ABSTRACT
Whether NF-κB promoter transactivation by the human T-cell leukemia virus type 1 (HTLV-1) Tax protein requires Tax SUMOylation is still a matter of debate. In this study, we revisited the role of Tax SUMOylation using a strategy based on the targeting of Ubc9, the unique E2 SUMO-conjugating enzyme. We show that either a catalytically inactive form of Ubc9 (Ubc9-C93S) or Ubc9 small interfering RNA (siRNA) dramatically reduces Tax conjugation to endogenous SUMO-1 or SUMO-2/3, demonstrating that as expected, Tax SUMOylation is under the control of the catalytic activity of Ubc9. We further report that a non-SUMOylated Tax protein produced in 293T cells is still able to activate either a transfected or an integrated NF-κB reporter promoter and to induce expression of an NF-κB-regulated endogenous gene. Importantly, blocking Ubc9 activity in T cells also results in the production of a non-SUMOylated Tax that is still fully functional for the activation of a NF-κB promoter. These results provide the definitive evidence that Tax SUMOylation is not required for NF-κB-driven gene induction.
IMPORTANCE Human T-cell leukemia virus type 1 is able to transform CD4+ T lymphocytes. The viral oncoprotein Tax plays a key role in this process by promoting cell proliferation and survival, mainly through permanent activation of the NF-κB pathway. Elucidating the molecular mechanisms involved in NF-κB pathway activation by Tax is therefore a key issue to understand HTLV-1-mediated transformation. Tax SUMOylation was initially proposed to be critical for Tax-induced NF-κB promoter activation, which was challenged by our later observation that a low-level-SUMOylated Tax mutant was still functional for activation of NF-κB promoters. To clarify the role of Tax SUMOylation, we set up a new approach based on the inhibition of the SUMOylation machinery in Tax-expressing cells. We show that blocking the SUMO-conjugating enzyme Ubc9 abolishes Tax SUMOylation and that a non-SUMOylated Tax still activates NF-κB promoters in either adherent cells or T cells.
INTRODUCTION
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia/lymphoma (ATLL), a highly aggressive malignant proliferation of CD4+ T lymphocytes. About 5% of HTLV-1-infected individuals develop ATLL, and this cancer occurs after a prolonged latency period (1). The oncogenic capacity of HTLV-1 is mainly due to the regulatory Tax protein, which promotes permanent T-cell proliferation through various mechanisms (2). In particular, Tax promotes permanent activation of the NF-κB pathway (3), inducing thereby the expression of a large series of proteins governing cell growth and survival (4). Importantly, Tax-induced NF-κB activation was shown to be critical for HTLV-1-induced immortalization of primary CD4+ T lymphocytes (5).
In previous studies, we and others demonstrated that Tax is conjugated to ubiquitin (Ub), notably to K63-linked Ub chains, and to either SUMO-1 or SUMO-2/3 molecules (6–12). In an initial study based on the analysis of a set of ubiquitin- and SUMO-deficient lysine Tax mutants fused or not to either Ub or SUMO, we concluded that the two modifications were each required for NF-κB activation. Indeed, we proposed that Tax ubiquitination is required for the activation of the cytoplasmic IκB kinase (IKK) complex and that Tax SUMOylation facilitates promoter activation in the nucleus (7). However, our subsequent finding that a Tax mutant that was properly ubiquitinated but poorly SUMOylated was still transcriptionally active supported the opposite view that Tax SUMOylation could be dispensable for NF-κB promoter activation (12). In contrast with this conclusion, other groups then argued that even a low level of Tax SUMOylation might be critical for NF-κB promoter activation by Tax (13–15), by acting through a threshold effect (14), as reported in other models (16).
To further explore the importance of Tax SUMOylation to NF-κB activation, we designed a new strategy to generate a totally non-SUMOylated Tax without introducing mutations in Tax. We took advantage of the fact that SUMOylation relies on a unique E2-conjugating enzyme, namely, Ubc9 (16, 17), allowing easy inhibition of the SUMOylation process. We found that blocking either the activity or the expression of endogenous Ubc9 allows the production of a non-SUMOylated Tax and that this non-SUMOylated Tax is still able to activate NF-κB promoters in both adherent cells and T cells.
MATERIALS AND METHODS
Cell culture and transfection.
