Abstract
Glycogen storage disease type I (GSD-I) consists of two subtypes: GSD-Ia, a deficiency in glucose-6- phosphatase-α (G6Pase-α) and GSD-Ib, which is characterized by an absence of a glucose-6-phosphate (G6P) transporter (G6PT). A third disorder, G6Pase-β deficiency, shares similarities with this group of diseases. G6Pase-α and G6Pase-β are G6P hydrolases in the membrane of the endoplasmic reticulum, which depend on G6PT to transport G6P from the cytoplasm into the lumen. A functional complex of G6PT and G6Pase-α maintains interprandial glucose homeostasis, whereas G6PT and G6Pase-β act in conjunction to maintain neutrophil function and homeostasis. Patients with GSD-Ia and those with GSD-Ib exhibit a common metabolic phenotype of disturbed glucose homeostasis that is not evident in patients with G6Pase-β deficiency. Patients with a deficiency in G6PT and those lacking G6Pase-β display a common myeloid phenotype that is not shared by patients with GSD-Ia. Previous studies have shown that neutrophils express the complex of G6PT and G6Pase-β to produce endogenous glucose. Inactivation of either G6PT or G6Pase-β increases neutrophil apoptosis, which underlies, at least in part, neutrophil loss (neutropenia) and dysfunction in GSD-Ib and G6Pase-β deficiency. Dietary and/or granulocyte colony-stimulating factor therapies are available; however, many aspects of the diseases are still poorly understood. This Review will address the etiology of GSD-Ia, GSD-Ib and G6Pase-β deficiency and highlight advances in diagnosis and new treatment approaches, including gene therapy.
Introduction
Glycogen storage disease type I (GSD-I), also known as von Gierke disease, consists of a group of autosomal recessive disorders that occur with an overall incidence of approximately 1 in 100,000 individuals.1,2 The subtype GSD-Ia is caused by an inactivating mutation in the gene G6PC, which encodes glucose-6-phosphatase-α (G6Pase-α),3,4 whereas the subtype GSD-Ib results from an inactivating mutation in SLC37A4,5,6 the gene that encodes a glucose-6-phosphate transporter (G6PT).5–8 Two additional subtypes, GSD-Ic and GSD-Id, were originally categorized as GSD-I disorders,1,2 but most, if not all, reported cases of GSD-Ic and GSD-Id have been genotyped and were shown to harbor the SLC37A4 mutations also found in patients with GSD-Ib.9–12 G6PT deficiency is, therefore, implicated in all reported cases of GSD-Ib, GSD-Ic and GSD-Id. This notion is consistent with the biochemistry of the disease and the finding that G6PT is a eukaryotic antiporter that transports glucose-6-phosphate (G6P) into the lumen of the endoplasmic reticulum in exchange for inorganic phosphate.13
Interprandial (between meals) blood glucose homeostasis is maintained primarily by a complex of G6PT and G6Pase-α that catalyzes the hydrolysis of the intracellular G6P to glucose in the terminal step of gluconeogenesis and glycogenolysis in the liver, kidney and intestine (Figures 1 and 2). In this complex, G6Pase-α and G6PT are coupled functionally, rather than physically.1,2 G6PT transports G6P from the cytoplasm into the lumen of the endoplasmic reticulum, where it is hydrolyzed to glucose and inorganic phosphate by G6Pase-α.1,2 A detrimental mutation in either protein disrupts this functional process and causes the same metabolic phenotype.1,2
Figure 1.
The primary anabolic and catabolic pathways of glucose-6-phosphate in gluconeogenic organs. G6Pase-α and G6PT are shown embedded within the membrane of the endoplasmic reticulum. GLUT2, the transporter responsible for the transport of glucose in and out of the cell in liver, kidney and intestine, is shown embedded in the plasma membrane. Abbreviations: G6P, glucose-6-phosphate; G6Pase-α, glucose-6-phosphatase-α; G6PT, glucose-6-phosphate transporter; GLUT2, solute carrier family 2, facilitated glucose transporter member 2; P, phosphate; Pi, inorganic phosphate; UDP, uridine diphosphate.
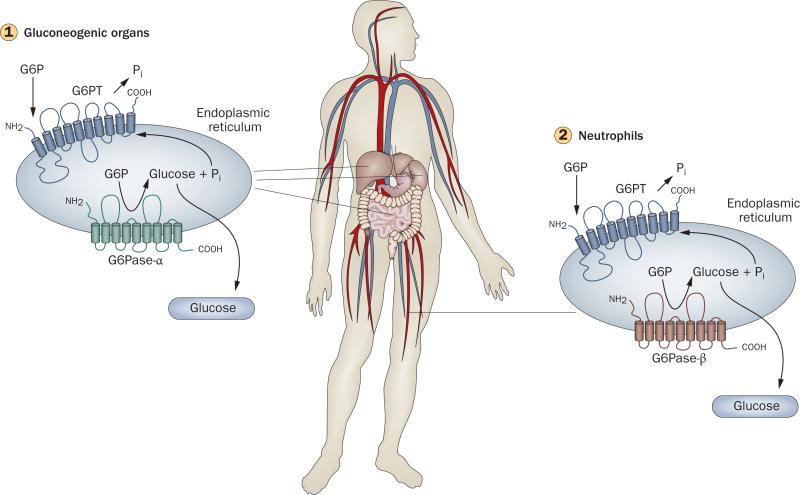
Figure 2.
Glucose homeostasis in gluconeogenic organs and neutrophils. (1) Interprandial glucose homestasis is maintained by the endoplasmic reticulum-associated G6PT/G6Pase-α complex.1,2 Disruption of G6PT results in glycogen storage disease (GSD) type Ib, whereas disruption of G6Pase-α results in GSD-Ia. G6Pase-α is expressed primarily in the liver, kidney and intestine and hydrolyzes G6P to glucose and phosphate, whereas G6PT is ubiquitously expressed and transports G6P from the cytoplasm into the lumen of the endoplasmic reticulum. Glucose generated in the gluconeogenic organs is released into the blood for use by nongluconeogenic organs between meals. without this pathway, blood glucose homeostasis is lost. (2) Neutrophil homeostasis is maintained by the endoplasmic reticulum-associated G6PT/G6Pase-β complex.19,20,37 Unlike G6Pase-α, G6Pase-β is ubiquitously expressed. The G6PT/G6Pase-β complex acts in a manner similar to the G6PT/G6Pase-α complex, with G6PT transporting G6P from the cytoplasm into the lumen of the endoplasmic reticulum and G6Pase-β hydrolyzing G6P to glucose to maintain energy homeostasis in neutrophils. Deficiencies in G6PT (GSD-Ib) or G6Pase-α (GSD-Ia) result in a disturbed blood glucose homeostasis. Deficiencies in G6PT (GSD-Ib) or G6Pase-β result in neutrophil dysfunction. The common role of G6PT explains the neutrophil dysfunction seen in GSD-Ib but not GSD-Ia. Abbreviations: G6P, glucose-6-phosphate; G6Pase-α, glucose-6-phosphatase-α; G6Pase-β, glucose-6-phosphatase-β; G6PT, glucose-6-phosphate transporter.
An additional, characteristic myeloid dysfunction is unique to patients with GSD-Ib. G6Pase-α is expressed primarily in gluconeogenic organs—the liver, kidney and intestine14—whereas G6PT is expressed ubiquitously.15 Myeloid dysfunction in patients with G6PT deficiency, thus, suggested either other biological activities of G6PT in the immune system or that a second G6Pase exists in myeloid tissues. The latter is now known to be true.16,17 A second G6Pase, G6Pase-β, is encoded by the gene G6PC3 and exhibits a ubiquitous expression pattern similar to that of G6PT. In analogy to the complex formed by G6Pase-α and G6PT, G6Pase-β also couples functionally with G6PT to hydrolyze G6P to glucose and inorganic phosphate.16,18 A new framework has now emerged which shows that the G6PT/G6Pase-α complex maintains interprandial glucose homeostasis,1,2 whereas the G6PT/G6Pase-β complex maintains neutrophil homeostasis and function (Figure 2).19,20 Despite the structural and functional similarities between G6Pase-α and G6Pase-β, patients with G6Pase-β deficiency do not manifest the metabolic phenotype of individuals with GSD-I. Instead, these patients show a severe congenital neutropenia syndrome,19,21 which highlights the dif ferences between the overall phenotypes of GSD-Ia and GSD-Ib.
Genotype
GSd-Ia
GSD-Ia is the most prevalent subtype and represents approximately 80% of GSD-I cases.1 84 separate G6PC mutations have been identified (Table 1). The human G6PC gene is a single-copy gene composed of five exons on chromosome 17q21.3,4 It encodes the highly hydrophobic, 357-amino acid glycoprotein G6Pase-α.3 nine transmembrane helices anchor the protein in the endoplasmic reticulum, with the amino-terminus in the lumen and the carboxyl-terminus in the cytoplasm (Figure 1).22 Mutational and active-site labeling studies have elucidated the reaction mechanism of G6Pase-α (Figure 3): to hydrolyze G6P, His176 acts as a nucleo- phile on the phosphate bound by glucose to form a phosphohistidine- enzyme intermediate that is stabilized by hydrogen bonds with Arg83. His119 provides the proton that liberates the glucose molecule.23
Table 1.
Molecular genetics of GSD-I and G6Pase-β deficiency
| Characteristics | GSD-Ia | GSD-Ib | G6Pase-β deficiency |
|---|---|---|---|
| Protein | G6Pase-α | G6PT | G6Pase-β |
| Gene | G6PC | SLC37A4 | G6PC3 |
| Unique mutations identified | 84 in 550 patients24 | 80 in 160 patients37 | 11 in 15 patients21,46,47 |
| Missense mutations | 54 | 32 | 5 |
| Nonsense mutations | 10 | 11 | 3 |
| Insertions and/or deletions mutations | 17 | 20 (including 1 gross deletion) | 3 |
| Splicing mutations | 3 | 17 | 0 |
Abbreviations: G6Pase, glucose-6-phosphatase; G6PT, glucose-6-phosphate transporter; GSD-I, glycogen storage disease type I.
