-Blue control (Fig. 6A to C). Notably, Ad5-Blue induced some antiviral activity and the production of IFN-α or -β when it was used at a high dose (3 × 108 PFU/mouse). Mice treated with the high dose of Ad5-poIRF7/3(5D) produced, on average, 21,195 pg/ml of IFN-α, whereas 210 pg/ml was produced by mice treated with the Ad5-Blue control. Also, mice treated with the high dose of Ad-poIRF7/3(5D) produced, on average, 171 pg/ml of IFN-β, whereas 85 pg/ml was produced by mice treated with the Ad5-Blue control.
FIG 6.
Inoculation with Ad5-poIRF7/3(5D) induces high levels of IFN-α/β and total antiviral activity in mouse serum. Groups of mice (n = 5) were inoculated with Ad5-poIRF7/3(5D) or Ad5-Blue at the indicated doses. muIFN-α (A) or muIFN-β (B) protein levels in serum were tested by ELISA. Values are represented as the natural logarithm (ln) of the IFN concentration (in pg/ml). (C) Antiviral activity was measured in serum using a VSV bioassay. Values are represented as the natural logarithm of the IFN concentration (in U/ml). Statistical analyses were performed using Student's t test. ***, P < 0.001.
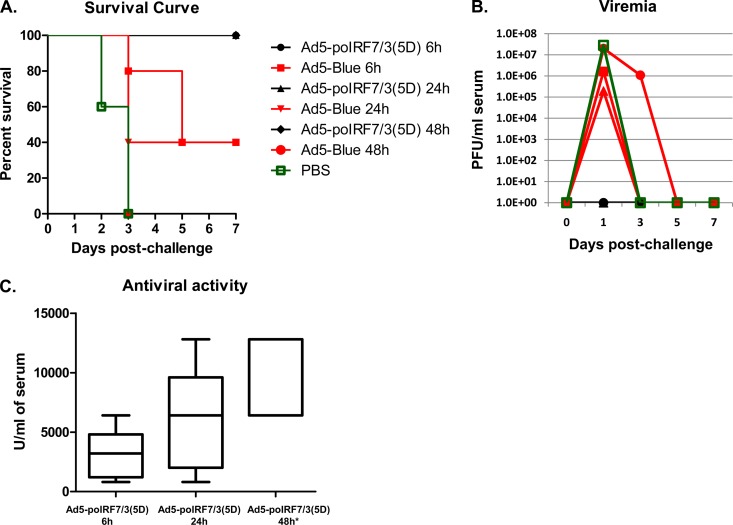
In a second experiment, groups of mice were treated with Ad5-poIRF7/3(5D) (3 × 108 PFU/mouse), Ad5-Blue (3 × 108 PFU/mouse), or PBS, followed by challenge with FMDV at 6 h, 24 h, or 48 h after treatment. All mice inoculated with Ad5-poIRF7/3(5D) were protected from FMDV challenge (Fig. 7A), none of the mice developed viremia (Fig. 7B), and all mice had high levels of antiviral activity in their sera (Fig. 7C). In contrast, partial protection was observed in the groups treated with Ad5-Blue at 6 h or 24 h prior to FMDV challenge (40% survival [Fig. 7A]), but all animals in the group treated with Ad5-Blue 48 h before challenge died. Consistent with these results, viremia was detected in the three Ad5-Blue-inoculated groups, with the highest levels being seen in the group inoculated 48 h prior to challenge (Fig. 7B); however, no systemic antiviral activity was detected. All animals treated with PBS died and developed high levels of viremia, and none of the animals displayed any antiviral activity in their sera.
FIG 7.
Ad5-poIRF7/3(5D) protects mice from FMDV challenge at 6, 24, or 48 h postinoculation. Groups of mice (n = 5) were inoculated with 3 × 108 PFU of Ad5-poIRF7/3(5D) or Ad5-Blue, followed by challenge with FMDV A24 (5 × 104 PFU/animal) at 6 h, 24 h, or 48 h. A control group was inoculated with PBS at 48 h before challenge. Animals were monitored for 7 days after challenge. (A) Survival curves; (B) FMDV titers in serum (viremia) determined by plaque assay; (C) antiviral activity in serum measured by VSV bioassay. *, the sera of groups inoculated with Ad5-Blue or PBS had antiviral activity below the detection levels.
