Abstract
Purpose
To assess whether diverse tumor location(s) show differences in percutaneous cryoablation (PCA) outcomes of cancer control, morbidity, and ablation volume reduction for many soft-tissue tumor types.
Materials and Methods
A total of 220 computed tomography (CT)– and/or ultrasonography-guided percutaneous cryotherapy procedures were performed for 251 oligometastatic tumors from multiple primary cancers in 126 patients. Tumor location was grouped according to regional sites: retroperitoneal, superficial, intraperitoneal, bone, and head and neck. PCA complications were graded according to Common Terminology Criteria for Adverse Events (version 4.0). Local tumor recurrence and involution were calculated from ablation zone measurements, grouped into 1-, 3-, 6-, 12-, 18-, and 24-month (or later) statistical bins.
Results
Tumor and procedure numbers for each site were 75 and 69 retroperitoneal, 76 and 62 superficial, 39 and 32 intraperitoneal, 34 and 34 bone, and 27 and 26 head and neck. Average diameters of tumor and visible ice during ablation were 3.4 and 5.5 cm, respectively. Major complications (ie, grade >3) attributable to PCA occurred after five procedures (2.3%). At 11 months average follow-up (range, 0–82 mo), a 10% total recurrence rate (26 of 251) was noted; three occurred within the ablation zone, for a local progression rate of 1.2%. Average time to recurrence was 4.9 months, and, at 21 months, the initial ablation zone had reduced in volume by 93%.
Conclusions
CT-guided PCA is a broadly safe, effective local cancer control option for oligometastatic disease with soft-tissue tumors in most anatomic sites. Other than bowel and nerve proximity, PCA also shows good healing if proper visualization and precautions are followed.
An estimated 1.6 million new cases of cancer were diagnosed in the United States in 2011, and 3%–68% will already have metastatic disease, depending on the site of origin (1–3). The most common cancers of lung, colon, breast, and prostate generally have metastatic rates of greater than 20% at presentation (1). Moreover, as many as 85% of patients who have breast, prostate, or lung cancer have bone metastases at the time of death (2). As many as 5% of all cancers may also develop cutaneous metastases (3). Improved low-morbidity treatment options are therefore needed for many patients with advanced-stage cancer of a broad spectrum of nonorgan metastatic sites.
Surgical resection has been the gold standard for local treatment of most newly diagnosed cancer cases. However, for patients with stage IV disease, resection of oligometastases in nonorgan locations produces quality-of-life concerns, and may limit most surgery to isolated resections of liver and pulmonary metastases. Chemotherapy is generally ineffective in treating pain in bone and recurrent soft-tissue metastases (4), and radiation therapy, although effective when used before surgery on small tumors, is limited for many sites (5). Other forms of heat-based percutaneous treatment include laser-induced thermotherapy (6) and radiofrequency (RF) ablation (7); however, these targeted treatment modalities have not emerged as desirable options for palliative care of many soft-tissue locations, particularly near skin (8).
RF ablation and cryoablation are effective thermal percutaneous treatment options when proper ablation parameters are followed to generate thorough cytotoxic temperatures (ie, greater than 60°C and less than −20°C, respectively) throughout the entire tumor volume. Today, RF is more commonly used for lung and liver ablation, but percutaneous cryoablation (PCA) series have noted lower PCA periprocedural pain and better involution of the ablation zone, or healing, especially for nonorgan soft-tissue sites (4,9–20). The well visualized ice treatment zone, seen by computed tomography (CT) and ultrasound (US) imaging (10–15,18–20), also estimates the underlying cytotoxic margin, lying 5–10 mm behind the leading edge (10). Local cancer control rates comparable to those of surgery or radiation were first noted with prostate cryoablation, even though the prostate is surrounded by vasculature and lies in close proximity to the rectum (12). The unique benefits offered by cryoablation then continued to expand to other sites, with similar safety and efficacy (4,9–20). Good cosmetic outcomes and soft-tissue healing with breast cryoablation have been noted for the treatment of fibroadenomas and locally recurrent and primary breast cancers (13,18). Similarly, our recent studies on disease-based survival for oligometastatic renal-cell carcinoma (21), non–small-cell lung cancer (22), and colorectal cancer (23) contained 79%, 52%, and 8% of tumors in soft-tissue locations, respectively. PCA appears to have the potential to cost-effectively augment many palliative-care situations (21–24) if it could be determined to be safe and effective for most locations.
In the present study, we assessed whether diverse tumor location(s) showed any relative differences in local procedural outcomes of cancer control, morbidity, and ablation volume reduction for a wide variety of soft-tissue tumors.
MATERIALS AND METHODS
All PCA procedures were performed between May 2000 and April 2011. Data were prospectively collected from consecutive patients to assess PCA feasibility, complications, and recurrence rates. PCA efficacy and healing were assessed by local tumor recurrence rate and ablation zone volume reduction, respectively.