293T cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies, France) supplemented with 10% fetal bovine serum, 2 mM glutamine, and antibiotics (Life Technologies, France). The 293T cell lines containing an integrated NF-κB reporter construct were generated following transfection of the pGL4.32 plasmid (see below) and hygromycin selection. These cells were cultured in the same medium as 293T cells with addition of 200 μg/ml of hygromycin. 293T cells were transfected using either the calcium phosphate procedure (luciferase assays) or the Lipofectamine reagent (nickel pulldown experiments) according to the manufacturer's procedure. The HTLV-1-negative MOLT4 T cells were cultured in RPMI 1640 medium (Life Technologies, France) supplemented as described above along with 0.5% glucose. MOLT4 T cells were transfected using the DMRIE-C reagent (Life Technologies, France) according to the manufacturer's procedure.
Plasmids and siRNA.
The Tax, Tax-6His, and Tax-M22 constructs cloned into the pSG5m empty vector and the HA-SUMO-3 and HA-Ub constructs have been described previously (7). The His-SUMO-3 plasmid was kindly provided by V. Lallemand-Breitenbach (INSERM UMR 944, Paris, France). The T7-Ubc9-C93S, which encodes a T7-tagged version of an Ubc9 mutant in which a cysteine residue of the active site was changed to a serine (17), was kindly provided by S. Nisole (INSERM UMR-S 747, Paris, France). The pGL4.32 plasmid, which contains the Luc2P gene under the control of five κB response elements and a hygromycin resistance gene, and the pRL-TK normalization plasmid, which encodes the Renilla luciferase under the control of the constitutive thymidine kinase promoter, were obtained from Promega (France). Control (siRNA-A, sc-37007) and Ubc9 (sc-36773) small interfering RNAs (siRNAs) were from Santa Cruz (Heidelberg, Germany).
Antibodies.
The following primary antibodies were used: anti-SUMO-1 (no. 4930S) and anti-Ub-K63 (no. 5621S) (Cell Signaling, Ozyme, France), anti-SUMO-2/3 (ab3742; Abcam, France), anti-Ubc9 (sc-10759; Santa Cruz, Heidelberg, Germany), and antihemagglutinin (anti-HA) (12CA5; Roche, France). Tax was detected with a serum obtained from an HTLV-1-infected individual. Horseradish peroxidase (HRP)-conjugated anti-human, anti-mouse, and anti-rabbit IgGs (Promega, France) were used as secondary antibodies in Western blotting.
Nickel pulldown experiments.
293T cells (3.5 × 106 cells seeded the day before in a 100-mm dish) and MOLT4 T cells (1.5 × 106 cells/well in a 6-well culture plate) were transfected with 3 μg of Tax-6His construct and either the control or Ubc9-C93S construct (3 μg) or the control or Ubc9 siRNA (50 nM). At 48 h or 72 h posttransfection, cells were lysed under reducing and highly stringent conditions in buffer A (6 M guanidium HCl, 0.1 M NaH2PO4, 10 mM imidazole, pH 8) and incubated with 25 μl of Ni2+-nitrilotriacetic acid (NTA) beads (Sigma, France) for 3 h at room temperature. The beads were washed three times in buffer A, then twice in buffer B (buffer A diluted 1:4 in buffer C), and finally twice in buffer C (25 mM Tris-HCl [pH 6.8], 10 mM imidazole). Bound proteins were eluted by adding 2× Laemmli buffer (Sigma, France), and purified proteins were analyzed by Western blotting as described below.
Western blotting.
Cells were lysed on ice for 20 min in radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-HCl [pH 8], 1% NP-40, 0.5% deoxycholate, 0.1% SDS, and 150 mM NaCl) supplemented with protease and phosphatase inhibitors (Roche, France), and lysates were centrifuged at 4°C for 15 min at 14,000 × g. Total proteins or proteins purified by nickel pulldown were mixed with 2× Laemmli buffer (Sigma, France), subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were then blocked in 5% nonfat dry milk and incubated with primary and secondary antibodies. Chemiluminescence reagents (Thermo Scientific, France) were finally used for signal detection.
Luciferase-based reporter assays.
293T cells (3 × 104 cells/well seeded the day before in 24-well plates) and MOLT4 T cells (4 × 105 cells/well in 24-well plates) were transfected with 500 ng of Tax-6His and with either the control or Ubc9-C93S construct (500 ng) or the control or Ubc9 siRNA (50 nM). After a 48-hour period of culture, cells were subjected to a second round of transfection with 200 ng of the pGL4.32 plasmid and the pRL-TK normalization plasmid for 293T cells (5 ng) and MOLT4 (20 ng) and with only the pRL-TK plasmid (5 ng) for the pGL4.32 stable 293T cell lines. Luciferase activity was quantified at 24 h after the second transfection using the dual-luciferase reporter assay system (Promega, France), and values were normalized with Renilla activity.
RNA extraction and real-time quantitative PCR (RT-qPCR).
