Machesky studies the role of the actin cytoskeleton in cancer invasion and metastasis.
Machesky studies the role of the actin cytoskeleton in cancer invasion and metastasis.
The actin cytoskeleton is the main driver of cell motility, an essential function of normal body tissues. However, the same processes are also at the heart of cancer cell metastasis and invasion. Understanding the normal regulation of actin-dependent cell motility and how this is altered in cancer cells should leave us better equipped to tackle the disease.

Laura Machesky
PHOTO COURTESY OF THE BEATSON INSTITUTE
Laura Machesky’s research is aimed at uncovering these mechanisms. Her work on the Arp2/3 complex, which nucleates the growth of new actin filaments, has elicited important mechanistic insights into the complex’s function (1) and the upstream pathways that regulate it (2, 3). Now her lab is looking at how these pathways are modulated during development and in invading cancer cells (4, 5). We called her at her lab at the UK’s Beatson Institute to discuss her work.
DEMYSTIFICATION
What are you passionate about?
If we’re talking about my work, I love spending time looking for new connections between systems, either in the literature or in various databases. I’m passionate about finding out new things and looking for new ways of connecting ideas that we were already aware of but didn’t know how to fit together.
“It was just an amazing atmosphere to be a student in.”
Outside the lab, I’m passionate about learning, education and discovery. I have 12-year-old twin girls and a 10-year-old boy, and I love the fact that they are interested in what I do at work. I love that they want to know about how cells move and how this relates to cancer. That’s not because I necessarily want my kids to become scientists, but I think that understanding science and research helps them understand how the world works. That helps make the challenges one faces in life less bewildering and confusing.
Do you discuss your own work very much with your family?
My husband, Robert Insall, is working in a very closely related field. He works on cell migration, mainly in Dictyostelium, whereas I work with mammalian cells and mice. So we actually do a lot of work together, and we talk about it at home. We don’t necessarily launch into a lot of jargon at the dinner table, but we do talk about our research sometimes, so our kids get to hear about it.
NUCLEATION
You did your PhD with Tom Pollard…
At first I thought I wanted to be a crystallographer, but after doing some lab rotations in graduate school at Johns Hopkins I realized that I more enjoyed biochemistry and cell biology. I joined Tom’s lab based on my rotation with him. It was an interesting time to be in the lab because Tom was very ill. He was in the hospital, and he would sneak out to do journal clubs and lab readings. He would say, “Don’t tell the nurses I’ve sneaked out!” Despite his illness, he was just full of energy, positive, and supportive. I knew that was the lab I wanted to work in. When I look back on my career, I think my time in Tom’s lab was probably the most fun that I ever had as a scientist. It was just an amazing atmosphere to be a student in.
I was doing biochemistry in Acanthamoeba, trying to figure out how the actin cytoskeleton worked. My main project concerned profilin’s interactions with membranes and lipids. As part of that project, we put profilin on a column to look for binding proteins and came up with the Arp2/3 complex. This happened about the same time the genes for the actin-related proteins Arp2 and Arp3 were cloned in yeast, so we knew that these unconventional actins existed. But nobody had any idea about what they really did yet. Ours was the first description of the Arp2/3 complex.
At the time, many labs were looking into how actin filaments get initiated or nucleated. We knew that could be a point where the cell might want to control actin dynamics. Arp2/3 was one of the first factors found to nucleate actin and leave a filament’s fast-growing end free for polymerization. Later, it became clear that the Arp2/3 complex and WASP family proteins cooperate to initiate branching growth of new actin filaments from the side of existing polymers.
I continued to work on Arp2/3 after I finished my PhD. I was passionate about it and wanted to find out what it did. For my postdoc, though, I decided I wanted to learn a bit more about Dictyostelium because I thought it would be useful to be able to do genetics—which I couldn’t do in the amoeba.
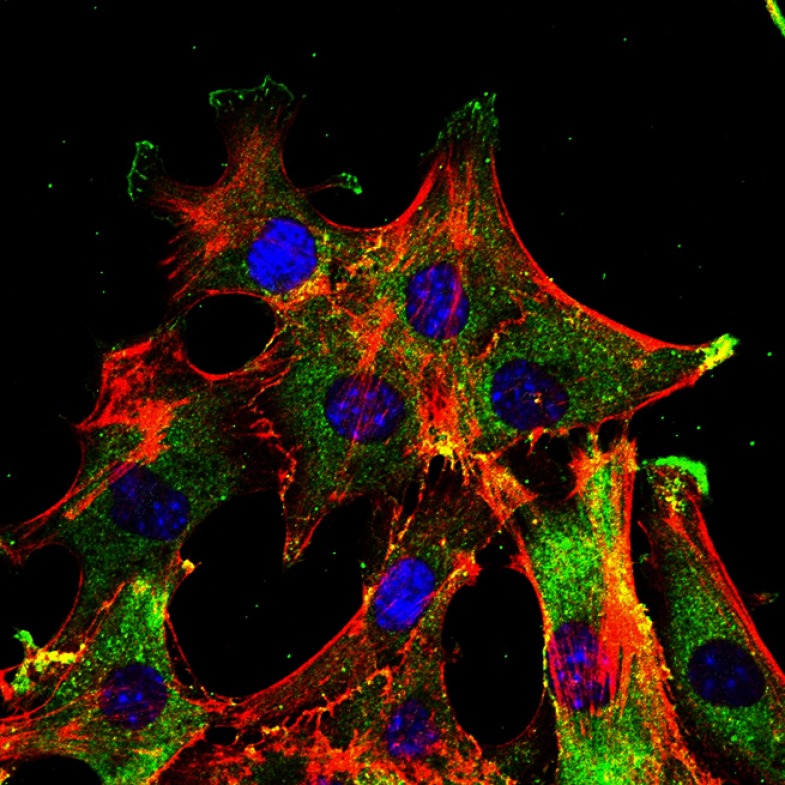
Pancreatic cancer cells (nuclei in blue, cortactin in green, actin in red) invading Matrigel.