In a third experiment, groups of mice were treated with 2 lower doses of Ad5-Blue or Ad5-poIRF7/3(5D) (3 × 106 or 3 × 107 PFU/mouse) in combination with 100 IU of muIFN-α, followed by FMDV challenge (Fig. 8). We found that 100% of mice treated with 3 × 107 PFU of Ad5-poIRF7/3(5D) or 3 × 107 PFU of Ad5-poIRF7/3(5D) plus 100 IU muIFN-α and 80% of mice treated with 3 × 106 PFU of Ad5-poIRF7/3(5D) plus 100 IU muIFN-α survived FMDV challenge, developed viremia (∼103 to 105 PFU/ml), and had high levels of antiviral activity in their sera (Fig. 8A to C). In contrast, 100% mortality, high viremia (∼107 PFU/ml), and antiviral activity below the levels of detection were found in all groups treated with Ad5-Blue alone or in combination with 100 IU muIFN-α (Fig. 8A and B). Unexpectedly, treatment with Ad5-poIRF7/3(5D) by itself or in combination with 100 IU muIFN-α induced similar protection. However, treatment with 100 IU of muIFN-α alone did not protect against FMDV replication, and no animals survived the challenge.
FIG 8.
Efficacy studies after treatment with Ad5-poIRF7/3(5D) in combination with muIFN-α. Groups of mice (n = 5) were inoculated with Ad5-poIRF7/3(5D), Ad5-Blue, recombinant muIFN-α protein, or combinations of these treatments at the indicated doses. Animals were challenged with FMDV A24 (5 × 104 PFU/animal at 48 h after Ad5 inoculation and/or 24 h after muIFN-α [100 IU] treatment). Clinical signs were monitored for 7 days. (A) Survival curve; (B) FMDV titers in serum (viremia) determined by plaque assay; (C) antiviral activity in serum measured by a VSV inhibition bioassay. *, the sera of groups inoculated with Ad5-Blue or PBS had antiviral activity below the detection levels.
DISCUSSION
In order to prevent or limit the spread of FMDV during outbreaks, it is imperative to develop methods that rapidly enhance innate immune responses in susceptible animals. It has been shown that overexpression of IFNs delivered with an Ad5 vector is effective in protecting swine and cattle against different serotypes of FMDV (25, 26, 28, 35). However, large amounts of Ad5-IFNs are required to fully protect swine and partially protect bovines. Thus, use of this strategy to protect large animals can be very expensive. To circumvent this limitation, a number of strategies have been examined, including use of a combination of type I and II IFNs which results in enhanced activity at lower doses (35), use of type III IFN (24, 48), use of adjuvants/modulators of innate immunity such as poly-ICLC [a synthetic complex of poly(I·C), poly-l-lysine, and carboxymethylcellulose] (8), or use of Venezuelan equine encephalitis replicon particles (VRPs) (42). Here, we report an additional strategy that involves a fusion construct generated from porcine sequences of IRF7 and IRF3, namely, poIRF7/3(5D). The resulting protein induced the activation of type I IFNs and, consequently, ISGs. We demonstrate that poIRF7/3(5D) is a powerful inducer of antiviral activity against FMDV and VSV.
Even though a small amount (25 ng) of the poIRF7/3(5D) construct was transfected into porcine cells, IRF7 transcript levels were significantly increased (Fig. 1C) and there was a significant induction of ISGs (Fig. 1D) and antiviral activity. These results demonstrate that even a low level of expression of this fusion protein is sufficient to induce innate responses in porcine cells. Importantly, the expression of this construct in vitro or in vivo did not result in cytotoxicity at doses that drastically reduced viral replication (∼6 log10 units).
Administration of an inactivated FMD vaccine or an Ad5 vector expressing the FMDV capsid requires approximately 7 days to induce protective immunity (49, 50). As a result, vaccinated animals exposed to virus within the first 7 days after vaccination are still susceptible to the disease (42, 51, 52). Here, we show that administration of sufficient amounts of Ad5-poIRF7/3(5D) can completely protect mice against FMD as early as 6 h and for at least 48 h after treatment. Furthermore, studies in vitro indicated that inhibition of viral replication was sustained for at least 5 days posttreatment. These results suggest that Ad5-poIRF7/3(5D) may induce not only rapid innate immunity but also a relatively long-lasting response that is needed for protecting animals until the vaccine-induced adaptive immunity is effective. Moreover, our results suggest that coadministration of Ad5-poIRF7/3(5D) might enhance the antiviral activity of a particular IFN, such as poIFN-β. This is expected, as Ad5-poIRF7/3(5D) stimulates several subtypes of type I IFNs, such as IFN-α, -β, and -ω, that could enhance the antiviral properties of a single type/subtype of porcine IFN. This information is instrumental in supporting future experiments to explore if the use of Ad5-poIRF7/3(5D) will allow Ad5-poIFN dose sparing to protect animals from FMD.
