Abstract
Objective
Apolipoprotein (apo) A-V is a low abundance plasma protein that modulates triacylglycerol (TG) homeostasis. Gene transfer studies were undertaken in apoa5 (−/−) mice to define the mechanism underlying the correlation between the single nucleotide polymorphism (SNP) c.553G>T in APOA5 and hypertriglyceridemia (HTG).
Approach and Results
Adeno-associated virus (AAV) 2/8 mediated gene transfer of wild type (WT) apoA-V induced a dramatic lowering of plasma TG in apoa5 (−/−) mice while AAV2/8-Gly162Cys apoA-V (corresponding to the c.553G>T SNP: rs2075291) had a modest effect. Characterization studies revealed that plasma levels of WT- and G162C apoA-V in transduced mice were similar and within the physiological range. Fractionation of plasma from mice transduced with AAV2/8-G162C apoA-V indicated that, unlike WT apoA-V, >50% of G162C apoA-V was recovered in the lipoprotein-free fraction. Non-reducing SDS-PAGE immunoblot analysis provided evidence that G162C apoA-V present in the lipoprotein-free fraction, but not that portion associated with lipoproteins, displayed altered electrophoretic mobility consistent with disulfide-linked hetero-dimer formation. Immunoprecipitation followed by liquid chromatography/mass spectrometry of human plasma from subjects homozygous for WT APOA5 and c.553G>T APOA5 revealed that G162C apoA-V forms adducts with extraneous plasma proteins including fibronectin, kininogen-1 and others.
Conclusion
Substitution of Cys for Gly at position 162 of mature apoA-V introduces a free cysteine that forms disulfide bonds with plasma proteins such that its lipoprotein binding and TG modulation functions are compromised.
Keywords: apolipoprotein A-V, triacylglycerol, lipoprotein, gene therapy, single nucleotide polymorphism
Apolipoprotein (apo) A-V was discovered by comparative genome analysis and shown to function as a modulator of plasma triacylglycerol (TG) levels.1 ApoA-V is a low abundance plasma protein (25 – 400 ng/ml) that associates with VLDL and HDL.2,3,4 It has been proposed that apoA-V modulates plasma TG-rich lipoprotein metabolism by binding to glycosylphosphatidylinositol high-density lipoprotein binding protein 1 (GPIHBP1), thereby promoting lipoprotein lipase (LPL) mediated TG hydrolysis.5
Genome wide association studies have identified SNPs in APOA5 that correlate with elevated plasma TG.6 Kao et al.7 described a c.553G>T APOA5 SNP in a Taiwanese population that gives rise to a Cys for Gly substitution at position 185 of the pre-protein, corresponding to residue 162 in mature, circulating apoA-V. This polymorphism introduces a second cysteine into apoA-V, with the other occurring at position 204. Minor allele frequency in control subjects is 0.042 compared to 0.27 (P<0.001) in subjects with hypertriglyceridemia (HTG). Serum TG levels showed a gene dosage effect, corresponding to 92 ± 38 mg/dl in G/G subjects, 107 ± 35 mg/dl in G/T individuals and 183 mg/dl in individuals with the T/T genotype (P=0.014). In another study, Pullinger et al.8 reported that the T allele frequency was 4 times higher in Chinese subjects with HTG compared to those with low TG (15.1% vs. 3.7%). Among those with HTG, heterozygosity was associated with doubling of plasma TG while homozygotes manifest severe HTG (mean TG = 2,292 ± 447 mg/dl). More recently, the c.553G>T SNP has been identified in subjects of European ancestry9,10, indicating worldwide occurrence.
Insofar as HTG is an independent risk factor for coronary artery disease11 and is a component of the metabolic syndrome12 there is great interest in understanding the molecular basis whereby APOA5 SNPs lead to elevated plasma TG.13 Indeed, given the frequency of the c.553G>T APOA5 SNP, it may be anticipated that millions of people worldwide are carriers. Dorfmeister et al.14 reported that G162C apoA-V forms multimers in vitro, although the bulk of the protein is monomeric. Binding studies with members of the low-density lipoprotein receptor family indicated G162C apoA-V is functional, leading these authors to propose defective LPL activation as the underlying mechanism. In another study, Huang et al.15 investigated the effect of different amino acid substitution mutations at position 162 in apoA-V on its LPL activation properties with the finding that Gly at this position is important in terms of LPL activation in vitro.