Patients
All patients treated with cryotherapy read and signed an authorization form issued under the Health Insurance Portability and Accountability Act of 1996. All patients also signed a separate consent form detailing the procedure, as well as an approved investigational review board consent form covering the PCA procedure and prospective data collection for the present study. Patients were generally referred by oncologists for local tumor control and/or pain control of oligometastatic disease. The following inclusion criteria are given, with our intent to fully treat visible target tumor and/or relieve pain: (i) no more than six separate masses to avoid excessive morbidity, (ii) percutaneous access and safety (eg, no adherent bowel or nerve), (iii) largest mass smaller than 8 cm, (iv) no widespread metastases, (v) growing or positron emission tomography–positive tumors received ablation priority, (vi) potential need for local pain control of tumor site, (vii) no severe bleeding dyscrasia or active anticoagulant therapy, and (viii) ability to give consent and expressed desire for local tumor control was required.
Primary tumor types were noted (Table 1), and PCA procedures were grouped into five locations: retroperitoneal, superficial, intraperitoneal, bone, and head and neck (Table 2). All cases were reviewed and performed by a radiologist with more than 15 years of interventional and cross-sectional imaging experience or an interventional oncologist with more than 5 years of experience. Patients received prophylactic antibiotic treatment, local anesthesia, and moderate sedation as needed during the procedure (13–14,18). All patients were planned as out-patients and monitored after PCA to assess the need for additional pain control or monitoring requiring overnight hospitalization.
Table 1.
Primary Tumor Histology of Soft-Tissue Metastases (N = 251)
| Tumor Type | Patients | Procedures | Tumors |
|---|---|---|---|
| Renal-cell carcinoma | 29 | 54 | 63 |
| Ovarian | 16 | 35 | 40 |
| NSCLC | 16 | 27 | 31 |
| Colorectal | 8 | 13 | 13 |
| Breast | 8 | 9 | 16 |
| Other | 7 | 10 | 11 |
| Genitourinary (other) | 6 | 7 | 7 |
| Head and neck (other) | 4 | 6 | 8 |
| Melanoma | 4 | 9 | 9 |
| Esophageal | 3 | 3 | 3 |
| Head and neck (squamous cell) | 3 | 7 | 7 |
| HCC | 3 | 3 | 4 |
| Osteosarcoma | 3 | 4 | 7 |
| Sarcoma (other) | 3 | 8 | 7 |
| Cervical | 2 | 4 | 5 |
| Uterine | 2 | 2 | 2 |
| ASPS | 2 | 3 | 2 |
| Leiomyosarcoma | 2 | 5 | 3 |
| GIST | 1 | 2 | 3 |
| Endometrial | 1 | 1 | 1 |
| Prostate | 1 | 1 | 1 |
| Multiple myeloma | 1 | 2 | 2 |
| Liposarcoma | 1 | 5 | 6 |
ASPS = alveolar soft part sarcoma, GIST = gastrointestinal stromal tumor, HCC = hepatocellular carcinoma, NSCLC = non–small-cell lung cancer.
Table 2.
Distribution among Procedural Locations of Soft-Tissue Metastases
| Outcome | Retroperitoneal | Superficial | Intraperitoneal | Bone | Head/Neck | Total |
|---|---|---|---|---|---|---|
| Patients | 47 | 41 | 21 | 21 | 12 | 126* |
| Procedures | 69 | 62 | 32 | 34 | 26 | 220* |
| Tumors | 75 | 76 | 39 | 34 | 27 | 251 |
| Mean Diameter (cm) | Volume (cm3) | Mean Diameter (cm) | Volume (cm3) | Mean Diameter (cm) | Volume (cm3) | Mean Diameter (cm) | Volume (cm3) | Mean Diameter (cm) | Volume (cm3) | Mean Diameter (cm) | Volume (cm3) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor | 3.6 | 48.9 | 3.0 | 34.2 | 2.6 | 57.2 | 4.6 | 82.6 | 2.7 | 24.8 | 3.3 | 47.3 |
| Ablation | 5.4 | 111.7 | 5.0 | 99.7 | 4.5 | 82.3 | 6.6 | 203.8 | 4.2 | 58.4 | 5.1 | 109.4 |
Retroperitoneal tumors included postnephrectomy recurrences, adrenal masses, and para-aortic/pericaval masses or adenopathy. Superficial tumor locations consisted of predominantly muscular, subcutaneous, or skin metastases, or all, within the extremities, chest, or abdominal wall, including desmoid tumors. Intraperitoneal tumors were within the abdominal cavity and included epicardial nodes interposed between the liver and heart. Tumors with bone location were limited metastatic deposits in non–weight-bearing locations with an epicenter in osseous structures. Head and neck foci were nodal recurrences of definitively treated (ie, surgery, chemotherapy, and radiation therapy) squamous-cell carcinoma or cancers residing in/around the mandible or clavicle.