IBRS-2 cells have traditionally been used to grow FMDV in cell culture. Apparently, high levels of viral replication are achieved in this cell line because no induction of IFN-α/β mRNA is detected (53). In this study, we confirmed that IBRS-2 cells are somewhat impaired in their ability to induce IFN-α/β compared to cells of another porcine cell line, SK6. Unexpectedly, we detected antiviral activity in the supernatants of IBRS-2 cells transfected with poIRF7/3(5D), suggesting that other IFNs or IFN-independent genes with direct antiviral activity might have been induced by the fusion protein.
Previous reports found that expression of IRF3 alone does not induce the synthesis of endogenous IFN-α1 and IFN-β (47, 54). However, a subset of genes was activated in cells expressing the constitutively active form, IRF3(5D), combined with neutralizing antibodies against IFN-α/β (54). This result led us to investigate whether poIRF7/3(5D) could induce genes with antiviral function independently of IFN. We neutralized the antiviral activity in the supernatants of IBRS-2 cells by combining an anti-swine IFN-α (clone K9) antibody and the inhibitor B18R, a product of vaccinia virus that prevents the binding of type I IFN to its natural receptor (the IFN-α/β receptor) (55). The B18R protein has broad activity across species (56), is soluble outside the cell, and is present on the cell surface; thus, it blocks type I IFN autocrine and paracrine functions (55). In the presence of B18R and anti-swine IFN-α at doses that fully neutralized antiviral activity in the supernatants of cells infected with Ad5-poIRF7/3(5D), the induction of IFN-α, -β, and -ω transcripts was still highly upregulated. In accordance with findings from Grandvaux et al. (54), we found that ISG54 and ISG56 were still highly upregulated in the presence of IFN neutralization treatment. The genes for other ISGs, including the GBP4 (but not GBP2), IP-10, MDA-5, Mx1, and OAS1 genes, were also highly upregulated in the presence of the IFN neutralization treatment after transduction with Ad5-poIRF7/3(5D). This is consistent with the predominantly positive transcription signature described for IRF7 (57) or a STAT1-independent mechanism of induction, as previously reported for IP-10 during HIV infection of astrocytes (58). A possible explanation for the high degree of upregulation of IFN transcription in the presence of the inhibitor B18R could be the two-step positive-feedback loop that IFN-α/β employs to amplify its own expression (59, 60). While B18R inhibits signaling through the IFN-α/β receptor (second wave), the earlier expression of IFN-β and IFN-α4 (59) remains unaffected by the use of the inhibitor B18R. Alternatively, our treatments with B18R and anti-IFN-α might not have been sufficient to neutralize all subtypes of type I IFN induced by the fusion protein. The high level of induction of IFN transcripts in the presence of IFN-neutralizing treatment does not necessarily imply that the induction of genes with antiviral function by poIRF7/3(5D) is fully independent of IFN.
Another study using B18R to block the IFN response has shown the induction of a lipid raft-associated protein, BST-2 (also known as tetherin or CD317), independently of IFN. Tetherin inhibits viral infection by preventing the diffusion of virus particles after budding from infected cells (61). Here, we confirmed that the levels of the BST-2 transcript were induced by 16-fold in the presence of Ad5-poIRF7/3(5D) and IFN-neutralizing treatment. This antiviral protein inhibits the release of diverse enveloped virus particles, and it plays a role in neutralizing VSV (62). However, a role in controlling infection of nonenveloped viruses, such as FMDV, remains to be elucidated.
Similarly, we have also demonstrated that poIRF7/3(5D) induces potent antiviral effects in cell lines of multiple species, including bovine, hamster, and murine cell lines. In the case of murine (L929) cells, Ad5-poIRF7/3(5D) seemed to be less efficient at reducing the viral yield, a result that was also observed when Ad5-poIRF7/3(5D) was tested in mouse embryonic fibroblasts (data not shown). This observation is consistent with a more distant phylogenetic relationship between mouse and porcine sequences. However, contrary to the case in murine cells, a reduction in the antiviral properties of Ad5-poIRF7/3(5D) in hamster cells was not observed. In fact, it was surprising to detect the strong antiviral activity induced by Ad5-poIRF7/3(5D) in cell lines that have previously been reported to be defective in IFN-α/β sensing or signaling (63, 64) and that are routinely used for viral expansion and production of FMD vaccines (65). BHK-21 cells did not respond to poly(I·C) stimulation (or viral infection) but responded to treatment with the poIRF7/3(5D) protein, suggesting that expression of this protein bypasses certain limitations in antiviral pathways. Treatment with poIRF7/3(5D) in BHK-21 or IBRS-2 cells might bypass a defect in pathogen-associated molecular pattern (PAMP) sensing or transduction pathways and directly induce strong transcription of IFN or other genes with antiviral activities. Further studies are required to characterize the plethora of responses that could be induced by the fusion protein in these cell lines.
