Abstract
Well-characterized membrane protein detergent complexes (PDC) that are pure, homogenous and stable with minimized excess detergent micelles are essential for functional assays and crystallization studies. Procedural steps to measure the mass, size, shape, homogeneity and molecular composition of PDCs and their host detergent micelle using size exclusion chromatography (SEC) with a Viscotek tetra detector array (TDA; absorbance, refractive index, light scattering and viscosity detectors) are presented. The value of starting with a quality PDC sample, the precision and accuracy of the results, and the use of a digital bench top refractometer are emphasized. An alternate and simplified purification and characterization approach using SEC with dual absorbance and refractive index detectors to optimize detergent and lipid concentration while measuring the PDC homogeneity are also described. Applications relative to purification and characterization goals are illustrated as well.
Keywords: membrane proteins, tetra detector array and analysis, differential pressure viscometer, intrinsic viscosity, refractive index
Purification and Characterization of Membrane Proteins Using Size Exclusion Chromatography with Tetra and Dual Configurations of Absorbance, Refractive Index, Static Light Scattering and Viscosity Detectors
On-line SEC triple detection, composed of absorbance, refractive index and static light scattering (LS) detectors, has proven useful for measuring membrane protein and detergent hydrodynamic properties, molecular composition and polydispersity (Takagi, 1990; Slotboom et al., 2008; Kunji et al., 2008). With the successful design of an on-line and absolute differential pressure (DP) viscometer (Haney 1985) that was shown to measure the shape or intrinsic viscosity (IV) and the radius of hydration (Rh) of soluble proteins (Dutta et al., 1991), multi-detection for soluble proteins advanced from three to four detectors (Hartmann et al., 2004; Chenal et al., 2009) and in this report for membrane proteins, taking full advantage of SEC.
SEC is a fundamental tool for the production of quality membrane protein (Newby et al., 2009) and is crucial for purifying the PDC from contaminating protein, lipids and other molecules, and for systematically finding buffer conditions that support a homogenous and stable oligomeric state with minimized detergent (Figure 1). When there are no column interactions, SEC separates proteins according to their hydrodynamic volumes [Vh = (M × IV)/2.5NA where Vh is the hydrodynamic volume, M is the molar mass, IV is the intrinsic viscosity, and NA is Avogadro’s number] (Fish et al., 1970; Frigon et al., 1983), and they elute as a Gaussian distribution of uniform molecules. For estimating molecular mass, mapping SEC retention volume verses Vh is considered the “Universal Calibration Method” for SEC (Potschka, 1987).
Figure 1.
Workflow from membranes to quality membrane protein. Following over-expression, the production of quality membrane protein starts with Membrane Preparation, and moves through Solubilization, Purification & Characterization and protein Concentration. In addition to purification, SEC with ion exchange (or other binding matrices) serves a crucial role in systematically working through the seven Key Parameters (top right) to find buffer conditions that maintain a single Gaussian peak shape at low and high protein concentrations. Since PDC oligomerization is one of the major problems encountered during purification, key to this approach is analyzing all protein fractions generated throughout the purification by SEC. Absorbance (200–600 nm) detection is used initially for purification, and is followed by dual absorbance and refractive index detection to optimize the micelle concentration while following PDC homogeneity. Dual and Tetra Detector Analysis is used during purification post SEC and/or ion exchange (IE), and during Concentration. Once the sample is pure, homogenous and stable with minimized detergent it goes to Function and Crystallization experiments. Thin layer chromatography (TLC) is used for lipid and detergent analysis, and mass spectrometry (MS) is used to measure protein molecular weights. Three chromatography workstations with computer interfaces are simultaneously used: 1) an affinity (usually Ni-NTA) station with absorbance detector, manual fraction collection and gravity flow; 2) a dual pump SE and IE station with diode array absorption and refractive index detectors, auto sampler and fraction collector; and 3) a TDA station.
The Basic Protocol of this Unit describes the use of a Viscotek on-line tetra detector array (Figure 2), and is designed for full characterization with good statistics of the whole purified PDC sample that will be subjected to function and crystallization experiments. A simplified purification and characterization Alternate Protocol using a dual array composed of absorbance and refractive index detectors is used to address sample homogeneity, detergent concentration, and the relative retentions times of the PDC and excess detergent or detergent-lipid micelle. Supporting Protocols include the purification of a protein standard used to measure detector response factors, and measuring solvent refractive index (RIsol) and molecular dn/dc using a digital and temperature controlled bench top refractometer. Several applications relative to purification and characterization goals are presented in the Commentary section.
Figure 2.
Description of Viscotek’s Tetra Detector Array. Tetra Detection is composed of an absorbance detector for quantifying protein weight concentration (CUV) according to their extinction coefficient (dA/dc) and absorbance (UV), and a differential refractometer for quantifying concentration of all molecules (CRI) at 660 nm according to their specific refractive index increment (dn/dc) and refractive index (RI), and the solvent refractive index (RIsol). The weight averaged mass (Mavg) detector uses a 670 nm LED laser to measure static light scattering (LS) at 90° (Right Angle LS) and/or 7° (Low Angle LS); however, due to lower sensitivity and S/N at 7°, LALS is usually not used. Kopt is an optical constant and is dependent on the laser wavelength λ and Avogadro’s number NA. The RALS detector, which is valid for essentially all proteins and PDCs, can accurately measure molar Mavg for molecules that have an averaged diameter of less than ~30 nm (< 1/20 of λ670nm = 33 nm). Detector response or calibration factors (KUV, KLS and KRI) are measured using a stable protein with defined M, dn/dc and dA/dc. The fourth detector is an absolute differential viscometer for measuring differential pressure (DP) and calculating molecular shape or intrinsic viscosity (IV). For proteins, IV can vary from 0.017 dl/g for globular shapes to 0.355 dl/g for elongated shapes to 0.499 dl/g for denatured or random shapes (Scheraga & Mandelkern 1953, Dutta et al 1991, Chenal et al 2009).
Basic Protocol 1 Tetra detector (absorbance, refractive index, light scattering and viscosity) analysis for complete characterization of purified PDC and host detergent micelle
This method uses four Viscotek detectors (connected in series from UV-LS-RI-DP) and is designed to thoroughly characterize the whole purified PDC sample with maximum precision and accuracy. Results include the mass, size, shape, homogeneity and molecular composition of the membrane protein detergent complex (PDC) and its host detergent micelle, and the concentration of excess micelles. It requires a SEC column and buffer system that maintains single Gaussian PDC peaks, PDC and micelle peaks which do not overlap, and a Gaussian calibration standard peak that does not elute near the inclusion limit. To help insure Gaussian RI, LS and DP peaks and improved accuracy, the starting PDC sample must minimally exhibit a single Gaussian UV optical peak (and RI peak if available; see Alternate Protocol 1). This will promote efficiency and the collection of complete TDA data sets that allow whole-peak analysis with good statistics and accurate characterization of the total PDC sample. Partial peak analysis can be used for estimations, but results are only valid if the molecular weight is constant throughout the whole peak. Starting with a quality PDC sample, the protocol involves 1) system equilibration with water and then column buffer, 2) measuring detector response factors (KUV, KRI and KLS) using a freshly purified standard in column buffer, 3) measuring detergent dn/dc and micelle properties using a stock detergent solution, and 4) measuring the PDC properties.
Materials
Pure 18 MOhm water (e.g., Milli-Q Biocel water purification system from Millipore).
SEC column buffer used to produce and support pure, homogenous and stable PDC. Measure the refractive index (RIsol) of the buffer using a bench top refractometer according to Support Protocol 1 The volume of column buffer required will depend on the total number of column injections planned and run time for each injection. Two liters of 20 mM Hepes, 100 mM NaCl, 1 mM DDM, pH 7.0 was used for the 21 injections presented in this protocol.
All solutions used for chromatography must be 0.22 μm filtered (e.g., Millipore vacuum filtration assembly and Magna 47mm nylon membranes) and degassed under vacuum for >6 hours with stirring at room temperature. It is crucial to make all the TDA buffer required for the complete experiment at one time, for it is impossible to remake the buffer with an identical RIsol (which affect KRI and KLS), or identical 280 nm absorbance (which affects KUV) when reducing agent is present.
Preparation of TDA Standard
Purified Ovalbumin Fraction VII (Sigma) at 4–7 mg/ml was used as a standard for measuring detector response factors (KUV, KRI and KLS), and the standard’s IV, Rh and homogeneity Mw/Mn. It is prepared in column buffer immediately before injections according to Support Protocol 2. Ovalbumin VII mass is 44,300 Da (Sigma Product Information sheet and references therein) with a theoretical 0.701 dA280nm/dc (Pace et al., 1995) and 0.187 dn/dc (Maezawa et al., 1983; Hayashi et al., 1989). Injections volumes range from 30 – 150 μl, and are typically100 μl.
The standard must be prepared in detergent-containing column buffer to insure the RI peak area is accurate; if it is not, a negative dip in the RI peak will be observed due to a local decrease in detergent concentration.
Preparation of stock detergent in column buffer
Detergent stock in the SEC column buffer (see above) for measuring detergent dn/dc and micelle M, Rh, IV and homogeneity Mw/Mn. Use analytical balance to measure detergent weight directly into a vial, add SEC buffer without detergent, solubilize, and measure exact volume using a volumetric pipet to determine concentration. Filter using a 0.2 μm syringe filter (e.g., Pall Acrodisc), and store at 4°C for up to two months. Final stock detergent concentration and volume is dependent on the number of injections, injection volumes and reasonable amplitudes of detector signals; 10 mL of 40 mM DDM stock when using a 1 mM DDM column buffer (used in this presentation), 140 mM OG stock with a 40 mM OG column buffer for 20–100 μl injections, and 30 mM FC12 or FC14 with a 4 mM FC12 or 0.5 mM FC14 column buffer concentration are adequate concentrations for injections ranging from 30–180 μl.
Sample preparation and injection
Quality PDC (pure, homogenous and stable with minimized detergent) in column buffer at > 0.6 mg/ml. Total mg of PDC required for a single injection is dependent on signal strengths of all detectors but 0.3 mg is a good starting point, and injection volume should be < 500 μl. Quantitate the protein concentration at 280 nm absorbance (or any wavelength identical to the TDA absorption detector wavelength used) using a spectrophotometer and measured (e.g., using amino acid analysis) or theoretical dA/dc at 280 nm (Pace et al., 1995). Unless a visible absorption band is available, 280 nm is the preferred wavelength for measuring protein concentration, and unless stated otherwise it is always used in this presentation when referring to absorbance, UV, dA/dc and optical density (OD). Independently measure the protein OD 3–4 times with a dilution factor to give ~ 0.1 OD/ml (10 cm path), average, correct for the dilution factor and divide by dA/dc.