Total RNAs were prepared with the NucleoSpin RNAII kit (Macherey-Nagel, France), and 1 μg of RNA was reverse transcribed using the Maxima first-strand cDNA synthesis kit (Thermo Scientific, France), according to the manufacturer's procedure. Real-time PCR was performed in a LightCycler 2.0 (Roche, France) on 10 ng of reverse-transcribed RNA using the real-time-ready ICAM-1 primers (no. 04685105001; Roche, France) and primers for Tax (forward, 5′TTCCCAGGGTTTGGACAGAG3′; reverse, 5′GATGGGGTCCCAGGTGATCT3′) and for the hypoxanthine phosphoribosyltransferase (HPRT) housekeeping gene (forward, 5′TGACACTGGCAAAACAATGCA3′; reverse, 5′GGTCCTTTTCACCAGCAAGCT3′) (used for normalization). ICAM-1 PCR was conducted using the TaqMan technology with the following conditions: a first step of denaturation at 95°C for 10 min, followed by 45 cycles of denaturation (95°C for 10 s), annealing (60°C for 30 s), and extension (72°C for 1 s) and a final extension at 72°C for 5 min. The Sybr green technology was employed for amplification of Tax and HPRT with the following conditions: a first step of denaturation at 95°C for 8 min, followed by 40 cycles of denaturation (95°C for 0 s), annealing (60°C for 10 s), and extension (72°C for 8 s) and a final melting curve (95°C for 0 s, then 65°C for 15 s, and finally 95°C for 60 s).
RESULTS
Blocking Ubc9 abolishes Tax SUMOylation.
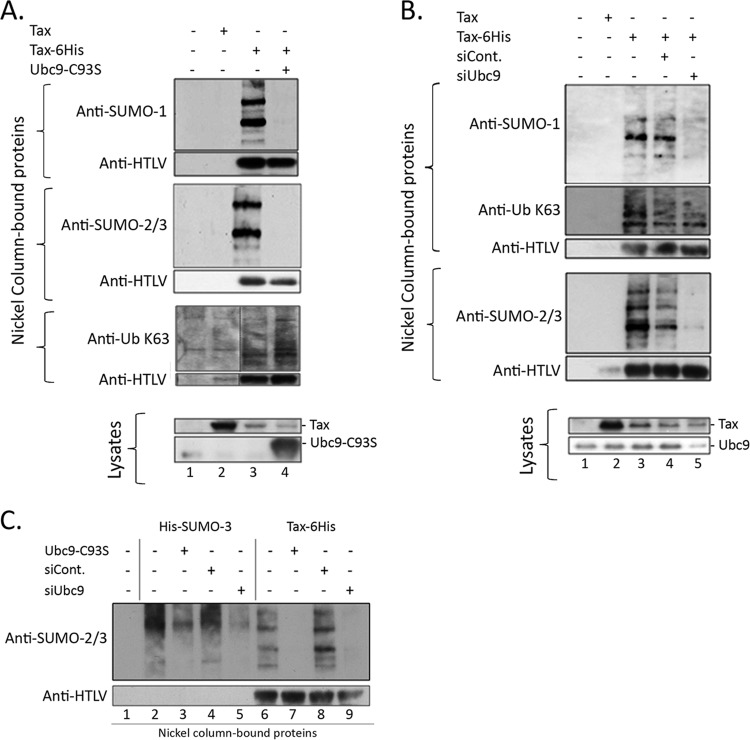
As a strategy to abolish Tax SUMOylation, we evaluated the effect of either blocking or silencing Ubc9, the unique SUMO E2-conjugating enzyme. To block the function of endogenous Ubc9, we used a construct producing a dominant negative mutant (T7-Ubc9-C93S) shown to be able to block endogenous SUMOylation (17). For Ubc9 silencing, Tax-6His was transfected together with anti-Ubc9 siRNA. 293T cells were transfected with the Tax-6His construct in the presence of the Ubc9 mutant or Ubc9 siRNA, and the level of endogenous Tax SUMOylation was analyzed by nickel pulldown performed under highly stringent conditions followed by Western blotting using anti-SUMO or, as control, antiubiquitin antibodies, as described previously (7). SUMO-1- or SUMO-2/3-conjugated Tax species were found in cells transfected with Tax-6His (Fig. 1A and B, lanes 3) but not in those transfected with the untagged Tax construct (Fig. 1A and B, lanes 2) or the control plasmid (lanes 1), validating the experiment. Expressing Tax-6His together with Ubc9-C93S lowered Tax conjugation to either SUMO-1 or SUMO-2/3 to background levels, while the amount of either unconjugated Tax or K63-linked ubiquitinated Tax was not affected (Fig. 1A, lane 4). Strong reduction of Ubc9 was observed in cells treated with the Ubc9 siRNA compared to control cells (Fig. 1B, lysates, lanes 4 and 5). This coincided with a massive reduction of Tax conjugation to either SUMO-1 or SUMO-2/3 with no effect on Tax K63 ubiquitination (Fig. 1B, lane 5). Parallel experiments showed a massive reduction of conjugation of total proteins to transfected His-SUMO-3 upon either expression of Ubc9-C93S or treatment with siUbc9 (Fig. 1C, lanes 3 and 5), confirming the blockage of the endogenous SUMOylation machinery.