Characterization of the antiviral activity induced by poIRF7/3(5D) in porcine cells led us to identify type I IFNs but not type III IFN (IFN-λ3 or IL-28B) as major players in the induced antiviral effect. Type III IFN includes three IFN-λ molecules (IFN-λ1, -λ2, and -λ3, which are also known as IL-29, IL-28A, and IL-28B, respectively) (39). These cytokines induce innate antiviral responses similar to those induced by type I IFNs, but they have different structures and bind a different cell surface receptor (9, 24). Contrary to our findings using poIRF7/3(5D) in porcine cells, it has been reported that human IFN-λ2/3 gene expression is mainly controlled by IRF7 (66). In accordance with our results, another study suggested that type III IFNs are induced through independent actions of IRFs and nuclear factor kappa B (NF-κB) (67). Another report also suggested that the c-REL/p65 NF-κB heterodimer and IRF1 are the main transcriptional regulators of type III IFNs (68). Further studies are needed to study the regulation of type III IFN in porcine cells.
We found that expression of poIRF7/3(5D) induced the expression of various but not all type I IFN mRNAs, including IFN-α, -β, or -ω mRNA. We are currently working to identify the subtypes of IFN type I with higher antiviral activity during FMDV infection in the presence and absence of the Ad5-poIRF7/3(5D) stimulation. These results are consistent with previous reports in which IFN-ε was mainly associated with cells of reproductive function and IFN-κ expressed in epidermal keratinocytes (69). IFN-δ has been shown to have high levels of antiviral activity in porcine cells and a relevant biological role during early pregnancy when it is secreted by the trophectoderm of the pig conceptus (70).
Finally, the results obtained in vitro prompted us to evaluate the effectiveness of poIRF7/3(5D) in protecting mice from FMDV infection. We found that mice challenged with FMDV A24 at 6, 24, or 48 h postinoculation with Ad5-poIRF7/3(5D) fully survived viral challenge and did not develop viremia. To evaluate if expression of the chimeric protein poIRF7/3(5D) could potentially allow sparing of the amount of Ad5-IFN required to induce protection, we also performed experiments in mice. Since poIFN-α is not active in the murine system, we combined recombinant muIFN-α at a dose known not to be protective by itself (100 IU/animal) with Ad5-poIRF7/3(5D). Unfortunately, we could not detect an enhancement of the antiviral activity in the sera of the treated animals or an increase in the rate of survival after inoculation with the combination treatment. Presumably, the antiviral activity of muIFN-α protein decayed rapidly after administration. Even though further standardization of the dose of type I IFN is required to demonstrate potentiation between IFN and Ad5-poIRF7/3(5D), here we report that low doses (3 × 106 PFU/mouse) of Ad5-poIRF7/3(5D) fully or partially protected mice from FMDV challenge. These findings support future work in which the potentiation ability of Ad5-poIFNs and Ad5-poIRF7/3(5D) will be assessed using swine.
Confirming the results from our in vitro studies, mice treated with Ad5-poIRF7/3(5D) at a high dose (3 × 108 PFU/mouse) produced on average of 100 times more IFN-α than the control group. Ad5-poIRF7/3(5D) at a high dose also induced the upregulation of IFN-β to a lesser extent than it induced the upregulation of IFN-α. These results are consistent with the rapid antiviral activity detected in mouse sera at 24 hpt. In contrast, IFN-β transcripts were induced at approximately 10-fold higher levels than IFN-α transcripts in cultured epithelial porcine cells infected with Ad5-poIRF7/3(5D). Nevertheless, when IFN-β transcripts were highly upregulated in vitro, antiviral activity accounted for only less than 10% after neutralization with IFN-α-specific antibodies. Further analysis of IFN transcripts and protein from in vivo experiments using porcine tissues and serum is necessary to make a more relevant comparison.
Altogether, our results demonstrate that poIRF7/3(5D) is a robust inducer of innate immunity in porcine cells. Furthermore, poIRF7/3(5D) inhibits viral replication in cell lines from several species, including porcine, murine, and bovine cell lines, suggesting that a single poIRF7/3(5D) construct might hold biotherapeutic properties across species of interest, thus potentially inducing protection against FMDV, a virus that affects a wide range of livestock and more than 70 species of wildlife. A more precise understanding of the role of poIRF7/3(5D) in blocking FMDV replication in vivo will come from future studies in the natural host.
