ABSTRACT
Recently, we identified a novel receptor, CD134, which interacts with the human herpesvirus 6B (HHV-6B) glycoprotein (g)H/gL/gQ1/gQ2 complex and plays a key role in the entry of HHV-6B into target cells. However, details of the interaction between the HHV-6B gH/gL/gQ1/gQ2 complex and CD134 were unknown. In this study, we identified a cysteine-rich domain (CRD), CDR2, of CD134 that is critical for binding to the HHV-6B glycoprotein complex and HHV-6B infection. Furthermore, we found that the expression of HHV-6B gQ1 and gQ2 subunits was sufficient for CD134 binding, which is different from the binding of human herpesvirus 6A (HHV-6A) to its receptor, CD46. Finally, we identified a region in gQ1 critical for HHV-6B gQ1 function. These results contribute much to our understanding of the interaction between this ligand and receptor.
IMPORTANCE We identified the domain in HHV-6B entry receptor CD134 and the components in the HHV-6B gH/gL/gQ1/gQ2 complex required for ligand-receptor binding during HHV-6B infection. Furthermore, we identified domains in gQ1 proteins of HHV-6A and -6B and a key amino acid residue in HHV-6B gQ1 required for its function. These data should be the basis for further investigation of ligand-receptor interaction in the study of HHV-6A and -6B.
INTRODUCTION
Shortly after its discovery, human herpesvirus 6 (HHV-6) was recognized as a single virus species comprised of two variants, HHV-6A and HHV-6B (1–4). Recently, HHV-6 variants have been reclassified as two virus species based on the different biocharacteristics of these two viruses with respect to the fields of biology, immunology, and epidemiology, and so on (5).
One striking difference between HHV-6A and HHV-6B is their cell tropism. HHV-6A infects a wider range of cells than HHV-6B (5–7). Although determinants of viral cell tropism lie in each step of the virus life cycle in target cells, the differences in receptor preference of HHV-6A and -6B may contribute much to their cell tropism. Human CD46 was identified as the cell receptor for HHV-6 (8) and binds the HHV-6A gH/gL/gQ1/gQ2 complex (9, 10). Interestingly, we found that a similar glycoprotein complex exists in HHV-6B but does not bind to CD46 (11, 12), even though it shares a relatively high sequence identity with the glycoprotein in HHV-6A (especially the gH and gL components). Recently, we found that human CD134 is a specific cellular receptor for HHV-6B and binds to the HHV-6B gH/gL/gQ1/gQ2 complex (13). While CD46 is expressed on all nucleated cells (14, 15), CD134 is expressed mainly on activated T cells (16). The different expression profiles of these two cellular receptors may be one of the key determinants of the differences in cell tropism of HHV-6A and -6B. However, it is still unknown how these two similar viral ligands bind to different cellular receptors.
Analysis of the receptor-ligand binding process would contribute not only to our understanding of the viral life cycle itself but also to the development of treatment methods to block the virus life cycle at the first step. Much is known about the receptor-ligand binding of HHV-6A. Short consensus repeats 2 and 3 (SCR2 and -3) of CD46 are required for its binding to the HHV-6A gH/gL/gQ1/gQ2 complex (17, 18), and complex formation is required for CD46 binding to its viral ligand (19, 20). However, no such analysis of the binding of HHV-6B ligand to CD134 had been performed.
Thus, in the present study, we focused first on the domain(s) of CD134 required for HHV-6B ligand binding.
Comparison of the two gH/gL/gQ1/gQ2 complexes of HHV-6A and -6B revealed higher sequence identities between either the gH or gL subunits of HHV-6A and -6B (21–23). We reported previously that the chimeric complex composed of gQ1 and gQ2 of HHV-6A and gH and gL of HHV-6B could bind to CD46 (24). Furthermore, the chimeric complex composed of gQ1 and gQ2 of HHV-6B and gH and gL of HHV-6A could bind to CD134 (13). Thus, it could be inferred that the gH and gL subunits of HHV-6A and -6B do not contribute to the binding of the tetrameric complexes to different receptors. On the other hand, the gQ1 and gQ2 subunits of HHV-6A and -6B share relatively low sequence identity (21–23), and we have confirmed that a chimeric complex composed of gQ2, gH, and gL of HHV-6B and gQ1 of HHV-6A could bind CD46, although at a lower affinity (24). Therefore, it is highly likely that the gQ1 subunit is responsible for the differential receptor binding of the gH/gL/gQ1/gQ2 complexes of HHV-6A and -6B. Because the gQ1 subunits share approximately 70% sequence identity (21–23), it was possible to test which residues (or regions containing these residues) determine receptor binding.
In the present study, we analyzed the CD134 domain and identified the components required for receptor-ligand binding of HHV-6B. We found that CRD2 of CD134 is required for binding to the HHV-6B gH/gL/gQ1/gQ2 complex. Moreover, the HHV-6B gQ1 and gQ2 subunits (in the absence of gH and gL) were sufficient for CD134 binding. In addition, we identified a region in HHV-6B gQ1 required for its function.
MATERIALS AND METHODS
Cells and virus strains.
The T-cell lines JJhan and MT4 were cultured in RPMI 1640 medium with 8% fetal bovine serum. 293T cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 8% fetal bovine serum. The HHV-6B strain HST was prepared as described previously (25). Umbilical cord blood mononuclear cells (CBMCs) used for virus propagation were prepared as described previously (26). CBMCs were kindly provided by H. Yamada (Kobe University Graduate School of Medicine) and purchased from RIKEN (the Institute of Physical and Chemical Research, Japan). Regarding CBMC usage, this study was approved by the ethical committee of the Kobe University Graduate School of Medicine.