The total number of patients is not reflected as a sum of each location because some patients underwent several procedures in different locations.
Imaging Equipment and Protocols
Real-time US (Logiq 700; GE Medical Systems, Milwaukee, Wisconsin) was solely used to place and monitor cryoprobes during procedures in superficial locations if US guidance could provide clear visualization of all tumor margins. However, US artifacts frequently degrade imaging after placement of more than one cryoprobe (10,13,14), and the posterior extent of the ice frequently cannot be visualized because of shadowing. CT (SOMATOM Plus 4; Siemens, Erlangen, Germany) was therefore used as the primary imaging modality for planning, procedure guidance, and treatment follow-up. CT or magnetic resonance (MR) imaging diagnostic studies before and after cryotherapy consisted of helical scans reconstructed at 3-mm increments before, during, and after intravenous enhancements (14,18) based on standard criteria for tumor recurrence (25). Tumor and ablation volumes were calculated by using the common prolate ellipse formula: π/6 × transverse measurement × anterior–posterior measurement × cranio-caudal measurement.
Cryotherapy Equipment and Procedure
Cryoprobes and the system were connected to a Joule Thompson-based gas cryogen control unit by using established probe requirement and placement techniques (10,13–15,18). Patients were placed in supine and/or lateral decubitus position according to procedure access and patient comfort. At least one cryoprobe 1.7 or 2.4 mm in diameter was placed for each centimeter of tumor diameter to achieve the goal of an approximate 1-cm rim of CT-visible ice extending beyond all apparent tumor margins (10). When safely possible, probes were asymmetrically placed closer to adjacent central vasculature to mitigate heat-sink effect and higher risk of tumor recurrence (16). An initial freeze cycle of 8–15 minutes was followed by 5–10 minutes of partial thaw (ie, only the ice ball periphery) and then 5–15 minutes of refreeze, tailored to cover tumor margins. Because of the fourfold greater thermal conductivity of solid ice than tissue liquid (26), the second freeze cycle rapidly recooled the ice volume for the shorter second freeze times.
Protection of adjacent crucial tissues from freezing temperatures was achieved by hydrodissection and/or warm sterile saline solution bags placed directly on the skin surface (13,14). For tumors involving the skin epidermis, cryoablation zones were visibly allowed to progress approximately 1 cm beyond apparent tumor margins without additional skin protection because it was part of the tumor target. Percutaneously placed, air-filled esophageal dilator balloons (2 × 4 cm; Boston Scientific, Boston, Massachusetts) were used for intra-peritoneal cases to move bowel, stomach, bladder, and/or colon when continuous hydrodissection could not be achieved (27).
Complications
All treatment-related complications were categorized in accordance with the Common Terminology Criteria for Adverse Events (Version 4.0) of the National Cancer Institute (28).
Recurrences
Follow-up imaging consisted of enhanced CT or MR at 1, 3, 6, 12, 18, and 24 months and then yearly thereafter. Follow-up time was noted only from the last available CT or MR imaging study, not including the complete time from the procedure. Any ablation size increase or distinct development of asymmetric and/or nodular enhancement was considered a local treatment failure (25) and then separated into local tumor progression and recurrence to better differentiate incomplete tumor ablation from satellite or adjacent disease recurrence (Fig 1).
Figure 1.
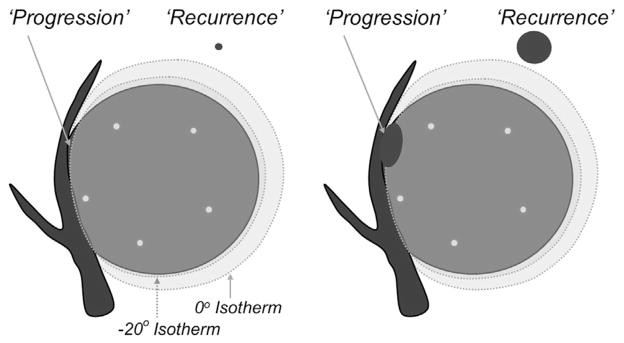
Graphical representation of ablation nomenclature (25) for local tumor recurrences: “progression” versus “recurrence.” Left image shows a 4- to 5-cm tumor with five cryoprobes (small black circles) placed within 1 cm of the tumor margin and less than 2 cm apart (10). A small portion of residual unablated tumor grows as local tumor progression in the right image. A subclinical focus or “satellite” may lie less than 1 cm beyond the visible ice margin and grow as a mass adjacent to the involuting PCA zone but is still considered a local tumor recurrence.
Involution Statistics
The involution data were put into six time-point categories, or statistical bins, of 0.001–4.499 months, 4.500–8.999 months, 9.000–14.999 months, 15.000–20.999 months, 21.000–26.999 months, and 27.000 months or more. These were noted as time points 1.5, 4.5, 9, 15, 21, and 27 months, respectively. The average involution was subsequently calculated at each of these points with respect to the average baseline ablation volume measured (ie, final ice measurements) on completion of all cryoablation procedures.