In a recent study, Sharma et al. reported that adeno-associated virus (AAV) 2/8 mediated gene transfer of wild type (WT) apoA-V improved the HTG phenotype of apoa5 (−/−) mice.16 In the present study it is demonstrated that gene transfer of G162C apoA-V results in a protein that forms disulfide-linked heterodimers with extraneous plasma proteins resulting in an unusual plasma distribution pattern, defective lipoprotein binding and compromised TG-lowering activity.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
ApoA-V expression following AAV2/8-mediated gene transfer into apoa5 (−/−) mice
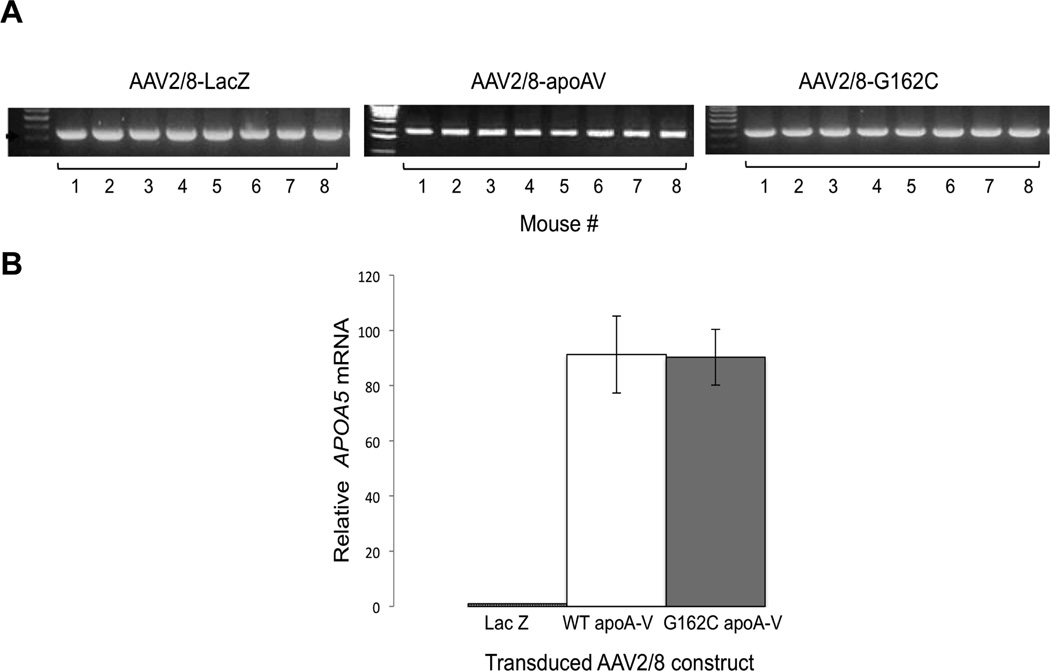
Previous gene transfer studies with AAV2/8 showed the virus homes to liver tissue.16 Consistent with this, gene amplification of cytomegalovirus promoter DNA in liver extracts from each of 8 apoa5 (−/−) mice transduced with AAV2/8-LacZ, AAV2/8-WT apoA-V or AAV2/8-G162C apoA-V provided evidence of vector delivery to the target organ (Figure 1a). Whereas no apoA-V mRNA was detected in liver extracts of mice transduced with AAV2/8-LacZ, similar levels of apoA-V mRNA were present (Figure1b) in liver extracts of mice injected with AAV2/8-WT apoA-V or AAV2/8-G162C apoA-V.
Figure 1. Characterization of AAV2/8 transduced livers.
Four weeks after administrating 1 × 1012 vg AAV2/8-LacZ, AAV2/8-WT apoA-V or AAV2/8-G162C apoA-V to apoa5 (−/−) mice, livers were harvested. Panel A) Gene amplification of cytomegalovirus promoter DNA from individual mouse liver extracts. Panel B) qPCR analysis of APOA5 mRNA expression levels in livers of AAV2/8-LacZ, AAV2/8-WT apoA-V and AAV2/8-G162C apoA-V transduced mice, normalized to GAPDH expression. Values are the mean ± SEM (n = 8).
Effect of AAV2/8-G162C apoA-V on plasma apoA-V and TG levels
To assess relative plasma levels of WT- and G162C- apoA-V protein, blood was drawn each week for 4 weeks following gene transfer and immunoblot analysis performed (Figure 2a). ApoA-V was detected in both groups after 1 week and increased in subsequent weeks, reaching a maximum at 4 weeks post transduction. From comparison of relative band intensities, it may be concluded that plasma concentrations of WT apoA-V and G162C apoA-V are in the same range. The TG content of individual mouse plasma samples as a function of time following gene transfer of AAV2/8-LacZ, AAV2/8-WT apoA-V and AAV2/8-G162C apoA-V were measured (Figure 2b). Following injection with AAV2/8-LacZ, plasma TG levels gradually increased, reaching 20% above baseline after 4 weeks. On the other hand, plasma TG levels steadily declined in mice injected with AAV2/8-WT apoA-V. Indeed, after 4 weeks, plasma TG levels were 69% lower than the corresponding values for AAV2/8-LacZ transduced mice (p=0.0003). By contrast, plasma TG levels in mice transduced with AAV2/8-G162C apoA-V remained relatively constant over time. As a result, at 4 weeks, TG levels in these mice were 21% lower than AAV2/8-LacZ transduced mice (p=0.32; not significant) and 50% higher (p=0.005) than AAV2/8-WT apoA-V transduced mice. Thus, despite the fact that their corresponding mRNA and plasma protein concentrations are very similar, G162C apoA-V was significantly different from WT apoA-V in terms of in vivo TG-lowering activity.
Figure 2. Effect of gene transfer on plasma apoA-V and TG content.
Apoa5 (−/−) mice (eight mice per group) were transduced with AAV2/8-LacZ, AAV2/8-WT apoA-V or AAV2/8-G162C apoA-V and plasma samples collected each week for four weeks. Panel A) anti-apoA-V immunoblot of pooled plasma samples (2 µl aliquot) from indicated time points. At the left is recombinant apoA-V standard (1.5 ng). Panel B) Plasma TG content in individual plasma samples reported as mean ± SEM (n=8) of percent change versus week 0 values. Black bars, AAV2/8-LacZ; marbled bars, AAV2/8-WT apoA-V; gray bars, AAV2/8-G162C apoA-V. At week 4, TG values for WT apoA-V transduced mice were 69% lower (p=0.0003) than LacZ transduced mice and 50% lower (p=0.005) than G162C apoA-V transduced mice.
