Summary
In lung cancer, targeted therapies depend on accurate histological subclassification of the tumor. The majority of lung cancers can be subclassified based on hematoxylin and eosin staining; however, classification may be difficult in small biopsies. In this study, we investigated the utility of a newly developed triple marker (combination of TTF1/Napsin A/p40) and compared the sensitivity and specificity of this novel marker with individual markers in the subclassification of non–small cell lung carcinomas. Lung cancer tissue microarrays were constructed using surgical resection material from the Johns Hopkins Hospital. They included 77 adenocarcinomas (ADCs), 77 squamous cell carcinomas (SqCCs), and 46 cases of metastatic lung ADCs. Immunostaining patterns of all markers were scored semi-quantitatively and compared. In ADCs, the sensitivity and specificity of the triple marker were 93.5% and 77.5%, respectively. The sensitivity and specificity of TTF1 and Napsin A were 85.7% and 75.0%, and 89.6% and 90.0%. In SqCCs, the sensitivity and specificity of the triple marker were 88.3% and 92.5%, while the p40, p63 and CK5/6 showed 80.5% and 90.0%; 93.5% and 80.0%; and 89.6% and 80.0%. In addition, the sensitivity and specificity of the triple marker in metastatic ADCs showed 71.7% and 73.5%, respectively. Our triple marker (combination of TTF1/Napsin A/p40) showed a similar sensitivity and specificity for the subclassification of NSCLC when compared to individual markers. Our study not only demonstrates a useful combination of immunomarkers but also optimally conserves tissue for molecular marker testing.
Keywords: Non–small cell lung carcinoma (NSCLC), Immunohistochemical markers, Novel triple stain marker, Napsin A, P40
1. Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States and worldwide [1]. Non–small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers [1] and [2]. Among them, adenocarcinoma (ADC) and squamous cell carcinoma (SqCC) are predominate histological subtypes [1] and [2]. Recently, therapeutic strategy has changed from traditional tumor stage–based approaches to histomorphology and genetic mutation–guided targeted therapies [3], [4], [5], [6] and [7]. The discovery of epidermal growth factor receptor (EGFR) gene mutations in ADC and the subsequent success in targeting these tumors with tyrosine kinase inhibitors highlight the critical role of accurate subclassification of the tumor [8], [9], [10] and [11]. Furthermore, the identification of the echinoderm microtubule-associated protein-like 4 and the anaplastic lymphoma kinase gene (EML4-ALK) rearrangement in a subset of lung ADCs has led to the targeted therapy using crizotinib [12]. These targeted therapies have advanced the treatment of lung cancer into the era of personalized medicine.
Clinical application of targeted therapies depends on accurate histological subclassification of NSCLC. This information is particularly important in patients with advanced disease (stage III and IV NSCLC) and in patients with metastatic NSCLC, since the majority of these patients are not candidates for surgical resection of the tumor. In these patients, fine needle aspiration (FNA) biopsy of the tumor is frequently performed to obtain tumor tissue for the diagnosis, histologic and molecular testing of the tumor [13] and [14]. The majority of NSCLC can be subclassified based on histomorphologic examination using hematoxylin and eosin (H&E) stained slides [2], [13] and [15].
However, an accurate classification can be difficult in small biopsy specimens due to a variety of reasons: such as scant tumor cells, lack of characteristic architecture in small biopsy specimens, artifacts in specimen preparations, and differentiation and heterogeneity of the tumor. Poorly differentiated carcinomas are particularly difficult to classify, since they lack specific architectural or cytological features of either ADC or SqCC differentiations. Under these circumstances, immunohistochemical (IHC) study of the tumor plays an invaluable role in the subclassification of NSCLC, that is, the determination of ADC or SqCC.
Conventionally, the most commonly used markers for identification of lung ADC are cytokeratin 7, thyroid transcription factor 1 (TTF1), Napsin A and mucin, whereas for SqCC, cytokeratin 5/6 (CK5/6), p63 and p40 are employed [16], [17], [18], [19], [20], [21], [22], [23] and [24]. These panels, however, require multiple sections of tumor tissue, are time consuming and cost-inefficient. It is also very common that no more tumor tissue is left for molecular analyses after IHC studies are performed. Therefore, subclassification of NSCLC using minimal tumor material in order to preserve tumor tissue for molecular tests and to be more cost-efficient, has been increasingly demanded.
