Long-range signaling by Wingless in the Drosophila ovary requires the glypican Dally-like and is antagonized by Dally-like cleavage by the extracellular metalloproteinase Mmp2.
Abstract
Ligand-based signaling can potentiate communication between neighboring cells and between cells separated by large distances. In the Drosophila melanogaster ovary, Wingless (Wg) promotes proliferation of follicle stem cells located ∼50 µm or five cell diameters away from the Wg source. How Wg traverses this distance is unclear. We find that this long-range signaling requires Division abnormally delayed (Dally)-like (Dlp), a glypican known to extend the range of Wg ligand in the wing disc by binding Wg. Dlp-mediated spreading of Wg to follicle stem cells is opposed by the extracellular protease Mmp2, which cleaved Dlp in cell culture, triggering its relocalization such that Dlp no longer contacted Wg protein. Mmp2-deficient ovaries displayed increased Wg distribution, activity, and stem cell proliferation. Mmp2 protein is expressed in the same cells that produce Wg; thus, niche cells produce both a long-range stem cell proliferation factor and a negative regulator of its spreading. This system could allow for spatial control of Wg signaling to targets at different distances from the source.
Introduction
Stem cells are supported by microenvironments called niches, which provide a combination of soluble signals and physical interactions to regulate stem cell behaviors. Although in many cases niche cells abut the stem cells directly, many stem cell regulatory factors, such as bone morphogenetic proteins (BMPs), Hedgehog, and Wnt, can signal over long ranges in other developmental contexts (Rogers and Schier, 2011). Indeed, the intersection patterns of long-range signaling molecules may define the spatial positioning of stem cells within a tissue; this appears to be the case for Drosophila melanogaster follicle stem cells (FSCs; Vied et al., 2012) and perhaps for stem cells of the mammalian olfactory epithelium (for review see Lander et al., 2012). The potential for long-range signaling molecules to regulate stem cell behaviors has implications in tumor progression and metastasis: the greater the range of heterotypic signals, the larger the potential tumor microenvironment for both primary and secondary tumors (Hanahan and Weinberg, 2011).
Both mammalian and Drosophila ovaries contain somatic stem cells that give rise to differentiated cells encapsulating the developing germ cells (Margolis and Spradling, 1995; Flesken-Nikitin et al., 2013). In the fly ovary, each germarium contains two FSCs, which give rise to a monolayer follicle epithelium encasing each developing egg (Fig. 1 A; Margolis and Spradling, 1995; Nystul and Spradling, 2007; Nystul and Spradling, 2010). After the initial stem cell division, daughters become follicle precursor cells, transit-amplifying cells that actively proliferate before differentiating into three cell types: stalk cells and polar cells, both of which immediately exit mitosis, and encasing follicle cells that proliferate through stage 6 (Horne-Badovinac and Bilder, 2005). FSCs are positioned midway along the germarium, and they appear to be approximately five cell diameters (∼50 µm) away from the cells that produce signaling ligands (Wingless [Wg] and Hedgehog [Hh]) regulating their behavior. Thus, FSCs are subject to long-range stem cell regulation (Forbes et al., 1996a,b; Song and Xie, 2003; Vied et al., 2012). Whether and how these signals traverse that distance is unclear (Sahai-Hernandez and Nystul, 2013).
Figure 1.
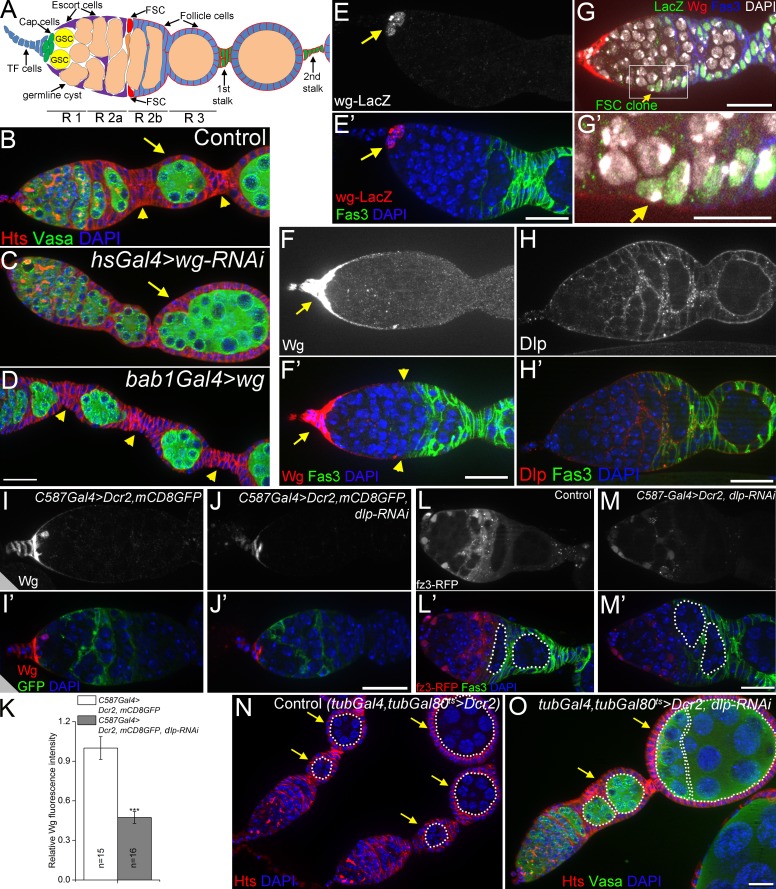
Dlp promotes long-range Wg signaling to FSCs. (A) A schematic diagram of the germarium. FSCs reside at the border of regions 2a (R 2a) and 2b. A cross-migrating FSC daughter is shown in orange. Follicle precursor cells are located in R 2b. TF cells (blue) and cap cells (green) are collectively called apical cells. GSC, germline stem cell. (B–D) Loss-of-function of wg (C) caused fused egg chambers (compound follicles, arrows; 60% penetration was observed in 35 ovarioles), whereas wg overexpression (D) resulted in stalks with increased cell numbers (arrowheads). (E) In wild-type germaria, wg was expressed only in cap cells (arrows) as shown by the wg-lacZ enhancer trap line. 3–5 cells were stained in 39/47 germaria. (F) In wild-type germaria, a continuous path of extracellular Wg (arrows), visualized at high exposure, spread to the FSCs (arrowheads). (G and G′) Wg spreading to a lacZ-labeled FSC clone (arrows). (H) Anti-Dlp in wild-type germaria. (I–K) Loss of function of dlp (with RNAi) reduced the levels of anti-Wg extracellular staining. mCD8GFP shows the pattern of C587Gal4 expression (strong in escort cells and weak in follicle cell precursors in region 2b). Dicer-2 (Dcr2) enhanced RNAi efficiency. ***, P < 0.001 (Student’s t test). Error bars represent SEM. n = number of germaria imaged. (L–M) dlp RNAi in escort cells decreased activity of the Wg signaling reporter fz3-RFP in posterior escort cells and FSCs, and caused encapsulation defects (58.3% of 84 germaria examined). 16-cell germline cysts in region 2b and region 3 are outlined. Note the two side-by-side cysts in dlp RNAi indicating an encapsulation defect. (N and O) Ubiquitous knockdown of dlp with tubGal4 resulted in fused egg chambers in 22.1% of 68 dlp RNAi germaria (arrows in O), compared with 0% in 42 control germaria (N). 16-cell germline cysts are outlined, egg chambers are indicated by arrows. DAPI labels DNA in blue. Anti-Hts labels follicle cell plasma membranes, spectrosomes, and fusomes. Anti-Vasa labels the germline. Anti-Fas3 labels follicle cell borders. Bars: (G′) 10 µm; (all other panels) 20 µm.
In this study we establish a continuous path of Wg ligand and signaling activity emanating from the anterior end of the germarium and extending to the FSCs, which lie on the shallow end of this observed ligand gradient. When the amount of Wnt signal is increased from the source, the stem cell proliferation rate increases. We identify a positive and negative regulator of Wnt long-range signaling to the FSC, and these collaborate to regulate the level and distribution of ligand sensed by the FSCs. The positive regulator is the glypican Division abnormally delayed (Dally)-like protein (Dlp), known to promote the spreading of Wg ligand in the wing disc; the negative regulator is a matrix metalloproteinase (MMP), a novel antagonist of canonical Wnt signaling, expressed in cells of the FSC niche. As a Wnt signaling antagonist, the MMP cleaves the glypican, reducing the ability of Dlp to interact with the Wnt ligand and promote its distribution. Thus, the niche produces both a long-range proliferative signal and the machinery to regulate the distribution of that signal.