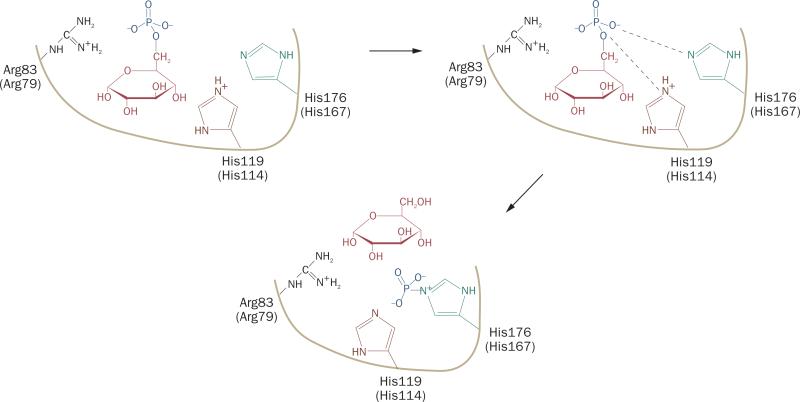
Figure 3.
Proposed roles of the active-site residues in G6Pase-α and G6Pase-β during catalysis. To hydrolyze G6P, His176 of G6Pase-α acts as a nucleophile on the phosphate bound by glucose to form a phosphohistidine-enzyme intermediate that is stabilized by hydrogen bonds with Arg83. His119 provides the proton that liberates the glucose molecule.23 The single thick line represents the general backbone of the protein, which lies within the membrane of the endoplasmic reticulum. The active-site residues in G6Pase-α are Arg83, His119 and His17623 and in G6Pase-β are Arg79, His114, and His16718 (in parenthesis). Abbreviation: G6Pase, glucose-6-phosphatase.
GSD-Ia is not restricted to any one racial or ethnic group; however, mutations that seem unique to white patients with GSD-Ia, as well as to those of Hispanic, Chinese, Japanese, Korean and Jewish ethnicity have been described (Table 2).24 50 missense, two nonsense and two codon deletion mutations have been functionally characterized by site-directed mutagenesis and transient expression assays.3,4,24–29 of the missense mutations, 32 mutations completely abolish G6Pase-α activity, whereas the other 18 mutations retain varying degrees of residual enzymatic acti vity.24 Several studies have investigated the relationship between residual enzyme activity and disease severity, but the overall consensus is that few, if any, stringent genotype– phenotype relationships for G6PC mutations exist.27,30–32 nakamura et al.33 studied 19 patients with GSD-Ia who carry the homo zygous c.648G>T splicing mutation and found that four patients (21%) developed hepatocellular carcinoma at a mean age of 48.3 years. In the general patient population, only 10% of patients with hepatocellular adenomas undergo malignant trans- formation to hepatocellular carcinoma. The high incidence of carcinoma observed by nakamura et al. must be considered cautiously. Such single site studies can suffer substantial patient selection bias from factors such as the presenting symptoms, severity of disease and patient age. Akanuma et al.34 have also suggested a genotype–phenotype correlation on the basis of a study of 40 patients with GSD-Ia who carry the homozygous c.648G>T splicing mutation. Common to this group of patients was the absence of recurrent episodes of severe hypoglycemia in infancy as opposed to the majority of patients with GSD-Ia. This group of patients exhibited marked variability in the age of onset of the disease and the severity of symptoms and complications, including 11 cases with hepatocellular adenoma with two progressing to hepatocellular carcinoma. This genotype–phenotype relationship may arise from a leaky splice mutation.
Table 2.
Prevalence of mutations in GSD-I
| Patient ethnicity | Mutation | Frequency (%) |
|---|---|---|
| GSD-Ia (Mutations in G6PC)24 | ||
| White (676)* | Arg83Cys | 33 |
| Gln347X‡ | 18 | |
| Chinese (112) | c.648G>T | 54 |
| Arg83His | 26 | |
| Japanese (172) | c.648G>T | 91 |
| Korean (28) | c.648G>T | 75 |
| Hispanics (24) | c.380–381insTA | 54 |
| Jewish (94) | Arg83Cys | 98 |
| Gln347X | 2 | |
| GSD-Ib (Mutations in SLC37A4)37 | ||
| White (216) | c.1042–1043delCT | 31 |
| Gly339Cys | 14 | |
| Japanese (38) | Trp118Arg | 37 |
Number of analzyed alleles is presented in brackets.
X represents a stop codon.
Abbreviation: GSD-I, glycogen storage disease type I.
The overall lack of genotype–phenotype correlation indicates that one or more as yet unidentified modifiers may exist that can compensate for, or stabilize, low level expression in vivo. evidence for the presence of such factors is suggested by one report of patients with GSD-Ia who carry a homozygous Gly188Arg mutation in the G6PC gene, as these individuals exhibit a myeloid phenotype, similar to that of patients with GSD-Ib, which is characterized by neutropenia and impaired neutrophil respiratory burst, chemotaxis and bacterial killing activities.35 However, mutations in the G6PT gene were not detected in these individuals. Furthermore, patients with compound heterozygous mutations in G6PC, with one Gly188Arg mutation, do not show this unusual phenotype.
GSd-Ib
The human SLC37A4 gene is a single-copy gene that consists of nine exons5,8 on chromosome 11q23.7 It encodes G6PT, a 429-amino acid protein with 10 transmembrane domains in the membrane of the endoplasmic reticulum (Figures 1 and 4).36 G6PT belongs to the organophosphate–phosphate antiporter family of the major facilitator superfamily and has been shown to be a phosphate-linked transporter that exchanges cytoplasmic G6P for inorganic phosphate stored in the lumen of the endoplasmic reticulum.13 of 80 identified mutations (Table 1), 31 missense and two codon deletion mutations have been functionally charac terized by site-directed mutagenesis and by both cell-based G6P transport activity assays5,37–39 and reconstituted proteoliposome transport assays.13,40 G6P uptake activity is completely abolished by 21 missense mutations and the two codon deletions, whereas the other mutations only partially inactivate the transporter. GSD-Ib is not restricted to any one racial or ethnic group, but the mu tations show some racial and ethnic variability (Table 2).
Figure 4.
Proposed pathways for glucose-6-phosphate metabolism in neutrophils. Glucose transported into the cytoplasm via GLUT1, the main glucose transporter in neutrophils, is metabolized by hexokinase to G6P, which can participate in glycolysis, hexose monophosphate shunt or glycogen synthesis or can be transported into the lumen of the endoplasmic reticulum by G6PT. In wild-type neutrophils, G6P localized within the lumen of the endoplasmic reticulum can be hydrolyzed by G6Pase-β, and the resulting glucose is transported back into the cytoplasm to re-enter any of the previously mentioned cytoplasmic pathways. (1) In G6PT-deficient neutrophils, cytoplasmic G6P cannot be transported into the lumen of the endoplasmic reticulum and blocks glucose/G6P recycling. (2) In G6Pase-β-deficient neutrophils, endoplasmic reticulum-localized G6P cannot be hydrolyzed to glucose, thus also blocks glucose/G6P recycling. The GLUT1 transporter, responsible for the transport of glucose in and out of the cell, is shown embedded in the plasma membrane. The G6PT transporter, responsible for the transport of G6P into the endoplasmic reticulum and G6Pase-β, responsible for hydrolyzing G6P to glucose and phosphate, are shown embedded in the endoplasmic reticulum membrane with 10 and nine transmembrane helices, respectively. Abbreviations: G6P, glucose-6-phosphate; G6Pase-β, glucose-6-phosphatase-β; G6PT, glucose-6-phosphate transporter, GLUT1, solute carrier family 2, facilitated glucose transporter member 1; HMS, hexose monophosphate shunt.
Comparable to the G6PC mutations, a strict genotype–phenotype relationship has not been determined. Indeed, a study of 22 patients with GSD-Ib with 16 different SLC37A4 mutations that included nine patients homozygous or compound heterozygous for nonsense mutations failed to show any correlation between any individual mutation and the presence of disease characteristics, such as neutropenia, bacterial infections and systemic complications.41 notably, not all patients with GSD-Ib seem to develop neutropenia, as shown by case reports of at least seven patients with deleterious SLC37A4 mutations who did not develop this complica- tion.41–44 Together, these data suggest that, as with G6PC mutations, yet unidentified factors can compensate or stabilize low level expression of G6PT in vivo.
G6Pase-β deficiency
The human, single-copy G6PC3 gene consists of six exons on chromosome 17q2145 and encodes G6Pase-β, a highly hydrophobic, 346-amino acid polypeptide.16 Despite marked structure similarity between G6Pase-α and G6Pase-β, the two enzymes share only 36% amino acid homology. Similar to G6Pase-α,22 G6Pase-β18 is a protein in the endoplasmic reticulum with nine transmembrane domains (Figure 4).18 Both enzymes exhibit a similar Michaelis–Menten constant KM—the concentration of G6P that leads to half of the maximum reaction rate (vmax)—but the vmax of G6Pase-α is about sixfold greater than that of G6Pase-β.16 Sequence alignment with G6Pase-α suggests the active center of G6Pase-β is comprised of Arg79, His114 and His167, which are inside the lumen of the endoplasmic reticulum. Site-directed mutagenesis and transient expression assays support this notion, as mutations in any of these proposed catalytic-site residues abolish enzyme activity.16 Active-site labeling has also established His167 as the nucleophile that covalently binds the glucose-bound phosphate to form the phosphohistidine-G6Pase-β intermediate during catalysis (Figure 3),18 analogous to His176 in G6Pase-α.23
To date, 11 mutations, including five missense, three nonsense and three insertions and/or deletions, have been identified in 15 G6Pase-β-deficient patients (Table 1).21,46,47 The Arg253His mutant was shown to have little or no phosphohydrolase activity in a yeast expression system.21 This finding must be interpreted with caution, as the yeast expression system is a relatively insensitive assay that contains background activity >40% of wild-type activity.21 Further functional charac- terization of G6Pase-β mutations is required, preferably in sensitive, low-background assays such as those based on the adenoviral expression system that were used to characterize the active-site mutants of G6Pase-β.16
Phenotype
Metabolic phenotype
Both GSD-Ia and GSD-Ib are lethal in childhood, if untreated. These disorders share a common metabolic pheno type,1,2 the hallmark of which is hypoglycemia follow ing a short fast, associated with increased levels of G6P in the cytoplasm. This increase stimulates alternative metabolic pathways (Figure 1), thus leading to the syn thesis and excessive accumulation of glycogen in the liver and kidney, a process that promotes progressive hepatomegaly and nephromegaly. Hepatomegaly is further exacerbated by an accumulation in liver neutral lipids. other major metabolic consequences of elevated cyto plasmic G6P are hypercholesterolemia, hyper triglyceridemia, hyperuricemia and lactic acidemia.1,2 long-term consequences include growth retardation, osteoporosis, gout, pulmonary hypertension, hepato cellular adenomas with risk of malignan cy and renal disease.