IMAGE COURTESY OF AMELIE JUIN, MACHESKY LAB
So you moved to Cambridge…
That was partly because I wanted to go abroad and have a few years of life experience. And then I ended up staying over here. [Laughs]
I learned a lot about cloning and DNA work during that postdoc, but then I went to a Dictyostelium conference and heard a talk by Alan Hall and was just blown away. His lab had discovered that small GTPases in the Rho family were major regulators of actin organization and actin dynamics. I thought, “I have to work on this!” I up and left my postdoc in Cambridge, went to Alan’s lab in London, and started working to try to find a connection between Rho GTPases and the Arp2/3 complex.
I had met Robert, my husband, at Johns Hopkins, and he had also come to London. Together we published a paper showing that the WASP-related protein Scar/WAVE activates the Arp2/3 complex. And we hypothesized that small GTPases like Cdc42 and Rac could regulate the ability of Scar/WAVE and WASP to activate Arp2/3. We collaborated with Tom on the biochemistry showing that the Scar/WAVE and WASP proteins activate Arp2/3-mediated actin nucleation.
We were working with Arp2/3 when no one else was studying it, and then suddenly there were hundreds of posters on Arp2/3 and WASP proteins at cell biology meetings. It was so cool to have our work validated and extended into new areas by so many other labs and to see their excitement about it.
I applied for and received a career development fellowship that allowed me to stay a while longer in Alan’s lab to pursue more of my independent work on Arp2/3. Then I applied to the University of Birmingham. They offered me lab space and a proper job and offered Robert the same, so we were lucky to solve the two-body problem that way.
BRANCHING OUT
Arp2/3 has roles in cell motility but also other processes…
Yes, that’s right. Three out of the five WASP family proteins that we have characterized to any extent are found on vesicles or on internal membranes in the cell. For example, we’ve studied actin nucleation by WASH, a vesicle-associated WASP family protein. When we first started working on WASH, we were surprised that it didn’t seem to have any role in 2D cell migration. But we noticed that it localized to vesicles inside the cell and started wondering what it was doing there. Then we had another surprise, which was that knockdown of WASH did impair migration in a 3D environment. To us, that smelled like trafficking. So together with Jim Norman at the Beatson Institute we looked at integrin trafficking, and we found that WASH was important for transporting α5 integrin when cells invade into 3D matrices. We think that WASH nucleates actin on vesicles that are trafficked into invading pseudopods and are used, at least partially, to help cells remodel the matrix and make their adhesions function properly. We’re still actively working on which cargos are found in WASH vesicles and are delivered by them to the plasma membrane.
“I made a conscious decision to work on invasion and 3D migration.”
Machesky and her children exploring the countryside together.
PHOTO COURTESY OF ROBERT INSALL
What got you interested in the involvement of the actin cytoskeleton in invasive behavior?
When I moved from Birmingham to the Beatson Institute for Cancer Research in 2007, I made a conscious decision to work on invasion and 3D migration. We had found that there was a lot of Arp2/3 and a lot of N-WASP in invadopodia. That made me think that invadopodia may be specialized structures where Arp2/3 was doing something really interesting. I also started working on the actin-bundling protein fascin at the same time. Fascin isn’t expressed in epithelial cells, but it is overexpressed in many types of cancer and seems to be specifically associated with metastasis and invasion. We found that fascin is present in invadopodia, where we think it acts to stabilize the actin cytoskeleton.
Right now about half our time is spent working with mouse cancer models. We’re looking at the role of fascin and N-WASP in pancreatic and colorectal cancers. We’re also studying the importance of Rac, Scar/WAVE, and Arp2/3 in the migration of melanoblasts through the epithelium in the mouth epidermis, in the hope that this will lead to insights into cancer invasion and metastasis in melanoma. These are rather ambitious, long-term projects, but a nice feature of working at the Beatson is that we can do these mouse experiments that might be too expensive and long-term to do if I were working in a university lab.
References
- 1.Machesky, L.M., et al. 1994. J. Cell Biol. 127:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machesky, L.M., and Insall R.H.. 1998. Curr. Biol. 8:1347–1356. [DOI] [PubMed] [Google Scholar]
- 3.Machesky, L.M., et al. 1999. Proc. Natl. Acad. Sci. USA. 96:3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li, A., et al. 2010. Curr. Biol. 20:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zech, T., et al. 2011. J. Cell Sci. 124:3753–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]