ABSTRACT
The HIV-1 surface glycoprotein gp120 has been reported to bind and signal through α4β7 by means of a tripeptide motif in the V2 loop that mimics structures present in the natural ligands for α4β7, suggesting that α4β7 may facilitate HIV-1 infection of CD4+ T cells in the gut. Furthermore, immune correlates in the RV144 vaccine efficacy trial generated the hypothesis that V1V2 antibodies to an epitope near the putative α4β7 binding motif may play a role in protection against HIV-1 infection. In the interest of developing an assay to detect antibodies that block gp120 binding to α4β7, we used retinoic acid (RA)-activated human peripheral blood mononuclear cells (PBMCs) and transfected HEK293T (293T) cells expressing the integrin complex to study the α4β7 binding properties of 16 HIV-1 envelope glycoproteins. The natural ligand for α4β7, mucosal addressin cell adhesion molecule-1 (MAdCAM-1), bound efficiently to RA-activated PBMCs and transfected 293T cells, and this binding was blocked by antibodies to α4. gp120 from multiple HIV-1 subtypes bound to RA-activated PBMCs from three donors in a CD4-dependent manner, but little or no α4β7 binding was detected. Similarly, little or no binding to α4β7 on transfected 293T cells was detected with multiple gp120s and gp140s, including gp120s from transmitted/founder strains, or when gp120 was produced in CHO, 293T, and 293S/GnT1−/− cells. Finally, we found no evidence that infectious HIV-1 virions produced in either PBMCs or 293T cells could bind α4β7 on transfected 293T cells. Infectious HIV-1 virions and most gp120s/gp140s appear to be poor ligands for the α4β7 integrin complex under the conditions tested here.
IMPORTANCE Certain HIV-1 gp120 envelope glycoproteins have been shown to bind the gut-homing receptor α4β7, and it has been suggested that this binding facilitates mucosal transmission and virus replication in the gut mucosa. Additional evidence has generated the hypothesis that antibodies that bind near the putative α4β7 binding motif in the V2 loop of gp120, possibly disrupting gp120-α4β7 binding, may be important for HIV-1 vaccines. Our evidence indicates that infectious HIV-1 virions and many gp120s lack detectable α4β7 binding activity, suggesting that this homing receptor may play a limited role in direct HIV-1 infection of cells.
INTRODUCTION
Integrins are cell receptors that play important immunomodulatory functions, such as cell adhesion, cellular trafficking, immune responses, as well as control of tumor growth and metastasis (1). These integrin receptors are composed of α and β subunits, and their surface expression plays a key role in the migration of cells to different tissues (2). The α4 subunit is expressed on T and B lymphocytes, monocytes, natural killer cells, and dendritic cells, where it can associate with β1 or β7 subunits (2, 3). The α4β7 heterodimer acts as a gut-homing receptor, mediating lymphocyte migration to the intestinal mucosa through interaction with the mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which is predominantly expressed on venules in the gut-associated lymphoid tissue (GALT) and intestinal lamina propria (4–6).
Following mucosal exposure, human immunodeficiency virus type 1 (HIV-1) replicates at low levels due to the insufficient numbers of CD4+ T cell targets to infect. Once the virus reaches the GALT, where large amounts of activated CD4+ T cells are present, a high level of replication takes place, resulting in immune dysfunction and the massive depletion of CD4+ T cells during acute infection (7–12).
The HIV-1 surface gp120 envelope glycoprotein has been reported to bind and signal through the α4β7 integrin complex. This interaction is mediated by a tripeptide motif in the V2 loop of gp120 that mimics the binding motif of the natural ligands for α4β7 (13). Although binding of α4β7 to gp120 is not a prerequisite for HIV-1 entry, it has been suggested that strong α4β7 reactivity may provide an increased fitness for mucosal transmission (13, 14). Additional evidence has shown that scaffold proteins containing the HIV-1 V1V2 loop can block α4β7-gp120 binding (15). These studies also showed that gp120 binding to α4β7 results in the rapid activation of LFA-1, the central integrin that mediates the formation of virological synapses, an event that could also facilitate HIV-1 cell-to-cell transmission (16). These findings have provided a plausible explanation for the massive infection of CD4+ T cells in the gut. Consistent with this hypothesis are the results of studies showing that the loss of β7-expressing CD4+ T cells in blood closely parallels the loss of CD4+ T cells in the intestine of rhesus macaques after simian immunodeficiency virus (SIV) infection (12). Moreover, administration of an α4β7 monoclonal antibody (MAb) to rhesus macaques just prior to and during acute SIV infection resulted in a significant decrease in the plasma and gastrointestinal tissue viral load and a marked reduction in the gut tissue proviral DNA load compared with the loads in control SIV-infected animals (17).
The finding that antibodies to the V1V2 region of gp120 inversely correlated with infection risk in the RV144 HIV-1 vaccine efficacy trial (18) has generated the hypothesis that these antibodies may have contributed to protection against HIV-1 infection. A plausible mechanism of protection by plasma anti-V2 antibodies does not appear to be dependent on their neutralizing activity (19). An alternative possible mechanism is that V2 antibodies block virus binding to α4β7 integrin in a way that impedes the establishment of chronic infection (20).
Notably, only a small group of gp120s has been shown to possess measurable α4β7 binding properties. In addition, removal of potential N-linked glycosylation sites in V1V2 or the use of proteins expressed in glycosylation-deficient mutant cell lines is often required to demonstrate an interaction of α4β7 with gp120s (14, 21, 22). More recently, the infectivity of transmitted/founder (T/F) HIV-1 subtype C viruses was found to be unaffected by anti-α4β7 antibodies, suggesting that the α4β7 binding properties of gp120 seen in vitro are not seen with native Env trimers on infectious viral particles (23). In addition, two independent studies have shown that non-gut-homing resting memory CD4+ T cells (integrin β7 negative) are preferential targets for productive HIV infection (24, 25).
Motivated by these observations and in an attempt to develop an assay to monitor vaccine-elicited antibodies that block α4β7-gp120 binding, we used retinoic acid (RA)-treated human peripheral blood mononuclear cells (PBMCs) as well as CD4-negative HEK293T (293T) cells transiently expressing this integrin complex to determine the optimal conditions for gp120 binding to α4β7. Our results suggest that α4β7 binding is not a general property of most gp120 proteins.
MATERIALS AND METHODS
Cells.
PBMCs from three different healthy HIV-1-naive donors were obtained from the local Red Cross and were purified by Ficoll-Hypaque separation from buffy coats. They were selected for these studies on the basis of the quantity of the cryopreserved stocks and susceptibility to HIV infection. After thawing, the PBMCs were cultured in RPMI containing 20% heat-inactivated fetal bovine serum (FBS) containing 5% interleukin-2 (IL-2; Advanced Biotechnologies, Inc.) and 5 μg/ml phytohemagglutinin (PHA; Sigma) for 24 h. After this period, PBMCs were cultured in RPMI containing 20% FBS and 5% IL-2 without PHA. 293T cells were obtained from the American Type Culture Collection (ATCC) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated FBS, 25 mM HEPES, and 50 μg/ml of gentamicin (Sigma).
Antibodies.
Allophycocyanin (APC)-α4 and phycoerythrin (PE)-β7 conjugated MAbs were obtained from BD Pharmingen. Purified CD49d (α4) MAbs, clones HP2/1 and L25, were obtained from Beckman Coulter and BD Pharmingen, respectively. A recombinant human MAdCAM-1 Fc chimera was obtained from R&D systems. The CD4 MAb SK313 was obtained from BD Pharmingen.