Results
Long-distance Wg signaling promotes FSC proliferation
Wnt signaling regulates proliferation and self-renewal in many types of stem cells across species (de Lau et al., 2007; Clevers and Nusse, 2012). The Wnt ligand is understood to act at short range, signaling to a stem cell from a neighboring cell source. However, in the Drosophila ovary, the relationship between the Wnt ligand and FSC proliferation is unclear because of the distance between them. FSC proliferation can be assessed by stalk cell number: in mutants where the FSCs over-proliferate, excess cells are shunted off to the stalks where they stop proliferating, resulting in increased numbers of stalk cells between egg chambers; in contrast, fewer FSC divisions result in not enough follicle cells, so that egg chambers are fused with fewer or no stalk cells (Forbes et al., 1996a; Song and Xie, 2003). Wnt downstream signaling is required for normal FSC proliferation, as ectopic activation of the Wnt pathway in FSCs (through loss of Axin or shaggy) triggers excess stalk cells, and loss of canonical Wnt signaling components (dishevelled or armadillo [arm]/β-catenin) autonomously in the FSCs results in stem cell loss (Song and Xie, 2003). We confirmed the role of the Wg ligand (one of seven Wnts in Drosophila) in FSC proliferation: RNAi-mediated loss of Wg resulted in fused egg chambers typical of reduced FSC proliferation (Fig. 1, B and C; Song and Xie, 2003). Conversely, overexpression of Wg in the somatic cells with bab1Gal4 (FBal0242651; Bolívar et al., 2006) resulted in increased stalk cell numbers (Fig. 1, B and D). As others have observed, wg transcription was limited to the apical cells (Fig. 1 E; Forbes et al., 1996b), raising the question of how—or even whether—this ligand can traverse the 50-µm distance between the source and the FSCs. We stained germaria for extracellular Wg protein, and, consistent with previous studies (Song and Xie, 2003), Wg protein was most abundant around the apical cells (arrows in Fig. 1 F). However, at high exposures we observed Wg protein in a continuous path extending to the FSCs (arrowheads in Fig. 1 F′; see Fig. S1 for antibody specificity control). Although there is no reliable marker for FSCs, they were unambiguously identified by lineage tracing (Fig. 1, G and G′, arrows). This direct visualization strongly suggested that Wg directly regulates FSCs, rather than triggering a signaling relay.
Dlp promotes long-distance Wg signaling in the germarium
The best-understood example of long-distance Wg signaling is in the fly wing disc. In the wing, although Wg is produced in a thin stripe of cells, the extracellular protein is distributed over a distance of ∼50 µm (Strigini and Cohen, 2000). Recent work has called into question the requirement for Wg spreading in patterning the wing, as flies that have all Wg ligand tethered to the cell membrane still develop normally patterned wings; however, this work also demonstrates that long-distance Wg signaling is required for normal cell proliferation in the wing and has other functions outside the wing that increase fitness (Alexandre et al., 2014). The spreading of Wg away from its source in the wing requires the glypican Dlp, a heparan sulfate proteoglycan tethered to the cell surface by a glycosylphosphatidylinositol (GPI) anchor. Dlp promotes Wg signaling in cells far from the source while simultaneously reducing short-range Wg signaling (Baeg et al., 2004; Kirkpatrick et al., 2004; Kreuger et al., 2004; Franch-Marro et al., 2005). Dlp binds Wg in cell culture (Yan et al., 2009), and in the wing disc Dlp protects extracellular Wg from endocytosis and degradation, allowing more Wg to be used for long-range signaling (Marois et al., 2006). Although Dlp is not known to regulate stem cell function or Wg signaling in the ovary, we stained ovaries for Dlp and confirmed previous reports of Dlp expression in the germarium (Fig. 1 H; Guo and Wang, 2009; Hayashi et al., 2012). As in the wing disc, the pattern of Dlp staining was inverse to that of Wg ligand (compare Fig. 1, F and H; Han et al., 2005). To address whether Dlp promotes Wg distribution in the ovary, we took an RNAi approach, as dlp mutants are lethal. When dlp was knocked down within escort cells and early follicle cells (C587-Gal4), we observed a decrease in the total amount and the spread of extracellular Wg protein (Fig. 1, I–K). To determine the effect of Dlp on Wg signaling activity, we identified a Wg signaling reporter, as none has been characterized in the germarium. Testing seven candidate reporters from other tissues (Fig. S2), we identified fz3-RFP, which expresses red fluorescent protein (RFP) under the frizzled 3 (fz3) promoter (Olson et al., 2011), as a Wg signaling reporter activated in a domain from the escort cells to the FSCs (Fig. 1 L), the same domain that contacts the Wg ligand we visualized (Fig. 1 F). We validated this reporter using mutations that turned on and off the Wg pathway (Fig. S2, A and B). In dlp knockdown germaria, this Wg activity domain was sharply reduced (Fig. 1 M). Importantly, loss of dlp also caused encapsulation defects (Fig. 1 M) and the appearance of compound follicles without intervening stalk cells (Fig. 1 O), similar to the wg LOF phenotype (Fig. 1 C). Thus, knockdown of dlp reduces Wg ligand spreading, Wg signaling, and stalk cell numbers. We conclude that Dlp promotes the spread of Wg from the apical cells to the FSCs.
Mmp2 limits long-distance Wg signaling in the germarium
Conditional mutations in the extracellular protease Mmp2 also disrupted the number of follicle cells in ovarian stalks. Two temperature-sensitive (ts) alleles, Mmp2Y53N and Mmp2Y675N (Fig. S3 A), isolated in a previous ethyl methanesulfonate (EMS) screen (Page-McCaw et al., 2003) displayed conditional Mmp2 phenotypes. When in trans to a strong Mmp2 allele (ts mutants), both alleles were lethal at nonpermissive temperatures and viable at permissive temperatures (see Fig. S3 B). Ts mutant females showed reduced fertility after the switch to nonpermissive temperatures, and upon dissection, Mmp2 ts mutant ovaries exhibited extra stalk cells (Fig. 2 A, arrows), with about twice as many cells in the first and second stalks as controls (Figs. 2 B and S3 C). Similar results were obtained for each Mmp2 ts allele in trans to several strong alleles: Mmp2W307* and Mmp2218ss (EMS alleles, see Fig. S3 A), Df(2R)BSC132 (a deletion removing the entire Mmp2 gene), and Mmp202353 (P element insertion in the third intron; Page-McCaw et al., 2003; see Fig. S3 C for Y675N data). Phospho-histone H3 staining revealed increased follicle cell proliferation specifically in region 2b, where the stem and precursor cells reside, but not in later stages (Fig. 2 C). Thus, Mmp2 appeared to inhibit early follicle cell proliferation.
Figure 2.
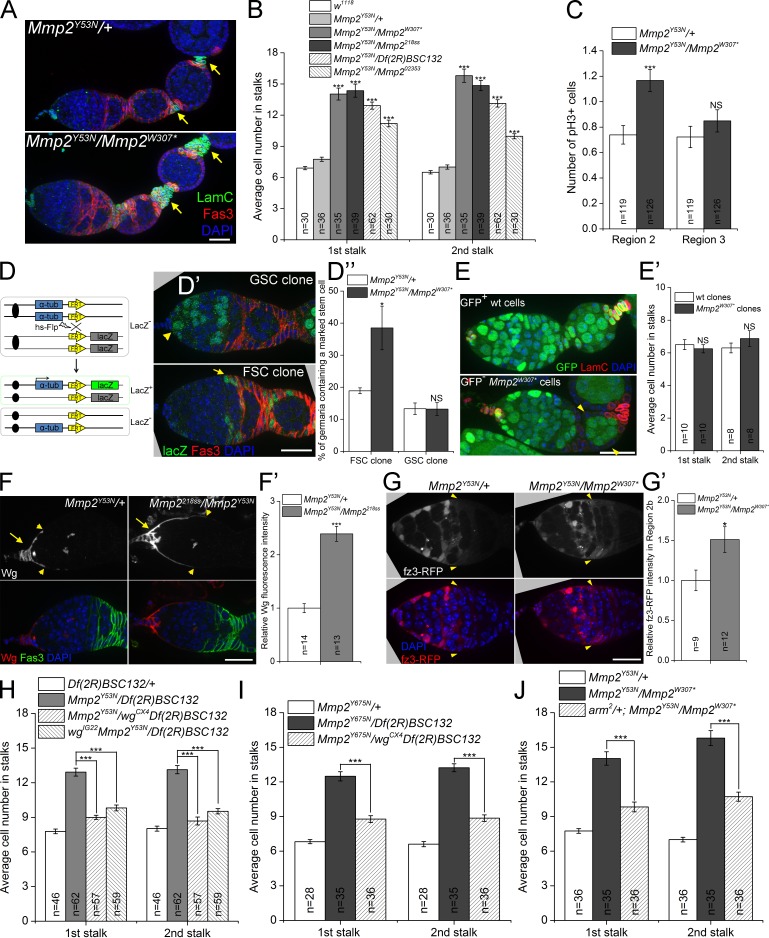
Mmp2 restricts long-range Wg signaling to FSCs. (A) Mmp2 ts mutant ovaries had stalks with increased cell numbers at a nonpermissive temperature. Arrows, first and second stalks. (B) Quantification of stalk cell numbers in Mmp2 ts mutants 7–9 d after temperature shift. Significance is relative to Mmp2Y53N/+ controls. (C) Mmp2 ts mutants showed increased proliferation of follicle precursor cells in region 2b. The number of mean pH3-positive follicle cells (including FSCs) per germarium is shown. (D–D″) FSCs divide more frequently in Mmp2 ts mutants. (D) Scheme for lineage-marking mitotic cells: hsFlp recombines the α-tub promoter (blue) in cis with the lacZ ORF (green). (D′) A GSC clone (arrowhead) and an FSC clone (arrow), each positively marked by lacZ (green). (D″) The percentage of germaria with at least one lacZ+ FSC clone or GSC clone for control and Mmp2 ts mutants (four independent experiments). (E and E′) Mmp2 was not required in follicle cells: Mmp2w307*mitotic clones (GFP-negative, arrowheads) had comparable stalk cell numbers to wt controls. (F and F′) Mmp2 ts mutants showed increased extracellular Wg staining in cap cells (arrows). Wg staining extended further in a posterior direction along the basement membrane (arrowheads denote the edge of visible Wg). F′ quantifies Wg intensity. (G and G′) Mmp2 ts mutants had increased Wg signaling in FSCs (arrowheads) and follicle precursor cells, visualized by the fz3-RFP reporter. G′ quantifies fz3-RFP intensity in FSCs and follicle precursor cells in region 2b. (H–J) Reducing wg with either of two alleles (H and I) or arm (J) dominantly suppressed the increased stalk cell number in Mmp2 ts mutants. *, P < 0.05; ***, P < 0.001; NS, not significant; Student’s t test. Error bars represent SEM. n = number of germaria counted or imaged. Bars, 20 µm.
