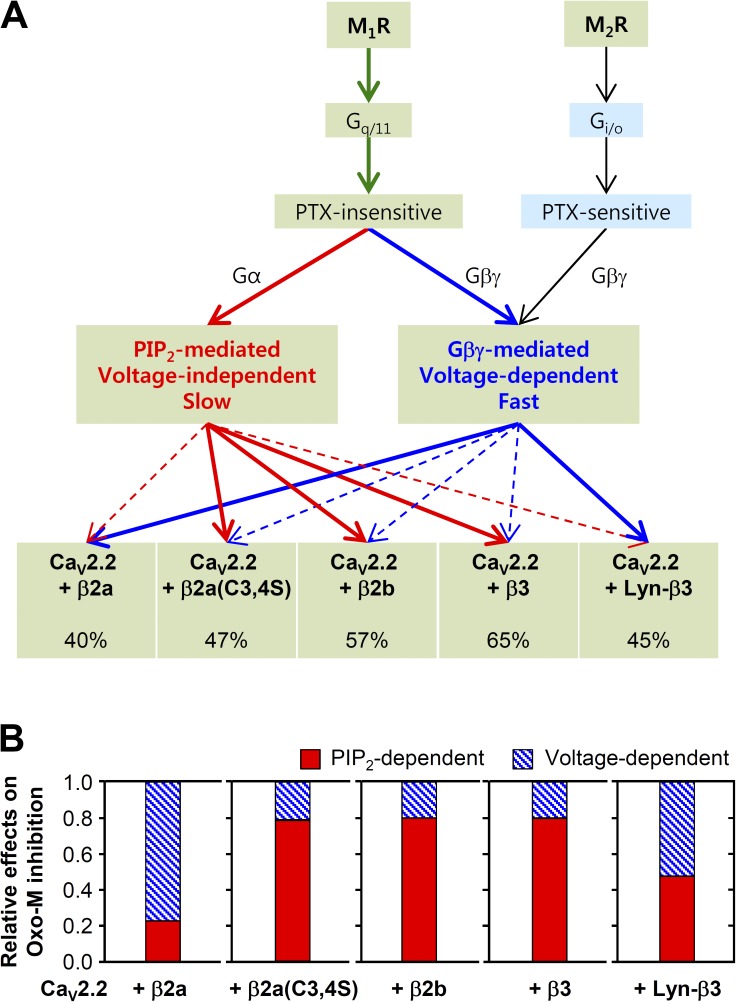
The pathway through which preferentially GqPCRs inhibit CaV2.2 channels depends on which β subunits are present.
Abstract
G protein–coupled receptors (GPCRs) signal through molecular messengers, such as Gβγ, Ca2+, and phosphatidylinositol 4,5-bisphosphate (PIP2), to modulate N-type voltage-gated Ca2+ (CaV2.2) channels, playing a crucial role in regulating synaptic transmission. However, the cellular pathways through which GqPCRs inhibit CaV2.2 channel current are not completely understood. Here, we report that the location of CaV β subunits is key to determining the voltage dependence of CaV2.2 channel modulation by GqPCRs. Application of the muscarinic agonist oxotremorine-M to tsA-201 cells expressing M1 receptors, together with CaV N-type α1B, α2δ1, and membrane-localized β2a subunits, shifted the current-voltage relationship for CaV2.2 activation 5 mV to the right and slowed current activation. Muscarinic suppression of CaV2.2 activity was relieved by strong depolarizing prepulses. Moreover, when the C terminus of β-adrenergic receptor kinase (which binds Gβγ) was coexpressed with N-type channels, inhibition of CaV2.2 current after M1 receptor activation was markedly reduced and delayed, whereas the delay between PIP2 hydrolysis and inhibition of CaV2.2 current was decreased. When the Gβγ-insensitive CaV2.2 α1C-1B chimera was expressed, voltage-dependent inhibition of calcium current was virtually abolished, suggesting that M1 receptors act through Gβγ to inhibit CaV2.2 channels bearing membrane-localized CaV β2a subunits. Expression of cytosolic β subunits such as β2b and β3, as well as the palmitoylation-negative mutant β2a(C3,4S), reduced the voltage dependence of M1 muscarinic inhibition of CaV2.2 channels, whereas it increased inhibition mediated by PIP2 depletion. Together, our results indicate that, with membrane-localized CaV β subunits, CaV2.2 channels are subject to Gβγ-mediated voltage-dependent inhibition, whereas cytosol-localized β subunits confer more effective PIP2-mediated voltage-independent regulation. Thus, the voltage dependence of GqPCR regulation of calcium channels can be determined by the location of isotype-specific CaV β subunits.
INTRODUCTION
Voltage-gated calcium (CaV) channels play fundamental roles in mediating calcium influx upon depolarization (Hille, 2001). They regulate many physiological responses ranging from neurotransmission to muscle contraction. Dysfunction in CaV channels is associated with many pathological conditions such as pain, epilepsy, migraine, and autism (Catterall, 2011). A CaV channel consists of three protein subunits, CaV α1, α2δ, and β (Hofmann et al., 1999; Catterall, 2000). CaV α1 and α2δ subunits are transmembrane proteins responsible for forming the voltage-sensitive pore of the channel and promoting CaV α1 subunit stabilization at the plasma membrane, respectively. CaV β subunits are intracellular components that play an essential role in regulating gating properties and receptor modulation of CaV channels. The CaV β subunit sets the sensitivity of CaV channels to the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2). Lipidation (palmitoylation) of the β subunit results in a plasma membrane localization and a decrease in current inactivation and PIP2 sensitivity of CaV2.2 channels (Hurley et al., 2000; Suh et al., 2012).
As CaV channels are critical in virtually all excitable cells, they are also intensely and dynamically modulated by an array of receptor-dependent signals. This includes regulation by G proteins after G protein–coupled receptor (GPCR) activation (Zamponi and Currie, 2013). For the GPCRs coupled to pertussis toxin (PTX)–sensitive Gi/o protein, it is the Gβγ subunit that acts at the cytoplasmic surface of the membrane to bind directly to the CaV2.2 α subunit after Gi/oPCR activation, consequently inhibiting CaV current (Herlitze et al., 1996; Ikeda, 1996). This is the most extensively studied mechanism, characterized by its fast and membrane-delimited inhibition (Bernheim et al., 1991), slowed activation kinetics, and a positive shift in the voltage dependence of the channel (Bean, 1989; Elmslie et al., 1990). This inhibition can be transiently relieved by large-step depolarizations that elicit dissociation of Gβγ from the channel (Boland and Bean, 1993) and is thus also known as the voltage-dependent pathway (Dolphin, 2003; Currie, 2010). Contrastingly, a slow, voltage-independent inhibition occurs mostly through the activation of GqPCRs. In this case, the Gq/PLC-mediated depletion of PIP2 and/or arachidonic acid generation is an important signaling messenger (Wu et al., 2002; Liu and Rittenhouse, 2003; Gamper et al., 2004; Suh et al., 2010). However, it seems that, depending on the receptor type, the voltage dependency of channel suppression would be determined by different messengers and showed different shape of regulation (see review, Tedford and Zamponi, 2006; Kisilevsky et al., 2008). Although Gi/oPCRs are widespread in the presynaptic neurons, GqPCRs are known to inhibit CaV2.2 in somata of sensory and sympathetic neurons (Filippov et al., 1998; Haley et al., 2000; Liu et al., 2004). Muscarinic acetylcholine receptor stimulation inhibits CaV channels through both of the described pathways (Hille, 1994). M2 and M4 receptor subtypes are coupled to Gi/o and engage the voltage-dependent pathway to inhibit CaV channels, whereas the M1, M3, and M5 subtypes are coupled to Gq and modulate CaV channels through the voltage-independent, second-messenger pathway. However, the molecular mechanism underlying the latter GqPCR modulation has been questioned and needs further clarification.
Recent studies have developed and implemented useful techniques to further dissect the different modes of CaV channel modulation. Through the use of zebrafish voltage-sensitive phosphatase (Dr-VSP), reversible depletion of membrane PIP2 became possible by applying a large depolarizing pulse that activates the enzyme (Murata et al., 2005; Suh et al., 2010). This allows exclusive analysis of PIP2 depletion effects on channel modulation without any other production of second messengers or the activation of receptors (Okamura et al., 2009). Furthermore, genetically expressible inhibitors and real-time indicators have helped identify the molecular mechanisms by which inhibition of CaV2.2 occurs after muscarinic receptor activation (Koch et al., 1994; van der Wal et al., 2001; Jensen et al., 2009).
Using these techniques, we continued our mechanistic study of Gq protein inhibition of N-type CaV2.2 channels. In superior cervical ganglion (SCG) neurons of the rat, it has been established that Gαq/11/PLC activation and subsequent PIP2 hydrolysis produce the major voltage-independent regulation of CaV2.2 channel after M1 muscarinic receptor activation; however, there also is some voltage-dependent regulation, raising the possibility of a role for Gβγ as well (Kammermeier et al., 2000; Melliti et al., 2001; Gamper et al., 2004; Suh et al., 2010; Vivas et al., 2013). Also arguing for a second signaling pathway, Suh et al. (2012) found much less current inhibition after direct depletion of PIP2 through the use of Dr-VSP than was seen by activation of M1 muscarinic receptors. In the present study, we propose that inhibition of N-type CaV channels after GqPCR activation occurs not only through the familiar PIP2-dependent and voltage-independent pathway, but also through the phospholipid-independent, Gβγ-dependent pathway. Furthermore, we find that the relative predominance of these two pathways changes according to the CaV β subunit present.
