ABSTRACT
Respiratory syncytial virus (RSV) is the single most important cause of serious lower respiratory tract infections in young children, yet no highly effective treatment or vaccine is available. In the present study, we investigated the effect of prophylactic treatment with the intact and F(ab′)2 forms of an anti-G protein monoclonal antibody (MAb), 131-2G, on the humoral and cellular adaptive immune responses to RSV rA2-line19F (r19F) challenge in BALB/c mice. The F(ab′)2 form of 131-2G does not decrease virus replication, but intact 131-2G does. The serum specimens for antibodies and spleen cells for memory T cell responses to RSV antigens were analyzed at 30, 45, 75, and 95 days postinfection (p.i.) with or without prior treatment with 131-2G. The ratios of Th2 to Th1 antibody isotypes at each time p.i indicated that both forms of MAb 131-2G shifted the subclass response from a Th2 (IgG1 and IgG2b) to a Th1 (IgG2A) bias. The ratio of IgG1 to IgG2A antibody titer was 3-fold to 10-fold higher for untreated than MAb-treated mice. There was also some increase in IgG (22% ± 13% increase) and neutralization (32% increase) in antibodies with MAb 131-2G prophylaxis at 75 days p.i. Treatment with 131-2G significantly (P ≤ 0.001) decreased the percentage of interleukin-4 (IL-4)-positive CD4 and CD8 cells in RSV-stimulated spleen cells at all times p.i., while the percentage of interferon gamma (IFN-γ) T cells significantly (P ≤ 0.001) increased ≥75 days p.i. The shift from a Th2- to a Th1-biased T cell response in treated compared to untreated mice likely was directed by the much higher levels of T-box transcription factor (T-bet) (≥45% versus <10%) in CD4 and CD8 T cells and lower levels of Gata-3 (≤2% versus ≥ 6%) in CD4 T cells in peptide-stimulated, day 75 p.i. spleen cells. These data show that the RSV G protein affects both humoral and cellular adaptive immune responses, and induction of 131-2G-like antibodies might improve the safety and long-term efficacy of an RSV vaccine.
IMPORTANCE The data in this report suggest that the RSV G protein not only contributes to disease but also dampens the host immune response to infection. Both effects of G likely contribute to difficulties in achieving an effective vaccine. The ability of MAb 131-2G to block these effects of G suggests that inducing antibodies similar to 131-2G should prevent disease and enhance the adaptive immune response with later RSV infection. The fact that 131-2G binds to the 13-amino-acid region conserved among all strains and that flanking sequences are conserved within group A or group B strains simplifies the task of developing a vaccine to induce 131-2G-like antibodies. If our findings in mice apply to humans, then including the 131-2G binding region of G in a vaccine should improve its safety and efficacy.
INTRODUCTION
Respiratory syncytial virus (RSV) is a leading cause of serious lower respiratory tract disease in infants and young children worldwide (1) and leads to as many as 200,000 deaths (a very rough estimate of a total that could be less or more) and 3 to 4 million hospitalizations in children <5 years of age each year (1, 2). Unfortunately, there is no safe and effective licensed vaccine to prevent RSV disease. A variety of vaccine candidates have been evaluated since the first candidate vaccine (i.e., formalin-inactivated RSV [FI-RSV] vaccine). The FI-RSV vaccine caused vaccine-enhanced illness and two deaths in young vaccinees (3–6), and since its failure, multiple live virus vaccines have been developed, as well as other vaccine platforms, including virus-like particles, peptide-based vaccines, protein subunit vaccines, and plasmid DNA-based vaccines (7–14). Many of these vaccines have been evaluated in animals, and a few have been studied in humans (13, 15). None, however, has shown sufficient promise to move toward licensure. It is likely that a better understanding of virus and host factors that contribute to both disease and protective immunity will help develop a safe and effective RSV vaccine.
Natural RSV infection induces only limited and short-term protective immunity as humans experience repeat infections throughout life (16, 17). The components of RSV that may provide protective immunity are not clearly understood, although it is clear that neutralizing antibody is an important contributor to protection from disease. Partial protection is afforded the young infant by a higher titer of maternally acquired neutralizing antibodies, the elderly by a higher titer of serum neutralizing antibodies, and high-risk infants by administration of immune prophylaxis (18–23). The increased risk of serious complications and death and prolonged virus replication in immunocompromised patients (24) suggests that cellular immunity is important to clearing infection and disease outcome.
RSV has been shown to evade immunity through a number of mechanisms, including inhibition of type I interferon responses by the NS1 and NS2 proteins (25) and modification of Toll-like receptor 4 (TLR4) and the shared component of CD14 responses by the F protein (26). It is becoming evident that the G protein also plays a substantial role in evading the host immune response to infection (27, 28) as well as inducing immune responses that contribute to disease. For example, the G protein is associated with a lower frequency of interferon gamma (IFN-γ) and a higher frequency of interleukin-5 (IL-5)- and IL-6-expressing T cells (29) and alterations in the production of cytokines in infected mice (30). Examples of G-associated host responses that contribute to disease pathogenesis include enhanced inflammation in FI-RSV-vaccinated mice challenged with RSV (29, 31) and increased pulmonary levels of the tachykinin substance P (32) and mucous production and breathing effort in RSV-challenged mice (33). The G protein also decreased respiratory rates in mice (34).
Many of these links between the G protein and RSV disease have been identified by the decrease in the disease manifestation after administration of the anti-G protein monoclonal antibody (MAb) 131-2G (35, 36). This MAb has been shown to block G-associated enhanced pulmonary inflammation and mucous production and increased breathing effort and decreased respiratory rate in the mouse model (33, 34, 37–39). Since this MAb neutralizes virus in an Fc-dependent fashion in vivo (35), the F(ab′)2 form of 131-2G does not neutralize RSV in vivo. Consequently, treatment with 131-2G F(ab′)2 provides a means to study 131-2G's effect on disease or immunity independent of virus replication (33, 37, 38).
Given the effect of G protein on disease and immune responses, we chose to investigate its effect on the adaptive immune response to RSV rA2-line19F (r19F) infection in the mouse with the intact and F(ab′)2 form of MAb 131-2G. We chose the r19F strain because in mice it induces a G-associated Th2-biased response not seen with the parent A2 strain (33, 40). The present study shows the adaptive immune response to r19F also has a substantial Th2 bias, and this bias can be switched to a Th1 bias by administration of the anti-G protein MAb 131-2G.
MATERIALS AND METHODS
Animals.
Animal studies were performed according to a protocol approved by the Emory University (Atlanta, GA) Institutional Animal Care and Use Committee. Six- to 8-week-old, specific-pathogen-free, female BALB/c mice (Charles River Laboratory, Wilmington, MA) were used in all experiments. Mice were housed in microisolator cages and fed sterilized water and food ad libitum. Mice were intraperitoneally treated with 300 μg anti-RSV G MAb 131-2G intact and F(ab′)2 forms or normal mouse IgG intact and F(ab′)2 forms (Pierce Protein Research Products, Rockford, IL). Two days later, they were challenged intranasally with 1 × 106 50% tissue culture infective doses (TCID50) of r19F in serum-free minimal essential medium (MEM) (50 μl). At each time point, five mice were examined in each group (Fig. 1).
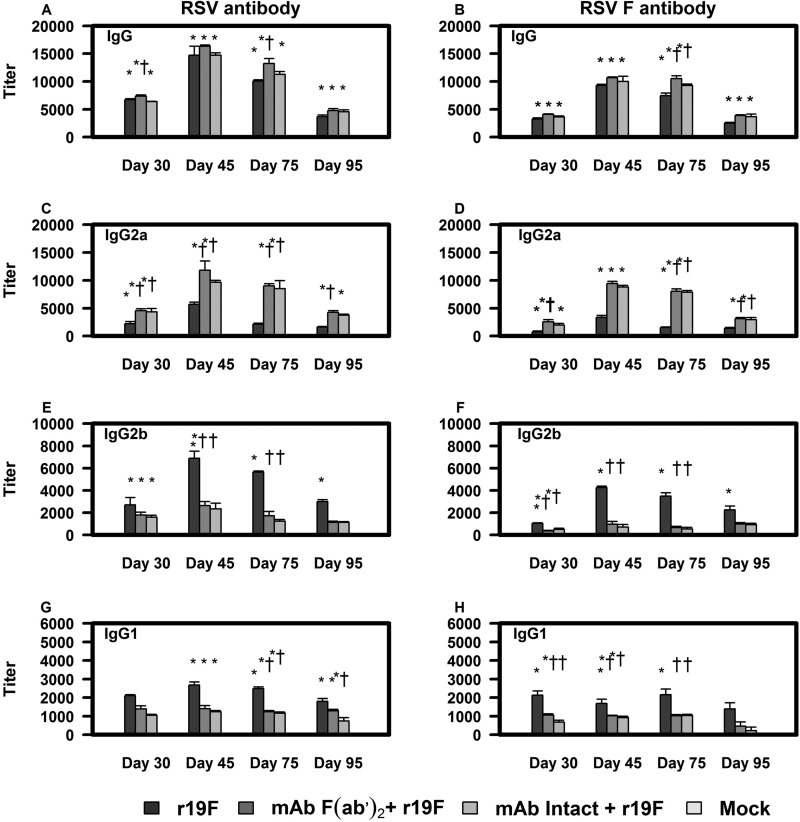
FIG 1.
Effect of MAb 131-2G prophylaxis on serum antibody responses to RSV (left column) and F protein (right column) in BALB/c mice. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock). RSV-specific antibody isotype IgG (A and B), IgG2a (C and D), IgG2b (E and F), and IgG1 (G and H) were measured with ELISA against RSV tissue culture lysate or F protein plasmid-transfected HEK 293 cells as described in Materials and Methods. Results are presented as an average of data from three separate experiments. Error bars represent the SEM from n = 5 mice per group. * or †, significant difference (P < 0.05), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-challenged mice (*) or untreated, r19F-challenged mice (†).
Virus preparation.
Briefly, r19F was propagated in HEp-2 cells at 0.01 multiplicity of infection (MOI), and at day 5 postinfection (p.i.), loosely capped flasks were transferred to a −80°C freezer overnight. After thawing at 4°C, cells were scraped down, and the cell lysates were transferred to 50-ml conical tubes. The cell debris was removed by centrifugation at 3,000 rpm for 7 min at 4°C. The supernatants were pooled and purified by centrifugation through a sucrose cushion (20% sucrose) at 16,000 × g for 4 h. After centrifugation, the supernatant and sucrose cushion were removed, and the pellet was dissolved in serum-free MEM. The viral pools were divided into aliquots, quick-frozen in liquid nitrogen, and stored at −80°C until they were used. A mock control was prepared in a similar fashion but without purification through a sucrose cushion.