Source of HIV-1 envelope glycoproteins.
gp120s from the subtype B isolate SF162 (SF162gp120) and the subtype C isolate TV-1 (TV-1gp120) were produced in Chinese hamster ovary (CHO) cells by Novartis. HIV-1 gp120s from strains 92UG037 (subtype A), BaL (subtype B), 92US715.6 (subtype B), and 96ZM651 (subtype C) were produced in CHO cells by ABL Inc. HIV-1 AN-1gp120 protein (subtype B) was provided by J. Arthos and was produced in cells of the CHO lec1.1−/− cell line, a CHO cell derivative that lacks N-acetylglucosamine glycosyltransferase activity (14, 26). HIV-1 gp140 proteins from strains CN54 (subtype C) and UG37 (subtype A) were made in CHO cells by Polymun Scientific Inc. The gp140 glycoproteins from the T/F strain C.1086 (subtype C), Q23.17 (subtype A), and a consensus sequence of group M Env (M-CONS) were made in CHO cells. The gp120s for SF162 (subtype B) and Q23.17 (subtype A) were produced in CHO, 293T, and 293S/GnT1−/− cells, all of which were prepared at the Duke Human Vaccine Institute, Duke University Medical Center.
T/F HIV-1 Env proteins.
Genes for four HIV-1 T/F gp120s, including three subtype B proteins (B.040, B.65321, and B.6240) and one subtype C protein (C.1086) obtained from subjects acutely infected with HIV-1 by single-genome amplification, were described previously (27, 28). All gp120 genes were codon optimized by converting amino acid sequences to nucleotide sequences by employing the codon usage of highly expressed human housekeeping genes (29) synthesized de novo for expression as gp120 with deletion of either 7 (C.1086Δ7gp120) or 11 (B.040Δ11gp120, B.65321Δ11gp120, and B.6240Δ11gp120) amino acid residues at the N terminus of the mature HIV-1 Env proteins (30, 31). These genes were then cloned into a mammalian expression plasmid, pcDNA3.1/hygromycin (Invitrogen, Grand Island, NY). The recombinant Env glycoproteins were purified from the supernatants of CHO cell cultures transfected with the HIV-1 gp120-expressing pcDNA3.1 plasmids by using DEAE–ion-exchange resins and Galanthus nivalis lectin-agarose (Vector Laboratories, Burlingame, CA) column chromatography and stored at −80°C until use.
Transient expression of α4β7 integrins in 293T cells.
HEK293T cells (grown to 80% confluence in a 75-cm2 flask) were transfected with 6 μg each of the cDNA expression clones for the α4 and β7 human integrin subunits (Origene Technologies, Inc.) using the Fugene reagent (Promega). Prior to use for gp120 binding and infectious virion capture studies, cells were assessed for α4β7 surface expression by flow cytometry at 48 h posttransfection, using APC- or PE-conjugated integrin-specific MAbs.
Biotin labeling of proteins.
HIV-1 gp120 and gp140 envelope glycoproteins and the recombinant human MAdCAM-1 Fc chimera proteins were labeled with biotin using a Biotin-XX microscale protein labeling kit (Molecular Probes/Invitrogen). In brief, 50 μg of protein was labeled with Biotin-XX sulfosuccinimidyl ester in a 50-μl reaction mixture for 15 min at 20°C, according to the instructions provided by the manufacturer. Unbound biotin was removed from the sample using a spin filter packed with Bio-Gel P-6.
Assay to measure α4β7 binding to gp120.
PBMCs were cultured for 7 days in RPMI containing 20% heat-inactivated FBS, 5% IL-2 (Advanced Biotechnologies, Inc.), and 10 nM RA (all-trans; Sigma). Expression of α4β7 integrins was monitored by flow cytometry with integrin-specific MAbs. Similarly, 293T cells transiently transfected with α4β7 cDNAs were removed from flasks using a nonenzymatic cell dissociation solution (Millipore) and tested for integrin expression by flow cytometry. The binding assay was performed as previously described by Arthos et al. (13). Cells (2 × 106) were washed and resuspended in 200 μl of binding buffer (HEPES-buffered saline supplemented with 100 μM Ca2+, 1 mM Mn2+, and 0.5% bovine serum albumin). For blocking experiments, cells were preincubated for 10 min at 4°C with saturating concentrations of either anti-α4 MAbs, unlabeled MAdCAM-1, or a combination of them. Saturating concentrations were predetermined by binding studies using serial dilutions of each of the MAbs. Biotinylated gp120 and MAdCAM-1 (400 ng) were added, and the mixture was incubated at 4°C for 15 min. Cells were washed and incubated with PE-streptavidin (BD Pharmingen) in binding buffer at 4°C for 10 min. After two final washings, cells were fixed in 1% formaldehyde–phosphate-buffered saline and analyzed by flow cytometry using a BD FACSCalibur analyzer (BD Biosciences). Between 12,000 and 100,000 live events were acquired per sample. Compensation and analysis were performed by using FlowJo software (Tree Star).
Infectious virions binding to α4β7-expressing 293T cells.
293T cells transfected with the cDNA expression vectors for α4 and β7 integrins were seeded at 50,000 cells per well in 96-well culture plates at 1 day posttransfection and incubated overnight at 37°C. The cells were then incubated with serial dilutions of infectious molecular clones of HIV-1, clones R2184c4.IMC.LucR (subtype CRF01_AE) and WEAU3-3.IMC.LucR (subtype B) (32), for 1 h at 37°C. Control wells containing nontransfected 293T cells were treated in the same manner. The cells were gently washed three times with culture medium, overlaid with10,000 TZM-bl cells per well, and incubated for 48 h at 37°C. Infection of TZM-bl cells was measured with a Britelite luminescence reporter gene assay system (PerkinElmer Life Sciences).
RESULTS
Retinoic acid upregulates the α4β7 integrin complex on human PBMCs.
Dendritic cells from mesenteric lymph nodes and Peyer's patches produce the vitamin A metabolite retinoic acid, which enhances the expression of α4β7 and CCR9 on T cells and imprints them with gut tropism (33). To study the α4β7 reactivity of different HIV-1 gp120s, human PBMCs from three donors were cultured in RA for 7 days. After this treatment, the level of α4β7 expression was determined by flow cytometry using APC- or PE-conjugated MAbs specific for the human integrins. As shown in Fig. 1A, untreated PBMCs from the three donors expressed different levels of both integrins. After culturing in RA, the levels of α4 remained mostly unchanged, but the level of β7 was increased for all three donors, particularly donors 2 and 3. Using Quantibrite PE beads, we estimated that for donor 3, the number of β7 molecules per cell (inferred with the β7-PE MAb) increased from 590 in untreated cells to 3,367 in RA-treated cells. Next, we tested the binding of the natural ligand for α4β7, MAdCAM-1, in the absence and presence of two anti-α4 antibodies, CD49d (anti-VLA-α4) clone L25 and CD49d clone HP2/1, which have been reported to block the gp120-α4β7 interaction (13). RA-treated PBMCs from donor 3 readily bound biotinylated MAdCAM-1, and this binding was blocked to near background levels by either anti-α4 MAb at a concentration of 100 μg/ml (Fig. 1B).
FIG 1.
RA upregulation of α4β7 integrins on three donor human PBMCs and MAdCAM-1 binding. (A) Expression of α4β7 integrins on PBMCs from three donors (D1, D2, and D3) after RA treatment was analyzed by flow cytometry. Cells were cultured with and without RA for 7 days in medium containing IL-2 and PHA. Cells were stained with an APC-conjugated anti-α4 MAb or PE-conjugated anti-β7 MAb, washed, and fixed with formaldehyde before analysis with a BD FACSCalibur analyzer. The mean fluorescence intensity (MFI) obtained from the analysis of the data with FlowJo software is shown. α4 and β7, the anti-human integrin MAbs used. (B) Binding of MAdCAM-1, the natural ligand for the α4β7 integrin complex, was measured by flow cytometry using RA-treated PBMCs from donor 3 incubated with a biotinylated recombinant human MAdCAM-1 Fc chimera and PE-avidin at 4°C. In parallel assays, RA-treated PBMCs were preincubated with the anti-α4 MAbs HP2/1 and L25 prior to the addition of MAdCAM-1. Black bars, mean fluorescence intensity obtained by analysis with FlowJo software.
CD4-dependent binding of gp120 to RA-treated human PBMCs.
To establish the integrity of our experimental system, we first aimed to demonstrate gp120 binding to CD4 on the surface of the donor PBMCs to be used in subsequent experiments. As shown in Fig. 2, biotin-labeled SF162gp120 and TV-1gp120 both bound to PBMCs from all three donors with the same magnitude, despite different levels of α4β7, and this binding could be completely blocked by the CD4 MAb Leu3a.