MATERIALS AND METHODS
Cell culture and transfection
Human embryonic kidney tsA-201 cells were maintained in DMEM supplemented with 10% FBS and 0.2% penicillin/streptomycin in 100-mm culture dishes. Subculture was accomplished every 7 d as cell density reached 75–80% using Ca2+-free DPBS for detaching and suspending the cells. For transfection, Lipofectamine 2000 (Invitrogen) was used when the confluency of cells reached 40–70%. In all experiments on CaV channels, cells were cotransfected with α1B, α2δ1, and various β subunits in a 1:1:1 molar ratio. Transfected cells were detached by trypsin and then moved onto poly-l-lysine–coated chips of coverslip 24 h after transfection, 12–24 h before the experiments. The cDNAs used were the channel subunits α1B of rat CaV2.2e[37b], rat β3, and rat α2δ1 (from D. Lipscombe, Brown University, Providence, RI), rat β2a (from W.A. Catterall, University of Washington, Seattle, WA), chimeric rat β2a(C3,4S) (from J. Hurley, Indiana University, Bloomington, IN), rat β2a(C3,4S)-GFP (subcloned by D. Kim), chimeric CaV2.2(α1C-1B) (from D. Yue, Johns Hopkins University, Baltimore, MD), Dr-VSP with IRES EGFP (from Y. Okamura, Osaka University, Osaka, Japan), human ECFP-PH(PLCδ1) and EYFP-PH(PLCδ1) (from K. Jalink, The Netherlands Cancer Institute, Amsterdam, Netherland), M1 muscarinic receptor (from N. Nathanson, University of Washington), human M2 muscarinic receptor (from Guthrie Resource Center, Rolla, MO), and C terminus of β-adrenergic receptor kinase (βARK-ct; from R. Lefkowitz, Duke University, Durham, NC).
Current recording
The whole-cell configuration was used to record currents carried by Ba2+ in transfected tsA-201 cells using patch clamp amplifier EPC-9 or EPC-10 USB (HEKA) at room temperature (22–25°C). Pipette resistance was 1–4 MΩ, and a series resistance of 2–6 MΩ was compensated by 60%. Ba2+ currents were measured with p/5 subtraction with a membrane holding potential of −80 mV, followed by 10-ms step depolarization to 10 mV. For full experiments, voltage pulses were repeated every 2 or 4 s. Application of step depolarization to 120 mV for 1 s induced full activation of Dr-VSP. A conditioning depolarizing prepulse was used to test the involvement of Gβγ.
Förster resonance energy transfer (FRET)
Regular pulses of indigo light (438 ± 12 nm) from a monochromator (Polychrome V; TILL Photonics) excited the fluorescent proteins. Emission, which passed through a 40×, NA 0.95 dry immersion objective lens (Olympus), was separated into short (460–500 nm) and long (520–550 nm) wavelengths by appropriate filters and then acquired by two photomultipliers. Donor and acceptor signals obtained by photometry (TILL Photonics) were transferred to the data acquisition board (PCI-6221; National Instruments). Signal acquisition and real-time calculation of FRET ratio were conducted by a homemade program. To correct bleed-through of emission of CFP into the YFP detector, cells expressing only CFP were used to obtain the ratio of the detected signal in short and long wavelength emission channels (Jensen et al., 2009). The calculated ratio (cFactor = CFP/YFP = 0.55) was used to correct the raw YFP emission signal. The bleed-through of YFP light into the CFP detector was only 0.02 and was neglected. The FRET ratio was thus calculated as follows:
where YFPC is the signal from YFP excited as result of FRET (YFP emission by CFP excitation), CFPC is CFP emission detected by the short wavelength photomultiplier, and YFPC is YFP emission detected by long wavelength photomultiplier.
Confocal imaging
TsA-201 cells were transfected on poly-l-lysine–coated coverslips and imaged within the next 24–48 h. The bath solution contained 160 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 8 mM glucose adjusted to pH 7.4 with NaOH. Images were taken with a confocal microscope (LSM 700; Carl Zeiss) at room temperature every 5 s and processed with ZEN 2009 Light Edition (Carl Zeiss) and Igor Pro (WaveMetrics).
Solutions and materials
The bath solution used for recording Ba2+ current contained 150 mM NaCl, 10 mM BaCl2-2H2O, 1 mM MgCl2, 10 mM HEPES, and 8 mM glucose and was titrated to pH 7.4 with NaOH. The pipette solution contained 160 mM CsCl, 5 mM MgCl2, 5 mM HEPES, 0.1 mM 1,2-bis(2-aminophenoxy)ethane N,N,N′N′-tetraacetic acid (BAPTA), 3 mM Na2ATP, and 0.1 mM Na3GTP and was titrated to pH 7.4 with CsOH. Reagents used were oxotremorine-M (Oxo-M; Research Biochemicals); BAPTA, DMEM, FBS, Lipofectamine 2000, and penicillin/streptomycin (Invitrogen); and ATP, GTP, and other chemicals (Sigma-Aldrich).
Data analysis
Pulse/Pulse Fit 8.11 software and the patch clamp amplifier (HEKA) were used for data acquisition and analysis. Supplementary data processing used Excel (Microsoft) and Igor Pro. Exponential fits were used to measure the time constants. All quantitative data were expressed as the mean ± SEM. Comparison between two groups was analyzed using the Student’s t test, and differences were considered significant at the P < 0.05 level. Comparison among more than two groups was analyzed using one-way ANOVA followed by a post hoc test.
RESULTS
M1 muscarinic receptors may suppress N-type CaV2.2 through two modulatory pathways
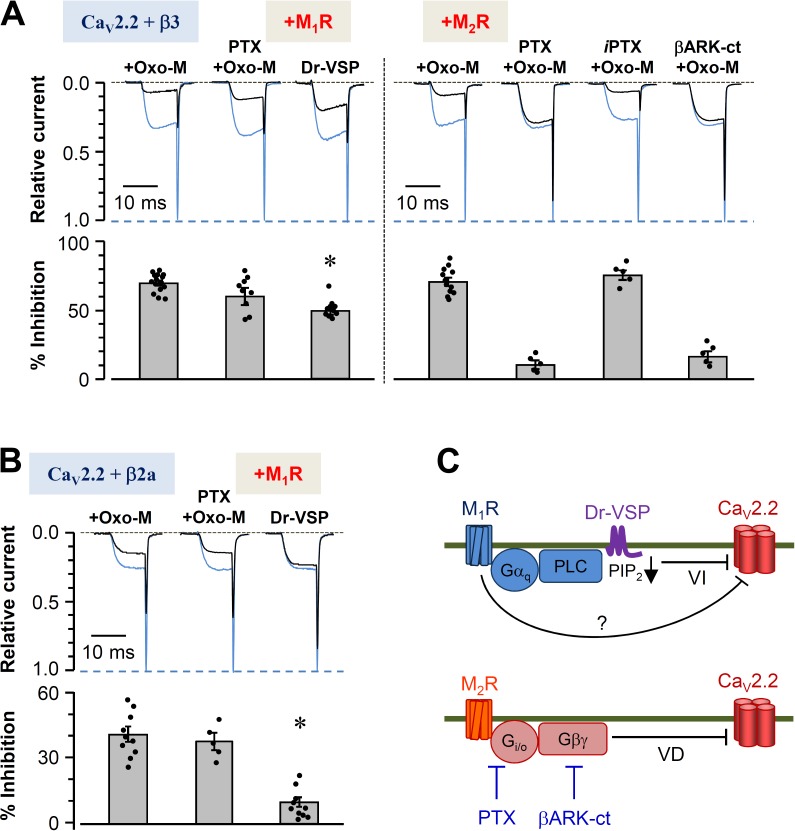
To dissect pathways by which CaV2.2 channels are modulated by muscarinic receptor activation, tsA-201 cells were transfected with CaV subunits α1B, α2δ1, and β3 and either M1 or M2 receptors. Barium currents were evoked by depolarizing voltage steps. Perfusion of the muscarinic agonist Oxo-M inhibited the current by 68 ± 3% for M1R and by 72 ± 5% for M2R (Fig. 1 A). The differential modulatory pathways of these receptors were first isolated with the use of PTX, which inactivates Gi/o by ADP ribosylation. As expected, preincubation in PTX (300 ng/ml, 12 h) strongly reduced Oxo-M–mediated current inhibition in cells expressing the Gi/o-coupled M2R (9 ± 2%; Fig. 1 A, right). Denatured PTX did not reduce the current. Furthermore, coexpressing βARK-ct, which chelates free Gβγ subunits, prevented CaV2.2 current inhibition in a manner similar to PTX (Koch et al., 1994). These experiments confirmed that the primary mechanism by which M2R inhibits CaV2.2 is through Gβγ subunits released after activation of Gi/o (Fig. 1 C, bottom).