Virus infectivity titers were determined by microinfectivity assays as previously described (41). Briefly, serial dilutions of the virus preparation in MEM supplemented with 5% fetal bovine serum (FBS) (Fisher, Pittsburgh, PA), 2 mM l-glutamine (Gibco, Grand Island, NY), and 5,000 U/ml penicillin-streptomycin (Gibco, Grand Island, NY) solution were inoculated onto subconfluent HEp-2 cells in 96-well microtiter plates. After 2 h of adsorption at a 37°C temperature, 180 μl of tissue culture medium was added, and 5 days later, the cells were fixed with 80% acetone in phosphate-buffered saline (PBS). Replication of virus was determined by an RSV enzyme-linked immunosorbent assay (ELISA) using plates with the acetone-fixed RSV-infected HEp-2 cells. The plates were blocked with 5% bovine serum albumin (BSA) in PBS for 1 h at 37°C, and then goat anti-RSV antibody (Millipore, Billerica, MA) was added, and the plate was incubated for 1 h at 37°C. The plates were washed with PBS containing 0.05% Tween 20 and then incubated for 1 h at 37°C with horseradish peroxidase (HRP)-conjugated donkey anti-goat antibody (Jackson ImmunoResearch, West Grove, PA). After a second washing, color was developed with o-phenylenediamine (OPD) substrate (Sigma-Aldrich, St. Louis, MO), as indicated by the manufacturer. The enzyme-substrate reaction was terminated by the addition of a sulfuric acid solution, and absorbance was measured at a wavelength of 450 nm. The Reed-Muench method (42) was used to estimate the highest dilution titer that infected 50% of wells (TCID50).
Monoclonal antibody preparation.
Monoclonal antibody 131-2G (3, 28) was purified from mouse ascitic fluid or hybridoma supernatant on a protein G column. The F(ab′)2 fragments were generated by pepsin digestion (Sigma-Aldrich). Briefly, the digested MAb was passed through a protein G-Sepharose column (GE Healthcare, Alpharetta, GA) to eliminate Fc fragments and undigested antibodies. Purified F(ab′)2 fragments were dialyzed and concentrated using a Centricon spin column (Millipore, Temecula, CA) with a 30-kDa cutoff. The purity of the F(ab′)2 fragments was determined by SDS-PAGE (Bio-Rad, Hercules, CA) under nonreducing conditions with only a band at 110 kDa detected. The band at 110 kDa indicates IgG F(ab′)2, while a band at 150 kDa would indicate intact IgG. The protein concentration was determined by the micro-bicinchoninic acid (micro-BCA) protein assay (Pierce Protein Research Products, Rockford, IL). Endotoxin concentrations were determined using a Limulus amebocyte lysate chromogenic endpoint assay, in accordance with the manufacturer's instructions (Lonza, Atlanta, GA).
Mammalian expression plasmids.
The mammalian expression plasmids under the control of a cytomegalovirus (CMV) promoter for r19F F protein-encoding genes were constructed using the pcDNA 3.1+ vector (Invitrogen, Grand Island, NY). The human codon bias-optimized line 19F cDNA (GeneArt, Invitrogen) was transferred from the provided pMArt shuttle vector to pcDNA3.1 by standard molecular biology methods. The pMArt plasmids containing the F gene and pcDNA 3.1+ vector were digested with KpnI and XhoI restriction enzymes (New England BioLabs, Ipswich, MA). Following digestion, the pcDNA 3.1 vector was dephosphorylated using Antarctic phosphatase (New England BioLabs, Ipswich, MA) and purified using the QIAquick gel extraction kit (Qiagen, Valencia, CA). The dephosphorylated pcDNA 3.1 vector and r19F F gene were ligated at a 1: 3 molar ratio using T4 DNA ligase and transformed in competent Escherichia coli DH5α cells. The bacterial colonies were selected on Luria broth agar-ampicillin plates. Single colonies were grown in LB-ampicillin medium, and plasmids were purified using the Qiagen plasmid miniprep kit (Qiagen, Valencia, CA). The positive clones were screened by KpnI and XhoI restriction endonuclease analysis, and authenticity of positive clones was confirmed by nucleotide sequence studies. The recombinant clones were grown in large volumes of LB-ampicillin, and plasmids were purified using the Qiagen EndoFree Giga kit (Qiagen, Valencia, CA). The plasmids were quantified using a Nanodrop 800 spectrophotometer (Thermo Scientific, Waltham, MA), and aliquots were frozen at −80°C until used for transfection of HEK 293cells.
Cell transfection.
Calcium phosphate coprecipitates (CaP) loaded with r19F F plasmid DNA (pDNA) were prepared according to the method described by Lindell et al. (43) with some modifications. In brief, sufficient volumes of stock solutions of pDNA (1.5 μg/ml) and CaCl2 (2 M) were mixed and incubated for 10 min at room temperature. Then the suspension was mixed with 2× Hanks buffered salt solution (HBSS) and added into MEM–5% FBS medium. The transfection suspension was lightly vortexed and inoculated onto subconfluent HEK 293 cells in 96-well microtiter plates. After 12 h of transfection at 37°C, tissue culture medium was replaced with 200 μl MEM–5% FBS, and 48 h later, the cells were fixed with 70% ethanol in PBS. The plates were used for antibody response analysis.
To determine the efficiency of F transfection in HEK 293 cells, we used the immunofluorescence staining method. Briefly, the fixed plates were blocked with 5% BSA in PBS for 1 h at 37°C, and then goat anti-RSV antibody (Millipore, Billerica, MA) was added, and the plate was incubated for 1 h at 37°C. The plates were washed with PBS containing 0.05% Tween and then incubated for 1 h at 37°C with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-goat antibody (Jackson ImmunoResearch, West Grove, PA). After a second washing, cells were stained with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Grand Island, NY) at room temperature. The cells were visualized with an AMG EVOS* fl digital inverted fluorescence microscope (Fisher Scientific, Pittsburgh, PA) with a 20× objective and analyzed using ImageJ software. Fifteen to 20 fields (20× magnification) were examined per well.
Antibody responses.
The kinetics and pattern of neutralizing and binding antibodies were determined at 30 ± 3, 45 ± 3, 75 ± 3, and 95 ± 3 days after infection for the various treatment groups. Anti-RSV IgG binding antibodies were determined by enzyme immune assay (EIA) against r19F (1 × 106 TCID50/well) lysate (or control lysate) or F protein-transfected HEK 293 cells (or control HEK 293 cells) incubated at 4°C overnight. After blocking (5% BSA in PBS) for 1 h at 37°C, 100 μl of 2-fold serial dilutions of serum samples in PBS containing 0.05% Tween 20 (PBST) was added to appropriate wells and incubated for 2 h at 37°C. Next, the plates were washed with PBST and then incubated for 1 h at 37°C with HRP-conjugated goat anti-mouse immunoglobulin (diluted 1:1,000) antibody (Thermo Scientific, Waltham, MA). After a second washing, color was developed with o-phenylenediamine (OPD) substrate (Sigma-Aldrich), as indicated by the manufacturer. The enzyme-substrate reaction was terminated by the addition of a sulfuric acid solution, and absorbance was measured at a wavelength of 450 nm. IgG isotypes IgG1, IgG2a, and IgG2b were detected with mouse anti-subtype antibodies (SouthernBiotech, Birmingham, Alabama) and HRP-conjugated goat anti-mouse immunoglobulin. Serial dilutions (starting at 1:500) of serum samples for each time points were tested by the ELISA. The cutoff value (absorbance of 0.3678) was chosen as the mean optical density (OD) + 3 standard deviations for the 1:500 dilution of serum from mock-infected mice for various IgG ELISAs. The highest dilution with absorbance above the cutoff value was estimated from a standard curve generated from standards for the various IgG ELISAs using linear regression.
The RSV neutralizing antibody titer was determined using 2-fold serially diluted heat-inactivated (56°C for 30 min) sera. Serum specimens (75 μl) were mixed with 100 TCID50 of RSV (25 μl) in MEM–5% FBS, and added to wells of a 96-well microtiter plate with HEp-2 cells (1.5 × 104/well), and incubated for 3 days in a 5% CO2 incubator at 37°C. The plates were washed with PBS, and the cells were fixed with 80% acetone followed by blocking with PBS–5% BSA blocking buffer. RSV replication was detected with goat anti-RSV antibody (Millipore, Billerica, MA) and HRP-conjugated donkey anti-goat antibody (Jackson ImmunoResearch, West Grove, PA) as noted above for determination of the infectivity titer of the virus preparation (33). Neutralization titers are expressed as the reciprocal of the serum dilution giving a 50% reduction in number of RSV-positive wells.
Preparation of splenocytes.
Mice were euthanized at the time points indicated in the figures. The spleens were removed and placed separately into complete RPMI supplemented with 10% FBS (Fisher Scientific, Pittsburgh, PA), 2 mM l-glutamine (Gibco, Grand Island, NY), 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), 1 mM HEPES (Thermo Scientific, Waltham, MA), 5,000 U/ml penicillin-streptomycin (Gibco, Grand Island, NY), and 0.5 mM sodium pyruvate (Sigma-Aldrich, St. Louis, MO). The tissues were minced and ground through a sterile steel mesh to obtain a single-cell suspension. The splenocytes were treated with red blood cell (RBC) lysing buffer (Sigma-Aldrich, St. Louis, MO), and cells were isolated by centrifugation at 3,000 × g. Cells were counted and resuspended at the stated cell concentration for the appropriate in vitro assay.
Stimulation of splenocytes with r19F.
The spleen cells (1 × 106 cells/well) were incubated for 24 h at 37°C in 96-well culture plates in the presence of mock-infected tissue culture (negative control), concanavalin A (1 μg/ml) (positive control), an r19F MOI of 0.1, or UV-inactivated r19F. The stimulated spleen cells (1 × 106 cells/well) were treated with brefeldin A (BD GolgiPlug, San Jose, CA) for last 4 h, incubated with FcR-blocking antibody (anti-CD16/CD32), and then stained for surface markers with anti-CD3, anti-CD4, and anti-CD8 (Table 1). Before intracellular staining, cells were fixed and permeabilized with CytoFix/CytoPerm (BD Biosciences, Mountain View, CA) solution and Perm/Wash buffer (BD Biosciences, Mountain View, CA). Intracellular cytokines were detected with anti-IFN-γ and anti-IL-4 (Table 1). The distribution and pattern of cell surface markers were determined for 100,000 lymphocyte-gated events analyzed on a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA), and the data were analyzed using FlowJo software (TreeStar, Ashland, OR). A significant increase in the percentage of IFN-γ- or IL-4-positive RSV-stimulated splenocytes compared to the level in mock-stimulated splenocytes was considered a positive RSV response.