Recently, several studies have addressed this issue by investigating the potential utility of the combination of several IHC markers as a single marker for NSCLC subclassification [25], [26], [27] and [28]. A dual marker combining TTF1 and Napsin A showed 74% sensitivity and 87% specificity [25], and 74% sensitivity and 88% to 96% specificity [26] in the identification of lung ADC using FNA material. A dual marker of p63 and CK5 showed 100% sensitivity and 100% specificity in the identification of lung SqCC using FNA material [27]. Lung tumor tissue microarray (TMA) data showed that a dual marker of TTF1 and p40 had 93% sensitivity and 92% specificity in diagnosing SqCC [28]. A marker combining desmoglein 3 and CK5 showed 100% sensitivity and 100% specificity in the diagnosing SqCC [28]. Taken together, all of these studies demonstrate that the dual or triple markers have a similar sensitivity and specificity as individual markers, and also have the advantage of using minimal tumor tissue for the immune-histological subclassification of the tumor.
In this study, we have investigated the utility of a novel triple marker (combination of TTF1, Napsin A and p40) and compared its sensitivity and specificity with individual marker in the subclassification of NSCLC using lung tumor TMAs.
2. Materials and methods
2.1. NSCLC TMA construction
NSCLC cases were retrieved from the department of pathology archive at the Johns Hopkins Medical Institutes. The World Health Organization and International Association for the Study of Lung Cancer/American Thoracic Society classification criteria were used to determine histological subtypes of lung NSCLC [2] and [13], and the American Joint Committee on Cancer 7th edition was used to determine the pathological stage (pT) of the primary tumor at the time of initial diagnosis [29]. The tumor area for TMA construction was selected based on the review of the H&E stained tissue section by two American Board of Pathology certified pathologists (Q.K.L. and E.G.). The lung carcinoma tissue microarray (0.6 mm in diameter, 3–4 cores per case) and tumor-matched normal lung tissue (0.6 mm in diameter, 2 cores per case) were constructed using surgically resected specimens. All tissues were fixed in 10% buffered formalin and embedded in paraffin.
Three lung carcinoma tissue microarrays were constructed, including ADCs (n = 77 cases), SqCCs (n = 77 cases), and metastatic primary lung ADCs to other sites (n = 46 cases). All tumor cases were annotated with available clinical information in a manner that protected patient identity. The use of human tumor tissue was approved by the Johns Hopkins Institutional Review Board.
2.2. IHC stain of individual markers
IHC was performed on TMAs. The sections were cut at 4 microns and deparaffinized prior to incubation with primary antibodies. Heat antigen retrieval at 98°C for 40 minutes was used to enhance signal detection. Primary antibodies were diluted according to standard protocols and/or manufacturer suggestions. The working conditions for primary antibodies were summarized in Table 1.
Table 1.
Summary of sources and working conditions of antibodies
| Antibody | Clone | Clonality | Dilution | Pre-treatment | Source |
|---|---|---|---|---|---|
| Napsin A | IP64 | Monoclonal | 0.5972222 | N/A | Novocastra, Newcastle, UK |
| TTF1 | 8G7G3/1 | Monoclonal | 0.3888889 | CC1 | Ventana, Tucoso, AZ |
| p63 | 4a4 | Monoclonal | Prediluted | CC1 | BioCare, Concord CA |
| p40 | rabbit | Polyclonal | 1.4305556 | CC1 | Oncogene, Cambridge, MA |
| CK5/6 | D5/16 B4 | Monoclonal | Prediluted | CC1 | Ventana, Tucoso, AZ |
| Triple marker | N/A | Monoclonal | Prediluted | CC | Intelli PATH, BioCare, Concord CA |
Abbreviations: N/A, not applicable; CC, cell conditioning.
All markers, except Napsin A, were stained using a Ventana XT autostainer (Ventana Medical System, Tucson, AZ). Napsin A was stained using a Leica Bondmax autostainer (Leica Microsystem, Bannockburn IL). TTF1, p63 and p40 have well-known nuclear staining patterns, while Napsin A, and CK5/6 have well-known cytoplasmic staining patterns. The staining pattern and intensity of these markers were scored semi-quantitatively using a four tier system: 0, undetectable (0% positive cells); 1+, focally positive (<10% positive cells); 2+, moderately positive (>10% to <50% positive cells), and 3+, intensely positive (more than 50% positive cells). Care was taken not to interpret entrapped normal bronchial epithelium or alveolar macrophages as positive for tumor cell staining. Appropriate positive and negative controls were also included in the assay. χ2 and Fisher’s test were used to calculate the P value. If the α value was less than .05, it was considered statistically significant (P < .05).
All immunostained TMAs were scanned using Aperio’s ImageScope (Vista, CA).