A number of other consequences of metabolic disruption, although not extensively documented, have been reported. A study of 20 patients with GSD-Ia and six patients with GSD-Ib showed that 61.5% of study participants had suboptimal levels of 25- hydroxyvitamin D, and six of seven patients had clinical evidence of osteopenia or osteoporosis.48 Bandsma et al.49 examined lipoprotein kinetics, lipogenesis and cholesterogenesis, as well as whole-body lipolysis in seven patients with GSD-Ia and showed that hyperlipidemia is associated with marked increases in lipogenesis, cholesterogenesis and a slower relative conversion of vlDl cholesterol to lDl cholesterol compared with that in healthy individuals. Melis et al.50 evaluated the growth hormone (GH)–insulin-like growth factor (IGF) system in 10 patients with GSD-Ia and seven individuals with GSD-Ib and showed that the impairment of the GH–IGF system differed between these disorders. In GSD-Ia, the prevalence of short stature was low, whereas in GSD-Ib an increased prevalence of short stature, partially related to GH deficiency caused by the presence of antipituitary antibodies, was found.
Myeloid phenotype
Except for a small number of patients with the homozygous Gly188Arg mutation,35 myeloid dysfunction is not observed in patients with GSD-Ia.1,2 By contrast, neutro penia and neutrophil dysfunction are major clinical presentations in patients with GSD-Ib.1,2 neutrophils from this patient population exhibit impairment of chemotaxis, calcium mobilization, respiratory burst and phagocytotic activities.51,52 As a result, recurrent bacterial infections are a characteristic of GSD-Ib. up to 77% of patients that exhibit neutropenia also develop inflammatory bowel disease, now more often described as enterocolitis, which is indistinguishable from idiopathic Crohn disease.53,54 A study of seven patients with GSD-Ib that evaluated the hypothalamus–pituitary–thyroid axis also showed that 57% of study participants had thyroid autoimmunity and hypothyroidism.55
For decades, the etiology of neutropenia and/or neutro phil dysfunction in GSD-Ib and its relationship to glucose homeostasis was unknown. In 2003, Kuijpers et al.56 showed that neutrophils of patients with GSD-Ib exhibit enhanced apoptosis, which suggested a causal relationship between apoptosis and neutro penia, but the underlying cause of neutropenia remained undetermined. with the discovery of G6Pase-β and its expression in neutrophils,19 suggestions arose that endo genous glucose production by G6PT and G6Pase-β in the endoplasmic reticulum of neutrophils was critical for neutrophil homeostasis and function. As was found for the functional complex of G6PT and G6Pase-α, an inactivating mutation in either G6PT or G6Pase-β prevents the other protein functioning efficiently and thus explains the myeloid phenotype in both patients with GSD-Ib and those with G6Pase-β deficiency. Three studies support this hypothesis.19–21 one study showed that transgenic mice deficient in G6Pase-β (G6pc3–/–) are unable to hydrolyze endoluminal G6P to glucose (Figure 4) and, therefore, show neutropenia and neutrophil dysfunction. Their neutrophils exhibit impaired respiratory burst, chemotaxis, calcium flux and phagocytosis,19 mimicking the immune phenotype seen in humans with GSD-Ib.1,2 The study also showed that neutrophils from G6pc3–/– mice exhibited enhanced stress of the endoplasmic reticulum and apoptosis that underlie, at least in part, neutropenia in murine G6Pase-β deficiency. This finding correlated with a report that a severe human con genital neutropenia syndrome, characterized by enhanced endoplasmic reticulum stress and apoptosis, was the result of mutations in the human G6PC3 gene.21 A third study showed that neutrophils from G6pt–/– mice57— a transgenic mouse model of GSD-Ib that lacks the G6PT protein and is thus unable to translocate G6P from the cytoplasm into the lumen of the endoplasmic reticulum (Figure 4)—also exhibit enhanced endoplasmic reticulum stress and apoptosis.20 The neutrophil apoptosis in G6pt–/– mice was shown to result from increased oxidative stress and to be mediated, at least in part, by the intrinsic mitochondrial stress pathway.20
These studies, although hinting at a mechanism for neutrophil dysfunction do not explain why endogenous glucose production is vital for normal neutrophil function. neutrophils are known to require a constant supply of glucose for their function and survival and are unable to produce glucose via gluconeogenesis.58 The primary source of glucose, therefore, is the circulating blood. Consistent with this notion, neutrophils from patients with GSD-Ib have a decreased rate of glucose transport across the cell membrane,59 which results in intracellular concentrations of G6P that are only 25% of normal levels, even in the presence of exogenous glucose.60 The falling levels of intracellular G6P are predicted to impair glyco-lysis, which leads to decreases in neutrophil lactate and ATP levels (Figure 4). In normal neutrophils, increased cytoplasmic levels of lactate61 and ATP62 stimulate uptake of blood glucose by inducing the translocation of GluT1 (solute carrier family 2, facilitated glucose transporter member 1) to the plasma membrane, which provides one explanation for the lower glucose uptake and G6P levels of neutrophils in patients with GSD-Ib. Pathologic neutrophils also undergo accelerated apoptosis,19–21,56 which could further decrease glucose uptake and G6P levels. Clearly a pattern is emerging, but a need remains for further functional studies focused on neutrophils depleted of apoptotic cells in order to fully understand the biology.
Complications in GSd-I
Renal disease
Renal disease is a long-term complication of GSD-Ia and GSD-Ib.63,64 whether renal disease is a long-term complication of G6Pase-β deficiency, which was only identified in 2009, remains to be determined.21 The early sign of renal dysfunction in GSD-I is glomerular hyperfiltration, followed by microalbuminuria and subsequent proteinuria.64 Patients with GSD-I can also develop hypocitraturia and hypercalciuria, which increases the risk of nephrocalcinosis and nephrolithiasis.65 This complication is further exacerbated in the absence of adequate control of hyperuricemia, hyperlipidemia and lactic acidemia.
Renal biopsies of patients with GSD-Ia show tubular atrophy, focal segmental glomerulosclerosis and interstitial fibrosis,66–68 but the underlying pathogenic mechanism is unknown. An increased expression of transforming growth factor-β1 (TGF-β1) was reported in a renal biopsy of a patient with GSD-Ia and renal dysfunction,69 which suggests one potential pathway. Two further studies showed that renal fibrosis70 and oxidative stress,71 mediated by upregulation of the angiotensin and TGF-β1 pathway, contribute to nephropathy in GSD-Ia. Mechanistically, the angiotensin and TGF-β1 pathway increases the expression of nADPH oxidase, activates the Akt and Forkhead box protein o pathway and suppresses superoxide dismutase and catalase.71 Yiu et al.70 showed that in kidneys of a mouse model of GSD-Ia, renal fibrosis was characterized by a marked increase in the synthesis and deposition of extracellular matrix proteins in the renal cortex, as well as by thickening of the tubular basement membrane, tubular atrophy and dilation and multifocal interstitial fibrosis.
Hepatocellular adenoma
Hepatocellular adenoma is a severe long-term complication of GSD-I that develops in 70–80% of patients >25 years old.63,72–74 Hepatocellular adenomas in patients with GSD-I are small, multiple, nonencapsulated adenomas that produce excess hepcidin that contributes to anemia.75 Additional complications from hepato cellular adenoma include local compression, intratumoral hemorrhage and, in 10% of patients, malignant trans formation to hepatocellular carcinoma.63,74,76 The under lying etiology of hepatocellular adenoma is un determined, and its management in GSD-I remains difficult owing to the absence of predictive signs. Good metabolic control does not seem to prevent the development of hepatocellular adenoma in GSD-Ia,77 and the signaling that leads to transformation into hepatocellular carcinoma is yet to be elucidated. Patients with GSD-Ia exhibit subclinical neutrophilia and elevated serum concentrations of interleukin 8, a cytokine that is more prominent in hepatocellular adenoma-bearing patients compared with the general population and nonhepatocellular adenomabearing patients with GSD-Ia.78 Moreover, serum concentrations of interleukin 8 in patients with GSD-Ia that do not have hepatocellular adenomas are higher than the general population. These observations suggest that chronic hepatic injury may predispose patients with GSD-Ia to develop hepatocellular adenoma. Consistent with this notion, a new study79 showed that murine GSD-Ia livers exhibited an enhanced rate of apoptosis.