This increase in follicle cell proliferation could be caused by an increase in stem cell divisions or by an increase in precursor cell divisions. To determine if stem cell divisions increased in Mmp2 ts mutants, we analyzed the frequency of generating mitotic clones. Dividing cells and their descendants can be permanently labeled by a mitotic recombination lineage-tracing method shown in Fig. 2 D. In brief, heat shock-induced Flippase (hsFlp) triggers homologous recombination between two FRT sites during mitosis, bringing the α-tub promoter and lacZ ORF in cis, which turns on lacZ expression (Harrison and Perrimon, 1993). The frequency of lacZ labeling depends on the length of heat shock and the rate of cell division. Although recombination can occur in any mitotic cell, only recombination in stem cells results in a permanent labeling, visualized 1 wk after heat shock. The rate of mitotic recombination in FSCs was significantly increased in Mmp2 mutants compared with heterozygote controls. In contrast, the incidence of mitotic labeling of germline stem cells (GSCs) did not change in mutants compared with controls (Fig. 2, D′ and D″). Thus, Mmp2 negatively regulates the proliferation of FSCs in the germarium.
To determine if Mmp2 was required in the follicle cells or stem cells to regulate FSC proliferation, we generated Mmp2w307* mitotic clones that included FSCs and the entire follicle epithelia. Despite the loss of Mmp2 from these cells, no change in stalk cell number was found (Fig. 2, E and E′). Because Mmp2 did not act in follicle cells directly, we asked whether it limited FSC proliferation by regulating niche signals including Hh and Wg. After staining for extracellular Wg protein, we observed a clear increase in the level of extracellular Wg protein in Mmp2 mutants compared with heterozygote controls, with the Wg distribution extending further posterior toward the FSCs (Fig. 2, F and F′, arrowheads). The fz3-RFP Wg signaling reporter confirmed increased Wg activity in FSCs and follicle precursor cells in region 2b in Mmp2 ts mutants (Fig. 2, G and G′). Mmp2 negatively regulated Wg levels through a posttranscriptional mechanism, as a wg transcriptional reporter (wg-lacZ enhancer trap) displayed lower rather than higher activity in Mmp2 mutants, which indicates feedback regulation of wg transcription (Fig. S3 D). Although Hh signaling can also regulate FSC proliferation (Forbes et al., 1996a; Zhang and Kalderon, 2001), Hh signaling was not affected, as Mmp2 mutants expressed comparable levels of the Hh reporter Patched (Ptc; Fig. S3 E). Thus, in Mmp2 mutants, Wg signaling is increased, and FSCs increase their proliferation.
To determine if there is a causal link between increased Wg signaling and FSC overproliferation in Mmp2 mutants, we asked if reducing the dose of wg suppressed the Mmp2-increased stalk cell numbers. Two independent alleles of wg (wgCX4 and wgIG22) resulted in dominant suppression of stalk cell numbers when wg was heterozygous in several different Mmp2 ts backgrounds (Fig. 2, H and I). Furthermore, mutating one copy of β-catenin (with the arm2 allele, which compromises Wg signaling activity without affecting adherens junctions; Cox et al., 1999) also suppressed the stalk cell phenotype of Mmp2 mutants (Fig. 2 J), which indicates that Mmp2 acts through canonical Wnt signaling to regulate FSC proliferation (Niehrs, 2012). Thus, Mmp2 limits Wg signaling to FSCs.
Mmp2 antagonizes Dlp in regulating long-range Wg signaling
We explored genetic interactions between Mmp2 and wg in gain-of-function studies. Although adult onset of Mmp2 overexpression with several different Gal4 drivers (bab1GalAgal4-5 and ptcGal4) resulted in lethality, we found that females could survive more than a week after Gal80ts-based conditional overexpression with C587-Gal4, expressed strongly in escort cells and weakly in follicle precursor cells in region 2b (Fig. 1 I; Manseau et al., 1997; Kirilly et al., 2011). This Mmp2 overexpression resulted in fused egg chambers lacking the stalk structure (Fig. 3, A, B, and F), which is reminiscent of wg loss of function (Fig. 1 C), supporting the model that Mmp2 negatively regulates Wg. Furthermore, this negative regulation requires the proteolytic activity of Mmp2, as coexpression of tissue inhibitor of metalloproteinase (Timp) suppressed the fused egg chamber phenotype and rescued the stalk cell numbers to wild-type levels (Fig. 3, C and F). Interestingly, when Mmp2 and wg were co-overexpressed, the stalk cell numbers were increased to an extent similar to when wg was overexpressed alone (Fig. 3, D–F). Thus, wg is epistatic to Mmp2 in this system, which suggests that Mmp2 does not act directly on the Wg ligand. In support of a “middleman” between Mmp2 and Wg, we did not observe cleavage of Wg protein by Mmp2 in cell culture (Fig. S5 A).
Figure 3.
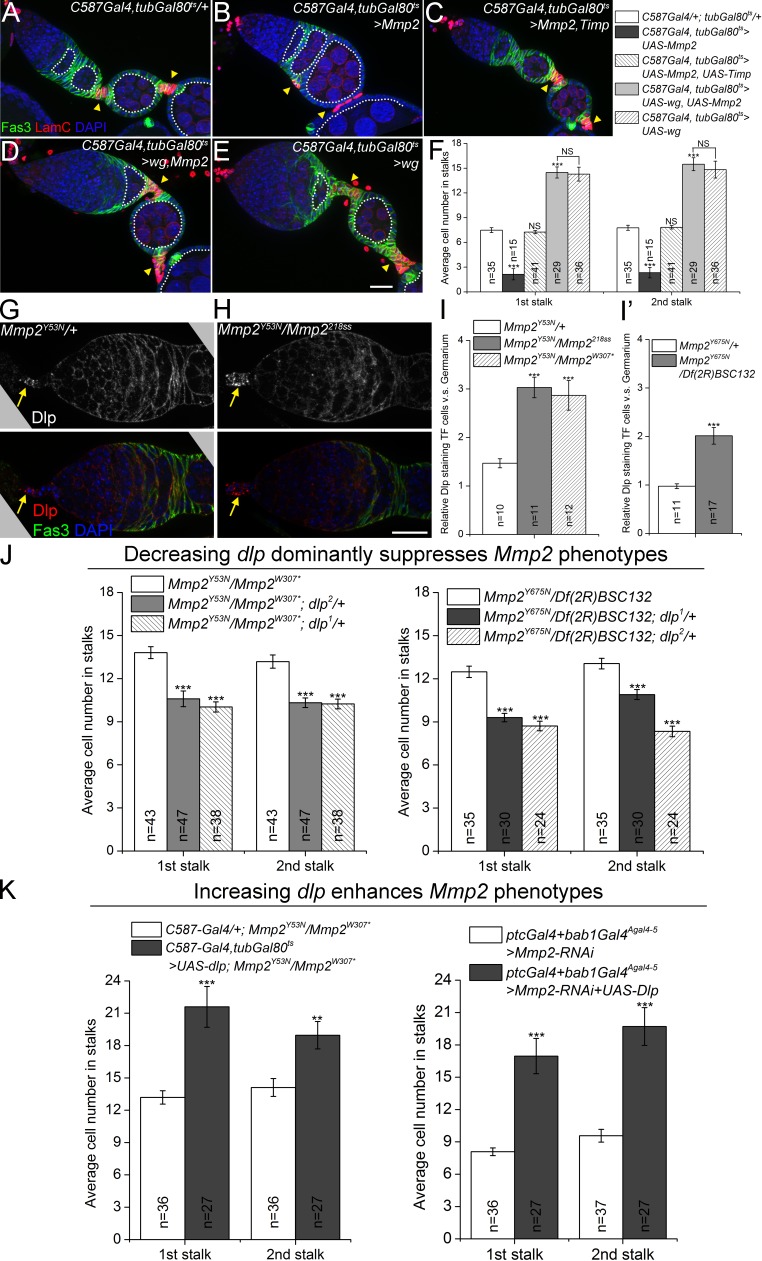
Mmp2 negatively regulates Dlp to restrict long-range Wg signaling to FSCs. (A–F) wg overexpression is epistatic to Mmp2 overexpression. Mmp2 overexpression caused fused egg chambers in 100% of 19 ovarioles examined (B), which was suppressed by coexpression of the Mmp2 catalytic inhibitor, Timp (C). Co-expression of wg and Mmp2 suppressed the Mmp2 phenotype, with fused egg chambers observed in 9.8% of 51 ovarioles (D). (E) Co-expression of Mmp2 and wg increased stalk cell numbers, phenocopying wg-alone overexpression. 16-cell germline cysts are outlined. (F) Quantification of stalk cell numbers. Significances were relative to control (C587Gal4/+; tubGal80ts/+) unless otherwise specified. (G–I) Mmp2 ts mutants showed changes in anti-Dlp staining in the germarium. Note the accumulation of Dlp in TF cells in Mmp2 mutants (H, arrows). Samples were not permeabilized. Quantification of Dlp localization change is shown in I and I′. (J) Two alleles of dlp dominantly suppressed stalk cell increases in Mmp2 ts mutants. Left: Mmp2Y53N trans-heterozygotes. Right: Mmp2Y675N trans-heterozygotes. (K) Dlp overexpression in escort cells enhances Mmp2 phenotypes. Left: Mmp2Y53N trans-heterozygotes. Right: Mmp2 RNAi. **, P < 0.01; ***, P < 0.001 (Student’s t test). Error bars represent SEM. n = number of germaria counted or imaged. Bars, 20 µm.