To insure there is a good absorption band shape and that the optical density at 280 nm is not artificially increased due to light scattering or skewed due to binding co-factors, it is important to measure the absorption spectrum of the protein (generally from 220 to 600 nm) instead of just reading the absorption at one wavelength.
SEC column identical to that used to produce and support a homogenous and stable (uniform population of molecules that do not change over time) PDC. Recommended columns are the silica-based TSK G3000SW 0.75 × 60 cm with a 0.75 × 5 cm guard column (10 μm particle size; 250 Å pores; 735 maximum psi; 2.5–7.5 pH range), and the linked agarose/dextran-based Superdex (SDX) 200 10/300 (1 × 30 cm; 13 μm average particles size, unspecified pore sizes, 218 maximum psi; 3–12 pH range) without a guard column. The TSK column was used for this Basic Protocol presentation.
To inhibit matrix compression and band broadening, the manufacturer’s recommended maximum pressure across the column (post sample injector to post detectors) must not be exceeded. Column pressure is dependent on column chemistry, solvent viscosity, tubing diameter, and on the number of detectors and their flow cell size and geometry. The final TDA flow rates tend to be just below the column maximum, and usually range from 0.3 to 0.6 ml/min. Even though a replaceable filter is installed by the manufactured in front of the LS detector flow cell, a new column should not be used in order to further minimize any possible effects of column shedding.
Tetra Detection Work Station composed of a stable isocratic pump with piston backflush and non-leaking seals and pistons, four properly maintained detectors (absorption, differential refractometer, static light scattering and absolute differential pressure viscometer), calibrated syringe or auto sampler for accurate sample volume injections, an on-line degasser to insure stable signals with minimal spikes and noise, and a computer interface for data collection and analysis. Tubing used to connect the pump and injector to the tetra detector array should be short and of small diameter to minimize band broadening. To insure a constant temperature for accurate and reproducible results (usually 4°C), all components except the degasser (which generally will not work at cold temperatures due to incompatible seals) and computer are placed in a temperature-controlled chromatography room or box.
All our direct experiences and presented data and results used a Viscotek system composed of a VE 1122 dual piston isocratic pump, manual 7725i Rheodyne sample injector valve, 500 μl Peek sample loading loop, blue (0.01″ ID × 1/16″ OD) PEEK tubing and a tetra detector array (model 302 triple array and Knauer K-2501 absorbance detector), all residing in a Puffer Hubbard dedicated 4°C chromatography refrigerator. The VE 7510 GPC degasser (Viscotek) and PC computer running Windows both reside outside the refrigerated box at room temperature. To accommodate the degasser the solvent resides at room temperature, and is connected to the degasser using 0.093″ID × 1/8″ OD tubing and 10 μm stainless steel solvent inlet stone; a coiled 10 foot piece of 0.25 mm diameter ID stainless steel tubing placed in the refrigerator and between the de-gasser and pump insures the solvent is temperature equilibrated before it enters the pump. Using this configuration, the temperature at the LS detector cell is a constant 6°C (temperature probe located at LS flow cell). A 100, 200 or 500 μl Hamilton gas-tight syringe, calibrated using water and an analytical balance, is used for sample injections.
This absolute viscometer measures DP between eluting peak and solvent by splitting the incoming SEC liquid stream and delaying one stream with an internal size exclusion delay column, and then comparing the output pressure of each side of the stream; with this design, the DP profile for each eluting component consists of a positive peak followed by a delayed negative peak.
An on-line degasser may not be required when properly degassed column buffer is used, and a calibrated auto-sampler will minimize total time required to complete a TDA experiment. Samples and syringes are always kept on ice or in the 4°C chromatography refrigerator.
Viscotek’s OmniSEC software version 4.1 running on a Windows-based PC computer used for data collection and analysis. Every tetra detector chromatogram (TDAgram) has a Data file with associated Run Parameters (sample name, date and time, injection volume, sample concentration and column flow rate). Using the Run Parameters and a created Method file, the TDAgram is calibrated to establish calibration response factors and Executed to output results using defined integration limits and baselines. Each Method file includes a defined Method Type; for a single-component molecule like a soluble protein or detergent micelle, the Multi-detector Homopolymer method type (mdHP) is utilized for data analysis, while the Multi-detector Copolymer (mdCP) method type is used to analyze a two-component, single scattering particle such as a PDC (Figure 3). Other method types within the OmniSEC data analysis module are available and useful depending on detectors used and knowledge of molecules under study. Even though only one mdHP method type is used to analyze each single-component TDAgram, for clarity four different sequential iterations are described and termed Individual mdHP K Method, Average mdHP K Method, RI mdHP Area Method and Micelle mdHP Method. The final PDC mdCP Method incorporates the mdCP method type and uses concentration data from the UV as well as the RI detectors for the final analysis of the PDC. Microsoft Excel and GraphPad Prism (or any similar spreadsheet programs) can be used to assist in data analysis and result summaries. For convenience, during data collection, a second Windows-based PC running OmniSEC software is used for data analysis.
Equations used for accumulated statistics for each data set are 1) relative error or error in average = SD/sqrt N, where SD and N represents the standard deviation and number of measurements, respectively, 2) absolute error or % error in average =(relative error/average) × 100, and 3) % average error = ((average-predicted)/predicted) × 100.
To properly use all capabilities of the extensive OmniSEC software, it is advised to study and understand the Method types, equations used, input parameters and output results before experiments are considered and planned.
Figure 3.
TDA equations used by OmniSEC for the analysis of a two-component, single scattering particle. Summary of the (A) required input parameters and (B) broken down equations used by Viscotek’s OmniSEC software to analyze a two-component, single scattering molecule like a PDC. Equations for the results are based on each 5 Hz slice i (left side), and then integrated throughout the whole or partial peak as a weighted average w (right side). (C) Polydispersity (Pd) in mass equals the weighted mass average (Mw) divided by the number mass average (Mn), with a value of 1.000 representing a 100% monodisperse or homogenous distribution.
Steps
-
Equilibrate SEC-TDA workstation (pumps, injector, column and detectors).
Turn triple array and UV power on, boot computer, turn LS laser and UV lamp on, and start OmniSEC software. The power to the triple array is kept on unless it will not be used for ~ 1 month. First activate the Quick Run tab to verify all detectors are active and stable with minimal noise by washing extensively with water (>12hr). Once baselines settle, sequentially “purge” the RI detector and then purge the viscometer for 15 min. Continue running until baselines stabilize and repeat purges. Check for stable baselines and lack of signal spikes due to solvent air bubbles and column shedding. Once the workstation is equilibrated and working correctly in water, re-equilibrate in SEC column buffer (125– 200 ml). Zero UV and RI detectors (zeroing detectors upon equilibration is not required since it is the detector change in mV which is being measured). To insure smooth and stable baselines during the experiment, all detectors power and lamps/laser remain on, and the flow is never turned off once the column is equilibrated. Overnight, when manual injections are not being made, the flow is reduced to 0.1 ml/min to conserve buffer and the absorbance detector lamp can be turned off to conserve the lamp.
To insure steady baselines and to minimize signal spikes especially in the viscometry signal (DP), it is important that the final liquid chromatography output tubing is stable to any motion or pressure; it should be clamped tight into position above the liquid waste beaker (which will allow manual fraction collection) or always immersed in the liquid. The chromatography refrigerator door must also be carefully opened and closed to avoid jarring the detectors. -
Measure detector response factors KUV, KRI and KLS.
Make ≥5 injections of purified stock Ovalbumin VII in column buffer. For each injection, open the Quick Run tab and input Run Parameters (data file name and storage directory, sample and injection volume, trigger on and total run time). Data acquisition is automatically started (triggered) upon each injection and stopped when the run time has elapsed. Analyze each Ovalbumin TDAgram using OmniSEC. Open the first data file and “Create New Method”. Select mdHP as Method Type. Under General, define detectors used (RI, RALS, DP are on by default; LALS is turned off), use conservative RALS de-spike and use defaults for Advanced features. Define calibration Standard used (protein name, dn/dc, dA/dc, M, Mw/Mn = 1 by default, injection volume and concentration), enable Peak Broadening Correction (corrects for tubing volume between detectors), and define Sample exactly like Standard (including RIsol). Automatically set baselines and peak limits for each detector peak using “Find Baselines”, and then manually insure the proper whole peak is selected and adjust baselines and peak limits as needed. Insure that the complete SEC peak is included within the limits. “Calibrate” the Method to output Calibration Factors and Standard Input Parameters (Figure 4A). “Execute” the Method using those detector response factors to output physical Ovalbumin Results and Sample input Parameters (Figure 4B), and Save as an Individual mdHP K Method. This Method’s subheadings (General, Sample, Standard, Calibration, Peak Detections) can now be visualized, and edited as needed. Repeat this analysis for each Ovalbumin injection, average all results with errors and verify that the results are reasonable (input values reproduced) and acceptable (small averaged errors) (Figure 5C,D). Input averaged Ks into an Individual mdHP K Method, Rename and Save to make the Average mdHP K Method.
To insure standard sample integrity (stable and monodisperse) and therefore quality data, the purified standard is injected immediately after purification and quantitation, and results (calculated M, Mw/Mn; K errors) verified. If measured M andMw/Mnare different from input values, insure quality of the TDAgram, and recheck detector peak limits. Large averaged errors among the different response factor data sets indicate standard is not compatible (unstable, insoluble, binding to column) in the column or column buffer, and a different standard must be used for calibration.LS data can also be corrected for spikes during acquisition, but is not preferred since it masks potential problems. -
Measure detergent dn/dc and micelle properties.