TABLE 1.
Flow cytometer surface staining antibodies
| Type of staining and cell | Staining patterna | Clone | Source |
|---|---|---|---|
| Surface | |||
| CD3 | CD3 (efluor450) | 17A2 | eBioscience |
| CD4 | CD4 (efluor650NC) | GK1.5 | eBioscience |
| CD8 | CD8a (efluor605NC) | 53-6.7 | eBioscience |
| Memory T cells | |||
| Effector memory (TMEM) | Anti-mouse CD62L-PE-Cy7 | MEL-14 | eBioscience |
| Central memory (TCM) | Anti-mouse CD44-Alexa Fluor 700 | IM7 | eBioscience |
| Regulatory T cells (Tregs) | Anti-mouse CD28-PerCP-Cy5.5 | 37.51 | eBioscience |
| Anti-mouse CD25-PE-Cy7 | PC61 | eBioscience | |
| Effector T cells (Tfh) | Anti-human/mouse CD45R-Alexa Fluor 700 | RA3-6B2 | Biolegend |
| Anti-mouse CD185 (CXCR5)-PE-Cy7 | L138D7 | Biolegend | |
| Intracellular | |||
| Memory T cells | Anti-mouse IFN-γ–PE | XMG1.2 | eBioscience |
| Anti-mouse IL-4–FITC | BVD6-24G2 | eBioscience | |
| Anti-human/mouse T-bet–PerCP–Cy5.5 | 4B10 | eBioscience | |
| Anti-human/mouse Gata-3–eFluor660 | TWAJ | eBioscience | |
| Regulatory T cell (Tregs) | Anti-FoxP3-Alexa Fluor 488 | MF-14 | Biolegend |
| Anti-mouse IL-10–APC | JES5-16E3 | Biolegend | |
| Effector T cells (Tfh) | Anti-mouse Bcl-6–PE | BCL-DWN | eBioscience |
| Isotype controls | Rat Ig2b κ isotype control-APC | eB149/10H5 | eBioscience |
| Rat IgG1 isotype control-PE | eBRG1 | eBioscience | |
| Rat IgG1 κ isotype control-FITC | eBRG1 | eBioscience | |
| Rat IgG2b κ isotype control-Alexa Fluor 488 | RTK4530 | Biolegend | |
| Mouse IgG1 κ isotype control-PerCP-Cy5.5 | MOPC-21 | Biolegend | |
| Mouse IgG2b κ isotype control-eFluor 660 | MPC-11 | Biolegend |
Conjugate abbreviations: PE, phycoerythrin; PerCP, peridinin chlorophyll protein; FITC, fluorescein isothiocyanate; APC, allophycocyanin.
Stimulation of splenocytes with RSV CD4- and CD8-specific peptides.
The spleen cells (1 × 106cells/well) were incubated for 6 h at 37°C in 96-well culture plates in the presence of RSV peptides for CD4 or CD8 epitopes. The CD4 peptide is aa 183 to 197 of the A2 G protein (44), the CD8 peptide (M282–90) is aa 82 to 90 of the A2 M2 protein (45), and the ovalbumin control peptide is at aa 323 to 339 (46). The stimulated spleen cells (1 × 106 cells/well) were treated with brefeldin A (BD GolgiPlug, San Jose, CA) for the last 4 h, incubated with FcR-blocking antibody (anti-CD16/CD32), and then stained for surface markers (Table 1). Before intracellular staining, cells were fixed and permeabilized with CytoFix/CytoPerm (BD Biosciences, Mountain View, CA) solution and Perm/Wash buffer (BD Biosciences, Mountain View, CA). Intracellular cytokines were detected using designated antibodies (Table 1). The distribution and pattern of cell surface markers were determined for 100,000 lymphocyte-gated events analyzed on a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA), and data were analyzed using FlowJo software (TreeStar, Ashland, OR).
M2 tetramer staining.
The spleen cells (1 × 106 cells/well) were seeded in 96-well culture plates, incubated with FcR-blocking antibody, and stained for surface markers with anti-CD3 and anti-CD8 (Table 1) in the presence of M2 tetramer (allophycocyanin [APC]-conjugated H-2Kd RSV M2) (Beckman Coulter, Indianapolis, IN) for 30 min at 4°C. The cells were then additionally stained for intracellular IFN-γ and IL-4 as described for peptide-stimulated splenocytes. The distribution and pattern of cell surface markers were determined for 100,000 lymphocyte-gated events analyzed on a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA), and data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistical analyses.
Unless otherwise indicated, groups were compared by one-way analysis of variance (ANOVA) and post hoc Tukey's honestly significant difference (HSD) test (with P ≤ 0.05 considered statistically significant). All statistical analyses were performed using the statistical package R (R Developmental Core Team 2012). Data are shown as means ± standard errors of the means (SEM).
RESULTS
Viral load.
As previously noted, r19F consistently infects BALB/c mice, with the peak of pulmonary infection occurring on day 5 p.i. Consistent with our previous results (33, 37, 38), the F(ab′)2 form of 131-2G does not decrease virus replication, but intact 131-2G does (Table 2).
TABLE 2.
Effect of prophylactic administration of anti-RSV G protein MAb 131-2G on RSV load in the lungs of BALB/c micea
| Group | RSV titer (TCID50/g of lung)b |
||
|---|---|---|---|
| Study 1 | Study 2 | Study 3 | |
| r19F | 15,774.5 ± 1,435.1 | 37,704.5 ± 28,113.9 | 26,511.1 ± 9,733.2 |
| MAb F(ab′)2 + r19F | 15,247.2 ± 961.7 | 35,510.2 ± 34,405.4 | 25,899.6 ± 7,864.2 |
| MAb Intact + r19F | 130.1 ± 84.4* | 389.1 ± 220.9* | 208.3 ± 11.7* |
| Mock | 0 | 0 | 0 |
BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2 + r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F (r19F)- or mock-infected tissue culture material (mock) (n = 3 mice/group). Lung samples were collected on day 5 p.i., and the viral titer was determined using the Reed-Muench method (42) as described in Materials and Methods.
Data are means ± SEM. *, significant decrease (P ≤ 0.001, ANOVA) in virus titer of intact 131-2G-treated mice compared to those for 131-2G-untreated and 131-2G MAb F(ab′)2-treated mice.
The effect of MAb 131-2G prophylaxis on the antibody response.
We hypothesized that since r19F induced a Th2-biased acute response to infection, it might also induce a Th2-biased antibody response. Similarly, because both the F(ab′)2 form and the intact form of MAb 131-2G shifted the acute response to a Th1-type response (33), we thought it might also do so for the antibody response. Serum was collected from mice of various treatment groups and control mice on days 0, 30, 45, 75, and 95 p.i. All r19F-infected mice developed RSV-specific antibodies, and the mock-infected mice did not. The peak values for total IgG and for IgG subclass antibodies were detected in serum collected on day 45 p.i. (Fig. 1). There was a slightly higher titer in serum from 131-2G F(ab′)2-treated compared to untreated r19F-infected mice that was significant on days 30, 75, and 95 for both the RSV and anti-F protein antibody ELISAs (Fig. 1A and B).
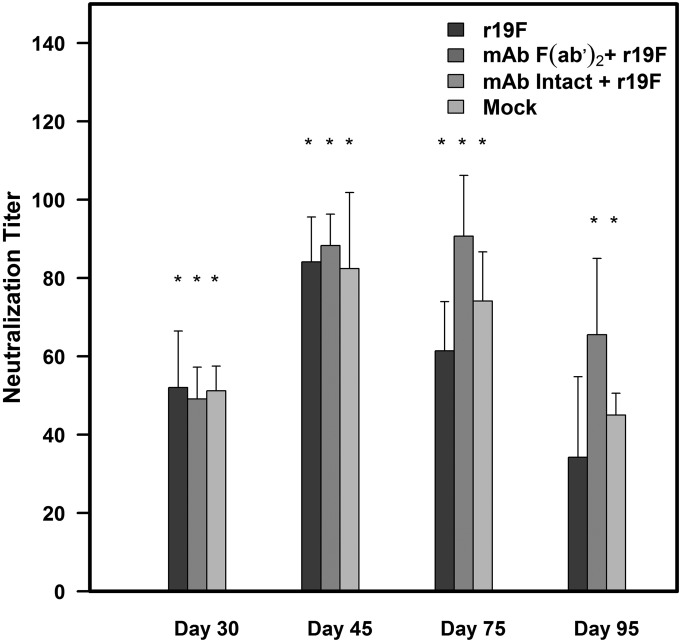
The patterns of anti-RSV (Fig. 1A, C, E, and G) and anti-F protein (Fig. 1B, D, F, and H) antibody responses were similar. For both, the subclass antibody responses showed the greatest differences between untreated and MAb-treated infected mice. As we hypothesized, r19F-infected mice had a Th2 IgG antibody subclass pattern (i.e., higher titers of IgG1 and IgG2b than IgG2a antibodies), while the 131-2G-treated mice had a Th1 pattern response (Fig. 1C, E, and G). The ratio of IgG1 to IgG2a antibody titers was 3-fold to 10-fold higher for untreated compared to F(ab′)2 MAb-treated mice. For untreated mice, this ratio was >1.0, except for day 45 p.i., when the ratios were 0.59 and 0.56 for anti-RSV and anti-F protein antibodies, respectively. The values for F(ab′)2 MAb-treated, infected mice were always <0.5 and 0.13 (anti-RSV) and 0.11 (anti-F protein) on day 45 p.i. (Table 3). Mice treated with either form of 131-2G also had higher, although not significantly higher, serum neutralizing antibody titers than untreated, r19F-infected mice at 30, 75, and 95 days p.i. (Fig. 2). Thus, binding G with MAb 131-2G shifted the antibody response from a Th2 to Th1 pattern and maintained a higher titer of antibody longer than the untreated, r19F-infected mice.
TABLE 3.