Because Mmp2 negatively regulated Wg signaling, and Dlp promoted Wg signaling, we next asked if Mmp2 negatively regulated Dlp. Supporting this model, anti-Dlp immunostaining showed altered protein distribution in Mmp2 mutants (Fig. 3, G and H). Significantly, in two different Mmp2 ts mutants the Dlp pattern was altered, resulting in Dlp accumulation in apical cells relative to the rest of the germarium (Fig. 3 H; quantified in Fig. 3, I and I′). To test if Mmp2 negatively regulates Dlp function, we removed one copy of dlp from Mmp2 mutants; in these ovaries, the Mmp2 phenotype of increased stalk cells was dominantly suppressed by dlp (Fig. 3 J). This suppression was specific to dlp, as two strong mutant alleles each led to dominant suppression (dlp1, a deletion mutant; and dlp2, an EMS allele; Kirkpatrick et al., 2004); each was tested in two different Mmp2 ts backgrounds (Fig. 3 J). Reciprocally, overexpression of dlp with C587-Gal4 enhanced the increases in stalk cell numbers in both an Mmp2 ts mutant and in an Mmp2 RNAi knockdown line (Fig. 3 K). Thus, Mmp2 limits Wg signaling and FSC proliferation by antagonizing the function of Dlp.
Mmp2 is required in niche cells
It has not been possible to determine Mmp2 protein localization in any Drosophila tissue because no known antibody labels endogenous Drosophila Mmp2. To determine Mmp2 protein localization in the ovary, we recombineered a genomic bacterial artificial chromosome (BAC; CH321-81G18) containing Mmp2 and all endogenous cis-regulatory sequences, including the entire 73-kb Mmp2 gene region, as well as ∼4 kb in the 3′ direction and ∼17 kb in the 5′ direction (Venken et al., 2008, 2009). The unmodified BAC completely rescued Mmp2 mutants (unpublished data). We inserted an EGFP sequence before the putative GPI attachment sequence so that the resulting BAC construct (P[acman]-Mmp2-EGFP-GPI) expressed EGFP-tagged Mmp2 under endogenous regulatory sequences (Fig. S4 A). After transformation into flies, the Mmp2-EGFP patterns matched those of previously identified in situ hybridization patterns for Mmp2 (Fig. S4 B; Llano et al., 2002; Page-McCaw et al., 2003). P[acman]-Mmp2-EGFP-GPI completely rescued the pupal lethality of Mmp2 mutants, restoring Mendelian ratios to the progeny classes; however, the lifespan, fertility, and follicle cell phenotypes of adult flies were rescued only in weak allelic combinations (Mmp2Y53N/Df(2R)BSC132) but not in strong ones (Mmp2W307*/Mmp2218ss; unpublished data). Given the complete rescue by the untagged Mmp2 BAC construct, we suspect that the EGFP tag is partially interfering with some Mmp2 functions.
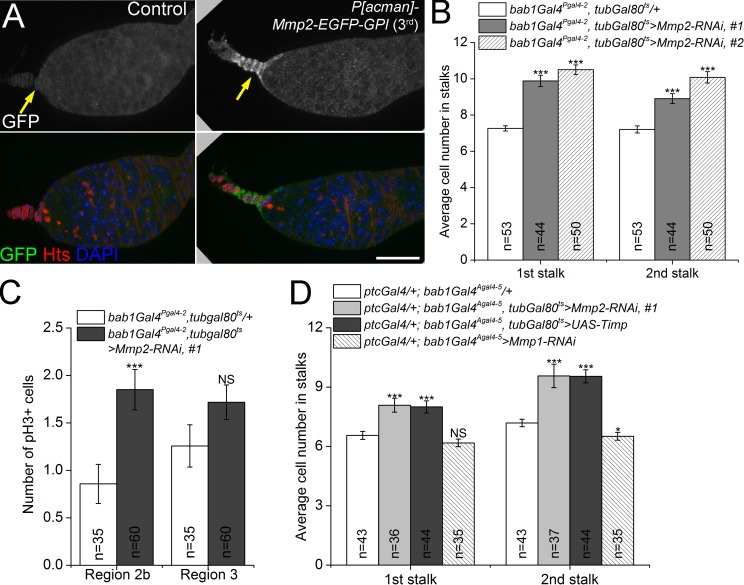
We analyzed the expression pattern of P[acman]-Mmp2-EGFP-GPI in the ovary and found that Mmp2-EGFP was expressed most strongly in the apical cells at the anterior of the germarium, which produce the Wg signal, and was also expressed in a few anterior escort cells (Fig. 4 A). Two independent insertions of the construct displayed similar patterns (Fig. S4 C). To determine the cell- and tissue-specific requirements for Mmp2, we knocked down Mmp2 expression with several Gal4 lines expressed in different patterns. When expressed with bab1Gal4Pgal4-2 in apical, escort, follicle, and stalk cells (Fig. S4 D), two different Mmp2-dsRNA constructs phenocopied the Mmp2 ts mutants, with significant increases in stalk cell numbers and follicle cell proliferation in region 2b (Fig. 4, B and C). To further refine the requirements for Mmp2, we used drivers specific to apical cells (bab1Gal4Agal4-5, distinct from the previously described bab1Gal4Pgal4-2), escort cells (ptcGal4), or follicle cells (109-30 Gal4) to knock down Mmp2. See Fig. S4 (D–G) for the expression domains of these Gal4 lines. Puzzlingly, none of these resulted in the Mmp2 phenotype of increased stalk cells (Fig. S4 G, right; and unpublished data). The stalk cell phenotype was recapitulated, however, when we knocked down Mmp2 in the apical and escort cells simultaneously with both bab1Gal4Agal4-5 and ptcGal4 (Fig. 4 D). Mmp2 catalytic activity is required for FSC proliferation, as expressing the MMP catalytic inhibitor Timp (Page-McCaw et al., 2003; Wei et al., 2003) with the same drivers caused a similar increase in stalk cell numbers (Fig. 4 D). In contrast, knockdown of Mmp1, the only other fly MMP, with a functional RNAi (Stevens and Page-McCaw, 2012) did not cause changes in stalk cell number. These results indicate that Mmp2 is supplied from both apical and escort cells to regulate FSC proliferation via its proteolytic activity, and that either population is sufficient. This is the same region where Dlp accumulates in Mmp2 mutants (Fig. 3, G and H) and where Wg is expressed (Fig. 1 E).
Figure 4.
The FSC niche cells produce a negative regulator of Wg signaling. (A) Mmp2 protein was localized to apical cells (arrows) by a GFP-tagged BAC genomic construct. Bar, 20 µm. (B) Knocking down Mmp2 with bab1Gal4Pgal4-2 resulted in increased stalk cell numbers. Two RNAi lines targeting different regions of the Mmp2 ORF gave similar results. (C) Knocking down Mmp2 with bab1Gal4Pgal4-2 resulted in overproliferation of follicle precursor cells in region 2b. (D) The Mmp2 ts phenotype of increased stalk cell numbers was phenocopied by expressing Mmp2 RNAi or Timp, but not Mmp1 RNAi, in both apical cells and escort cells. *, P < 0.05; ***, P < 0.001; NS, not significant (Student’s t test). Error bars represent SEM. n = number of germaria counted.