Figure 1.
Differential modulation of CaV2.2 channels by muscarinic receptors depends on the CaV β subunit. (A and B) Cells transfected with α1B (CaV2.2), α2δ1, and β3 (A) or β2a (B) subunits were cultured in the presence or absence of PTX or heat-inactivated PTX (iPTX) for 12 h. The cells were stimulated with 10 µM Oxo-M to activate muscarinic receptors or depolarized to 120 mV for 1 s to activate the coexpressed Dr-VSP. (A) CaV2.2 currents before and after the stimulation of M1 (left) and M2 (right) muscarinic receptors or the activation of Dr-VSP were measured in cells expressing β3, and the currents were superimposed. Blue traces are the control, and black traces are after stimulation. Current regulation by M2 receptor was also measured in cells cotransfected with βARK-ct. (bottom) Summary of current inhibition (percentage) by the activation of muscarinic receptors or Dr-VSP. Dots indicate the individual data points for each experiment (n = 5–15). Analysis was performed by one-way ANOVA followed by a post hoc test. (B) CaV2.2 currents were measured before and after the stimulation in cells expressing β2a. (bottom) Summary of current inhibition (percentage) by the activation of M1 receptor or Dr-VSP. (A and B) Mean ± SEM is shown. *, P < 0.01, compared with current inhibition by Oxo-M. (C) Diagram of inhibitory signaling to CaV2.2 channels by M1 and M2 muscarinic receptors. VD, voltage-dependent inhibition; VI, voltage-independent inhibition.
In contrast, the GqPCR M1R is thought to inhibit CaV2.2 mainly via PLC and depletion of PIP2 (Fig. 1 C, top; Gamper et al., 2004; Suh et al., 2010; Vivas et al., 2013). Preincubation with PTX did not change the inhibition of current by M1 muscarinic receptor stimulation (Fig. 1, A [left] and B). Thus M1R function does not involve Gi/o. The PIP2-dependent pathway can be studied in isolation using activation of the voltage-sensitive lipid phosphatase Dr-VSP, which can deplete PIP2 from the plasma membrane quickly during a strong depolarizing pulse. The cotransfected Dr-VSP showed different extents of CaV2.2 current inhibition depending on the type of CaV β subunit used, as reported in our previous study (Suh et al., 2012). Depolarization with the expressed Dr-VSP inhibited current more strongly in cells cotransfected with β3 subunits (Fig. 1 A, left) than in those cotransfected with β2a subunits (Fig. 1 B). Such observations led to the main hypothesis of this study: if Dr-VSP depletes PIP2 from the membrane yet sometimes leads to only weak current inhibition through this phospholipid-dependent pathway, other signals must contribute to the remaining significant portion of current inhibition by M1 muscarinic stimulation. A summary of the known signaling pathways is given in Fig. 1 C, with a question mark designating this presumed additional pathway from M1Rs.
Gβγ scavenger attenuates M1 muscarinic receptor–induced CaV2.2 current inhibition
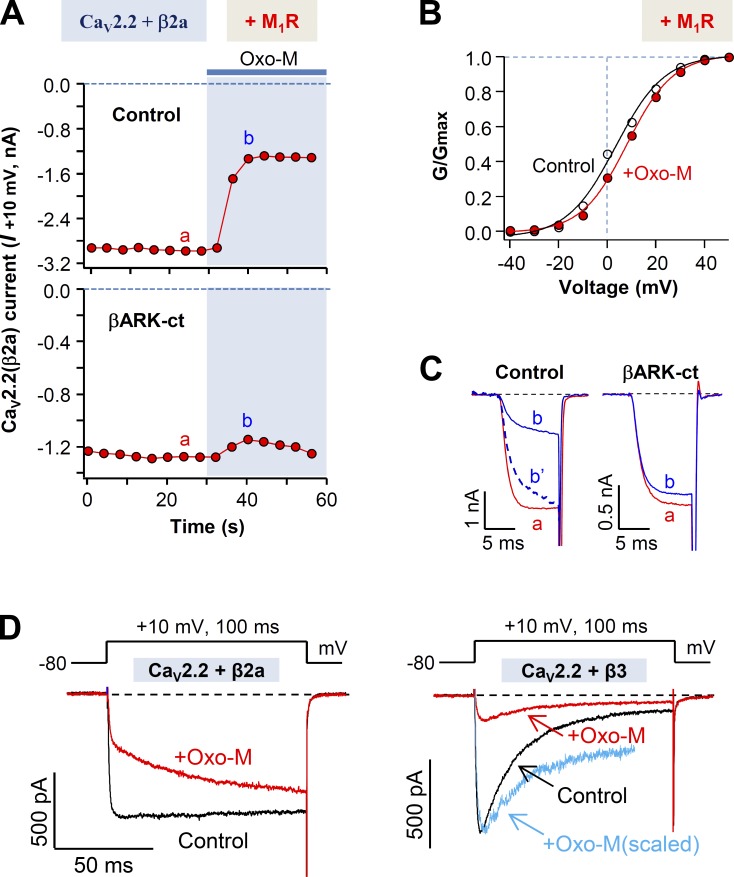
A series of studies was performed to determine whether Gβγ subunits might play a role in CaV2.2 modulation by M1 muscarinic receptor. Several results were consistent with this hypothesis, but as we eventually show, the outcomes depended on which CaV β subunit was used. Fig. 2 A compares CaV2.2 (β2a) current inhibition in control cells with that in cells expressing the Gβγ scavenger βARK-ct. The scavenger attenuates inhibition strongly, as if Gβγ is needed for the M1 muscarinic inhibition, when the CaV β2a channel subunit is present. There are additional hallmarks of inhibition by Gβγ subunits. The voltage-dependent activation curves showed a shift to the right by ∼5 mV during the M1 receptor activation (Fig. 2 B). Furthermore, comparison of single traces of current recorded before and after Oxo-M treatment also revealed differences in the activation kinetics of control cells but not in cells expressing βARK-ct (Fig. 2 C). The control cells transfected with α1B and β2a subunits displayed slowing of activation during Oxo-M (also see Fig. 2 D, left), whereas cells cotransfected with βARK-ct showed little change of activation. This observation makes it seem as if the inhibition of CaV2.2 (β2a) by Oxo-M involves Gβγ. In contrast, for cells expressing β3 subunits, the Gβγ hallmark changes in current activation were much less prominent (Fig. 2 D, right).
Figure 2.
βARK-ct attenuates M1 muscarinic receptor–induced inhibition of CaV2.2(β2a) currents. (A) Cells transfected with CaV2.2, α2δ1, and β2a in the presence and absence of βARK-ct were stimulated with Oxo-M, and the CaV2.2(β2a) current suppression was measured. (B) Voltage dependence of activation of the CaV2.2(β2a) channel before and during M1 receptor stimulation with Oxo-M. Dashed line is the I-V relation during Oxo-M application, which is scaled to the peak amplitude of the control. (C) Superimposed CaV2.2(β2a) current traces a and b from A. In control, the b′ dashed trace is a scaled version of b. (D) Superimposed CaV2.2(β2a) and CaV2.2(β3) current traces for control and during the stimulation of M1 receptor. Blue line in right panel shows the scaled trace of CaV2.2(β3) current after Oxo-M application (red). Note that during the Oxo-M application, activation of CaV2.2(β2a) channels (left) but not CaV2.2(β3) channels (right) is dramatically slowed.
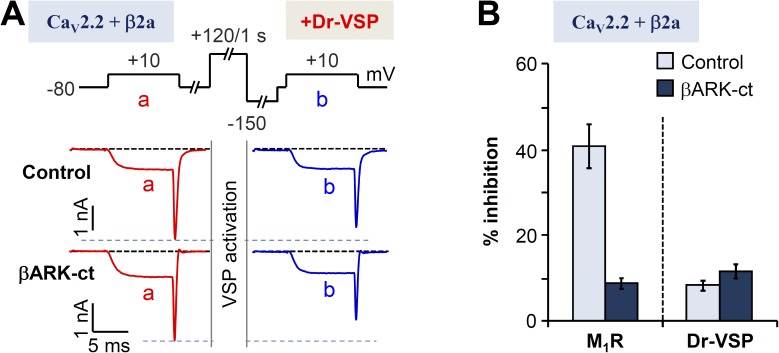
We next tested the effects of Gβγ on channel inhibition by depleting PIP2 by means of Dr-VSP (Okamura et al., 2009), which is appropriate for experimental designs involving reversible PIP2 depletion after an activating depolarization. The standardized voltage protocol to deplete PIP2 from the membrane by activating Dr-VSP was applied to cells expressing βARK-ct (Fig. 3 A, top). Compared with control cells, the expression of βARK-ct did not diminish the current inhibition mediated by Dr-VSP (Fig. 3 A). Thus, we conclude that βARK-ct does not impede the PIP2-dependent pathway of CaV2.2 (β2a) inhibition, but it does block the Gβγ-dependent pathway. As is summarized in Fig. 3 B, M1 muscarinic current inhibition in β2a-expressing cells is significantly decreased by coexpressing βARK-ct, again as if Gβγ plays an important role in CaV2.2 (β2a) modulation. In contrast, the smaller current inhibition upon activation of Dr-VSP was not changed by coexpressing βARK-ct. This suggests that the M1R-induced inhibition of CaV2.2 (β2a) could involve a direct action of Gβγ on the channel itself rather than an action through the phospholipid-sensitive pathway.