FIG 2.
CD4-specific binding of gp120 to RA-treated human PBMCs. Biotinylated gp120 from HIV-1 isolates SF162 (subtype B) and TV-1 (subtype C) was incubated with RA-treated PBMCs at 4°C for 15 min in HEPES buffer containing Ca2+ and Mn2+. Bound gp120 was detected with PE-avidin and flow cytometry analysis. The binding of gp120 to RA-treated PBMCs preincubated with a saturating concentration (25 μg/ml) of the mouse anti-human CD4 MAb Leu3a was also measured in this assay. Assays were performed with PBMCs from three donors. Black curves in the histograms, unstained PBMCs; gray curves, PBMCs incubated with avidin-PE only; red curves, PBMCs incubated with biotinylated gp120 from the indicated HIV-1 isolate.
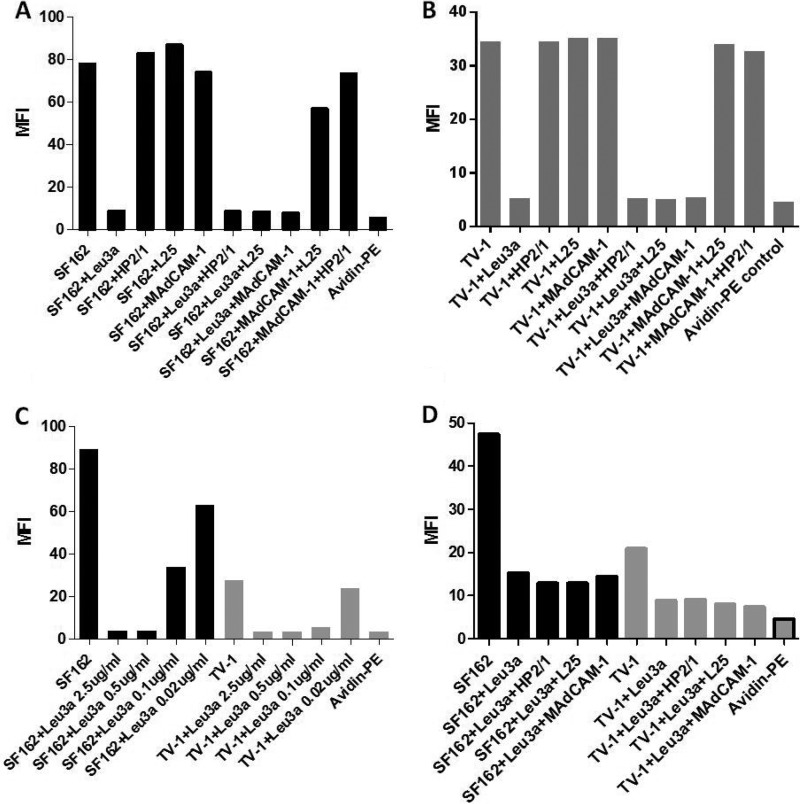
Attempts to detect α4β7-specific binding of SF162gp120 and TV-1gp120 to RA-treated human PBMCs.
It was previously reported that gp120 binding to α4β7 can be blocked with anti-α4 MAbs HP2/1 and L25 (13). We tested whether these MAbs and the α4β7 natural ligand MAdCAM-1 would block the binding of SF162gp120 and TV-1gp120 to donor 3 PBMCs. As shown in Fig. 3A and B, Leu3a clearly competed with both gp120s for binding to PBMCs. Marginal inhibition of SF162gp120 binding was seen in the presence of MAdCAM-1 (5% inhibition) and a combination of MAdCAM-1 plus L25 (27% inhibition), whereas no inhibition was seen in the presence of either HP2/1 or L25. We saw no decrease in TV-1gp120 binding in the presence of HP2/1, L25, MAdCAM-1, or combinations of these potential blocking agents.
FIG 3.
Lack of α4β7-specific binding of gp120 from two different HIV-1 isolates and effect of subsaturating concentrations of Leu3a. (A and B) Binding of biotinylated SF162gp120 (A) and TV-1gp120 (B) to RA-treated PBMCs from donor 3 with and without preincubation with either unlabeled MAdCAM-1, the anti-α4 MAbs HP2/1 and L25, or the anti-CD4 MAb Leu3a at a saturating concentration or combinations of these reagents. (C) Titration of Leu3a for suboptimal inhibition of gp120 binding to donor 3 RA-treated PBMCs. (D) Effect of subsaturating concentrations of Leu3a on the binding of SF162gp120 or TV-1gp120 to donor 3 RA-treated PBMCs in the presence and absence of either HP2/1, L25, or MAdCAM-1. A subsaturating concentration of 0.08 μg/ml of Leu3a was chosen for both gp120s. Binding was assessed by flow cytometry. The mean fluorescence intensity obtained for each reagent and combinations is shown. Black bars, SF162gp120; gray bars, TV-1gp120. The same avidin-PE control was used for both gp120s in panels C and D (gray bar with a black border).
Although the binding of gp120 to α4β7 is CD4 independent, previous studies have demonstrated a close association of CD4 and α4β7 on the surface of T cells (34). To investigate the possibility that gp120 engages α4β7 and CD4 in a cooperative manner, we tested the α4β7 reactivity of the gp120s with RA-treated PBMCs preincubated with a subsaturating concentration of the anti-CD4 MAb Leu3a. As shown in Fig. 3C, a subsaturating concentration of Leu3a that partially blocked the binding of SF162gp120 and TV-1gp120 to PBMCs was identified. Using a combination of a subsaturating concentration of Leu3a and saturating amounts of α4 MAbs or MAdCAM-1, the binding of SF162gp120 was reduced either 6% (MAdCAM-1 plus Leu3a) or 16% (α4 MAbs plus Leu3a) relative to the level of binding seen in the presence of the subsaturating concentration of Leu3a alone (Fig. 3D). Similarly, TV-1gp120 binding was reduced either 10% (Leu3a plus L25) or 17% (Leu3a plus MAdCAM-1) relative to the level of binding seen in the presence of the subsaturating concentration of Leu3a alone. These results again indicate that binding of these gp120s to the integrin complex is negligible.
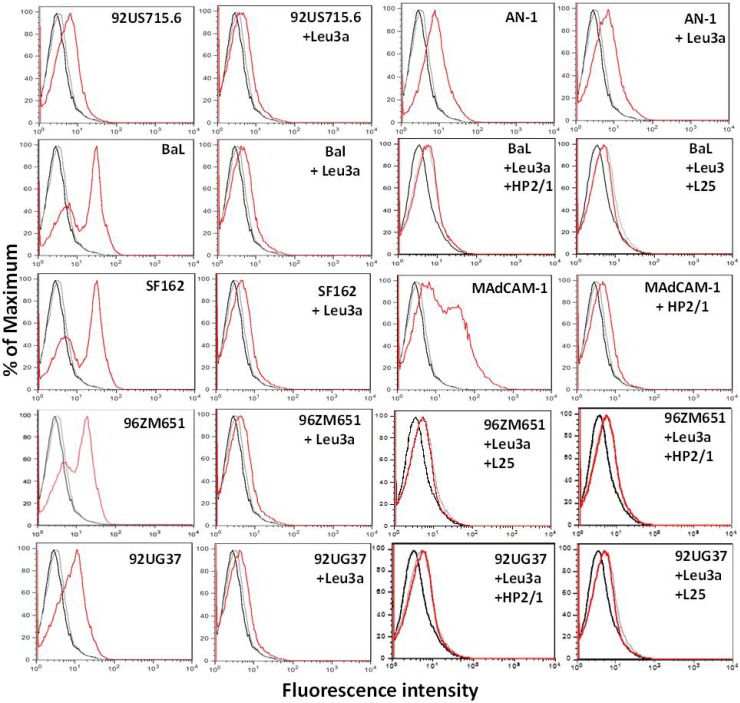
Binding of gp120s from multiple HIV-1 isolates to RA-cultured PBMCs.
