ABSTRACT
African green monkeys (AGMs; genus Chlorocebus) are a natural host of simian immunodeficiency virus (SIVAGM). As they do not develop simian AIDS, there is great interest in understanding how this species has evolved to avoid immunodeficiency. Adult African green monkeys naturally have low numbers of CD4 T cells and a large population of major histocompatibility complex class II-restricted CD8αdim T cells that are generated through CD4 downregulation in CD4+ T cells. Mechanisms that drive this process of CD4 downregulation are unknown. Here, we show that juvenile AGMs accelerate CD4-to-CD8αα conversion upon SIV infection and avoid progression to AIDS. The CD4 downregulation induced by SIV infection is not limited to SIV-specific T cells, and vaccination of an adult AGM who had a negligible number of CD4 T cells demonstrated that CD4 downregulation can occur without antigenic exposure. Finally, we show that the T cell homeostatic cytokines interleukin-2 (IL-2), IL-7, and IL-15 can induce CD4 downregulation in vitro. These data identify a mechanism that allows AGMs to generate a large, diverse population of T cells that perform CD4 T cell functions but are resistant to SIV infection. A better understanding of this mechanism may allow the development of treatments to induce protective CD4 downregulation in humans.
IMPORTANCE Many African primate species are naturally infected with SIV. African green monkeys, one natural host species, avoid simian AIDS by creating a population of T cells that lack CD4, the human immunodeficiency virus/SIV receptor; therefore, they are resistant to infection. However, these T cells maintain properties of CD4+ T cells even after receptor downregulation and preserve immune function. Here, we show that juvenile AGMs, who have not undergone extensive CD4 downregulation, accelerate this process upon SIV infection. Furthermore, we show that in vivo, CD4 downregulation does not occur exclusively in antigen-experienced T cells. Finally, we show that the cytokines IL-2, IL-7, and IL-15, which induce homeostatic T cell proliferation, lead to CD4 downregulation in vitro; therefore, they can provide signals that lead to antigen-independent CD4 downregulation. These results suggest that if a similar process of CD4 downregulation could be induced in humans, it could provide a cure for AIDS.
INTRODUCTION
African green monkeys (AGMs) are a natural host of simian immunodeficiency virus (SIVAGM), and approximately 50% of adult AGMs in the wild are SIV infected (1). Although plasma SIV viral loads are high, progression to AIDS is very rare in this species (1). However, SIVAGM is pathogenic in pigtail macaques when they are experimentally infected (2, 3). Therefore, there is much interest in understanding how AGMs have evolved to avoid progressive disease.
Adult AGMs have low CD4+ T cell counts (4, 5) and a large population of CD8αdim T cells that express CD8αα homodimers and are distinct from the classical CD8 T cells that express the α and β chain of CD8 (4, 6, 7). These CD8αα T cells arise postthymically through CD4 downregulation by CD4+ T cells (4). The resulting CD8αα T cells retain many characteristics of CD4+ T cells, including major histocompatibility complex (MHC) class II restriction and expression of FoxP3, CD40 ligand, IL-17, and/or IL-2 (4). However, because these T cells do not express CD4, they are resistant to SIV infection in vivo, and viral replication is restricted to CD4+ T cells (4, 8). The existence of this population of CD8αα T cells, which can perform functions associated with CD4+ T cells but are not infected with SIV, is hypothesized to underlie the nonprogressive phenotype of SIV infection in AGMs.
The process that induces CD4 downregulation is unknown. Downregulation on some CD4+ T cells can be induced in vitro by stimulatory signals, such as mitogens or superantigens, that stimulate through the T cell receptor (4, 6, 7), but it remains unknown why some dividing cells downregulate CD4 while others maintain expression. In vivo, nearly all CD8αα T cells have a memory phenotype (4), so it is thought that CD4 downregulation may be driven by cellular maturation. This is consistent with the fact that CD8αα T cells numerically are vastly dominant to CD4+ T cells in tissues, especially in the gastrointestinal (GI) tract, and with the fact that juvenile AGMs, who have had limited immunological stimulation in vivo, have few CD8αα T cells and high CD4+ T cell counts relative to adults (4). It should be noted that vertical transmission of SIVAGM has rarely been observed, and, given the high CD4+ T cell counts in infant AGMs, the block to vertical transmission in this species may be an important consideration in understanding the absence of disease progression in these animals (4, 9).
Downregulation of CD4 appears to be evolutionarily conserved in other natural host species, which also exhibit CD4− T cells that can perform functions of CD4+ T cells (10). In sooty mangabeys, double-negative T cells, which express neither CD4 nor CD8, are thought to be responsible for the lack of disease progression in a subset of animals that undergo severe CD4+ T cell depletion (11–13). Furthermore, there is evidence that CD4 downregulation and the large population of CD8αα T cells observed in Patas monkeys led to the extinction of a Patas-specific SIV (14). Like the immunologically similar AGMs, Patas monkeys do not develop AIDS after experimental infection with SIVAGM despite ongoing viral replication and postinfection CD4+ T cell depletion (14). One Patas monkey that had almost no CD4+ T cells did not have any signs of immunodeficiency but was unable to be infected intravenously with SIVAGM due to the lack of target cells (14).
It is clear that CD4 downregulation in AGMs dramatically influences their immune phenotype by creating a large population of CD8αα T cells that are able to perform functions of CD4+ T cells but are resistant to SIV infection. However, the mechanisms by which this downregulation occurs are unclear. Here, we studied juvenile and adult vervet AGMs to understand the biological significance of CD4 downregulation and mechanisms by which this protective phenotype is induced. Our data demonstrate that an antigen-independent mechanism drives the formation of a diverse repertoire of CD8αα T cells, which are able to preserve CD4-like functions in the absence of CD4+ T cells.
MATERIALS AND METHODS
Ethics.
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Office of Animal Welfare, and the United States Department of Agriculture (15). All animal work was approved by the NIAID Division of Intramural Research Animal Care and Use Committees (IACUC) in Bethesda, MD (protocols LMM-12E and LMM-6). The animal facility is accredited by the American Association for Accreditation of Laboratory Animal Care. All procedures were carried out under ketamine anesthesia by trained personnel under the supervision of veterinary staff, and all efforts were made to maximize animal welfare and to minimize animal suffering in accordance with the recommendations of the Weatherall report on the use of nonhuman primates (29). Animals were housed in adjoining individual primate cages, allowing social interactions, under controlled conditions of humidity, temperature, and light (12-h light/12-h dark cycles). Food and water were available ad libitum. Animals were monitored twice daily and fed commercial monkey chow, treats, and fruit twice daily by trained personnel. Early endpoint criteria, as specified by the IACUC approved score parameters, were used to determine when animals should be humanely euthanized.
Animals.