Figure 3.
Differential effects of βARK-ct on M1R- and Dr-VSP–induced inhibition of CaV2.2(β2a) currents. (A) CaV2.2(β2a) current inhibition by Dr-VSP activation in control or cells expressing βARK-ct. Cells received a 10-ms test pulse (a) and then a 1-s depolarization to 120 mV for activating the expressed VSP, followed by the second 10-ms test pulse (b). Note that current inhibition by Dr-VSP activation was not significantly different between control and βARK-ct–expressing cells. (B) Summary of the current inhibition (percentage) after the activation of M1 receptors (Fig. 2 A) or Dr-VSP in control and βARK-ct–expressing cells. Data are mean ± SEM (n = 5–7).
Single-cell assay reveals separation of fast and slow pathways in M1R-induced current modulation
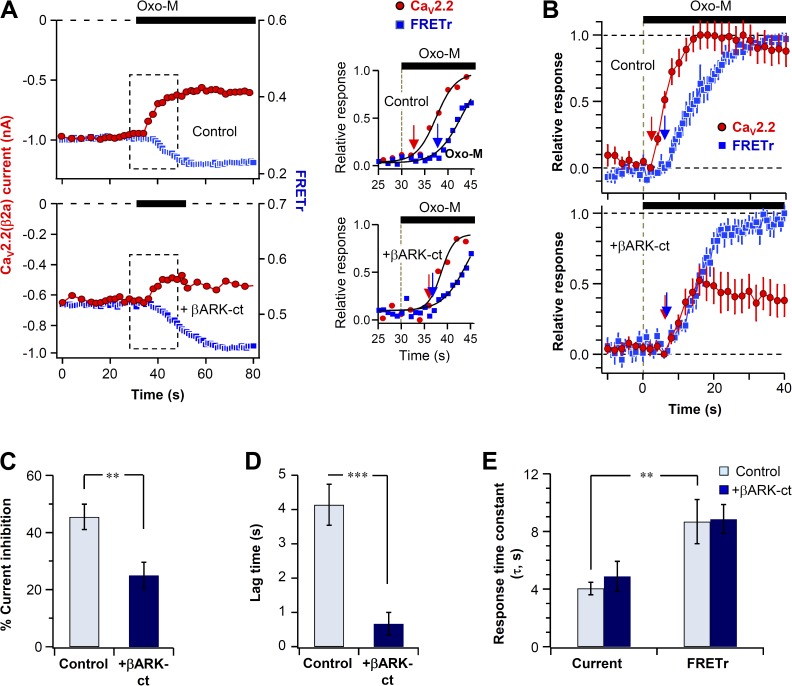
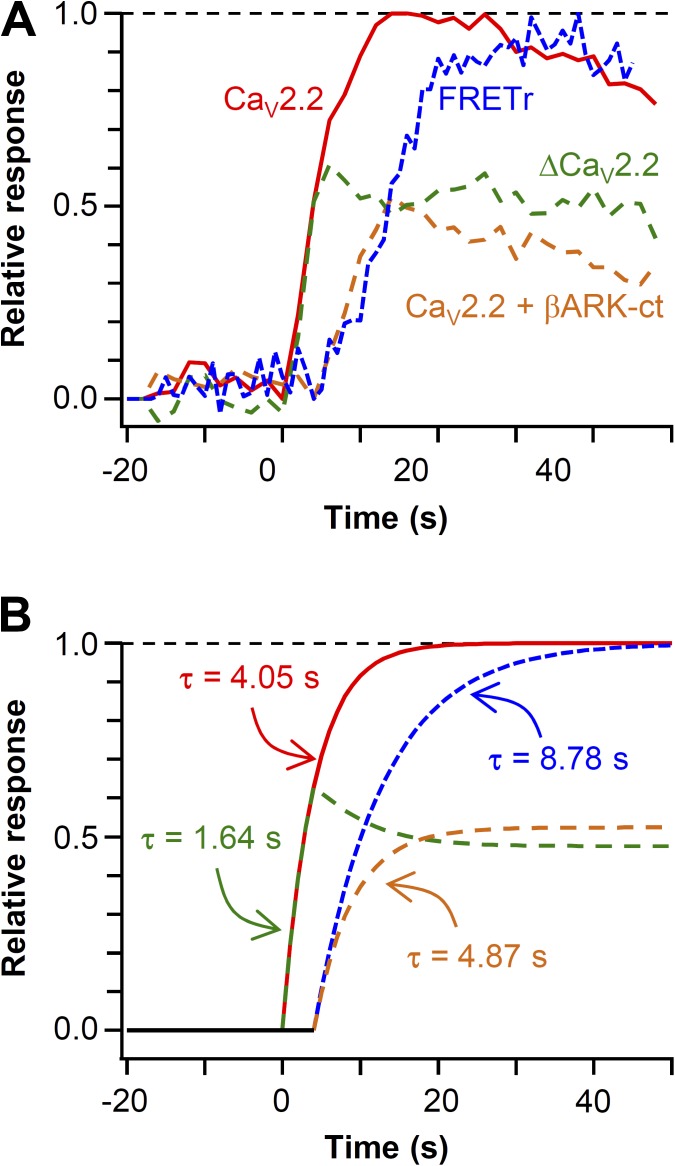
We simultaneously measured the current modulation and PIP2 hydrolysis in single control and βARK-ct–expressing cells. Plasma membrane PIP2 was measured by FRET between CFP- and YFP-labeled probes that selectively bind to membrane PIP2 (van der Wal et al., 2001; Jensen et al., 2009; Suh et al., 2010; Falkenburger et al., 2013). A decrease of their FRET interaction indicates depletion of PIP2 that releases the probe from the membrane. Fig. 4 A plots representative time courses of CaV2.2 current and the FRETr change in single control and βARK-ct–expressing cells. After perfusion of Oxo-M, the decrease of FRETr (blue trace) was comparable in the two cells, whereas the current inhibition (red trace) showed several differences. On average, current inhibition was 46 ± 4% in control cells (n = 7) and only 25 ± 5% in βARK-ct–expressing cells (n = 6; Fig. 4 C). Furthermore, the latency for initiation of current inhibition was less than that for the FRETr decrease (Fig. 4, A [right] and B). The mean lag time between the initiation of CaV current inhibition and PIP2 hydrolysis was 4.1 ± 0.6 s in control cells and 0.7 ± 0.3 s in βARK-ct–expressing cells (Fig. 4 D). The variability of fluorescent protein expression was compensated by normalizing the FRET change between 0 and 1 and averaging the traces (Fig. 4 B). As expected, the time constant of FRET change (PIP2 hydrolysis) was not affected by βARK-ct (Fig. 4 E, τ = 8.7 ± 1.5 s for control and τ = 8.9 ± 1.1 s for βARK-ct–expressing cells). Fig. 5 summarizes the main results of Fig. 4. First, we estimated a putative component of current inhibition by G protein βγ subunits by subtracting the averaged current of the two groups (Fig. 5 A, dashed green line). Then we mimicked the observed time courses with exponential curves Fig. 5 B. This model showed that, as would be appropriate for direct G protein action, the difference component is fast with an exponential time constant of 1.6 s (Fig. 5 B, dashed green line). The remaining component, attributed to PIP2 signaling, has a slow time course like the FRETr change.
Figure 4.
Simultaneous measurement of current inhibition and PIP2 hydrolysis in single cells. All cells coexpressed CaV2.2(β2a) channel subunits, PH domain probes, and M1 receptors. (A) CaV2.2(β2a) current and PIP2 (FRET ratio, FRETr) were measured simultaneously in single cells in the absence (top) or presence (bottom) of βARK-ct. 10 µM Oxo-M was applied during solid bars. (right) Scaled responses from the dashed boxes shown in the left panels. The initiation of the muscarinic response is indicated by arrows. (B) Normalized mean time courses of current suppression and PIP2 hydrolysis (FRETr) from single cells without or with βARK-ct expression. (C) Summary of maximum current inhibition by Oxo-M in control and cells expressing βARK-ct. **, P < 0.01, compared with control. (D) Effect of βARK-ct on lag time between the initiation of current inhibition and the FRETr change. ***, P < 0.001. (B–D) Data are mean ± SEM. (E) Analysis of the onset time (τ) for current inhibition and PIP2 hydrolysis (FRETr) in control and βARK-ct–expressing cells. Data are mean ± SEM (n = 6–7). **, P < 0.01.
Figure 5.