Lipid and apoA-V distribution in transduced mouse plasma
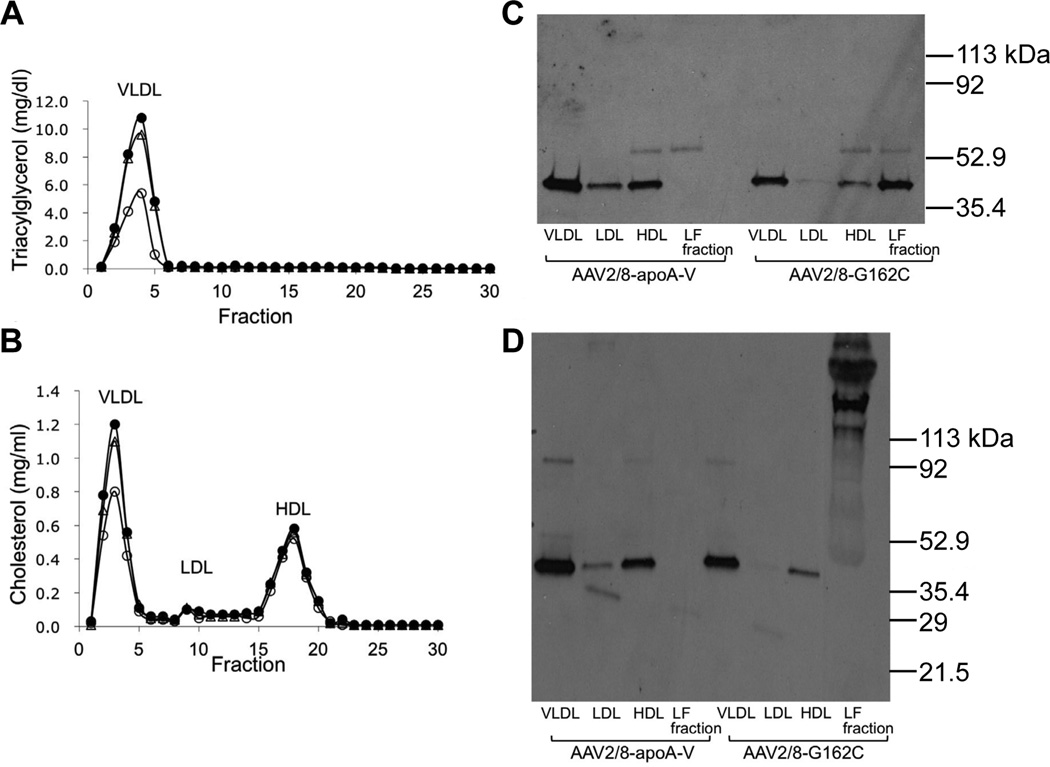
Four weeks after injection of AAV2/8-LacZ, AAV2/8-WT apoA-V or AAV2/8-G162C apoA-V into apoa5 (−/−) mice, the animals were sacrificed and blood collected. Pooled plasma samples from each group were subjected to FPLC to separate individual lipoprotein classes and the lipoprotein-free fraction. Lipid analysis confirmed that TG lowering in mice transduced with WT apoA-V was substantial and confined to VLDL (Figure 3a). By contrast, gene transfer of AAV2/8-G162C apoA-V induced only a slight decline in VLDL TG, as compared to AAV2/8-LacZ injected mice. Cholesterol lowering in mice transduced with WT apoA-V was confined to VLDL (Figure 3b). SDS-PAGE-immunoblot analysis of fractionated lipoproteins under reducing conditions revealed that virtually all WT apoA-V was recovered in association with lipoprotein particles, primarily VLDL followed by HDL (Figure 3c). By contrast, G162C apoA-V showed a very different distribution pattern with large amounts of this protein recovered in the lipoprotein-free fraction. Densitometry analysis indicated that >50% of G162C apoA-V was present in the lipoprotein-free fraction of plasma, as compared to <1% for WT apoA-V. To assess whether G162C apoA-V in the lipoprotein-free fraction of plasma may be the result of homo- or hetero-disulfide bond formation via the Cys residue introduced by the c.553G>T SNP, SDS-PAGE–immunoblot analysis was performed under non-reducing conditions (Figure 3d). Whereas all WT apoA-V, as well as that portion of G162C apoA-V recovered in lipoprotein fractions, migrated as a monomeric protein, G162C apoA-V recovered in the lipoprotein-free fraction displayed much slower electrophoretic mobility, with several discrete higher molecular weight bands present.
Figure 3. Effect of AAV2/8 gene transfer on plasma lipid and apolipoprotein distribution.
Panel A) Plasma samples collected from mice 4 weeks after injection of AAV2/8-LacZ, AAV2/8-WT apoA-V or AAV2/8-G162C apoA-V were pooled (8 mice/group) and subjected to FPLC. Panels A) and B): TG and cholesterol content, respectively, of separated lipoprotein fractions from AAV2/8-LacZ plasma (filled circles), AAV2/8-WT apoA-V plasma (open circles) and AAV2/8-G162C apoA-V plasma (open triangles). Panel C) Anti-apoA-V SDS-PAGE (reducing conditions) immunoblot analysis of fractions obtained following FPLC of indicated plasma samples. Panel D) Non-reducing SDS-PAGE anti-apoA-V immunoblot of fractions obtained from FPLC of indicated plasma samples. VLDL = very low-density lipoprotein; LDL = low-density lipoprotein and HDL = high-density lipoprotein. FPLC elution fractions 22–30 were pooled as lipoprotein-free plasma; labeled as “LF fraction” in panels C and D. An equivalent volume of each sample was applied to the gel after concentration by ultrafiltration.