2.3. IHC of the triple marker (combination of TTF1, Napsin A, and P40)
Triple immunostaining for lung tumors was developed on an automated system (Intelli PATH, Biocare Medical, Concord, CA) using a novel antibody combination and a multiplex detection technology. The antibody combination of p40 (pink color nuclear stains), TTF1 (brown color nuclear stains), and Napsin A (brown color cytoplasmic stains) was applied simultaneously. Subsequently, mouse-HRP and rabbit-AP polymer detection systems were used to achieve triple immunostaining. Slides were deparaffinized, and antigen retrieval was performed with cell conditioning solution, pH 6.2 (Diva Decloaker, Biocare Medical). Primary antibody cocktail incubation was done for 45 minutes followed by polymer detection (MACH2 Double stain 2), and the test was visualized using chromogenic DAB and Wrap Red. Slides were counterstained with hematoxylin and dehydrated for permanent mounting. Unless specified all reagents were supplied from Biocare Medical, Concord, CA.
3. Results
3.1. Clinical information
In our study, the patients’ median age was 63.8 years (range, 44–86 years). On primary lung ADC TMAs, a total of 77 cases were included. The male and female ratio was: 1:0.97. Among tumors, 40 cases were pT1, 31 cases were pT2, and 6 cases were pT3/4 tumors. The mean tumor size was 3.07 cm, ranging from 0.5 to 9.0 cm. The subtypes of ADCs were as follows: mixed (44 cases), acinar (19 cases), mucinous (6 cases), true papillary (4 cases), solid (3 cases), and non-mucinous ADC with lepidic pattern (formerly bronchioloalveolar adenocarcinoma, 1 case). On SqCC TMA, a total of 77 cases were included. The male and female ratio was: 1:0.60. Among tumors, 21 cases were pT1, 23 cases were pT2, 23 cases were pT3 and 10 cases were pT4 tumors. The mean tumor size was 4.17 cm, ranging from 0.43 to 13.0 cm. In addition, we also constructed a TMA with cases of metastatic lung ADC to other sites, including 36 cases of brain, 6 cases of pleura, 2 cases of lymph nodes, and 1 case each of bone and liver metastases.
3.2. The immunostaining of the triple marker in primary lung ADCs
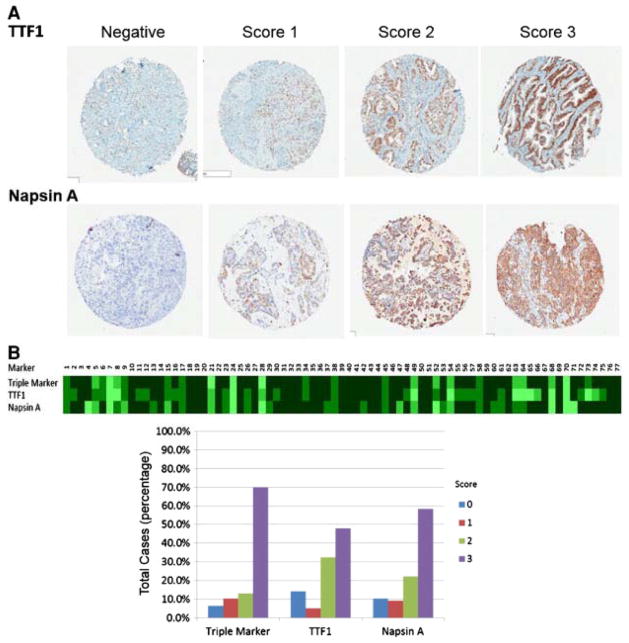
In ADCs, the triple marker showed the following staining patterns: 6.5% were negative, 10.4% were scored 1, 13.0% were scored 2, and 70.1% were scored 3 (Fig. 1 and Table 2). We also examined the staining pattern of the triple marker in different subtypes of ADCs (Table 3). We further compared the immunostain pattern of the triple marker with TTF1 and Napsin A (Fig. 2 and Table 2). The sensitivity and specificity of the triple marker were 93.5% and 77.5%, respectively. The sensitivity and specificity of TTF1 were 85.7% and 75.0%, while the sensitivity and specificity of Napsin A were 89.6% and 90.0%, respectively. The triple marker showed a slightly higher sensitivity than that of TTF1 and Napsin A alone, whereas the specificity of the triple marker was lower than that Napsin A. However, the statistical analyses did not show significant differences among the triple marker, TTF1 and Napsin A (P > .05, P = .185 and P = .564, respectively).