ACKNOWLEDGMENTS
This research was supported by CRIS 1940-32000-057-00D, ARS-DHS IAA HSHQDC-11-X-00189, the ORISE-PIADC Research Participation Program, and the Texas A&M University Graduate Student Trainee and Agrilife Programs.
We thank Neetu Singh, Diego Sturza, Elizabeth Ramirez-Medina, Gisselle Medina, X. Lu, and Marla Koster for professional and technical support and the PIADC animal caretakers for their assistance with the animal experiments. We also thank Marvin Grubman for critical discussions and review of the manuscript.
Footnotes
Published ahead of print 16 July 2014
REFERENCES
- 1.Grubman MJ, Baxt B. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17:465–493. 10.1128/CMR.17.2.465-493.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez LL, Gay CG. 2011. Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert Rev. Vaccines 10:377–387. 10.1586/erv.11.4 [DOI] [PubMed] [Google Scholar]
- 3.Whitton JL, Cornell CT, Feuer R. 2005. Host and virus determinants of picornavirus pathogenesis and tropism. Nat. Rev. Microbiol. 3:765–776. 10.1038/nrmicro1284 [DOI] [PubMed] [Google Scholar]
- 4.Ehrenfeld E, Domingo E, Ross RP. 2010. The picornaviruses. ASM Press, Washington, DC [Google Scholar]
- 5.Mason PW, Grubman MJ, Baxt B. 2003. Molecular basis of pathogenesis of FMDV. Virus Res. 91:9–32. 10.1016/S0168-1702(02)00257-5 [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez LL, Grubman MJ. 2009. Foot and mouth disease virus vaccines. Vaccine 5(Suppl 4):D90–D94. 10.1016/j.vaccine.2009.08.039 [DOI] [PubMed] [Google Scholar]
- 7.Ludi A, Rodriguez LL. 2013. Novel approaches to foot-and-mouth disease vaccine development. Dev. Biol. (Basel) 135:107–116. 10.1159/000313913 [DOI] [PubMed] [Google Scholar]
- 8.Dias CC, Moraes MP, Weiss M, Diaz-San Segundo F, Perez-Martin E, Salazar AM, de los Santos T, Grubman MJ. 2012. Novel antiviral therapeutics to control foot-and-mouth disease. J. Interferon Cytokine Res. 32:462–473. 10.1089/jir.2012.0012 [DOI] [PubMed] [Google Scholar]
- 9.Platanias LC. 2005. Mechanisms of type-I- and type-II-interferon-mediated signaling. Nat. Rev. Immunol. 5:375–386. 10.1038/nri1604 [DOI] [PubMed] [Google Scholar]
- 10.Sen GC, Sarkar SN. 2007. The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA, and viruses. Curr. Top. Microbiol. Immunol. 316:233–250 [DOI] [PubMed] [Google Scholar]
- 11.Sin WX, Li P, Yeong JP, Chin KC. 2012. Activation and regulation of interferon-beta in immune responses. Immunol. Res. 53:25–40. 10.1007/s12026-012-8293-7 [DOI] [PubMed] [Google Scholar]
- 12.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737. 10.1038/ni1087 [DOI] [PubMed] [Google Scholar]
- 13.Bartlett NW, Slater L, Glanville N, Haas JJ, Caramori G, Casolari P, Clarke DL, Message SD, Aniscenko J, Kebadze T, Zhu J, Mallia P, Mizgerd JP, Belvisi M, Papi A, Kotenko SV, Johnston SL, Edwards MR. 2012. Defining critical roles for NF-kappaB p65 and type I interferon in innate immunity to rhinovirus. EMBO Mol. Med. 4:1244–1260. 10.1002/emmm.201201650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanos D, Maniatis T. 1995. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83:1091–1100. 10.1016/0092-8674(95)90136-1 [DOI] [PubMed] [Google Scholar]
- 15.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777. 10.1038/nature03464 [DOI] [PubMed] [Google Scholar]
- 16.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485. 10.1038/nature09907; 10.1038/nature09907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grubman MJ, Moraes MP, Diaz-San Segundo F, Pena L, de los Santos T. 2008. Evading the host immune response: how foot-and-mouth disease virus has become an effective pathogen. FEMS Immunol. Med. Microbiol. 53:8–17. 10.1111/j.1574-695X.2008.00409.x [DOI] [PubMed] [Google Scholar]
- 18.Nfon CK, Ferman GS, Toka FN, Gregg DA, Golde WT. 2008. Interferon-alpha production by swine dendritic cells is inhibited during acute infection with foot-and-mouth disease virus. Viral Immunol. 21:68–77. 10.1089/vim.2007.0097 [DOI] [PubMed] [Google Scholar]