Because we found very little binding of SF162gp120 to α4β7, we extended our studies to include an additional panel of gp120s from HIV-1 subtypes A, B, and C. As a positive control for α4β7 binding, we included the deduced ancestral AN-1gp120 (26) that had previously been shown to bind α4β7 (13, 14). With the exception of AN-1gp120, all Env glycoproteins exhibited clear CD4-dependent binding to RA-treated PBMCs; this was demonstrated by specific inhibition of binding to near background levels by Leu3a (Fig. 4). Little or no additional blocking of binding was seen with combinations of the anti-α4 MAb L25 or HP2/1 and Leu3a. Binding of AN-1gp120 to the surface of these PBMCs was blocked very poorly by Leu3a, suggesting that binding was mostly CD4 independent and presumably due in part to α4β7 binding. The integrity of α4β7 expression on these cells was confirmed by showing strong binding of MAdCAM-1 and the inhibition of this binding by HP2/1.
FIG 4.
CD4-dependent binding of gp120 from multiple HIV-1 subtypes to human PBMCs. Biotinylated gp120s from HIV-1 subtypes A, B, and C were tested for binding to RA-treated PBMCs by flow cytometry. In addition, PBMCs preincubated with a saturating concentration of Leu3a prior to the addition of biotinylated gp120 were tested in parallel. Biotinylated MAdCAM-1 was used as a positive control to confirm the presence of the α4β7 integrins on the PBMCs. Black curves in the histograms, unstained PBMCs; gray curves, PBMCs incubated with avidin-PE only; red curves, PBMCs incubated with gp120 from HIV-1 isolates 92US715.6 (subtype B), 92UG37 (subtype A), BaL (subtype B), AN-1 (deduced ancestral subtype), SF162 (subtype B), and 96ZM651 (subtype C), in which binding was detected with avidin-PE. The results also correspond to those for PBMCs incubated with biotinylated MAdCAM-1, in which binding was detected with avidin-PE.
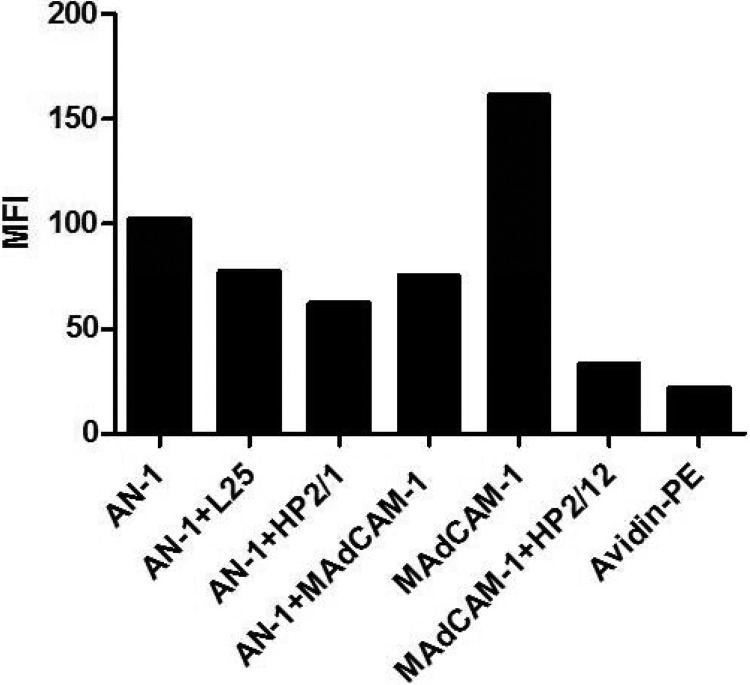
AN-1gp120 binding to α4β7 on RA-treated PBMCs.
We next tested the ability of MAdCAM-1 and the anti-α4 MAbs L25 and HP2/1 to block the binding of biotinylated AN-1gp120 to RA-treated PBMCs. As shown in Fig. 5, AN-1gp120 bound to PBMCs weakly, and this weak binding could be partially blocked by all three reagents and was blocked to an extent greater than that seen with Leu3a, indicating that this gp120 binds α4β7, as previously reported (13, 14). Notably, a substantial amount of binding could not be blocked by any of these reagents, suggesting that a certain level of binding to unknown surface constituents occurs. As before, the integrity of α4β7 expression was confirmed by showing nearly complete inhibition of MAdCAM-1 binding to PBMCs in the presence of HP2/1.
FIG 5.
Weak inhibition of AN-1gp120 binding to RA-treated PBMCs in the presence of anti-α4 MAbs and MAdCAM-1. Binding of biotinylated AN-1gp120 to RA-treated human PBMCs preincubated with either Leu3a, L25, HP2/1, or MAdCAM-1 was tested by flow cytometry. Binding of biotinylated MAdCAM-1 to the same PBMCs preincubated with HP2/1 was used as a control. Black bars, the mean fluorescence intensity obtained by analysis with FlowJo software for each reagent.
Testing of gp120 binding to 293T cells expressing high levels of α4β7 integrins.
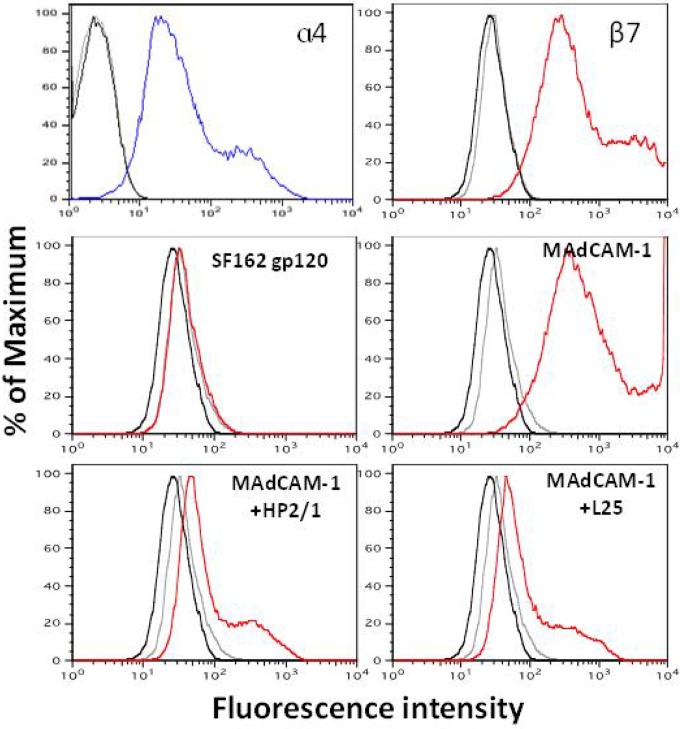
To study gp120 binding to α4β7 in the absence of CD4, we cotransfected cDNA expression vectors for the human α4 and β7 integrin subunits into 293T cells. As shown in Fig. 6, high levels of each of the α4β7 integrin subunits were detected on the surface of 293T cells by flow cytometry at 48 h posttransfection. Furthermore, these cells readily bound biotinylated MAdCAM-1, and this binding was blocked by pretreatment of the cells with the anti-α4 MAbs HP2/1 and L25, confirming the integrity of the α4β7 complex. Despite the high levels of α4β7 expression, no significant binding of biotinylated SF162gp120 could be detected.
FIG 6.
Binding of SF162gp120 and MAdCAM-1 to α4β7 expressed on 293T cells. 293T cells were transfected with expression vectors for the cDNA of the human α4 and β7 integrins. The cell surface expression levels of the integrins were detected by flow cytometry 48 h later (top; blue and red for α4 and β7, respectively). (Middle) Binding of biotinylated SF162gp120 and MAdCAM-1 to transfected cells (red plots); (bottom) specificity of binding determined by testing the ability of HP2/1 and L25 to block the binding of biotinylated MAdCAM-1. Black curves, untreated cells; gray curves, cells incubated with avidin-PE only.
AN-1gp120 binding to 293T cells expressing high levels of α4β7 integrins.
We next tested the binding of AN-1gp120 to 293T cells expressing α4β7. As shown in Fig. 7, AN-1gp120 readily bound to these cells, and this binding could be partially blocked by either MAdCAM-1 or the anti-α4 MAbs, suggesting a modest level of α4β7-specific binding. Nonetheless, as seen in experiments with RA-treated PBMCs as described above, a substantial level of binding was not blocked by these reagents, indicating that this gp120 binds other cell surface constituents that remain to be identified.