Perform ≥8 different volume injections of stock detergent in column buffer using Quick Run as described above in Step 2. Detergent data analysis for each data file involves two steps: A) To calculate detergent dn/dc, open a detergent data file and the Average mdHP K Method; insure that the RI detector is chosen for Sample instead of UV, verify that the Run Parameters are correct, save Method as RI mdHP Area Method, set baselines and integration limits, and Execute to obtain the RI area. Note that the sample run parameters are saved with the data file before the injection is made, but they can be changed at any time. Repeat analysis of all other detergent data files using the RI mdHP Area Method. Plot the RI Area (mvml) verses weight (grams) of detergent injected, and calculate slope to give dn/dc (ml/g). Even though OmniSEC will plot and calculate the slope (rate of change along the best-fit linear regression line through measured data points), manually inputting data into a spreadsheet is preferred for better plotting capabilities and measured fit statistics. B) To measure other micelle properties, open a data file, input measured detergent sample dn/dc into RI mdHP Area Method, save to new Micelle mdHP Method and Execute (Figure 3C). Repeat data analysis for all the other detergent data files. Overlay the RI traces for visualization of linearity, tabulate results and summarize (Figure 6).
If a detergent has a useful absorption band, the absorbance concentration detector can also be utilized. -
Measure PDC properties.
Inject ≥ 5 different injections of the same sample using identical or different volumes of purified PDC in column buffer using Quick Run, and analyze each resulting TDAgram with OmniSEC. Open a PDC data file and create new PDC mdCP Method using Multi-detector Copolymer Method Type. Every time a new method is created it must be calibrated. Calibrate the mdCP method then manually input average Ks into the calibration panel and save PDC Method. Both UV and RI detectors are used for concentration as default; use Ovalbumin VII as the Standard; the PDC is the sample. In this method Component A = protein and Component B = detergent (input dA/dc and dn/dc for both protein and detergent, and RIsol). Run baseline and peak detection, adjust baseline and peak limits, and Execute on PDC (Figure 3D). Repeat for all PDC data files, tabulate results and summarize (Figure 7).
For users who wish to use their own software for data and/or error analysis, the inputs, outputs and equations outlined in Figure 3 and grouped by steps in Table 2 would be used. When considering a single PDC peak, the protein dA/dc input defines the protein concentration and the protein dn/dc input measures how much of the RI detergent peak area belongs to the protein, while the remaining RI area is therefore due to the detergent and quantitated by it’s own dn/dc. -
Clean SEC-TDA workstation.
Wash system with water for 12–24 hrs followed by >12 hrs with 0.025% sodium azide in water (or other reagents which prevent bacterial growth such as 20% ethanol) for storage. Insure that the RI and DP reference cells are also washed (purged) and stored in sodium azide.
Figure 4.
Examples of OmniSEC output tables at different stages during the TDA analysis of the PDC presented in the Basic Protocol. (A) Calibrated and (B) Executed Individual mdHP K Method outputs for a single Ovalbumin injection (Step 2). (C) Executed RI mdHP Area Method output for a single DDM injection using the SEC measured dn/dc (Step 3). (D) Executed PDC mdCP Method output for a single PDC injection (Step 4). Types of output files or Reports are extensive, and are at the discretion of the user.
Figure 5.
Measuring TDA detector calibration factors. Five different column injections of Ovalbumin where performed to measure averaged detector response factors K, and Ovalbumin’s IV, Rh and Mw/Mn. (A) TDAgram of 0.6 mg (100 μl) Ovalbumin injected onto a TSK column using 20 mM Hepes, 100 mM NaCl, 1 mM DDM, pH 7.4 running buffer at 0.6 ml/min. Tables summarizing (A) Ovalbumin input parameters, (B) measured Ks and (C) Ovalbumin properties, respectively. The highly reproducible profiles (Ks have errors less than 0.3%, and shape and size errors are less than 1%) prove that the standard is stable with no column interactions in this buffer/column combination.
Figure 6.
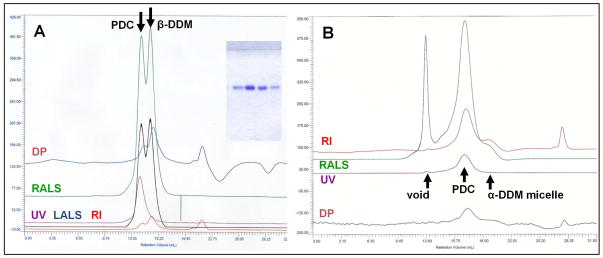
Measuring detergent dn/dc and micelle properties using TDA. DDM TDAgrams where collected as in Figure 4. (A) RI overlays of 10 out of 16 different DDM injections ranging from 20 to 100 μl and −0.05 to 1.970 mg DDM/injection. The second negative RI peak represents the inclusion limit and is due to a slight mismatch of Hepes and/or NaCl concentrations between the sample and column buffers. (B) Plot of DDM mass (g) verses RI area (mvml), and a calculated dn/dc (= slope × (RIsol/KRI)) of 0.130 ml/g (N = 16). (C) Table summarizing measured properties of DDM micelles. There is a good dn/dc fit and errors for micelle calculations are less than 3%. The micelle is spherical with a Mavg of 90,000 Da and perfectly homogenous.
Figure 7.
Measuring PDC properties using TDA. (A) Table of PDC results averaged from five different column injections performed as in Figure 4. (B) PDC input parameters. (C) TDAgram of a 150 μl injection containing 0.71 mg PDC in column buffer. By comparing the RI retention time of the PDC and DDM micelle control (↓ and Figure 5), the concentrated sample contains no excess micelles. (D) RI peak (red) contained within integration limits overlaid with measured mass (black) at each 5hz data point. Since the final peak is a weighted average according to intensity, the mass averaged from the total peak of 5 different injections is precise to less than 0.5%. (E) SDS-PAGE profile of PDC used for the injections shows that it is a pure heterodimer; two different heavy loads on the left and two different light loads on the right flank the center lane of standards. Even though all of the data sets for this experiment are precise, the PDC mass maps to 1.19 dimers/PDC indicating a 19% error in accuracy due to the values of one or more PDC and calibration input parameters (Figure 3). For this pure, homogenous, stable and globular shaped PDC sample, each dimer is bound to 220 DDM molecules with minimized detergent. Such samples represent our Gold Standard for quality and well-characterized PDCs.
Table 2.
Percent change in TDA output parameters for a single analysis step as a function of a single 5% change in each input parameter. Blank cells represent no effect.
| Analysis Step | Input | Output | ||||
|---|---|---|---|---|---|---|
| KLS | KRI | KUV | IV | Rh | ||
| Calibration | Injection mg/ml | 5.0 | −4.8 | −4.8 | −4.8 | −1.6 |
| Injection volume | 5.0 | −4.8 | −4.8 | −4.8 | −1.6 | |
| M | 5.0 | 1.6 | ||||
| dn/dc | 10.3 | −4.8 | ||||
| dA/dc | −4.8 | |||||
| dA/dc, mg/ml | −4.8 | 5.0 | 5.0 | 5.2 | 1.6 | |
| RIsol | 10.2 | 5.0 | ||||
| RI area | dn/dc | |||||
| Detergent dn/dc | Injection mg/ml | 5 | −5 | |||
| Injection volume | 5 | −5 | ||||
| KRI | −5 | |||||
| RIsol | 5 | |||||
| M | IV | Rh | ||||
| Micelle properties | dn/dc | −4.8 | 4.9 | |||
| KRI | 5.0 | 4.9 | 3.3 | |||
| KLS | 5.0 | 1.6 | ||||
| RIsol | −13.6 | −4.9 | −6.3 | |||
| M | IV | Rh | MA | MB | ||
| PDC properties | Protein dn/dc | −4.0 | 4.1 | −0.1 | −8.6 | |
| Protein dA/dc | 1.4 | −1.2 | −4.8 | 8.3 | ||
| Detergent dn/dc | −2.1 | 2.1 | −4.7 | |||
| KUV | 1.1 | −1.2 | −4.8 | 8.3 | ||
| KRI | 3.8 | 6.2 | 3.3 | 10.2 | 4.0 | |
| KLS | 5.0 | 1.6 | 5.0 | 5.0 | ||
| RIsol | −12.6 | −5.9 | −6.3 | −17.7 | −6.5 | |
Alternate Protocol Dual absorbance and refractive index detection to enhance PDC homogeneity measurements, optimize detergent concentration, define SEC purification limitations and insure sample quality for tetra detection
A combination of dual on-line absorbance and refractive index detectors allows the simultaneous measurement of all molecules such as proteins, detergent and contaminants. To insure that UV and RI peaks are Gaussian, and that the RI excess micelles peak does not overlap with the protein RI and UV peak, this dual configuration is recommended before performing the Basic Protocol. This simplified two-detector method can be performed with two stand-alone detectors or on a TDA workstation. It requires no detector response factors, molecular dn/dc or stable light scattering and viscosity baselines. However, a control injection of known detergent weight is needed to estimate excess detergent concentration. Due to its high sensitivity to all molecules including any contaminants or salts in an impure sample, the RI detector is generally not used until the PDC has been partially purified (such as post affinity, purification tag cleavage, removal of cleavage enzyme and initial SEC runs). By comparing both the UV and RI profiles of the PDC and its host micelle, optical and refractive index homogeneity measurements of the PDC are made, and the host micelle concentration can be monitored and optimized while following its effect on the PDC homogeneity. If both these peaks are Gaussian and separated, the LS and DP peaks will most likely also be Gaussian, making the sample ideal for maximum tetra detection analysis (Basic Protocol). By comparing the retention volumes of the micelles to the PDC, key SEC abilities and limitations for a particular protein-detergent system are determined; if they are baseline separated, lipids and excess micelles (both which co-elute) can be removed from the PDC, the PDC can be concentrated before SEC injection, and SEC may be used to remove excess micelles. While using this dual detector configuration, it is a prime time to also verify that a potential standard used for measuring the detector response factors is compatible in the particular SEC column and running buffer (remains stable and monodisperse with no column binding), and does not co-elute with small molecules (e.g., salts, reducing agents, osmolytes) eluting at or near the inclusion limit.
Materials
Solutions (water, column buffer, detergent stock) and SEC column are identical to those defined for the Basic Protocol.
PDC sample in SEC running buffer as described in the Basic Protocol, but the PDC does not need necessarily need to be pure, homogenous and stable.
Equipment is an isocratic chromatography workstation composed of a pump, sample injector, absorbance and RI detectors, fraction collector, and computer software. Currently in use is a Shimadzu binary workstation composed of an SCL-10Avp system controller, two LC-10ADvp pumps, SPD-M20A diode array detector, RID-10A refractive index detector, SIL-10AP automatic sample injector, FRC-10A fraction collector, windows-based computer and Class-VP 7.4 software.