We housed a total of 36 vervet African green monkeys (Chlorocebus pygerythrus) and 9 rhesus macaques (Macaca mulatta); the AGMs included 9 experimentally infected adults, 8 naturally infected adults, 9 uninfected adults, 3 experimentally infected juveniles, and 7 uninfected juveniles at the NIH NIAID Animal Center. The rhesus macaques included 8 uninfected adults and 1 experimentally infected juvenile. Eight of the SIV+ AGM vervets were infected in the wild, and nine were experimentally infected intravenously with 50% tissue culture infectious doses (TCID50) of SIVAGMver1 or 1,000 TCID50 of SIVAGMver90 (4). We isolated virus as previously described (16).
Juvenile infections.
Juvenile AGMs were infected intravenously with 1,000 TCID50 of SIVAGMver90. The ages at infection were the following: AG35, 460 days old; AG37, 203 days old; AG38, 186 days old. One juvenile rhesus macaque was infected intravenously with 250 TCID50 of SIVmac239 at 176 days of age.
MML vaccination.
Animals were vaccinated subcutaneously with a mixture of 50 μg of MML polyprotein and 1 mg of poly ICLC (Oncovir, Washington, DC) at each vaccination time point. MML (also known as Leish-111f) is a recombinant polyprotein derived from Leishmania species that has been shown to be protective in vivo and is comprised of three proteins: TSA (also known as MAPS), LmSTI1 (also known as M15), and LeIF (17).
Absolute cell counts.
Absolute cell counts were calculated from flow cytometry frequencies and complete blood count (CBC) absolute lymphocyte counts (Antech, Irvine, CA). Data from the 2008 time point were previously reported by Beaumier et al. (4).
Flow cytometry.
Cellular frequency and activation status were determined through ex vivo staining of isolated peripheral blood mononuclear cells (PBMC). Cells were washed twice with PBS and incubated with Live/Dead fixable aqua dead cell stain (Invitrogen, Carlsbad, CA) for 5 min at room temperature. Cells then were stained with fluorescently conjugated monoclonal antibodies to CCR5 (clone 3A9, conjugated to PE; BD Bioscience, San Jose, CA) and CCR7 (clone 3D12, conjugated to Cy7PE; BD Bioscience) and incubated for 15 min at 37°C, after which antibodies to CD3 (clone SP34-2, conjugated to Alexa 700; BD Bioscience), CD4 (clone L200, conjugated to APC; BD Bioscience), CD8 (clone RPA-T8, conjugated to Pacific Blue; BD Bioscience), CD28 (clone 28.2, conjugated to ECD; Beckman Coulter, Brea, CA), CD95 (clone DX2, conjugated to Cy5PE; BD Bioscience), and HLA-DR (clone L243, conjugated to APC-H7; BD Bioscience) were added and incubated for an additional 30 min at 4°C. Cells were washed with PBS and permeabilized with Cytofix/Cytoperm buffer (BD Bioscience) for 20 min at 4°C. After washing twice with 1× perm/wash buffer (BD Bioscience), we then intracellularly stained the cells with FITC-conjugated monoclonal antibody to Ki67 (clone B56; BD Bioscience) and incubated them for 30 min at 4°C. We washed the cells with 1× perm/wash buffer and then fixed them in a 1% paraformaldehyde solution (Electron Microscopy Sciences, Hatfield, PA).
For intracellular cytokine staining, after stimulation cells were washed twice with PBS and incubated with Live/Dead fixable aqua dead cell stain (Invitrogen) for 5 min at room temperature. Cells then were stained with fluorescently conjugated monoclonal antibodies to CD3 (clone SP34-2, conjugated to Alexa 700; BD Bioscience), CD4 (clone L200, conjugated to PE; BD Bioscience), CD8 (clone RPA-T8, conjugated to Pacific Blue; BD Bioscience), CD28 (clone 28.2, conjugated to ECD; Beckman Coulter), and CD95 (clone DX2, conjugated to Cy5PE; BD Bioscience) for 30 min at 4°C. Cells were washed with PBS and permeabilized with Cytofix/Cytoperm buffer (BD Bioscience) for 20 min at 4°C. After washing twice with 1× perm/wash buffer (BD Bioscience), we intracellularly stained the cells with fluorescently conjugated monoclonal antibodies to gamma interferon (clone 4S.B3, conjugated to Cy7PE; BD Bioscience), IL-2 (clone MQ1-17H12, conjugated to APC; BD Bioscience), CD40L (clone TRAP1, conjugated to APC-e780; BD Bioscience), and TNF (clone MAb11, conjugated to FITC; BD Bioscience) and incubated them for 30 min at 4°C. We washed the cells with 1× perm/wash buffer and then fixed them in a 1% paraformaldehyde solution (Electron Microscopy Sciences).
Antigen stimulation of PBMC.
For intracellular cytokine staining, we incubated PBMC overnight at 37°C with medium alone, 1 mg/ml of SEB (Sigma, St. Louis, MO), 2.5 μg/ml of SIVAGM Gag peptides, or 20 μg/ml MML protein in the presence of 5 μl/ml of CD28 ECD monoclonal antibody (28.2; Beckman Coulter) and 10 μg/ml brefeldin A (Sigma), which was added after 2 h. For some experiments, we pretreated PBMC for 1 h at 37°C with antibodies against MHC-I (G46-2.6; BD Bioscience) or MHC-II (TU39; BD Bioscience) at a concentration of 25 μg/ml.
The SIV Gag peptides were 15mers overlapping by 11 amino acids that were synthesized by New England Peptide. The sequence was based on that for SIVAGM9063 (accession number L40990.1). The peptides corresponding to SIV Gag were pooled for use in stimulation experiments, and each peptide was used at a final concentration of 2.5 μg/ml.
Cellular proliferation.
We labeled PBMC with 0.25 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen). We then sorted CD4+ T cells (defined as CD3+ CD4+ live lymphocytes) and APCs (defined as CD3− NKG2A− HLA− DR+ live cells) at the CD4 T cell/APC ratio found in the unsorted PBMC and stimulated the sorted cells with 25 ng/ml of IL-7 (Peprotech, Rocky Hill, NJ) for 7 days, 25 ng/ml of IL-15 (Peprotech) for 7 days, 50 ng/ml IL-2 (Peprotech) or 10% IL-2-containing medium (Advanced Biotechnologies, Columbia, MD) for 6 days, or 1 mg/ml of SEB (Sigma) for 7 days. Sorted cells were stained for sorting with Live/Dead fixable aqua dead cell stain (Invitrogen) and monoclonal antibodies to CD3 (clone SP34-2, conjugated to Alexa 700; BD Bioscience), CD4 (clone L200, conjugated to APC; BD Bioscience), NKG2A (clone Z199, conjugated to PE; Beckman Coulter), and HLA-DR (clone L243, conjugated to APC-H7; BD Bioscience). At the end of the stimulation period, cells were stained with monoclonal antibodies to CD3 (clone SP34-2, conjugated to Alexa 700; BD Bioscience), CD4 (clone L200, conjugated to APC; BD Bioscience), CD8α (clone RPA-T8, conjugated to Pacific Blue; BD Bioscience), CD8β (clone 2ST8.5H7, conjugated to PE; BD Bioscience), CD28 (clone 28.2, conjugated to ECD; Beckman Coulter), CD95 (clone DX2, conjugated to Cy5PE; BD Bioscience), HLA-DR (clone L243, conjugated to APC-H7; BD Bioscience), and CD69 (clone FN50, conjugated to Cy7PE, BD Bioscience) for 30 min at 4°C, washed, fixed in 1% paraformaldehyde solution (Electron Microscopy Sciences), and collected within 2 h.