FIG 7.
Binding of AN-1gp120 to α4β7 expressed on 293T cells. Flow cytometry was used to test the binding of biotinylated AN-1gp120 to 293T cells transiently transfected with the human α4 and β7 cDNAs. In parallel, AN-1gp120 was tested for binding to α4β7-expressing cells preincubated with either L25, HP2/1, or MAdCAM-1. Biotinylated MAdCAM-1was used as a control to monitor α4β7 expression levels. Black bars, the mean fluorescence intensity obtained from the analysis of the data with FlowJo software.
Binding of gp140 Env glycoproteins to 293T cells expressing the α4β7 complex.
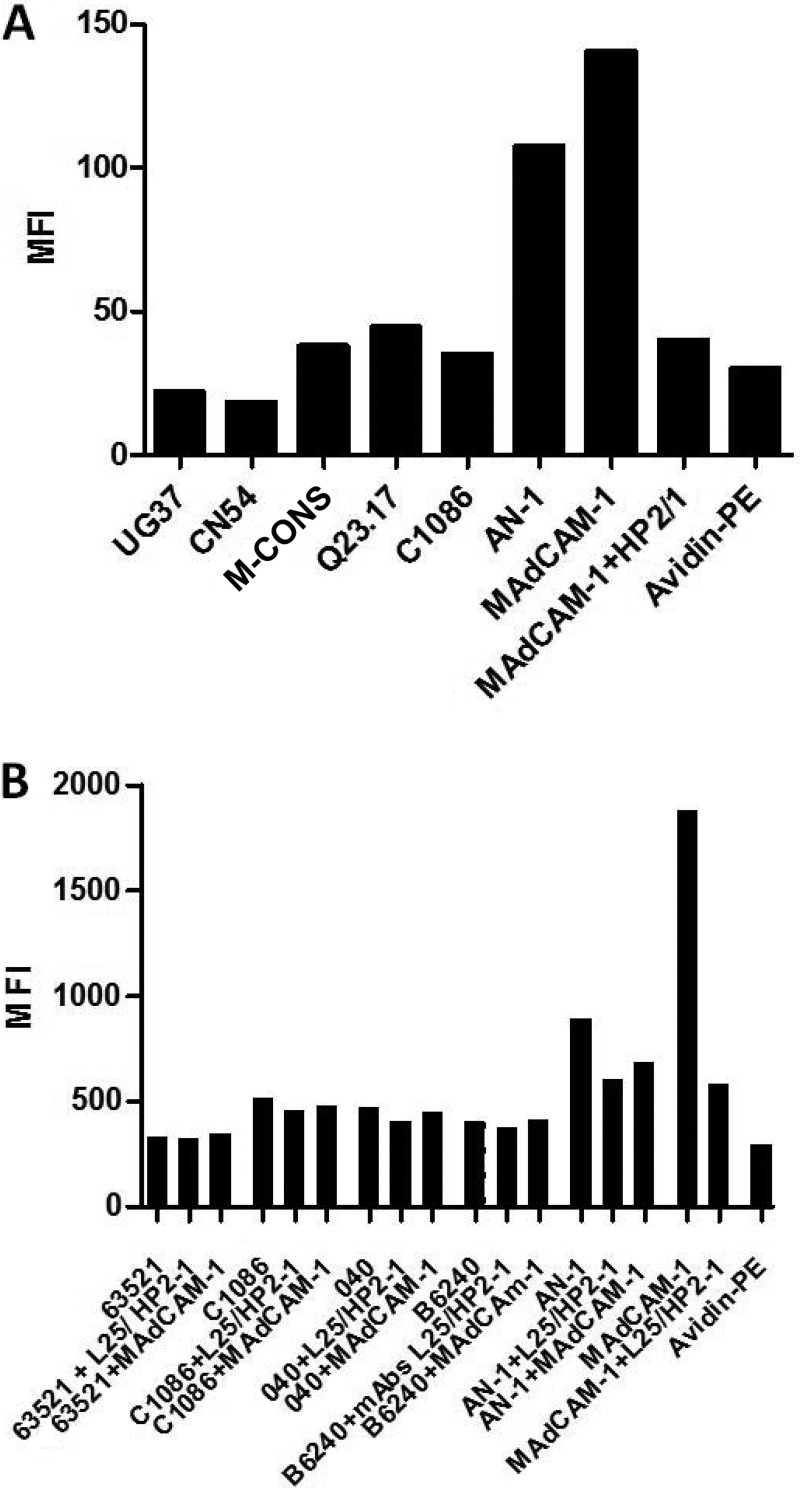
The recombinant uncleaved gp140 glycoproteins from the subtype C/B strain CN54, subtype A strains 92UG037 and Q23.17, subtype C strain C.1086, and M-CONS (a consensus sequence of group M Env) (35) were biotinylated and tested for binding to CD4-negative 293T cells transiently expressing the α4β7 integrin complex. As shown in Fig. 8A, some of these gp140 Env products bound only marginally to 293T cells expressing high levels of the α4β7 complex, whereas AN-1gp120 and MAdCAM-1 showed much more substantial binding. The marginal α4β7 binding observed for some of the gp140s did not change with pretreatment of the cells with the α4 MAbs (not shown).
FIG 8.
Binding of gp140 and T/F gp120s to α4β7 expressed on 293T cells. (A) The gp140s of HIV-1 strains CN54 (subtype C), 92UG037 (UG37; subtype A), M-CONS (a consensus sequence of group M Env), the transmitted/founder subtype C strain C.1086, and Q23.17 (subtype A), all produced in CHO cells, were biotinylated and tested for binding to α4β7-expressing 293T cells by flow cytometry. The mean fluorescence intensity representing the binding of the different gp140s to the integrin-expressing cells, in which binding was detected by avidin-PE, is shown. (B) gp120s from the transmitted/founder subtype B isolates B6240D11 (B6240), B040D11 (040), and 63521D11 (63521) and from the subtype C isolate C.1086 were biotinylated and tested in parallel for binding to α4β7-expressing cells by flow cytometry. Black bars, the mean fluorescence intensity obtained by analysis of the data with FlowJo software. The basal background level of fluorescence determined for cells incubated only with avidin-PE is also shown.
Binding of gp120 from transmitted/founder HIV isolates to α4β7-expressing cells.
It was previously suggested that the genotype of T/F HIV gp120s promotes α4β7 binding. We tested the integrin binding capacity of four T/F gp120s, three subtype B gp120s (B.040Δ11gp120, B.65321Δ11gp120, and B.6240Δ11gp120) and one subtype C gp120 (C.1086Δ7gp120). As shown in Fig. 8B, some of the T/F gp120s bound marginally to α4β7-expressing cells. Pretreatment of the cells with α4 MAbs or the integrin natural ligand had very little or no effect on the observed binding. In contrast, binding of AN-1gp120 and MAdCAM-1 to the same α4β7-expressing cells was substantially higher and was partially inhibited by a combination of the two α4 MAbs and by MAdCAM-1.