Characterization of G162C apoA-V in T/T human plasma
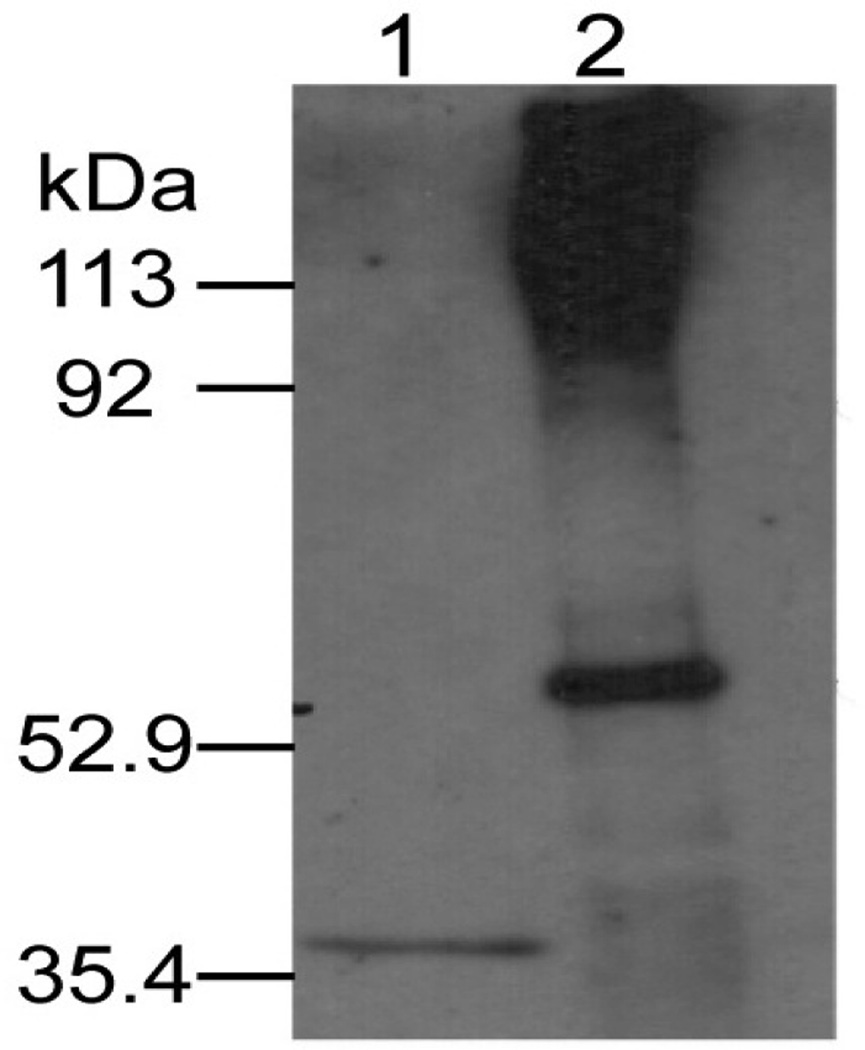
To evaluate the extent to which observations made in AAV2/8-G162C apoA-V transduced apoa5 (−/−) mice recapitulate physiological processes in humans harboring the c.553G>T SNP, pooled plasma from individuals homozygous for the variant (T/T) allele were subjected to density ultracentrifugation to obtain the lipoprotein-free bottom fraction. Following dialysis, immunoprecipitation was performed with anti-apoA-V IgG. Precipitated proteins were subjected by non-reducing SDS-PAGE, transferred to PVDF and probed with α-apoA-V (Figure 4). The data show that, as with plasma from apoa5 (−/−) mice transduced with G162C apoA-V (Figure 3), T/T plasma possessed abundant apoA-V protein with slower electrophoretic mobility in the lipoprotein-free fraction. To assess whether some portion of apoA-V in T/T plasma is associated with the lipoprotein-rich top fraction, immunoblots for apoA-V were carried out as described in Supplement Figure I. The data reveals that some apoA-V associates with lipoproteins and that high molecular adducts are present. Unlike T/T results, the lipoprotein-free bottom fraction from pooled G/G plasma showed no apoA-V (data not shown). Based on these findings, studies designed to identify the binding partner(s) with which apoA-V interacts were performed with human plasma samples.
Figure 4. Immunoblot analysis of human T/T lipoprotein-free plasma fraction.
Pooled plasma samples from human subjects homozygous for the T/T allele was adjusted to 1.21 g/ml with NaBr and subjected to density ultracentrifugation. The lipoprotein-free bottom was collected, dialyzed against PBS and immunoprecipitated with anti-apoA-V IgG. The precipitated proteins were solubilized in sample treatment buffer, electrophoresed under non-reducing conditions, transferred to a PVDF membrane and probed for apoA-V as described in Materials and Methods. Lane 1, recombinant apoA-V standard (1.5 ng); lane 2, T/T sample immunoprecipitate (molecular weights indicated on left).
Identification of G162C apoA-V interaction partners in T/T human plasma
To identify proteins with which G162C apoA-V interacts in human plasma, immunoprecipitation and LC/MS analysis were performed. Using the positive staining region of a T/T plasma sample immunoblot as guide, the corresponding low mobility regions from a parallel gel were excised, digested with trypsin and subjected to LC/MS. The sample from subjects homozygous for the T/T allele yielded numerous interacting protein candidates (Table 1) that were absent or detected at far lower levels in the G/G sample or a T/T plasma sample subjected to IP with beads lacking Ab. The relative abundance of proteins in each sample was approximated by calculating its contribution to a sum of the exponentially modified protein indices (emPAI) assigned to each sample component (mol %).17 The generated mol % values were then compared across samples. Only proteins that demonstrated at least 4 fold higher relative abundance in the T/T sample vs. controls (G/G plasma or T/T plasma subjected to IP with beads lacking Ab) were considered potential apoA-V interacting partners. As expected, apoA-V was detected only in the T/T sample. Among the confidently identified proteins, that met the above acceptance criteria, were the extracellular matrix protein, fibronectin, kininogen-1 (a constituent of the blood coagulation system) and the immune-related proteins, complement C1q, Ig gamma-2 chain C region and complement factor B. Of note, relative abundances of all candidate proteins were either very close to or lower than that of apoA-V itself (Molar Fraction = 0.28%). In studies designed to obtain independent verification of the mass spectrometry results presented, an antibody directed against one of the candidate proteins (apoE) was used to probe T/T and G/G plasma sample apoA-V immunoprecipitates (Supplement Figure II). The data reveal apoE immunoreactivity only in the T/T plasma lipoprotein-rich fraction.
Table 1.