- 19.de los Santos T, de Avila Botton S, Weiblen R, Grubman MJ. 2006. The leader proteinase of foot-and-mouth disease virus inhibits the induction of beta interferon mRNA and blocks the host innate immune response. J. Virol. 80:1906–1914. 10.1128/JVI.80.4.1906-1914.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Pulido M, Borrego B, Sobrino F, Saiz M. 2011. RNA structural domains in noncoding regions of the foot-and-mouth disease virus genome trigger innate immunity in porcine cells and mice. J. Virol. 85:6492–6501. 10.1128/JVI.00599-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Fang L, Bi J, Chen Q, Cao L, Luo R, Chen H, Xiao S. 2011. Foot-and-mouth disease virus leader proteinase inhibits dsRNA-induced RANTES transcription in PK-15 cells. Virus Genes 42:388–393. 10.1007/s11262-011-0590-z [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Fang L, Luo R, Ye R, Fang Y, Xie L, Chen H, Xiao S. 2010. Foot-and-mouth disease virus leader proteinase inhibits dsRNA-induced type I interferon transcription by decreasing interferon regulatory factor 3/7 in protein levels. Biochem. Biophys. Res. Commun. 399:72–78. 10.1016/j.bbrc.2010.07.044 [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Fang L, Li P, Sun L, Fan J, Zhang Q, Luo R, Liu X, Li K, Chen H, Chen Z, Xiao S. 2011. The leader proteinase of foot-and-mouth disease virus negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. J. Virol. 85:3758–3766. 10.1128/JVI.02589-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Martin E, Weiss M, Diaz-San Segundo F, Pacheco JM, Arzt J, Grubman MJ, de los Santos T. 2012. Bovine type III interferon significantly delays and reduces the severity of foot-and-mouth disease in cattle. J. Virol. 86:4477–4487. 10.1128/JVI.06683-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Brum MC, Caron L, Koster M, Grubman MJ. 2003. Adenovirus-mediated type I interferon expression delays and reduces disease signs in cattle challenged with foot-and-mouth disease virus. J. Interferon Cytokine Res. 23:359–368. 10.1089/107999003322226014 [DOI] [PubMed] [Google Scholar]
- 26.Chinsangaram J, Moraes MP, Koster M, Grubman MJ. 2003. Novel viral disease control strategy: adenovirus expressing alpha interferon rapidly protects swine from foot-and-mouth disease. J. Virol. 77:1621–1625. 10.1128/JVI.77.2.1621-1625.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-San Segundo F, Moraes MP, de los Santos T, Dias CC, Grubman MJ. 2010. Interferon-induced protection against foot-and-mouth disease virus infection correlates with enhanced tissue-specific innate immune cell infiltration and interferon-stimulated gene expression. J. Virol. 84:2063–2077. 10.1128/JVI.01874-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias CC, Moraes MP, Segundo FD, de los Santos T, Grubman MJ. 2011. Porcine type I interferon rapidly protects swine against challenge with multiple serotypes of foot-and-mouth disease virus. J. Interferon Cytokine Res. 31:227–236. 10.1089/jir.2010.0055 [DOI] [PubMed] [Google Scholar]
- 29.Lin R, Genin P, Mamane Y, Hiscott J. 2000. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol. Cell. Biol. 20:6342–6353. 10.1128/MCB.20.17.6342-6353.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bramson JL, Dayball K, Hall JR, Millar JB, Miller M, Wan YH, Lin R, Hiscott J. 2003. Super-activated interferon-regulatory factors can enhance plasmid immunization. Vaccine 21:1363–1370. 10.1016/S0264-410X(02)00694-1 [DOI] [PubMed] [Google Scholar]
- 31.Graham FL, Prevec L. 1991. Manipulation of adenovirus vectors. Methods Mol. Biol. 7:109–128. 10.1385/0-89603-178-0:109 [DOI] [PubMed] [Google Scholar]
- 32.Rieder E, Bunch T, Brown F, Mason PW. 1993. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J. Virol. 67:5139–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grubman MJ, Baxt B, Bachrach HL. 1979. Foot-and-mouth disease virion RNA: studies on the relation between the length of its 3′-poly(A) segment and infectivity. Virology 97:22–31. 10.1016/0042-6822(79)90369-6 [DOI] [PubMed] [Google Scholar]
- 34.Moraes MP, Mayr GA, Grubman MJ. 2001. pAd5-Blue: direct ligation system for engineering recombinant adenovirus constructs. Biotechniques 31:1050, 1052, 1054–1056 [DOI] [PubMed] [Google Scholar]