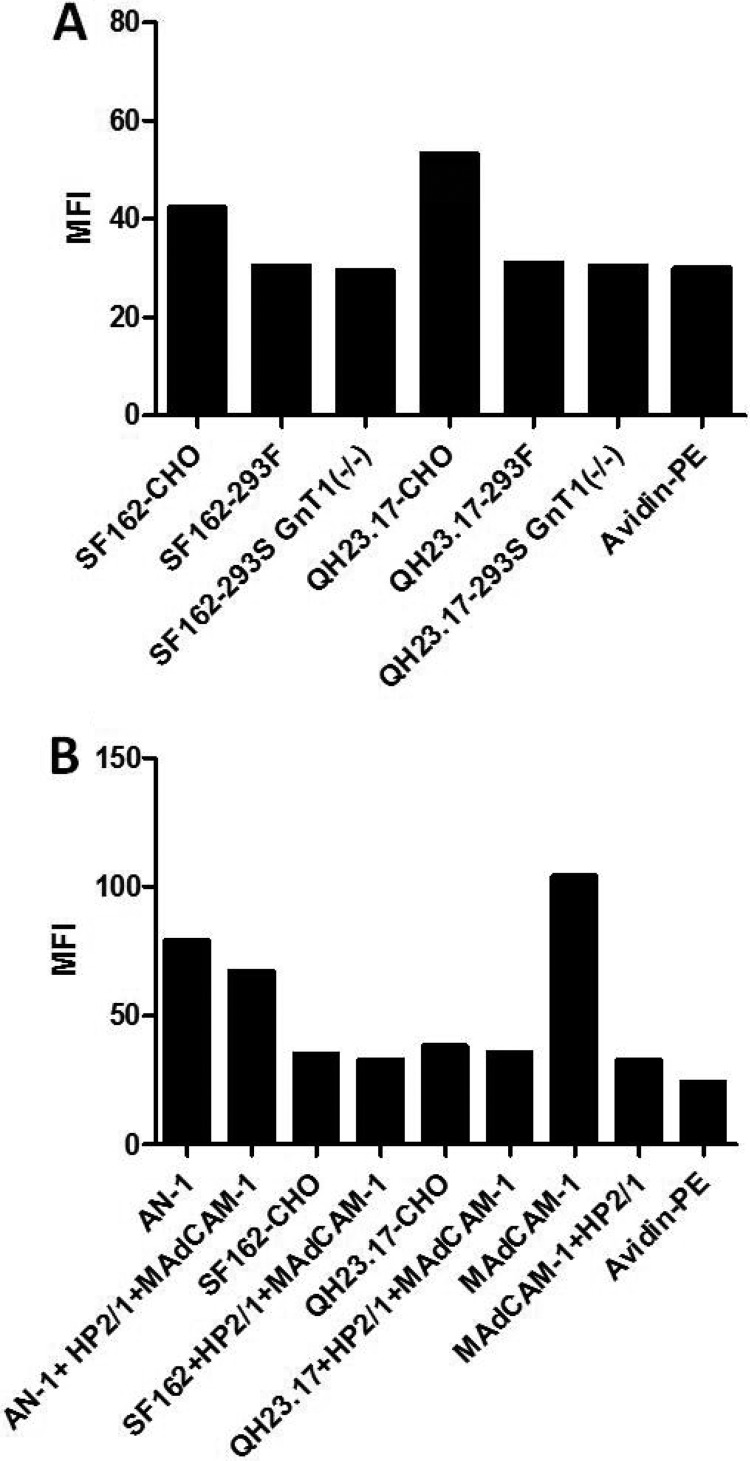
α4β7 binding of gp120 produced in different cell lines.
During mucosal transmission of HIV-1 subtype C, the T/F viruses have gp120s with a reduced number of N-linked glycosylation sites and shorter V1V2 variable loops. It has been suggested that a lower number of N-linked glycosylation sites gives a transmission advantage by facilitating α4β7 binding (14). Because in our assays a gp120 lacking complex glycans (AN-1) was the only glycoprotein with detectable α4β7 binding, we next compared the integrin binding capacity of other gp120s grown in three different cell lines that possess different glycosylation machineries. SF162 and Q23.17 gp120s produced in CHO, 293F, and 293S/GnT1−/− cell lines were tested for binding to α4β7-expressing 293T cells. As shown in Fig. 9A, only the gp120s produced in CHO cells demonstrated binding at levels that were above the background levels. This marginal binding could not be blocked by a combination of HP2/1 and MAdCAM-1 (Fig. 9B). As a control, the combination of HP2/1 and MAdCAM-1 showed partial blocking of binding of AN-1gp120 produced in CHO lec1.1−/− cells (Fig. 9B).
FIG 9.
The α4β7 reactivity of gp120s produced in three different cell lines. (A) Biotinylated SF162 and Q23.17 gp120s produced in CHO, 293F, and 293S/GnT1−/− cell lines were tested in parallel for binding to 293T cells transiently expressing α4β7 integrins. (B) The same gp120s described for panel A were produced in CHO cells and tested for binding to α4β7-expressing 293T cells in the presence and absence of a combination of anti-α4 MAb HP2/1 and MAdCAM-1. Binding of biotinylated MAdCAM-1 to α4β7-expressing 293T cells in the presence and absence of the anti-α4 MAb HP2/1 was used as a control. Black bars, the mean fluorescence intensity obtained by analysis with FlowJo software. The basal background level of fluorescence determined for cells incubated only with avidin-PE is also shown.
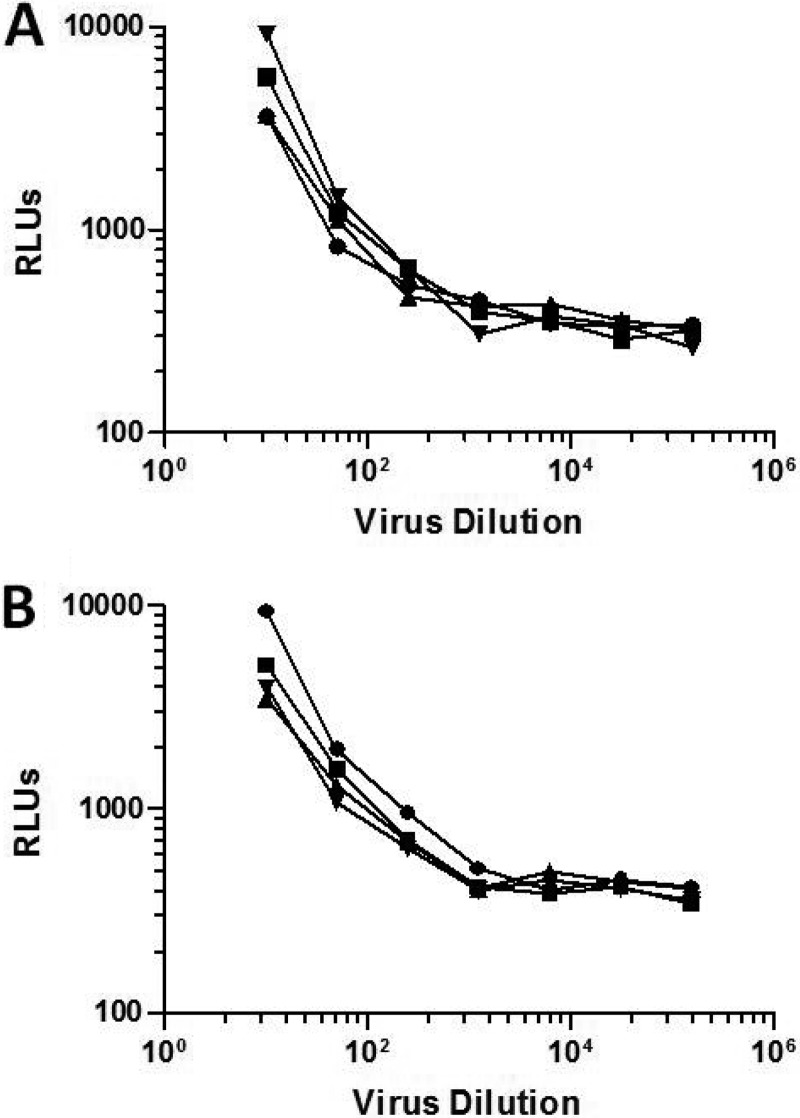
Binding of infectious virions to α4β7-expressing 293T cells.
We next tested whether infectious virus produced in either human PBMCs or 293T cells was capable of binding the α4β7 integrin complex on transfected 293T cells. For these experiments we used as molecular clones two HIV-1 isolates carrying a Renilla luciferase (Luc) reporter gene (Env.IMC.LucR viruses): the subtype AE isolate R2184c4.IMC.LucR and the subtype B isolate WEAU3-3.IMC.LucR. As shown in Fig. 10, some infectious virus was retained on 293T cells in a virus dose-dependent manner, but no measurable differences in the amount of virus that bound to nontransfected 293T cells and α4β7-expressing 293T cells were observed for either virus. Since these cells do not express CD4 or any known coreceptors for HIV-1, the nonspecific binding may be attributed to any number of cell adhesion molecules that are known to bind HIV-1 (36).