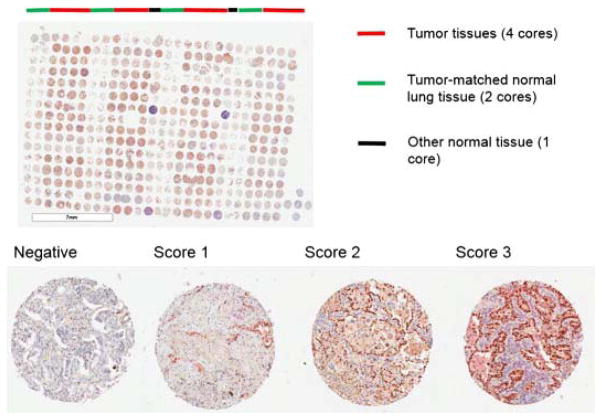
Fig. 1.
Staining pattern of the triple marker in TMA of primary ADCs. The TMA was scanned using Aperio’s ImageScope. The photo of entire TMA was taken at original magnification (1×), and photos of individual scores were taken at 4× magnification.
Table 2.
Immunostaining patterns of different markers in lung adenocarcinomas and squamous cell carcinomas
| Markers | IHC score | Sensitivity | Specificity | P | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| A. Staining patterns of lung adenocarcinomas | |||||||
| Lung adenocarcinomas (n = 77) | |||||||
| Triple marker | 5 (6.5%) | 8 (10.4%) | 10 (13.0%) | 54 (70.1%) | 93.50% | 77.50% | |
| TTF1 | 11 (14.3%) | 4 (5.2%) | 25 (32.5%) | 37 (48.1%) | 85.70% | 75.00% | 0.19 |
| Napsin A | 8 (10.4%) | 7 (9.1%) | 17 (22.1%) | 45 (58.4%) | 89.60% | 90.00% | 0.56 |
| B. Staining patterns of lung squamous cell carcinomas | |||||||
| Lung squamous cell carcinomas (n = 77) | |||||||
| Triple marker | 9 (11.7%) | 7 (9.1%) | 12 (15.6%) | 49 (63.6%) | 88.30% | 92.50% | |
| p40 | 15 (19.5%) | 29 (37.7%) | 26 (33.8%) | 7 (9.1%) | 80.50% | 90.00% | 0.75 |
| p63 | 5 (6.5%) | 5 (6.5%) | 25 (32.5%) | 42 (54.5%) | 93.50% | 80.00% | 0.4 |
| CK5/6 | 8 (10.4%) | 11 (14.3%) | 23 (29.9%) | 35 (45.5%) | 89.60% | 80.00% | 0.46 |
Table 3.
Immunostaining patterns of the triple marker in subtypes of primary lung adenocarcinomas
| Subtype of adenocarcinoma | IHC score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Mixed (n = 44) | 3 (6.8%) | 4 (9.1%) | 7 (15.9%) | 30 (68.2%) |
| Acinar (n = 19) | 0 (0%) | 2 (10.5%) | 1 (5.3%) | 16 (84.2%) |
| Mucinous (n = 6) | 2 (33.3%) | 2 (33.3%) | 1 (16.7%) | 1 (16.7%) |
| True papillary (n = 4) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (100%) |
| Solid (n = 3) | 0 (0%) | 0 (0%) | 1 (33.3%) | 2 (66.7%) |
| Lepidic (n = 1) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) |
| Total (n = 77) | 5 (6.5%) | 8 (10.4%) | 10 (13.0%) | 54 (70.1%) |
Fig. 2.
Comparison of triple marker stains with TTF1 and Napsin A in TMA of primary ADCs. A, Immunoscores of TTF1 and Napsin A in TMA of primary ADC (4×, Aperio’s ImageScope). B, The heat map and bar graph of IHC stains.