Quantitative PCR.
We sorted cell populations by flow cytometry and lysed them with 25 μl of a 1:100 dilution of proteinase K (Roche, Indianapolis, IN) in 10 mM Tris buffer. We performed quantitative PCR with 5 μl of cell lysates per reaction. Reaction conditions were the following: 95°C for 5 min and 40 cycles of 95°C for 15 s, followed by 60°C for 1 min. We used the Taq DNA polymerase kit (Invitrogen). The sequence of the forward primer for SIVAGM was 5′-GTCCAGTCTCAGCATTTACTTG-3′. The reverse primer sequence was 5′-CGGGCATTGAGGTTTTTCAC-3′. The probe sequence was 5′-CAGATGTTGAAGCTGACCATTTGGG-3′. For cell number quantification, we measured monkey albumin gene copy number as previously described (18). We used the StepOnePlus PCR machine (Applied Biosystems, Foster City, CA), and we performed the analysis with StepOne software (Applied Biosystems).
Plasma viral load.
A real-time RT-PCR assay for quantitation of viral RNA in plasma was performed as previously described (19).
RESULTS
SIV infection of juvenile AGMs is comparable to that of adults.
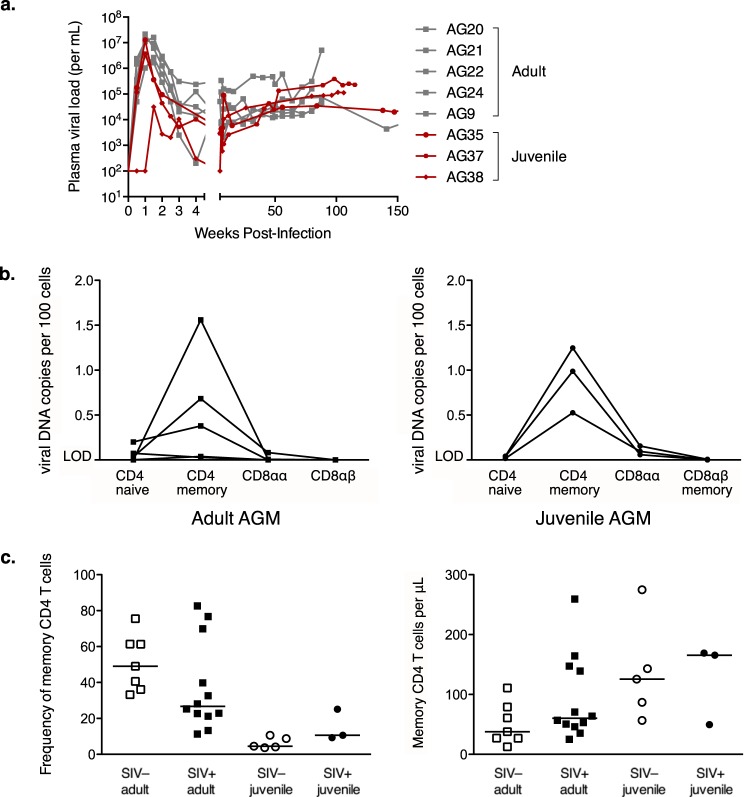
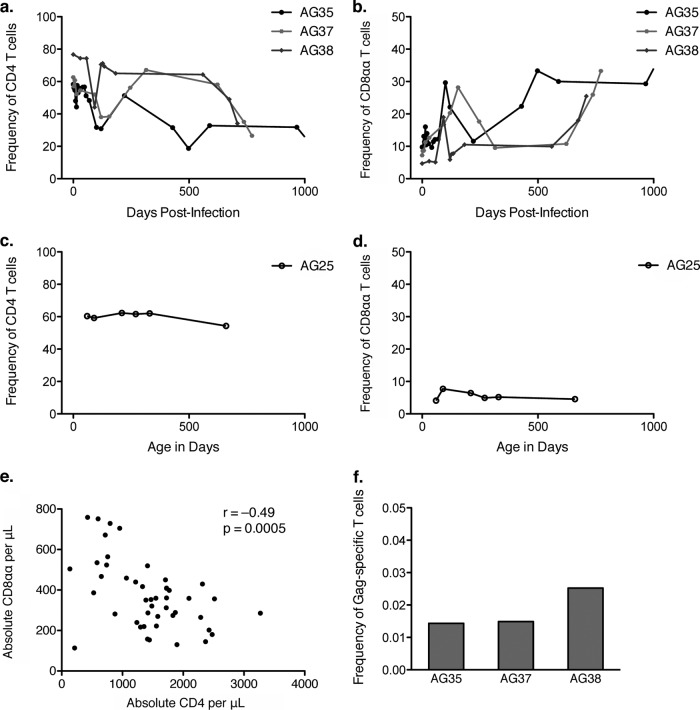
In stark contrast to adult AGMs, who have low frequencies and absolute numbers of CD4+ T cells, juvenile AGMs have high levels of CD4+ T cells (P < 0.0015 in all cases) (Fig. 1a and b). This can be attributed to the fact that juveniles are immunologically inexperienced and have not yet undergone significant CD4 downregulation to form the CD8αα T cell population, which is of low frequency and absolute size in juvenile animals (Fig. 1c and d). When we examined the longitudinal changes in absolute CD4+ T cell count in a diverse cohort of vervet AGMs, there was a significant decrease in the number of CD4+ T cells over 5 years (P = 0.002) (Fig. 1e), which is consistent with CD4+-to-CD8αα conversion being driven by immunologic experience. This cohort included both SIV+ and SIV− animals, but the groups were not matched for CD4+ T cell count or age at baseline, precluding us from drawing any conclusions about the effect of SIV status on CD4+ T cell loss.
FIG 1.
CD4+-to-CD8αα conversion in AGMs is driven by immunologic experience. The frequency of a cell population out of the total population of CD3+ T cells (a and c) and absolute number of cells per microliter of blood (b and d) of T cell populations were measured by flow cytometry, and absolute numbers were calculated from complete blood counts. (a and b) CD4+ T cells gated by CD3+ CD4+ expression. (c and d) CD8αα T cells gated by CD3+ CD4− CD8αdim expression. The Mann-Whitney test was used for panels a to d. For panels a and b, P < 0.0015 for comparisons to all juveniles. (e) Change in CD4+ T cell count over 5 years (Wilcoxon matched-pair signed-rank test). Adult animals are denoted by squares, juvenile animals by circles, SIV+ animals by filled symbols, and SIV− animals by open symbols. Median values for each population are shown.