Hepatocellular adenoma has been classified into four subtypes on the basis of varying etiology:80,81 mutations in the hepatic nuclear factor 1α (HNF-1α) gene; mutations in the β-catenin gene; no mutations in HNF-1α or β-catenin, but with inflammatory infiltrates; and no mutations in HNF-1α or the β-catenin gene and no inflammatory infiltrates. Two patients with GSD-I and hepatocellular adenomas have been characterized, one with mutations in β-catenin and one without mutations and without inflammatory infiltrates.80 A genome-wide, high-density analysis of single nucleotide poly morphisms in 10 patients with GSD-Ia and hepatocellular adenomas and in seven patients in the general population with hepatocellular adenoma showed that chromosomal aberrations were detected in approximately 60% of all hepatocellular adenomas.82 In patients with GSD-Ia, the most common genetic aberration is found on chromo- some 6—a simultaneous gain of chromosome 6p and loss of chromosome 6q82—which is frequently identified in hepatocellular carcinomas. whether chromosome 6 aberrations are an early event in liver tumorigenesis in GSD-I remains to be clarified.
Animal models
Both transgenic mouse models and naturally occurring dog models for GSD-Ia are currently available. G6pc–/– mice show all of the symptoms of human GSD-Ia— hypoglycemia, growth retardation, hepatomegaly, nephro megaly, hyperlipidemia, hyperuricemia and mild lactic acidemia.83,78 A naturally occurring dog model of GSD-Ia, which was generated by cross breeding a wildtype Beagle with a Maltese with a Met121Ile mutation in the G6PC gene, similarly manifests all the symptoms typical of patients with GSD-Ia, including marked lactic acidosis.84
For GSD-Ib57 and G6Pase-β deficiency,19 only transgenic mouse models are currently available. The mouse model for GSD-Ib (G6pt–/–)57 exhibits all the metabolic defects of human GSD-I, as well as the myeloid dysfunction of GSD-Ib, including neutropenia and neutrophil dysfunction. The G6pc3–/– mice19 show neutropenia, defects in neutrophil respiratory burst, chemotaxis and calcium flux and display an increased susceptibility to bacterial infection, thereby also mimicking myeloid dysfunction of GSD-Ib. These animal models are useful to further our understanding of the biology and pathophysiology of these disorders, to develop novel therapies and to monitor the long-term complications of GSD-I and G6Pase-β deficiency.
Diagnosis
Some patients with GSD-I present with hypoglycemia and lactic acidosis in the neonatal period. nevertheless, typically, clinical symptoms are not detected until the patient is a few months old. The first symptom is usually hepatomegaly or symptomatic hypoglycemia that develops after a short fast.1 Traditionally, GSD-I was diagnosed primarily by clinical and biochemical evaluation and subsequently confirmed by measurements of G6Pase-α activity in liver biopsy samples.1 Patients with GSD-Ia characteristically lack G6Pase activity in both intact and disrupted liver microsomes, whereas individuals with GSD-Ib lack G6Pase activity in intact liver microsomes but exhibit normal activity in disrupted microsomes. After the cloning of the G6PC and SLC37A4 genes, DnA-based diagnostic tests for GSD-Ia and GSD-Ib became available that are now used for disease confirmation, carrier testing of families at risk and prenatal diagnosis.
The guidelines from the european Study on GSD-I (eSGSD-I)63.85 recommend that diagnosis of GSD-I should be made initially on the basis of clinical presentation and biochemical abnormalities, such as hypoglycemia, hepatomegaly, hyperlactacidemia, stunted growth, doll face, hypotrophic muscles, diarrhea, hyper-lipidemia, hyperuricemia and impaired platelet function.85 The presence or absence of neutropenia should then be used to judge if final mutational analysis of the G6PC or the SLC37A4 gene is warranted for a definitive diagnosis.63,85 However, this triage may not be effective for all patients. neutropenia is not a clinical presentation of all patients with GSD-Ib,41–44 and individuals with GSD-Ia who carry the homozygous Gly188Arg muta- tion display mild neutropenia.35 A definitive diagnosis should, therefore, be confirmed by mutational analysis of both genes.
Initial diagnosis of G6Pase-β deficiency should be made on the basis of clinical presentation with neutropenia associated with congenital heart defects, urogenital malformations and an increased visibility of superficial veins,21 although a firm diagnosis requires mutational analysis of the G6PC3 gene.
Treatment
Diet
Metabolic disruption in patients with GSD-Ia and GSD-Ib can be adequately managed with diet86,87 and adjuvant drug therapy.1,88 Infants typically receive nocturnal nasogastric infusion of glucose to avoid hypoglycemia.86 Patients ≥3 years are prescribed uncooked cornstarch, a slow release carbohydrate, to prolong the length of euglycemia between meals.87 The levels of pancreatic amylase that hydrolyzes raw starch are low in children <3 years of age, and the ability to utilize raw cornstarch should be tested before the decision is made to use glucose infusion or prescribe dietary cornstarch. Dietary therapies achieve near normal glucose levels, growth and pubertal development, with few deaths, although complications are still common.63,89 In a retrospective study of the collaborative eSGSD-I with a cohort of 231 patients with GSD-Ia and 57 patients with GSD-Ib, dietary therapy was able to maintain normo glycemia during the night, and few patients died from consequences of acute metabolic derangement.63 However, hypercholesterolemia was identified in 53% and 14% of patients with GSD-Ia and GSD-Ib, respectively, and hypertriglyceridemia was still reported in 91% of individuals with GSD-Ia and 79% of patients with GSD-Ib. 57% of patients with GSD-I continued to have hyperuricemia and 89% exhibited hepatomegaly. The prevalence of hepatocellular adenomas in the population with GSD-I was 16%, but increased to 70–80% for patients aged >25 years.63 All patients >25 years exhibited microalbuminuria, and 50% of patients developed proteinuria.63 A study that evaluated an experimental, modified cornstarch showed increased effectiveness for the prevention of hypoglycemia.90 A small pilot study on milk supplemented with medium-chain triglycerides showed that the supplement reduced serum levels of lactate and triglyceride but increased HDl cholesterol levels.91 The results suggest that despite the control of hypoglycemia, the underlying pathological process remains uncorrected and long-term complications, including renal disease and liver adenomas, two major causes of morbidity and mortality in patients with GSD-I, remain with current dietary therapies.
Drugs
Adjunct drug therapies,88 such as lipid-lowering drugs for hyperlipidemia, potassium citrate for hypocitraturia, and angiotensin-converting enzyme (ACe) inhibitors92,93 for renal dysfunction, have improved patient management. In a 10-year retrospective study of 95 patients with GSD-Ia, the progression from glomerular hyperfiltration to microalbuminuria was markedly delayed by ACe inhibitors.92 This finding was supported by a second study that reported a decreased glomerular filtration rate with ACe inhibitor therapy in patients with GSD-Ia.93 Both studies suggest early intervention with ACe inhibitors should be used to prevent renal damage in GSD-I. Melis et al.,92 however, showed that the progression from microalbuminuria to proteinuria was not improved by ACe inhibitors. Given that an antioxidant treatment appears to normalize renal function and delay renal damage and fibrosis in a mouse model of GSD-Ia,71 a combination therapy of antioxidant with ACe inhibitor therapy may be more beneficial.
GCSF therapy
Therapy with granulocyte colony-stimulating factor (GCSF) is used in patients with GSD-Ib who have neutropenia and/or neutrophil dysfunctions and are, therefore, predisposed to frequent infections and enterocolitis.53,54,94 Calderwood et al.95 reported that GCSF treatment of 13 patients with GSD-Ib increased absolute neutrophil count from 0.46 ± 0.09 × 109/l to 2.43 ± 0.30 × 109/l, a level close to the range of un affected individuals (2.5–7.5 × 109/l). GCSF therapy also improved neutrophil function and reduced the incidence and severity of infection. Similarly, in a retro spective study of the eSGSD-I patient cohort,94 all 18 patients treated with GCSF responded with a decrease in the number and severity of infections and reduction in the severity of enterocolitis. In GSD-Ib, enterocolitis can be treated with a combination of GCSF and 5-aminosalicylic acid (5-ASA), provided renal function is carefully monitored.96 Gastrointestinal inflammation in one patient with GSD-Ib who was refractory to GCSF and 5-ASA treatment has been successfully treated with adalimumab, a monoclonal antibody that targets tumor necrosis factor.97 GCSF therapy shows no substantial short-term toxicity, although all patients on GCSF therapy develop spleno megaly.95 one major complication of GCSF therapy in patients with severe chronic neutropen ia is myelodysplasia or acute myeloid leukemia.98
No cases of acute myeloid leukemia have been found in patients with GSD-Ib who receive short-term GCSF therapy;95 however, three separate studies have reported the development of acute myeloid leukemia in this patient population in the long term.99–101 Acute myeloid leukemia observed after 14 years of continuous GCSF therapy is the result of a classical monosomy of chromosome 7 and a translocation event.101 This finding indicates that patients with GSD-Ib treated with GCSF over a long time period should receive regular bone marrow examinations. The current guideline for GSD-Ib96 recom mends limiting the use of GCSF to patients with persistent neutro phil count below 0.2 × 109/l, a single life- threatening infection requiring intravenous antibiotics, serious enterocolitis documented by abnormal colonoscopy and biopsies or severe diarrhea that requires hospitalization. In patients with G6Pase-β deficiency, neutropenia is also treated with GCSF.21 Given that this disorder was only identi-fied in 2009, the long-term safety and efficacy of GCSF therapy and its impact on the management of other clinic al sy mptoms is unknown.