Plasmid construction.
Plasmids for the expression of gQ1, gQ2, gH, and gL of HHV-6A and -6B and the plasmids for the expression of human CD134 and the soluble forms of CD46 and CD134 were described previously (10, 11, 13, 19, 20, 24). Murine CD134 was amplified from a murine cDNA library using the primers 5′-ACACTCGAGAATTCGCCACCATGTATGTGTGGGTTCAG-3′ and 5′-ACACTCGAGTCAGATCTTGGCCAGAGTAAAG-3′ and cloned into a pCAGGS-MCS plasmid (27). All CD134 mutants and gQ1 mutants (deletion, chimeric, and soluble forms) were constructed by two-step PCR, as described elsewhere (28). To construct the plasmids for stable expression in JJhan cells, CD134 or chimeric CD134s were subcloned into a pCAGGS-MCS-puro plasmid (29).
Generation of stable expression cell lines and infection with HHV-6B.
JJhan cells were transfected with the plasmids (pCAGGS-MCS-puro) for the expression of CD134 or chimeric CD134. The cells were selected in medium containing 1 μg/ml puromycin. The surviving cells were cloned by endpoint dilution. The cells from a single clone were infected with the HHV-6B virus, and at 24 h postinfection, the cells were harvested and prepared for Western blot analysis with anti-IE1 and anti-α-tubulin antibodies.
Antibodies.
Antibodies to gO, gQ1, gQ2, gH, and gL of HHV-6A and -6B and the antibody to HHV-6B IE1 were described previously (11, 18, 20, 30). Monoclonal antibodies (MAbs) to CD134 were produced as described previously (20). Anti-CD46 MAb and anti-α-tubulin and Alexa Fluor 488 goat anti-human IgG (heavy plus light chain [H+L]) antibodies were purchased from Immunotech, Sigma-Aldrich, and Invitrogen, respectively.
Purification of the Fc fusion protein.
293T cells were transfected with plasmids for the expression of Fc fusion proteins with a His tag at their carboxyl termini using Lipofectamine 2000 (Invitrogen) according the manufacturer's instructions. The secreted Fc fusion proteins in the medium were purified using nickel-nitrilotriacetic acid (Ni-NTA) (Qiagen) affinity chromatography.
Flow cytometry.
Cell surface expression of selected proteins and the binding of Fc or Fc fusion proteins to the cell surface were analyzed by flow cytometry, as described previously (13).
Infection inhibition assay.
Cell-free HHV-6B virus was incubated with soluble Fc, human CD134Fc, murine CD134Fc, or chimeric CD134Fc at 37°C for 30 min, and then the viruses were used to infect MT4 cells or CBMCs (5 × 105 for each sample) at 37°C for 1 h at a multiplicity of infection of 0.01. The cells were cultured in 1 ml of medium for 24 h and then lysed with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.4], 150 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) for immunoblot analysis with anti-IE1 and anti-α-tubulin antibodies.
Pulldown assay.
Ni-NTA (Qiagen) was incubated with the soluble forms of CD134 or CD46 secreted into the culture medium of 293T cells. After a washing with phosphate-buffered saline (PBS), Ni-NTA was incubated with the cell lysates of 293T cells transfected with the plasmid(s) for the expression of the proteins of interest. These cells were lysed with TNE buffer (10 mM Tris-HCl [pH 7.8], 0.15 M NaCl, 1 mM EDTA, 1% NP-40; Nacalai Tesque). After a washing with TNE buffer, the proteins bound to Ni-NTA were eluted with 250 mM imidazole and prepared for Western blot analysis.
Immunoprecipitation assay and immunoblotting.
The methods for the immunoprecipitation assay and immunoblotting were described previously (20).
RESULTS
CRD2 of CD134 is essential for binding to the HHV-6B ligand.
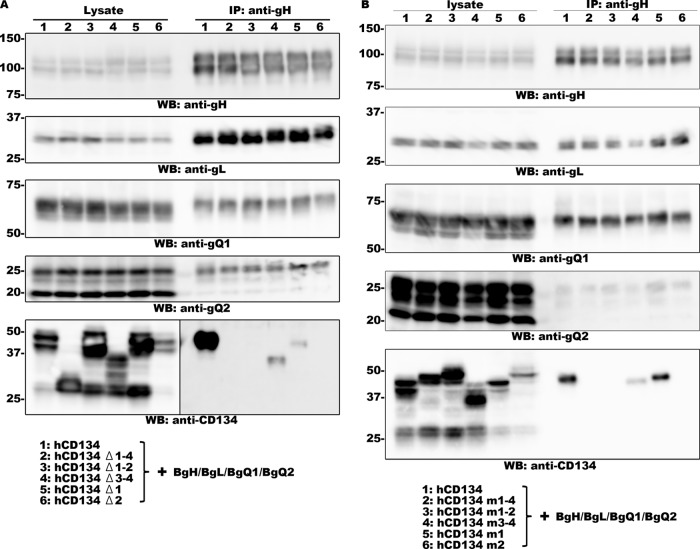
CD134 belongs to the tumor necrosis factor (TNF) receptor superfamily, and its ectodomain contains four CRDs and a stalk domain (31). To identify which domain(s) in CD134 is required for HHV-6B glycoprotein complex binding, we constructed several CD134 deletion mutants and transfected 293T cells with plasmids expressing these mutants together with the gH, gL, gQ1, and gQ2 subunits of HHV-6B. Interaction of the HHV-6B glycoprotein complex with each CD134 mutant was then analyzed by immunoprecipitation assay. Deletion of CRD1 to -4, CRD1 and -2, or CRD2 completely abolished the interaction, and deletion of CRD3 and -4 or CRD1 partially affected the interaction (Fig. 1A). To confirm this result, we repeated these experiments using chimeric CD134s, in which one segment of human CD134 had been replaced with the corresponding region of murine CD134 (hCD134 mX). In agreement with the previous results, replacement of CRD1 to -4, CRD1 and -2, or CRD2, but not CRD1, completely abolished the interaction (Fig. 1B). In all experiments, we used anti-gO antibody as a negative control for immunoprecipitation assays, and none of these proteins could be precipitated by this antibody (data not shown). These results indicate that CRD2 of human CD134 is critical for its interaction with the HHV-6B gH/gL/gQ1/gQ2 complex.