Kinetic assays reveal participation of Gβγ in M1 receptor–induced CaV2.2(β2a) current inhibition. (A) Summary of Fig. 4 B. The estimated effect of M1R-mediated release of Gβγ on CaV2.2 current (ΔCaV2.2, green) was calculated by subtracting the mean current of βARK-ct (orange) from that of control (red). Blue trace indicates M1R-induced PIP2 hydrolysis observed by FRET change. (B) Interpretation of the CaV2.2 current inhibition as a series of exponential curves in control and βARK-ct–expressing cells. Icontrol = exp(−t/4.05) (t > 0; red), IβARK-ct = 0.52*exp(−(t − 4)/4.87) (t > 4; orange), FRETr = exp(−(t − 4)/8.78) (t > 4; blue). Predicted Gβγ-induced CaV current inhibition (dashed green) was calculated by subtracting the mean current of βARK-ct from that of control. The amplitude of IβARK-ct is determined by obtaining the relative current amplitude between control and βARK-ct–expressing cells.
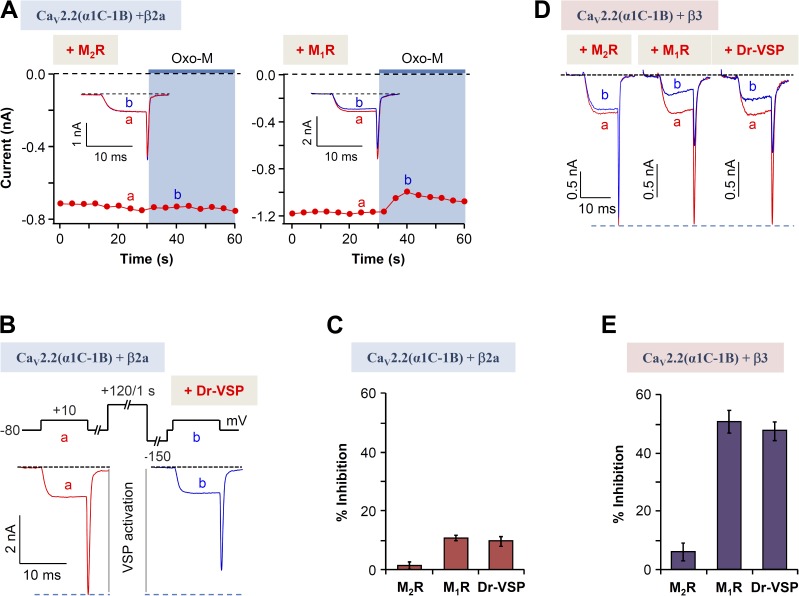
Gβγ-dependent, but not PIP2-dependent, modulation is absent in a chimeric N-type channel
The effects of Gβγ on CaV2.2 regulation were investigated through a more direct approach. We used a mutated CaV2.2 α subunit that does not bind Gβγ subunits. In this chimera, called CaV2.2 α1C-1B, the N terminus of the CaV2.2(α1B) subunit was replaced by the N terminus of the CaV1.2(α1C) subunit, which lacks the N-terminal Gβγ-binding site of α1B (Agler et al., 2005). Fig. 6 A illustrates the modulation of N-type currents in cells expressing this α1 construct with β2a upon activation of either M2R or M1R receptors. The chimera shows a smaller response to either receptor, as is summarized in Fig. 6 C. The M2R, a Gi/oPCR, is anticipated to signal through the direct binding of Gβγ, so the chimera should lack modulation, exactly as seen. Inhibition dropped from 60 to 2%. However, now we find that signaling from the M1R, a GqPCR, is also decreased by the chimera, giving only ∼10% inhibition of current instead of the >40% seen in control cells (Fig. 1 B), consistent with the concept that M1Rs also can signal by the Gβγ pathway. Continuing on, as expected, CaV2.2(α1C-1B) (β2a) current inhibition by activation of Dr-VSP was not changed compared with control conditions (Fig. 6, B and C).
Figure 6.
Voltage-dependent muscarinic modulation disappears in Gβγ-insensitive chimeric CaV2.2(α1C-1B) channels. (A) Effects on CaV2.2(α1C-1B) (β2a) currents of M1 and M2 muscarinic receptor stimulation. The current amplitude was measured at 10 mV every 4 s. Insets show currents a and b superimposed. (B) Inhibition by Dr-VSP activation of CaV2.2(α1C-1B) (β2a) currents. Cells received a test pulse (a) and then were depolarized to 120 mV for 1 s, followed by a second test pulse (b). Current traces before and after the Dr-VSP activation in cells expressing the α1C-1B and β3 subunits are shown. (C) Summary of current suppression by muscarinic stimulation or Dr-VSP activation. (D) Current traces before (a) and during (b) the Oxo-M application (left and middle) or Dr-VSP activation (right) in cells expressing the α1C-1B and β3 subunits. Effects on CaV2.2(α1C-1B) (β3) currents of M1 and M2 muscarinic receptor stimulation and Dr-VSP activation were traced as above. (E) Summary of current suppression by muscarinic stimulation or Dr-VSP activation. (C and E) Data are mean ± SEM (n = 5 for each bar).
We now consider whether switching from the CaV β2a subunit to the CaV β3 subunit alters the modulation of the CaV2.2(α1C-1B) chimera. Qualitatively, the modulation had similar features with either β subunit (Fig. 6 D). As expected, activation of M2R, which acts primarily through the Gβγ pathway, produced very little inhibition (Fig. 6 E). However, interestingly, with the CaV β3 subunit, the inhibition upon activation of M1 muscarinic receptors or upon PIP2 depletion by VSP was much higher than with β2a-containing chimeric channels. Indeed it was more like the inhibition of wild-type CaV2.2 channels. This fits well with the concept that M1 muscarinic inhibition of CaV2.2 channels with β3 is voltage independent and does not need Gβγ subunits.
M1R-induced, voltage-dependent modulation of CaV2.2 currents is dependent on CaV β subtypes
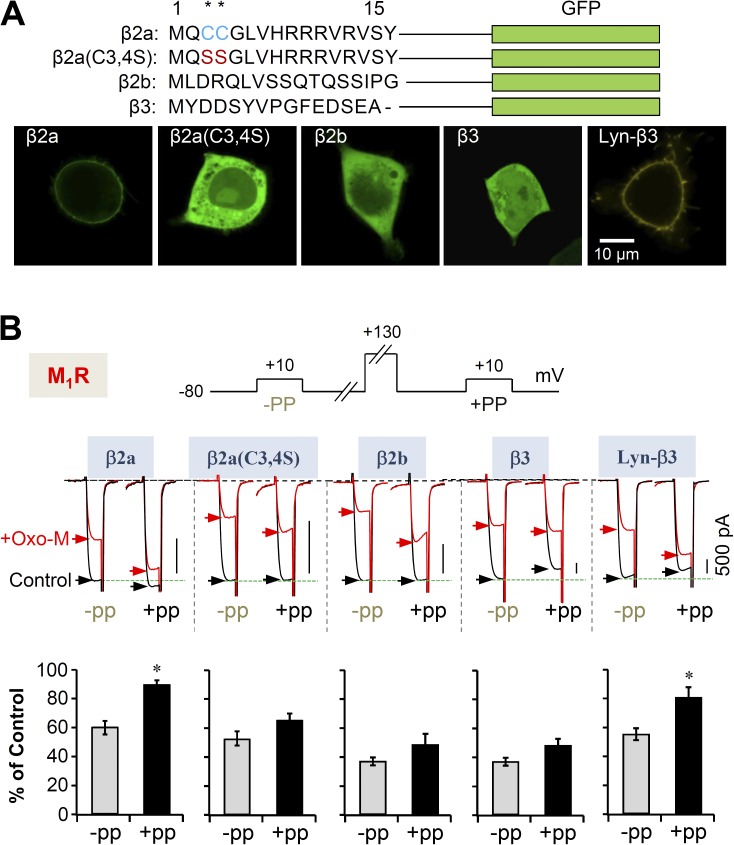
Our results reveal that M1Rs use two pathways to suppress CaV2.2 currents. We now examine further whether the choice between inhibitory pathways might depend on the CaV channel β subunit. Fig. 7 A shows that different β subunits localize differently. Expressed by themselves, β2a subunits are membrane localized and β2b and β3 subunits are soluble in the cytosol (Fig. 7 A). Palmitoylation on two consecutive N-terminal cysteines makes β2a subunits membrane resident (Chien et al., 1995; Hurley et al., 2000), and when the cysteines are substituted by serines, the mutant β2a(C3,4S) moves to the cytosol. Appending a membrane-targeting Lyn sequence to β3 makes the chimeric Lyn-β3 subunit localize at the plasma membrane (Suh et al., 2012).
Figure 7.
Cytosolic β subunit decreases the Gβγ-mediated, voltage-dependent suppression of CaV2.2 currents. (A) N-terminal amino acid sequences of β2a, β2a(C3,4S), β2b, and β3 subunit with GFP as a fluorescent label. In the palmitoylation-resistant mutant β2a(C3,4S), both palmitoylated cysteine residues (*, blue) are replaced with serine (red). Lyn-β3 is labeled with YFP. (bottom) Confocal images of the β subunits expressed in tsA-201 cells. (B) Inhibition of CaV2.2 current by M1 receptors is significantly relieved by a prepulse (+PP) in cells with membrane-localized β subunits but not in cells with cytosolic β subunits. Cells were given a test pulse (−PP) and then depolarized to 130 mV for 20 ms, followed by the second test pulse after 20 ms (+PP). The experiments were performed before (control) and during the Oxo-M application (+Oxo-M). (bottom) Summary of the prepulse experiments in control and Oxo-M–perfused cells with different CaV β subunits. The current amplitude after Oxo-M application is given as percentage of the initial control. Data are mean ± SEM (n = 5–6). *, P < 0.01.