FIG 10.
Capture of HIV-1 infectious virions by α4β7 expressed on 293T cells. Serial dilutions of the HIV-1 infectious molecular clones R2184c4.IMC.LucR (A) and WEAU3-3.IMC.LucR (B) were incubated with either α4β7-expressing 293T cells or mock-transfected 293T cells for 1 h at 37°C. Cells were washed and overlaid with TZM-bl cells. Activation of the Luc reporter gene in the TZM-bl cells was measured 48 h later using a Britelite luminescence reaction kit. Circles, virus grown in PBMCs and incubated with mock-transfected 293T cells; squares, virus grown in 293T cells and incubated with mock-transfected 293T cells; triangles, virus grown in PBMCs and incubated with α4β7-expressing 293T cells; inverted triangles, virus grown in 293T cells and incubated with α4β7-expressing 293T cells. RLU, relative light units.
DISCUSSION
We aimed to develop an assay to detect antibodies that block gp120 binding to the α4β7 integrin complex. The first step in developing such an assay was to identify optimal conditions that reproducibly generate an adequate signal for gp120-α4β7 binding. Intestinal dendritic cells and stromal cells produce retinoic acid, which has been shown to act as an extrinsic factor that controls the expression of α4β7 and the generation of gut-homing T cells (33, 37, 38). In our experiments, we reproduced this finding in vitro by culturing human PBMCs in the presence of RA for 7 days. This treatment imprints the gut-homing phenotype, as PBMCs from three donors showed increased surface expression of the β7 integrin and they efficiently bound the natural ligand for this complex, MAdCAM-1. The latter event indicates that the α4β7 complex expressed on these cells is in the extended/activated conformation, as previously described for this complex (34, 39). Indeed, our assay conditions generated a strong binding signal for the natural ligand, MAdCAM-1, where the majority of this binding could be inhibited by the anti-α4 MAbs L25 and HP2/1, confirming the specificity of MAdCAM-1 binding to the α4β7 complex on RA-treated PBMCs.
Using these optimal conditions with gp120 glycoproteins from two HIV-1 strains (SF162 and TV-1) and RA-treated PBMCs from three donors expressing the α4β7 complex, we detected strong CD4-dependent binding but very little or no α4β7-dependent binding to these cells. Moreover, we saw no evidence of increased binding of these gp120s or four additional gp120s to α4β7 when the anti-α4 MAbs and MAdCAM-1 were tested in the presence of subsaturating concentrations of Leu3a, suggesting no cooperative effect between CD4 and the integrin complex, which are thought to localize in close proximity on the cell membrane (34, 40).
A second assay was developed with CD4-negative 293T cells transiently expressing the activated form of the α4β7 integrin complex. High levels of surface expression of the integrins could be demonstrated by staining with MAbs to α4 and β7. Similar to the findings of the PBMC assay, a strong MAdCAM-1 binding signal was obtained with these transfected 293T cells, and this binding was nearly completely blocked by anti-α4 MAbs, confirming the activated form of the integrin complex. Using these transfected 293T cells, we detected very little or no α4β7 binding activity for another 11 gp120s/gp140s from multiple HIV-1 subtypes. Importantly, all gp120s used in these assays contain the LDV/LDI tripeptide. This tripeptide motif has been shown to mediate the binding of MAdCAM-1 to α4β7 (13). It was previously reported that the gp120 of HIV-1 SF162 binds α4β7 (14, 34). In our assays, this gp120 exhibited very weak binding, regardless of whether the glycoprotein was produced in CHO, 293T, or 293S/GnT1−/− cells (the last cell type lacks N-acetylglucosamine glycosyltransferase activity). AN-1gp120 produced in CHO lec 1.1−/− cells (which also lack N-acetylglucosamine glycosyltransferase activity), which has been shown to exhibit high levels of binding to α4β7 (13, 14), exhibited the best α4β7-specific binding activity in our assays, although this binding activity remained relatively low.
In contrast to the other 15 glycoprotein tested here, AN-1gp120 is not from a natural isolate (26) and was produced in CHO lec1.1−/− cells. Glycoproteins produced in this glycosylation-deficient cell line are devoid of complex carbohydrates and are enriched with oligomannose-type glycans (14). It has been previously reported that this modified glycan profile results in a 100-fold increase in α4β7 binding over that for AN-1gp120 produced in CHO cells (14). Thus, AN-1gp120 produced in CHO lec1.1−/− cells may be structurally different from the gp120s/gp140s used in our study. Support for this conclusion comes from two lines of evidence. First, we detected very little blocking of AN-1gp120 binding to RA-treated PBMCs by the anti-CD4 MAb Leu3a, whereas binding of CHO-derived gp120s was strongly blocked by this MAb, suggesting that the AN-1gp120 used here is far less CD4 dependent than the gp120 from most other strains of the virus. Second, anti-α4 MAbs and MAdCAM-1 only partially competed the binding of AN-1gp120 to α4β7-expressing cells under conditions in which the binding of the natural ligand, MAdCAM-1, to the same cells was nearly completely blocked by the same MAbs. The latter observations suggest that AN-1gp120 binds to a molecule other than CD4, in addition to the α4β7 complex. In fact, in our binding assay, AN-1gp120 bound 293T cells in the absence of CD4 and α4β7 expression (data not shown).
Binley et al. (41) examined the role of complex carbohydrates in HIV-1 infectivity and resistance to antibody neutralization. Using HIV-1 isolates grown in glycosylation-deficient cell lines, those studies demonstrated that the antennae of N-glycans serve to protect the integrity of both the V3 loop and the CD4 binding site, while the stems regulate the native trimer conformation. Those studies also showed that N-glycan removal can sometimes compromise viral infectivity. N-glycans also constitute a protective shield against antibodies, where their removal makes viruses more susceptible to neutralization by certain antibodies (42). It is possible that the modified N-glycan moieties on AN-1gp120 result in a change in protein conformation that exposes the V2 loop LDV/LDI in a conformation optimal for α4β7 reactivity while reducing CD4 binding. A possible explanation for why the gp120s produced in 293S/GnT1−/− cells did not bind α4β7 could be that CHO lec1.1−/− cells carry an additional glycosylation defect (43). Our attempts to produce gp120s in CHO lec1.1−/− cells were unsuccessful. Because of this, we cannot rule out the possibility that the production of gp120 in CHO lec1.1−/− cells is a strict requirement for efficient α4β7 binding.
HIV infection in most cases is established by one or a very few founder viruses, and the gp120s of these T/F viruses have V1-V4 domains with reduced numbers of N-linked glycosylation sites (44, 45). Based on the findings of Nawaz et al. (14) that T/F gp120s have an increased affinity for α4β7 and that this affinity is influenced by N-linked glycosylation sites in C3/V4, we tested four T/F gp120s expressed in CHO cells. In our assay, these T/F gp120s had poor or no binding to α4β7-expressing cells compared to the level of binding of AN-1gp120 or MAdCAM-1 under similar conditions. We also could not detect any specific binding of infectious virions to α4β7-expressing cells. This lack of an integrin binding capacity of native envelope trimers on infectious virions parallels the results that we obtained with purified gp120/gp140 glycoproteins and are in agreement with recent findings that anti-α4β7 integrin antibodies cannot block the infectivity of transmitted/founder and chronic subtype C HIV-1 isolates (23).
In conclusion, under optimal conditions for α4β7 binding to its natural ligand, MAdCAM-1, we detected very little or no α4β7-specific binding activity of multiple gp120s and gp140s, including gp120s from T/F strains of HIV-1. Moreover, we found no evidence that infectious HIV-1 virions produced in either PBMCs or 293T cells are capable of binding α4β7. Robust α4β7 binding assays for gp120 may require the use of special envelope glycoproteins that have specific and as yet poorly defined glycan moieties, such as those that arise in CHO lec1.1−/− cells. They may also require glycoproteins with certain N-linked glycans removed altogether (14). Except for AN-1gp120, which showed the best binding to α4β7 in our assays, we used only naturally occurring gp120s and gp140s. We encourage additional studies to clarify the possible role of the α4β7 integrin complex in direct HIV-1 infection of cells.