Identification of candidate G162C apoA-V interaction partners1
| Entry Name |
Identified Protein Name | Protein Score2 |
Molar Fraction (%)2 |
Molecular Mass (Da) |
[mol %] TT/GG ratio3 |
[mol %] TT/no Ab TT ratio3 |
|---|---|---|---|---|---|---|
| APOA5_HUMAN | Apolipoprotein A-V | 183 | 0.28 | 41244 | ||
| FINC_HUMAN | Fibronectin4 | 942 | 0.22 | 266052 | 5.3 | |
| KNG1_HUMAN | Kininogen-1 | 252 | 0.23 | 72996 | 10.3 | 0.9 |
| ZA2G_HUMAN | Zinc-alpha-2-glycoprotein | 141 | 0.15 | 34465 | ||
| C1QB_HUMAN | Complement C1q subcomp. subunit B | 127 | 0.20 | 26933 | ||
| IGHG2_HUMAN | Ig gamma-2 chain C region | 125 | 0.23 | 36505 | ||
| IGHG1_HUMAN | Ig gamma-1 chain C region | 93 | 0.23 | 36596 | 4.6 | |
| CASPE_HUMAN | Caspase-14 | 78 | 0.09 | 27947 | ||
| CFAB_HUMAN | Complement factor B | 71 | 0.03 | 86847 | ||
| BLMH_HUMAN | Bleomycin hydrolase | 50 | 0.10 | 53155 | ||
| HBB_HUMAN | Hemoglobin subunit beta | 47 | 0.16 | 16102 | ||
| S10A7_HUMAN | Protein S100-A7 | 44 | 0.23 | 11578 | ||
| HRG_HUMAN | Histidine-rich glycoprotein | 37 | 0.04 | 60510 | ||
| APOE_HUMAN | Apolipoprotein E | 35 | 0.07 | 36246 |
Proteins were identified from specified human plasma samples following immunocapture with anti-apoA-V IgG- or control- Direct-IP beads. Immunoprecipitated proteins were separated by non-reducing SDS-PAGE and excised gel slices digested with trypsin prior to LC/MS, as described in Materials and Methods.
Protein Score and Molar Fraction were calculated as described in Materials and Methods. Protein Score is the sum of the highest ions score for each distinct peptide sequence. Molar Fraction (mol %) for each protein represents a contribution of its emPAI value to the total emPAI values of all proteins identified in the sample. emPAI relies on an assumption that the extent to which the theoretically observable peptides derived from a protein identified (observed) in the analysis directly correlates with the relative abundance of a protein in a sample. emPAI values were estimated by the Mascot search engine using protein molecular mass, the average amino acid composition of the database and enzyme specificity to derive a number of theoretically observable peptides.
Molar ratio values are indicated when a given protein was also detected in G/G plasma or T/T plasma immunoprecipitated with beads lacking Ab. Only proteins with either ratio > 4 are shown.
Fibronectin peptides cover sequence: G/G plasma: 1435 to 2176; T/T plasma: 370 to 2356.
Discussion
ApoA-V is a member of the class of exchangeable apolipoproteins that is synthesized only in liver1,18. Following signal sequence cleavage, mature apoA-V is secreted into plasma where it associates with plasma lipoproteins, particularly VLDL and HDL.3 A unique aspect of apoA-V is a very low abundance in plasma (between 25 and 400 ng/ml). Despite this low concentration, genome wide association studies have consistently identified APOA5 as a risk factor for dyslipidemia and cardiovascular disease. Moreover, population studies have identified SNPs in APOA5 that correlate with elevated plasma TG. Among these, the c.553G>T SNP (rs2075291) has attracted attention owing to its common occurrence among those of East Asian ancestry and strong association with increased TG levels.
Vaessen et al.19 evaluated the TG lowering activity of WT human apoA-V versus G162C apoA-V using adenovirus mediated gene transfer in C57Bl/6 mice. In their study, at viral doses that increase apoA-V levels approximately 50- to 100-fold over those present in normal human plasma, both WT apoA-V and G162C apoA-V exerted a plasma TG lowering effect. No differences in other plasma parameters, including free fatty acids and glycerol concentrations or post-heparin LPL activity, were noted. The authors concluded that, despite its strong correlation with HTG, G162C apoA-V is functionally able to reduce plasma TG levels in mice. Two problems associated with this study include a) extreme overexpression of apoA-V and b) the use of WT mice that express endogenous apoA-V. The former problem results in supra-physiological levels of apoA-V that may over-ride normal homeostatic processes. The second problem is that plasma TG levels in the recipient mice are low to begin with. Thus, any TG lowering effect caused by transduced apoA-V will be relatively small. In the present study, the apoa5 (−/−) mice employed have HTG, permitting evaluation of possible mechanisms underlying the correlation between the c.553G>T APOA5 SNP and elevated plasma TG. Unlike Vaessen et al.19, we found that when expressed in apoa5 (−/−) mice apoA-V levels resemble that of human plasma and G162C apoA-V displays defective TG lowering activity compared to WT apoA-V.
The results suggest that alterations in the plasma distribution of G162C apoA-V, as compared to WT apoA-V, may account for the observed loss of function. Whereas lipoprotein association is considered an absolute requirement for manifestation of apoA-V TG lowering activity4, a large proportion of G162C apoA-V in plasma of transduced mice was recovered in the lipoprotein-free fraction. When associated with TG-rich lipoproteins, WT apoA-V directs these particles to GPIHBP1 via a specific binding interaction.20 A positively charged sequence motif, located between residues 186 – 227 in apoA-V, mediates interaction with the highly acidic N-terminal segment of GPIHBP1, effectively sequestering lipoproteins at the endothelial cell surface. Insofar as LPL also binds GPIHBP121, current models propose that apoA-V indirectly activates lipolysis by promoting TG-rich lipoprotein binding to sites at the endothelial cell surface (i.e. GPIHBP1) where LPL resides22. Recently, Gonzales et al.23 reported that apoA-V also directs lipoproteins to proteoglycans on the surface of hepatocytes, thereby promoting whole particle uptake by receptor mediated endocytosis, a complementary means whereby TG lowering can be achieved.