Using the Gβγ-resistant chimera CaV2.2(α1C-α1B), we saw that coexpression with β2a makes channels that are more sensitive to the Gβγ-dependent pathway and less sensitive to the PIP2-dependent pathway, whereas coexpression with β3 makes channels more sensitive to the PIP2-dependent pathway relative to the Gβγ-dependent pathway. The same switch applies to wild-type CaV2.2 channels. Using diverse β constructs, we further analyzed the voltage-dependent and -independent modulation of CaV2.2 currents by M1 muscarinic receptors. By applying a prepulse of 130 mV (in the absence of VSP), the Gβγ-dependent portion of inhibition upon Oxo-M treatment could be estimated (Fig. 7 B). M1R activation before the prepulse gave rise to the expected inhibition percentage as in Fig. 1 A. However, after the prepulse, the inhibition percentage upon Oxo-M perfusion was much reduced in β2a expressing cells, ∼40 to ∼10%, and only slightly reduced in β2b-, β3-, and β2a(C3,4S)-expressing cells (Fig. 7 B). Thus, the voltage-dependent inhibition of CaV2.2 depends on the subcellular location of the β subunits and is stronger in channels with membrane-binding β subunits. The voltage-independent inhibition is stronger in channels with cytosolic β subunits.
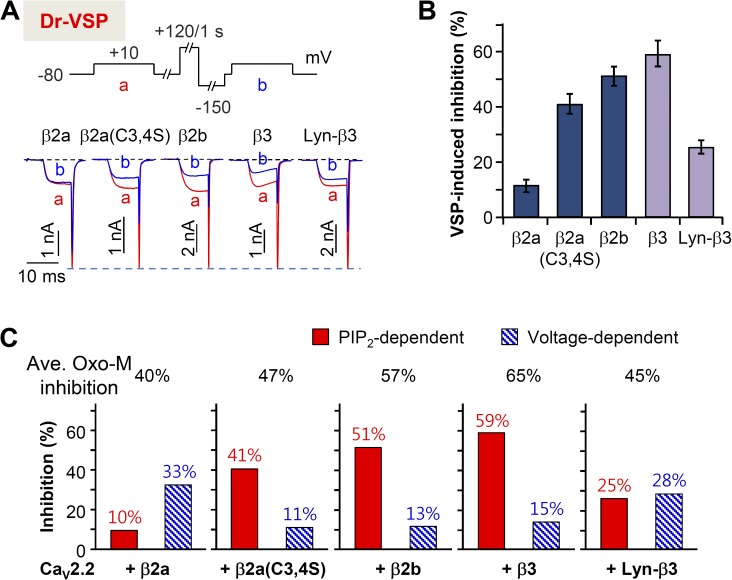
The PIP2-dependent portion of inhibition was tested in cells with different β subunits (Fig. 8 A) using the potent PIP2 5-phosphatase Dr-VSP. Cells transfected with CaV2.2, various β subunits, and Dr-VSP were depolarized to 120 mV for 1 s. The PIP2 depletion–dependent inhibition of current was low at 10% with β2a compared with 40–60% with cytosolic β2b, β3, and β2a(C3,4S). When membrane-targeted Lyn-β3 was expressed, the PIP2 sensitivity decreased to ∼20% (Fig. 8 B). Fig. 8 B contrasts the inhibition percentages of PIP2-dependent, voltage-independent and Gβγ-mediated, voltage-dependent pathways. Though M1 receptor stimulation suppresses all combinations of CaV2.2 and β subunits, depending on the types of CaV β subunit, the modulatory mechanism by M1 receptor is clearly different.
Figure 8.
Cytosolic β subunit increases the PIP2 depletion–mediated suppression of CaV2.2 currents. (A) Current inhibition by Dr-VSP activation in cells expressing different β subunits. Cells received a test pulse (a) and then were depolarized to 120 mV for 1 s, followed by the second test pulse (b). The a and b currents are superimposed. (B) Summary of the Dr-VSP–induced inhibition of CaV2.2 current. Data are mean ± SEM (n = 6–8). (C) Differential effects (mean percent inhibition) of Dr-VSP-induced, voltage-independent (A) and Gβγ-mediated, voltage-dependent (Fig. 7 B) pathways on the Oxo-M suppression of CaV2.2 channels with different CaV β subunits. Mean maximal inhibition by M1 receptor activation is presented in the top.
DISCUSSION
We have shown that with an appropriate choice of CaV β subunit, a GqPCR can signal by Gβγ subunits to suppress N-type Ca2+ currents. This contrasts with the simpler view that PTX-insensitive, GqPCRs modulate Ca2+ channels exclusively by actions downstream of PLC and that only PTX-sensitive Gi/oPCRs can modulate through Gβγ, and it extends earlier clear suggestions of Gβγ roles in M1R signaling (Kammermeier et al., 2000; Melliti et al., 2001; Gamper et al., 2004; Suh et al., 2010; Vivas et al., 2013). Our adjusted working hypothesis is summarized as a flow chart in Fig. 9 A. M1Rs inhibit CaV2.2 not only through Gαq and PLC but also through the Gβγ pathway, whereas M2Rs suppress principally through the Gβγ pathway. Furthermore, for M1Rs, the choice between PLC and Gβγ pathways is biased by the subtype of CaV β subunit expressed. Channels with the membrane–lipid-interacting β subunit β2a were more sensitive to the Gβγ-dependent pathway and less to the PIP2 depletion, whereas channels with cytosolic β subunits, including β2b, β3, and β2a(C3,4S), were more sensitive to PIP2 depletion (Fig. 9 B). Our data also showed that even though the maximum inhibition of N-type CaV current by M1 receptors ranged from 40 to 65% for different cytosolic CaV β subunits, the relative proportion of the total inhibition mediated by PIP2 and Gβγ was almost the same for each of the cytosolic β subtypes. For M1 muscarinic inhibition with cytosolic β subunits, the fractional distribution between the Gβγ-dependent pathway and the PIP2-dependent pathway was ∼20 and ∼80% of the total (Fig. 9 B). In contrast, in cells expressing the membrane-localized β2a subunits, the fractional distribution was reversed, ∼80 and ∼20%, and in cells expressing the membrane-targeted form of β3, Lyn-β3, the distribution was equal, ∼50 and 50%. This intermediate effect of Lyn-β3 is consistent with its weaker effects on current inactivation and on PIP2 depletion–mediated suppression compared with control β3 (Suh et al., 2012).
Figure 9.
M1R suppresses CaV2.2 currents through two separate pathways. (A) Diagram shows two separate signaling pathways independently modulating CaV2.2 channels. The predominance of each type of modulation is controlled by the β subunit. Solid lines indicate major inhibitory pathways. Dashed lines indicate minor or weak inhibitory pathways. (B) Relative effects of PIP2- and Gβγ-dependent pathways on the Oxo-M regulation of CaV2.2 channels with different β subunits. Membrane localization of β subunits decreases the PIP2-dependent regulation, whereas it enhances the effects of Gβγ subunit–mediated, voltage-dependent regulation.
Several of our findings support Gβγ as one of the inhibitory signals in M1 muscarinic suppression of CaV2.2 channels. (a) M1 receptor activation shifts the voltage dependence of activation of channels rightward by ∼5 mV and slows the activation kinetics, comparable with Gβγ-dependent regulation of N-type channels in sympathetic neurons (Elmslie et al., 1990; Beech et al., 1992; Boland and Bean, 1993). (b) Coexpression of the Gβγ chelator reduced inhibition of the Ca2+ currents by M1R stimulation (Kammermeier et al., 2000; Melliti et al., 2001). (c) M1 receptor activation induces a fast component of channel inhibition in addition to a slow one (Melliti et al., 2001). The fast, βARK-ct–sensitive component precedes the slow one by 3 s, about the time difference between Gq activation and PIP2 depletion (Jensen et al., 2009). (d) A chimeric α1 calcium channel subunit unable to bind to Gβγ showed much less M1 receptor–induced inhibition. Lastly (e), the suppression of CaV2.2 current by M1Rs could be reversed partially by applying a strong positive prepulse. Thus, for M1R signaling, a Gβγ-mediated, voltage-dependent pathway coexists with the well-known slow PLC and PIP2-sensitive voltage-independent pathway that is not affected by the expression of βARK-ct, chimeric α1 subunits, or depolarizing prepulses (Fig. 6 A; Melliti et al., 2001; Gamper et al., 2004; Suh et al., 2010). With M1Rs, neither pathway is sensitive to PTX.
