Abstract
In varicella-zoster virus (VZV)-infected primary human brain vascular adventitial fibroblasts (BRAFs), levels of beta interferon (IFN-β,) STAT1, and STAT2 transcripts as well as STAT1 and STAT2 protein were decreased. IFN-α transcript levels were increased but not secreted IFN-α protein levels. Compared to IFN-α-treated control results, in VZV-infected BRAFs, phosphorylated STAT1 did not translocate to the nucleus, resulting in impaired downstream expression of interferon-inducible antiviral Mx1. Overall, VZV interference with the type I interferon pathway may promote virus persistence in cerebral arteries.
TEXT
Varicella-zoster virus (VZV) vasculopathy is often protracted, perhaps due in part to persistent infection of arterial adventitial fibroblasts (1). A potential mechanism of virus persistence is evasion of the antiviral type I interferon (interferon alpha [IFN-α] and IFN-β) response (2) which induces antiviral interferon-inducible genes such as Mx1. Mx1 belongs to the dynamin superfamily of large GTPases (3, 4), is present in all vertebrates in one to three copies, and is strictly controlled by type I and type III interferons. Mx1 encodes the human MxA protein, which accumulates in the cytoplasm, recognizes viral nucleocapsids, and blocks viral replication. In human lung embryonic fibroblasts, VZV immediate-early protein (IE) 62 blocks phosphorylation of interferon regulatory factor 3 (IRF3) and subsequent induction of IFN-β (5). Similarly, in HEK 293T and MeWo cells, VZV IE61 degrades activated IRF3 (6), and in VZV-infected epidermal cells, IFN-α is downregulated and IFN-α-induced phosphorylated STAT1 (pSTAT1) is absent (7). Thus, we examined interference with type 1 IFN signaling in VZV-infected cerebrovascular adventitial fibroblasts as a potential mechanism for virus persistence.
Primary human brain vascular adventitial fibroblasts (BRAFs) (ScienCell, Carlsbad, CA) were seeded at 5,000 cells/cm2 in basal fibroblast medium supplemented with 2% fetal bovine serum (FBS), 1% fibroblast growth serum, and 1% 100× penicillin-streptomycin (ScienCell). After 24 h, medium was changed to basal fibroblast medium supplemented with 0.1% FBS and 1% 100× penicillin-streptomycin and replenished every 48 h for 1 week to establish quiescence. Quiescent BRAFs were cocultivated with VZV-infected or uninfected BRAFs or treated for 24 h with 1,000 U of IFN-α (PBL Interferon Source, Piscataway, NJ). VZV-infected BRAFs were analyzed at the height of the cytopathic effect (CPE) 3 days later and positive controls 24 h after treatment in 4 independent experiments. mRNA was analyzed by reverse-transcription PCR (RT-PCR) using SYBR green (8) and primers for IFN-α, IFN-β, STAT1, STAT2, Mx1, and GAPDH (glyceraldehyde 3-phosphate dehydrogenase; Fig. 1A). Primer efficiencies were 104%, 93%, 92%, 89%, 107%, and 102%, respectively. Data were normalized to GAPDH and analyzed using the delta delta threshold cycle (CT) method (9).
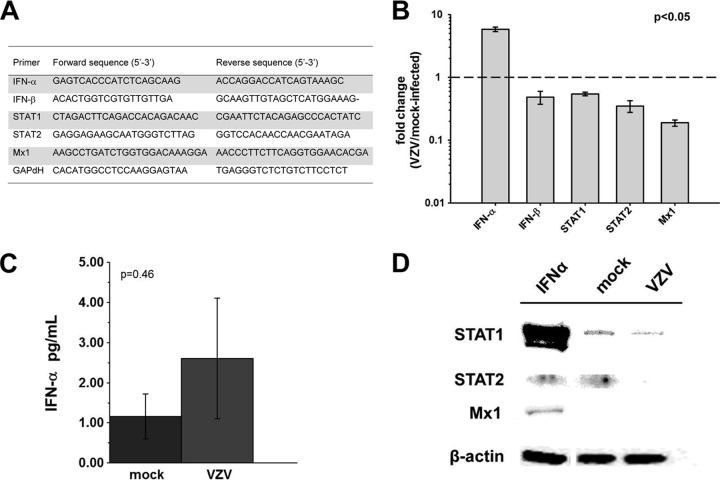
FIG 1.