ACKNOWLEDGMENTS
We thank J. Arthos for AN-1gp120 and his helpful advice, Susan Barnett for SF162gp120 and TV-1gp120, and the Duke CFAR Flow Cytometry Core for the cell analyses. We also thank Christina Ochsenbauer and John Kappes for the Env.IMC.LucR viruses.
This work was supported by grant 1032144 from the Bill and Melinda Gates Foundation's Collaboration for AIDS Vaccine Discovery and by the Duke Center for AIDS Research (CFAR), an NIH-funded program (P30 AI 64518).
Footnotes
Published ahead of print 9 July 2014
REFERENCES
- 1.Desgrosellier JS, Cheresh DA. 2010. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10:9–22. 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynes RO. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687. 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- 3.DeNucci CC, Pagán AJ, Mitchell JS, Shimizu Y. 2010. Control of α4β7 integrin expression and CD4 T cell homing by the β1 integrin subunit. J. Immunol. 184:2458–2467. 10.4049/jimmunol.0902407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. 1995. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80:413–422. 10.1016/0092-8674(95)90491-3 [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Picker LJ. 1996. Lymphocyte homing and homeostasis. Science 272:60–67. 10.1126/science.272.5258.60 [DOI] [PubMed] [Google Scholar]
- 6.Campbell DJ, Butcher EC. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135–141. 10.1084/jem.20011502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770. 10.1084/jem.20041196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57–64. 10.1128/JVI.74.1.57-64.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759. 10.1084/jem.20040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haase AT. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783–792. 10.1038/nri1706 [DOI] [PubMed] [Google Scholar]
- 11.Margolis L, Shattock R. 2006. Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper' problem resolved? Nat. Rev. Microbiol. 4:312–317. 10.1038/nrmicro1387 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Xu H, Gill AF, Pahar B, Kempf D, Rasmussen T, Lackner AA, Veazey RS. 2009. Monitoring α4β7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol. 2:518–526. 10.1038/mi.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. 2008. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301–309. 10.1038/ni1566 [DOI] [PubMed] [Google Scholar]
- 14.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei D, Fauci AS, Arthos J. 2011. The genotype of early-transmitting HIV gp120s promotes α4β7-reactivity, revealing α4β7/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 7:e1001301. 10.1371/journal.ppat.1001301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang G-Y, Diwanji D, Georgiev I, Do Kwon Y, Lee D, Louder MK, Moquin S, Schmidt SD, Yang Z-Y, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang L-X, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. 10.1038/nature10696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker TN, Cimakasky LM, Coleman EM, Madison MN, Hildreth JE. 2013. Antibody against integrin lymphocyte function-associated antigen 1 inhibits HIV type 1 infection in primary cells through caspase-8-mediated apoptosis. AIDS Res. Hum. Retroviruses 29:371–383. 10.1089/aid.2011.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, Wang X, Li J, Price AA, Little DM, Zaidi M, Lyles R, Villinger F. 2011. Blocking of α4β7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J. Immunol. 186:1044–1059. 10.4049/jimmunol.1003052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H-X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 206:431–441. 10.1093/infdis/jis367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorny MK, Pan R, Williams C, Wang X-H, Volsky B, O'Neal T, Spurrier B, Sampson JM, Li L, Seaman MS, Kong X-P, Zolla-Pazner S. 2012. Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from HIV-1-infected individuals. Virology 427:198–207. 10.1016/j.virol.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura GR, Fonseca DPAJ, O'Rourke SM, Vollrath AL, Berman PW. 2012. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of α4β7 binding. PLoS One 7:e39045. 10.1371/journal.pone.0039045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez Y, Tuen M, Shen G, Nawaz F, Arthos J, Wolff MJ, Poles MA, Hioe C. 2013. Preferential HIV infection of CCR6+ Th17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands. J. Virol. 87:10843–10854. 10.1128/JVI.01838-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. 2012. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7. PLoS Pathog. 8:e1002686. 10.1371/journal.ppat.1002686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBride K, Xu Y, Baily M, Seddiki N, Suzuki K, Murray JM, Gao Y, Yan C, Cooper DA, Kelleher AD, Koelsch KK, Zaunders J. 2013. The majority of HIV type 1 DNA in circulating CD4+ T lymphocytes is present in non-gut-homing resting memory CD4+ T cells. AIDS Res. Hum. Retroviruses 29:1330–1339. 10.1089/aid.2012.0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteiro P, Gosselin A, Wacleche VS, El-Far M, Said EA, Kared H, Grandvaux N, Boulassel M-R, Routy J-P, Ancuta P. 2011. Memory CCR6+ CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin β7. J. Immunol. 186:4618–4630. 10.4049/jimmunol.1004151 [DOI] [PubMed] [Google Scholar]
- 26.Doria-Rose NA, Learn GH, Rodrigo AG, Nickle DC, Li F, Mahalanabis M, Hensel MT, McLaughlin S, Edmonson PF, Montefiori D, Barnett SW, Haigwood NL, Mullins JI. 2005. Human immunodeficiency virus type 1 subtype B ancestral envelope protein is functional and elicits neutralizing antibodies in rabbits similar to those elicited by a circulating subtype B envelope. J. Virol. 79:11214–11234. 10.1128/JVI.79.17.11214-11224.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557. 10.1073/pnas.0802203105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, Lu X, Sutherland LL, Scearce RM, Bowman C, Parks R, Chen H, Blinn JH, Lapedes A, Watson S, Xia SM, Foulger A, Hahn BH, Shaw GM, Swanstrom R, Montefiori DC, Gao F, Haynes BF, Korber B. 2013. Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1. J. Virol. 87:4185–4201. 10.1128/JVI.02297-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barouch DH, O'Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, Perry JR, Seaman MS, Carville A, Mansfield KG, Szinger JJ, Fischer W, Muldoon M, Korber B. 2010. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat. Med. 16:319–323. 10.1038/nm.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang K, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL, Jr, Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. 2013. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J. Virol. 87:1554–1568. 10.1128/JVI.00718-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. 10.1016/j.immuni.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. 10.1016/j.virol.2010.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21:527–538. 10.1016/j.immuni.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 34.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. 2009. The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:20877–20882. 10.1073/pnas.0911796106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao H-X, Sutherland LL, Xia S-M, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho ZT, Ma B-J, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353:268–282. 10.1016/j.virol.2006.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Z, Roos JW, Hildreth JE. 2000. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res. Hum. Retroviruses 16:355–366. 10.1089/088922200309232 [DOI] [PubMed] [Google Scholar]
- 37.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Förster R, Pabst O. 2008. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205:2483–2490. 10.1084/jem.20080039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, Agace WW. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202:1063–1073. 10.1084/jem.20051100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424:88–93. 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- 40.Cicala C, Arthos J, Fauci A. 2010. HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV. J. Transl. Med. 9:S2. 10.1186/1479-5876-9-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binley JM, Ban Y-EA, Crooks ET, Eggink D, Osawa K, Schief WR, Sanders RW. 2010. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J. Virol. 84:5637–5655. 10.1128/JVI.00105-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, Wood B, Nathe C, Richman D, Tomaras GD, Bibollet-Ruche F, Robinson JE, Morris L, Shaw GM, Montefiori DC, Mascola JR. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651–11668. 10.1128/JVI.01762-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Vries E, de Vries RP, Wienholts MJ, Floris CE, Jacobs M-S, van den Heuvel A, Rottier PJM, de Haan CAM. 2012. Influenza A virus entry into cells lacking sialyted N-glycans. Proc. Natl. Acad. Sci. U. S. A. 109:7457–7462. 10.1073/pnas.1200987109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019–2022. 10.1126/science.1093137 [DOI] [PubMed] [Google Scholar]
- 45.Go PE, Hewawasam G, Liao H-X, Chen H, Ping L-H, Anderson JA, Hua DC, Haynes BF, Desaire H. 2011. Characterization of glycosylation profiles of HIV-1 transmitted/founder envelopes by mass spectrometry. J. Virol. 85:8270–8284. 10.1128/JVI.05053-11 [DOI] [PMC free article] [PubMed] [Google Scholar]