3.3. The immunostaining of the triple marker in primary lung SqCCs
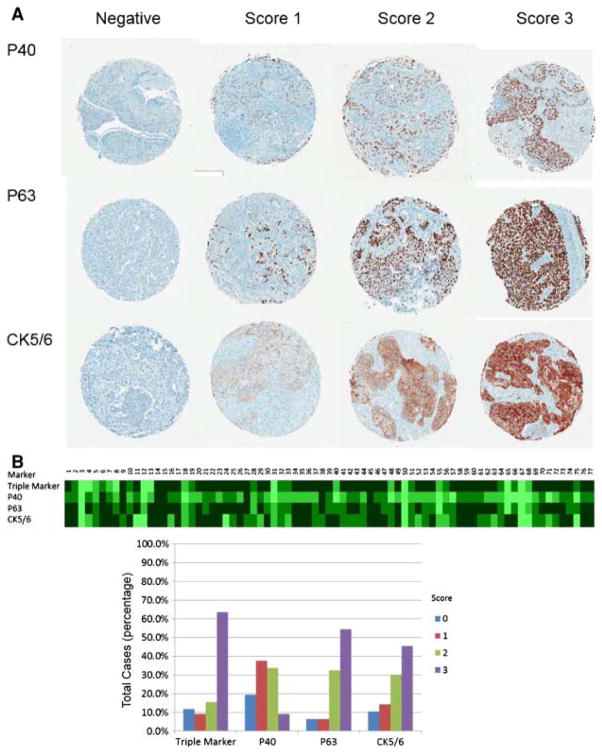
In SqCCs, triple marker showed the following staining patterns: 11.7% were negative, 9.1% were scored 1, 15.6% were scored 2, and 63.6% were scored 3 (Fig. 3 and Table 2). We compared the immunostain pattern of the triple maker with p40, p63 and CK5/6 (Fig. 4 and Table 2). The sensitivity and specificity of the triple marker were 88.3% and 92.5%, respectively, whereas the sensitivity and specificity of p40, p63 and CK5/6 were 80.5% and 90.0%; 93.5% and 80.0%, and 89.6% and 80.0%. The statistical analyses did not show significant differences among the triple marker and individual markers (P > .05, P = .749, P = .395, and P = .456).
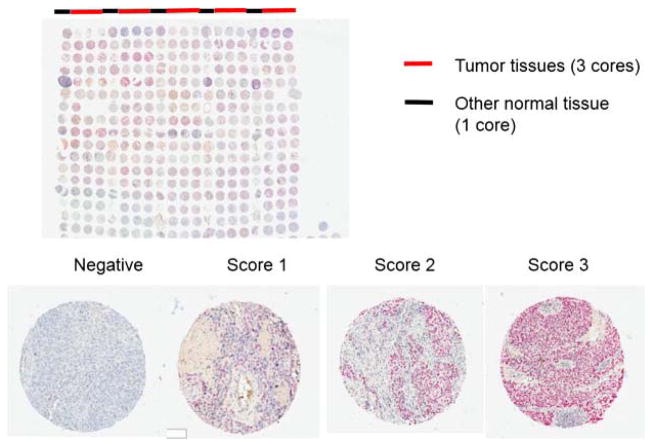
Fig. 3.
Staining pattern of the triple marker in TMA of primary SqCCs. The TMA was scanned using Aperio’s ImageScope. The photo of entire TMA was taken at original magnification (1×), and photos of individual scores were taken at 4× magnification.
Fig. 4.
Comparison of triple marker stains with p63, p40 and CK5/6 in TMA of primary SqCCs. A, Immunoscores of p63, p40 and CK5/6 in TMA of primary SqCCs (4×, Aperio’s ImageScope). B, The heat map and bar graph of IHC stains.
3.4. Immunostaining of the triple marker in metastatic lung carcinomas
We also examined the immunostaining pattern of the triple marker in the metastatic lung ADCs and compared its results with patterns of TTF1 and Napsin A (Table 4). The triple marker showed the following staining patterns: 28.3% were negative, 6.5% were scored 1, 19.6% were scored 2, and 45.7% were scored 3 (Table 4). The triple marker showed a slightly lower negative rate and higher positive rates than that of TTF1 and Napsin A alone. However, the statistical analyses did not show significant differences of the sensitivity and specificity among the triple marker, TTF1, and Napsin A (P > .05, P = 1.000 and P = .378, respectively).
Table 4.
Immunostaining patterns of different markers in metastatic lung adenocarcinomas
| Total Cases (n = 46) | |||||||
|---|---|---|---|---|---|---|---|
| Markers | IHC score | Sensitivity | Specificity | P | |||
| 0 | 1 | 2 | 3 | ||||
| Triple marker | 13 (28.3%) | 3 (6.5%) | 9 (19.6%) | 21 (45.7%) | 71.70% | 73.50% | |
| TTF1 | 14 (30.4%) | 6 (13%) | 13 (28.3%) | 13 (28.3%) | 69.60% | 72.00% | 1 |
| Napsin A | 18 (39.1%) | 4 (8.7%) | 9 (19.6%) | 15 (32.6%) | 60.90% | 89.00% | 0.38 |
4. Discussion
In this study, we combined three commonly used immunomarkers (TTF1, Napsin A and p40) into a triple marker. We then evaluated the staining pattern of this triple marker using TMAs of primary lung ADCs, primary lung SqCCs, and metastatic lung ADCs. Our data showed that the triple marker had comparable sensitivities and specificities as individual markers. Our data also demonstrated that the combination TTF1/Napsin A/p40 panel is cost-efficient and could conserve valuable tumor tissues during the immunohistochemical study and the subclassification of NSCLCs.