RSV-specific and RSV F protein-specific IgG1/IgG2a and IgG2b/IgG2a ratios of serum antibody responses
| Type of response and day | IgG1/IgG2a ratio |
IgG2b/IgG2a ratio |
||
|---|---|---|---|---|
| r19F | MAb F(ab′)2 + r19F | r19F | MAb F(ab′)2 + r19F | |
| RSV specific | ||||
| Day 30 | 1.09 ± 0.37 | 0.32 ± 0.14 | 1.20 ± 0.40 | 0.39 ± 0.08 |
| Day 45 | 0.56 ± 0.22 | 0.13 ± 0.05 | 1.24 ± 0.31 | 0.23 ± 0.08 |
| Day 75 | 1.68 ± 0.19 | 0.14 ± 0.01 | 2.71 ± 0.53 | 0.20 ± 0.12 |
| Day 95 | 1.11 ± 0.22 | 0.31 ± 0.08 | 1.84 ± 0.21 | 0.26 ± 0.08 |
| RSV F protein specific | ||||
| Day 30 | 3.59 ± 1.97 | 0.48 ± 0.21 | 1.80 ± 0.84 | 0.16 ± 0.07 |
| Day 45 | 0.59 ± 0.42 | 0.11 ± 0.02 | 1.36 ± 0.43 | 0.10 ± 0.06 |
| Day 75 | 1.40 ± 0.52 | 0.13 ± 0.03 | 2.23 ± 0.46 | 0.08 ± 0.02 |
| Day 95 | 1.01 ± 0.62 | 0.15 ± 0.19 | 1.65 ± 0.59 | 0.32 ± 0.09 |
FIG 2.
Effect of MAb 131-2G prophylaxis on neutralizing antibody response to r19F infection in BALB/c mice. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before infection with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock). Neutralizing antibody titers were determined at 30 ± 3, 45 ± 3, 75 ± 3, and 95 ± 3 days after infection by a microneutralization assay with r19F as described in Materials and Methods. The error bar represents the SEM from n = 5 mice per group. *, significant difference (P < 0.05), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-infected mice.
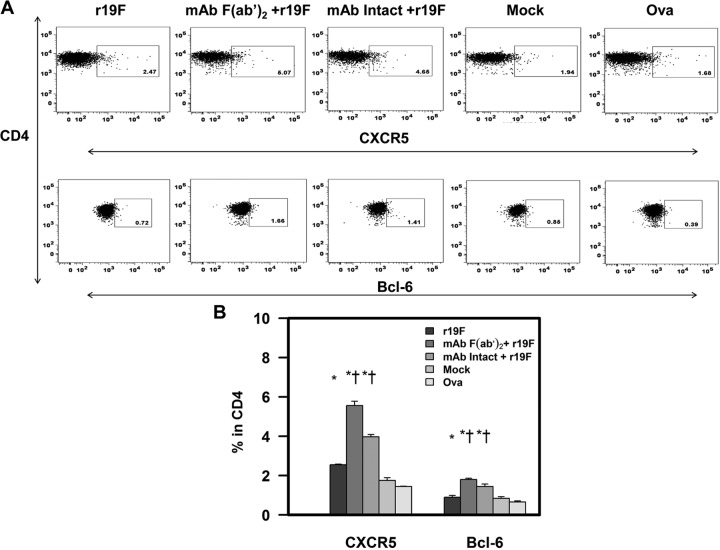
To identify possible mechanisms for the effect of 131-2G treatment on antibody responses, we studied follicular helper T cells (Tfh) induced by the various treatments. The initial antibody response is linked to antibody-secreting cells and the later antibodies to long-lived plasma cells (LLPCs) and the memory B cells. B cells develop into LLPCs and memory B cells in germinal centers (GCs). Tfh cells facilitate B cell trafficking to GCs and subsequent development into LLPCs and memory B cells in the GCs (47). Tfh cells are identified by the presence of the surface marker CXCR5 and the transcription factor Bcl6 (48, 49). The increase in the percentage of Tfh cells (i.e., CXCR5+ and Bcl6+ CD4 T cells) in peptide-stimulated, day 75 p.i. spleen cells in treated compared to untreated mice suggests a mechanism for a higher titer of antibodies late p.i. (Fig. 3). The increase in Tfh T cells might have increased the number of B cells migrating to GCs and developing into LLPCs and memory B cells.
FIG 3.
Effect of anti-RSV G protein MAb 131-2G treatment on the frequency of Tfh cells. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock) (n = 5 mice/group). Spleen cells were harvested at day 75 p.i. and stimulated with RSV-specific CD4 G peptide for 6 h. Tfh cells were determined by staining with CD4, CXCR5, and Bcl-6 antibodies. (A) Representative dot plots from flow cytometric analysis of CXCR5 and Bcl-6. (B) Percentage of CXCR5+ and Bcl-6+ CD4 T cells from corresponding dot plot quadrants. The error bar represents the SEM for n = 5 mice. * or †, significant difference (P < 0.05), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-infected mice (*) or untreated, r19F-infected mice (†). The values for ovalbumin (Ova) stimulation are for MAb-treated mice, but values are similar for all groups.
The effect of MAb 131-2G prophylaxis on CD4 and CD8 T cell responses.
The T cell response induced by RSV-stimulated splenocytes was assessed by intracellular cytokine staining. The splenocytes were stimulated with (i) an MOI of 0.1 of purified r19F RSV, UV-inactivated r19F, or mock-infected tissue culture material (negative control) or 1 μg/ml concanavalin A (positive control) or (ii) RSV major histocompatibility complex class I (MHC-I)- and MHC-II-restricted peptides or an albumin negative-control peptide antigen. We also stained unstimulated splenocytes with an RSV MCH-I tetramer. We hypothesized that the memory T cell response induced by r19F infection would be Th2 biased, and prophylaxis with the F(ab′)2 or intact form of MAb 131-2G would switch this Th2 bias to a Th1 bias.
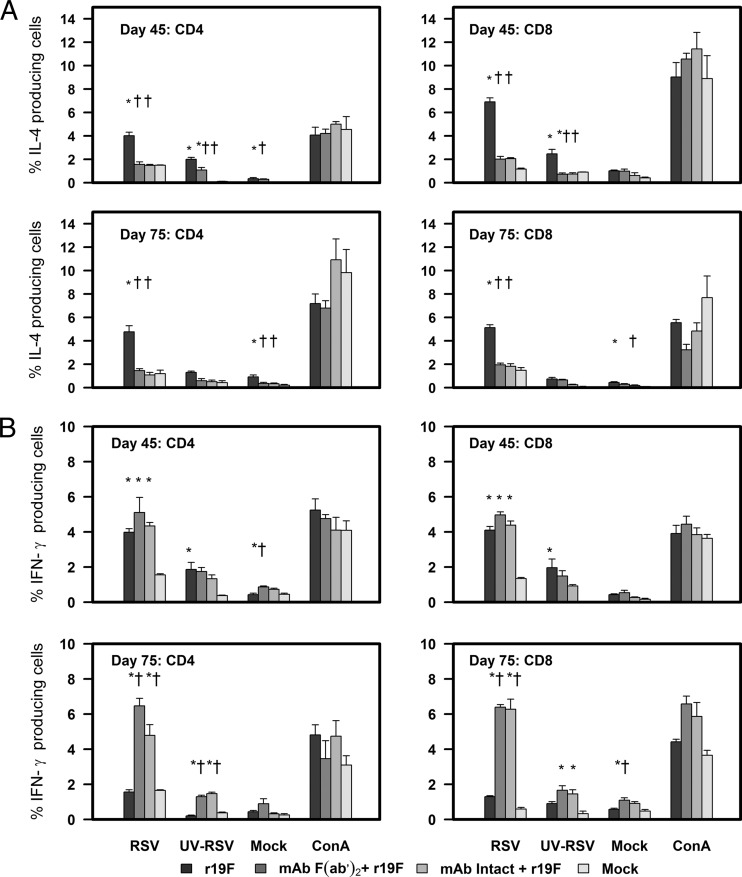
The r19F-stimulated splenocytes from days 45 and 75 p.i from untreated, infected mice had a significant increase in the percentage of IL-4-positive CD4 and CD8 T cells compared to negative controls (i.e., RSV-stimulated splenocytes from mock-infected mice or negative-control-stimulated splenocytes from RSV-infected mice). Of note, the total number of CD8 T cells was decreased in MAb-treated mice, which is consistent with previous work showing the RSV G protein can increase CD8 T cells (50). In contrast, infected mice treated prophylactically with either form of MAb 131-2G compared to untreated mice had a significantly lower percentage of IL-4-positive T cells. The percentage of IL-4-positive T cells was similar to that for the controls. Both treated and untreated infected mice had similar levels of IFN-γ expression in CD4 and CD8 T cells on day 45 p.i., but the percentage of IFN-γ-positive T cells was significantly higher for MAb-treated mice on day 75 p.i. The percentage of IFN-γ-positive T cells remained at ∼day 45 levels for MAb-treated mice and substantially decreased in untreated, r19F-infected mice (Fig. 4).
FIG 4.
Effect of anti RSV G protein MAb 131-2G treatment on the frequency of the indicated cells after RSV stimulation. (A) IL-4-positive T cells. (B) IFN-γ-positive T cells. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock) (n = 5 mice/group). Spleen cells were harvested at the indicated time points and stimulated with r19F, UV-inactivated r19F, mock-infected tissue culture, or ConA for 24 h. The error bar represents the SEM from n = 5 mice. * or †, significant difference (P < 0.05), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-infected mice (*) or untreated, r19F-infected mice (†).
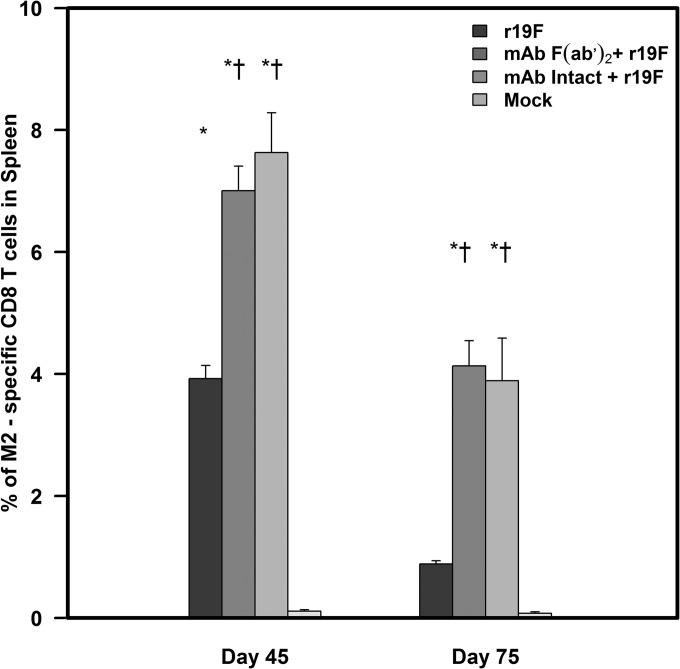
For the tetramer studies, we stained unstimulated splenocytes with the M282–90 tetramer that is specific for the immune dominant MHC class I H-2Kd restricted epitope (SYIGSINNI) of the RSV M2-1 protein (45) (Fig. 5). With MAb 131-2G prophylaxis, we noted an increase in the percentage of tetramer-positive CD8 T cells (Table 4). Although there were relatively few tetramer-positive cells, with MAb 131-2G treatment, there was a noticeable decrease (61.54% ± 21.81%) in the percentage of IL-4-positive and increase (31.42% ± 23.12%) in the percentage of IFN-γ-positive tetramer-positive CD8 T cells compared to untreated, r19F-infected mice at day 45 p.i. (Fig. 5).