Transplantation
Liver and kidney
In 2002, guidelines from the eSGSD-I recommended liver transplantation in patients with GSD-I and unresect able hepatocellular adenomas that are unresponsive to dietary therapy, particularly if these tumors are associated with serious compression or hemorrhage or show signs of transformation into hepatocellular carcinomas.63 Although liver transplantation corrects most metabolic derangements in patients with GSD-I,102–105 its effectiveness on renal disease and neutropenia or neutro phil dysfunction remains undetermined. As a result, patients with impending renal failure are currently recommended to consider combined liver and kidney transplantation.106
A study that evaluated efficacy of tumor resection showed that hepatocellular adenomas remained in all patients after liver resection, and all patients experienced adenomatous disease progression.107 on the other hand, in children with GSD-I, living-donor or reduced-size liver transplantation108,109 has corrected metabolic abnormalities in all nine cases of GSD-Ia and four cases of GSD-Ib documented and improved neutropenia and decreased infectious episodes in children with GSD-Ib.109 In a 21-year-old patient with GSD-I and multiple hepato-cellular adenomas, reduced-size liver transplantation was also successful,110 which suggests that living-donor liver transplantation is a viable option to restore normal metabolic balance in some patients with GSD-I. Despite the transplant guidelines, the liver transplant priority for patients with GSD-I, calculated on the basis of a score for end-stage liver disease, is extremely low. Petitioning review boards to include GSD-I as an additional model for end-stage liver disease may increase the priority for liver transplantation in this patient population.105
Bone marrow
Bone marrow transplantation has been investigated for treatment of GSD-Ib-related myeloid deficiencies. In animal studies, transplantation of bone marrow cells from G6pt–/– mice into wild-type mice showed that the absence of G6PT expression in the bone marrow and resident neutrophils led to abnormal myeloid function, which suggested that the transplanted bone marrow cells affected overall myeloid function.111 Bone marrow trans- plantation into a patient with severe enterocolitis and recurrent infections owing to GSD-Ib improved neutrophil function and reduced the severity of the entero-colitis, although mild neutropenia persisted.112 Although an isolated case, this promising outcome may support further exploration of this approach in addressing severe myeloid complications in GSD-Ib.
Gene therapy
Gene therapy for GSD-Ia
Somatic gene therapy is a promising therapeutic approach for a hydrophobic, transmembrane protein such as G6Pase-α. Gene therapy studies with adeno-viral vectors that express murine G6Pase-α113 or canine G6Pase-α,114 as well as studies with an adeno-associated virus (AAv) vector carrying murine,115 canine116 or human117,118 G6Pase-α have been developed in animal models of GSD-Ia (Table 3).
Table 3.
Gene therapies in GSD-I
| Study reference | vector | Gene | Promoter and enhancer | Results | Complications |
|---|---|---|---|---|---|
| GSD-Ia | |||||
| Zingone et al.113 | Adenoviral | Murine G6PC |
RSV | Transient correction of metabolic abnormalities | Little or no transgene expression in the kidney |
| Koeberl et al.114 | Adenoviral, helper-dependent | Canine G6PC |
Human apolipoprotein AI | Improved survival Hepatic G6Pase-α activity restored to 20–40% of wild-type enzyme activity at age 7 months Normalized fasting hypoglycemia Reduced hepatic glycogen storage |
Increased risk of dose-limiting hepatotoxicity Little or no transgene expression in the kidney |
| Ghosh et al.115 | AAV1 | Murine G6PC |
Hybrid CBA/CMV | Improved survival Normalized levels of blood glucose and metabolic profiles Normal liver histology with mild glycogen storage Hepatic and renal G6Pase-α activity restored to 11% and 7% of wild-type enzyme activity, respectively, at age 24–57 weeks |
Mice were infused with AAV1 neonatally then at age 1 week, but glycogen accumulation increased and histological abnormalities were observed in the kidneys of mice that aged |
| Koeberl et al.116 | AAV8 | Canine G6PC |
Nucleotides –1372 to –11 of the canine G6PC 5′-flanking sequences |
Improved survival Normalized levels of blood glucose and metabolic profiles Hepatic G6Pase-α activity restored to 21% of wild-type enzyme activity at age 7 months |
Subnormal fed and fasting levels of blood glucose Little or no transgene expression in the kidney |
| Koeberl et al.117 | AAV8 | Human G6PC |
Nucleotides –298 to +128 of the human G6PC 5′-flanking sequence |
Hepatic G6Pase-α activity restored to wild-type levels in mice and heterozygous levels in dogs Treated mice show improved survival and normoglycemia Treated dogs show improved survival, normal fasting glucose and lactate levels |
Treated mice show lower hepatic G6Pase-α expression, reduced levels of fed and fasted blood glucose and increased hepatic glycogen content accompanied by mild hepatomegaly compared with wild-type mice Little or no transgene expression in the kidney |
| Yiu et al.118 | AAV8 | Human G6PC |
Nucleotides –2864 to –1 of the human G6PC 5′-flanking sequence |
Improved survival Complete restoration to wild-type levels of hepatic G6Pase-α activity in mice aged 6 months, measured by phosphohydrolase and enzyme histochemical assays Blood glucose levels indistinguishable from those of control mice No fasting hypoglycemia |
Little or no transgene expression in the kidney |
| GSD-Ib | |||||
| Yiu et al.121 | Adenoviral vector | Human SLC37A4 |
CMV | Transient correction of metabolic and myeloid abnormalities | Little or no transgene expression in the kidney |
| Yiu et al.122 | AAV8 | Human SLC37A4 |
Hybrid CBA/CMV | Transient correction of myeloid dysfunction Long-term metabolic normalization, normalized serum glucose and metabolite profiles |
Hepatic G6PT activity dropped from 50% of wild-type levels at age 2 weeks after infusion to 3% by age 6–72 weeks Accumulation of excessive hepatic glycogen and neutral lipids in five mice aged >50 weeks Steatohepatitis and multiple hepatocellular adenomas in two mice, one with malignant transformation |
Abbreviations: AAV, adeno-associated virus pseudotype 2; CBA, chicken β-actin; CMV, cytomegalovirus; G6Pase-α, glucose-6-phosphatase-α; G6PT, glucose-6-phosphate transporter; GSD-I, glycogen storage disease type I; RSV, respiratory syncytial virus.
In both mouse and dog models, hepatic G6Pase-α deficiency has been successfully corrected in the long term with the use of AAv vectors, in the absence of detectable toxicity. In the mouse model, AAv1-mediated G6Pase-α transfer115 also restored renal G6Pase-α acti vity to 7% of wild-type levels at age 24–57 weeks. Kidney histology at 49–57 weeks after infusion showed a lot of variability in the four animals examined, ranging from no abnormality in one animal to histological abnormalities including glomerular sclerosis in another.115 By contrast, AAv8-mediated gene transfer showed little or no renal G6Pase-α expression, and all infused mice showed abnormal renal pathology.116–118 These findings highlight that different AAv serotypes display distinct tissue tropism, potentially owing to the distribution of their receptors on target cells.119,120 Investigation of other AAv serotypes may lead to a combination of recombinant vectors that are more effective than a single-vector gene therapy for GSD-Ia. This strategy remains an experimental area to be explored.
Gene therapy for GSD-Ib
Somatic gene therapy is also a promising therapeutic approach for the hydrophobic, transmembrane protein G6PT. Gene therapy in GSD-Ib has been evaluated in G6pt–/– mice with both adenoviral121 and AAv122 vectors that express human G6PT (Table 3). The adenoviral vector transiently corrects metabolic and myeloid abnor-malities in infused mice,121 although promising, long-term efficacy studies and extensive safety evaluations are needed.
An AAv8 vector delivered the G6PT transgene to the liver and bone marrow and transiently corrected myeloid dysfunction in infused neonatal G6pt–/– mice.122 long-term metabolic correction was achieved; however, five mice that lived >50 weeks accumulated excessive hepatic glycogen and neutral lipids, and two of these mice developed steatohepatitis and multiple hepato-cellular adenomas with malignant transformation in one mouse, despite metabolic normalization.122 This outcome reinforces the concern that normoglycemia alone cannot prevent the development of hepato-cellular adenomas in GSD-I. This finding also explains why patients with GSD-I who maintain normo glycemia under intense dietary therapy continue to be at risk of long-term complications. Further investigation is needed to determine whether gene therapy can correct long-term myeloid dysfunction in GSD-Ib.
Conclusions
Endogenous glucose is produced by G6PT and G6Pase-α in gluconeogenic organs and by G6PT and G6Pase-β in neutrophils. Molecular genetic studies have confirmed that inactivating mutations in G6PC, SLC37A4 and G6PC3 cause GSD-Ia, GSD-Ib and G6Pase-β deficiency, respectively. Patients with GSD-Ia and GSD-Ib display metabolic abnormalities with long-term complications of renal disease and hepatocellular adenomas that are prone to malignant transformation. renal fibrosis and oxidative stress mediated by activation of the angiotensin and TGF-β1 pathway underlie, at least in part, renal disease in patients with in GSD-Ia, and chromosome 6 aberrations are the most common genetic aberrations in hepato cellular adenomas in this patient population. Patients with GSD-Ib or G6Pase-β deficiency have neutropenia and neutrophil dysfunction as a result of enhanced neutrophil apoptosis. The metabolic abnor-malities in GSD-I are currently treated by dietary thera- pies. Despite the sympto matic relief provided by these therapies, the underlying pathological processes remain uncorrected and long-term complications persist. The myeloid abnormalities in GSD-Ib and G6Pase-β deficiency can be treated with GCSF therapy, although acute myeloid leukemia can be a major long-term complication of this approach. Animal models of the three disorders are available and are now being exploited to delineate the disease more precisely and develop new therapies. However, although gene therapies have shown promising results in animal models, further investigations and refinements are required.
Key points.
■ Glycogen storage disease type I (GSD-I) comprises GSD-Ia, a deficiency in glucose-6-phosphatase-α (G6Pase-α) and GSD-Ib, a deficiency in a glucose-6-phosphate transporter (G6PT)
■ G6Pase-α is expressed primarily in gluconeogenic tissues, whereas G6PT is ubiquitously expressed; G6Pase-α couples functionally to G6PT to form a complex that maintains interprandial blood glucose homeostasis
■ Patients with GSD-I display a metabolic phenotype characterized by hypoglycemia, hepatomegaly, nephromegaly, hyperlipidemia, hyperuricemia, lactic acidemia, growth retardation and long-term renal and liver disease
■ G6Pase-β is ubiquitously expressed and couples functionally to G6PT to form a complex that maintains neutrophil homeostasis and function
■ Patients with GSD-Ib and those with G6Pase-β-deficiency exhibit a common myeloid phenotype characterized by neutropenia and neutrophil dysfunction
■ Patients with GSD-Ia or GSD-Ib are treated by dietary therapies to correct metabolic abnormalities; to correct myeloid dysfunction, individuals with GSD-Ib or G6Pase-β deficiency require therapy with granulocyte colony- stimulating factor
Acknowledgments
The authors were supported by the Intramural Research Program of the National Institute of Child Health & Human Development (NICHD), NIH.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
J. Y. Chou and H. S. Jun researched the data for the article. J. Y. Chou and B. C. Mansfield provided a substantial contribution to discussions of the content and contributed equally to writing the article. All authors reviewed and/or edited the manuscript before submission.