Mmp2 cleaves Dlp
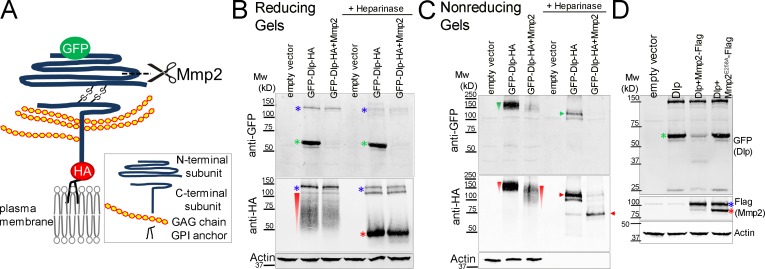
Mmp2 is an extracellular endopeptidase, and both Mmp2 and Dlp are predicted to have GPI anchors. To determine if Mmp2 cleaves Dlp, we coexpressed Mmp2 and Dlp in S2R+ cultured cells (Yanagawa et al., 1998) and examined the electrophoretic mobility of Dlp. Like mammalian glypicans, Dlp is obligately cleaved by a furin-like pro-protein convertase into two subunits that remain connected by disulfide bonds. The C-terminal subunit contains all the predicted heparan sulfate glycosaminoglycan (GAG) attachment sites (De Cat et al., 2003; Eugster et al., 2007; Kim et al., 2011). We obtained a Dlp construct where the N terminus was tagged with GFP at G69, and the C terminus was tagged with HA at S732 proximal to the GPI anchoring site, allowing us to visualize both subunits (Kreuger et al., 2004; Fig. 5 A). When lysates from cells expressing this Dlp construct were immunoblotted from reducing gels, which disrupted the disulfide bridge, anti-GFP recognized the N-terminal subunit at ∼60 kD (green asterisks in Fig. 5 B), in agreement with previous observations (Eugster et al., 2007), whereas anti-HA recognized the C-terminal subunit as a smear caused by the heterogeneity of the GAG modifications (red wedge in Fig. 5 B). Heparinase treatment removed the GAG modifications and resolved the smear into a band at ∼50 kD (red asterisks in Fig. 5 B). Two faint Dlp bands were observed between 100 and 150 kD (Fig. 5 B, blue asterisks), which probably represent unprocessed Dlp (Fig. S5 C).
Figure 5.
Mmp2 cleaves the N-terminal subunit of Dlp in cultured cells. All Western blots were performed on transiently transfected S2R+ cell lysates. (A) Diagram of a Dlp construct. Mature Dlp is processed by furin-like convertase into N- and C-terminal subunits that are linked by two disulfide bonds. GFP labels the N terminus and HA labels the C terminus. The cleavage by Mmp2 as deduced from experiments in B and C is shown. (B) On reducing gels, the N-terminal subunit ran at ∼60 kD (green asterisks); the GAG modifications on the C-terminal subunit caused it to run as a smear (red wedge), resolved by heparinase treatment to a ∼50 kD band (red asterisk). Mmp2 overexpression eliminated detection of the GFP-labeled N-terminal subunit (lanes 3 and 6). The high molecular weight bands (blue asterisk) probably represent unprocessed Dlp precursor polypeptide (see also Fig. S5 C). (C) On nonreducing gels, the disulfide-bonded Dlp protein ran as a smear at ∼150 kD (green and red wedges), resolved by heparinase into doublets (green and red arrow heads) between 100 kD and 150 kD. Mmp2 overexpression reduced the sizes of the Dlp smear (lane 3, red wedge) and band (lane 6, red arrowhead) recognized by HA. (D) Dlp cleavage required Mmp2 catalytic activity, as Mmp2E258A did not decrease the level of GFP-labeled N-terminal subunit (compare lanes 3 and 4). Flag-tagged Mmp2 ran as doublets (the blue asterisk represents the likely zymogen and the red asterisk represents the likely activated enzyme without the pro-domain). Actin, loading control.
Strikingly, when Mmp2 was coexpressed with Dlp, the N-terminal band was nearly eliminated (Fig. 5 B, green asterisks, compare lanes 2 and 3, and lanes 5 and 6). In contrast, neither the mobility nor the abundance of the C-terminal band was affected (Fig. 5 B, red wedge and red asterisk). On nonreducing gels where the subunits remain associated, the HA-tagged Dlp smear exhibited increased mobility in the presence of Mmp2 (Fig. 5 C, compare the red wedges in lanes 2 and 3); when heparinase treatment was applied to resolve the smear, the predominant ∼115-kD HA-tagged band shifted to ∼75 kD in the presence of Mmp2 (Fig. 5 C, compare the red arrowheads). These results indicate that Mmp2 cleaves the N-terminal subunit of Dlp, removing a GFP-containing piece of ∼40 kD. Adjusting for the GFP tag, it appears that Mmp2 cleaves Dlp ∼10–15 kD, or ∼100–140 amino acids, after the signal sequence. A similar loss of the N-terminal Dlp band was observed when the N terminus was tagged with Myc, thus ruling out the possibility that Mmp2 was cleaving the GFP moiety itself (Fig. S5, D and D′). Further supporting the model that Mmp2 cleaves Dlp, we found that a catalytically inactive mutant, Mmp2E258A, did not alter Dlp mobility (Fig. 5 D). These data show that Mmp2 is responsible for cleaving Dlp and suggest that the cleaved N-terminal Dlp fragment is either unstable or shed outside the cell. Because Mmp2 and Dlp were overexpressed proteins in these cell culture experiments, we cannot rule out the possibility that one or both are localized to an ectopic location or have lost specificity in some way. However, our cell culture result finding that Mmp2 cleaves Dlp is consistent with our in vivo data showing that reducing the level of dlp attenuates Mmp2 loss-of-function phenotypes (Fig. 3 J) and that in Mmp2 mutants Dlp protein accumulates excessively (Fig. 3 H).
Another glypican, Dally, is known to alter the spread of Wg in the wing disc (Han et al., 2005), and Dally is expressed in the germarium where it functions to maintain GSCs (Guo and Wang, 2009; Hayashi et al., 2009). We considered the possibility that Mmp2 may also cleave Dally, but in S2R+ cells we did not observe proteolysis of Dally upon cotransfection with Mmp2 (Fig. S5 B). Consistent with the lack of cleavage, the Mmp2 phenotype of increased stalk cells does not seem related to the dally phenotype of germline loss. Thus we conclude that Dally is not a target of Mmp2 in regulating FSCs.
Mmp2-dependent cleavage inhibits Dlp localization on the cell surface
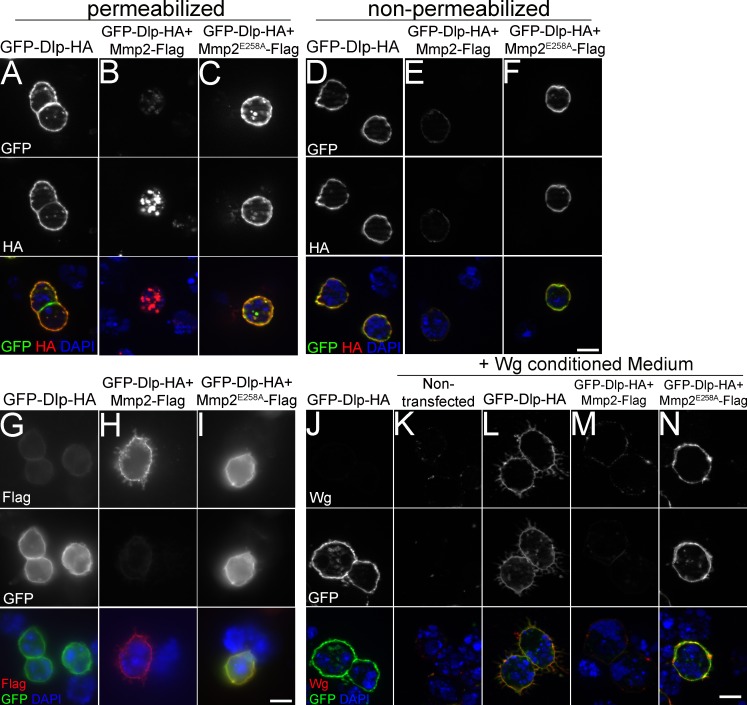
To determine how Mmp2 cleavage modifies Dlp function, we examined the subcellular distribution of Dlp. When expressed in S2R+ cells, Dlp localized predominantly to the outside of the plasma membrane, with staining visible under permeabilizing and nonpermeabilizing conditions (Fig. 6, A and D). However, when Mmp2 was coexpressed, the cell surface staining of Dlp was dramatically reduced (Fig. 6 E), replaced by prominent intracellular localization visible only under permeabilizing conditions (Fig. 6 B). The loss of Dlp from the cell surface was dependent on the catalytic activity of Mmp2 (Fig. 6, C and F). Interestingly, Mmp2 localized to the outside of the plasma membrane (Fig. 6 H), which suggests that Dlp could interact with Mmp2 on the plasma membrane. When catalytically inactive Mmp2E258A was coexpressed with Dlp, colocalization was detected (Fig. 6 I). Dlp has been shown to bind Wg on the cell surface (Yan et al., 2009), so we next tested if Dlp was capable of binding the Wg ligand after cleavage by Mmp2. Wg accumulation on the cell surface was dependent on Dlp (Fig. 6, K and L), and this accumulation was greatly compromised by coexpression of Mmp2 (Fig. 6 M) but not inactive Mmp2E258A (Fig. 6 N). The lack of Wg binding seems likely to be caused by the loss of Dlp from the cell surface; it is also possible that the Wg-binding domain of Dlp has been disrupted, as Dlp binds Wg through its N-terminal domain (Yan et al., 2009). By cleaving Dlp, Mmp2 limits the capacity of Wg to bind to a protein that promotes its extracellular distribution and protects it from internalization and degradation (Marois et al., 2006; Yan and Lin, 2009). Thus, in the absence of Mmp2, excess Wg signals FSC proliferation.
Figure 6.