RESULTS
PCA outcomes were not analyzed according to primary tumor type, in part because of the diverse histologic types noted in Table 1. The anatomic distribution of the 251 soft-tissue tumors treated during 220 PCA procedures involving 126 patients were classified into the five defined procedural locations in Table 2. The average tumor diameter of 3.3 cm was 1.8 cm less than the ablation diameter, compatible with the goal of extending visible ice as much as 1 cm beyond all apparent tumor margins (ie, nonlethal margin) (10). The average patient age at the time of the first procedure was 60.4 years (range, 18.4–91.7 y). Overnight monitoring beyond the standard 2–6-hour postprocedural observation period occurred in 33% of patients, and appeared limited to patients with larger retroperitoneal masses (> 4 cm) and/or patients with tumor locations causing initial pain (eg, bone).
Follow-up
Average follow-up times were 9.0 months for retroperitoneal tumors (range, 0–82 mo), 10.6 months for superficial tumors (range, 0–71 mo), 12.7 months for intraperitoneal tumors (range, 0–70 mo), 14.5 months for bone tumors (range, 0–61 mo), and 11.4 months for head and neck tumors (range, 0–55 mo). The overall average follow-up for all five tumor sites was 11 months (range, 0–82 mo).
Complications
Table 3 shows tumor locations and complications according to Common Terminology Criteria for Adverse Events. A total of seven complications of grade 3 or more severe (ie, major) were noted, but the two deaths that occurred were considered unrelated to treatment and to have resulted from preexisting comorbidity and cardiovascular events. Deaths were still included because they occurred within the standard 1-month postprocedure time course. Therefore, five major complications (2.3%) were attributable to the procedure. Two of the grade 4 complications from the superficial tumor group were related to underlying bowel damage requiring surgical intervention (Fig 2). One grade 4 complication related to bladder damage during an early intraperitoneal case required bladder wall repair and temporary suprapubic drainage. A chest wall ablation, or bone case, directly abutted the pericardium and resulted in a reactive pericardial effusion that required a surgical pericardial window. One grade 3 complication consisted of a patient having prolonged foot drop from ablation of a sacral metastasis from renal-cell carcinoma, but this was anticipated based on its location in a nonsurgical candidate.
Table 3.
Complications per Procedure by Anatomic Location
| Soft Tissue Location | No. of Procedures | Grade
|
||||
|---|---|---|---|---|---|---|
| 1/2 | 3 | 4 | 5 | > 3 | ||
| Retroperitoneal | 69 | 9 | 0 | 0 | 2* | 2 (3) |
| Superficial | 62 | 4 | 0 | 2 | 0 | 2 (3) |
| Intraperitoneal | 32 | 2 | 0 | 1 | 0 | 1 (3) |
| Bone | 34 | 4 | 1 | 1 | 0 | 2 (7) |
| Head/neck | 26 | 3 | 0 | 0 | 0 | 0 |
| Total | 220† | 22 | 1 | 4 | 2 | 7 (3.2) |
Values in parentheses are percentages.
The two deaths that occurred were considered unrelated to the procedure.
The total number of patients is not reflected as a sum of each location because some patients underwent several procedures in different locations.
Figure 2.
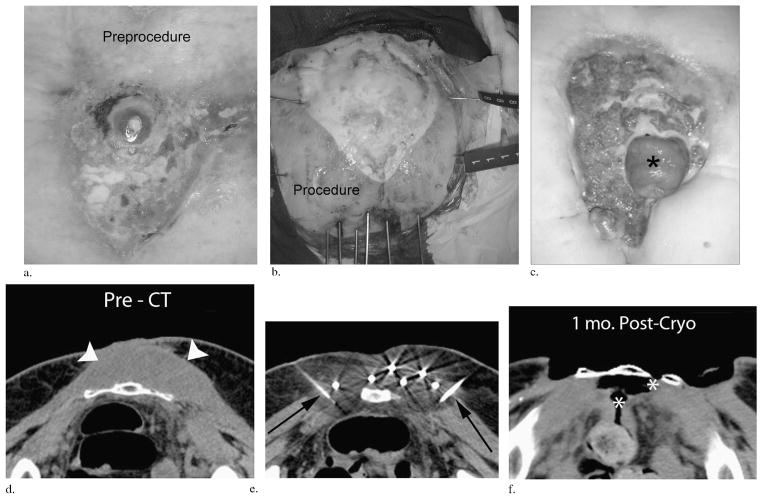
(a–c) Clinical images over time in a patient with a superficial melanosarcoma invading the sacrum that had repeatedly recurred after multiple surgeries. The initial open tumor wound (a), the grossly visible ice extension beyond all tumor margins at the completion of the first freeze with six inferior cryoprobes and lateral saline solution injection needles (b), and the 3-month follow-up (c) after resection of the lower portion of the sacrum finally show a herniated underlying loop of small bowel (black asterisk) and a new satellite tumor recurrence along its inferior margin. (d–f) Companion CT images: pre-CT image through the upper mid-sacrum (d) at the maximum tumor extent (arrowheads). During PCA (e), ice rapidly extended posteriorly into the rectal wall when the lateral saline solution injection needles were occluded by ice (black arrows). This resulted in an eventual fistula track at 1-month follow-up CT (white asterisks, f) before surgical repair and resection of the lower sacrum. In addition to this avoidable grade 4 complication, this patient accounted for four repeated cryoablations of satellite recurrences beyond the visible treatment margin but remained pleased with the outcome, as the debrided wound was easier to manage than the continual weeping and bleeding initial tumor.