REVIEWS
- 1.Chou JY, Matern D, Mansfield BC, Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr. Mol. Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- 2.Chou JY, Mansfield BC. In: Membrane Transporter Diseases. Broer S, Wagner CA, editors. Springer; New York: 2003. pp. 191–205. Ch. 13. [Google Scholar]
- 3.Lei K-J, Shelly LL, Pan C-J, Sidbury JB, Chou JY. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993;262:580–583. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- 4.Lei K-J, Pan C-J, Shelly LL, Liu J-L, Chou JY. Identification of mutations in the gene for glucose-6-phosphatase, the enzyme deficient in glycogen storage disease type 1a. J. Clin. Invest. 1994;93:1994–1999. doi: 10.1172/JCI117192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiraiwa H, Pan C-J, Lin B, Moses SW, Chou JY. Inactivation of the glucose 6-phosphate transporter causes glycogen storage disease type 1b. J. Biol. Chem. 1999;274:5532–5536. doi: 10.1074/jbc.274.9.5532. [DOI] [PubMed] [Google Scholar]
- 6.Gerin I, Veiga-da-Cunha M, Achouri Y, Collet J-F, van Schaftingen E. Sequence of a putative glucose 6-phosphate translocase, mutated in glycogen storage disease type Ib. FEBS Lett. 1997;419:235–238. doi: 10.1016/s0014-5793(97)01463-4. [DOI] [PubMed] [Google Scholar]
- 7.Annabi B, et al. The gene for glycogen-storage disease type 1b maps to chromosome 11q23. Am. J. Hum. Genet. 1998;62:400–405. doi: 10.1086/301727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerin I, Veiga-da-Cunha M, Noël G, van Schaftingen E. Structure of the gene mutated in glycogen storage disease type Ib. Gene. 1999;227:189–195. doi: 10.1016/s0378-1119(98)00614-3. [DOI] [PubMed] [Google Scholar]
- 9.Veiga-da-Cunha M, et al. A gene on chromosome 11q23 coding for a putative glucose-6-phosphate translocase is mutated in glycogen-storage disease types Ib and Ic. Am. J. Hum. Genet. 1998;63:976–983. doi: 10.1086/302068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veiga-da-Cunha M, et al. The putative glucose 6-phosphate translocase gene is mutated in essentially all cases of glycogen storage disease type I non-a. Eur. J. Hum. Genet. 1999;7:717–723. doi: 10.1038/sj.ejhg.5200366. [DOI] [PubMed] [Google Scholar]
- 11.Galli L, et al. Mutations in the glucose-6-phosphate transporter (G6PT) gene in patients with glycogen storage diseases type 1b and 1c. FEBS Lett. 1999;459:255–258. doi: 10.1016/s0014-5793(99)01248-x. [DOI] [PubMed] [Google Scholar]
- 12.Janecke AR, et al. Mutation analysis in glycogen storage disease type 1 non-a. Hum. Genet. 2000;107:285–289. doi: 10.1007/s004390000371. [DOI] [PubMed] [Google Scholar]
- 13.Chen SY, et al. The glucose-6-phosphate transporter is a phosphate-linked antiporter deficient in glycogen storage disease type Ib and Ic. FASEB J. 2008;22:2206–2213. doi: 10.1096/fj.07-104851. [DOI] [PubMed] [Google Scholar]
- 14.Pan C-J, Lei K-J, Chen H, Ward JM, Chou JY. Ontogeny of the murine glucose-6-phosphatase system. Arch. Biochem. Biophys. 1998;358:17–24. doi: 10.1006/abbi.1998.0849. [DOI] [PubMed] [Google Scholar]
- 15.Lin B, Annabi B, Hiraiwa H, Pan C-J, Chou JY. Cloning and characterization of cDNAs encoding a candidate glycogen storage disease type 1b protein in rodents. J. Biol. Chem. 1998;273:31656–31660. doi: 10.1074/jbc.273.48.31656. [DOI] [PubMed] [Google Scholar]
- 16.Shieh J-J, Pan C-J, Mansfield BC, Chou JY. A glucose-6-phosphate hydrolase, widely expressed outside the liver, can explain age-dependent resolution of hypoglycemia in glycogen storage disease type Ia. J. Biol. Chem. 2003;278:47098–47103. doi: 10.1074/jbc.M309472200. [DOI] [PubMed] [Google Scholar]
- 17.Guionie O, Clottes E, Stafford K, Burchell A. Identification and characterisation of a new human glucose-6-phosphatase isoform. FEBS Lett. 2003;551:159–164. doi: 10.1016/s0014-5793(03)00903-7. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, Shieh J-J, Pan C-J, Chou JY. Histidine-167 is the phosphate acceptor in glucose-6-phosphatase-β forming a phosphohistidine-enzyme intermediate during catalysis. J. Biol. Chem. 2004;279:12479–12483. doi: 10.1074/jbc.M313271200. [DOI] [PubMed] [Google Scholar]
- 19.Cheung YY, et al. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-β. J. Clin. Invest. 2007;117:784–793. doi: 10.1172/JCI30443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SY, Jun HS, Mead PA, Mansfield BC, Chou JY. Neutrophil stress and apoptosis underlie myeloid dysfunction in glycogen storage disease type Ib. Blood. 2008;111:5704–5711. doi: 10.1182/blood-2007-12-129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boztug K, et al. A syndrome with congenital neutropenia and mutations in G6PC3. N. Engl. J. Med. 2009;360:32–43. doi: 10.1056/NEJMoa0805051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan C-J, Lei K-J, Annabi B, Chou JY. Transmembrane topology of glucose-6-phosphatase. J. Biol. Chem. 1998;273:6144–6148. doi: 10.1074/jbc.273.11.6144. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh A, Shieh J-J, Pan C-J, Sun M-S, Chou JY. The catalytic center of glucose-6-phosphatase: His176 is the nucleophile forming the phosphohistidine-enzyme intermediate during catalysis. J. Biol. Chem. 2002;277:32837–32842. doi: 10.1074/jbc.M201853200. [DOI] [PubMed] [Google Scholar]
- 24.Chou JY, Mansfield BC. Mutations in the glucose-6-phosphatase-α (G6PC) gene that cause type Ia glycogen storage disease. Hum. Mutat. 2008;29:921–930. doi: 10.1002/humu.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei K-J, et al. Genetic basis of glycogen storage disease type 1a: prevalent mutations at the glucose-6-phosphatase locus. Am. J. Hum. Genet. 1995;57:766–771. [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni N, et al. Enzymatic characterization of four new mutations in the glucose-6 phosphatase (G6PC) gene which cause glycogen storage disease type 1a. Ann. Hum. Genet. 1999;63:141–146. doi: 10.1046/j.1469-1809.1999.6320141.x. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K, et al. Heterogeneous mutations in the glucose-6-phosphatase gene in Japanese patients with glycogen storage disease type Ia. Am. J. Med. Genet. 2000;92:90–94. [PubMed] [Google Scholar]
- 28.Shieh J-J, et al. The molecular basis of glycogen storage disease type 1a: structure and function analysis of mutations in glucose-6-phosphatase. J. Biol. Chem. 2002;277:5047–5053. doi: 10.1074/jbc.M110486200. [DOI] [PubMed] [Google Scholar]
- 29.Angaroni CJ, et al. Glycogen storage disease type Ia in Argentina: two novel glucose-6-phosphatase mutations affecting protein stability. Mol. Genet. Metab. 2004;83:276–279. doi: 10.1016/j.ymgme.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Keller KM, et al. A new mutation of the glucose-6-phosphatase gene in a 4-year-old girl with oligosymptomatic glycogen storage disease type 1a. J. Pediatr. 1998;132:360–361. doi: 10.1016/s0022-3476(98)70463-9. [DOI] [PubMed] [Google Scholar]
- 31.Rake JP, et al. Identification of a novel mutation (867delA) in the glucose-6-phosphatase gene in two siblings with glycogen storage disease type Ia with different phenotypes. Hum. Mutat. 2000;15:381. doi: 10.1002/(SICI)1098-1004(200004)15:4<381::AID-HUMU13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Matern D, Seydewitz HH, Bali D, Lang C, Chen YT. Glycogen storage disease type I: diagnosis and phenotype/genotype correlation. Eur. J. Pediatr. 2002;161(Suppl. 1):S10–S19. doi: 10.1007/s00431-002-0998-5. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, Ozawa T, Kawasaki T, Nakamura H, Sugimura H. Glucose-6-phosphatase gene mutations in 20 adult Japanese patients with glycogen storage disease type 1a with reference to hepatic tumors. J. Gastroenterol. Hepatol. 2001;16:1402–1408. doi: 10.1046/j.1440-1746.2001.02645.x. [DOI] [PubMed] [Google Scholar]
- 34.Akanuma J, et al. Glycogen storage disease type Ia: molecular diagnosis of 51 Japanese patients and characterization of splicing mutations by analysis of ectopically transcribed mRNA from lymphoblastoid cells. Am. J. Med. Genet. 2000;91:107–112. [PubMed] [Google Scholar]
- 35.Weston BW, et al. Glucose-6-phosphatase mutation G188R confers an atypical glycogen storage disease type 1b phenotype. Pediatr. Res. 2000;48:329–334. doi: 10.1203/00006450-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Pan C-J, Lin B, Chou JY. Transmembrane topology of human glucose 6-phosphate transporter. J. Biol. Chem. 1999;274:13865–13869. doi: 10.1074/jbc.274.20.13865. [DOI] [PubMed] [Google Scholar]
- 37.Chou JY, Jun HS, Mansfield BC. Neutropenia in type Ib glycogen storage disease. Curr. Opin. Hematol. 2010;17:36–42. doi: 10.1097/MOH.0b013e328331df85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L-Y, Lin B, Pan C-J, Hiraiwa H, Chou JY. Structural requirements for the stability and microsomal transport activity of the human glucose 6-phosphate transporter. J. Biol. Chem. 2000;275:34280–34286. doi: 10.1074/jbc.M006439200. [DOI] [PubMed] [Google Scholar]