Mmp2 negatively regulates the cell surface localization of Dlp and reduces Wg binding. (A–F) Dlp localization was analyzed in S2R+ cells transiently transfected with Dlp labeled by N-terminal GFP and C-terminal HA (see Fig. 5 A), in the absence or presence of Mmp2-Flag or catalytically inactive Mmp2E258A-FLAG. Cell-surface localization of Dlp revealed by anti-GFP and anti-HA staining under permeabilized (A) and nonpermeabilized conditions (D). Dlp coexpression with active Mmp2 caused the loss of the Dlp N terminus labeled by GFP (B and E, top); in the presence of Mmp2, the Dlp C terminus labeled with HA was no longer present on the cell surface (E, middle) but instead was detected inside the cell (B, middle), dependent on Mmp2 catalytic activity (C and F). (G–I) Mmp2 localized to the surface of S2R+ cells, shown in nonpermeabilized cells by anti-Flag staining (H, top). Inactive Mmp2E258A colocalized with Dlp on the cell surface (I). (J–N) Transfected S2R+ cells were incubated with Wg-conditioned media. Wg was detected by anti-Wg, and Dlp was detected by anti-GFP in nonpermeabilized cells. Wg accumulation at the cell surface was Dlp dependent (K and L) and inhibited by active Mmp2 coexpression (M and N). Bars, 10 µm.
Discussion
Wnt ligands act in both a short-range and long-range manner. In the fly ovary, it has been known that FSCs lie ∼50 µm from the Wg source at the anterior tip of the germarium (Forbes et al., 1996b; Song and Xie, 2003), which places them as long-range Wg signaling targets. We observed that Wg is produced in cap cells and spreads extracellularly in a visible path extending to the FSCs in wild-type germaria. Although a recent study suggests that Wg may be produced by escort cells, this population of Wg ligand cannot be visualized and at most represents a small fraction of the Wg ligand expressed by cap cells (Sahai-Hernandez and Nystul, 2013). We directly visualized extracellular Wg ligand as specific antibody staining that extends from the cap cells to the FSCs, and we observed a parallel domain of activity with a Wg signaling reporter.
We find that Mmp2 limits the Wg range in the germarium. The range of Wg spreading is controlled by interactions between Wg and Dlp on the cell surface. Our results are consistent with Dlp having the same function in the germarium as it does in the wing disc, where Dlp retains Wg on the cell surface and protects Wg from endocytosis and degradation or from extracellular loss (Marois et al., 2006; Yan and Lin, 2009). Based on our fly and cell culture data, we propose that Mmp2 cleaves Dlp near the Wg source, causing Dlp to relocalize inside the cell where it can no longer interact with or protect extracellular Wg. In wild-type ovaries, Mmp2 serves to limit Wg signaling and FSC proliferation. In Mmp2 mutants, we observed increased Wg protein levels and a distribution extending further toward the stem cells, and we show that increased stem cell proliferation is dependent on the dose of wg in Mmp2 mutants. Our model of how Mmp2 cleaves Dlp to limit Wg stem cell signaling is sketched in Fig. 7; this model is supported by the following observations. First, Mmp2 cleaves Dlp and induces its loss from the cell surface to an intracellular location in cultured cells. Second, in the ovary, Dlp is required for long-range Wg signaling, and reducing Dlp attenuates Mmp2 loss-of-function stem cell proliferation phenotypes. Third, Mmp2 mutants exhibit accumulation of Dlp at apical cells, sites where Mmp2 is expressed.
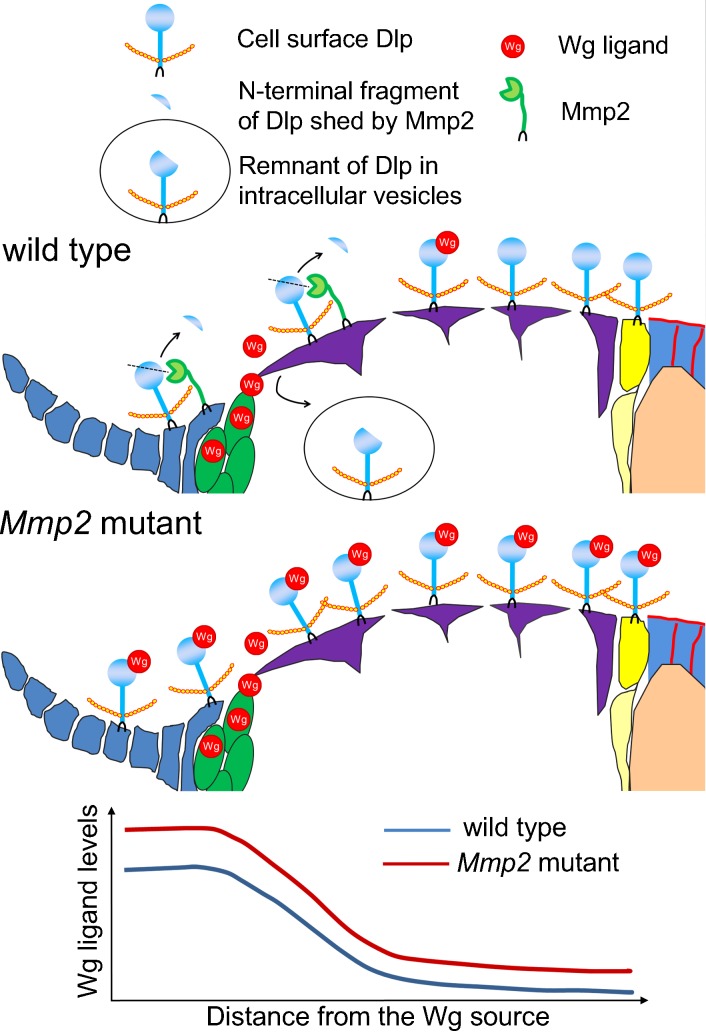
Figure 7.
Model for how Mmp2 regulates Dlp and the distribution of Wg ligand in the germarium. In the wild-type germarium, Mmp2 is produced near the Wg source (cap cells) and mediates Dlp cleavage caused by the relocalization of Dlp from cell surface to intracellular vesicles. In Mmp2 mutants, more Dlp is retained at the cell surface to stabilize and facilitate Wg transport, resulting in increased levels of extracellular Wg ligand across the germarium and increased follicle cell divisions.
It would seem that Wg signaling to FSCs could be controlled more easily by altering the level of released ligand. What could be the advantage of these cells expressing Mmp2 as a negative regulator of Wg signaling? Interestingly, we do not find a role for Mmp2 in Wg signaling in the wing disc (unpublished data, see Materials and methods). In the wing disc, Notum appears to play a similar role to Mmp2. Notum antagonizes Dlp by cleaving the GPI anchor from Dlp, and like Mmp2, it is expressed in the Wg domain. Notum appears to act as a feedback regulator of Wg, and Mmp2 may also play this role in the germarium. Alternatively, the role of Mmp2 may reflect differences between the germarium and the wing disc in size regulation. Unlike the wing, which is highly stereotyped in terms of size regulation, the germarium changes size during development, probably cyclically with the production of new egg chambers; the distance between the Wg source and the FSCs changes with it. When the germarium is bigger, the cell surface concentration of Mmp2 and Dlp may be reduced, resulting in fewer cleavage events and more Wg spreading, able to reach the more distant FSCs; conversely, when the germarium is smaller, more cleavage events may limit the spread of Wg to the closer FSCs. Another possibility is that Mmp2 may act to coordinate the development of follicle epithelium with the development of the germline, two tissues that arise from distinct ovarian stem cells that appear to be regulated by niche signals emanating from the same cell types (Li and Xie, 2005).
Implications for other tissues
It has been known that Dlp function is controlled at multiple levels, including transcription, shedding from cell surface, and intracellular trafficking (Baeg et al., 2004; Kreuger et al., 2004; Han et al., 2005; Gallet et al., 2008). Regulatory mechanisms have been identified that govern transcription (Han et al., 2005) and shedding by the lipase Notum, which cleaves the GPI anchor (Kreuger et al., 2004), yet no regulatory mechanism controlling trafficking has been previously discovered. Our study unveils a novel mechanism of Dlp regulation: proteolytic cleavage by Mmp2, which causes a change in Dlp localization from the cell surface to intracellular sites. Dlp is known to regulate ligand availability and/or signaling reception in several signaling pathways in Drosophila including Hh, BMP, FGF, and JAK/STAT in addition to Wg (Desbordes and Sanson, 2003; Belenkaya et al., 2004; Yan and Lin, 2007; Gallet et al., 2008; Hayashi et al., 2012; Zhang et al., 2013). The region of Dlp cleaved by Mmp2 is highly conserved in mammalian glypicans, and similarly, vertebrate glypicans regulate many signaling pathways including Wnt, BMP, Hh, and FGF (Filmus et al., 2008; Fico et al., 2011). Additionally, human glypicans participate in tumor susceptibility and progression (DeBaun et al., 2001; Capurro et al., 2005; Jakubovic and Jothy, 2007; Fico et al., 2012). Thus, the regulation of Dlp function by Mmp2 may have widespread significance.