IFN-α, IFN-β, STAT1, STAT2, and Mx1 expression in VZV-infected BRAFs and mock-infected controls. (A) mRNA was extracted from VZV-infected quiescent BRAFs and analyzed by RT-PCR 3 days later using primers for IFN-α, IFN-β, STAT1, STAT2, Mx1, and GAPDH; transcripts were normalized to GAPDH, and delta-delta CT analysis was performed. (B) Compared to mock-infected cells, VZV-infected BRAFs contained increased IFN-α transcript levels and decreased IFN-β, STAT1, STAT2, and Mx1 transcript levels (P < 0.05 for all transcripts analyzed). Data represent means ± standard errors of the means (SEM) of the results from 4 independent experiments. The dashed line represents a 1-fold change or no change in levels of transcripts from VZV-infected and mock-infected BRAFs. (C) Tissue culture medium from quiescent BRAFs infected with VZV was analyzed for IFN-α protein at 3 dpi using Mesoscale Discovery IFN-α plates. Compared to mock-infected cells, VZV-infected BRAFs did not contain significantly increased IFN-α protein levels. Data represent means ± SEM of the results from 4 independent experiments (P = 0.46). (D) Western blot analysis revealed bands corresponding to STAT1, STAT2, and Mx1 protein in IFN-α-treated BRAFs; STAT1 and STAT2, but not Mx1, in mock-infected cells; and no STAT2 or Mx1 and less STAT1 in VZV-infected cells than in mock-infected cells.
BRAFS were propagated on coverslips, fixed, and permeabilized (10). Mouse anti-VZV gE (Santa Cruz Biotechnology, Santa Cruz, CA) (1:500) was added in addition to another primary antibody: rabbit anti-STAT1 (Epitomics, Burlingame, CA) (1:200); rabbit anti-pSTAT1 (Epitomics) (1:300); or rabbit anti-Mx1 (Abcam, Cambridge, MA) (1:100). Secondary antibodies were 1:1,000 dilutions of both Alexa Fluor 594 donkey anti-mouse IgG and Alexa Fluor 488 donkey anti-rabbit IgG 488 (Life Technologies, Grand Island, NY). Coverslips were mounted with Vectashield containing DAPI (4′,6-]diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA) and visualized by microscopy.
For Western blotting, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Roche), and 5 μg of the reaction mixture was electrophoresed through a 12% Tris-glycine gel, transferred to polyvinylidene difluoride (PVDF) membranes, and blocked using 1× casein (Vector Laboratories) diluted in 1× Tris-buffered saline (TBS)–0.05% Tween for 30 min at room temperature. Membranes were incubated overnight at 4°C with antibodies against STAT1, STAT2, and Mx1 (Abcam) diluted 1:100 in blocking buffer. β-Actin was diluted 1:5,000 for loading controls. After washing in blocking buffer at room temperature was performed, blots were incubated with secondary antibody horseradish peroxidase-labeled goat anti-rabbit IgG (H+L) (Jackson ImmunoResearch) diluted 1:10,000 for 30 min, washed in blocking buffer, and developed using Pico West chemiluminescent substrate (Thermo). Bands on blots were quantitated using an Alpha Innotech digital imaging system and ImageJ software (NIH).
Comparison of transcripts in VZV-infected BRAFs versus mock-infected BRAFs at 3 days postinfection (dpi) (Fig. 1B) revealed increased IFN-α mRNA (5.88-fold ± 0.50-fold) and decreased IFN-β (0.49-fold ± 0.12-fold), STAT1 (0.54-fold ± 0.03-fold), STAT2 (0.35-fold ± 0.08-fold), and Mx1 mRNA (0.19-fold ± 0.02-fold) levels (P < 0.05 for all transcripts analyzed). At 3 dpi, secreted IFN-α protein levels in VZV-infected BRAFs were not significantly higher (1.93-fold ± 1.3-fold) than in mock-infected BRAFs (1.16 pg/ml ± 0.57 in mock-infected BRAFs versus 2.6 pg/ml ± 1.51; P = 0.46; Fig. 1C). Western blotting detected bands corresponding to STAT1, STAT2, and Mx1 in IFN-α-treated BRAFs (Fig. 1D, lane 1). In mock-infected BRAFs, STAT1 and STAT 2 were detected (lane 2) but not Mx1; in VZV-infected BRAFs, only STAT1 was detected (lane 3).