Given our model that the ability of AGMs to form CD8αα T cells through CD4 downregulation underlies the nonprogressive phenotype of SIV infection in this species, we hypothesized that juvenile AGMs would be susceptible to progressive disease, because these animals have not yet undergone CD4 downregulation in vivo. To test this hypothesis, we intravenously infected 3 juvenile AGMs with SIVAGMver90 to determine whether the infection dynamics would differ from those of adult experimental infections and whether the infected juveniles would progress to AIDS. All three were infected after a single inoculation, and the acute and chronic plasma viral loads were fairly similar to those of adults (Fig. 2a). Furthermore, during the chronic phase of infection, the amounts of viral DNA within different subsets of T cells by SIVAGM also was similar in juveniles and adults (Fig. 2b). CD4+ memory T cells were the main targets of viral replication in the juveniles and adults (Fig. 2b), which raised the question of how the immunologically inexperienced juveniles had sufficient CD4+ memory T cells to produce viral loads comparable to those of adults. Although all juvenile animals had low frequencies of CD4+ memory T cells (Fig. 2c), because their CD4+ T cell counts were so much higher than those of adult animals (Fig. 1b), they had as many or more CD4+ memory T cells per microliter of blood (Fig. 2c).
FIG 2.
Viral replication dynamics are similar in juvenile and adult AGMs. (a) Plasma viral load in adult (gray squares) and juvenile (red circles) AGMs at time points measured in weeks after inoculation with SIVAGM. (b) Infection frequencies of sorted lymphocyte subsets from SIVAGM-infected adult (left) and juvenile (right) AGMs, as determined by quantitative PCR for viral DNA. Naive and memory subsets were gated based on expression of CD28 and CD95. LOD denotes samples at or below the assay limit of detection. (c) Frequency of memory CD4+ T cells out of total CD4+ T cells (left) and absolute number of memory CD4+ T cells per microliter of blood (right) measured by flow cytometry and absolute number calculated from complete blood count. Memory CD4+ T cells were gated by CD3+ CD4+ CD95+ and CD28hi or CD28low expression. Adult animals are denoted by squares, juvenile animals by circles, SIV+ animals by filled symbols, and SIV− animals by open symbols. Median values for each population are shown.
Despite readily detectable viral replication, there was no evidence of disease progression in the SIVAGM-infected juvenile AGMs. No opportunistic infections or other indications of AIDS were observed even though the animals had been infected for 2 to 3 years. In contrast, a juvenile rhesus macaque infected with SIVMAC239 in parallel to AG37 had to be euthanized within 5 months of infection due to progressive disease (data not shown).
SIV infection of juvenile AGMs induces CD4 downregulation.
Notably, SIV infection of the juvenile AGMs appeared to cause accelerated CD4+-to-CD8αα conversion, beginning after the acute phase between day 50 and day 100 postinfection and continuing until at least day 150 (Fig. 3a and b). This pattern of decreased CD4+ T cell frequency and increased CD8αα T cell frequency continued across the several years of follow-up (Fig. 3a and b). The difference from the preinfection time point at day 0 to the last available time point between days 700 and 1,000 was not significant; however, this is likely because of the small sample size. Consistent longitudinal CD4+ T cell and CD8αα T cell frequencies for an uninfected juvenile, AG25, are shown for comparison, with axes of the same scale showing the animal's age in days (Fig. 3c and d). That the changes in CD4+ and CD8αα frequency are driven primarily by CD4+-to-CD8αα conversion rather than by CD4+ T cell loss is supported by the inverse correlation between absolute CD4+ and CD8αα T cells per microliter of blood after SIV infection in these animals (Spearman r = −0.49; P = 0.0005) (Fig. 3e).
FIG 3.
Accelerated CD4+-to-CD8αα conversion in SIV-infected juvenile AGMs. (a) Frequency of CD4+ T cells out of total T cells in 3 SIV-infected juvenile AGMs relative to time postinfection. (b) Frequency of CD8αα T cells out of total T cells in 3 SIV-infected juvenile AGMs relative to time postinfection. (c) Frequency of CD4+ T cells out of total T cells in an SIV-uninfected juvenile AGM relative to time postbirth. (d) Frequency of CD8αα T cells out of total T cells in an SIV-uninfected juvenile AGM relative to time postbirth. (e) CD4+ T cell count versus CD8αα T cell count in SIV-infected juvenile AGMs. All time points from panels a and b are shown as individual data points (n = 46). The Spearman correlation was calculated; r and P values are shown. (f) SIV Gag-specific T cell response magnitude measured by flow cytometry and intracellular cytokine staining. PBMC were stimulated with the SIV Gag peptide pool or media control overnight in the presence of brefeldin A and CD28 and then stained for intracellular cytokines. The background-subtracted frequency of Gag-specific T cells out of total T cells is shown for 3 SIV-infected juvenile AGMs.
T cells specific for a neoantigen can downregulate CD4 prior to antigen exposure.
The dramatic degree of CD4-to-CD8αα conversion after SIV infection of the juvenile AGMs was not limited to SIV Gag-specific T cells, which make up less than 1% of the total T cell pool (Fig. 3f). This raised the question of what signals in the infected animals were driving a significant fraction of the CD4+ T cells to downregulate CD4. To investigate the ability of repeated antigen stimulus to drive in vivo CD4 downregulation, we used a vaccination strategy that elicits MHC class II-restricted CD4+ T cell responses in rhesus macaques and mice (20, 21). We subcutaneously vaccinated 2 juvenile AGMs, one SIV+ and one SIV−, with the MML protein of Leishmania major and poly ICLC as an adjuvant. Each animal received 5 doses, with boosts 4 weeks apart. The MML-specific T cell responses were measured at each time point by flow cytometry.