MMPs as Wnt regulators
The concept of MMPs regulating both Wnt signaling and stem cell proliferation was recently introduced in a study in the mouse mammary gland (Kessenbrock et al., 2013). The authors showed that MMP3 can sequester or cleave Wnt5a, a noncanonical Wnt ligand, which acts as an inhibitor of canonical Wnt signaling, resulting in increased canonical Wnt signaling and stem cell function. In contrast, we show that DmMmp2 inhibits canonical Wnt signaling and stem cell proliferation via cleavage of the glypican Dlp. These two distinct mechanisms reflect the versatility of the Wnt signaling machinery and the variety of its outputs. In the mammary gland, the number of mammary stem cells is variable and controlled by Wnt signaling; the architecture of the resulting epithelial ductal structure is variable and reflects the local signaling environment. Generally, Wnt signaling acts in a canonical fashion through β-catenin/Arm to regulate cell cycle or cell fate; Wnt signaling acts in noncanonical fashion to regulate planar cell polarity or alternative transcriptional responses. In the mammary gland, it appears that MMP3 acts as a switch between canonical and noncanonical Wnt signaling. MMP3 may even act as a feedback inhibitor, switching between these two types of Wnt signaling (Kessenbrock et al., 2013).
The biology of the Drosophila ovary reflects different aspects of Wnt signaling. The Wg signal emanates from cells that are 50 µm away from the FSCs, raising the possibility that many cells may respond to the Wg gradient at different concentrations. Indeed, Wg is known to act in a concentration-dependent manner, morphogen-style, so that short-range and long-range targets have distinct signaling responses. The FSCs are long-range Wg targets; closer targets exist for the apical cell Wg signal, as indicated by our Wg reporter Fz3. In this tissue, DmMmp2 acts to tune the levels of Wg ligand, regulating the Wg concentration that reaches the FSCs to trigger proliferation. Perhaps Mmp2 in concert with Dlp, which is known to have opposing effects on short-range and long-range targets, acts as a rheostat to selectively alter the strength of signaling to distinct targets along the gradient. In both the mammary gland and in the fly ovary, hyperactive canonical Wnt signaling gives rise to epithelial overgrowth. The results from these two studies highlight not only the diversity of Wnt signaling, but also the need to understand thoroughly the regulatory mechanisms governing Wnt signaling in order to develop effective therapeutic strategies to halt pathological Wnt-driven cell proliferation
DmMmp2 represents a class of MMPs that are attached to the cell surface by a GPI anchor. Two mammalian GPI-anchored MMPs exist, MMP17 and MMP25, and unlike secreted MMPs and MMPs with transmembrane domains, functional studies of this GPI-anchored class of MMPs remain very limited. The GPI anchor is expected to confer MMPs with a unique subcellular localization and offer access to distinct substrates (Sohail et al., 2008). Our results show that GPI-anchored MMPs do indeed play a unique role, as DmMmp2 is not redundant with or compensated by the other Drosophila MMP, the secreted Mmp1 (Page-McCaw et al., 2003); and our finding of a GPI-anchored substrate, Dlp, supports the concept that GPI-anchored MMPs have unique substrates based on their cellular localization. Although other heparan sulfate proteoglycans including syndecan are known targets of MMP-mediated proteolysis (Li et al., 2002; Page-McCaw et al., 2007), glypicans have not been previously identified as MMP substrates in any system.
Mammalian MMPs are up-regulated in cancer, and they are considered to promote tumor progression in various stages from initiation to metastasis. The protumorigenic function of MMP3 in breast cancer may involve its activating role in mammary stem cells (Kessenbrock et al., 2013). However, the roles of MMPs in cancer are further complicated by studies that indicate that some MMP family members have tumor-suppressor, protective activities (Decock et al., 2011). Our studies showing that Mmp2 inhibits proliferation of somatic stem cells may advance our understanding of the protective roles of MMPs in cancer.
Materials and methods
Fly stocks
Flies were maintained on cornmeal-molasses media at 25°C. Females harvested for ovaries were fed with fresh wet yeast changed every other day until dissection. Ts alleles of Mmp2 were generated by treating cn bw sp males with 25 mM EMS and selecting for lethal noncomplementation of an Mmp2 P-insertion at 29°C (Page-McCaw et al., 2003). All Mmp2 mutant alleles were backcrossed to w1118 for four to five generations.
The following stocks are described in Flybase and obtained from Bloomington Drosophila Stock Center: Df(2R)BSC132, wgCX4, wgIG22, arm2, UAS-wg-HA, UAS-dlp, bab1Gal4Pgal4-2 (#6803), bab1Gal4Agal4-5 (#6802), bab1Gal4 (FBal0242651; provided by A. Gonzalez-Reyes, Universidad Pablo de Olavide, Sevilla, Spain; Bolívar et al., 2006), ptcGal4 (#2017), and tubGal80ts. Other lines include Mmp202353 (FBal0007998), UAS-Timp (FBal0150584), UAS-Mmp2 (FBal0150748; Page-McCaw et al., 2003), wg-lacZ (FBal0042267; a gift from G. Struhl, Columbia University, New York, NY); C587-Gal4 (FBal0150629; a gift from D. Drummond-Barbosa, Johns Hopkins University, Baltimore, MD), fz3-RFP (FBal0267159; provided by R. DasGupta, New York University, New York, NY; Olson et al., 2011), dlp1 (FBal0190800), and dlp2 (FBal0191142; both provided by S. Selleck, Pennsylvania State University, University Park, PA; Kirkpatrick et al., 2004). RNAi lines used were dlp-RNAi (Vienna Drosophila RNAi Center, 10299), wg-RNAi (#4889R-4), and Mmp2-RNAi #1 (#1794-1R-1, both from National Institute of Genetics Fly Stock Center, Japan); as well as Mmp2-RNAi, #2 (Transgenic RNAi project, JF01337), and Mmp1-RNAi (FBal0212915; Uhlirova and Bohmann, 2006).
Detailed genotypes of animals in each experiment
Figure 1.
(B) w1118; wg-RNAi/+. (C) w/w1118; wg-RNAi/+; hsGal4/+. (D) w; bab1Gal4/UAS-wg-HA. (E) w1118; wglacZ/CyO. (F) w; bab1Gal4Agal4-5, tubGal80ts/+. (G) y w/w hsFlp; X15-33/X15-29. (H) w1118; UAS-dlp/+. (I) w C587Gal4/w; UAS-Dcr2/UAS-mCD8GFP. (J) w C587Gal4/w; UAS-Dcr2/UAS-mCD8GFP; dlp-RNAi/+. (L) w C587Gal4/w; fz3-RFP/+. (M) w C587Gal4/w; fz3-RFP/UAS-Dcr2; dlp-RNAi/+. (N) w; UAS-Dcr2/+; tubGal4, tubGal80ts/+. (O) w; UAS-Dcr2/+; tubGal4, tubGal80ts/dlp-RNAi.
Figure 2.
(A–C and F) All mutants and heterozygote controls were in a w1118 background. (D″) Control, y w/w hsFlp; X15-33, Mmp2Y53N/X15-29. Mutant, y w/w hsFlp; X15-33, Mmp2Y53N/X15-29, Mmp2W307*. (E) hsflp/+; FRT13A ubiGFP/FRT13A Mmp2W307*. (G) Control, w1118; Mmp2Y53N/fz3-RFP. Mutant, w1118; Mmp2Y53N/fz3-RFP, Mmp2W307*. (H and I) All genotypes were in a w1118 background. (J) From left to right: w1118; Mmp2Y53N/+. w1118; Mmp2Y53N/Mmp2W307*. arm2 FRT101/w1118; Mmp2Y53N/Mmp2W307*.
Figure 3.
(A) w C587Gal4/w; tubGal80ts/+. (B) w C587Gal4/w; tubGal80ts/UAS-Mmp2. (C) w C587Gal4/w; UAS-Timp, tubGal80ts/UAS-Mmp2. (D) w C587Gal4/w; tubGal80ts/UAS-Mmp2, UAS-wg-HA. (E) w C587Gal4/w; tubGal80ts/UAS-wg-HA. (G–I′) All mutants and heterozygote controls were in a w1118 background. (J, left) From left to right: w1118; Mmp2Y53N/Mmp2W307*. w1118; Mmp2Y53N/Mmp2W307*; ru h dlp2 st ry506 es/+. w1118; Mmp2Y53N/Mmp2W307*; dlp1 st1 ry506/+. (J, right) From left to right: w1118; Mmp2Y675N/Df(2R)BSC132. w1118; Mmp2Y675N/Df(2R)BSC132; dlp1 FRT2A/+. w1118; Mmp2Y675N/Df(2R)BSC132; ru h dlp2 st ry506 es/+. (K, left, from left to right) w C587Gal4/w1118; Mmp2Y53N/Mmp2W307*. w C587Gal4/w1118; Mmp2Y53N/Mmp2W307*, tubGal80ts, UAS-dlp/+. (K, right, from left to right) w; ptcGal4/+; bab1Gal4Agal4-5/Mmp2-RNAi. w; ptcGal4/+; bab1Gal4Agal4-5/Mmp2-RNAi, UAS-dlp.
Figure 4.
(A, left) y w; VK33 (Control). (A, right) y w; P{P[acman]-Mmp2-EGFP-GPI}VK33. (B) From left to right: w; bab1Gal4Pgal4-2, tubGal80ts/+. w; bab1Gal4Pgal4-2, tubGal80ts/UAS-Mmp2-RNAi, #1. w; bab1Gal4Pgal4-2, tubGal80ts/UAS-Mmp2-RNAi, #2. (C) From left to right: w; bab1Gal4Pgal4-2, tubGal80ts/+. w; bab1Gal4Pgal4-2, tubGal80ts/UAS-Mmp2-RNAi, #1. (D) w; ptcGal4/+; bab1Gal4Agal4-5/+. w; ptcGal4/+; bab1Gal4Agal4-5, tubGal80ts/Mmp2-RNAi, #1. w; ptcGal4/+; bab1Gal4Agal4-5, tubGal80ts/UAS-Timp. w; ptcGal4/+; bab1Gal4Agal4-5/Mmp1-RNAi.