Steps
Equilibrate workstation in water and then column buffer, similar to the description in the Basic Protocol but for a shorter period of time. Generally, the system is equilibrated in water overnight, and then approximately 2 hours in column buffer the following day.
-
Measure and compare the PDC and micelle UV and RI retention times and peak shapes (Figure 8). Inject the PDC sample and overlay the Abs and RI traces to visually estimate PDC optical plus refractive homogeneity. Then inject detergent stock to measure the micelle retention time. If the amount of detergent injected is known, integrate its total peak area using the workstations software to quantitate RI peak area per detergent weight; then integrate the PDC sample RI micelle peak, and quantitate. It is useful to tabulate detergent retention time as a function of SEC column type and column buffer used (Table 1).
For estimation of excess detergent in the PDC sample, the detergent concentration in the running buffer must be subtracted from the concentration of the detergent control.
Figure 8.
Dual UV and RI analysis of two PDCs and their host detergent OG. UV (purple traces) and RI (red traces) SDX chromatograms of (A) 40 μl concentrated tAqp4 and (B) 1.97 mg of it’s host detergent OG (75 μl, 90 mM). The tAqp4/OG PDC is highly homogenous (Gaussian UV and RI peaks) and contains 10.5 mM (0.122 mg) excess OG. The tAqp4 sample was concentrated to about 40 mg/ml using a 50 kDa cut-off filter, and then diluted two fold with identical buffer containing no detergent. Running buffer was 25 mM citrate, 50 mM NaCl, 5% (v/v) glycerol, 40 mM OG, pH 6.0, 2 mM DTT. Since the PDC and excess micelles are baseline resolved, the sample can be concentrated before running SEC and is ideal for a complete TDA experiment. Similar samples were ultimately used for complete TDA analysis (not shown), and for successful function and structure studies (Ho et al., 2009). SDX profiles of (C) 20 μl of ~ 16 mg/ml PDC and (D) 2.92 mg (100 μl, 100 mM) of its host detergent OG. The PDC is optical homogenous (Gaussian UV peak) and contains about 270 mM OG (1.6 mg), but since the micelle and PDC co-elute, it is not a good sample for TDA. The PDC sample was concentrated to about 16 mg/ml using a 50 kDa cut-off filter, and running buffer was 20 mM MES, 100 mM NaCl, 40 mM OG, pH 6.0, 2 mM DTT. Excess micelles were ultimately removed by ion exchange allowing for complete TDA analysis (not shown). Insets are the Coomassie stained SDS-PAGE profiles of each injected PDC showing that they are pure, and that their depicted oligomeric states (verified by antibody staining) are affected by the SDS assay conditions.
Table 1.
Measured RI peak retention volumes (Rv) of detergent micelles in different SEC columns and running buffers.
| Detergent1 | Column2 | Rv (ml)3 | Column Running Buffer |
|---|---|---|---|
| Cymal 5 | SDX | 15.50 | 5 mM Cymal 5, 25 mM Tris Cl, 25 mM NaCl, 10%4 glycerol, pH 8.0, 2 mM DTT |
| DDM | SDX | 15.06 | 2 mM DDM, 20 mM Tris Cl, 150 mM NaCl, pH 8.0 |
| DM | SDX | 15.00 | 2 mM DM, 50 mM Tris Cl, 50 mM NaCl, 2 mM βME, pH 8.31 |
| FC-12 | SDX | 15.86 | 4 mM FC12, 20 mM Tris Cl, 100 mM NaCl, 10% glycerol, pH 8.5 |
| FC-14 | SDX | 14.33 | 0.5 mM FC14, 20 mM HEPES, 150 mM NaCl, 10% glycerol, pH 7.4 |
| FC-14 | SDX | 14.66 | 0.5 mM FC14, 20 mM HEPES, 150 mM NaCl, pH 7.5 |
| NG | SDX | 10.98 | 10 mM NG 25 mM Citrate, 250 mM NaCl, 10% glycerol, pH 6.0, 2 mM DTT |
| OM | SDX | 17.30 | 40 mM OM, 40 mM NaAcetate, 200 mM NaCl, pH 5.0, 10 mM DTT |
| OG | SDX | 16.24 | 40 mM OG, 20 mM HEPES, 200 mM NaCl, pH 7.4 |
| OG | SDX | 15.51 | 40 mM OG, 25 mM Citrate, 50 mM NaCl, 5% glycerol, pH 6.0, 2 mM DTT |
| DDM | TSK | 17.60 | 1 mM DDM, 20 mM HEPES, 100 mM NaCl, pH 7.0 |
| DDM | TSK | 18.64 | 1 mM DDM, 20 mM HEPES, 150 mM NaCl, 10% glycerol, pH 7.3, |
| α-DDM | TSK | 19.89 | 0.5 mM α-DDM, 20 mM HEPES, 100 mM NaCl, 10% glycerol, pH 7.3 |
| OG | TSK | 19.63 | 40 mM OG, 20 mM MES, 100 mM NaCl, pH 6.0 |
Cymal 5, Cyclohexyl Pentyl Maltoside; DDM, Dodecyl maltoside; DM, Decyl maltoside; FC-12, Fos-Choline 12; FC-14, Fos-Choline 14; NG, Nonyl glucoside; OM, Octyl glucoside.
SDX: Superdex 200 10/300 GL; TSK: Tosoh 0.75 × 60 cm G3000SW + 0.75 × 5 cm guard column.
Peak retention volumes (Rv) are listed, but retention volume of whole peak must be considered.
All % glycerol concentrations are v/v.
Support Protocol 1 Using a digital bench top refractometer to measure molecular dn/dc and refractive index of SEC column buffer (RIsol)
To complement any on-line refractive index efforts, a bench top refractometer is required for measuring the refractive index of solvents (RIsol). It is also the preferred method for measuring the dn/dc of the calibration standard and detergent, and is useful for comparison to the dn/dc measured on-line using SEC.
Materials
Solutions are as described in the Basic Protocol.
A properly calibrated bench top refractometer is required. The refractometer should have a digital processor with digital outputs to at least five decimal places (Stratt and Forrest, 1939), stable temperature control and possible variable wavelengths (RI is dependent on both temperature and wavelength). An analog Abbe-3L refractometer (Fisher Scientific) placed at 4° C originally used was found to have inadequate resolution and accuracy for RI analysis. But a single wavelength λ = 589.29 nm; considered standard; Anton Paar Abbemat RXA156 digital refractometer (now called Abbemat HP, High Precision) and Windows-based PC computer used in this presentation is precise and accurate to 6 decimal places with fast Peltier temperature changes (10–70° C range; 2 min equilibration for 2° changes), and a 150 μl sample cell (50 μl sample cell available) (Figure 9). Calibration is performed using water and can be easily corrected for small differences in wavelength and temperature if desired (Tilton and Taylor, 1938).
Figure 9.
Refractive index (nD) of water verses temperature using a RXA156 refractometer. To test the capabilities of the Anton Paar Abbemat RXA156 (λ = 589.29 nm), water temperature profiles from 10 to 26°C in 2° increments were measured starting with 20° calibration shown in blue and 10°C calibration shown in green. For comparison and validity, the original 589.29 nm calibration data from is shown in red (Tilton and Taylor, 1938). The RXA156 was calibrated using water (1.333690/10°C; 1.332988/20°C). A single 150 μl water sample was used for each temperature curve, and re-equilibration time between temperatures was 2 min. 13–17 data points where automatically collected every 5 sec for each temperature, with standard deviations of 0.000001 for all temperatures. Data for all three curves fit well to a 2nd order polynomial: (red) y = −0.0000019x2 − 0.0000110x + 1.3339897, R2 = 0.9999305; (blue) y = −0.000002x2 − 0.000022x + 1.334188, R2 = 0.999905; (green) y = −0.000002x2 − 0.000018x + 1.334077, R2 = 0.999905. The water standards are valid, and for the most accurate results, the RXA156 should be calibrated with water at the temperature used for sample measurements.
Steps
-
Initialize the refractometer.
Turn on the RXA156 and PC, start the Abbemat PC software and set the measuring temperature. Depending on temperature desired, place the calibration standard and samples to be measured on ice or at RT.
-
Perform a one-point calibrate using pure water.
Insure that the instrument has been switched on for at least 30 min. Clean the measuring prism (sample holder) with a tissue (e.g., Kimwipe) and water, and insure that it is dry. Place 150 μl water in measuring prism, close the cover, and wait for the temperature and signal to stabilize (less than 2 min), and set calibration (go to Menu, then point calibration, and enter name of calibration liquid and its refractive index (nD); for example, use 1.333690 for 10°C. Calibration and reproducibility can be verified by repeating the measurement using fresh samples. The RI value between measurements should be within +/− 0.000005 nD.
-
Measure RIsol or any single sample.
Set the desired temperature, pipette 130 μl sample in the measuring prism and close lid, set sampling frequency to 5 sec and total acquisition time to 3 min, and start acquisition once temperature and readings have stabilized. When complete, note final measured RI and save data file. Import data file into Excel and average measurements. Repeat data acquisition and averaging two more times, and more if desired. The RI value between automatic measurements should be within +/− 0.000005 nD.
The RXA156 is a sophisticated refractometer with many methods available for data collection. Sampling frequency and total time used for computer data acquisition, and determination of whether a data file is automatically saved or averaged is at the discretion of the user. Most important is that the sample in the sample holder is at the correct temperature, and its RI signal is stabilized. Sample sizes as low as 100μlhave been successfully used. -
Measure dn/dc.
Repeat measurements as in step 3 for at least six different concentrations including its solvent control. Samples of different concentrations should be made using serial dilutions from a single concentrated stock. Between samples, wash measuring prism with water and a Kimwipe and insure it is dry before loading the next sample. Plot measured nD verses weight concentration (grams/ml) and calculate the dn/dc slope. To evaluate data quality, overlay plot with fitted linear slope and compare measured buffer dn/dc to Y-intercept (Figure 10).
Clean and dry measuring prism and lid before storing the instrument.
Figure 10.