FIG 1.
Endogenous Tax SUMOylation is dramatically reduced upon Ubc9 inhibition. Nickel pulldown and Western blot analyses were performed to study the effect of Ubc9 inhibition or silencing on endogenous Tax SUMOylation. (A) 293T cells were transfected with the Tax-6His construct and with the control or T7-Ubc9-C93S construct. Cells were lysed at 2 days posttransfection, and total proteins were either purified by nickel pulldown (nickel column-bound proteins) or directly analyzed by Western blotting to study Ubc9 and Tax expression (lysates). For nickel column-purified proteins, three separate gels were prepared that were blotted successively with the anti-SUMO-1 and HTLV-1 serum, the anti-SUMO-2/3 and HTLV-1 serum, or the anti-Ub-K63 and HTLV-1 serum. (B) 293T cells were transfected with the Tax-6His construct and with the control or Ubc9 siRNA, and total proteins were prepared as described above. For nickel column-purified proteins, two separate gels were prepared that were blotted successively with the anti-SUMO-1, anti-Ub-K63, and HTLV-1 serum or with the anti-SUMO-2/3 and HTLV-1 serum. (C) Nickel pulldown experiment to study the effect of Ubc9 inhibition or silencing on the SUMOylation of total proteins. 293T cells were transfected with either a His-SUMO-3 or the Tax-6His construct in the presence or absence of T7-Ubc9-C93S or siUbc9. Cells were lysed at 2 days posttransfection, and total proteins purified by nickel pulldown were analyzed by Western blotting using successively an anti-SUMO-2/3 antibody or the HTLV serum.
These data show that Tax SUMOylation is under the control of Ubc9 and that blocking or silencing Ubc9 is a valuable strategy for generating an ubiquitinated but not SUMOylated Tax protein without mutating Tax.
A non-SUMOylated Tax is able to activate a transfected or integrated NF-κB reporter gene.
Luciferase-based reporter assays to evaluate NF-κB activation by non-SUMOylated Tax were performed in 293T cells. Cells were transfected with the Tax-6His construct and either the Ubc9-C93S construct or siUbc9 and cultivated for a 48-hour period, ensuring inhibition of Tax SUMOylation, as shown in Fig. 1. Cells were then subjected to a second round of transfection with the reporter plasmids, and luciferase activities were assessed after an additional 24-hour period. Similar levels of NF-κB promoter activation were found in cells producing Tax-6His alone (set to 100%) or in combination with Ubc9-C93S (Fig. 2A, left side). Moreover, higher NF-κB promoter activation was found in cells treated with the Ubc9 siRNA than in control cells (Fig. 2B, left side). To confirm these data, experiments were performed using two 293T cell lines in which the κB reporter construct was integrated into chromatin (Fig. 2C and D). As above, Ubc9-C93S had no effect on Tax-induced NF-κB promoter activation (Fig. 2C, left side) while higher activation was observed with the Ubc9 siRNA (Fig. 2D, left side). Western blot analyses performed in parallel confirmed similar levels of Tax expression under all conditions as well as strong expression of Ubc9-C93S (Fig. 2A and C, right sides) and potent silencing of Ubc9 upon siRNA treatment (Fig. 2B and D, right sides).
FIG 2.
Non-SUMOylated Tax activates both transfected and integrated NF-κB reporter constructs. Luciferase reporter assays were performed in 293T cells to analyze the ability of non-SUMOylated Tax to transactivate either a transfected (A and B) or an integrated (C and D) κB reporter construct. (A and B) 293T cells were transfected with the Tax-6His construct and either the control or T7-Ubc9-C93S construct (A) or the control or Ubc9 siRNA (B) together with the pGL4.32 (κB-Luc2P) construct and the pRL-TK normalization plasmid. (C and D) 293T cells containing the integrated κB-Luc2P reporter construct were transfected with the Tax-6His construct and either the control or T7-Ubc9-C93S plasmid (C) or the control or Ubc9 siRNA (D) together with only the pRL-TK normalization plasmid. Data represent the Luc2P/Renilla ratio normalized to that of Tax alone (100%) and are the means and standard deviations from two independent experiments performed in duplicates. The outline of each experiment and the Western blot analyses showing Tax and Ubc9 expression are included in all panels.