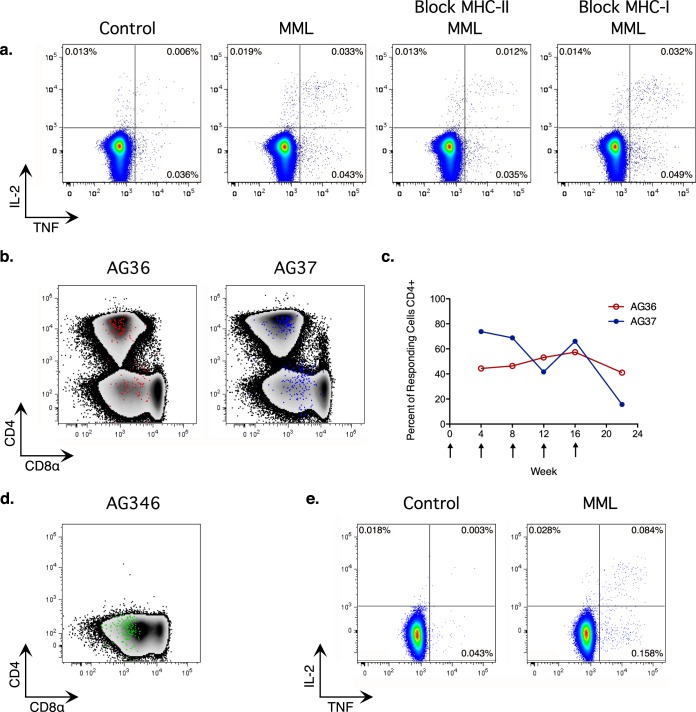
As expected based on the results in other species, the vaccine elicited a T cell response restricted by MHC class II, which was demonstrated by pretreating the stimulated cells with blocking antibodies to MHC class I or class II (Fig. 4a). At the earliest time point after vaccination, a large proportion of the MML-specific T cells were within the CD4− CD8αα+ population (Fig. 4b and c). The proportion of responding MML-specific T cells that was CD4+ did not change upon repeated boosts in the SIV− animal, AG36 (Fig. 4c), and the proportion decreased in the SIV+ animal, AG37, only at the final time point 6 weeks after the last boost (Fig. 4c).
FIG 4.
MML vaccination of AGMs elicits an MHC class II (MHC-II)-restricted T cell response. (a) Flow cytometry intracellular cytokine staining data showing live CD3+ T cells from vaccinated SIV+ juvenile AG37 at week 8. PBMC were stimulated for 14 h, with brefeldin A added after 2 h. The control tube was incubated with CD28 only. The MML tube was incubated with CD28 and MML protein. The MHC-II tube was preincubated with blocking antibody to MHC class II, and then CD28 and MML protein were added. The MHC-I tube was preincubated with blocking antibody to MHC class I, and then CD28 and MML protein were added. The frequency of cells expressing IL-2 (y axis) and TNF (x axis) is shown. (b) Density plot showing CD4 (y axis) and CD8α expression (x axis) of live CD3+ T cells with MML-specific T cells overlaid as a dot plot. SIV-uninfected juvenile AG36 (left), shown in red, and SIV+ juvenile AG37 (right), shown in blue, from the week 12 time point. (c) Percent MML-specific T cells that were CD4+ at each time point. Vaccine dose timing is indicated by arrows. (d) Density plot showing CD4 (y axis) and CD8α expression (x axis) of live CD3+ T cells with MML-specific T cells overlaid as a dot plot at week 2 for adult AG346 who had consistently low CD4+ T cell counts. (e) Flow cytometry intracellular cytokine staining data showing live CD3+ T cells from vaccinated SIV+ adult AG346 at week 2. The frequency of cells expressing IL-2 (y axis) and TNF (x axis) is shown.
We observed evidence of CD4 downregulation in the fact that we were able to find T cells that responded to the neoantigen, MML, that were restricted by MHC class II and were CD4− CD8αα+. However, because the MML-specific T cell response at the earliest time point already contained a high percentage of CD4− T cells, we were unable to conclude that the initial response was elicited exclusively from the CD4+ population. To determine whether there is a mechanism by which MML-specific T cells could enter the CD8αα population prior to antigen encounter (an antigen-independent mechanism), we took advantage of the fact that there was one AGM in our colony, AG346, who appeared to have driven the process of CD4 downregulation almost to completion. This SIV-infected AGM has had a CD4+ T cell count below 20 cells per microliter for over 5 years (Fig. 4d is an example of one time point). Consistent with our model that viral replication is limited to CD4+ T cells and the CD8αα T cell population provides the majority of CD4-like immune function in adult AGMs, AG346 had no indications of AIDS and, concomitant with the loss of CD4+ T cells, its plasma viral load became consistently undetectable. The absence of detectable viral load in this animal suggests that CD4+ T cell depletion occurred throughout its tissues and not simply in the periphery. Indeed, this finding raises the intriguing possibility that complete downregulation of CD4 by CD4+ T cells could purge reservoirs of latently SIVAGM-infected cells in vivo.
We reasoned that if CD4-to-CD8αα conversion were driven exclusively by antigen-dependent mechanisms, the CD8αα repertoire would be circumscribed by the identity of antigens the animal had already encountered, limiting the ability of AG346 to respond to MHC class II-restricted neoantigens. Therefore, if AG346 were able to respond to MML vaccination, it would indicate that an antigen-independent mechanism of CD4+-to-CD8αα conversion had produced a more diverse T cell repertoire within the CD8αα pool. Consistent with this premise, a single dose of the MML and poly ICLC vaccine elicited a robust and polyfunctional T cell response in AG346 (Fig. 4e), and the responding cells were CD4− CD8αα+ T cells (Fig. 4d). This result strongly suggested that there was an antigen-independent mechanism that could induce CD4-to-CD8αα conversion in vivo.
Homeostatic cytokines can induce CD4 downregulation.
To investigate antigen-independent stimuli that might drive CD4 downregulation in AGMs, we sorted CD4+ T cells from SIV-uninfected AGMs and rhesus macaques and cultured them with human IL-2, IL-7, or IL-15, which are cytokines involved in homeostatic proliferation of T cells. To track proliferation, the PBMC first were labeled with CFSE and then sorted for CD4+ CD3+ T cells and CD3− NKG2A− HLA-DR+ APCs, followed by a 6-day culture with 50 ng/ml of IL-2 or 7-day culture with 25 ng/ml of IL-15 or IL-7. Four SIV+ AGMs were included in the IL-2 stimulation experiment to determine whether the results differed in samples from infected and uninfected animals. The sorted cells contained both naive and memory CD4+ T cells in the proportion they were found in the sample.