Recurrences
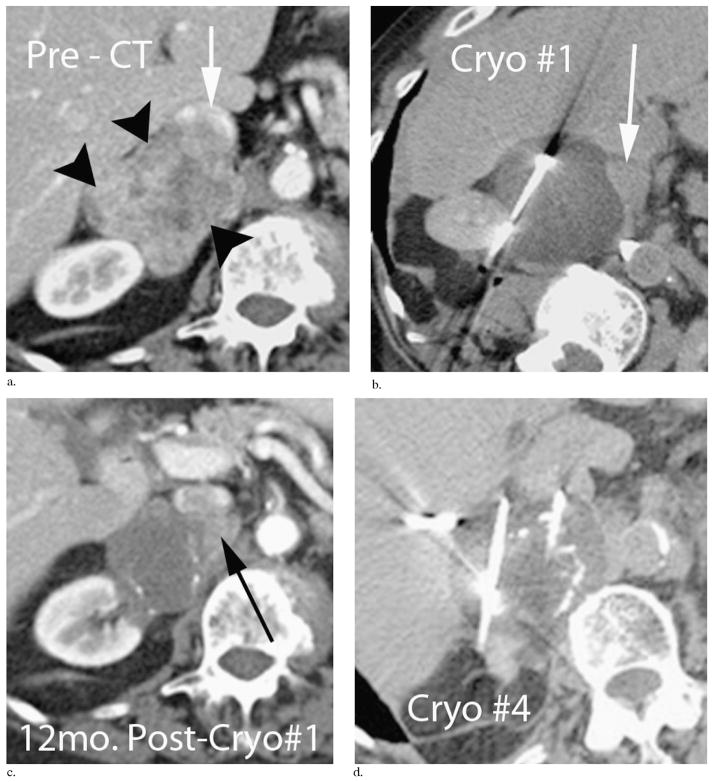
Table 4 shows local recurrences after the procedure, separated according to likely local tumor progression or recurrence (25) for the five tumor location groups. Several observations are notable despite insufficient numbers to generate statistical significance. The retroperitoneal recurrences are more dominated by the tumor progression subgroup and appear related to abutting major vasculature of the aorta and/or inferior vena cava (IVC). Figure 3 demonstrates three local tumor progressions over a 3-year time span, abutting the IVC in a 90-year-old patient with a leiomyosarcoma of the IVC wall that replaced the right adrenal gland. Conversely, local tumor recurrences were predominantly related to subclinical satellite lesions, and four of these occurred repeatedly in one patient with an original 7-cm melanosarcoma (Fig 2). The average time to recurrence for all patients was 4.1 months.
Table 4.
Rates of local Tumor Progression and Recurrence by Anatomic Group
| Procedure Site | No. of Tumors | Total Local Recurrences | Average Time to Recurrence (mo) | Type
|
|
|---|---|---|---|---|---|
| Procedural | Satellite | ||||
| Retroperitoneal | 75 | 12 (16) | 5.5 | 3/12 (25) | 9/12 (75) |
| Superficial | 76 | 6 (8) | 1.1 | 0/6 | 6/6 |
| Intraperitoneal | 39 | 2 (13) | 2.3 | 0/2 | 2/2 |
| Bone | 34 | 1 (3) | 42* | 0/1 | 1/1 |
| Head/neck | 27 | 5 (19) | 3.4 | 0/0 | 5/5 |
| Total | 251 | 26 (10)† | 4.9 | 3/26 (12) | 23/26 (88) |
Values in parentheses are percentages.
The 42-month “average” time to recurrence is not an accurate depiction, as this value was derived from a single tumor.
Of the total 26 recurrences (10%), three of 251 were within initial tumor margins, for a local tumor progression rate of 1.2%.
Figure 3.
Repeated local tumor progressions near the IVC (arrow, a) following PCA procedures for a 5-cm leiomyosarcoma involving the posterior wall of the IVC and replacing the right adrenal (black arrowheads, a). This 90-year-old patient tolerated repeated outpatient PCA procedures (b, d) and maintained a 100% Karnofsky performance status. Progressive calcifications along the involuted tumor rim developed between 12 months (c) and 24 months (d), which also helped define the original tumor margin from newly enhancing adjacent local tumor recurrences (black arrow, c).