FIG 5.
Enumeration of RSV M2-specific CD8+ T cells using MHC class I tetramers. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock) (n = 5 mice/group). Spleen cell suspensions obtained from the study groups were stained with anti-mouse CD3e-Alexa Fluor 700-, anti-mouse CD8-FITC-, and APC-labeled M2-specific H-2Kd tetramer. Error bars represents the SEM from n = 5 mice per group. * or†, significant difference (P < 0.05), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-infected mice (*) or untreated, r19F-infected mice (†).
TABLE 4.
Changes in the unstimulated spleen T cells after prophylaxis with intact or F(ab′)2 forms of MAb 131-2G 75 days after infectiona
| Group | %/1 × 106 splenocytes ofb: |
|||
|---|---|---|---|---|
| CD3 | CD4 | CD8 | M282–90 + CD8 | |
| r19F | 23.78 ± 1.87 | 53.86 ± 1.02 | 37.58 ± 1.00 | 0.80 ± 0.12 |
| MAb F(ab′)2 + r19F | 24.92 ± 1.19 | 62.86 ± 1.76 | 28.18 ± 0.96* | 4.12 ± 0.28 |
| MAb Intact + r19F | 24.38 ± 2.18 | 68.52 ± 0.92 | 26.22 ± 1.16* | 3.60 ± 0.46 |
| Mock infected | 25.64 ± 1.90 | 69.74 ± 2.14 | 24.44 ± 2.42 | 0.01 ± 0.04 |
BALB/c mice were treated with intact (MAb Intact + r19F) or F(ab′)2 [MAb F(ab′)2 + r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F-infected or mock-infected tissue culture material (n = 5 mice/group). The spleen cells were harvested on day 75 postchallenge.
*, significant difference (P < 0.05) as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, comparing MAb 131-2G-treated mice with untreated r19F-challenged mice.
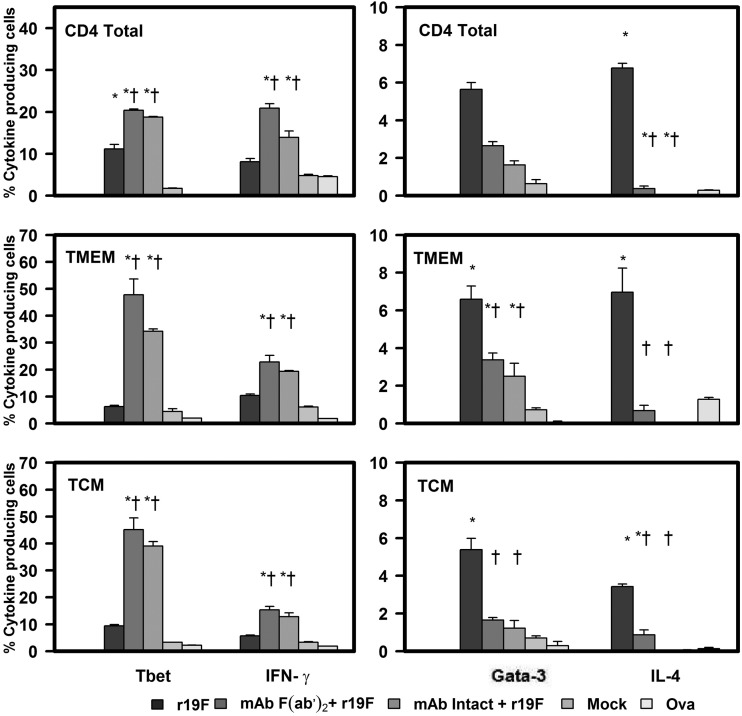
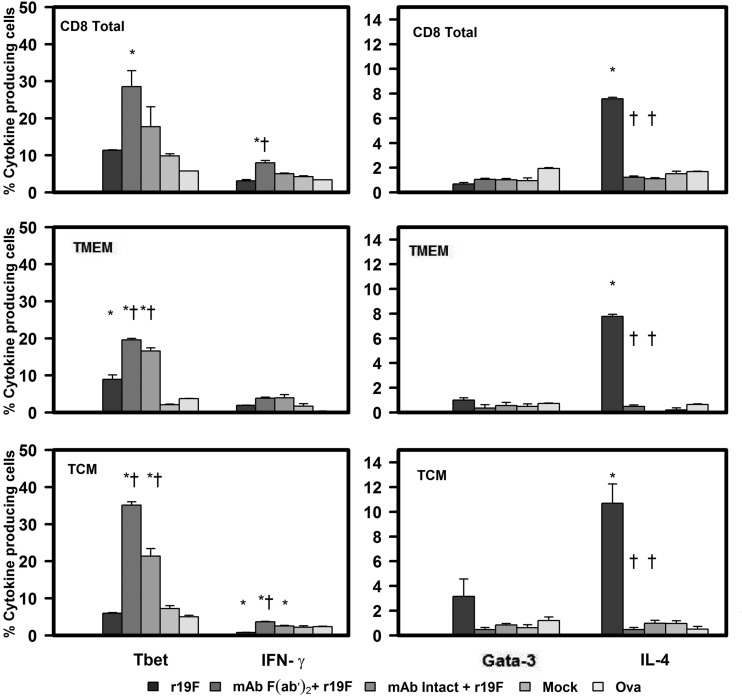
The studies of splenocytes stimulated with RSV MHC class I and II restricted peptides confirmed the findings with RSV stimulation and tetramer staining. With peptide stimulation, we also found that r19F infection induces a Th2-biased response that changes to a Th1 bias with MAb 131-2G prophylaxis. Stimulation of splenocytes collected 75 days p.i. showed the percentage of IFN-γ-positive CD4 and CD8 T cells was significantly increased in MAb-treated compared to untreated, r19F-infected mice. The most notable difference between the two groups, however, was the significant decrease in the percentage of IL-4-positive cells with MAb treatment (Fig. 5, 6, and 7).
FIG 6.
Effect of anti RSV G protein MAb 131-2G treatment on the frequency of peptide-induced T-bet and IFN-γ or Gata-3 and IL-4 secretion in CD4, TMEM, or TCM T cells. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2 + r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock) (n = 5 mice/group). Spleen cells were harvested at day 75 p.i. and stimulated with RSV G protein-specific CD4 epitope peptide or ovalbumin (Ova) control peptide. Error bars represent the SEM from n = 5 mice per group. * or †, significant difference (P ≤ 0.001), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-infected mice (*) or untreated, r19F-infected mice (†).
FIG 7.
Effect of anti-RSV G protein MAb 131-2G treatment on the frequency of peptide-induced T-bet and IFN-γ or Gata-3 and IL-4 secretion in CD8, TMEM, or TCM T cells. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before challenge with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock) (n = 5 mice/group). Spleen cells were harvested at day 75 p.i. and stimulated with the peptides or ovalbumin (Ova) control peptide. Error bar represents the SEM from n = 5 mice per group. *or †, significant difference (P ≤ 0.001), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-infected mice (*) or untreated, r19F-infected mice (†).
To understand the mechanism associated with this switch to a Th1-type response with 131-2G prophylaxis, we looked at expression of the transcription factors T-bet and Gata-3 in central memory (TCM, CD62L highCD44+), and effector memory (TEM, CD62L lowCD44+) CD4+ and CD8+ cells. T-bet regulates differentiation to the Th1 lineage by inducing lineage-restricted target genes, such as the IFN-γ and IL-12 genes (51, 52), and Gata-3 regulates differentiation to Th2 by inducing genes coding for IL-4, IL-5, and IL-13 (52–54). Peptide stimulation of day 75 p.i. splenocytes showed that the prophylactic treatment with 131-2G significantly (P ≤ 0.001) increased the percentage of T-bet+ cells, coincident with the increase in IFN-γ+, CD4 and CD8 TCM and TEM cells (Fig. 5 and 6). In contrast, the treated, r19F-infected mice had a marked decrease in Gata-3+ and IL-4+ CD4 TCM and TEM T cells. CD8 TCM and TEM T cells showed no increase in Gata-3 expression for treated or untreated r19F-infected mice (Fig. 5, 6, and 7).
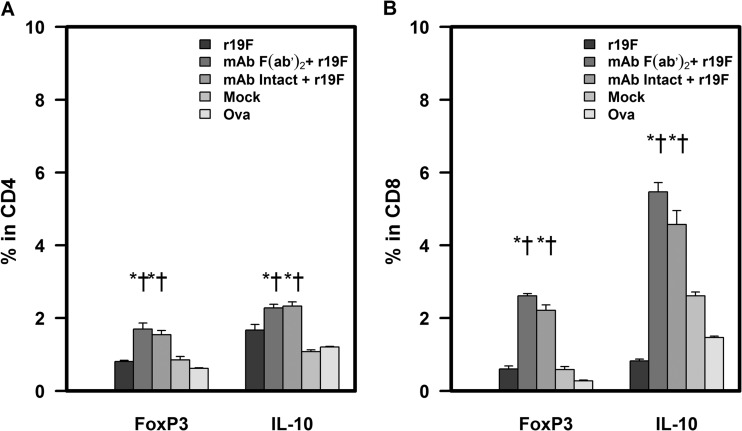
Next, we studied the regulatory T cells (Tregs), which are associated with controlling the immune-response and avoiding autoimmune- and immune response-induced disease (55, 56). During RSV infection, Treg cells limit antigen-specific T cell responses, suppress inflammation, and may help to control viral replication (57–60). Our results show that prophylaxis with MAb 131-2G slightly (P ≤ 0.05) increased the percentages of FoxP3+ and Foxp3+ IL-10+ CD4 T cells compared to untreated r19F-infected mice (Fig. 8 and 9).
FIG 8.