FIG 1.
CRD2 of CD134 is required for binding to HHV-6B gH/gL/gQ1/gQ2. 293T cells were transfected with HHV-6B gH, gL, gQ1, and gQ2, (BgH, BgL, BgQ1, and BgQ2, respectively) and the CD134 deletion mutant (A) or chimeric mutant (B). Cells were harvested 2 days after transfection and prepared for immunoprecipitation (using anti-gH antibody) and Western blot (WB) analysis. The samples applied to each lane are listed at the bottom.
CRD2 of human CD134 is required for HHV-6B infection.
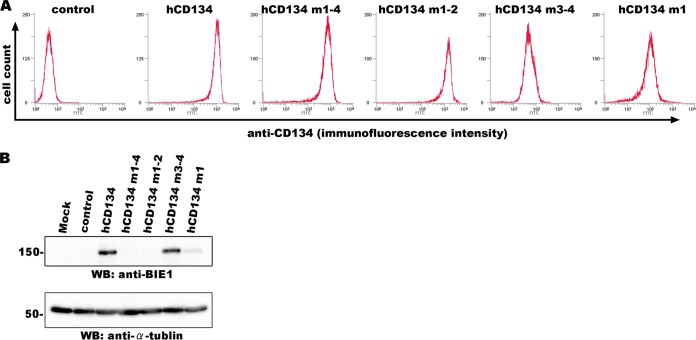
To further confirm whether CRD2 is required for HHV-6B infection, we constructed JJhan cell lines in which wild-type human CD134 or chimeric mutants of human and murine CD134 were expressed. In our previous study, we showed that CD134 is expressed at low levels in the JJhan cells, which are rarely permissive for HHV-6B infection (13). Expression of each protein on the cell surface was confirmed by flow cytometry (Fig. 2A). These cells were then infected with HHV-6B, and the infection efficiency was analyzed at 24 h postinfection by Western blotting using an anti-IE1 antibody. As shown in Fig. 2B, cells expressing human CD134 and CD134 mutants with CRD1 and -2 of human CD134 (hCD134 m3-4) showed similar levels of sensitivity to HHV-6B infection. Cells expressing the CD134 mutants containing CRD1 to -4 or CRD1 and -2 of murine CD134 (hCD134 m1-4 or hCD134 m1-2) showed little sensitivity to HHV-6B infection, and cells expressing the CD134 mutant with murine CRD1 and human CRD2 (hCD134 m1) exhibited reduced sensitivity to HHV-6B. Although we also tried to generate JJhan cells expressing a CD134 mutant with human CRD1 and murine CRD2 (hCD134 m2), the expression level of this mutant on the cell surface was extremely low, so this mutant was not included in the analyses (data not shown). These results indicate that CRD2 of human CD134 is essential for HHV-6B infection.
FIG 2.
CRD2 of CD134 is required for HHV-6B infection. (A) Expression of human CD134 or its chimeric mutants in stable expression cell lines (JJhan cells) was confirmed by flow cytometry using an antibody for CD134. (B) JJhan cells, human-CD134-expressing JJhan cells, and chimeric-CD134-expressing JJhan cells were infected with HHV-6B. The cells were harvested at 24 h postinfection and prepared for Western blot (WB) analysis.
Soluble forms of CD134 containing CRD2 block HHV-6B infection.
We also constructed several soluble CD134 mutants to test whether mutants containing the CRD2 of human CD134 could block HHV-6B infection. The ectodomains of the CD134 mutants described above (chimeric CD134) were fused with the Fc fragment of human IgG1 and tagged with a His tag at their carboxyl termini. The secreted soluble forms of the CD134 mutants were purified and confirmed by Western blot analysis (Fig. 3, top). These soluble proteins were incubated with the HHV-6B virus, and the viruses were then used to infect to MT4 cells. The ability of each soluble form to inhibit HHV-6B infection was analyzed at 24 h postinfection by Western blot analysis using anti-IE1 antibody. As shown in Fig. 3 (middle and bottom), soluble proteins containing CRD1 and -2 of human CD134 (hCD134 m3-4FcHis) or CRD1 of murine CD134 and CRD2 of human CD134 (hCD134 m1FcHis) completely blocked HHV-6B infection. Conversely, the mutant containing murine CRD2 (hCD134 m2FcHis) had little effect on HHV-6B infection. Finally, the murine CD134 mutant containing CRD1 and -2 of human CD134 (mCD134 h1-2FcHis) also efficiently blocked HHV-6B infection. We obtained similar results when CBMCs were used (data not shown). Together, these results strongly suggest that CRD2 of human CD134 is crucial for HHV-6B infection. These results are summarized in Table 1.