TTF1 has been the predominant immunohistochemical marker used for the identification of lung origin, with a reported 75% to 80% sensitivity for lung ADCs [16], [18] and [19]. However, TTF1 is also known to stain other tissues and tumors, such as thyroid tissue, metastatic breast carcinoma, neuroendocrine tumors such as small cell lung carcinoma and carcinoid [16], [18], [19], [25] and [26]. In addition, its expression is known to decrease inversely to the degree of tumor differentiation, ie, poorly differentiated ADC are less likely to express TTF1 compared to those which are well differentiated [18] and [19]. Recently, Napsin A, an aspartic proteinase involved in the maturation of the surfactant protein B, has been used as a novel marker of lung ADCs [16], [19] and [26]. Previous studies using resected tumor tissue and biopsy material have shown that Napsin A was equal to or better than TTF1 for the determination of lung origin among well to moderately differentiated ADCs [16] and [19]. In our previous study of 75 cytological cases, we found that 81% (61/75 cases) stained for TTF1 and 65% (49/75 cases) stained for Napsin A [16]. The overall sensitivity of TTF1 was 81%, while Napsin A was 65% within our study cases [16]. Napsin A is also expressed in the cytoplasm of normal lung (type II pneumocytes and Clara cells) and kidney cells (proximal and convoluted tubules), as well as in renal cell carcinomas [16].
In this study, the sensitivity and specificity of the triple marker showed 93.50% and 77.5% for the primary lung ADCs. Whereas, the sensitivity and specificity of TTF1 and Napsin A were: 85.7% and 75.0%; and 89.6% and 90.0%. We also evaluated the performance of the triple marker in the metastatic lung ADCs. The dominate type of metastatic tumors in our study were lung ADC metastases to the brain (78.3%, 36/46 cases). In our study cases, the sensitivity and specificity of the triple marker was 71.7% and 73.5%, whereas, the TTF1 and Napsin A showed comparable sensitivity and specificity of 69.6% and 72.0%; and 60.9% and 89.0%. The triple marker showed a slightly higher sensitivity than that of TTF1 and Napsin A alone; however, the statistical analyses did not show significant differences of the sensitivity and specificity among the triple marker, TTF1 and Napsin A. The different sensitivity and specificity of the triple marker between primary and metastatic lung ADC could be related to several factors. First of all, metastatic tumor may gain or lose protein expressions in comparison to its primary tumor. Secondly, tumor heterogeneity may play a potential role in variable IHC staining patterns. Finally, a recent study has shown that chemotherapy may alter the phenotype of the tumor [5]. Further study using paired tumor tissue (metastatic tumor matched with its original tumor) may help us to better understand the differential staining pattern of metastatic versus primary tumors.
The human p63 gene is located on chromosome 3q27–29 and contains 2 promoters that generate 2 types of proteins: the full-length protein TAp63 (containing the N-terminal transactivation domain) and the truncated protein ΔNp63 (N-terminal-truncated protein isoform of TA63). The ΔNp63 lacks the transactivation domain and can be identified by the antibody designated as p40 [21], [22] and [23], whereas the full-length protein TAp63 can be identified using antibody 4A4 (p63). Recent studies have shown that the p40 has a sensitivity and specificity of 100% and 98%-100% in SqCCs [21], [22] and [23]. In our study, the sensitivity and specificity of the triple marker showed 88.3% and 92.5%, respectively, whereas the sensitivity and specificity of p40, p63 and CK5/6 were 80.5% and 90.0%; 93.5% and 80.0%, and 89.6% and 80.0%. The statistical analyses did not show significant differences among the triple marker and individual markers.