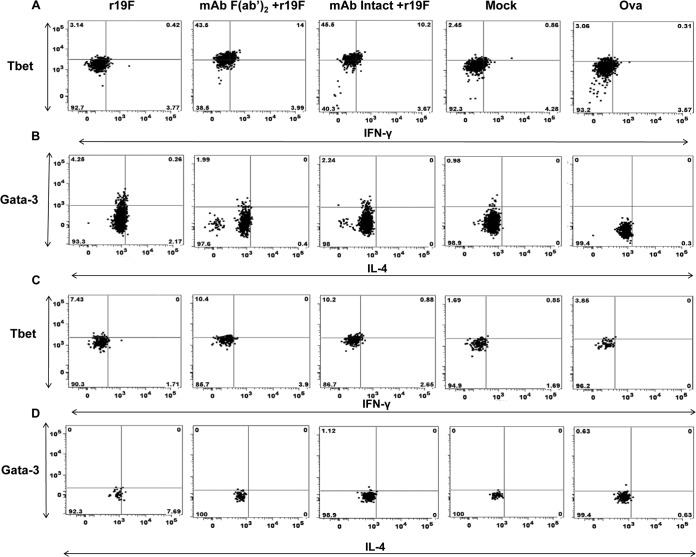
Representative dot plots from flow cytometric analysis of the effect of anti RSV G protein MAb 131-2G treatment on the frequency of T-bet and IFN-γ or Gata-3 and IL-4 secretion in TCM T cells. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before infection with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock) (n = 5 mice/group). Spleen cells were harvested at day 75 p.i. and stimulated for 6 h with (A) G peptide specific for CD4 epitope (B) M2 peptide specific for CD8 epitope. The ovalbumin (Ova) peptide is used as a negative control for the peptide stimulations.
FIG 9.
Effect of anti-RSV G protein MAb 131-2G treatment on the frequency of RSV-specific Treg cells. BALB/c mice were treated with intact (MAb Intact+r19F) or F(ab′)2 [MAb F(ab′)2+ r19F] 131-2G 2 days before infection with 1 × 106 TCID50 of r19F (r19F) or mock-infected tissue culture material (mock) (n = 5 mice/group). Spleen cells were harvested at day 75 p.i. and stimulated with CD4 peptide or ovalbumin (Ova) control peptide for 6 h. Treg cells were determined by staining with CD4, CD8, FoxP3, and IL-10 antibodies. Error bars represent the SEM from n = 5 mice per group. * or †, significant difference (P < 0.05), as determined by one-way analysis of variance (ANOVA) and post hoc Tukey's HSD test, compared with mock-challenged mice (*) or untreated, r19F-challenged mice (†).
DISCUSSION
One obstacle to developing an RSV vaccine has been the difficulty in inducing long-term protective immunity, as evidenced by the repeated infections throughout life and the incomplete protection afforded recipients of immune prophylaxis. We and others have previously shown that the G protein induces host immune responses that contribute to pulmonary inflammation, pulmonary eosinophilia, and/or weight loss during primary infection in mice or RSV-challenged FI-RSV-vaccinated mice (29, 31, 37–39). We also showed that the RSV G protein in r19F-infected mice contributes to increased pulmonary mucous production, pulmonary Th2 cytokine levels, and breathing effort, and administration of the anti-G protein MAb 131-2G blocked many of these effects (33). In the present report, we show that binding the G protein with MAb 131-2G also shifts the adaptive immune response from a Th2 to a Th1 bias and likely increases the duration of the antibody response, as indicated by higher antibody titers >75 days p.i.
The decrease in Th2-associated cytokines and antibodies was the most prominent effect of prophylactic treatment with either form of MAb 131-2G. MAb 131-2G treatment led to a Th1 pattern with higher IgG2a and lower IgG1 and IgG2b antibody titers in infected mice, while untreated infected mice had a Th2 pattern with higher IgG1 and IgG2b and lower IgG2a antibody titers. This difference in antibody subclass patterns was evident at all times studied and for both anti-RSV and anti-F protein antibodies. The shift from Th2- to Th1-biased CD4 T cell and CD8 T cell responses with MAb treatment was evident for spleen cells stimulated with purified RSV and with class I-and class II-restricted peptides and unstimulated cells stained with a class I restricted tetramer. The data for expression of the transcription factors T-bet and Gata-3 in CD4 cells and T-bet in CD8 T cells suggest these transcription factors direct the Th1 (T-bet)- or Th2 (Gata-3)-biased responses. T-bet regulates differentiation to the Th1 lineage by inducing lineage-restricted target genes, such as IFN-γ and IL-12 (51, 52), and Gata-3 regulates differentiation to Th2 by inducing genes for IL-4, IL-5, and IL-13 (52–54). The peptide-stimulated splenocytes from MAb-treated, r19F-infected mice preferentially expressed T-bet, and those from untreated, r19F-infected mice preferentially expressed Gata-3. Since T-bet in T cells helps direct B cell switching to the IgG2a subclass, the increase in T-bet with MAb treatment likely contributed to the increase in IgG2a titers. Since we did not detect Gata-3 in CD8 T cells from r19F-infected, untreated mice, it does not appear to be involved in the increase in IL-4 levels in CD8 T cells.
The switch from a Th2- to a Th1-biased response with 131-2G treatment is consistent with our earlier studies that showed 131-2G prophylactic treatment in FI-RSV-vaccinated, infected mice or in unvaccinated, r19F-infected mice shifted the pulmonary inflammatory response from a Th2 to a Th1 bias (33, 37). It also is consistent with a recent report by Jorquera et al. that described the response in mice to a nanoparticle G peptide vaccine that includes the binding site for MAb 131-2G. In this report, RSV-challenged mice vaccinated with the nanoparticle peptide vaccine compared to an intact G vaccine had a higher percentage of M282–90 tetramer-positive T cells in the lung and in the spleen (36). They also noted nanoparticle-vaccinated mice had more IFN-γ than IL-4-expressing T cells (Th1-type response) in splenocytes stimulated with the M282–90 peptide. In contrast, there were more IL-4- than IFN-γ-expressing T cells (Th2-type response) in mice vaccinated with intact G.
The suggestion of a more durable antibody response in MAb-treated mice was seen for anti-RSV antibodies, anti-RSV F protein, and RSV neutralizing antibodies. This difference was most prominent later after infection (i.e., ≥day 75 p.i.). The data on T follicular helper (Tfh) cells suggest a mechanism by which this occurred. The late antibody response is governed by long-lived plasma cells (LLPC) and memory B cells that develop in germinal centers (GCs) in secondary lymphoid organs (61). The Tfh cells play a key role in B cell trafficking to GCs and GC B cells developing into LLPCs and memory B cells. Tfh cells develop from CD4 T cells and are characterized by expression of chemokine (C-X-C motif) receptor 5 (CXCR5) and express the transcription factor Bcl-6 (47, 49, 62–64). Bcl-6-deficient mice display defective T-dependent antibody responses and have limited antibody affinity maturation due to the absence of GCs (65–67). Bcl-6 expression is also associated with decreased Gata-3 and IL-4 expression by T cells (65, 68). Our data suggest that the presence of G protein during infection (i.e., not bound by antibody) may hamper development of the T-dependent, long-lived, high-affinity antibodies.
RSV has been noted to affect a number of immune responses, including impairment of lymphoproliferation (69), decreased CD8+ T cell response by human peripheral blood mononuclear cells (PBMCs) when infected in vitro (70), increased apoptosis of peripheral lymphocytes during acute infection in infants (71), increase in IL-10 and regulatory T cells in lungs of infected mice (72–75), and altered dendritic cell stimulation of T cells in mice (76, 77) and in human PBMCs or cord blood mononuclear cells in vitro (78–80). The G protein specifically has been associated with suppressing a number of immune responses, including induction of Toll-like receptor 3 (TLR3) or 4, IFN-β (81), proinflammatory responses of lung epithelial cells (82), lymphoproliferation (83), and activation of dendritic cells (84, 85). G has also been noted to downregulate type I IFN production by inducing suppression of cytokine signaling (SOCS) (86) and to enhance cytotoxic T cell responses (87, 88).
The finding that G protein affects the Th1 versus Th2 bias of the later humoral and cellular memory responses and duration of antibody responses is novel. These effects of the G protein have not been previously noted, probably in part because few studies have assessed memory responses late after infection. In addition, unlike the more commonly used A2 virus, the r19F virus used in this study induces a strong Th2-biased response and the shift from a Th2 to a Th1 response was the most prominent finding in this study.
RSV G protein is approximately 50% conserved among predominant RSV strains, but contains two conserved regions: the cytoplasmic/transmembrane region (aa 1 to 66) and a central conserved region (CCR) from aa 148 to 198 (89, 90). Within the central conserved region of RSV G protein is a CX3C chemokine motif between aa 182 and 186 that functionally mimics the CX3C chemokine fractalkine (FKN [CX3CL1]) (91) and is one possible contributor to these effects of the G protein. The CX3CL1-CX3CR1 interaction has been found to induce chemotaxis and leukocyte migration, as well as affect proliferation and survival of immune cells. The CX3CL1-CX3CR1 interaction has been linked to a number of inflammation-associated diseases, including Crohn's disease, rheumatoid arthritis, allergic asthma, systemic lupus erythematosis, and atherosclerosis (92). In animal studies, the CX3CR1-CX3CL1 interaction has been associated sometimes with increased disease and other times with a decrease in disease. For example, in studies of LACK (Leishmania homolog of receptors for activated C kinase) antigen or ovalbumin-induced allergic pulmonary inflammation, CX3CR1−/− mice did not develop disease, while CX3CR1+/+ mice developed Th2-biased responses and disease (93). In these experiments, the presence of CX3CR1 was also associated with maintenance of both Th1 and Th2 T cells in the lungs. In a study of experimental autoimmune encephalitis (EAE), Garcia et al. (94) found that CX3CR1−/− mice developed more inflammation, more severe neurologic disease, and more IFN-γ-positive and IL-17-positive T cells than CX3CR1+/+ mice. Thus, the CX3CL1-CX3CR1 interaction has been noted to affect the type, magnitude, and maintenance of adaptive immune responses, but how this interaction affects a specific disease varies. In RSV infection, we have previously shown that G protein-CX3CR1 interaction increases inflammation in RSV-challenged FI-RSV-vaccinated mice (31), decreased migration of CX3CR1+ T cells to the lungs of infected mice (34), and decreased rate of breathing in mice given the G protein intravenously (34). We have not yet determined what, if any, role the G protein-CX3C motif plays in downregulation of adaptive immune responses.
There are other immune active sequences in this region of G, and their function would likely be blocked by 131-2G. For example, T cell epitopes that induce a Th2-biased response that includes induction of pulmonary eosinophilia and increased pulmonary IL-4, IL-5, IL-10, and IL-13 levels have been described in this region of the G protein (95–98). Polack et al. (85) noted that amino acid sequences in the G central conserved region (CCR-G) inhibited proinflammatory responses to RSV infection and inhibited Toll-like receptor (TLR) induction of cytokines. They noted that this inhibition was not affected by treatment with anti-CX3CR1 antibodies and, therefore, was not associated with binding to CX3CR1. This region of G is also associated with increased cytotoxic T cell activity in mice (87, 99). Interestingly, we noted a decrease in the percentage of CD8 compared to CD4 cells but more tetramer-positive CD8+ T cells when G is bound by 131-2G. Further study is needed to determine which regions of the G protein affect the adaptive immune response to RSV infection and the mechanisms for the effect.