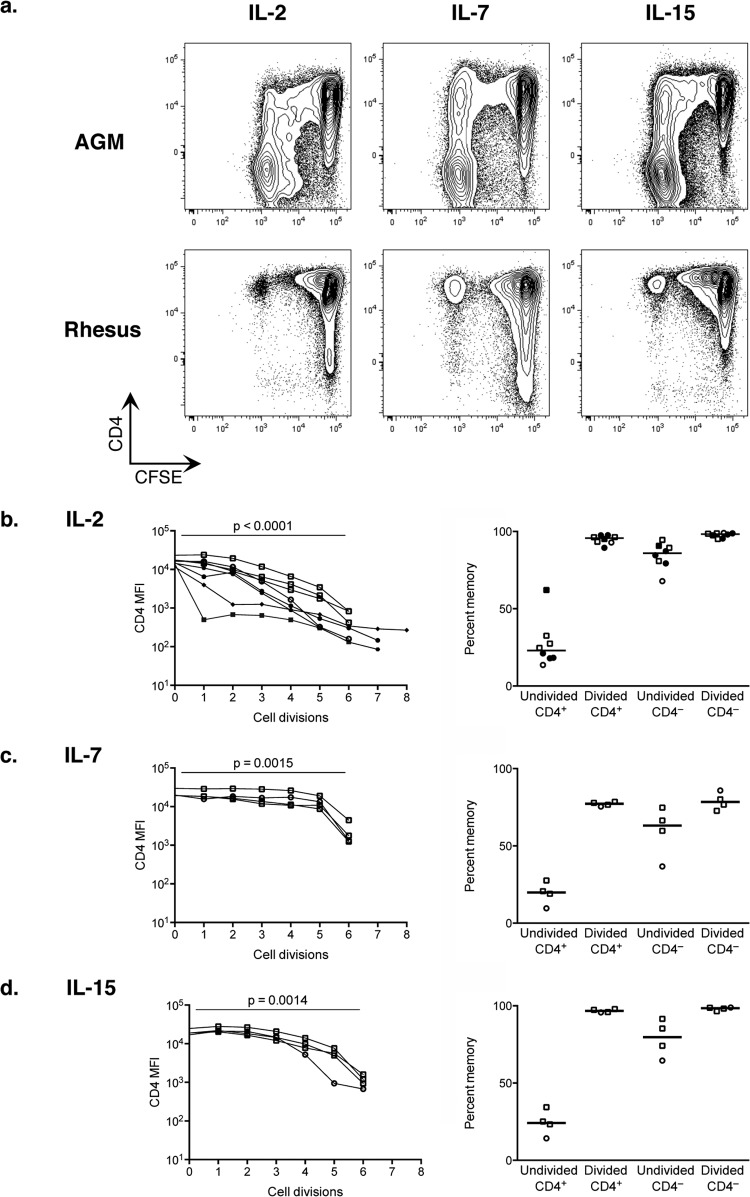
Each of the three cytokines induced proliferation of some CD4+ T cells, and a large proportion of the proliferating T cells from the AGMs (but not the rhesus macaques) downregulated CD4 (Fig. 5a). CD4 surface expression, measured by the median fluorescence intensity for each generation of dividing cells, decreased on average with each cell division in AGM cells (Fig. 5b to d, left) independent of SIV infection and was significantly lower in cells that had divided 6 times than in cells that did not divide (P < 0.0015). That proliferating cells downregulated CD4 (Fig. 5a) and CD8αα T cells proliferated well in response to homeostatic cytokines (data not shown) may explain how this population becomes such a large proportion of the total T cell pool.
FIG 5.
CD4 downregulation induced by homeostatic cytokines. AGM and rhesus macaque PBMC were CFSE labeled and sorted for CD4+ CD3+ T cells and CD3− NKG2A− HLA-DR+ antigen-presenting cells. These were cultured with IL-2 for 6 days or IL-7 or IL-15 for 7 days and analyzed by flow cytometry. (a) Plots gated on live CD3+ T cells showing CD4 (y axis) and CFSE (x axis). (b to d, left) Median fluorescence intensity of CD4 plotted for AGM cells that have divided the indicated number of times based on CFSE dilution. MFI of CD4 in undivided cells (zero divisions) compared to cells that had divided six times using a paired t test. (b to d, right) Percentage of AGM T cells in each population that expressed a memory phenotype by CD28 and CD95 staining. Undivided CD4+ T cells were compared to each of the other populations by paired t test (P < 0.004 in all cases). Adult animals are denoted by squares, juvenile animals by circles, SIV+ animals by filled symbols, and SIV− animals by open symbols. Median values for each population are shown.
Notably, the cells that responded to the homeostatic cytokines had a memory phenotype based on expression of CD28 and CD95, including cells that divided and downregulated to become CD8αα cells, cells that maintained CD4 expression while dividing, and those that downregulated CD4 but did not divide (Fig. 5b to d, right). This suggests either that memory cells preferentially respond to homeostatic cytokines in AGMs or that their signaling in AGMs imparts a memory phenotype. Results were similar in cells from SIV-infected and uninfected AGMs stimulated with IL-2 (Fig. 5b). Of the T cells that divided in response to homeostatic cytokines, 30% to 90% downregulated CD4 (data not shown), and all of them upregulated CD8α (see Fig. S1a in the supplemental material). These results indicate that homeostatic cytokines can induce CD4+-to-CD8αα conversion in AGM T cells.
To determine whether CD4 downregulation was sensitive to cytokine concentration, we cultured sorted, CFSE-labeled CD4+ CD3+ T cells and CD3− NKG2A− HLA− DR+ APCs with various concentrations of IL-2 spanning two orders of magnitude and measured the frequency of downregulation among cells that divided. The number of cells that downregulated CD4 when responding to 1 to 100 ng/ml of IL-2 was similar in both SIV-uninfected animals at the concentrations tested (see Fig. S1 in the supplemental material). This result suggests that CD4 downregulation in response to the homeostatic cytokine IL-2 occurs for a wide range of cytokine concentrations.
DISCUSSION
Our previous work produced a model for how AGMs avoid SIV disease progression through CD4 downregulation to produce a population of CD8αα T cells that maintain the immunological functions of CD4+ T cells but are resistant to SIV infection. Here, we sought to test this model and the role of the CD8αα T cells in maintaining immunity in AGMs with differing levels of immune experience and to identify the signals that lead to CD4 downregulation. We found that immunologically inexperienced juvenile AGMs could be infected with SIVAGM and had viral replication dynamics similar to those of adults. Like adult AGMs, the juvenile animals did not progress to AIDS; instead, they showed an accelerated conversion of CD4+ to CD8αα T cells after SIV infection, giving them a more adult-like immune phenotype. CD4 downregulation was not limited to SIV-specific T cells. Vaccination studies with an antigen that the animals had not previously encountered demonstrated that adult and juvenile AGMs made robust T cell responses to neoantigens even after SIV infection, and that CD4 downregulation could occur prior to antigen exposure. Finally, we showed that IL-2, IL-7, or IL-15 treatment led to CD4 downregulation in vitro. These data are consistent with the hypothesis that CD8αα T cells play a central role in avoiding SIV disease and identify, for the first time, homeostatic signals that lead to the formation of this important population.
We investigated juvenile AGMs in order to test our model that CD8αα T cells are responsible for the nonprogressive phenotype of SIV infection in this species. A previous study had examined infection of newborn AGMs and found no evidence of disease progression (22), but as the study did not distinguish between CD8αα T cells and classical CD8αβ T cells, we were unable to draw conclusions about the role of CD8αα T cells in nonprogression. A recent study identified infected juvenile AGMs in the wild and found no evidence of AIDS, but they were unable to obtain T cell counts from specimens taken in the wild (23). Although the juvenile animals that we infected were immunologically inexperienced and had low numbers of CD8αα T cells at the time of infection, their accelerated conversion of CD4+ to CD8αα T cells after SIV infection precluded our determination of whether the absence of CD8αα T cells would lead to progressive disease. However, that the accelerated generation of CD8αα T cells occurred in SIV+ juveniles and that there was no evidence of disease progression is consistent with our model. Furthermore, the accelerated conversion observed after SIV infection in T cells not specific for SIV suggests that the processes that drive downregulation are robust. It is consistent with a role for homeostatic cytokines inducing CD4 downregulation that immunologically inexperienced juvenile AGMs have few CD8αα T cells, as naive CD4 T cells are known to have relatively low turnover in humans and nonhuman primates (24).