- 35.Moraes MP, de los Santos T, Koster M, Turecek T, Wang H, Andreyev VG, Grubman MJ. 2007. Enhanced antiviral activity against foot-and-mouth disease virus by a combination of type I and II porcine interferons. J. Virol. 81:7124–7135. 10.1128/JVI.02775-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng G, Chen W, Li Z, Yan W, Zhao X, Xie J, Liu M, Zhang H, Zhong Y, Zheng Z. 2006. Characterization of the porcine alpha interferon multigene family. Gene 382:28–38. 10.1016/j.gene.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 37.Cheng G, Zhao X, Chen W, Yan W, Liu M, Chen J, Zheng Z. 2007. Detection of differential expression of porcine IFN-alpha subtypes by reverse transcription polymerase chain reaction. J. Interferon Cytokine Res. 27:579–587. 10.1089/jir.2006.0126 [DOI] [PubMed] [Google Scholar]
- 38.Sang Y, Rowland RR, Hesse RA, Blecha F. 2010. Differential expression and activity of the porcine type I interferon family. Physiol. Genomics 42:248–258. 10.1152/physiolgenomics.00198.2009 [DOI] [PubMed] [Google Scholar]
- 39.Sang Y, Rowland RR, Blecha F. 2010. Molecular characterization and antiviral analyses of porcine type III interferons J. Interferon Cytokine Res. 30:801–807. 10.1089/jir.2010.0016 [DOI] [PubMed] [Google Scholar]
- 40.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 42.Diaz-San Segundo F, Dias CC, Moraes MP, Weiss M, Perez-Martin E, Owens G, Custer M, Kamrud K, de los Santos T, Grubman MJ. 2013. Venezuelan equine encephalitis replicon particles can induce rapid protection against foot-and-mouth disease virus. J. Virol. 87:5447–5460. 10.1128/JVI.03462-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 44.U.S. Office of Science and Technology Policy. 1985. Laboratory animal welfare: U.S. Government principles for utilization and care of vertebrates animal used in testing, research and training, notice. Fed. Regist. 50:20864–20865 [PubMed] [Google Scholar]
- 45.Salguero FJ, Sánchez-Martín MA, Díaz-San Segundo F, deAvila A, Sevilla N. 2005. Foot-and-mouth disease virus (FMDV) causes an acute disease that can be lethal for adult laboratory mice. Virology 332:384–396. 10.1016/j.virol.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 46.De Ioannes P, Escalante CR, Aggarwal AK. 2011. Structures of apo IRF-3 and IRF-7 DNA binding domains: effect of loop L1 on DNA binding. Nucleic Acids Res. 39:7300–7307. 10.1093/nar/gkr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heylbroeck C, Balachandran S, Servant MJ, DeLuca C, Barber GN, Lin R, Hiscott J. 2000. The IRF-3 transcription factor mediates Sendai virus-induced apoptosis. J. Virol. 74:3781–3792. 10.1128/JVI.74.8.3781-3792.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz-San Segundo F, Weiss M, Perez-Martin E, Koster MJ, Zhu J, Grubman MJ, de los Santos T. 2011. Antiviral activity of bovine type III interferon against foot-and-mouth disease virus. Virology 413:283–292. 10.1016/j.virol.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 49.Golde WT, Pacheco JM, Duque H, Doel T, Penfold B, Ferman GS, Gregg DR, Rodriguez LL. 2005. Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: use in emergency outbreak response. Vaccine 23:5775–5782. 10.1016/j.vaccine.2005.07.043 [DOI] [PubMed] [Google Scholar]
- 50.Pacheco JM, Brum MC, Moraes MP, Golde WT, Grubman MJ. 2005. Rapid protection of cattle from direct challenge with foot-and-mouth disease virus (FMDV) by a single inoculation with an adenovirus-vectored FMDV subunit vaccine. Virology 337:205–209. 10.1016/j.virol.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 51.Mayr GA, Chinsangaram J, Grubman MJ. 1999. Development of replication-defective adenovirus serotype 5 containing the capsid and 3C protease coding regions of foot-and-mouth disease virus as a vaccine candidate. Virology 263:496–506. 10.1006/viro.1999.9940 [DOI] [PubMed] [Google Scholar]
- 52.Mayr GA, O'Donnell V, Chinsangaram J, Mason PW, Grubman MJ. 2001. Immune responses and protection against foot-and-mouth disease virus (FMDV) challenge in swine vaccinated with adenovirus-FMDV constructs. Vaccine 19:2152–2162. 10.1016/S0264-410X(00)00384-4 [DOI] [PubMed] [Google Scholar]