A non-SUMOylated Tax is able to activate an NF-κB-dependent endogenous gene.
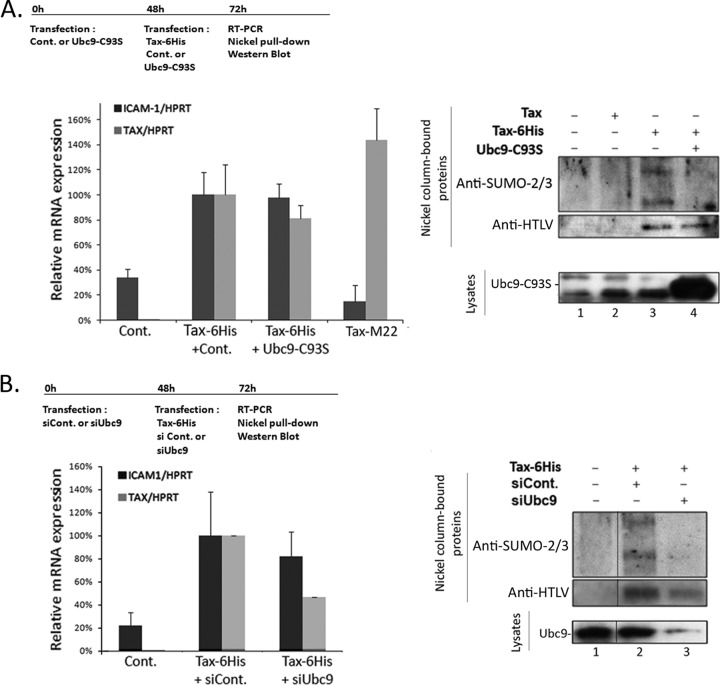
To study the impact of Tax SUMOylation on the activation of a natural NF-κB-regulated promoter, we quantified by real-time PCR the expression of ICAM-1, which has been reported to be induced by Tax in an NF-κB-dependent manner (18). 293T cells were first transfected with the Ubc9-C93S mutant (Fig. 3A) or the Ubc9 siRNA (Fig. 3B) and cultivated for 2 days to block the SUMOylation machinery prior to Tax expression. These cells then were transfected with the Tax-6His plasmid together with the control or Ubc9-C93S construct or with the control or Ubc9 siRNA for an additional 24-hour period. As a negative control for ICAM-1 induction, cells were also transfected with the NF-κB-defective Tax-M22 mutant (19). As shown in Fig. 3A (left side), ICAM-1 mRNA was induced by wild-type Tax but not by the M22 mutant, confirming that ICAM-1 induction by Tax in 293T cells indeed relied on NF-κB pathway activation. Expression of neither Ubc9-C93S nor siUbc9 had an impact on the level of induction of ICAM-1 (Fig. 3A and B, left sides). Nickel pulldown experiments were performed on the same day as the RT-PCR analysis to evaluate the level of Tax SUMOylation (Fig. 3A and B, right sides). Compared to that in control cells, total inhibition of Tax SUMOylation was found in both cell expressing Ubc9-C93S (Fig. 3A, right sides, lanes 3 and 4) and siUbc9-treated cells (Fig. 3B, right sides, lanes 2 and 3). Western blot analyses showed potent expression of Ubc9-C93S and silencing of Ubc9 upon siRNA treatment (Fig. 3, right sides, lysates). In contrast, no signal was found for Tax in Western blotting (data not shown). This is very likely due to the fact that the Tax-6His plasmid was transfected at day 2 in already-transfected cells, reducing plasmid expression. However, Tax-6His was produced under each condition, as shown by the Tax signals detected by either RT-PCR or nickel pulldown.
FIG 3.
Non-SUMOylated Tax induces the expression of the NF-κB regulated ICAM-1 gene. Real-time PCR experiments were performed in 293T cells to quantify ICAM-1 mRNA induction by Tax in the presence or absence of Ubc9-C93S or siUbc9. 293T cells were transfected with the control or T7-Ubc9-C93S construct (A) or the control or Ubc9 siRNA (B) and 48 h later with the Tax-6His plasmid, and total RNA was extracted after 24 h. For panel A the Tax-M22 mutant, which is defective for NF-κB activation, was also included as a negative control for ICAM-1 induction. Left sides show the amount of Tax or ICAM-1 mRNA normalized to the amount of HPRT mRNA and are the means and standard deviations from at least two independent experiments performed in duplicates. Right sides show the level of Tax SUMOylation after purification of Tax-6His proteins (nickel column-bound proteins) and the level of expression of Ubc9 analyzed by Western blotting (lysates). The outline of each experiment is indicated at the top of each panel.