Several recent studies have proposed to combine individual markers as a dual or triple marker for subtyping NSCLC; and showed variable sensitivities and specificities [25], [26], [27] and [28]. Fatima et al have studied the combination of TTF1 and Napsin A in diagnosing lung ADC using FNA cell block material, and they showed a sensitivity and specificity of 74% and 87%, respectively [25]. Brown et al have studied combinations of two antibodies. In the combination of TTF1 and p40, the sensitivity and specificity for the SqCC and ADC were 93% and 92%, and 77% and 100% [28]. It is interesting that, in the same study, the marker of combination of desmoglein 3 and CK5 showed 100% sensitivity and specificity in subtyping of SqCC. [28]. None of the ADCs in their study expressed desmoglein 3 and CK5. Although Brown’s study demonstrated an optimal performance of the desmoglein 3 plus CK5 in subtyping SqCC, a recent study by Sakai et al showed that desmoglein 3 was also variably expressed by a subset of lung ADCs [30]. Sethi et al have reported that the combination of TTF1 and Napsin A had 100% sensitivity and specificity in ADC, and the combination of p63 and CK5 had 100% sensitivity and specificity in SqCC [27]. Even though a few studies suggested 100% sensitivity and 100% specificity of certain markers, sampling error and bias should be considered when interpreting the data, particularly when studies were performed using a small set of selected samples. The perfect sensitivity and specificity may not be the case when larger-scale studies are performed. Nevertheless, all these prior studies of diagnostic panels with the inclusion of TTF1, Napsin A, squamous markers (p40, p63, or CK5/6), and other markers showed comparable sensitivities and specificities in the subclassification of NSCLC. Our study also showed a comparable sensitivity and specificity of our novel triple marker. Taken together, all studies demonstrate that the clinical usefulness of a dual or triple marker for subtyping NSCLCs.
Finally, lung carcinomas, particularly NSCLCs, are a heterogeneous group of tumors at the histologic, immunophenotypic and molecular genetic levels. The accurate classification of NSCLC is becoming increasingly important due to recent advances in targeted therapies. For example, EGFR tyrosine kinase inhibitors erlotinib (Tarceva) and gefitinib (Iressa) are currently recommended as the first-line treatment for ADCs with EGFR mutations. EML4-ALK alteration in ADCs predicts sensitivity to PF-02341066 (Crizotinib). Bevacizumab (Avastin) is contraindicated in SqCCs due to the risk for pulmonary hemorrhage [31], [32] and [33]. Pemetrexed (Alimta) is also contraindicated in SqCCs because of the lack of effectiveness [31], [32] and [33]. Although most NSCLCs can be subclassified based on the H&E-stained sections, a small number of tumors can only be further classified using immunomarkers due to several reasons, such as a small amount of diagnostic material available from a cytology or biopsy specimen, and/or the lack of identifiable differentiation (eg, keratinization and intercellular bridges for SqCCs, and luminal formation and intracytoplasmic mucin for ADCs). Therefore, the triple marker is a cost-efficient alternative option that could be used to conserve tumor tissues during the immunohistochemical subclassification of difficult cases.
In summary, our triple marker, the combination of TTF1/Napsin A/p40, showed a similar specificity and sensitivity for the subclassification of NSCLC when compared with individual markers. Our study not only provides a useful combination of immunomarkers but also yields optimal conservation of tissue for molecular marker testing.
Acknowledgments
This work is partially supported by Drs Ji and Li Family Cancer Research Foundation (Q.K.L.).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch FR, Spreafico A, Novello S, Wood MD, Simms L, Papotti M. The prognostic and predictive role of histology in advanced non-small cell lung cancer: a literature review. J Thorac Oncol. 2008;3:1468–81. doi: 10.1097/JTO.0b013e318189f551. [DOI] [PubMed] [Google Scholar]
- 4.Janku F, Garrido-Laguna I, Petruzelka LB, Stewart DJ, Kurzrock R. Novel therapeutic targets in non-small cell lung cancer. J Thorac Oncol. 2011;6:1601–12. doi: 10.1097/JTO.0b013e31822944b3. Review. [DOI] [PubMed] [Google Scholar]