Measuring dn/dc of Ovalbumin and detergent using a RXA150 refractometer at 10°C. (A) dn/dc measurements of Ovalbumin from 0–2 mg/ml in 20mm Hepes, 150 mM NaCl, pH 7.0 with (shown) and without (7 data points) 0.1 mM TFA-1. The detergent TFA-1 was provided by Pil Chae and Sam Gellman (Chae et al 2010). (B) dn/dc measurements of Ovalbumin from 0–2.5 mg/ml in 25 mM Na citrate and 50 mM NaCl, pH 6.0 (A) with (shown) and without (7 data points) 40 mM OG. Using the same solutions, the dn/dc plot with OG was repeated (+ OG repeat; 8 data points). 2 mg/ml and 2.5 mg/ml stocks were first made using the appropriate 1x buffers, and then diluted again with 1x buffer to make each Ovalbumin concentration. (C) OG dn/dc measurements from 0–29 mg/ml (11 data points) in 25 mM citrate and 50 mM NaCl, pH 6.0 buffer plus 5% (v/v) glycerol (shown), plain buffer and water. 100 mM (29.24 mg/ml) OG solutions where made from 400 mM OG in water, 2x buffer and water, and decreasing concentrations where made by mixing 100 mM OG with 1x buffer or water. (D) dn/dc measurements of DDM from 0–10 mg/ml in 20mm Hepes, 150 mM NaCl, pH 7.0 buffer (shown) and water (8 data points). 20 mM (102.12 mg/ml) DDM solutions where made from 200 mM DDM in water, 2x buffer and water, and decreasing concentrations where made by mixing 20 mM DDM with 1x buffer and water. The inset tables of each plot show the measured dn/dc (slope), y intercept (calculated solvent dn/c) and the measured dn/dc of the started solvent. The Anton Paar RXA150 refractometer was calibrated using water RI of 1.333690, equilibrated at each concentration for 3–5 min and 30–60 data points automatically collected every 5 sec. Sample volume was 130–150 μl and data collected every 5 sec for each concentration. The standard deviation of each data point was 0.000001.
Support Protocol 2 Purification of Ovalbumin Factor VII for measuring TDA detector response factors
A standard used for detector calibration not only needs a well defined M, dn/dc and dA/dc, but also must exhibit a single and stable SEC Gaussian peak. To insure a homogenous population with good detector signals, Ovalbumin VII is dissolved in TDA buffer at 20 to 30 mg/ml, filtered with a 0.2 μm syringe filter and purified by SEC; the top ~30% of the peak is pooled, quantitated using a spectrophotometer, and yields a purified standard at 4–7 mg/ml.
Solutions
SEC column buffer as described in the Basic Protocol.
Equipment
A temperature-controlled microcentrifuge such as an Eppendorf 5415 R Centrifuge.
SEC column identical to that used for TDA (Basic Protocol).
Isocratic SEC station with absorbance detector, sample injector and fraction collector. The authors currently use a Shimadzu SCL-10Avp system controller, LC-20AD pump, SPD-20A dual wavelength UV/Vis detector, FRC-10A fraction collector, manual 7725i Rheodyne sample injector valve and 0.5 ml PEEK sample loading loop.
Spectrophotometer with a good optical bench. A Shimadzu 2501-PC UV-Vis or Agilent 8453 UV-visible diode array is utilized with a quartz, masked, and 10 mm path cuvette.
Steps
-
Prepare Ovalbumin Fraction VII for purification.
Immediately before SEC purification, weigh protein using an analytical balance, add enough cold TDA buffer to make at least 1.1 ml at ~23 mg/ml, and stir on ice for 5–15 min or until clear. When working with detergent-containing samples, care must be taken not to generate detergent foam. Clarify by centrifugation in a pre-chilled tube for 10 min at 15,000 × g and 4°C.
-
Purify by SEC.
Make two sequential 500 μl injections using a pre-chilled 500 μl Hamilton gas-tight syringe (Figure 11). Collect the top 25–30% of each monomeric peak, and pool the fractions.
Even though one injection will usually produce enough purified sample, two are always made in case problems arise with the first injection. -
Quantitate concentration of pooled fractions using a spectrophotometer.
To insure accuracy, dilute the 3–5 OD sample (10 mm path) from SEC purification in column buffer to obtain a 280 nm signal of ~ 0.1 OD, and repeat 2–3 times to obtain an averaged concentration in mg/ml. Once the baseline is corrected using a blank, auto zero at 600 nm, scan from 600 to 250 nm and measure the maximum OD at 280 nm. Correct the measured OD for its dilution factor and covert to mg/ml using a 0.701 dA/dc; e.g., an Ovalbumin sample with a 0.070 OD 280 nm/ml and dilution factor of 51 (adding 20 μl sample to 1000 μl column buffer) has a concentration of 5.093 mg/ml.
Figure 11.
Purification and quantitation of Ovalbumin Fraction VII used for detector calibration. SEC chromatograms of Ovalbumin Fraction VII in different buffers using a TSK column at 0.6 ml/min. (A) Chromatograms +/−TFA-1 using 20 mM Hepes, 150 mM NaCl, +/− 0.1mM TFA-1, pH 7.0. These Ovalbumin purifications where used to characterize the novel tandem facial amphiphile TFA-1 by TDA (Chae et al 2010, supplemental material). (B) SEC profiles +/− OG (green with OG; blue and red without OG) using 25mM Citrate, 50mM NaCl, +/− 40 mM OG, pH 6.0. Very similar chromatograms have been produced using other detergents such as octyl maltoside (OM), decyl maltoside (DM), DDM, Fos-Choline-12 (FC12) and Fos-Choline-14 (FC14). Four individual absorption spectra from each pooled fraction using dilution factors from 21–51 had differences less than or equal to 0.002 OD 280 nm/ml.
Reagents and Solutions
β-DDM Anagrade (#D310; Anatrace, now Affymetrix-Anatrace). All other detergents are from Anatrace, and Anagrade is used if available. Unless stated otherwise, all detergents are the β isomer.
Ovalbumin Fraction VII (Sigma #A7641-1g).
Both solid reagents, and all other detergent solids, are stored at −20°C under desiccant (e.g., Drierite). To minimize condensation or water absorption, reagents must be brought to room temperature before opening the bottle. All detergent and protein solutions are stored at 4°C.
Commentary
Background Information
SEC coupled with a single UV-Vis absorbance concentration detector is a fundamental tool for the production of pure, optically homogenous and stable membrane protein. Although a Gaussian-shaped SEC optical peak using single (usually 280 nm), dual (280 and 220 nm) or multiple absorption wavelengths does not prove protein homogeneity, it is the simplest measurement for estimating PDC homogeneity (Figure 12).
Figure 12.
Using SEC absorbance detectors for estimating PDC homogeneity. (A) SEC chromatograms at 280 nm of a purified PDC using a SDX column and 20 mM Hepes, 100 mM NaCl, 10 % (v/v) glycerol, 2 mM DTT, pH 7.3 running buffer. The PDC remains monodisperse when dilute (black), concentrated 20x (blue) and concentrated after 1-month storage at 4° (red). (B) TSK 280 nm chromatograms of purified GlpF in 40 mM OG at pH 5 (red), 7 (blue) and 9 (green). Following detergent solubilization and affinity purification, GlpF is composed of two non-reversible oligomeric populations. Once the main peak at pH 5 is collected, it remains monodisperse at all concentrations, crystallized as a homo-tetramer and was used to solve its crystal structure (Fu et al 2000). (C) TSK chromatogram of a PDC in 1 mM alpha DDM using a diode array absorption detector. The normalized 280 nm peak due to absorption by aromatic residues (blue) and the 220 nm peak due to peptide backbone absorption (gray) overlay well. The inset for each panel shows that the injected samples consist of one Coomassie stained SDS-PAGE band. (D) Total peak purity from 200–400 nm shows that the peak is spectrally similar from 32–36 min. The threshold (red trace) defines the limits of integration and the similarity index (blue trace) shows the calculated purity relative to the reference spectrum at peak apex (gray line at 33.8 min).
In addition to being pure, homogenous and stable, the final detergent or detergent-lipid micelle concentration in the PDC sample is another critical parameter for functional assays and crystallization experiments. Excess micelles can sterically inhibit substrate or drug binding, prevent the proper formation of proteoliposomes, and obstruct the growth of well-ordered crystals due to micelle phase separation. Since most detergent micelles co-concentrate with the PDC using molecular weight cut-off filters (the main method for concentrating protein) and many detergents are not dialyzable due to their large micelle size, the majority of concentrated PDC targets contain high levels of detergent. If the detergent micelle concentration is excessive the ability to experiment at low detergent micelle concentration or by adding additional detergents and/or lipids is not possible.
The most common concentration detector used in chemistry is a refractometer, and it measures the concentration of all molecules according to their unique refractive index increment dn/dc (dl/g). By adding a second on-line RI detector, the PDC, micelles and all contaminants are simultaneously monitored, optical and refractive homogeneity measurements are made, and key SEC abilities and limitations for a particular protein-detergent system are determined. Coupled with a bench-top refractometer, it is the second most important detector for the production of quality membrane protein used for crystallization, function and drug binding experiments.
The addition of a third on-line static light scattering detector using right angle (RALS), low angle (LALS), dual RALS and LALS, or many angles (MALS) allows the molar average mass to be measured at every point on the SEC chromatogram, giving precise measurements of the PDC homogeneity by mass and important information on the protein’s oligomeric state in the PDC, the amount of detergent bound, and physical properties of the detergent micelle. The RALS detector, which is valid for essentially all proteins and PDCs, can accurately measure Mavg for molecules that have an average diameter of less than ~30 nm (≪ 1/20 of λ670nm = 33 nm) and therefore scatter light equally in all directions (isotropic scattering). A LALS (less than 10°) detector can measure the mass of molecules of all sizes (isotopic and anisotropic scattering particles), but has a lower signal to noise ratio due to the relatively small size of proteins and PDCs, and it has enhanced sensitivity to large, contaminating particles. A hybrid system can measure the M of all molecules, and uses the appropriate scattering data to maximize signal-to-noise where it is needed. A MALS detector can measure the molecular weight of all particles, and includes the angular dependence of scattering in its calculations (Wyatt, 1993; Folta-Stogniew and Williams, 1999).