- 39.Chen L-Y, Pan C-J, Shieh J-J, Chou JY. Structure-function analysis of the glucose-6-phosphate transporter deficient in glycogen storage disease type Ib. Hum. Mol. Genet. 2002;11:3199–3207. doi: 10.1093/hmg/11.25.3199. [DOI] [PubMed] [Google Scholar]
- 40.Chen S-Y, Pan C-J, Lee S, Peng W, Chou JY. Functional analysis of mutations in the glucose-6-phosphate transporter that cause glycogen storage disease type Ib. Mol. Genet. Metab. 2008;95:220–223. doi: 10.1016/j.ymgme.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melis D, et al. Genotype/phenotype correlation in glycogen storage disease type 1b: a multicentre study and review of the literature. Eur. J. Pediatr. 2005;164:501–508. doi: 10.1007/s00431-005-1657-4. [DOI] [PubMed] [Google Scholar]
- 42.Kure S, et al. Glycogen storage disease type Ib without neutropenia. J. Pediatr. 2000;137:253–256. doi: 10.1067/mpd.2000.107472. [DOI] [PubMed] [Google Scholar]
- 43.Martens DH, et al. A patient with common glycogen storage disease type Ib mutations without neutropenia or neutrophil dysfunction. J. Inherit. Metab. Dis. 2006;29:224–225. doi: 10.1007/s10545-006-0146-x. [DOI] [PubMed] [Google Scholar]
- 44.Angaroni CJ, et al. Glycogen storage disease type Ib without neutropenia generated by a novel splice-site mutation in the glucose-6-phosphate translocase gene. Mol. Genet. Metab. 2006;88:96–99. doi: 10.1016/j.ymgme.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Martin CC, et al. Identification and characterization of a human cDNA and gene encoding a ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J. Mol. Endocrinol. 2002;29:205–222. doi: 10.1677/jme.0.0290205. [DOI] [PubMed] [Google Scholar]
- 46.Aróstegui JI, et al. A novel G6PC3 homozygous 1-bp deletion as a cause of severe congenital neutropenia. Blood. 2009;114:1718–1719. doi: 10.1182/blood-2009-04-219451. [DOI] [PubMed] [Google Scholar]
- 47.Xia J, et al. Prevalence of mutations in ELANE, GFI1, HAX1, SBDS, WAS and G6PC3 in patients with severe congenital neutropenia. Br. J. Haematol. 2009;147:535–542. doi: 10.1111/j.1365-2141.2009.07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banugaria SG, Austin SL, Boney A, Weber TJ, Kishnani PS. Hypovitaminosis D in glycogen storage disease type I. Mol. Genet. Metab. 2010;99:434–437. doi: 10.1016/j.ymgme.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Bandsma RH, et al. Increased de novo lipogenesis and delayed conversion of large VLDL into intermediate density lipoprotein particles contribute to hyperlipidemia in glycogen storage disease type 1a. Pediatr. Res. 2008;63:702–707. doi: 10.1203/PDR.0b013e31816c9013. [DOI] [PubMed] [Google Scholar]
- 50.Melis D, et al. The growth hormone-insulin-like growth factor axis in glycogen storage disease type 1: evidence of different growth patterns and insulin-like growth factor levels in patients with glycogen storage disease type 1a and 1b. J. Pediatr. 2010;156:663–670. doi: 10.1016/j.jpeds.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 51.Kilpatrick L, et al. Impaired metabolic function and signaling defects in phagocytic cells in glycogen storage disease type 1b. J. Clin. Invest. 1990;86:196–202. doi: 10.1172/JCI114684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gitzelmann R, Bosshard NU. Defective neutrophil and monocyte functions in glycogen storage disease type Ib: a literature review. Eur. J. Pediatr. 1993;152(Suppl. 1):S33–S38. doi: 10.1007/BF02072085. [DOI] [PubMed] [Google Scholar]
- 53.Visser G, et al. Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J. Pediatr. 2000;137:187–191. doi: 10.1067/mpd.2000.105232. [DOI] [PubMed] [Google Scholar]
- 54.Dieckgraefe BK, Korzenik JR, Husain A, Dieruf L. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur. J. Pediatr. 2002;161(Suppl. 1):S88–S92. doi: 10.1007/s00431-002-1011-z. [DOI] [PubMed] [Google Scholar]
- 55.Melis D, et al. Increased prevalence of thyroid autoimmunity and hypothyroidism in patients with glycogen storage disease type I. J. Pediatr. 2007;150:300–305. doi: 10.1016/j.jpeds.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 56.Kuijpers TW, et al. Apoptotic neutrophils in the circulation of patients with glycogen storage disease type 1b (GSD1b). Blood. 2003;101:5021–5024. doi: 10.1182/blood-2002-10-3128. [DOI] [PubMed] [Google Scholar]
- 57.Chen L-Y, et al. Impaired glucose homeostasis, neutrophil trafficking and function in mice lacking the glucose-6-phosphate transporter. Hum. Mol. Genet. 2003;12:2547–2558. doi: 10.1093/hmg/ddg263. [DOI] [PubMed] [Google Scholar]
- 58.Stjernholm RL, Burns CP, Hohnadel JH. Carbohydrate metabolism by leukocytes. Enzyme. 1972;13:7–31. doi: 10.1159/000459647. [DOI] [PubMed] [Google Scholar]
- 59.Bashan N, Potashnik R, Hagay Y, Moses SW. Impaired glucose transport in polymorphonuclear leukocytes in glycogen storage disease Ib. J. Inherit. Metab. Dis. 1987;10:234–241. doi: 10.1007/BF01800068. [DOI] [PubMed] [Google Scholar]
- 60.Verhoeven AJ, et al. A convenient diagnostic function test of peripheral blood neutrophils in glycogen storage disease type Ib. Pediatr. Res. 1999;45:881–885. doi: 10.1203/00006450-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Medina RA, Southworth R, Fuller W, Garlick PB. Lactate-induced translocation of GLUT1 and GLUT4 is not mediated by the phosphatidyl-inositol-3-kinase pathway in the rat heart. Basic Res. Cardiol. 2002;97:168–176. doi: 10.1007/s003950200008. [DOI] [PubMed] [Google Scholar]
- 62.Kim MS, et al. ATP stimulates glucose transport through activation of P2 purinergic receptors in C2C12 skeletal muscle cells. Arch. Biochem. Biophys. 2002;401:205–214. doi: 10.1016/S0003-9861(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 63.Rake JP, et al. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur. J. Pediatr. 2002;161(Suppl. 1):S20–S34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- 64.Chou JY, Mansfield BC, Weinstein DA. In: Genetic Diseases of the Kidney Ch. 41. Lifton RP, et al., editors. Academic Press; New York: 2009. pp. 693–708. [Google Scholar]
- 65.Weinstein DA, Somers MJ, Wolfsdorf JI. Decreased urinary citrate excretion in type 1a glycogen storage disease. J. Pediatr. 2001;138:378–382. doi: 10.1067/mpd.2001.111322. [DOI] [PubMed] [Google Scholar]
- 66.Chen YT, Coleman RA, Scheinman JI, Kolbeck PC, Sidbury JB. Renal disease in type I glycogen storage disease. N. Engl. J. Med. 1988;318:7–11. doi: 10.1056/NEJM198801073180102. [DOI] [PubMed] [Google Scholar]
- 67.Verani R, Bernstein J. Renal glomerular and tubular abnormalities in glycogen storage disease type I. Arch. Pathol. Lab. Med. 1988;112:271–274. [PubMed] [Google Scholar]
- 68.Baker L, et al. Hyperfiltration and renal disease in glycogen storage disease, type I. Kidney Int. 1989;35:1345–1350. doi: 10.1038/ki.1989.133. [DOI] [PubMed] [Google Scholar]
- 69.Urushihara M, et al. Transforming growth factor-beta in renal disease with glycogen storage disease I. Pediatr. Nephrol. 2004;19:676–678. doi: 10.1007/s00467-004-1456-6. [DOI] [PubMed] [Google Scholar]
- 70.Yiu WH, et al. Angiotensin mediates renal fibrosis in the nephropathy of glycogen storage disease type Ia. Kidney Int. 2008;73:716–723. doi: 10.1038/sj.ki.5002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yiu WH, Mead PA, Jun HS, Mansfield BC, Chou JY. Oxidative stress mediates nephropathy in type Ia glycogen storage disease. Lab. Invest. 2010;90:620–629. doi: 10.1038/labinvest.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bianchi L. Glycogen storage disease I and hepatocellular tumours. Eur. J. Pediatr. 1993;52(Suppl. 1):S63–S70. doi: 10.1007/BF02072092. [DOI] [PubMed] [Google Scholar]
- 73.Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J. Pediatr. Gastroenterol. Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Lee PJ. Glycogen storage disease type I: pathophysiology of liver adenomas. Eur. J. Pediatr. 2002;161(Suppl. 1):S46–S49. doi: 10.1007/s00431-002-1002-0. [DOI] [PubMed] [Google Scholar]
- 75.Weinstein DA, et al. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 76.Franco LM, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J. Inherit. Metab. Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 77.Di Rocco M, et al. Hepatocellular adenoma and metabolic balance in patients with type Ia glycogen storage disease. Mol. Genet. Metab. 2008;93:398–402. doi: 10.1016/j.ymgme.2007.10.134. [DOI] [PubMed] [Google Scholar]