The data in this report suggest that the G protein not only contributes to disease but also dampens the host immune response to infection. Both effects of G likely contribute to difficulties in achieving an effective vaccine. The ability of MAb 131-2G to block these effects of G suggests that inducing antibodies similar to 131-2G should prevent disease and enhance the adaptive immune response with later RSV infection. Enhancing the adaptive immune response to later infection should increase duration of protection. The fact that 131-2G binds to the 13-aa region conserved among all strains and flanking sequences are conserved within group A or within group B strains simplifies the task of developing a vaccine to induce 131-2G-like antibodies. If our findings in mice apply to humans, then including the 131-2G binding region of G in a vaccine should improve its safety and efficacy. Studies of the role of the G protein human disease and immune responses are needed to determine how G peptides might be best used in a vaccine.
ACKNOWLEDGMENTS
This work was supported by NIH grant 1U19AI095227 awarded to M.L.M. and L.J.A., NIH grant 1R01AI087798 to M.L.M., funding from Children's Healthcare of Atlanta, support from the Immunology core and Flow core of Emory+Children's Pediatric Research Center, and Emory Vaccinology Training grant (VTP) T32 5T32AI074492-03.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 2 July 2014
REFERENCES
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422–434 [DOI] [PubMed] [Google Scholar]
- 4.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89:405–421 [DOI] [PubMed] [Google Scholar]
- 5.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 89:449–463 [DOI] [PubMed] [Google Scholar]
- 6.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 89:435–448 [DOI] [PubMed] [Google Scholar]
- 7.Power UF, Nguyen TN, Rietveld E, de Swart RL, Groen J, Osterhaus AD, de Groot R, Corvaia N, Beck A, Bouveret-Le-Cam N, Bonnefoy JY. 2001. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J. Infect. Dis. 184:1456–1460. 10.1086/324426 [DOI] [PubMed] [Google Scholar]
- 8.Piedra PA, Cron SG, Jewell A, Hamblett N, McBride R, Palacio MA, Ginsberg R, Oermann CM, Hiatt PW. 2003. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine 21:2448–2460. 10.1016/S0264-410X(03)00098-7 [DOI] [PubMed] [Google Scholar]
- 9.Munoz FM, Piedra PA, Glezen WP. 2003. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 21:3465–3467. 10.1016/S0264-410X(03)00352-9 [DOI] [PubMed] [Google Scholar]
- 10.Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, Gorse GJ, Edelman R, Hayden FG, McElhaney JE, Neuzil KM, Nichol KL, Simoes EA, Wright PF, Sales VM. 2008. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (rsv) vaccines—nonadjuvanted vaccine or vaccine adjuvanted with alum—given concomitantly with influenza vaccine to high-risk elderly individuals. J. Infect. Dis. 198:1317–1326. 10.1086/592168 [DOI] [PubMed] [Google Scholar]
- 11.Boyoglu S, Vig K, Pillai S, Rangari V, Dennis VA, Khazi F, Singh SR. 2009. Enhanced delivery and expression of a nanoencapsulated DNA vaccine vector for respiratory syncytial virus. Nanomedicine 5:463–472. 10.1016/j.nano.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 12.Barnum SB, Subbarayan P, Vig K, Pillai S, Dennis VA. 2012. Nano-encapsulated DNA and/or protein boost immunizations increase efficiency of DNA vaccine protection against RSV. J. Nanomed. Nanotechnol. 3:132. 10.4172/2157-7439.1000132 [DOI] [Google Scholar]
- 13.Graham BS. 2011. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol. Rev. 239:149–166. 10.1111/j.1600-065X.2010.00972.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karron RA, Thumar B, Schappell E, Buchholz UJ, Collins PL. 2013. Attenuation of live respiratory syncytial virus vaccines is associated with reductions in levels of nasal cytokines. J. Infect. Dis. 207:1773–1779. 10.1093/infdis/jit089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. 2013. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 31(Suppl 2):B209–B215. 10.1016/j.vaccine.2012.11.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falsey AR. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759. 10.1056/NEJMoa043951 [DOI] [PubMed] [Google Scholar]
- 17.Henderson FW, Collier AM, Clyde WA, Denny FW. 1979. Respiratory-syncytial-virus infections, reinfections and immunity: a prospective, longitudinal study in young children. N. Engl. J. Med. 300:530–534. 10.1056/NEJM197903083001004 [DOI] [PubMed] [Google Scholar]
- 18.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 98:708–715. 10.1016/S0022-3476(81)80829-3 [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163:693–698. 10.1093/infdis/163.4.693 [DOI] [PubMed] [Google Scholar]
- 20.Lamprecht CL, Krause HE, Mufson MA. 1976. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J. Infect. Dis. 134:211–217. 10.1093/infdis/134.3.211 [DOI] [PubMed] [Google Scholar]
- 21.Luchsinger V, Piedra PA, Ruiz M, Zunino E, Martinez MA, Machado C, Fasce R, Ulloa MT, Fink MC, Lara P, Avendano LF. 2012. Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clin. Infect. Dis. 54:905–912. 10.1093/cid/cir955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stensballe LG, Ravn H, Kristensen K, Agerskov K, Meakins T, Aaby P, Simoes EA. 2009. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 123:398–403. 10.1016/j.jaci.2008.10.043 [DOI] [PubMed] [Google Scholar]
- 23.Walsh EE, Falsey AR. 2004. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J. Med. Virol. 73:295–299. 10.1002/jmv.20090 [DOI] [PubMed] [Google Scholar]
- 24.Renaud C, Xie H, Seo S, Kuypers J, Cent A, Corey L, Leisenring W, Boeckh M, Englund JA. 2013. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol. Blood Marrow Transplant. 19:1220–1226. 10.1016/j.bbmt.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swedan S, Andrews J, Majumdar T, Musiyenko A, Barik S. 2011. Multiple functional domains and complexes of the two nonstructural proteins of human respiratory syncytial virus contribute to interferon suppression and cellular location. J. Virol. 85:10090–10100. 10.1128/JVI.00413-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlender J, Walliser G, Fricke J, Conzelmann KK. 2002. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J. Virol. 76:1163–1170. 10.1128/JVI.76.3.1163-1170.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripp RA. 2004. Pathogenesis of respiratory syncytial virus infection. Viral Immunol. 17:165–181. 10.1089/0882824041310513 [DOI] [PubMed] [Google Scholar]
- 28.Kauvar LM, Harcourt JL, Haynes LM, Tripp RA. 2010. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy 2:655–661. 10.2217/imt.10.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 73:7099–7107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripp RA, Jones L, Anderson LJ. 2000. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 74:6227–6229. 10.1128/JVI.74.13.6227-6229.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. 2003. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. J. Virol. 77:9831–9844. 10.1128/JVI.77.18.9831-9844.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripp RA, Moore D, Winter J, Anderson LJ. 2000. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J. Virol. 74:1614–1622. 10.1128/JVI.74.4.1614-1622.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyoglu-Barnum S, Gaston KA, Todd SO, Boyoglu C, Chirkova T, Barnum TR, Jorquera P, Haynes LM, Tripp RA, Moore ML, Anderson LJ. 2013. A respiratory syncytial virus (RSV) anti-G protein F(ab′)2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected BALB/c mice. J. Virol. 87:10955–10967. 10.1128/JVI.01164-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripp RA, Dakhama A, Jones LP, Barskey A, Gelfand EW, Anderson LJ. 2003. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. J. Virol. 77:6580–6584. 10.1128/JVI.77.11.6580-6584.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson LJ, Bingham P, Hierholzer JC. 1988. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J. Virol. 62:4232–4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson LJ, Hierholzer JC, Stone YO, Tsou C, Fernie BF. 1986. Identification of epitopes on respiratory syncytial virus proteins by competitive binding immunoassay. J. Clin. Microbiol. 23:475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. 2010. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J. Virol. 84:9632–9636. 10.1128/JVI.00451-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao C, Radu GU, Caidi H, Tripp RA, Anderson LJ, Haynes LM. 2009. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab′)2 components mediates reduced pulmonary inflammation in mice. J. Gen. Virol. 90:1119–1123. 10.1099/vir.0.009308-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, Anderson LJ. 2009. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J. Infect. Dis. 200:439–447. 10.1086/600108 [DOI] [PubMed] [Google Scholar]
- 40.Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE, Jr, Goleniewska K, Williams JV, Collins PL, Peebles RS., Jr 2009. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J. Virol. 83:4185–4194. 10.1128/JVI.01853-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson LJ, Hierholzer JC, Bingham PG, Stone YO. 1985. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J. Clin. Microbiol. 22:1050–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 43.Lindell J, Girard P, Muller N, Jordan M, Wurm F. 2004. Calfection: a novel gene transfer method for suspension cells. Biochim. Biophys. Acta 1676:155–161. 10.1016/j.bbaexp.2003.11.016 [DOI] [PubMed] [Google Scholar]
- 44.Varga SM, Wissinger EL, Braciale TJ. 2000. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 165:6487–6495. 10.4049/jimmunol.165.11.6487 [DOI] [PubMed] [Google Scholar]
- 45.Kulkarni AB, Morse HC, III, Bennink JR, Yewdell JW, Murphy BR. 1993. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J. Virol. 67:4086–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Ruckwardt TJ, Chen M, Johnson TR, Graham BS. 2009. Characterization of respiratory syncytial virus M- and M2-specific CD4 T cells in a murine model. J. Virol. 83:4934–4941. 10.1128/JVI.02140-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621–663. 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 48.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. 2000. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192:1553–1562. 10.1084/jem.192.11.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moser B, Schaerli P, Loetscher P. 2002. CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 23:250–254. 10.1016/S1471-4906(02)02218-4 [DOI] [PubMed] [Google Scholar]
- 50.Jorquera PA, Choi Y, Oakley KE, Powell TJ, Boyd JG, Palath N, Haynes LM, Anderson LJ, Tripp RA. 2013. Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease. PLoS One 8:e74905. 10.1371/journal.pone.0074905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655–669. 10.1016/S0092-8674(00)80702-3 [DOI] [PubMed] [Google Scholar]