The fourth on-line absolute viscometer measures homogeneity by shape and size, and is used extensively in plastic and polymer synthesis and production (Bohdanechy, M. and Kovar, J., 1992; Barth et al., 1994). Using SEC coupled with a refractometer, light scattering and viscometer (La Gatta et al., 2010), polymer concentration, mass, branching, and purity are simultaneously measured. The viscometer is also an old method for measuring hydrodynamic properties of protein biopolymers (Scheraga and Mandelkern 1953). The on-line viscometer as a single detector (Dutta et al 1991) and with triple detection has been used to study soluble protein denaturation (Qian et al., 1997; Ye, 2006), and in tetra detection to characterize antibodies (Hartmann et al., 2004) and substrate-dependent conformational changes (Chenal et al., 2009). Since viscometry had not been applied to membrane proteins and Viscotek (now part of Malvern Instruments) was the only provider at the time, the Viscotek Tetra Detector array was chosen to maximize the potential of SEC. Currently, Malvern and Wyatt Technology Corporation both provide differential viscometers and comparable tetra detector systems but differ in how static LS is measured.
Critical Parameters and Troubleshooting
Define multi-detector goals
Before considering a multi-detector experiment, first define the experimental goals and the desired precision and accuracy; whether it be a crude estimation or meticulous measurement of homogeneity, oligomeric state and detergent optimization of the whole or partial PDC sample. This will dictate what samples, methods and detectors are needed, and how many injections and how much buffer are required. The preferred and optimized Tetra Detection Method described in the Basic Protocol can easily be streamlined to particular goals and experimental styles.
Whole-peak versus partial-peak analysis
Fundamental to accurate quantification using chromatography is to run controls to determine the retention times and concentrations of all components in a sample being studied, and to verify that peaks from different sample components do not overlap. For TDA analysis of the total PDC sample, the whole SEC peak must be examined. When different constituent peaks of a sample overlap, partial peak analysis using a subset population can be performed but results are only valid if the molecular weight is constant throughout the whole peak. Be aware that for partial peak analysis, the results will vary and be highly dependent on the integration limits and baselines, with the variation in results being directly proportional to the heterogeneity within the integration limits (Figure 13).
Figure 13.
Measuring Sample Homogeneity (Mw/Mn) to optimize peak collection. TDA analysis of a provided monomeric PDC in DDM. Following the last SEC step for purification, the sample was concentrated 25x using a 100 kDa spin filter with intermittent manual mixing during concentration. (A) TSK TDAgram of a 70 μl injection (at ~10 mg/ml) using 20mM NaCl, 100mM NaCl, 0.33 mM DDM, pH 7 column buffer at 0.5ml/min. The profile is composed of single UV peak with an insignificant aggregate (large LS, no DP, small RI, and very small UV) at ~11 ml retention. The sample is 85 % monodisperse (goal is >95%). Excess DDM located at the backside of the RI peak is estimated to be <0.1 mg/0.07ml or 2.8 mM. (B) MABi at every 5 Hz slice overlaid onto the RIABi trace. Integration limits (represented by solid black line) are progressively narrowed going from left to right panels, and represent the Whole complete protein UV peak profile, Large center portion of the complete UV protein profile without the tails and shoulders and Small center peak, respectively. (C) Table of the TDA results. The small centered fraction from 13.9 to 15.1 ml is homogenous. Using the provided dA/dc (method for determination unknown), which is 28% higher than the theoretical dA/dc, the oligomeric state is 1.03 monomers/PDC, as expected. If the theoretical dA/dc was used, the change in CA, CB, MAB, MA, MB, IV and Rh would change respectively by 41, −11, −3, 38, −12, 2 and 0 %. The % error in averaged Ks from 5 calibration standard injections ranged from 0.1 to 0.4. (D) dn/dc plot of DDM from 0–2 mg/ml (N = 10; 0–100 μl injections of 40 mM DDM). The measured dn/dc of DDM was 0.1460 ml/g.
In general, samples which exhibit a Gaussian absorbance and RI peaks yield Gaussian LS and DP peaks and are ideal for whole-peak analysis; if this is not the case, further purification of the PDC is required before performing TDA. If the PDC has excess micelles and the PDC and micelle RI peaks overlap, the excess micelles should be removed (Figure 14) or exchanged to a more amenable detergent (Figure 15). However, multi-detector analysis of PDCs with overlapping protein oligomeric states or overlapping protein and micelle peaks is still useful, and will ultimately lead to producing an ideal PDC sample for TDA and downstream experiments.
Figure 14.
Using TDA and cation exchange chromatography to minimize DDM detergent concentration while concentrating protein and maintaining homogeneity. All TDA profiles of this PDC were obtained using a SDX 200 10/300 GL column in 2 mM DDM. (A) TDAgram after being concentrated 58x using a 50kDa XM filter and stirred cell. The PDC remains homogenous (Gaussian UV, RI, LS and DP peak) and is pure (inset). The PDC sample contains 61 mM excess DDM micelles, which represent 47% theoretical removal; only 25% was removed using an YM 50kDa spin filter. It produced major detergent phase separation during crystallization experiments and yielded no crystals. (B) TDAgram after being concentrated using S cation exchange and 2 mM β-DDM buffer. The PDC eluted from S at 9 mg/ml (inset; red trace is the NaCl step gradient; blue trace is the 280 nm absorbance profile) and contains −1.5 mM DDM (negative RI peak). Due to a low detergent: protein ratio the PDC forms larger oligomers. No detergent phase separation or crystals were formed during crystallization trials. (C) TDAgram after being concentrated to 7.5 mg/ml using S cation exchange and 4 mM β-DDM buffer (inset). The PDC remains homogenous and contains no excess detergent micelles. No crystals or detergent phase separation were produced from the first round of crystallizations. This project is now worthy to enter comprehensive crystallization trials and functional studies. (D) Table of TDA results using 7 independent preparations (defined by number in column 1 and 8 comprehensive TDA experiments using whole (when possible) and partial peak analysis; C0, Cconc and Cfinal represent starting concentration, concentrated by Mwt cut-off filters, and concentrated plus dialyzed post SEC samples, respectively, and Sconc is post concentration using cation exchange. Measured DDM dn/dc values, which were dependent on running conditions, ranged from 0.133 to 0.157 ml/g and the micelle Mavg ranged from 80–90 kDa. For analysis of the protein component, the theoretical dA/dc of 1.30 OD280nm/mg and dn/dc of 0.185 ml/g were used.
Figure 15.
Detergent concentration minimized using different detergent isomer. TDAgrams of a PDC in 0.5 mM DDM post 100 kDa spin concentration in β-DDM and α-DDM. (A) In β-DDM the PDC is homogenous (peak 1 with UV signal in purple), β-DDM micelles concentrate on 100 kDa (peak 2 with large RI in red and DP signal in blue), and extreme phase separation due to the ~ 20 mM excess β-DDM is obtained during crystallization trials. The LALS signal is shown in black. Interestingly, when repeated using a pure β-DDM control, β-DDM micelles did not concentrate. The inset shows that the protein is pure by SDS-PAGE. (B) When purified in α-DDM, α-DDM micelles barely concentrate (~ 2 mM excess) on a 100 kDa filter (peak 3; no UV and minimal RI and RALS), the PDC is mostly homogenous (peak 2) with a small void (peak 1, minimal 280 nm and maximum light scattering signal), and no phase separation dis observed during crystallization.
By insuring that the sample buffer is as identical as possible to the SEC column buffer, small molecules at slightly different concentrations (e.g., NaCl which has a dn/dc of 170 ml/g, and glycerol) are removed in the inclusion limit and will not interfere with peak analysis.
Obtaining stable baseline
A fundamental problem in using RI, LS and DP detectors is obtaining stable baselines, and being able to reside and operate at or near 4°C where PDCs are most stable. Performing maintenance procedures as outlined by the manufacturer is essential. It is crucial that bacteria, fungi and molds are not allowed to grow in the cold box and ultimately on the detector electronics or in the solvent pathways. The cold box should be routinely cleaned with bleach, and equipment thoroughly inspected for contamination, leaks, condensation and rust. Inlet solvent filters and waste containers should be routinely replaced. Detector lamps and filters, and pump seals and check valves should be periodically replaced. It is good practice to extensively (1–2 days) wash the detectors with water after every experiment, and every month with 10% methanol; however a stronger cleaning agent may be required, but should only be used in consultation with the manufacturer. To minimize signal spikes, solvents must be thoroughly degassed and in-line particle filters routinely changed. Without a column, an increase in backpressure indicates that in-line filters and tubing should be changed, while a decrease in pressure reveals leaky fittings that must be tightened or replaced. Backpressure fluctuations of the pump indicate a leaky piston seal and/or scratched piston that must be replaced. A rolling RI signal indicates that air is in the column buffer, and an in-line degasser must be installed or buffer further degassed under vacuum; but if the viscometer’s DP is stable and displayed inlet pressure (IP; pressure difference between flow in and flow out) constant, air is in the RI detector and it must be properly purged. If the IP signal is unstable, the delay column in the viscometer must be replaced. The IP transducer signal must be zeroed with the flow off and must not exceed its maximum output of 14.5 psi or internal leaks in the viscometer will develop. Optimal care and maintenance should be taken very seriously, and it is best to have one person responsible for these operations. Even with careful attention, unstable baselines can be detrimental, and in some cases can be corrected while setting the baseline and integration limits (Figure 16).
Figure 16.
Working with anomalous sample baselines to determine oligomeric state and % bound detergent. Three identical back-to-back PDC samples were injected (black, green and red traces are respectively the 1st, 2nd and 3rd runs) onto a SDX column running 50 mM Tris, 50 mM NaCl, 2 mM βME, 2mM DM, pH 8.3 buffer. A normalized decyl maltoside (DM) injection (0.96 mg in water) is shown in blue. (A) Overlay of the three RI traces show good reproducibility and reasonable baselines. A minor excess DM peak (~ 0.002 μg) and then a broad peak representing the mismatch in column and sample buffer follow the first PDC peak. (B) Overlay of the three LS profiles with fitted and corrected baseline. The anomalous LS baselines from 6.5 to 12.5 ml have no RI signal and are declining. Using these 4 traces and 3 calibration standards, the PDC is a dimer with about 65% detergent by weight (monomers and % detergent for the runs are 2.05/35.7%, 2.04/35.6% and 1.89/33.2%, respectively. Without multiple runs, there would have been no confidence in the measured oligomeric state.