- 53.Chinsangaram J, Koster M, Grubman MJ. 2001. Inhibition of L-deleted foot-and-mouth disease virus replication by alpha/beta interferon involves double-stranded RNA-dependent protein kinase. J. Virol. 75:5498–5503. 10.1128/JVI.75.12.5498-5503.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grandvaux N, Servant MJ, tenOever B, Sen GC, Balachandran S, Barber GN, Lin R, Hiscott J. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76:5532–5539. 10.1128/JVI.76.11.5532-5539.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colamonici OR, Domanski P, Sweitzer SM, Larner A, Buller RM. 1995. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J. Biol. Chem. 270:15974–15978. 10.1074/jbc.270.27.15974 [DOI] [PubMed] [Google Scholar]
- 56.Symons JA, Alcami A, Smith GL. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81:551–560. 10.1016/0092-8674(95)90076-4 [DOI] [PubMed] [Google Scholar]
- 57.Barnes BJ, Richards J, Mancl M, Hanash S, Beretta L, Pitha PM. 2004. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J. Biol. Chem. 279:45194–45207. 10.1074/jbc.M400726200 [DOI] [PubMed] [Google Scholar]
- 58.Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, Phillipson C, Lindsley K, Krucker T, Fox HS, Campbell IL. 2001. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J. Virol. 75:7067–7077. 10.1128/JVI.75.15.7067-7077.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marié I, Durbin JE, Levy DE. 1998. Differential viral induction of distinct interferon alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660–6669. 10.1093/emboj/17.22.6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441:106–110. 10.1016/S0014-5793(98)01514-2 [DOI] [PubMed] [Google Scholar]
- 61.Bego MG, Mercier J, Cohen EA. 2012. Virus-activated interferon regulatory factor 7 upregulates expression of the interferon-regulated BST2 gene independently of interferon signaling. J. Virol. 86:3513–3527. 10.1128/JVI.06971-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weidner JM, Jiang D, Pan XB, Chang J, Block TM, Guo JT. 2010. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 84:12646–12657. 10.1128/JVI.01328-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clarke JB, Spier RE. 1983. An investigation into causes of resistance of a cloned line of BHK cells to a strain of foot-and-mouth disease virus. Vet. Microbiol. 8:259–270. 10.1016/0378-1135(83)90078-0 [DOI] [PubMed] [Google Scholar]
- 64.Moutailler S, Roche B, Thiberge JM, Caro V, Rougeon F, Failloux AB. 2011. Host alternation is necessary to maintain the genome stability of Rift Valley fever virus. PLoS Negl. Trop. Dis. 5:e1156. 10.1371/journal.pntd.0001156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amadori M, Volpe G, Defilippi P, Berneri C. 1997. Phenotypic features of BHK-21 cells used for production of foot-and-mouth disease vaccine. Biologicals 25:65–73. 10.1006/biol.1996.0061 [DOI] [PubMed] [Google Scholar]
- 66.Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. 2007. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J. Immunol. 179:3434–3442. 10.4049/jimmunol.179.6.3434 [DOI] [PubMed] [Google Scholar]
- 67.Iversen MB, Paludan SR. 2010. Mechanisms of type III interferon expression. J. Interferon Cytokine Res. 30:573–578. 10.1089/jir.2010.0063 [DOI] [PubMed] [Google Scholar]
- 68.Siegel R, Eskdale J, Gallagher G. 2011. Regulation of IFN-λ1 promoter activity (IFN-λ1/IL-29) in human airway epithelial cells. J. Immunol. 187:5636–5644. 10.4049/jimmunol.1003988 [DOI] [PubMed] [Google Scholar]
- 69.Van Pesch V, Lanaya H, Renauld JC, Michiels T. 2004. Characterization of the murine alpha interferon gene family. J. Virol. 78:8219–8228. 10.1128/JVI.78.15.8219-8228.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefevre F, Guillomot M, D'Andrea S, Battegay S, La Bonnardiere C. 1998. Interferon-delta: the first member of a novel type I interferon family. Biochimie 80:779–788. 10.1016/S0300-9084(99)80030-3 [DOI] [PubMed] [Google Scholar]