FIG 3.
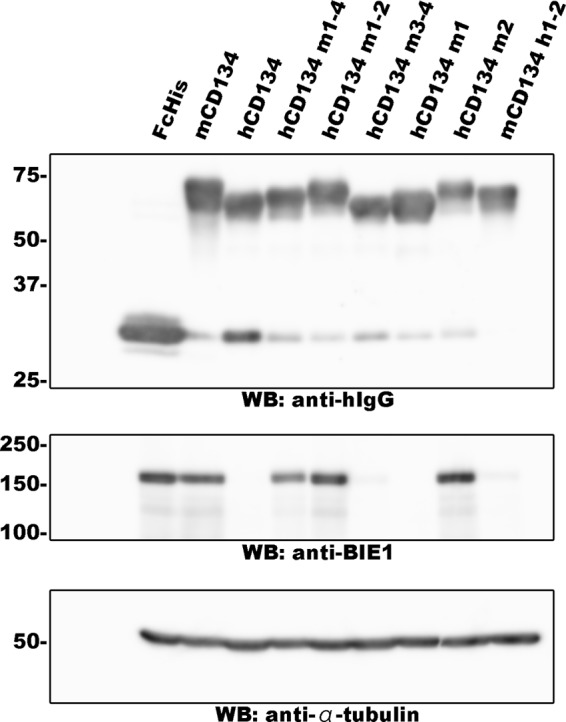
Soluble CD134 blocks HHV-6B infection. Soluble CD134 proteins were purified from the culture medium of 293T cells transfected with plasmids for the expression of these proteins, and expression was confirmed by Western blot (WB) analysis (top). These proteins were incubated with HHV-6B, which was then used to infect MT4 cells. The cells were harvested at 24 h postinfection and prepared for Western blot analysis (middle and bottom).
TABLE 1.
Summary of characteristics of CD134 mutantsa
| Protein | Binding to BgH/BgL/BgQ1/BgQ2 | HHV-6B sensitivity for JJhan cells in which applicable form is expressed | Blocking of HHV-6B by soluble form |
|---|---|---|---|
| hCD134 | + | + | + |
| hCD134 m1-4 | − | − | − |
| hCD134 m1-2 | − | − | − |
| hCD134 m3-4 | + | + | + |
| hCD134 m1 | + | + | + |
| hCD134 m2 | − | NT | − |
| mCD134 h1-2 | NT | NT | + |
hCD134, human CD134; mCD134, murine CD134; m1-4, cysteine-rich domains 1 to 4 of mCD134; h1-2, cystein-rich domains 1 and 2 of hCD134; NT, not tested.
HHV-6B gQ1 and gQ2 are required and sufficient for CD134 binding.
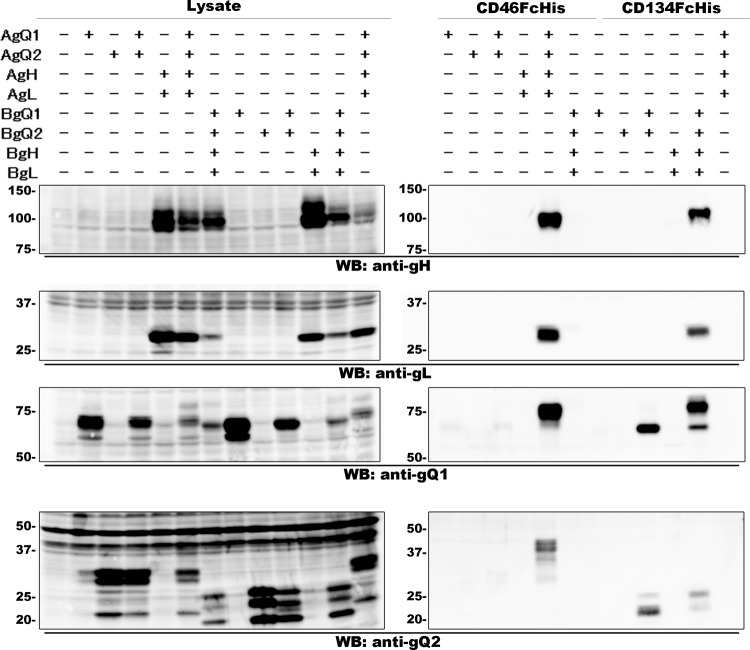
We reported previously that all of the components of the HHV-6A gH/gL/gQ1/gQ2 complex are required for CD46 binding (20). To determine whether this is also the case for the HHV-6B complex, we performed a pulldown assay. As shown previously (20), CD46 bound to the HHV-6A gH/gL/gQ1/gQ2 complex only when all of the glycoproteins were expressed together. In addition, CD46 did not bind to the complex of HHV-6B, even when all of the gH/gL/gQ1/gQ2 complex glycoproteins were expressed together. Interestingly, CD134 could bind the HHV-6B glycoprotein complex even when only two of the glycoproteins, gQ1 and gQ2, were coexpressed, although CD134 could not bind to gQ1 or gQ2 alone (Fig. 4). These results indicate that HHV-6B gQ1 and gQ2 are required and sufficient for CD134 binding.
FIG 4.
gQ1 and gQ2 of HHV-6B bind to CD134. The expression of each protein (indicated at the top of each lane) in the lysates of 293T cells transfected with an expression plasmid(s) was confirmed by Western blot (WB) analysis (left). Soluble forms of CD46 and CD134 (CD46FcHis and CD134FcHis, respectively) bound to Ni-NTA and were incubated with the cell lysates. The proteins bound to Ni-NTA were eluted and prepared for Western blot analysis (right).