- 5.Munfus-McCray D, Cui M, Zhang Z, Askin F, Gabrielson E, Li QK. Comparison of EGFR and KRAS mutations in primary and unpaired metastatic lung adenocarcinoma with potential chemotherapy effect. HUM PATHOL. 2011;42:1447–53. doi: 10.1016/j.humpath.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Li QK, Singh A, Biswal S, Askin F, Gabrielson E. KEAP1 gene mutations and NRF2 activation are common in pulmonary papillary adenocarcinoma. J Hum Genet. 2011;56:230–4. doi: 10.1038/jhg.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li QK, Gabrielson E, Zhang H. Application of glycoproteomics in the discovery of biomarkers for lung cancer. Proteomics: Clinical App. 2012;5–6:244–56. doi: 10.1002/prca.201100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–23. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 9.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 10.Tsao M-S, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer— molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 11.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–24. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 13.Sterlacci W, Savic S, Schmid T, et al. Tissue-sparing application of the newly proposed IASLC/ATS/ERS classification of adenocarcinoma of the lung shows practical diagnostic and prognostic impact. Am J Clin Pathol. 2012;137:946–56. doi: 10.1309/AJCP77KMKJXNMPMS. [DOI] [PubMed] [Google Scholar]
- 14.Yung RCW, Otell S, Illei P, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120:185–95. doi: 10.1002/cncy.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feller-Kopman D, Yung RCW, Burroughs F, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). A study of 135 cases with histology correlation. Cancer Cytopathol. 2009;117:482–90. doi: 10.1002/cncy.20049. [DOI] [PubMed] [Google Scholar]
- 16.Stoll LM, Johnson MW, Gabrielson E, Askin F, Clark DP, Li QK. The utility of Napsin A in the identification of primary and metastatic lung adenocarcinoma among cytologically “poorly differentiated carcinoma”. Cancer Cytopathol. 2010;118:441–9. doi: 10.1002/cncy.20108. [DOI] [PubMed] [Google Scholar]
- 17.Ring BZ, Seitz RS, Beck RA, et al. A novel five-antibody immunohistochemical test for subclassification of lung carcinoma. Mod Pathol. 2009;22:1032–43. doi: 10.1038/modpathol.2009.60. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF1, napsin A, p63, and CK5/6. Am J Surg Pathol. 2011;35:15–25. doi: 10.1097/PAS.0b013e3182036d05. [DOI] [PubMed] [Google Scholar]
- 19.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole tissue sections with validation in small specimens. Mod Pathol. 2011;24:1348–59. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 20.Tacha D, Yu C, Bremer R, Qi W, Haas T. A 6-antibody panel for the classification of lung adenocarcinoma versus squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:201–7. doi: 10.1097/PAI.0b013e31823d7f0e. [DOI] [PubMed] [Google Scholar]
- 21.Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405–15. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- 22.Nobre AR, Albergaria A, Schmitt F. p40: a p63 isoform useful for lung cancer diagnosis. A review of the physiological and pathological role of p63. Acta Cytol. 2012;57:1–8. doi: 10.1159/000345245. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka D. A study of ΔNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol. 2012;36(6):895–9. doi: 10.1097/PAS.0b013e3182498f2b. [DOI] [PubMed] [Google Scholar]
- 24.Whithaus K, Fukuoka J, Prihoda TJ, Jagirdar J. Evaluation of napsin A, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136:155–62. doi: 10.5858/arpa.2011-0232-OA. [DOI] [PubMed] [Google Scholar]
- 25.Fatima N, Cohen C, Lawson D, Siddiqui MT. TTF1 and Napsin A double stain: a useful marker for diagnosing lung adenocarcinoma on fine-needle aspiration cell blocks. Cancer Cytopathol. 2011;119:127–33. doi: 10.1002/cncy.20135. [DOI] [PubMed] [Google Scholar]
- 26.Johnson H, Cohen C, Fatima N, Duncan D, Siddiqui MT. Thyroid transcription factor 1 and napsin a double stain: utilizing different vendor antibodies for diagnosing lung adenocarcinoma. Acta Cytol. 2012;56:596–602. doi: 10.1159/000339793. [DOI] [PubMed] [Google Scholar]
- 27.Sethi S, Geng L, Shidham VB, et al. Dual color multiplex TTF1 + napsin A and p63 + CK5 immunostaining for subcategorizing of poorly differentiated pulmonary non-small carcinomas into adenocarcinoma and squamous cell carcinoma in fine needle aspiration specimens. Cytojournal. 2012;9:10. doi: 10.4103/1742-6413.94570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown AF, Sirohi D, Fukuoka J, et al. Tissue-preserving antibody cocktails to differentiate primary squamous cell carcinoma, adenocarcinoma, and small cell carcinoma of lung. Arch Pathol Lab Med. 2013;137:1274–81. doi: 10.5858/arpa.2012-0635-OA. [DOI] [PubMed] [Google Scholar]
- 29.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 30.Sakai Y, Nakai T, Ohbayashi C, et al. Immunohistochemical profiling of ALK fusion gene-positive adenocarcinomas of the lung. Int J Surg Pathol. 2013;21:476–82. doi: 10.1177/1066896913489345. [DOI] [PubMed] [Google Scholar]
- 31.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 32.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoSMed. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic KRAS mutations as a mechanism associated with resistance to EGFRtargeted agents: a systematic review and meta-analysis of studies in advanced non-small cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–72. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]