Our single-cell experiments combining FRET and patch clamp confirmed that M1 receptors can suppress the N-type current through the fast Gβγ-mediated signaling pathways and that the fast current inhibition is independent from and unable to be triggered by the slow PIP2 depletion. Many previous studies suggested that N-type channel suppression by GqPCRs occurs through both fast and slow pathways (Hille, 1994; Melliti et al., 2001; Mitra-Ganguli et al., 2009). Here, we clearly show that the M1 receptor–mediated channel inhibition and the PIP2 depletion are temporally separated (a lag time) in a live single cell. Current inhibition begins earlier than PIP2 depletion, and the lag time was almost completely abolished by the Gβγ scavenger βARK-ct, resulting in almost the same time constants for the PIP2 depletion and the current inhibition. This temporal separation can be interpreted as a Gβγ-dependent CaV current inhibition that occurs immediately after the receptor stimulation in synapse, followed by a PLC- and lipid-dependent slow current inhibition, if the receptor activation lasts longer than the lag time. Hence the lag time determines a threshold for diversity of signaling in synaptic transmission. For example, short (<2 s) M1 receptor stimulation may suppress the CaV currents only through the fast inhibitory pathway, whereas longer receptor stimulation may regulate slower signaling by PIP2 depletion, PKC activation, Ca2+ release from the ER, and gene expression by activating the downstream PLC signaling. Thus, our new finding would provide clues to elucidate the role of M1R and CaV channels in synaptic plasticity such as Gαq-mediated long-term depression (Kamsler et al., 2010; Collingridge et al., 2010).
CaV β subunit isoforms have profound effects on calcium channel trafficking, inactivation kinetics, and susceptibility to modulation. A key distinction governing the actions of isoforms is whether they are palmitoylated and membrane directed (β2a, Lyn-β3) or not (β2a(C3,4S), β2b, β3; Fig. 9; Chien et al., 1995; Hurley et al., 2000; Feng et al., 2001). Thus, Feng et al. (2001) showed that raising free Gβγ by transient expression of Gβ subunits induced kinetic slowing of activation in CaV2.2 channels expressed with lipidated β2a subunit, whereas it had little effect in channels with other types of CaV β subunit. Similarly, in our experiments, expression of β2a gave a stronger Gβγ-mediated, voltage-dependent inhibition, whereas expression of β3 gave a stronger PIP2-mediated, voltage-independent regulation. The dependence on subcellular localization was confirmed by reversing the targeting of the β2a and β3 subunits. A cytosolic β subunit conferred reduced voltage dependency and increased voltage independency of the M1 muscarinic inhibition of N-type calcium channels. So far, the mechanism of how the membrane-targeted CaV β subunit regulates the Gβγ signaling to CaV2.2 channel is not clear. However, it is well known that the intracellular I-II loop (as well as N and C termini) of the α1 subunit is the major target site for both Gβγ and CaV β subunits, and thus binding of CaV β2a to the I-II loop through the BID domain and the plasma membrane through N-terminal palmitoyl groups at the same time may affect the mobility of this region in an unfavorable way, making the CaV channel retain high Gβγ binding affinity and be more susceptible to βγ subunit–mediated inhibition (Zamponi and Currie, 2013).
Our findings are important to understand regulation of Ca2+ channels by neurotransmitter receptors that couple to Gq in excitable cells. Previous studies reported that the lipidated β2a subunit is highly expressed in chromaffin cells, form noninactivating N-type channel currents, and contribute to hormone release (Cahill et al., 2000). The β2a subunit is also expressed broadly in brain, heart, and aorta (Hullin et al., 1992; Pichler et al., 1997), though only a small proportion of endogenous CaV2.2 interacts with the β2a subunit (Scott et al., 1996). SCG neurons express mostly β2a subunits but also β3 and β4 (Heneghan et al., 2009), accounting for relatively slow N-type current inactivation and less sensitivity to Dr-VSP–mediated PIP2 depletion compared with expression systems with the β3 subunit alone (Suh et al., 2012). However, Gamper et al. (2004) and Vivas et al. (2013) clearly showed that the N-type Ca2+ current was still strongly suppressed by membrane PIP2 depletion in neurons, suggesting that a disproportionate fraction of β3 subunits become bound to N-type α1B subunits. This is supported by previous studies showing that the modulation of N-type currents by M1 receptors in SCG neurons appears as a mixture of voltage-dependent and -independent pathways (Kammermeier et al., 2000; Suh et al., 2010) and that β3 subunits are the predominant form associated with brain N-type Ca2+ channels (Vance et al., 1998). Furthermore, the temporal expression pattern of CaV β subunits varies across brain tissue and within a single cell type during the development (Vance et al., 1998; Wittemann et al., 2000). This implies that regulation of N-type channels in nerve might change with developmental stage.
In conclusion, our study provides some insight into the possible mechanism of how GqPCRs modulate the Ca2+ channel activity and thus regulate intracellular Ca2+ concentrations in excitable nerve terminals and tissues (Kubista et al., 2009). Our results showed that Gq/11PCRs can inhibit CaV2.2 channels through the Gβγ-mediated, voltage-dependent pathway and the PIP2-sensitive, voltage-independent pathway and that this dual mode of inhibition after GqPCR activation is tightly controlled by the type of CaV β subunit present. Considering the previous observations that CaV channels can be regulated by diverse intracellular signals, such as protein kinase C and SNARE proteins (Swartz et al., 1993; Zamponi et al., 1997; Hamid et al., 1999; Magga et al., 2000), our data provide further intricacy in the G protein modulatory mechanism of Ca2+ influx and therefore neurotransmitter release in the synaptic terminal.
Acknowledgments
We are grateful to Dr. Bertil Hille for valuable discussions. We thank many laboratories for providing the plasmids.
This work was supported by the Ministry of Education, Science, and Technology (grant no. 2012R1A1A2044699) and the DGIST R&D Program of the Ministry of Science, ICT, and Future Planning (grant no. 14-BD-06).
The authors declare no competing financial interests.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- βARK-ct
- C terminus of β-adrenergic receptor kinase
- CaV
- voltage-gated calcium
- Dr-VSP
- zebrafish voltage-sensitive phosphatase
- FRET
- Förster resonance energy transfer
- GPCR
- G protein–coupled receptor
- Oxo-M
- oxotremorine-M
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PTX
- pertussis toxin
- SCG
- superior cervical ganglion
References
- Agler, H.L., Evans J., Tay L.H., Anderson M.J., Colecraft H.M., and Yue D.T.. 2005. G protein-gated inhibitory module of N-type (CaV2.2) Ca2+ channels. Neuron. 46:891–904 10.1016/j.neuron.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Bean, B.P.1989. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 340:153–156 10.1038/340153a0 [DOI] [PubMed] [Google Scholar]
- Beech, D.J., Bernheim L., and Hille B.. 1992. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 8:97–106 10.1016/0896-6273(92)90111-P [DOI] [PubMed] [Google Scholar]
- Bernheim, L., Beech D.J., and Hille B.. 1991. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 6:859–867 10.1016/0896-6273(91)90226-P [DOI] [PubMed] [Google Scholar]
- Boland, L.M., and Bean B.P.. 1993. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J. Neurosci. 13:516–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, A.L., Hurley J.H., and Fox A.P.. 2000. Coexpression of cloned α(1B), β(2a), and α(2)/δ subunits produces non-inactivating calcium currents similar to those found in bovine chromaffin cells. J. Neurosci. 20:1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall, W.A.2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16:521–555 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- Catterall, W.A.2011. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3:a003947 10.1101/cshperspect.a003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, A.J., Zhao X., Shirokov R.E., Puri T.S., Chang C.F., Sun D., Rios E., and Hosey M.M.. 1995. Roles of a membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. J. Biol. Chem. 270:30036–30044 10.1074/jbc.270.50.30036 [DOI] [PubMed] [Google Scholar]
- Collingridge, G.L., Peineau S., Howland J.G., and Wang Y.T.. 2010. Long-term depression in the CNS. Nat. Rev. Neurosci. 11:459–473 10.1038/nrn2867 [DOI] [PubMed] [Google Scholar]
- Currie, K.P.M.2010. G protein inhibition of CaV2 calcium channels. Channels (Austin). 4:497–509 10.4161/chan.4.6.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin, A.C.2003. G protein modulation of voltage-gated calcium channels. Pharmacol. Rev. 55:607–627 10.1124/pr.55.4.3 [DOI] [PubMed] [Google Scholar]
- Elmslie, K.S., Zhou W., and Jones S.W.. 1990. LHRH and GTP-γ-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 5:75–80 10.1016/0896-6273(90)90035-E [DOI] [PubMed] [Google Scholar]