The finding that CD4 downregulation can occur prior to antigen exposure has key implications for the diversity of the CD8αα T cell pool and, consequently, the range of pathogens to which individual AGMs can respond. The CD8αα T cells make up the majority of T cells in the blood and at effector sites in adult AGMs; therefore, they are expected to be central players in AGM immunity, while the CD4+ T cells make up only a small fraction of T cells at important sites, such as the GI tract, even in SIV-uninfected AGMs (5). If a dramatic imbalance existed in the diversity of the T cell repertoire between the CD4+ and CD8αα T cell populations, it is hard to imagine that adequate immunity could be provided by CD8αα T cells in the absence of CD4+ T cells. A previous report suggested that there was not a clear difference in T cell receptor distribution among the CD4+ and CD8αα populations in 3 AGMs (6). Our finding that homeostatic cytokines induce CD4 downregulation in an antigen-independent manner provides a mechanism for this diversity. That the responding T cells in vitro have a memory phenotype suggests an explanation for the memory phenotype of CD8αα T cells in vivo. Furthermore, our finding that an AGM lacking almost any CD4+ T cells was able to make a robust MHC class II-restricted response to a vaccine antigen to which it had not been previously exposed strongly supports the model that the CD8αα T cell repertoire is diverse and not limited to clones previously primed with antigen, despite the memory phenotype of its constituent T cells.
Importantly, the CD8αα T cells do not protect the AGMs through a superior SIV-specific T cell response. Consistent with previous studies (7, 25), the SIV Gag-specific T cell response was not of exceptional magnitude, and the immune response to SIV clearly was not able to fully control viremia in the study animals. Instead, it is clear that natural host species are able to avoid progressive disease despite ongoing viral replication. In AGMs, the numerical dominance of the CD8αα population in the GI tract, even in the absence of SIV, suggests that it is likely the CD8αα T cells rather than the CD4+ T cells that are responsible for maintenance of the GI tract immune barrier and that their continued functioning in the presence or absence of SIV allows the AGMs to avoid chronic microbial translocation and its downstream ill effects. It has been shown previously that SIV infection does not cause preferential depletion of Th17 cells in AGMs, as it does in nonnatural host species, such as pigtail macaques, but that CD4+ T cells overall are lost in the colon after SIV infection, leading to an overall decrease in the number of IL-17-producing CD4+ T cells (26). Consistent with maintenance of this important function by CD4− T cells after SIV infection, the relative abundance of RORc mRNA in the colon of AGMs did not decrease after SIV infection despite the loss of CD4+ T cells in this anatomical site (26).
The ability of CD4− T cells to perform immunological functions normally associated with CD4+ T cells has been demonstrated in many species of natural hosts (10). The role of CD4− T cells in maintaining immunity during SIV infection of natural hosts has been identified in a subset of sooty mangabeys that experience severe CD4+ T cell depletion but do not develop opportunistic infections, likely through maintenance of immunological function by CD4− CD8− double-negative T cells (11, 13). Although it is not known how the double-negative T cells in sooty mangabeys develop, our results suggest that the role of homeostatic cytokines is an avenue of investigation to better define whether this mechanism is evolutionarily conserved in natural host species.
The existence of several sooty mangabeys with low CD4+ T cell counts but no evidence of immunodeficiency is an example of the current model of adaptive uncoupling of T cell populations that support viral replication from those that support immunity in natural host species but not in nonnatural host species (27). Our results and those of others suggest that the Cercopithecini lineage of AGMs and Patas monkeys followed this evolutionary path to a point where viral clearance could be the result of this uncoupling followed by loss of the cells that support viral replication. There is evidence that this has already occurred in the Patas, and there is the intriguing possibility that it can occur in AGMs as well. The existence of an AGM, AG346, with very few CD4+ T cells for multiple years and no evidence of immunodeficiency strongly suggests that in both steady-state and vaccination conditions, the CD8αα T cells in AGMs are sufficient to provide CD4-like functions. This is consistent with a previous report of another healthy AGM at steady state with few CD4+ T cells (7) and a similar Patas monkey who lacked evidence of immunodeficiency with a negligible number of CD4+ T cells (14). Moreover, that AG346 became aviremic concomitant with the loss of CD4+ T cells suggests that downregulation of CD4 by all CD4+ T cells would limit the ability of the host to support viral replication for prolonged periods of time. That CD4+ T cell availability limits viral replication is consistent with the finding that AGM susceptibility to mucosal infection is proportional to the number of target CD4+ T cells at the site of infection (28). Therefore, it is tempting to speculate that viral clearance results from the complete loss of CD4+ T cells, and the data suggest that this loss in AGMs would not lead to immunodeficiency due to the immunological functioning of the CD8αα T cells.
In the experiments described here, CD4 downregulation was most readily observed in T cells that divided in response to homeostatic cytokines. Because these cytokines act to cause division, it is difficult to experimentally separate the process of division from the signaling that may be required for CD4 downregulation. For this reason, we do not know whether CD4 downregulation is intrinsically linked to cellular division or whether it can occur in cells that do not divide.
We have identified the T cell homeostatic cytokines IL-2, IL-7, and IL-15 as antigen-independent signals that can induce CD4 downregulation in AGMs, which allows the creation of a diverse population of T cells that are MHC class II restricted but are resistant to SIV infection. As these T cells likely are responsible for the maintenance of immunological function in these animals, a better understanding of this mechanism will allow the development of treatments aimed at inducing this phenomenon in humans to cure AIDS.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Heather Cronise, Joanna Swerczek, Richard Herbert, and all of the veterinary staff at the NIH NIAID Animal Center. We also thank David Ambrozak, Rick Koup, and Mario Roederer of the NIH Vaccine Research Center for assistance with live cell sorts. We are grateful to Patricia Darrah and Barbara Flynn for providing the MML protein and poly ICLC used for vaccinations. We thank Martha Nason for assistance with statistical methods. We thank the CLIC/BBC for advice and helpful discussions.
Funding for this study was provided in part by the Division of Intramural Research/NIAID/NIH.
The content of this publication does not necessarily reflect the views or policies of DHHS, and the mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government.
Footnotes
Published ahead of print 2 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01331-14.