The secreted HHV-6B gQ1/gQ2 complex binds to CD134.
To confirm the above results, we constructed an HHV-6B gQ1 subunit with an Fc fragment and His tag at its C terminus. We then expressed the gQ1 construct with (or without) HHV-6B gQ2 in 293T cells. Secreted gQ1 was purified and confirmed by Western blot analysis (Fig. 5A), and binding of these proteins to CD134-expressing JJhan cells was confirmed by flow cytometry. As shown in Fig. 5B, secreted HHV-6B gQ1 alone could not bind to CD134-expressing cells. On the other hand, the secreted HHV-6B gQ1/gQ2 complex could bind to CD134-expressing cells. These data further confirm that HHV-6B gQ1 and gQ2 are required and sufficient for binding to CD134.
FIG 5.
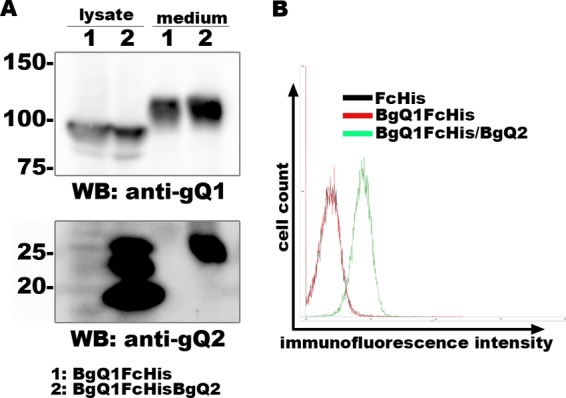
The secreted HHV-6B gQ1/gQ2 complex binds to CD134. 293T cells were transfected with a plasmid(s) for the expression of BgQ1 (tagged with Fc and His) alone or together with BgQ2. Secreted BgQ1 was purified using Ni-NTA. The expression and secretion (lysate and medium, respectively) of BgQ1 and BgQ2 were confirmed by Western blot (WB) analysis (A). The purified proteins were incubated with CD134-expressing JJhan cells. Binding of the purified proteins to the cells was analyzed by flow cytometry using Alexa Fluor 488 goat anti-human IgG antibody (B).
Identification of amino acid residues in HHV-6B gQ1 required for its function.
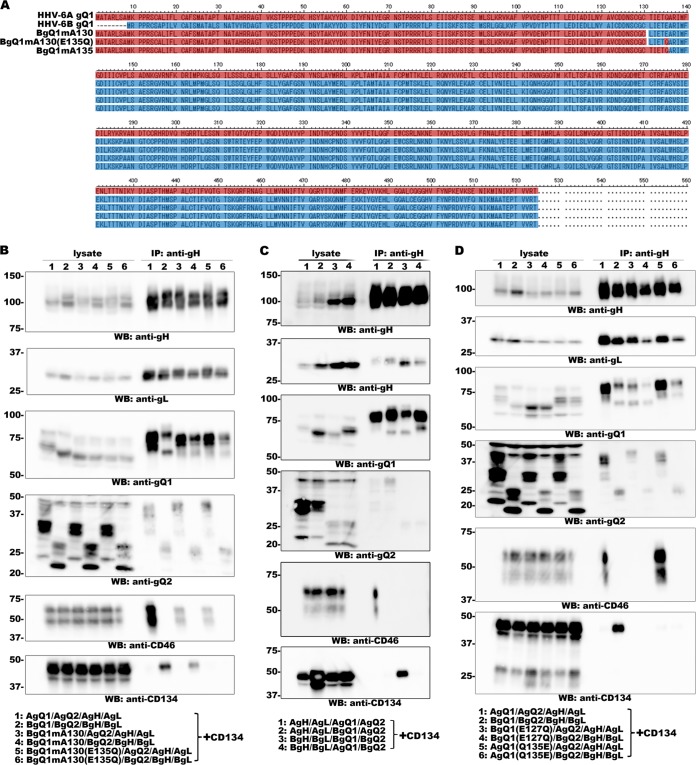
As described above, it is highly likely that gQ1 contributes to receptor selection of HHV-6A and -6B, even though the gQ1 subunits share 70% sequence identity. We partially replaced a segment of HHV-6B gQ1 with the homologous segment of HHV-6A gQ1 and tested whether the chimeric gQ1 was recognized by AgQ1 1-1 (19) and KH-1 (11), neutralizing antibodies for HHV-6 gQ1 that recognize HHV-6A gQ1 and HHV-6B gQ1, respectively. We also examined whether the complexes formed with the chimeric gQ1s could bind to CD134 or CD46. When the constructed chimeric gQ1s were used, several interesting results were obtained. When we replaced amino acid residues 1 to 122 of HHV-6B gQ1 with the homologous region of HHV-6A gQ1 (amino acid residues 1 to 130), the resultant gQ1 protein (BgQ1mA130) could be recognized by the MAb KH-1and form a complex to bind CD134 (Fig. 6A and B). However, when two more amino acid residues were replaced (L131T and E135Q), the resultant protein, BgQ1mA135, could be recognized by neither KH-1 nor AgQ1 1-1 and could not form a complex to bind to CD134 (data not shown). Furthermore, when only E135 of BgQ1mA130 was replaced with Q, the resultant protein, BgQ1mA130(E135Q), could not be recognized by KH-1 and could not form a complex to bind CD134 (Fig. 6A and B). More interestingly, both BgQ1mA130 and BgQ1mA130(E135Q) could form a complex with gH, gL, and gQ2 of HHV-6A, which binds to CD46 (Fig. 6B). As mentioned previously, the HHV-6B gH/gL/gQ1/gQ2 complex did not bind to CD46 (12), even when HHV-6B gQ1 formed a complex with HHV-6A gH, gL, and gQ2 (19) (Fig. 6C). These data indicate that the region containing amino acids 1 to 127 (the homologous region of amino acids 1 to 135 of HHV-6A gQ1) of HHV-6B gQ1 is crucial for its function and E127 of HHV-6B gQ1 may play a key role for ligand-receptor interaction. To confirm this, we replaced Q135 of HHV-6A gQ1 with E (AgQ1Q135E) and replaced E127 of HHV-6B gQ1 with Q (BgQ1E127Q), and then we tested their binding to CD46 and CD134. We found that the replacement had no effect on HHV-6A gQ1 function (formation of a complex to bind CD46 in the case of coexpression with HHV-6A gQ2, gH, and gL but not those of HHV-6B), but the resultant HHV-6B gQ1 could not form a complex to bind to CD134 and CD46 (in the case of coexpression both with HHV-6B gQ2, gH, and gL and with those of HHV-6A) (Fig. 6D). These data suggest that E127 is crucial for the function of HHV-6B gQ1, but the homologous position (Q135) of HHV-6A is not required for its function, at least for CD46 binding.