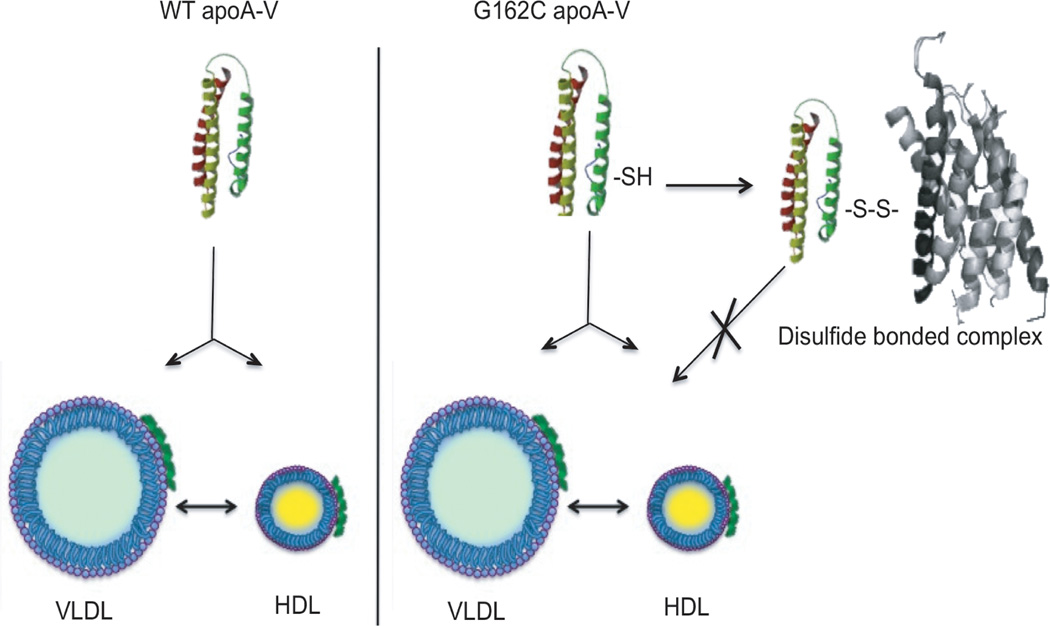
An obvious question is why does a large portion of G162C apoA-V distribute to the lipoprotein-free fraction of plasma? A clue to the underlying reason emerged from studies by Dorfmeister et al.14 who showed G162C apoA-V forms disulfide-linked dimers in vitro. In a similar manner, in the present study apoA-V recovered in the lipoprotein-free fraction displays altered electrophoretic mobility under non-reducing conditions. Taken together, the evidence indicates that the c.553G>T polymorphism introduces a free cysteine sulfhydryl into apoA-V that is available for homo- or hetero-disulfide bond formation. It appears that, upon secretion from hepatocytes, G162C apoA-V has two possible, yet mutually exclusive, fates. On one hand, G162C apoA-V may associate with lipoproteins in a functionally productive manner while, on the other, it may form a hetero-disulfide bond with seemingly unrelated plasma proteins. Previous studies have shown that, although 100% of WT apoA-V associates with lipoproteins, it is not secreted from cells in complex with nascent apoB containing lipoproteins.24 Thus, it is envisaged that apoA-V rapidly transitions from a lipid-free or lipid-poor state to a lipoprotein-associated state following secretion from hepatocytes. Formation of a disulfide bond between G162C apoA-V and extraneous plasma proteins appears to preclude lipoprotein binding by G162C apoA-V, rendering it ineffective in terms of TG lowering. The finding that, in transduced mice, the fraction of the G162C apoA-V pool recovered in association with lipoproteins does not display retarded electrophoretic mobility under non-reducing conditions suggests that competition between these two fates occurs (Figure 5). At present it is not known whether prior association with lipoproteins affects disulfide bond formation or if disulfide bond formation leads to dissociation of apoA-V from lipoproteins. It is also possible that newly secreted lipid-poor G162C apoA-V is prevented from lipoprotein association by prior disulfide bond-linked heterodimer formation. Regardless of the sequence of events, it is evident that introduction of this free cysteine leads to the appearance of a lipoprotein-free, non-functional pool of apoA-V. At the same time, it may be considered that the residual pool of lipoprotein-bound G162C apoA-V in plasma of transduced apoa5 (−/−) mice is responsible for the ~20% lowering of plasma TG versus LacZ transduced mice after 4 weeks. Whether lipoprotein associated G162C apoA-V is equivalent to WT apoA-V in terms of GPIHBP1 binding remains to be determined.
Figure 5. Model depicting opposing fates of G162C apoA-V in plasma.
Left) Upon secretion from hepatocytes, WT apoA-V associates with VLDL and HDL and remains 100 % lipoprotein bound. Right) In addition to lipoprotein association, G162C apoA-V forms disulfide bonds with extraneous plasma proteins, thereby abrogating its lipoprotein interaction capability (depicted by the X). As such, hetero-disulfide bonded G162C apoA-V constitutes a nonfunctional pool of apoA-V in terms of TG modulation.