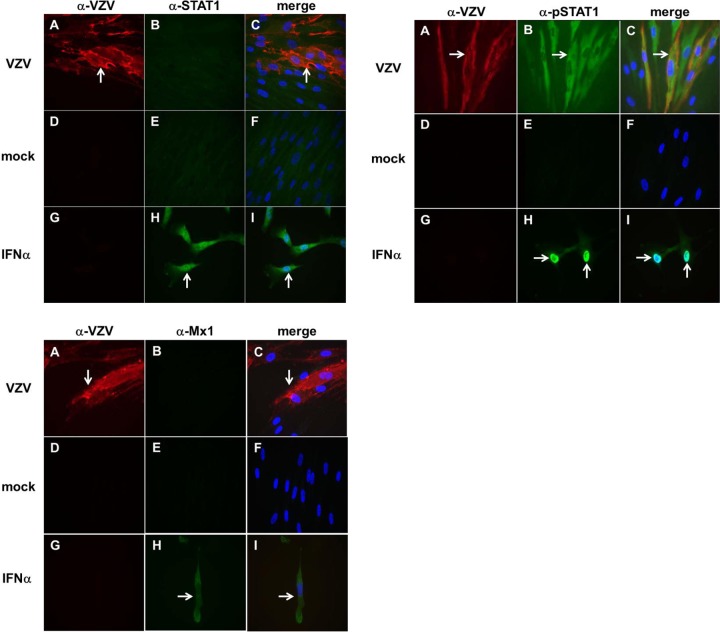
Immunohistochemical analysis revealed VZV antigen in infected BRAFs (Fig. 2, all panels, A and C) but not in mock-infected BRAFs (all panels, D and F) or IFN-α-treated BRAFs (all panels, G and I). STAT1 (top left panel, green) was absent in VZV-infected BRAFs (top left panel, B and C) and in mock-infected BRAFs (top left panel, E and F) but was seen in both the nucleus and cytoplasm of IFN-α-treated BRAFs (positive control) (top left panel, H and I). Dual staining with antibody to pSTAT1 and VZV revealed pSTAT1 exclusively in the cytoplasm of VZV-infected BRAFs (top right panel, B and C). pSTAT1 was absent in mock-infected BRAFs (top right panel, E and F), while in IFN-α-treated BRAFs, pSTAT1 was predominantly in the nucleus (top right panel, H and I). Dual staining with antibody to Mx1 and VZV revealed no Mx1 protein in VZV-infected BRAFs (bottom left panel, B and C) or in mock-infected BRAFs (bottom left panel, E and F), but Mx1 was present predominantly in the cytoplasm of IFN-α-treated BRAFs (H and I).
FIG 2.
Immunofluorescence analysis of STAT1, pSTAT1, and Mx1 in VZV-infected, mock-infected, and IFN-α-treated BRAFs. As shown in the top left panel, immunofluorescence confirmed the presence of VZV in infected cells (A and C, red, arrow) but not in mock-infected cells (D and F) or IFN-α-treated BRAFs (G and I) and the presence of STAT1 in IFN-α-treated BRAFs (positive control) (H and I, green, white arrow) but not in VZV-infected cells (B and C) or mock-infected cells (E and F). As shown in the top right panel, VZV was present in infected cells (A and C, red, white arrow) but not in mock-infected cells (D and F) or in IFN-α-treated BRAFs (G and I). pSTAT1 expression was abundant in the nucleus of IFN-α-treated BRAFs (H and I, white arrow), but pSTAT1 was present only in the cytoplasm of VZV-infected cells (B and C, green, white arrow) and not in mock-infected cells (E and F). As shown in the bottom left panel, VZV was present in infected cells (A and C, red, white arrow) but not in mock-infected cells (D and F) or IFN-α-treated cells (G and I). Mx1 protein was found predominantly in the cytoplasm of IFN-α-treated BRAFs (H and I, green, white arrow) but not in VZV-infected cells (B and C) or mock-infected cells (E and F). Blue coloring indicates cell nuclei. Magnification, ×600.