FIG 6.
Analysis of the interaction between the complexes containing gQ1 mutants and CD134. (A) Amino acid sequence alignment of gQ1 proteins. The HHV-6A gQ1 sequence is in red, and the HHV-6B gQ1 sequence is in blue. (B, C, and D) 293T cells transfected with plasmids for expression of the proteins (lane numbers are defined at the bottom of the figure) were lysed for immunoprecipitation assay using anti-gH antibody. Expression of each protein (lysate) and the proteins precipitated from the lysates (anti-gH immunoprecipitation [IP]) were confirmed by Western blot (WB) analysis.
DISCUSSION
Investigation of the interaction between viral ligands and their cellular receptors is important for elucidating virus entry events and developing therapies for the treatment of viral infections. Recently, we identified the HHV-6B entry receptor, CD134 (13); however, the detailed binding process of this ligand and receptor pair was unknown. In the present study, we determined that CRD2 of human CD134 is critical for its binding to the HHV-6B gH/gL/gQ1/gQ2 complex and found that HHV-6B gQ1/gQ2 complex formation is sufficient for binding to CD134. Finally, we also identified a region in HHV-6B gQ1 that is required for its function.
The interaction between CD134 and its natural ligand, OX40L, has been elucidated by X-ray crystallography. It is highly likely that an interaction interface formed by CRD1 to -3 of CD134, rather than the binding of specific amino acids, is required for its binding to OX40L (31). In contrast to binding of OX40L, we found that CRD2 of human CD134 is essential for its binding to the HHV-6B gH/gL/gQ1/gQ2 complex. Deletion or replacement of CRD2 with the homologous region from murine CD134 completely abolished its binding to the HHV-6B complex (Fig. 1), which corresponded to the results showing that the expression of a chimeric CD134 containing murine CRD1 and -2 completely prevented HHV-6B infection (Fig. 2).
Binding of HHV-6A gH/gL/gQ1/gQ2 to CD46 requires the coordinate expression of gH, gL, gQ1, and gQ2 (20). It remains unknown whether this is because of a requirement for the interaction interface formed by the four components or the conformational structure of the component(s) in the complex that is formed only during coexpression. Different from the case of HHV-6A, binding of the HHV-6B gH/gL/gQ1/gQ2 complex to CD134 requires only the coexpression of gQ1 and gQ2. This binding pattern is comparable to binding of the Epstein-Barr virus (EBV) gH/gL/gp42 complex to its receptor, HLA class II, during which gp42 can bind to HLA class II alone (32). Similar to HHV-6A binding, it remains unknown whether the interaction interface formed by gQ1 and gQ2 of HHV-6B or the conformational structure of gQ1 that is formed only during coexpression is required for CD134 binding. Interaction with gQ2 alone would be sufficient for exposure of the HHV-6B gQ1 binding domain to CD134; however, complex formation with gH, gL, and gQ2 would be required for exposure of the HHV-6A gQ1 domain for CD46 binding. The dynamic folding of the gQ1 protein may play an important role in determining HHV-6 entry.
The key amino acid residues required for the binding of viral ligands to their cellular receptors or maintaining their conformational structures in other viruses have been reported (33–37). Previously, we reported that amino acid residues 494 to 497 of HHV-6A gQ1 and 484 to 496 of HHV-6B gQ1 are important for the function of gQ1 proteins (11, 19). Here, we found that an E127Q mutation in HHV-6B gQ1 could completely abolish its function. Analysis of the conformational structures of the gH/gL/gQ1/gQ2 complexes of HHV-6A and -6B would contribute to our understanding of why these amino acid residues are important for the function of gQ1 in these complexes.
Previously, we reported that two MAbs, AgQ1 1-1 and KH-1, specifically recognize the gQ1 subunits of HHV-6A and HHV-6B, respectively. AgQ1 1-1 efficiently neutralizes HHV-6A (19) but not HHV-6B infection, and KH-1 efficiently neutralizes HHV-6B but not HHV-6A infection (11). It was unknown whether the epitopes of these MAbs overlap the receptor-binding domains within the gQ1 proteins. In the present study, BgQ1mA130(E135Q) and BgQ1mA130 could not be recognized by AgQ1 1-1 (data not shown) but did form a complex with HHV-6A gH, gL, and gQ2 that could bind to CD46, but their binding looked very weak (Fig. 6B). This may suggest that neutralization by AgQ1 1-1 does not occur via a direct effect on the interaction interface of the HHV-6A complex and CD46.