Non-reducing SDS-PAGE immunoblot analysis of plasma from apoa5 (−/−) mice transduced with G162C apoA-V provided evidence for unique apoA-V positive bands with lower electrophoretic mobility than monomeric apoA-V. On the basis of IP experiments with human plasma samples using anti-apoA-V IgG derivatized beads and LC/MS analyses, 13 unique candidate G162C apoA-V interacting proteins were identified. Whereas each of these proteins possess cysteine residues and hence, are physically capable of forming a disulfide bond with G162C apoA-V, four candidates identified may be regarded as intracellular proteins (bleomycin hydrolase, protein S100A7, caspase-14 and hemoglobin subunit beta). A candidate protein detected at much higher levels (Protein Score 942) than other proteins identified was fibronectin. Fibronectin is a relatively abundant (~300 µg/ml) plasma glycoprotein that also functions as an extracellular matrix component. Plasma fibronectin is a cysteine-rich heparin binding protein that, upon deposition at sites of injury, forms clots that protect underlying tissue. Among the many proteins with which fibronectin interacts is Lipoprotein(a), providing a potential connection to lipoprotein metabolism.25 Another candidate interaction partner is kininogen-1, a component of the blood coagulation cascade that possesses heparan sulfate proteoglycan binding activity.26 Unlike other proteins identified, this candidate was also detected in both control samples. Despite this, inclusion criteria were met for a specific G162C apoA-V binding interaction (Table 1). Other proteins identified as G162C apoA-V interacting partners are complement C1q subcomponent subunit B, Ig gamma 2-C and complement factor B. Although it was identified on the basis of a single peptide, a candidate interacting partner that has a well-known connection to lipoprotein metabolism is apoE. Because mouse apoE lacks cysteine, it is unlikely to be a binding partner for G162C apoA-V. Consistent with the fact that human apoE3 possesses a free sulfhydryl, it is not surprising that immunoblot analysis confirmed that it forms an adduct with G162C apoA-V, but not with WT apoA-V. Interestingly, apoE has previously been reported to form a disulfide bond with apoA-II.27 In considering the range of proteins identified by LC/MS as potential interaction partners with G162C apoA-V, no specific connection is readily apparent. Indeed, whereas certain candidate binding partners identified are relatively abundant plasma proteins, others are considered intracellular proteins such that, if present, their plasma concentration would be expected to be very low. It is conceivable that one or more of the proteins identified does not actually interact with G162C apoA-V but rather, is detected by the fact that it interacts with another protein on the list with which G162C apoA-V does interact. Furthermore, based on the fact that the most abundant proteins in plasma were not detected, it is unlikely that G162C apoA-V interactions are entirely random. In considering this, it is noteworthy that several candidates share with apoA-V28 an ability to bind sulfated glycosaminoglycans (i.e. fibronectin, kininogen-1, zinc alpha-2 glycoprotein, complement factor B, histidine-rich glycoprotein and apoE). Thus, it is conceivable that initial contact between interacting partners is facilitated by this shared property and, once a disulfide bond forms, the functional ability of apoA-V is compromised.
In summary, we conclude that the rs2075291 APOA5 SNP introduces a free sulfhydryl that forms disulfide bonds with various proteins it encounters in plasma. This alternate fate affects the lipoprotein binding properties of G162C apoA-V, thereby abrogating its ability to modulate plasma TG levels. Given the correlation between the c.553G>T APOA5 SNP and HTG in human subjects, the strong correlation between HTG and dyslipidemic disease and the apparent worldwide prevalence of this SNP, apoA-V gene therapy represents a potential disease prevention strategy.
Supplementary Material
Significance.
Genome wide association studies have consistently identified APOA5 as a modulator of plasma triacylglycerol (TG) and single nucleotide polymorphisms (SNP) in APOA5 correlate with elevated plasma TG. Gene transfer experiments in apoa5 (−/−) mice investigated the correlation between c.553G>T SNP and hypertriglyceridemia. Whereas wild type (WT) apoA-V induced a dramatic lowering of plasma TG, the SNP variant, Gly162Cys apoA-V, did not. Furthermore, unlike WT apoA-V, >50% of G162C apoA-V was recovered in the lipoprotein-free fraction of plasma. Plasma samples from human subjects homozygous for WT APOA5 and the c.553G>T SNP were analyzed, revealing that G162C apoA-V forms disulfide linked adducts with extraneous plasma proteins including fibronectin, kininogen-1 and others. Thus, SNP-mediated substitution of Cys for Gly at position 162 of mature apoA-V introduces a free cysteine thiol that forms disulfide bonds with plasma proteins such that its lipoprotein binding and TG modulation functions are compromised.
Acknowledgements
We acknowledge Maria Hassis for excellent technical assistance.
Sources of funding
Supported by NIH (HL-64159). VS is recipient of an American Heart Association Postdoctoral Fellowship Award (12POST12030008). JBS was supported by awards from the Danish VKR and “IMK Almene” Foundations. Mass spectrometry analysis was performed by the UCSF Sandler-Moore Mass Spectrometry Core Facility, which acknowledges support from the Sandler Family Foundation, the Gordon and Betty Moore Foundation, and NIH/NCI Cancer Center Support Grant P30 CA082103.