Overall, compared to mock-infected cells, VZV-infected cells have significantly decreased IFN-β transcript levels, consistent with studies in other cell types that show decreased IRF3-dependent IFN-β production due to VZV interference with IRF3 (5, 6). Unexpectedly, while IFN-α transcript levels were significantly increased, secreted IFN-α protein levels were unchanged, suggesting that VZV may interfere with translation or secretion of IFN-α, consistent with the absence of intracellular IFN-α in VZV-infected epidermal cells (7). Levels of STAT1 and STAT2 transcripts and protein were also decreased in VZV-infected cells. Although no remarkable increase in IFN-α or IFN-β levels was found, STAT1 was still phosphorylated in VZV-infected cells. However, pSTAT1 did not translocate to the nucleus, where it typically acts as a transcriptional activator of interferon-inducible genes such as Mx1. Indeed, levels of Mx1 transcripts were decreased and protein was absent by Western blotting and immunohistochemistry in VZV-infected cells. Failure of pSTAT1 to accumulate in the nucleus is consistent with analyses in which herpes simplex virus 1 (HSV-1) protein ICP27 (the VZV open reading frame [ORF] 4 homolog) prevented translocation of pSTAT1 to the nucleus (11). VZV interference with the type I interferon pathway decreases expression of interferon-responsive antiviral genes such as Mx1, possibly promoting virus persistence in cerebral arteries.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AG006127 and AG032958 (to D.G.) and NS 067070 (M.A.N.) from the National Institutes of Health. S.F.J. and N.L.B. are supported by training grant NS007321 to D.G. from the National Institutes of Health.
We thank Marina Hoffman for editorial review and Cathy Allen for manuscript preparation.
Footnotes
Published ahead of print 23 July 2014
REFERENCES
- 1.Nagel MA, Gilden D. 2014. Update on varicella zoster virus vasculopathy. Curr. Infect. Dis. Rep. 16:407. 10.1007/s11908-014-0407-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539–548. 10.1016/S1074-7613(00)00053-4 [DOI] [PubMed] [Google Scholar]
- 3.Haller O, Kochs G. 2011. Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. J. Interferon Cytokine Res. 31:79–87. 10.1089/jir.2010.0076 [DOI] [PubMed] [Google Scholar]
- 4.Verhelst J, Hulpiau P, Saelens X. 2013. Mx proteins: antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 77:551–566. 10.1128/MMBR.00024-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen N, Sommer M, Che Z, White K, Ruyechan WT, Arvin AA. 2010. Varicella-zoster virus immediate-early protein 62 blocks interferon regulatory factor 3 (IRF3) phosphorylation at key serine residues: a novel mechanism of IRF3 inhibition among herpesviruses. J. Virol. 84:9240–9253. 10.1128/JVI.01147-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF-3 mediated innate immune response through degradation of activated IRF3. J. Virol. 85:11079–11089. 10.1128/JVI.05098-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku C-C, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. 2004. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-α. J. Exp. Med. 200:917–925. 10.1084/jem.20040634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagel MA, Choe A, Traktinskiy I, Cordery-Cotter R, Gilden D, Cohrs RJ. 2011. Varicella zoster virus transcriptome in latently infected human ganglia. J. Virol. 85:2276–2287. 10.1128/JVI.01862-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird NL, Bowlin JL, Yu X, Jonjić S, Haas J, Cohrs RJ, Gilden D. 2014. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology 458–459:1–3. 10.1016/j.virol.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Seitz S, Pointon T, Bowlin JL, Cohrs RJ, Jonjić S, Haas J, Wellish M, Gilden D. 2013. Varicella zoster virus infection of highly pure terminally differentiated human neurons. J. Neurovirol. 19:75–81. 10.1007/s13365-012-0142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KE, Knipe DM. 2010. Herpes simplex virus-1 infection causes the secretion of a type 1 interferon-antagonizing protein and inhibits signaling at or before Jak-1 activation. Virology 396:21–29. 10.1016/j.virol.2009.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]