Replacement of amino acid residues 1 to 122 of HHV-6B with the homologous region of HHV-6A gQ1 renders it able to interact with CD46, although only in the case of coexpression with HHV-6A gH, gL, and gQ2 (Fig. 6A and B). This finding suggests that the domain of HHV-6A gQ1 responsible for binding to CD46 may lie in the replaced domain. However, we cannot rule out the possibility that replacement and coexpression events expose the CD46-binding domain in HHV-6B gQ1 (presuming that HHV-6B gQ1 has a CD46-binding domain).
In summary, we have identified the key domain in CD134 and components in the HHV-6B gH/gL/gQ1/gQ2 complex required for their interaction. We also identified the region in HHV-6B gQ1 required for its function. The present study provides the basis for further analysis of their interaction, such as conformational structure analysis of HHV-6A and -6B ligands in complex with their cellular receptors.
ACKNOWLEDGMENTS
We thank Hideto Yamada (Department of Obstetrics and Gynecology, Kobe University Graduate School of Medicine) for providing the CBMCs.
This study was supported in part by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS).
Footnotes
Published ahead of print 9 July 2014
REFERENCES
- 1.Aubin JT, Collandre H, Candotti D, Ingrand D, Rouzioux C, Burgard M, Richard S, Huraux JM, Agut H. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J. Clin. Microbiol. 29:367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campadelli-Fiume G, Guerrini S, Liu X, Foa-Tomasi L. 1993. Monoclonal antibodies to glycoprotein B differentiate human herpesvirus 6 into two clusters, variants A and B. J. Gen. Virol. 74:2257–2262. 10.1099/0022-1317-74-10-2257 [DOI] [PubMed] [Google Scholar]
- 3.Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, et al. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596–601. 10.1126/science.2876520 [DOI] [PubMed] [Google Scholar]
- 4.Wyatt LS, Balachandran N, Frenkel N. 1990. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J. Infect. Dis. 162:852–857. 10.1093/infdis/162.4.852 [DOI] [PubMed] [Google Scholar]
- 5.Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, Diluca D, Flamand L, Frenkel N, Gallo R, Gompels UA, Hollsberg P, Jacobson S, Luppi M, Lusso P, Malnati M, Medveczky P, Mori Y, Pellett PE, Pritchett JC, Yamanishi K, Yoshikawa T. 2014. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 159:863–870. 10.1007/s00705-013-1902-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ablashi DV, Balachandran N, Josephs SF, Hung CL, Krueger GR, Kramarsky B, Salahuddin SZ, Gallo RC. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545–552. 10.1016/0042-6822(91)90424-A [DOI] [PubMed] [Google Scholar]
- 7.Mori Y. 2009. Recent topics related to human herpesvirus 6 cell tropism. Cell Microbiol. 11:1001–1006. 10.1111/j.1462-5822.2009.01312.x [DOI] [PubMed] [Google Scholar]
- 8.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817–827. 10.1016/S0092-8674(00)81678-5 [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Yang X, Akkapaiboon P, Okuno T, Yamanishi K. 2003. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q. complex associates with human CD46. J. Virol. 77:4992–4999. 10.1128/JVI.77.8.4992-4999.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akkapaiboon P, Mori Y, Sadaoka T, Yonemoto S, Yamanishi K. 2004. Intracellular processing of human herpesvirus 6 glycoproteins Q1 and Q2 into tetrameric complexes expressed on the viral envelope. J. Virol. 78:7969–7983. 10.1128/JVI.78.15.7969-7983.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawabata A, Oyaizu H, Maeki T, Tang H, Yamanishi K, Mori Y. 2011. Analysis of a neutralizing antibody for human herpesvirus 6B reveals a role for glycoprotein Q1 in viral entry. J. Virol. 85:12962–12971. 10.1128/JVI.05622-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyaizu H, Tang H, Ota M, Takenaka N, Ozono K, Yamanishi K, Mori Y. 2012. Complementation of the function of glycoprotein H of human herpesvirus 6 variant A by glycoprotein H of variant B in the virus life cycle. J. Virol. 86:8492–8498. 10.1128/JVI.00504-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang H, Serada S, Kawabata A, Ota M, Hayashi E, Naka T, Yamanishi K, Mori Y. 2013. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc. Natl. Acad. Sci. U. S. A. 110:9096–9099. 10.1073/pnas.1305187110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lublin DM, Liszewski MK, Post TW, Arce MA, Le Beau MM, Rebentisch MB, Lemons LS, Seya T, Atkinson JP. 1988. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J. Exp. Med. 168:181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seya T, Turner JR, Atkinson JP. 1986. Purification and characterization of a membrane protein (gp45–70) that is a cofactor for cleavage of C3b and C4b. J. Exp. Med. 163:837–855. 10.1084/jem.163.4.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watts TH. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23:23–68. 10.1146/annurev.immunol.23.021704.115839 [DOI] [PubMed] [Google Scholar]