Involution Statistics
A total of 174 of 251 tumors had imaging follow-up with associated tumor measurements: 151 in the 1.5-month bin, 100 in the 4.5-month bin, 83 in the 9-month bin, 41 in the 15-month bin, 15 in the 21-month bin, and 30 in the 27-month-or-longer bin. Table 5 shows volumetric changes in the ablation zone over time by noting the percentage of the ablation area that remained. By 4.5 months after cryoablation, the ablation zone had reduced to less than the original tumor volume, compatible with thorough cryoablation effect, except for all tumor recurrences that occurred within 6 months.
Table 5.
Involution after Initial Ablation for All Tumors
| Parameter | Time since Cryotherapy
|
||||||
|---|---|---|---|---|---|---|---|
| Baseline | < 1.5 mo | 4.5 mo | 9 mo | 15 mo | 21 mo | ≥ 27 mo | |
|
| |||||||
| No. of tumors | 263* | 151 | 100 | 83 | 41 | 15 | 30 |
| Initial tumor volume (cm3) | 90.4 | – | – | – | – | – | – |
| Ablation zone volume (cm3) | 208.4 | 149.0 | 84.1 | 53.8 | 25.6 | 14.5 | 20.3 |
| Tumor volume reduction (cm3) | – | 59.5 | 124.4 | 154.6 | 182.8 | 193.9 | 188.09 |
| Resorption (%) | – | 28.5 | 59.7 | 74.2 | 87.7 | 93.0 | 90.3 |
Whereas ablation volume had been reduced to half at 4.5 mo, maximal involution was approached by 15 mo.
Baseline tumor count of 263 is higher than the actual number of tumors in the study because tumors treated repeatedly were used for resorption calculations only until the time of the subsequent procedure, at which time a new unique value was associated with that tumor and its respective involution observations.
DISCUSSION
The present study details the use of predominantly CT-guided PCA in diverse, nonorgan locations for a large number of difficult-to-treat patients requiring local control of recurrent and/or oligometastatic disease. Complication and local tumor progression and recurrence rates of 3% and 1.2%, respectively, corroborate our hypothesis that the visible edge of the ice ball and knowledge of underlying cytotoxic isotherms provide for safety and efficacy of PCA in many diverse soft-issue and nonorgan locations. Ablation zone visualization is the primary advantage of cryoablation over other ablation options, and helps ensure cytotoxic tumor coverage, assuming sufficient cryoprobe placement density (10). Real-time CT and US imaging of ice coverage during the procedure thereby provided a good surrogate for cryoablation outcomes. Such visual prognostication of safety and efficacy may not be possible for heat-based ablations, particularly near skin or nerves. CT provides better circumferential visualization of the entire ablation zone, particularly for deeper masses, similar to the findings of previous cryoablation studies (10,12,14,15,18). CT control of the cryoablation zone proved particularly important for planning and avoiding complications (Fig 2). Similarly, a previous study of cryoablation of breast cancer (18) used only US guidance without CT when a tumor was small and superficial and had thorough margin visualization.
Complications in the present series were generally anticipated and offered insight into special conditions. Complications that could have been prevented were likely part of the “learning curve” associated with any evolving procedure. In one example, a bladder dome perforation was related to inadequate intraperitoneal hydrodissection and/or balloon interposition, as it was presumed that it was potentially safe to freeze into the bladder, similar to prostate cryotherapy (12). However, the bladder neck near the prostate is retroperitoneal and unlikely to break down during healing. We now emphasize vigorous protection for any intraperitoneal portion of the bladder or bowel, which frequently requires balloon interposition because fluid injected during hydrodissection will likely not remain at the injection site (27). None of our patients with ablations near the adrenal gland had excessive hypertensive responses, presumably because the functional adrenal tissue was likely replaced by a tumor. However, α- and β-blockade are routinely advocated if cryoablation of liver, kidney, and/or retroperitoneal tumors may extend into normal-appearing, adjacent adrenal gland (29). No unintended skin complication was noted, and even large intended skin ablations healed well (Fig 2).
Evaluating cryoablation zone involution over time, along with local tumor recurrence data, provided insight. Namely, nearly all recurrences were identified within 6 months (Table 4). If the overall ablation zone is not smaller than the original tumor size by 6 months (Table 5), greater scrutiny and/or biopsy confirmation appears warranted. Involution of the well visualized treatment zone to less than the original tumor size by 6 months therefore appears to have prognostic significance, in addition to the standard imaging criteria (25). The 93% progressive reduction in ablation zone volume by 21 months is also comparable to earlier observations of cryoablation healing (13–15,18). Ablation zone reductions are likely related to underlying fibrous content of the tumor and associated ablation zone (13,14,18). The overall ablation zone reduction or healing potential of cryotherapy also appears greater than heat-based ablations, which denature protein. Perhaps the body’s natural healing processes (eg, inflammatory cell infiltrates [30]) cannot easily penetrate a denatured coagulum without an underlying collagenous architecture. Further work may address whether observed survival improvements after PCA (21–23) may relate to the immunotherapy potential associated with these infiltrates, rather than just simple debulking of disease burden.