Choosing a SEC column and running buffer
Key to SEC analysis is empirically finding a column and detergent buffer system that is compatible with the PDC of interest. The sample must not bind, and it should remain stable as a single Gaussian peak over time and at different protein concentrations. All buffer components must be considered and systematically studied with different columns to find the optimum match (Figure 1). Key SEC column parameters for any particular PDC and running buffer that must be considered are bead chemistry (which dictates buffer compatibility such as pH, and protein compatibility), particle and pore size (determines inclusion and exclusion limits; plate count), column length (resolution proportional to length), injection volume and solvent viscosity (resolution inversely proportional to injection volume and viscosity). Matrices composed of silica-, sugar- or polymer-based chemistries should all be considered. Rigid silica-based columns are our first choice but have a pH restriction of 3–7.5; soft sugar-based columns such as SDX in general have poorer resolutions and lower maximum pressure limits, but are stable between pH 3–12 and tend to bind less protein. For the 7.5 mm diameter G3000SW TSK column (300 Å pore size), the xl version is not recommended; the improved resolution gained by using a smaller particle size (5 vs.10 μm) is lost due to the shorter column length (30 vs. 60 cm), and the smaller particle size is more easily compressed with viscous membrane protein running buffers. Newer silica-based columns from Sepax Technologies, such as the SRT SEC-300 (7.8 mm × 60 cm; 10 μm particle size; 300 Å pore size), have excellent resolution, are stable from pH 2–8.5, and can tolerate much higher pressures; their Nanofilm SEC-250 columns (5 μm particles) appear to be popular among some membrane protein labs.
Choosing a standard for detector calibration
UV-Vis, RI and LS detectors are not absolute and require calibration for sample quantitation. Any standard used for measuring their detector response factors must have a well-defined mass, dn/dc and dA/dc, and must be soluble, homogenous and stable in column buffer and have no interactions with the column matrix or buffer and sample components. Lysozyme was an initial choice, but it was not stable in some detergents, and its small volume of hydration (Vh) makes it elute near the inclusion limit and therefore its peaks are skewed by small differences in buffer components such as salts and osmolytes. Bovine serum albumin is popular for the analysis of soluble protein, but is not used since it binds hydrophobic molecules such as detergents (Korepanova and Matayoshi, 2012). The most popular standard used in multi-detection analysis of membrane protein is Ovalbumin. It has been found to be stable for at least 2 days in many different detergent buffers, but its defined mass of 44,300 Da is not absolute. Values ranging from 42.75 to 49.9 kDa have been utilized, and purified Ovalbumin Fraction VII is composed of 8 different masses presumable due to variations in glycosylation ranging from 44,197 to 44,971 Da (Figure 17). Even though this ~1% variation in molecular weight is insignificant, other standards that are compatible with more types of detergent and detergent-lipid micelles or bicelles must be explored.
Figure 17.

LCMS of purified Ovalbumin Fraction VII used for TDA calibration. Mass spectrometry (kindly provided by David Maltby at the Mass Spectrometry Facility at UCSF, Mission Bay) of the SEC purified protein was obtained on an ABI QSTAR XL mass spectrometer, interfaced with an ABI 140B HPLC system. The column used was a 1 × 150 mm Vydac C4, 5 um particle size, 300 angstrom pore size. Solvent A was 0.1 % formic acid in water and solvent B was 0.1 % formic acid in acetonitrile. The sample was injected with the column at 5 % solvent B and a gradient was run up to 70 % solvent B in 35 minutes. Data was averaged over the elution time of the protein peak using the standard Analyst software of the QSTAR. Deconvoluted mass data were also calculated using the Analyst software. Ovalbumin was prepared according to Supporting Protocol 2 using a TSK column, 50 mM Ammonium Acetate pH 7.0 column buffer and a 0.6 ml/min flow rate. Two 500 μl of 4.4 mg/ml was injected yielding a fraction of 1.7 mg/ml.
Measuring RIsol and dn/dc using a bench top refractometer
For on-line multi-detector analysis of PDCs, data can be analyzed using published values for detergent and protein dn/dc, but it is best to measure dn/dc independently for improved accuracy (Kendrick et al., 2001). Reported dn/dc values vary and rarely state the sample source, purity, mass, and how the concentration was determined, how many data points were used or which refractometer, temperature, wavelength or calibration was used, or compare the measured versus calculated buffer dn/dc (which indicates the quality of measurements). Protein dn/dc is dependent on its buffer, and can range from 0.153 to 0.272 for hen egg white lysozyme using a bench top refractometer (Ball and Ramsden, 1998) and from 0.186 in 20 mM NaCl salt to 0.171 in 2 M NaCl for ovalbumin using on-line RI and UV ratios (Wen and Arakawa, 2000). Our own RXA156 dn/dc measurements in different solvents for ovalbumin, DDM and OG (Figure 9) ranged respectively from 0.171 to 0.19, 0.144 to 0.195, and 0.143 to 0.151.
All RIsol and dn/dc measurements should be made using a precise bench top digital refractometer calibrated with water at the temperature used for sample measurements (Figure 8). Precise dn/dc measurements can be made on-line (Figure 5B and 13D), but accuracy is dependent on many variables (calibration standard, accurate solutions, injection volumes, SEC, peak purity, integration limits), where a bench top refractometer only depends on the well-defined water calibration and the skill to make accurate solutions.
Differences in measured RIsol, which vary in the 4th and 6th decimal place respectively for analog and digital bench top instruments, are insignificant; but values have changed up to 2% between the two types of refractometers, and up to 17% in dn/dc measurements. Bench top RI measurements can be corrected for other temperatures and wavelengths (water varies in the 4th significant figure from 4–12°C, and in the 3rd significant figure from 589.26–667.81 nm); but these corrections have no effect on dn/dc (slope of g/ml vs. nD) measured at the bench, or on-line using SEC since the correction is automatically applied in KRI by using the RIsol input during the TDA calculation of detergent dn/dc. However, any correction or error in RIsol affects the calibration constants KLS and KRI, detergent dn/dc, micelle M and Rh, and all the PDC properties (Table 2).
Obtaining dA/dc
Since dA/dc is required to measure the UV detector response factor KUV and the membrane protein concentration CAi, it is the best way to also measure the concentration of the calibration standard and the PDC. However, obtaining an accurate dA/dc for any protein is difficult, and using amino acid analysis is probably the best route. In two attempts to measure the dA/dc of ovalbumin using Edman degradation outsourced to a core facility, precision among three measurements was excellent, but the difference between each data set was large and therefore neither was used. The next best route, and by far most popular, is using a theoretical dA/dc based on the amino acid sequence; but be aware that the differences between measured and theoretical can range from 16 to −17 % (Pace at al 1995), and even larger in some cases (Figure 13).
Precision and Accuracy
Multiple injections must be performed since reproducibility provides reliability and confidence in the instruments and the results. Measurements should be made a number of times (N) and precision computed from the data sets’ variation from the average or standard deviation (SD), relative error between measurements or error in average (=SD/sqrt N), and absolute error or % error in average (=(relative error/average) × 100). A predictive accuracy, which does not take into account the errors in the input parameters, should also be calculated when a strong prediction (e.g., protein mass from both its gene and mass spectrometry) is known using the % average error (= ((average-predicted)/predicted) × 100). As seen in Figures 4, 5, and 6, Viscotek’s TDA is highly precise when accurate injections and solutions are made; even measurements over many months using seven independent preparations are respectable (Figure 14D). With stable baselines, amendable SE column and buffer, and properly working TDA workstation, precision will depend on sample quality, accurate injection volumes and accurately prepared solutions.
Even though valid estimates can be made and are usually workable, especially in comparative studies, the goal should be to minimize the inherent error in each measurement. Meaningful TDA conclusions require a degree of accuracy for each measurement, and propagating those uncertainties throughout all parameters and final results. Minimally, all input parameter values used for any final result should be stated (e.g., Figure 5B and 7B) and their method(s) of measurement defined. Since the TDA results are weighted averages according to slice intensity (Figure 3B) and the equations are multi-termed with internal secondary parameters (exponents less than 1, negative constants and constants less than 1), error propagation (or values) cannot be determined accurately using the basic equations in Figure 2 and 3. For a simple estimation using an experimental data set, Table 2 lists all the required TDA input parameters grouped according to analysis step, and shows how a single change in input parameter affects the output parameters in that step.
The easiest parameters to measure, and therefore the parameters that introduce the smallest theoretical errors, are injection volume, injection concentration and RIsol. There should be no error in the input RIsol when using a precise bench top refractometer. Errors in injection volume should be insignificant if performed properly and can be easily seen when comparing whole-peak areas of identical injections or plotting peak areas versus injection weights (Figure 5B). dA/dc is the most difficult parameter to measure, and directly affects the measurement of the protein or PDC dn/dc.
Error propagation of a single input parameter or multiple input parameters throughout all the calculations can have significant effects on many properties, some which are additive and some which cancel each other out. For example, a change in Ovalbumin dn/dc affects all parameters: it directly affects a 1) KRI which directly affects detergent dn/dc, all micelle properties, and PDC M, IV and MB; the affected detergent dn/dc in turns effects Micelle M and IV, and all PDC properties except Rh and 2) directly affects KLS which affects micelle M and Rh, and all PDC properties except IV.
Fraction collection
Automated fraction collection during TDA is generally not performed since large spikes can be introduced electronically during activation or by slight pressure changes when the output flow backpressure is momentarily changed. However, manual fraction collection using extreme care can be accomplished by manually placing a tube or vial under the clamped out-flow tubing while not moving or disturbing the exit tubing in any way. The door to the cold box must be carefully opened and closed, and the temperature of the cold box and consequently the TDA must not be allowed to rise.
Anticipated Results
Starting with a pure and stable PDC and SEC system where the micelle and PDC peaks are baseline resolved (Figure 7A), a complete TDA analysis as shown in Figures 4, 5, and 6 can be expected. However, when the PDC and excess micelles co-elute, homogeneity and micelle concentration can be estimated by dual (Figure 7A,B) or tetra detection (Figures 13, 14, 15A, 16), but with high concentrations of detergent (Figure 7C and 15A) complete TDA analysis is not ideal and the excess detergent is best removed by ion exchange chromatography (Figure 14) or in some cases by changing detergent (Figure 15B).