- 17.Greenstone HL, Santoro F, Lusso P, Berger EA. 2002. Human herpesvirus 6 and measles virus employ distinct CD46 domains for receptor function. J. Biol. Chem. 277:39112–39118. 10.1074/jbc.M206488200 [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Seya T, Huang HL, Akkapaiboon P, Dhepakson P, Yamanishi K. 2002. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J. Virol. 76:6750–6761. 10.1128/JVI.76.13.6750-6761.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeki T, Hayashi M, Kawabata A, Tang H, Yamanishi K, Mori Y. 2013. Identification of the human herpesvirus 6A gQ1 domain essential for its functional conformation. J. Virol. 87:7054–7063. 10.1128/JVI.00611-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang H, Hayashi M, Maeki T, Yamanishi K, Mori Y. 2011. HHV-6 glycoprotein complex formation is required for folding and trafficking of the gH/gL/gQ1/gQ2 complex and its cellular receptor binding. J. Virol. 85:11121–11130. 10.1128/JVI.05251-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez G, Dambaugh TR, Stamey FR, Dewhurst S, Inoue N, Pellett PE. 1999. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J. Virol. 73:8040–8052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gompels UA, Nicholas J, Lawrence G, Jones M, Thomson BJ, Martin ME, Efstathiou S, Craxton M, Macaulay HA. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29–51. 10.1006/viro.1995.1228 [DOI] [PubMed] [Google Scholar]
- 23.Isegawa Y, Mukai T, Nakano K, Kagawa M, Chen J, Mori Y, Sunagawa T, Kawanishi K, Sashihara J, Hata A, Zou P, Kosuge H, Yamanishi K. 1999. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J. Virol. 73:8053–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasirwan C, Furusawa Y, Tang H, Maeki T, Mori Y. 2014. Human herpesvirus-6A gQ1 and gQ2 are critical for human CD46 usage. Microbiol. Immunol. 58:22–30. 10.1111/1348-0421.12110 [DOI] [PubMed] [Google Scholar]
- 25.Mori Y, Akkapaiboon P, Yang X, Yamanishi K. 2003. The human herpesvirus 6 U100 gene product is the third component of the gH-gL glycoprotein complex on the viral envelope. J. Virol. 77:2452–2458. 10.1128/JVI.77.4.2452-2458.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhepakson P, Mori Y, Jiang YB, Huang HL, Akkapaiboon P, Okuno T, Yamanishi K. 2002. Human herpesvirus-6 rep/U94 gene product has single-stranded DNA-binding activity. J. Gen. Virol. 83:847–854 [DOI] [PubMed] [Google Scholar]
- 27.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. 10.1016/0378-1119(91)90434-D [DOI] [PubMed] [Google Scholar]
- 28.Landt O, Grunert HP, Hahn U. 1990. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene 96:125–128. 10.1016/0378-1119(90)90351-Q [DOI] [PubMed] [Google Scholar]
- 29.Sadaoka T, Serada S, Kato J, Hayashi M, Gomi Y, Naka T, Yamanishi K, Mori Y. 2014. Varicella-zoster virus ORF49 functions in the efficient production of progeny virus through its interaction with essential tegument protein ORF44. J. Virol. 88:188–201. 10.1128/JVI.02245-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori Y, Akkapaiboon P, Yonemoto S, Koike M, Takemoto M, Sadaoka T, Sasamoto Y, Konishi S, Uchiyama Y, Yamanishi K. 2004. Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46. J. Virol. 78:4609–4616. 10.1128/JVI.78.9.4609-4616.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Compaan DM, Hymowitz SG. 2006. The crystal structure of the costimulatory OX40-OX40L complex. Structure 14:1321–1330. 10.1016/j.str.2006.06.015 [DOI] [PubMed] [Google Scholar]
- 32.McShane MP, Mullen MM, Haan KM, Jardetzky TS, Longnecker R. 2003. Mutational analysis of the HLA class II interaction with Epstein-Barr virus glycoprotein 42. J. Virol. 77:7655–7662. 10.1128/JVI.77.13.7655-7662.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed ML, Yen HL, DuBois RM, Bridges OA, Salomon R, Webster RG, Russell CJ. 2009. Amino acid residues in the fusion peptide pocket regulate the pH of activation of the H5N1 influenza virus hemagglutinin protein. J. Virol. 83:3568–3580. 10.1128/JVI.02238-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schader SM, Colby-Germinario SP, Quashie PK, Oliveira M, Ibanescu RI, Moisi D, Mesplede T, Wainberg MA. 2012. HIV gp120 H375 is unique to HIV-1 subtype CRF01_AE and confers strong resistance to the entry inhibitor BMS-599793, a candidate microbicide drug. Antimicrob. Agents Chemother. 56:4257–4267. 10.1128/AAC.00639-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duenas-Decamp MJ, Peters P, Burton D, Clapham PR. 2008. Natural resistance of human immunodeficiency virus type 1 to the CD4bs antibody b12 conferred by a glycan and an arginine residue close to the CD4 binding loop. J. Virol. 82:5807–5814. 10.1128/JVI.02585-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo D, Shi X, Arledge KC, Song D, Jiang L, Fu L, Gong X, Zhang S, Wang X, Zhang L. 2012. A single residue within the V5 region of HIV-1 envelope facilitates viral escape from the broadly neutralizing monoclonal antibody VRC01. J. Biol. Chem. 287:43170–43179. 10.1074/jbc.M112.399402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casali M, Banta S, Zambonelli C, Megeed Z, Yarmush ML. 2008. Site-directed mutagenesis of the hinge peptide from the hemagglutinin protein: enhancement of the pH-responsive conformational change. Protein Eng. Des. Sel. 21:395–404. 10.1093/protein/gzn018 [DOI] [PubMed] [Google Scholar]