Together with the above data, these findings provide the direct demonstration that non-SUMOylated Tax is as functional as wild-type Tax for NF-κB promoter activation in adherent cells.
Non-SUMOylated Tax is also functional for NF-κB promoter activation in T cells.
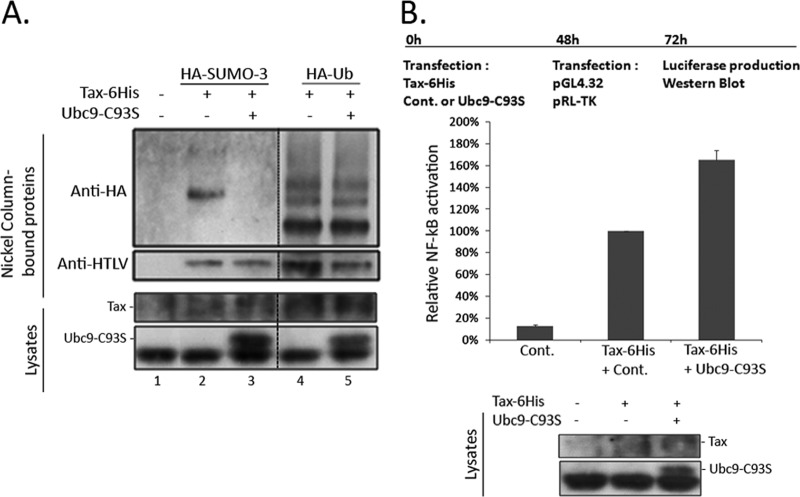
Experiments were finally performed in T cells, which represent the preferential target cells of HTLV-1 in vivo. We used the MOLT4 T-cell line, in which acceptable level of transfection (around 20%) could be achieved. However, even with this optimized protocol, we were not able to detect the endogenous SUMOylation of Tax in these cells (data not shown). To facilitate the detection of SUMOylated Tax in T cells, the Tax-6His construct was transfected together with an HA-SUMO-3 plasmid (or HA-Ub as control), as previously described (7). Analysis of proteins purified upon nickel pulldown at 2 days posttransfection showed anti-HA-reactive products in cells transfected with the Tax-6His (Fig. 4A, lanes 2 and 4) but not the control (lane 1), validating the experiment. Expressing Tax-6His together with Ubc9-C93S abolished Tax conjugation to HA-SUMO-3 (Fig. 4A, lane 3) with no effect on the level of conjugation to HA-Ub (Fig. 4A, lane 5). Only partial reduction of Tax SUMOylation was observed upon treatment with the siUbc9 (data not shown).
FIG 4.
Non-SUMOylated Tax activates a NF-κB promoter in T cells. (A) Nickel pulldown experiment to study the effect of Ubc9-C93S on Tax SUMOylation in T cells. MOLT4 T cells were transfected with the control or the Tax-6His plasmid together with either HA-SUMO-3 or HA-Ub and in the presence or absence of T7-Ubc9-C93S. Cells were lysed at 2 days posttransfection, and total proteins were either directly analyzed by Western blotting for Ubc9 and Tax expression (lysates) or purified by nickel pulldown (nickel column-bound proteins). Purified proteins were blotted successively with the anti-HA antibody and the HTLV-1 serum. (B) Luciferase reporter assays in MOLT4 T cells. Cells were first transfected with the Tax-6His construct and either the control or T7-Ubc9-C93S construct and then subjected to a second round of transfection 2 days later with the pGL4.32 (κB-Luc2P) and pRL-TK reporter plasmids. Data represent the Luc2P/Renilla ratio normalized to that of Tax alone (100%) and are the means and standard deviations from two independent experiments performed in duplicates. The outline of the experiment and the Western blot analysis showing Tax and Ubc9 expression are also presented.
Luciferase-based reporter assays were then performed in MOLT4 T cells. Cells were first transfected with the Tax-6His plasmid and the Ubc9-C93S construct to inhibit Tax SUMOylation prior to promoter transfection and then subjected to a second round of transfection 48 hours later with the reporter plasmids. Finally, luciferase activities were assessed after an additional 24-hour period. Compared to that in cells expressing Tax alone (set to 100%), a larger amount of luciferase was found in cells producing Tax-6His together with Ubc9-C93S (Fig. 4B). Western blot analyses confirmed similar levels of Tax expression under all conditions as well as strong expression of Ubc9-C93S (Fig. 4B, lysates).
These findings demonstrate that Tax SUMOylation is also dispensable for Tax-induced NF-κB promoter activation in T cells.