The separation of local tumor progression and recurrences in the present study conforms to anticipated updates to established ablation nomenclature (25), and helped identify differences in local heat sink and/or disease characteristics for each tumor type and/or location. The greater incidence of local tumor progression adjacent to major vasculature (Fig 3) corroborates the need for increased probe density (ie, closer spacing) and/or ablation power to achieve sufficient cytotoxic temperatures adjacent to major heat sinks. However, larger tumors (31) are more prone to satellite foci and local tumor recurrence (Fig 2), which likely will require combination with more effective systemic treatments.
Our total PCA local recurrence rate of 10% at a mean of 11 months of follow-up is comparable to the 10%–20% recurrence rates at 5 years in surgical series (32). The lack of long-term follow-up in our group is mitigated by the fact that all known recurrences (with the exception of one) occurred within 6 months, and there was only one known late recurrence, in a patient with up to 82 months as of preparation. In addition, only three of the 26 recurrences were deemed to have occurred within the initial tumor margins, meaning the remaining recurrences may have been caused by adjacent microscopic foci that proliferated over time, rather than procedural failure. The 3% complication rate seen in the present study involving patients with stage IV disease is also much lower than the 22% rate reported for sarcoma surgery of primary cancers (33). Cryoablation could become a useful palliative treatment for diverse anatomic sites, while providing cost-effective potential options for many patients with common cancers (21–23). A synergistic approach to palliative care that uses cryoablation in conjunction with chemotherapy and/or radiation therapy may expand treatment options for those unable to undergo surgery and reduce overall treatment morbidities and/or costs.
Despite the large size of the present series, numerous weaknesses of the study could be viewed as arising from the diversity of patients, anatomic sites, and tumor types. Separation by tumor type could have provided more disease-specific analyses and survival comparisons with historical controls, but have already been addressed for three of the five most common tumor types in Table 1 (21–23). Our large overall sample size therefore allowed greater statistical power to assess ablation zone reduction over time, which suggests minimal difference in complication and recurrence rates regardless of anatomic site, with appropriate precautions. The diverse tumor types and locations also did not allow extensive description of procedural techniques for each region. However, this may also be viewed as a strength of soft-tissue cryoablation because interventional oncologists should be able to reproduce our outcomes with attention to basic probe placement guidelines (10,13,14,18–23).
In summary, PCA for nearly any soft-tissue mass is a safe, predominantly outpatient technique that allows intraprocedural adjustments to ensure thorough treatment coverage. Visualization of the cryoablation zone helps provide safety and efficacy for soft-tissue tumors in difficult-to-treat patients requiring local tumor palliation for diverse anatomic sites.
Acknowledgments
This work was supported by grant sponsorship from Endocare (Malvern, Pennsylvania) and Galil Medical (Yokneam, Israel) for the Clinical Trials Office and associated costs and by Cancer Center Core Grant funding.
ABBREVIATIONS
- IVC
inferior vena cava
- PCA
percutaneous cryoablation
- RF
radiofrequency
References
- 1.American Cancer Society. Cancer facts & figures 2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509–524. doi: 10.1200/JCO.1991.9.3.509. [DOI] [PubMed] [Google Scholar]
- 3.Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 4.Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases-why cryoablation? Skeletal Radiol. 2009;38:835–839. doi: 10.1007/s00256-009-0736-4. [DOI] [PubMed] [Google Scholar]
- 5.Strander H, Turesson I, Cavallin-Stahl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol. 2003;42:516–531. doi: 10.1080/02841860310014732. [DOI] [PubMed] [Google Scholar]
- 6.Vogl TJ, Mack MG, Straub R, Engelmann K, Zangos S, Eichler K. Inter-ventionelle MR-gesteuerte laserinduzierte thermotherapie bei onkologischen fragestellungen. Radiologie. 1999;39:764–771. doi: 10.1007/s001170050573. [DOI] [PubMed] [Google Scholar]
- 7.Sanou R, Bazin C, Krakowski I, et al. Radiofrequency ablation for palliation of soft tissue tumor pain. J Radiol. 2010;91:281–286. doi: 10.1016/s0221-0363(10)70039-1. [DOI] [PubMed] [Google Scholar]