Nonstandard Abbreviations and Acronyms
- AAV
adeno-associated virus
- Apo
apolipoprotein
- GPIHBP1
glycosylphosphatidylinositol high density lipoprotein binding protein 1
- HTG
hypertriglyceridemia
- LC/MS
liquid chromatography / mass spectrometry
- TG
triacylglycerol
- SNP
single nucleotide polymorphism
- WT
wild type
Footnotes
Disclosures
None
References
- 1.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien PJ, Alborn WE, Sloan JH, Ulmer M, Boodhoo A, Knierman MD, Schultze AE, Konrad RJ. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 3.Nelbach L, Shu X, Konrad RJ, Ryan RO, Forte TM. Effect of apolipoprotein on plasma triglyceride, lipoprotein size, and composition in genetically engineered mice. J Lipid Res. 2008;49:572–580. doi: 10.1194/jlr.M700281-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara M, Kujiraoka T, Iwasaki T, et al. A sandwich enzyme-linked immunosorbent assay for human plasma apolipoprotein A-V concentration. J Lipid Res. 2005;46:2015–2022. doi: 10.1194/jlr.D500018-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Sharma V, Forte TM, Ryan RO. Influence of apolipoprotein A-V on the metabolic fate of triacylglycerol. Curr Opin Lipidol. 2013;24:153–159. doi: 10.1097/MOL.0b013e32835c8c1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen CT, Wang J, Lanktree MB, et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet. 2010;42:684–687. doi: 10.1038/ng.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao JT, Wen HC, Chien KL, Hsu HC, Lin SW. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum Mol Genet. 2003;12:2533–2539. doi: 10.1093/hmg/ddg255. [DOI] [PubMed] [Google Scholar]
- 8.Pullinger CR, Aouizerat BE, Movsesyan I, et al. An apolipoprotein A-V gene SNP is associated with marked hypertriglyceridemia among Asian-American patients. J Lipid Res. 2008;49:1846–1854. doi: 10.1194/jlr.P800011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(Suppl.):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 10.Johansen CT, Wang J, Lanktree MB, et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet. 2010;42:684–687. doi: 10.1038/ng.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D, Aberle J, Beil FU. Resequencing the apolipoprotein A5 (APOA5) gene in patients with various forms of hypertriglyceridemia. Atherosclerosis. 2011;219:715–720. doi: 10.1016/j.atherosclerosis.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Wierzbicki AS, Clarke RE, Viljoen A, Mikhailidis DP. Triglycerides: a case for treatment? Curr Opin Cardiol. 2012;27:398–404. doi: 10.1097/HCO.0b013e328353adc1. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Sun P, Guo D, Ferro A, Ji Y, Chen Q, Fan L. A genetic variant c.553G >T in the apolipoprotein A5 gene is associated with an increased risk of coronary artery disease and altered triglyceride levels in a Chinese population. Atherosclerosis. 2006;185:433–437. doi: 10.1016/j.atherosclerosis.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Dorfmeister B, Zeng WW, Dichlberger A, et al. Effects of six APOA5 variants, identified in patients with severe hypertriglyceridemia, on in vitro lipoprotein lipase activity and receptor binding. Arterioscler Thromb Vasc Biol. 2008;28:1866–1871. doi: 10.1161/ATVBAHA.108.172866. [DOI] [PubMed] [Google Scholar]
- 15.Huang YJ, Lin YL, Chiang CI, Yen CT, Lin SW, Kao JT. Functional importance of apolipoprotein A5 185G in the activation of lipoprotein lipase. Clin Chim Acta. 2012;413:246–250. doi: 10.1016/j.cca.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V, Beckstead J, Simonsen JB, Nelbach L, Watson G, Forte TM, Ryan RO. Gene transfer of apolipoprotein A-V improves the hypertriglyceridemic phenotype of apoa5 (−/−) mice. Arterioscler Thromb Vasc Biol. 2013;33:474–480. doi: 10.1161/ATVBAHA.112.301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishayama Y. Proteomic LC-MS systems using nanoscale liquid chromatography with tandem mass spectrometry. J Chromatography A. 2005;1067:73–83. doi: 10.1016/j.chroma.2004.10.107. [DOI] [PubMed] [Google Scholar]
- 18.Van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem. 2001;276:44512–44520. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 19.Vaessen SF, Sierts JA, Kuivenhoven JA, Schaap FG. Efficient lowering of triglyceride levels in mice by human apoAV protein variants associated with hypertriglyceridemia. Biochem Biophys Res Commun. 2009;379:542–546. doi: 10.1016/j.bbrc.2008.12.115. [DOI] [PubMed] [Google Scholar]
- 20.Gin P, Yin L, Davies BS, Weinstein MM, Ryan RO, Bensadoun A, Fong LG, Young SG, Beigneux AP. The acidic domain of GPIHBP1 is important for the binding of lipoprotein lipase and chylomicrons. J Biol Chem. 2008;283:29554–29562. doi: 10.1074/jbc.M802579200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adeyo O, Goulbourne CN, Bensadoun A, Beigneux AP, Fong LG, Young SG. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 and the intravascular processing of triglyceride-rich lipoproteins. J. Intern Med. 2012;272:528–540. doi: 10.1111/joim.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gin P, Beigneux AP, Voss C, Davies BSJ, Beckstead JA, Ryan RO, Bensadoun A, Fong LG, Young SG. Binding Preferences for Glycosylphosphatidylinositol HBP1, a Glycosylphosphatidylinositol-Anchored Protein of Capillary Endothelial Cells. Arterioscler Thromb Vasc Biol. 2011;31:176–182. doi: 10.1161/ATVBAHA.110.214718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzales JC, Gordts PL, Foley EM, Esko JD. Apolipoproteins E and AV mediate lipoprotein clearance by hepatic proteoglycans. J Clin Invest. 2013;123:2742–2751. doi: 10.1172/JCI67398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu X, Chan J, Ryan RO, Forte TM. Apolipoprotein A-V association with intracellular lipid droplets. J Lipid Res. 2007;48:1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Ehnholm C, Jauhiainen M, Metso J. Interaction of lipoprotein(a) with fibronectin and its potential role in atherogenesis. Eur Heart J. 1990;11(Suppl E):190–195. doi: 10.1093/eurheartj/11.suppl_e.190. [DOI] [PubMed] [Google Scholar]
- 26.Renne T, Dedlo J, David G, Muller-Esterl W. High molecular weight kininogen utilizes proteoglycans for accumulation on endothelial cells. J Biol Chem. 2000;375:33688–33696. doi: 10.1074/jbc.M000313200. [DOI] [PubMed] [Google Scholar]
- 27.Weisgraber KH. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J. Lipid Res. 1990;31:1503–1511. [PubMed] [Google Scholar]
- 28.Lookene A, Beckstead JA, Nilsson S, Olivecrona G, Ryan RO. Apolipoprotein A-V heparin interactions. Implications for plasma lipoprotein metabolism. J Biol Chem. 2005;280:25383–25387. doi: 10.1074/jbc.M501589200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.