- Falkenburger, B.H., Dickson E.J., and Hille B.. 2013. Quantitative properties and receptor reserve of the DAG and PKC branch of Gq-coupled receptor signaling. J. Gen. Physiol. 141:537–555 10.1085/jgp.201210887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Z.P., Arnot M.I., Doering C.J., and Zamponi G.W.. 2001. Calcium channel β subunits differentially regulate the inhibition of N-type channels by individual Gβ isoforms. J. Biol. Chem. 276:45051–45058 10.1074/jbc.M107784200 [DOI] [PubMed] [Google Scholar]
- Filippov, A.K., Webb T.E., Barnard E.A., and Brown D.A.. 1998. P2Y2 nucleotide receptors expressed heterologously in sympathetic neurons inhibit both N-type Ca2+ and M-type K+ currents. J. Neurosci. 18:5170–5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper, N., Reznikov V., Yamada Y., Yang J., and Shapiro M.S.. 2004. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. 24:10980–10992 (published erratum appears in J. Neurosci. 2005. 25:1 p following 757) 10.1523/JNEUROSCI.3869-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, J.E., Delmas P., Offermanns S., Abogadie F.C., Simon M.I., Buckley N.J., and Brown D.A.. 2000. Muscarinic inhibition of calcium current and M current in Gαq-deficient mice. J. Neurosci. 20:3973–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid, J., Nelson D., Spaetgens R., Dubel S.J., Snutch T.P., and Zamponi G.W.. 1999. Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N-type calcium channels. J. Biol. Chem. 274:6195–6202 10.1074/jbc.274.10.6195 [DOI] [PubMed] [Google Scholar]
- Heneghan, J.F., Mitra-Ganguli T., Stanish L.F., Liu L., Zhao R., and Rittenhouse A.R.. 2009. The Ca2+ channel β subunit determines whether stimulation of Gq-coupled receptors enhances or inhibits N current. J. Gen. Physiol. 134:369–384 10.1085/jgp.200910203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze, S., Garcia D.E., Mackie K., Hille B., Scheuer T., and Catterall W.A.. 1996. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 380:258–262 10.1038/380258a0 [DOI] [PubMed] [Google Scholar]
- Hille, B.1994. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 17:531–536 10.1016/0166-2236(94)90157-0 [DOI] [PubMed] [Google Scholar]
- Hille, B.2001. Ion Channels in Excitable Membranes. Third edition Sinauer Associates, Inc., Sunderland, MA: 814 pp [Google Scholar]
- Hofmann, F., Lacinová L., and Klugbauer N.. 1999. Voltage-dependent calcium channels: from structure to function. Rev. Physiol. Biochem. Pharmacol. 139:33–87 10.1007/BFb0033648 [DOI] [PubMed] [Google Scholar]
- Hullin, R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., and Flockerzi V.. 1992. Calcium channel β subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 11:885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley, J.H., Cahill A.L., Currie K.P., and Fox A.P.. 2000. The role of dynamic palmitoylation in Ca2+ channel inactivation. Proc. Natl. Acad. Sci. USA. 97:9293–9298 10.1073/pnas.160589697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, S.R.1996. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 380:255–258 10.1038/380255a0 [DOI] [PubMed] [Google Scholar]
- Jensen, J.B., Lyssand J.S., Hague C., and Hille B.. 2009. Fluorescence changes reveal kinetic steps of muscarinic receptor-mediated modulation of phosphoinositides and Kv7.2/7.3 K+ channels. J. Gen. Physiol. 133:347–359 10.1085/jgp.200810075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier, P.J., Ruiz-Velasco V., and Ikeda S.R.. 2000. A voltage-independent calcium current inhibitory pathway activated by muscarinic agonists in rat sympathetic neurons requires both Gαq/11 and Gβγ. J. Neurosci. 20:5623–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsler, A., McHugh T.J., Gerber D., Huang S.Y., and Tonegawa S.. 2010. Presynaptic m1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proc. Natl. Acad. Sci. USA. 107:1618–1623 10.1073/pnas.0912540107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisilevsky, A.E., Mulligan S.J., Altier C., Iftinca M.C., Varela D., Tai C., Chen L., Hameed S., Hamid J., Macvicar B.A., and Zamponi G.W.. 2008. D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron. 58:557–570 10.1016/j.neuron.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Koch, W.J., Hawes B.E., Inglese J., Luttrell L.M., and Lefkowitz R.J.. 1994. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gβγ-mediated signaling. J. Biol. Chem. 269:6193–6197 [PubMed] [Google Scholar]
- Kubista, H., Kosenburger K., Mahlknecht P., Drobny H., and Boehm S.. 2009. Inhibition of transmitter release from rat sympathetic neurons via presynaptic M1 muscarinic acetylcholine receptors. Br. J. Pharmacol. 156:1342–1352 10.1111/j.1476-5381.2009.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., and Rittenhouse A.R.. 2003. Arachidonic acid mediates muscarinic inhibition and enhancement of N-type Ca2+ current in sympathetic neurons. Proc. Natl. Acad. Sci. USA. 100:295–300 10.1073/pnas.0136826100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., Roberts M.L., and Rittenhouse A.R.. 2004. Phospholipid metabolism is required for M1 muscarinic inhibition of N-type calcium current in sympathetic neurons. Eur. Biophys. J. 33:255–264 10.1007/s00249-003-0387-7 [DOI] [PubMed] [Google Scholar]
- Magga, J.M., Jarvis S.E., Arnot M.I., Zamponi G.W., and Braun J.E.. 2000. Cysteine string protein regulates G protein modulation of N-type calcium channels. Neuron. 28:195–204 10.1016/S0896-6273(00)00096-9 [DOI] [PubMed] [Google Scholar]
- Melliti, K., Meza U., and Adams B.A.. 2001. RGS2 blocks slow muscarinic inhibition of N-type Ca2+ channels reconstituted in a human cell line. J. Physiol. 532:337–347 10.1111/j.1469-7793.2001.0337f.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra-Ganguli, T., Vitko I., Perez-Reyes E., and Rittenhouse A.R.. 2009. Orientation of palmitoylated CaVβ2a relative to CaV2.2 is critical for slow pathway modulation of N-type Ca2+ current by tachykinin receptor activation. J. Gen. Physiol. 134:385–396 10.1085/jgp.200910204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, Y., Iwasaki H., Sasaki M., Inaba K., and Okamura Y.. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 435:1239–1243 10.1038/nature03650 [DOI] [PubMed] [Google Scholar]
- Okamura, Y., Murata Y., and Iwasaki H.. 2009. Voltage-sensing phosphatase: actions and potentials. J. Physiol. 587:513–520 10.1113/jphysiol.2008.163097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler, M., Cassidy T.N., Reimer D., Haase H., Kraus R., Ostler D., and Striessnig J.. 1997. β subunit heterogeneity in neuronal L-type Ca2+ channels. J. Biol. Chem. 272:13877–13882 10.1074/jbc.272.21.13877 [DOI] [PubMed] [Google Scholar]
- Scott, V.E., De Waard M., Liu H., Gurnett C.A., Venzke D.P., Lennon V.A., and Campbell K.P.. 1996. β subunit heterogeneity in N-type Ca2+ channels. J. Biol. Chem. 271:3207–3212 10.1074/jbc.271.6.3207 [DOI] [PubMed] [Google Scholar]
- Suh, B.C., Leal K., and Hille B.. 2010. Modulation of high-voltage activated Ca2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 67:224–238 10.1016/j.neuron.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh, B.C., Kim D.I., Falkenburger B.H., and Hille B.. 2012. Membrane-localized β-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc. Natl. Acad. Sci. USA. 109:3161–3166 10.1073/pnas.1121434109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, K.J., Merritt A., Bean B.P., and Lovinger D.M.. 1993. Protein kinase C modulates glutamate receptor inhibition of Ca2+ channels and synaptic transmission. Nature. 361:165–168 10.1038/361165a0 [DOI] [PubMed] [Google Scholar]
- Tedford, H.W., and Zamponi G.W.. 2006. Direct G protein modulation of Cav2 calcium channels. Pharmacol. Rev. 58:837–862 10.1124/pr.58.4.11 [DOI] [PubMed] [Google Scholar]
- van der Wal, J., Habets R., Várnai P., Balla T., and Jalink K.. 2001. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J. Biol. Chem. 276:15337–15344 10.1074/jbc.M007194200 [DOI] [PubMed] [Google Scholar]
- Vance, C.L., Begg C.M., Lee W.L., Haase H., Copeland T.D., and McEnery M.W.. 1998. Differential expression and association of calcium channel α1B and β subunits during rat brain ontogeny. J. Biol. Chem. 273:14495–14502 10.1074/jbc.273.23.14495 [DOI] [PubMed] [Google Scholar]
- Vivas, O., Castro H., Arenas I., Elías-Viñas D., and García D.E.. 2013. PIP2 hydrolysis is responsible for voltage independent inhibition of CaV2.2 channels in sympathetic neurons. Biochem. Biophys. Res. Commun. 432:275–280 10.1016/j.bbrc.2013.01.117 [DOI] [PubMed] [Google Scholar]
- Wittemann, S., Mark M.D., Rettig J., and Herlitze S.. 2000. Synaptic localization and presynaptic function of calcium channel β4-subunits in cultured hippocampal neurons. J. Biol. Chem. 275:37807–37814 10.1074/jbc.M004653200 [DOI] [PubMed] [Google Scholar]
- Wu, L., Bauer C.S., Zhen X.G., Xie C., and Yang J.. 2002. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 419:947–952 10.1038/nature01118 [DOI] [PubMed] [Google Scholar]
- Zamponi, G.W., and Currie K.P.. 2013. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta. 1828:1629–1643 10.1016/j.bbamem.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi, G.W., Bourinet E., Nelson D., Nargeot J., and Snutch T.P.. 1997. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 385:442–446 10.1038/385442a0 [DOI] [PubMed] [Google Scholar]