- 8.Callstrom MR, York JD, Gaba RC, et al. Research reporting standards for image-guided ablation of bone and soft tissue tumors. J Vasc Interv Radiol. 2009;20:1527–1540. doi: 10.1016/j.jvir.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Tuncali, et al. MRI-Guided percutaneous cryotherapy for soft tissue and bone metastases: initial experience. AJR Am J Roentgenol. 2007;189:232–239. doi: 10.2214/AJR.06.0588. [DOI] [PubMed] [Google Scholar]
- 10.Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allaf ME, Varkarakis IM, Bhayani SB, et al. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation—initial observations. Radiology. 2005;237:366–370. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- 12.Bahn DK, Lee F, Badalament R, et al. Targeted cryoablation of the prostate: Seven year outcomes in the primary treatment of prostate cancer. Urology. 2002;60:3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- 13.Littrup PJ, Freeman-Gibb L, Andea A, et al. Cryotherapy for breast fibroadenomas. Radiology. 2005;234:63–72. doi: 10.1148/radiol.2341030931. [DOI] [PubMed] [Google Scholar]
- 14.Littrup PJ, Ahmed A, Aoun HD, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18:383–392. doi: 10.1016/j.jvir.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Littrup PJ, Duan Y, et al. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235:289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 16.Weber SM, Lee FT, Jr, Chinn DO, et al. Perivascular and intralesional tissue necrosis after hepatic cryoablation: Results in a porcine model. Surgery. 1997;122:742–747. doi: 10.1016/s0039-6060(97)90082-9. [DOI] [PubMed] [Google Scholar]
- 17.Berger WK, Poledna J. New strategies for the placement of cryoprobes in malignant tumors of the liver for reducing the probability of recurrences after hepatic cryosurgery. Int J Colorectal Dis. 2001;16:331–339. doi: 10.1007/s003840100317. [DOI] [PubMed] [Google Scholar]
- 18.Littrup PJ, Jallad B, Chandiwala-Mody P, et al. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329–1341. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lessard AM, Gilchrist J, Schaefer L, Dupuy DE. Palliation of recurrent Ewing sarcoma of the pelvis with cryoablation and somatosensory-evoked potentials. J Pediatr Hematol Oncol. 2009;31:18–21. doi: 10.1097/MPH.0b013e31818ab2b7. [DOI] [PubMed] [Google Scholar]
- 20.Schirmang TC, Davis LM, Nigri PT, Dupuy DE. Solitary fibrous tumor of the buccal space: treatment with percutaneous cryoablation. AJNR Am J Neuroradiol. 2007;28:1728–1730. doi: 10.3174/ajnr.A0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang HJ, Littrup PJ, Currier BP, et al. Percutaneous cryoablation of metastatic lesions from non-small-cell lung carcinoma: initial survival, local control, and cost observations. J Vasc Interv Radiol. 2012;23:761–769. doi: 10.1016/j.jvir.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bang HJ, Littrup PJ, Goodrich DJ, et al. Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost effectiveness for palliation. J Vasc Interv Radiol. 2012;23:770–777. doi: 10.1016/j.jvir.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang HJ, Littrup PJ, Currier BP, et al. Percutaneous cryoablation of metastatic lesions from colorectal cancer: Efficacy and feasibility with survival and cost-effectiveness observations. ISRN Minim Invas Surg. 2012:942364. doi: 10.5402/2012/942364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMenomy BP, Kurup AN, Johnson GB. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2012;24:207–213. doi: 10.1016/j.jvir.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765–778. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 26.Evans W, Fish J, Keblinski P. Thermal conductivity of ordered molecular water. J Chem Phys. 2007;126:154504. doi: 10.1063/1.2723071. [DOI] [PubMed] [Google Scholar]
- 27.Kam AW, Littrup PJ, Walther MM, et al. Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2004;15:753–758. doi: 10.1097/01.rvi.0000133535.16753.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trotti A, Colevas AD, Setser A, et al. CTCAE v4. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 29.Atwell TD, Wass CT, Charboneau JW, et al. Malignant hypertension during cryoablation of an adrenal gland tumor. J Vasv Interv Radiol. 2006;17:573–575. doi: 10.1097/01.RVI.0000197370.83569.33. [DOI] [PubMed] [Google Scholar]
- 30.Littrup PJ, Mody A, Sparschu R, et al. Prostatic cryotherapy: ultrasonographic and pathologic correlation in the canine model. Urology. 1994;44:175–184. doi: 10.1016/s0090-4295(94)80123-1. [DOI] [PubMed] [Google Scholar]
- 31.Ng IOL, Path MRC, Lai ECS, et al. Prognostic significance of pathologic features of hepatocellular carcinoma. Cancer. 1995;76:2443–2448. doi: 10.1002/1097-0142(19951215)76:12<2443::aid-cncr2820761207>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 33.Stoeckle E, Italiano A, Stock N, et al. Surgical margins in soft tissue sarcoma. Bull Cancer. 2008;95:1199–1204. doi: 10.1684/bdc.2008.0765. [DOI] [PubMed] [Google Scholar]