DISCUSSION
Based on the analysis of an ubiquitinated but weakly SUMOylated Tax mutant still able to activate an NF-κB reporter gene, we previously concluded that Tax SUMOylation is not required for Tax-induced NF-κB pathway activation (12). However, this conclusion was challenged by other groups arguing that this residual level of endogenous Tax SUMOylation could still represent an important functional determinant, presumably by targeting the nuclear population of Tax directly involved in transcription (13–15). Here, we set up a new strategy that relies not on Tax mutants but on the inhibition of the SUMOylation machinery via the targeting of the unique SUMO-conjugating enzyme Ubc9. This allowed us to show that Tax still activates NF-κB-driven gene expression at a wild-type level in adherent cells in which Ubc9 is inactivated, resulting in total disappearance of either SUMO-1- or SUMO-2/3-conjugated Tax species. It is noteworthy that potent NF-κB promoter activation by non-SUMOylated Tax was observed not only with a transfected NF-κB reporter promoter but also with an integrated reporter construct as well as with an endogenous NF-κB-regulated gene. This argues against a specific role of Tax SUMOylation in Tax interaction with promoter regions in the context of chromatin.
Since the impact of SUMOylation may be cell type dependent (20), it was important to also examine how the loss of Tax SUMOylation affected Tax-induced NF-κB activation in T cells, which constitute the natural target cells of HTLV-1. We found that expressing Tax together with Ubc9-C93S also abolished Tax SUMOylation in MOLT4 cells, showing that Tax SUMOylation is also under the control of Ubc9 in T cells. Furthermore, the absence of Tax SUMOylation also had no effect on the activation of a transfected NF-κB promoter in MOLT4 T cells.
Interestingly, reporter assays performed in 293T cells showed that while little change was observed upon blocking the catalytic activity of Ubc9, Ubc9 silencing led to increased Tax-induced NF-κB promoter activation. Moreover, expressing Ubc9-C93S in T cells also increased NF-κB promoter activation by Tax. Our findings therefore indicate not only that Tax SUMOylation is dispensable for Tax-induced NF-κB activation in adherent cells or T cells but that endogenous Ubc9 may exert a negative effect on this process via either a SUMOylation-dependent or -independent mechanism. It is noteworthy that Ubc9 has already been described to contribute to transcriptional repression mediated by several transcription factors via either SUMOylation-dependent (21) or -independent (22) mechanisms.
Another interesting issue is whether the SUMOylation of cellular proteins is required for Tax-induced NF-κB pathway activation. We observed that blocking or silencing Ubc9 massively reduced not only Tax SUMOylation but also conjugation of transfected SUMO-3 to total proteins (Fig. 1C). This suggests that de novo SUMOylation of cellular cofactors is not required for Tax-induced NF-κB activation. However, since SUMOylated proteins may have long half-lives, we cannot exclude the possibility that cellular proteins SUMOylated prior to Ubc9 inhibition may contribute to NF-κB activation by Tax.
In conclusion, we believe that this study clarifies the issue of the role of SUMOylation in regard to Tax by providing conclusive evidence that non-SUMOylated Tax proteins are still fully functional for NF-κB promoter activation.
ACKNOWLEDGMENTS
We thank Florence Margottin-Goguet for critical reading of the manuscript.
This work was funded by grants from the Ligue contre le Cancer (Comité de Paris) and the Institut National du Cancer (InCA)/Cancéropôle Lyon Auvergne Rhône Alpes (CLARA). S.P. is supported by a grant from University Paris Diderot.