REFERENCES
- 1.VandeWoude S, Apetrei C. 2006. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 19:728–762. 10.1128/CMR.00009-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein S, Ourmanov I, Brown CR, Plishka R, Buckler-White A, Byrum R, Hirsch VM. 2005. Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J. Virol. 79:5153–5162. 10.1128/JVI.79.8.5153-5162.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69:955–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 15:879–885. 10.1038/nm.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 179:3035–3046. 10.4049/jimmunol.179.5.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murayama Y, Amano A, Mukai R, Shibata H, Matsunaga S, Takahashi H, Yoshikawa Y, Hayami M, Noguchi A. 1997. CD4 and CD8 expressions in African green monkey helper T lymphocytes: implication for resistance to SIV infection. Int. Immunol. 9:843–851. 10.1093/intimm/9.6.843 [DOI] [PubMed] [Google Scholar]
- 7.Murayama Y, Mukai R, Inoue-Murayama M, Yoshikawa Y. 1999. An African green monkey lacking peripheral CD4 lymphocytes that retains helper T cell activity and coexists with SIVagm. Clin. Exp. Immunol. 117:504–512. 10.1046/j.1365-2249.1999.00999.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz JE, Ma ZM, Hagan EA, Wilks AB, Furr KL, Linde CH, Zahn RC, Brenchley JM, Miller CJ, Permar SR. 2012. Memory CD4(+) T lymphocytes in the gastrointestinal tract are a major source of cell-associated simian immunodeficiency virus in chronic nonpathogenic infection of African green monkeys. J. Virol. 86:11380–11385. 10.1128/JVI.01556-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandrea I, Onanga R, Souquiere S, Mouinga-Ondeme A, Bourry O, Makuwa M, Rouquet P, Silvestri G, Simon F, Roques P, Apetrei C. 2008. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J. Virol. 82:5501–5509. 10.1128/JVI.02555-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinton C, Klatt NR, Harris LD, Briant JA, Sanders-Beer BE, Herbert R, Woodward R, Silvestri G, Pandrea I, Apetrei C, Hirsch VM, Brenchley JM. 2011. CD4-like immunological function by CD4− T cells in multiple natural hosts of simian immunodeficiency virus. J. Virol. 85:8702–8708. 10.1128/JVI.00332-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA, Reeves JD, Anton E, O'Neill E, Butler E, Hancock K, Cole KS, Brenchley JM, Else JG, Silvestri G, Sodora DL. 2011. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J. Clin. Investig. 121:1102–1110. 10.1172/JCI44876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundaravaradan V, Saleem R, Micci L, Gasper MA, Ortiz AM, Else J, Silvestri G, Paiardini M, Aitchison JD, Sodora DL. 2013. Multifunctional double-negative T cells in sooty mangabeys mediate T-helper functions irrespective of SIV infection. PLoS Pathog. 9:e1003441. 10.1371/journal.ppat.1003441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, Miamidian JL, Paiardini M, Barry AP, Staprans SI, Silvestri G, Sodora DL. 2007. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J. Immunol. 179:3047–3056. 10.4049/jimmunol.179.5.3047 [DOI] [PubMed] [Google Scholar]
- 14.Apetrei C, Gaufin T, Gautam R, Vinton C, Hirsch V, Lewis M, Brenchley J, Pandrea I. 2010. Pattern of SIVagm infection in Patas monkeys suggests that host adaptation to simian immunodeficiency virus infection may result in resistance to infection and virus extinction. J. Infect. Dis. 202(Suppl 3):S371–S376. 10.1086/655970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 16.Goldstein S, Ourmanov I, Brown CR, Beer BE, Elkins WR, Plishka R, Buckler-White A, Hirsch VM. 2000. Wide range of viral load in healthy African green monkeys naturally infected with simian immunodeficiency virus. J. Virol. 74:11744–11753. 10.1128/JVI.74.24.11744-11753.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coler RN, Reed SG. 2005. Second-generation vaccines against leishmaniasis. Trends Parasitol. 21:244–249. 10.1016/j.pt.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 18.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097. 10.1038/nature03501 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein S, Brown CR, Ourmanov I, Pandrea I, Buckler-White A, Erb C, Nandi JS, Foster GJ, Autissier P, Schmitz JE, Hirsch VM. 2006. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J. Virol. 80:4868–4877. 10.1128/JVI.80.10.4868-4877.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, Seder RA. 2010. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J. Exp. Med. 207:1421–1433. 10.1084/jem.20092532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klatt NR, Vinton CL, Lynch RM, Canary LA, Ho J, Darrah PA, Estes JD, Seder RA, Moir SL, Brenchley JM. 2011. SIV infection of rhesus macaques results in dysfunctional T-and B-cell responses to neo and recall Leishmania major vaccination. Blood 118:5803–5812. 10.1182/blood-2011-07-365874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beer B, Denner J, Brown CR, Norley S, zur Megede J, Coulibaly C, Plesker R, Holzammer S, Baier M, Hirsch VM, Kurth R. 1998. Simian immunodeficiency virus of African green monkeys is apathogenic in the newborn natural host. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:210–220. 10.1097/00042560-199807010-00003 [DOI] [PubMed] [Google Scholar]
- 23.Ma D, Jasinska A, Kristoff J, Grobler JP, Turner T, Jung Y, Schmitt C, Raehtz K, Feyertag F, Martinez Sosa N, Wijewardana V, Burke DS, Robertson DL, Tracy R, Pandrea I, Freimer N, Apetrei C. 2013. SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations. PLoS Pathog. 9:e1003011. 10.1371/journal.ppat.1003011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Boer RJ, Perelson AS. 2013. Quantifying T lymphocyte turnover. J. Theor. Biol. 327:45–87. 10.1016/j.jtbi.2012.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahn RC, Rett MD, Korioth-Schmitz B, Sun Y, Buzby AP, Goldstein S, Brown CR, Byrum RA, Freeman GJ, Letvin NL, Hirsch VM, Schmitz JE. 2008. Simian immunodeficiency virus (SIV)-specific CD8+ T-cell responses in vervet African green monkeys chronically infected with SIVagm. J. Virol. 82:11577–11588. 10.1128/JVI.01779-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. 2009. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5:e1000295. 10.1371/journal.ppat.1000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenchley JM, Silvestri G, Douek DC. 2010. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity 32:737–742. 10.1016/j.immuni.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandrea I, Parrish NF, Raehtz K, Gaufin T, Barbian HJ, Ma D, Kristoff J, Gautam R, Zhong F, Haret-Richter GS, Trichel A, Shaw GM, Hahn BH, Apetrei C. 2012. Mucosal simian immunodeficiency virus transmission in African green monkeys: susceptibility to infection is proportional to target cell availability at mucosal sites. J. Virol. 86:4158–4168. 10.1128/JVI.07141-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weatherall D. 2006. The use of non-human primates in research. Available at https://royalsociety.org/policy/publications/2006/weatherall-report/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.