When using a precise bench top digital refractometer with pure and accurate stock solutions and serial dilutions, dn/dc liner plots with identical buffer dn/dc and Y-intercept are always produced (Figure 9). For purification of the Ovalbumin Fraction VII calibration standard, SEC chromatograms as shown in Figure 10 are expected if the standard is pure, soluble, stable and exhibit no column interactions.
Time Considerations
A full and complete TDA analysis of detectors, calibration standard, micelle and PDC takes a total time of roughly 1 week with about 35 hours of hands-on time shared between two people. Total hands-on time is dependent on number of sample injections, column flow rate and volume, and degree of equipment automation. Data is analyzed and summarized on a different PC during data acquisition. Using quality samples (pure protein; single Gaussian protein peaks which do not overlap with excess micelle peak), ideal data sets for a new PDC/micelle sample include approximately 5 calibration, 8 detergent and 5 PDC injections. If executed properly with good statistics, the experiment will not have to be repeated. The approximate day-to-day efforts can be broken down as follows:
Day 1 and 2: Pre-equilibrate 12–24 hrs with water to insure system is working properly.
Day 2 and 3: Equilibrate system overnight with column buffer at reduced flow rate (12 hrs).
Day 3, early: Prepare calibration standard on second chromatography station while TDA station continues to equilibrate at maximum flow rate (4 hrs).
Day 3: Inject calibration standards (5–7 hrs).
Day 4: Inject detergent standards (7–9 hrs).
Day 5: Inject purified and stable PDC (5–7 hrs).
Day 5, 6 and 7: Clean and properly store TDA system.
Asking a subset of questions using dual detection (UV and RI) usually requires little extra time since it is performed during routine purification and characterization procedures, and will help insure that the sample is ideal for TDA.
Acknowledgments
This work was supported by the Membrane Protein Expression Center (NIH Roadmap grant P50 GM073210) and Center for Structure of Membrane Proteins (1U54GM094625).
Footnotes
Internet Resources
http://www.malvern.com/ and http://www.wyatt.com/
Web sites for information on the technology, techniques, schematics, instrument care, and applications for tetra detector arrays and analysis.
Web site for information on bench top refractometers.
http://www.tosohbioscience.com/, http://www.gelifesciences.com/ and http://www.sepax-tech.com/
Web sites for information on TSK, SDX, SRT and Nanofilm SEC columns.
http://www.affymetrix.com and http://avantilipids.com/
Web sites for information on detergents and lipids.
Literature Cited
- Ball V, Ramsden JJ. Buffer dependence of refractive Index increments of protein solutions. Biopolymers. 1998;46:489–492. [Google Scholar]
- Barth HG, Boyes BE, Jackson C. Size exclusion chromatography. Analytical Chemistry. 1994;6(12):595R–620R. doi: 10.1021/ac00084a022. [DOI] [PubMed] [Google Scholar]
- Bohdanechy M, Kovar J. Viscosity of Polymer Solutions. Elsevier Scientific Publication; Amsterdam, Oxford, New York: 1982. [Google Scholar]
- Bohidar H. Light scattering and viscosity study of heat aggregation of insulin. Biopolymers. 1998;45:1–8. doi: 10.1002/(SICI)1097-0282(199801)45:1<1::AID-BIP1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Chae PS, Gotfryd K, Pacyna J, Miercke LJW, Rasmussen SG, Robbins RA, Rana RR, Loland CJ, Kobilka B, Stroud RM, Byrne B, Gether U, Gellman SH. Tandem facial amphiphiles for membrane protein stabilization. Journal of the American Chemical Society. 2010;132(47):16750–2. doi: 10.1021/ja1072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenal A, Guijarro JI, Raynal B, Delepierre M, Ladant D. RTX calcium binding motifs are intrinsically disordered in the absence of calcium: implication for protein secretion. Journal of Biological Chemistry. 2009;284(3):1781–1789. doi: 10.1074/jbc.M807312200. [DOI] [PubMed] [Google Scholar]
- Dutta PK, Hammons K, Willibe B, Haney MA. Analysis of protein denaturation by high-performance continuous differential viscometry. Journal of Chromatography. 1991;56:113–121. doi: 10.1016/s0021-9673(01)89241-4. [DOI] [PubMed] [Google Scholar]
- Fish WW, Reynolds JA, Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. Journal of Biological Chemistry. 1970;245(19):5166–8. [PubMed] [Google Scholar]
- Folta-Stogniew E, Williams KR. Determination of Molecular Masses of Proteins in Solution: Implementation of an HPLC Size Exclusion Chromatography and Laser Light Scattering Service in a Core Laboratory. Journal of Biomolecular Techniques. 1999;10:51–63. [PMC free article] [PubMed] [Google Scholar]
- Frigon RP, Leypoldt JK, Uyeji S, Henderson LW. Disparity between Stokes radii of dextrans and proteins as determined by retention volume in gel permeation chromatography. Analytical Chemistry. 1983;55(8):1349–54. doi: 10.1021/ac00259a037. [DOI] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM. The Structure of a Glycerol Conducting Channel Reveals the Basis for its Selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Hartmann WK, Saptharishi N, Yang XY, Mitra G, Soman G. Characterization and analysis of thermal denaturation of antibodies by size exclusion high-performance liquid chromatography with quadruple detection. Analytical Biochemistry. 2004;325(2):227–39. doi: 10.1016/j.ab.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Haney MA. The differential viscometer. I. A new approach to the measurement of specific viscosities of polymer solutions. II. On-line Viscosity Detector for Size-Exclusion Chromatography. Journal of Applied Polymer Science. 1985;30:3023–3049. [Google Scholar]
- Hayashi Y, Matsui H, Takagi T. Membrane protein molecular weight determined by low-angle laser light-scattering photometry coupled with high-performance gel chromatography. Methods Enzymology. 1989;172:514–28. doi: 10.1016/s0076-6879(89)72031-0. [DOI] [PubMed] [Google Scholar]
- Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, Miercke LJ, Stroud RM. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc Natl Acad Sci U S A. 2009;106(18):7437–42. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick BS, Kerwin BA, Chang BS, Philo JS. Online size-exclusion high-performance liquid chromatography light scattering and differential refractometry methods to determine degree of polymer conjugation to proteins and protein-protein or protein-ligand association states. Analytical Biochemistry. 2001;299(2):136–46. doi: 10.1006/abio.2001.5411. [DOI] [PubMed] [Google Scholar]
- Korepanova A, Matayoshi ED. HPLC-SEC Characterization of Membrane Protein-Detergent Complexes. Current Protocols in Protein Science. 2012:29.5.1–29.5.12. doi: 10.1002/0471140864.ps2905s68. [DOI] [PubMed] [Google Scholar]
- Kunji ERS, Harding M, Jonathan P, Butler G, Akamine P. Determination of the molecular mass and dimensions of membrane proteins by size exclusion chromatography. Methods. 2008;46(2):62–72. doi: 10.1016/j.ymeth.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Maezawa S, Hayashi Y, Nakae T, Ishii J, Kameyama K, Takagi T. Determination of molecular weight of membrane-proteins by the use of low-angle laser-light scattering combined with high-performance gel chromatography in the presence of a non-ionic surfactant. Biochemica et Biophysica Acta. 1983;747:291–297. doi: 10.1016/0167-4838(83)90108-5. [DOI] [PubMed] [Google Scholar]
- La Gatta A, De Rosa M, Marzaioli I, Busico T, Schiraldi C. A complete hyaluronan hydrodynamic characterization using a size exclusion chromatography–triple detector array system during in vitro enzymatic degradation. Analytical Biochemistry. 2010;404:21–29. doi: 10.1016/j.ab.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Newby ZER, O’Connell JD, III, Gruswitz F, Hays FA, Harries WEC, Harwood IM, Ho JD, Lee JK, Savage DF, Miercke LJW, Stroud RM. A general protocol for the crystallization of membrane proteins for X-ray structural investigation. Nature Protocols. 2009;4(5):619–637. doi: 10.1038/nprot.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Science. 1995;4(11):2411–23. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka M. Universal calibration of gel permeation chromatography and determination of molecular shape in solution. Analytical Biochemistry. 1987;162:47–64. doi: 10.1016/0003-2697(87)90009-1. [DOI] [PubMed] [Google Scholar]
- Qian RL, Mhatre R, Krull IS. Characterization of antigen-antibody complexes by size-exclusion chromatography coupled with low-angle light-scattering photometry and viscometry. Journal of Chromatography. 1997;787(1–2):101–9. doi: 10.1016/s0021-9673(97)00666-3. [DOI] [PubMed] [Google Scholar]
- Scheraga HA, Mandelkern L. Consideration of the hydrodynamic Properties of Proteins. Journal of Chemical Physics. 1953;75:179–184. [Google Scholar]
- Slotboom DJ, Duurkens RH, Olieman K, Guus BE. Static light scattering to characterize membrane proteins in detergent solution. Methods. 2008;46:73–82. doi: 10.1016/j.ymeth.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Straat HW, Forrest JW. The Accuracy Requirements in Fifth Place Refractometry. Journal of the Optical Society of America. 1939;29:240–247. [Google Scholar]
- Strop P, Brunger AT. Refractive index-based determination of detergent concentration and its application to the study of membrane proteins. Protein Science. 2005;14:2207–2211. doi: 10.1110/ps.051543805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T. Application of low-angle laser light scattering detection in the field of biochemistry: Review of recent progress. Journal of Chromatography. 1990;506:409–416. [Google Scholar]
- Tilton LW, Taylor JK. Refractive index and dispersion of distilled water for visible radiation, at temperatures from 0 to 60°C. Journal of Research of the National Bureau of Standards. 1938;20:419–477. [Google Scholar]
- Wen J, Arakawa T. Refractive index of proteins in aqueous sodium chloride. Analytical Biochemistry. 2000;280(2):327–9. doi: 10.1006/abio.2000.4524. [DOI] [PubMed] [Google Scholar]
- Wyatt PJ. Light scattering and the absolute characterization of macromolecules. Analytica Chimica Acta. 1993;272:1–40. [Google Scholar]
- Ye H. Simultaneous determination of protein aggregation, degradation, and absolute molecular weight by size exclusion chromatography-multiangle laser light scattering. Analytical Biochemistry. 2006;356(1):76–85. doi: 10.1016/j.ab.2006.05.025. [DOI] [PubMed] [Google Scholar]