Footnotes
Published ahead of print 2 July 2014
REFERENCES
- 1.Iwanaga M, Watanabe T, Yamaguchi K. 2012. Adult T-cell leukemia: a review of epidemiological evidence. Front. Microbiol. 3:322. 10.3389/fmicb.2012.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currer R, Van Duyne R, Jaworski E, Guendel I, Sampey G, Das R, Narayanan A, Kashanchi F. 2012. HTLV tax: a fascinating multifunctional co-regulator of viral and cellular pathways. Front. Microbiol. 3:406. 10.3389/fmicb.2012.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kfoury Y, Nasr R, Journo C, Mahieux R, Pique C, Bazarbachi A. 2012. The multifaceted oncoprotein Tax: sub-cellular localization, post-translational modifications and NF-κB activation. Adv. Cancer Res. 113:85–120. 10.1016/B978-0-12-394280-7.00003-8 [DOI] [PubMed] [Google Scholar]
- 4.Baldwin AS. 2012. Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol. Rev. 246:327–345. 10.1111/j.1600-065X.2012.01095.x [DOI] [PubMed] [Google Scholar]
- 5.Robek MD, Ratner L. 1999. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiari E, Lamsoul I, Lodewick J, Chopin C, Bex F, Pique C. 2004. Stable ubiquitination of human T-cell leukemia virus type 1 tax is required for proteasome binding. J. Virol. 78:11823–11832. 10.1128/JVI.78.21.11823-11832.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasr R, Chiari E, El-Sabban M, Mahieux R, Kfoury Y, Abdulhay M, Yazbeck V, Hermine O, de The H, Pique C, Bazarbachi A. 2006. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappaB activation. Blood 107:4021–4029. 10.1182/blood-2005-09-3572 [DOI] [PubMed] [Google Scholar]
- 8.Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. 2005. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol. Cell. Biol. 25:10391–10406. 10.1128/MCB.25.23.10391-10406.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shembade N, Harhaj NS, Yamamoto M, Akira S, Harhaj EW. 2007. The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-kappaB activation. J. Virol. 81:13735–13742. 10.1128/JVI.01790-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kfoury Y, Setterblad N, El-Sabban M, Zamborlini A, Dassouki Z, El Hajj H, Hermine O, Pique C, de Thé H, Saïb A, Bazarbachi A. 2011. Tax ubiquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood 117:190–199. 10.1182/blood-2010-05-285742 [DOI] [PubMed] [Google Scholar]
- 11.Fryrear KA, Guo X, Kerscher O, Semmes OJ. 2012. The Sumo-targeted ubiquitin ligase RNF4 regulates the localization and function of the HTLV-1 oncoprotein Tax. Blood 119:1173–1181. 10.1182/blood-2011-06-358564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet A, Randrianarison-Huetz V, Nzounza P, Favre-Bonvin A, Pene S, Nedelec M, Chazal M, Waast L, Bazarbachi A, Mahieux R, Bénit L, Pique C. 2012. Low sumoylation and nuclear body formation do not prevent Tax-induced NF-kB promoter activation in primary or HTLV-1-infected T cells. Retrovirology 9:77. 10.1186/1742-4690-9-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zane L, Jeang KT. 2012. The importance of ubiquitination and sumoylation on the transforming activity of HTLV Tax-1 and Tax-2. Retrovirology 9:103. 10.1186/1742-4690-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turci M, Lodewick J, Di Gennaro G, Rinaldi AS, Marin O, Diani E, Sampaio C, Bex F, Bertazzoni U, Romanelli MG. 2012. Ubiquitination and sumoylation of the HTLV-2 Tax-2B protein regulate its NF-κB activity: a comparative study with the HTLV-1 Tax-1 protein. Retrovirology 9:102. 10.1186/1742-4690-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shirinian M, Kfoury Y, Dassouki Z, El-Hajj H, Bazarbachi A. 2013. Tax-1 and Tax-2 similarities and differences: focus on post-translational modifications and NF-κB activation. Front. Microbiol. 4:231. 10.3389/fmicb.2013.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay RT. 2005. SUMO: a history of modification. Mol. Cell 18:1–12. 10.1016/j.molcel.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 17.Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET. 1997. Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J. Biol. Chem. 272:28198–28201. 10.1074/jbc.272.45.28198 [DOI] [PubMed] [Google Scholar]
- 18.Sanda T, Asamitsu K, Ogura H, Iida S, Utsunomiya A, Ueda R, Okamoto T. 2006. Induction of cell death in adult T-cell leukemia cells by a novel IkappaB kinase inhibitor. Leukemia 20:590–598. 10.1038/sj.leu.2404129 [DOI] [PubMed] [Google Scholar]
- 19.Smith MR, Greene WC. 1990. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875–1885. 10.1101/gad.4.11.1875 [DOI] [PubMed] [Google Scholar]
- 20.Treuter E, Venteclef N. 2010. Transcriptional control of metabolic and inflammatory pathways by nuclear receptor SUMOylation. Biochim. Biophys. Acta 2:909–918. 10.1016/j.bbadis.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Yu X, Lei Q, Ma L, Guo D. 2013. The SUMOylation of zinc-fingers and homeoboxes 1 (ZHX1) by Ubc9 regulates its stability and transcriptional repression activity. J. Cell. Biochem. 114:2323–2333. 10.1002/jcb.24579 [DOI] [PubMed] [Google Scholar]
- 22.Tomoiu A, Gravel A, Tanguay RM, Flamand L. 2006. Functional interaction between human herpesvirus 6 immediate-early 2 protein and ubiquitin-conjugating enzyme 9 in the absence of sumoylation. J. Virol. 80:10218–10228. 10.1128/JVI.00375-06 [DOI] [PMC free article] [PubMed] [Google Scholar]