- 78.Kim SY, Weinstein DA, Starost MF, Mansfield BC, Chou JY. Necrotic foci, elevated chemokines and infiltrating neutrophils in the liver of glycogen storage disease type Ia. J. Hepatol. 2008;48:479–485. doi: 10.1016/j.jhep.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun B, et al. Activation of glycolysis and apoptosis in glycogen storage disease type Ia. Mol. Genet. Metab. 2009;97:267–271. doi: 10.1016/j.ymgme.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Zucman-Rossi J, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 81.Bioulac-Sage P, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 82.Kishnani PS, et al. Chromosomal and genetic alterations in human hepatocellular adenomas associated with type Ia glycogen storage disease. Hum. Mol. Genet. 2009;18:4781–4790. doi: 10.1093/hmg/ddp441. [DOI] [PubMed] [Google Scholar]
- 83.Lei K-J, et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat. Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- 84.Kishnani PS, et al. Canine model and genomic structural organization of glycogen storage disease type Ia (GSD Ia). Vet. Pathol. 2001;38:83–91. doi: 10.1354/vp.38-1-83. [DOI] [PubMed] [Google Scholar]
- 85.Rake JP, et al. Glycogen storage disease type Ia: recent experience with mutation analysis, a summary of mutations reported in the literature and a newly developed diagnostic flowchart. Eur. J. Pediatr. 2000;159:322–330. doi: 10.1007/s004310051281. [DOI] [PubMed] [Google Scholar]
- 86.Greene HL, Slonim AE, O'Neill JA, Jr, Burr IM. Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N. Engl. J. Med. 1976;294:423–425. doi: 10.1056/NEJM197602192940805. [DOI] [PubMed] [Google Scholar]
- 87.Chen YT, Cornblath M, Sidbury JB. Cornstarch therapy in type I glycogen-storage disease. N. Engl. J. Med. 1984;310:171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- 88.Koeberl DD, Kishnani PS, Bali D, Chen YT. Emerging therapies for glycogen storage disease type I. Trends Endocrinol. Metab. 2009;20:252–258. doi: 10.1016/j.tem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Weinstein DA, Wolfsdorf JI. Effect of continuous glucose therapy with uncooked cornstarch on the long-term clinical course of type 1a glycogen storage disease. Eur. J. Pediatr. 2002;161(Suppl. 1):S35–S39. doi: 10.1007/s00431-002-1000-2. [DOI] [PubMed] [Google Scholar]
- 90.Correia CE, et al. Use of modified cornstarch therapy to extend fasting in glycogen storage disease types Ia and Ib. Am. J. Clin. Nutr. 2008;88:1272–1276. doi: 10.3945/ajcn.2008.26352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagasaka H, et al. Improvements of hypertriglyceridemia and hyperlacticemia in Japanese children with glycogen storage disease type Ia by medium-chain triglyceride milk. Eur. J. Pediatr. 2007;166:1009–1016. doi: 10.1007/s00431-006-0372-0. [DOI] [PubMed] [Google Scholar]
- 92.Melis D, et al. Efficacy of ACE-inhibitor therapy on renal disease in glycogen storage disease type 1: a multicentre retrospective study. Clin. Endocrinol. (Oxf.) 2005;63:19–25. doi: 10.1111/j.1365-2265.2005.02292.x. [DOI] [PubMed] [Google Scholar]
- 93.Martens DH, et al. Renal function in glycogen storage disease type I, natural course, and renopreservative effects of ACE inhibition. Clin. J. Am. Soc. Nephrol. 2009;4:1741–1746. doi: 10.2215/CJN.00050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Visser G, et al. Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease Type 1. Eur. J. Pediatr. 2002;161(Suppl. 1):S83–S87. doi: 10.1007/s00431-002-1010-0. [DOI] [PubMed] [Google Scholar]
- 95.Calderwood S, et al. Recombinant human granulocyte colony-stimulating factor therapy for patients with neutropenia and/or neutrophil dysfunction secondary to glycogen storage disease type 1b. Blood. 2001;97:376–382. doi: 10.1182/blood.v97.2.376. [DOI] [PubMed] [Google Scholar]
- 96.Visser G, et al. Consensus guidelines for management of glycogen storage disease type 1b—European Study on Glycogen Storage Disease Type 1. Eur. J. Pediatr. 2002;161(Suppl. 1):S120–S123. doi: 10.1007/s00431-002-1017-6. [DOI] [PubMed] [Google Scholar]
- 97.Davis MK, Rufo PA, Polyak SF, Weinstein DA. Adalimumab for the treatment of Crohn-like colitis and enteritis in glycogen storage disease type Ib. J. Inherit. Metab. Dis. doi: 10.1007/s10545-007-0774-9. doi:10.1007/s10545-077-0774-9. [DOI] [PubMed] [Google Scholar]
- 98.Donadieu J, et al. Analysis of risk factors for myelodysplasias, leukemias and death from infection among patients with congenital neutropenia. Experience of the French Severe Chronic Neutropenia Study Group. Haematologica. 2005;90:45–53. [PubMed] [Google Scholar]
- 99.Simmons PS, Smithson WA, Gronert GA, Haymond MW. Acute myelogenous leukemia and malignant hyperthermia in a patient with type 1b glycogen storage disease. J. Pediatr. 1984;105:428–431. doi: 10.1016/s0022-3476(84)80020-7. [DOI] [PubMed] [Google Scholar]
- 100.Pinsk M, et al. Acute myelogenous leukemia and glycogen storage disease 1b. J. Pediatr. Hematol. Oncol. 2002;24:756–758. doi: 10.1097/00043426-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 101.Schroeder T, Hildebrandt B, Mayatepek E, Germing U, Haas R. A patient with glycogen storage disease type Ib presenting with acute myeloid leukemia (AML) bearing monosomy 7 and translocation t(3;8)(q26;q24) after 14 years of treatment with granulocyte colony-stimulating factor (G-CSF): a case report. J. Med. Case Reports. 2008;2:319. doi: 10.1186/1752-1947-2-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faivre L, et al. Long-term outcome of liver transplantation in patients with glycogen storage disease type Ia. J. Inherit. Metab. Dis. 1999;22:723–732. doi: 10.1023/a:1005544117285. [DOI] [PubMed] [Google Scholar]
- 103.Matern D, et al. Liver transplantation for glycogen storage disease types, I, III, and IV. Eur. J. Pediatr. 1999;158(Suppl. 2):S43–S48. doi: 10.1007/pl00014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davis MK, Weinstein DA. Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr. Transplant. 2008;12:137–145. doi: 10.1111/j.1399-3046.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 105.Reddy SK, et al. Liver transplantation for glycogen storage disease type Ia. J. Hepatol. 2009;51:483–490. doi: 10.1016/j.jhep.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 106.Labrune P. Glycogen storage disease type I: indications for liver and/or kidney transplantation. Eur. J. Pediatr. 2002;161(Suppl. 1):S53–S55. doi: 10.1007/s00431-002-1004-y. [DOI] [PubMed] [Google Scholar]
- 107.Reddy SK, et al. Resection of hepatocellular adenoma in patients with glycogen storage disease type Ia. J. Hepatol. 2007;47:658–663. doi: 10.1016/j.jhep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Iyer SG, et al. Long-term results of living donor liver transplantation for glycogen storage disorders in children. Liver Transpl. 2007;13:848–852. doi: 10.1002/lt.21151. [DOI] [PubMed] [Google Scholar]
- 109.Kasahara M, et al. Living donor liver transplantation for glycogen storage disease type Ib. Liver Transpl. 2009;15:1867–1871. doi: 10.1002/lt.21929. [DOI] [PubMed] [Google Scholar]
- 110.Ji HF, et al. Reduced-size liver transplantation for glycogen storage disease. Hepatobiliary Pancreat. Dis. Int. 2009;8:106–108. [PubMed] [Google Scholar]
- 111.Kim SY, et al. Bone-marrow derived cells require a functional glucose-6-phosphate transporter for normal myeloid functions. J. Biol. Chem. 2006;281:28794–28801. doi: 10.1074/jbc.M604964200. [DOI] [PubMed] [Google Scholar]
- 112.Pierre G, et al. A. Bone marrow transplantation in glycogen storage disease type 1b. J. Pediatr. 2008;152:286–288. doi: 10.1016/j.jpeds.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 113.Zingone A, et al. Correction of glycogen storage disease type 1a in a mouse model by gene therapy. J. Biol. Chem. 2000;275:828–832. doi: 10.1074/jbc.275.2.828. [DOI] [PubMed] [Google Scholar]
- 114.Koeberl DD, et al. Efficacy of helper-dependent adenovirus vector-mediated gene therapy in murine glycogen storage disease type Ia. Mol. Ther. 2007;15:1253–1258. doi: 10.1038/sj.mt.6300188. [DOI] [PubMed] [Google Scholar]
- 115.Ghosh A, et al. Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther. 2006;13:321–329. doi: 10.1038/sj.gt.3302650. [DOI] [PubMed] [Google Scholar]
- 116.Koeberl DD, et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- 117.Koeberl DD, et al. AAv vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol. Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- 118.Yiu WH, et al. Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy. Mol. Ther. 2010;18:1076–1084. doi: 10.1038/mt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akache B, et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yiu WH, Pan C-J, Allamarvdasht M, Kim SY, Chou JY. Glucose-6-phosphate transporter gene therapy corrects metabolic and myeloid abnormalities in glycogen storage disease type Ib mice. Gene Ther. 2007;14:219–226. doi: 10.1038/sj.gt.3302869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yiu WH, et al. Normoglycemia alone is insufficient to prevent long-term complications of hepatocellular adenoma in glycogen storage disease type Ib mice. J. Hepatol. 2009;51:909–917. doi: 10.1016/j.jhep.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]