- 52.Yan L, Xiao-Ling S, Zheng-Yan C, Guo-Ping L, Sen Z, Zhuang C. 2013. HSP70/CD80 DNA vaccine inhibits airway remodeling by regulating the transcription factors T-bet and GATA-3 in a murine model of chronic asthma. Arch. Med. Sci. 9:906–915. 10.5114/aoms.2013.33180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook KD, Miller J. 2010. TCR-dependent translational control of GATA-3 enhances Th2 differentiation. J. Immunol. 185:3209–3216. 10.4049/jimmunol.0902544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kemp KL, Levin SD, Bryce PJ, Stein PL. 2010. Lck mediates Th2 differentiation through effects on T-bet and GATA-3. J. Immunol. 184:4178–4184. 10.4049/jimmunol.0901282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rouse BT, Sarangi PP, Suvas S. 2006. Regulatory T cells in virus infections. Immunol. Rev. 212:272–286. 10.1111/j.0105-2896.2006.00412.x [DOI] [PubMed] [Google Scholar]
- 56.Suvas S, Rouse BT. 2006. Treg control of antimicrobial T cell responses. Curr. Opin. Immunol. 18:344–348. 10.1016/j.coi.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 57.Ruckwardt TJ, Bonaparte KL, Nason MC, Graham BS. 2009. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J. Virol. 83:3019–3028. 10.1128/JVI.00036-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fulton RB, Meyerholz DK, Varga SM. 2010. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 185:2382–2392. 10.4049/jimmunol.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee DC, Harker JA, Tregoning JS, Atabani SF, Johansson C, Schwarze J, Openshaw PJM. 2010. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J. Virol. 84:8790–8798. 10.1128/JVI.00796-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durant LR, Makris S, Voorburg CM, Loebbermann J, Johansson C, Openshaw PJM. 2013. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J. Virol. 87:10946–10954. 10.1128/JVI.01295-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarlinton D, Good-Jacobson K. 2013. Diversity among memory B cells: origin, consequences, and utility. Science 341:1205–1211. 10.1126/science.1241146 [DOI] [PubMed] [Google Scholar]
- 62.McHeyzer-Williams LJ, Pelletier N, Mark L, Fazilleau N, McHeyzer-Williams MG. 2009. Follicular helper T cells as cognate regulators of B cell immunity. Curr. Opin. Immunol. 21:266–273. 10.1016/j.coi.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. 2011. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 34:947–960. 10.1016/j.immuni.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su C, Duan X, Zheng J, Liang L, Wang F, Guo L. 2013. IFN-α as an adjuvant for adenovirus-vectored FMDV subunit vaccine through improving the generation of T follicular helper cells. PLoS One 8:e66134. 10.1371/journal.pone.0066134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.King C, Tangye SG, Mackay CR.2008. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 26:741–766. 10.1146/annurev.immunol.26.021607.090344 [DOI] [PubMed] [Google Scholar]
- 66.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. 1997. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276:589–592. 10.1126/science.276.5312.589 [DOI] [PubMed] [Google Scholar]
- 67.Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RS, Rothman P, Stall AM, Pandolfi PP, Dalla-Favera R. 1997. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat. Genet. 16:161–170 [DOI] [PubMed] [Google Scholar]
- 68.Kusam S, Toney LM, Sato H, Dent AL. 2003. Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. J. Immunol. 170:2435–2441. 10.4049/jimmunol.170.5.2435 [DOI] [PubMed] [Google Scholar]
- 69.Preston FM, Beier PL, Pope JH. 1992. Infectious respiratory syncytial virus (RSV) effectively inhibits the proliferative T cell response to inactivated RSV in vitro. J. Infect. Dis. 165:819–825 [DOI] [PubMed] [Google Scholar]
- 70.Heidema J, de Bree GJ, De Graaff PM, van Maren WW, Hoogerhout P, Out TA, Kimpen JL, van Bleek GM. 2004. Human CD8(+) T cell responses against five newly identified respiratory syncytial virus-derived epitopes. J. Gen. Virol. 85:2365–2374. 10.1099/vir.0.80131-0 [DOI] [PubMed] [Google Scholar]
- 71.Roe MF, Bloxham DM, White DK, Ross-Russell RI, Tasker RT, O'Donnell DR. 2004. Lymphocyte apoptosis in acute respiratory syncytial virus bronchiolitis. Clin. Exp. Immunol. 137:139–145. 10.1111/j.1365-2249.2004.02512.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loebbermann J, Schnoeller C, Thornton H, Durant L, Sweeney NP, Schuijs M, O'Garra A, Johansson C, Openshaw PJ. 2012. IL-10 regulates viral lung immunopathology during acute respiratory syncytial virus infection in mice. PLoS One 7:e32371. 10.1371/journal.pone.0032371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, Varga SM. 2011. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J. Immunol. 187:3145–3154. 10.4049/jimmunol.1100764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fulton RB, Meyerholz DK, Varga SM. 2010. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 185:2382–2392. 10.4049/jimmunol.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun J, Cardani A, Sharma AK, Laubach VE, Jack RS, Muller W, Braciale TJ. 2011. Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus. PLoS Pathog. 7:e1002173. 10.1371/journal.ppat.1002173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez PA, Prado CE, Leiva ED, Carreno LJ, Bueno SM, Riedel CA, Kalergis AM. 2008. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 105:14999–15004. 10.1073/pnas.0802555105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuchida T, Matsuse H, Fukahori S, Kawano T, Tomari S, Fukushima C, Kohno S. 2012. Effect of respiratory syncytial virus infection on plasmacytoid dendritic cell regulation of allergic airway inflammation. Int. Arch. Allergy Immunol. 157:21–30. 10.1159/000324676 [DOI] [PubMed] [Google Scholar]
- 78.Le Nouen C, Hillyer P, Munir S, Winter CC, McCarty T, Bukreyev A, Collins PL, Rabin RL, Buchholz UJ. 2010. Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells. PLoS One 5:e15017. 10.1371/journal.pone.0015017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. 2003. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology 109:49–57. 10.1046/j.1365-2567.2003.01629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chi B, Dickensheets HL, Spann KM, Alston MA, Luongo C, Dumoutier L, Huang J, Renauld JC, Kotenko SV, Roederer M, Beeler JA, Donnelly RP, Collins PL, Rabin RL. 2006. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J. Virol. 80:5032–5040. 10.1128/JVI.80.10.5032-5040.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shingai M, Azuma M, Ebihara T, Sasai M, Funami K, Ayata M, Ogura H, Tsutsumi H, Matsumoto M, Seya T. 2008. Soluble G protein of respiratory syncytial virus inhibits Toll-like receptor 3/4-mediated IFN-beta induction. Int. Immunol. 20:1169–1180. 10.1093/intimm/dxn074 [DOI] [PubMed] [Google Scholar]
- 82.Arnold R, Konig B, Werchau H, Konig W. 2004. Respiratory syncytial virus deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology 330:384–397. 10.1016/j.virol.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 83.Ray R, Hoft DF, Meyer K, Brown R, Lagging LM, Belshe RB. 2001. Immunoregulatory role of secreted glycoprotein G from respiratory syncytial virus. Virus Res. 75:147–154. 10.1016/S0168-1702(01)00237-4 [DOI] [PubMed] [Google Scholar]
- 84.Johnson TR, McLellan JS, Graham BS. 2012. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J. Virol. 86:1339–1347. 10.1128/JVI.06096-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polack FP, Irusta PM, Hoffman SJ, Schiatti MP, Melendi GA, Delgado MF, Laham FR, Thumar B, Hendry RM, Melero JA, Karron RA, Collins PL, Kleeberger SR. 2005. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc. Natl. Acad. Sci. U. S. A. 102:8996–9001. 10.1073/pnas.0409478102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oshansky CM, Zhang W, Moore E, Tripp RA. 2009. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol. 4:279–297. 10.2217/fmb.09.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bukreyev A, Serra ME, Laham FR, Melendi GA, Kleeberger SR, Collins PL, Polack FP. 2006. The cysteine-rich region and secreted form of the attachment G glycoprotein of respiratory syncytial virus enhance the cytotoxic T-lymphocyte response despite lacking major histocompatibility complex class I-restricted epitopes. J. Virol. 80:5854–5861. 10.1128/JVI.02671-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melendi GA, Bridget D, Monsalvo AC, Laham FF, Acosta P, Delgado MF, Polack FP, Irusta PM. 2011. Conserved cysteine residues within the attachment G glycoprotein of respiratory syncytial virus play a critical role in the enhancement of cytotoxic T-lymphocyte responses. Virus Genes 42:46–54. 10.1007/s11262-010-0545-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson PR, Olmsted RA, Prince GA, Murphy BR, Alling DW, Walsh EE, Collins PL. 1987. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J. Virol. 61:3163–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sullender W. 1995. Antigenic analysis of chimeric and truncated G proteins of respiratory syncytial virus. Virology 209:70–79 [DOI] [PubMed] [Google Scholar]
- 91.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2:732–738. 10.1038/90675 [DOI] [PubMed] [Google Scholar]
- 92.D'Haese JG, Demir IE, Friess H, Ceyhan GO. 2010. Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin. Ther. Targets 14:207–219. 10.1517/14728220903540265 [DOI] [PubMed] [Google Scholar]
- 93.Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, Magnan A, Dombrowicz D, Glaichenhaus N, Julia V. 2010. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat. Med. 16:1305–1312. 10.1038/nm.2253 [DOI] [PubMed] [Google Scholar]
- 94.Garcia J, Pino PA, Mizutani M, Cardona SM, Charo IF, Ransohoff RM, Forsthuber TG, Cardona AE. 2013. Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J. Immunol. 191:1063–1072. 10.4049/jimmunol.1300040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Becker Y. 2006. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy—a review. Virus Genes 33:235–252. 10.1007/s11262-006-0064-x [DOI] [PubMed] [Google Scholar]
- 96.Sparer TE, Matthews S, Hussell T, Rae AJ, Garcia-Barreno B, Melero JA, Openshaw PJM. 1998. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp. Med. 187:1921–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tebbey P, Hagen M, Hancock G. 1998. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 188:1967–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elliott MB, Pryharski KS, Yu Q, Boutilier LA, Campeol N, Melville K, Laughlin TS, Gupta CK, Lerch RA, Randolph VB, LaPierre NA, Dack KM, Hancock GE. 2004. Characterization of recombinant respiratory syncytial viruses with the region responsible for type 2 T-cell responses and pulmonary eosinophilia deleted from the attachment (G) protein. J. Virol. 78:8446–8454. 10.1128/JVI.78.16.8446-8454.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melendi GA, Bridget D, Monsalvo AC, Laham FF, Acosta P, Delgado MF, Polack FP, Irusta PM. 2011. Conserved cysteine residues within the attachment G glycoprotein of respiratory syncytial virus play a critical role in the enhancement of cytotoxic T-lymphocyte responses. Virus Genes 42:46–54. 10.1007/s11262-010-0545-9 [DOI] [PMC free article] [PubMed] [Google Scholar]