Clonal analysis and temperature shift conditions
lacZ-labeled mitotic stem-cell clones were generated using previously published methods (Harrison and Perrimon, 1993). The yw, P[(hsFlp)12, ry+]; X.15.29 and yw; X.15.33 lines (a gift from N. Perrimon, Harvard Medical School, Boston, MA) were recombined with different Mmp2 mutant alleles. Flies were collected every 2 d upon eclosion, aged at 29°C for 5 d, exposed to a 1-h heat shock at 37°C, and dissected 7 d later. GFP-negative mitotic clones were generated using FLP-mediated mitotic recombination (Xu and Rubin, 1993). The genotype used was hsflp/+; FRT13A ubiGFP/FRT13A Mmp2W307*. 3 d after eclosion, flies were exposed to 1-h heat shocks at 37°C twice a day with an interval of at least 8 h for three consecutive days. Ovaries were dissected 10 d later.
Trans-heterozygous Mmp2 ts mutant adults were raised at a permissive temperature (18°C for Mmp2Y675N and 25°C for Mmp2Y53N); progeny were collected upon eclosion every 2 d and shifted to a nonpermissive temperature (29°C) for 7–10 d until dissection. For Gal4/Gal80ts controlled gene expression, flies were raised at 18°C, shifted 1–2 d upon eclosion to 29°C, and aged 7–10 d before dissection. For hs-Gal4 induced expression, 2 d after eclosion flies were heat shocked for 1 h each day for three consecutive days and dissected 3 d later.
Immunohistochemistry
Ovaries were stained as described previously (Song et al., 2002). For nonpermeabilized staining, PBS was used instead of PBST (PBS + 0.1% Triton X-100). Extracellular Wg staining was performed according to a published protocol (Strigini and Cohen, 2000). In brief, dissected ovaries were incubated with anti-Wg (1:3) on ice for 40 min, rinsed thoroughly with cold PBS, and then fixed and costained according to conventional ovary staining procedures. Antibodies used were as follows: mouse anti-Dlp (against Dlp V523-Q702, clone 13G8, 1:5), mouse anti-Fas3 (7G10, 1:8), mouse anti-Hts (1B1, 1:5), mouse anti-lacZ (40-1a, 1:50), mouse anti-LamC (LC28.26, 1:20), mouse anti-Wg (4D4, 1:3), and rat anti-Vasa (1:10). These antibodies were from Developmental Studies Hybridoma Bank (DSHB). Other primary antibodies used were rabbit anti–phospho-Histone H3 (1:1,000; EMD Millipore), mouse anti-GFP (clone N86/38, used 1:5; UC Davis/National Institutes of Health NeuroMab Facility). Secondary antibodies used were Cy3- or FITC-conjugated goat anti–mouse IgG1 or IgG2a, Dylight 649–conjugated donkey anti–rat IgG (all from Jackson ImmunoResearch Laboratories, Inc.), goat anti–rabbit IgG, and goat anti–rat IgG conjugated to Alexa Fluor 488 (Molecular Probes). Stained samples were mounted in Vectashield (Vector Laboratories).
Analysis of Wg signaling in the wing disc
To determine if Mmp2 affects Wg signaling in the wing disc, the following assays were performed. Extracellular Wg was assessed by staining (as described for ovaries) third instar wing discs from Mmp2W307*/+ and Mmp2W307*/Mmp2218ss larvae. The long-range Wg target Dll (Duncan et al., 1998) was assessed by anti-Dll staining of wing discs from w1118 and Mmp2W307*/Df(2R)BSC132 third instar larvae. For short-range Wg signaling, Mmp2 ts mutants (Mmp2Y53N/Df(2R)BSC132 and Mmp2Y53N/Mmp2W307*) were raised at 29°C for 5 d until pupariation, shifted to 25°C for 2 d, and then shifted back to 29°C through eclosion. Mechanosensory bristles on the wing margin were counted as an indicator of short-range Wg signaling. No differences were observed between control and Mmp2 mutants in any of these assays.
Plasmids and recombineering
pUAST-Mmp2-Flag was constructed from pUAST-Mmp2 (Page-McCaw et al., 2003) by introducing a BamHI site into Mmp2 (after S710) and subsequently inserting a 3× flag sequence flanked by BamHI sites. The E258A mutation was introduced by PCR into pUAST-Mmp2-Flag to generate pUAST-Mmp2E258A-Flag. Other plasmids used were pUAST-GFP-Dlp-HA-C (GFP was inserted in place of G69 via SphI and the HA tag was inserted at S732 via NheI into Dlp; provided by S. Cohen, Institute of Molecular and Cell Biology, Singapore; Kreuger et al., 2004), pAW-Dally-Myc (Myc was inserted at R50 via Aat II into Dally; provided by H. Nakato, University of Minnesota, Minneapolis, MN; Dejima et al., 2011), and pMT-Gal4 (FBmc0003005).
A genomic BAC construct for Mmp2 (CH321-81G18, 2R:9,607,564–9,701,338, Berkeley Drosophila Genome Project D. melanogaster Release_6) was obtained from the P[acman] libraries (Venken et al., 2009). To construct P[acman]-Mmp2-EGFP-GPI, an EGFP tag was inserted before the GPI anchor of Mmp2 (after S710) by recombineering using PL-452 C-EGFP as the template vector (Venken et al., 2008). Transgenic flies were generated for both untagged and tagged Mmp2 BAC constructs by ϕC31-mediated integration into yw; attP40; VK33 flies (Genetic Services Inc.). Integration events were identified by screening for w+ and confirmed by PCR.
Cell culture, transfection, and immunoblotting
Drosophila S2 cells (Drosophila Genomics Resources Center) and S2R+ cells (a gift of N. Perrimon) were maintained at 27°C in Schneider’s Drosophila medium (Gibco) containing 10% heat inactivated fetal bovine serum (BRL 16140; Gibco) and 100 U/ml penicillin/streptomycin. Transient transfection was performed as described previously (Broderick et al., 2012). Induction of protein expression was performed in the presence of 0.7 mM Cu2+ at 27°C for 2 d; or for any Dlp or Dally-related experiments, at 18°C for 4 d to promote better processing and folding of Dlp.
For immunoblotting, cell pellets were washed in PBS, resuspended in 1× NuPage LDS sample buffer (Invitrogen) with (for reducing gels) or without (for nonreducing gels) 5% β-mercaptoethanol, and heated at 75°C for 5 min. Lysates were run on 10% Mini-PROTEAN TGX precast gels (Bio-Rad Laboratories) and transferred onto Hybond-C Extra nitrocellulose membranes (GE Healthcare). Blots were probed with primary antibodies including rabbit anti-GFP (ab6556; Abcam), mouse anti-GFP (clones 7.1 and 13.1; Roche), rat anti-HA (3F10; Roche), anti-actin (MAB1501R; EMD Milllipore), and rabbit anti-Flag (F7425; Sigma-Aldrich). Secondary antibodies used were: donkey anti–rabbit or anti–mouse IgG conjugated to IRDye 680, and goat anti–rat IgG conjugated to IRDye 680 or IRDye 800CW (LI-COR Biosciences). Blots were developed and imaged with the Odyssey Infrared Imaging System (LI-COR Biosciences).
For immunostaining, S2R+ cells were harvested, reattached to a 12-well multi-test slide (MP Biomedicals), and stained as described previously (Broderick et al., 2012). PBST was used to permeabilize cells whereas PBS was used for nonpermeabilized staining. Primary antibodies used were: mouse anti-GFP (clone N86/38, 1:25; UC Davis/National Institutes of Health NeuroMab Facility), rat anti-HA (3F10, 1:500; Roche), mouse anti-Wg (4D4, 1:33; DSHB), and rabbit anti-Flag (1:500; Sigma-Aldrich). Secondary antibodies used were FITC-conjugated goat anti–mouse IgG2a, Dylight 649–conjugated donkey anti–rat, Cy3-conjugated goat anti–mouse IgG1, and Cy3-conjugated donkey anti–rabbit IgG (all from Jackson ImmunoResearch Laboratories, Inc.).
Heparinase treatment
For heparinase treatment, cells were lysed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.5% Triton X-100 plus complete proteinase inhibitor (EDTA free; Roche) on ice for 30 min. Supernatant was diluted with equal volumes of 20 mM Tris-HCl, pH 7.5, and 4 mM CaCl2 plus complete proteinase inhibitor (EDTA free; Roche). Heparinase III (Sigma-Aldrich) was added to a final concentration of 2 U/ml. After 4 h of incubation at 37°C, the reaction was halved and the two aliquots were mixed with NuPage LDS sample buffer (Invitrogen) with (for reducing gels) or without β-ME (for nonreducing gels) and run on 10% Mini-Protean TGX precast gels (Bio-Rad Laboratories).
Wg-binding assay
The Wg binding assay was performed as described previously (Wu et al., 2010). In brief, Wg-conditioned medium was collected from a S2-Tub-wg stable cell line (Drosophila Genomics Resources Center). To assay Wg binding, S2R+ cells were seeded onto poly-d-lysine–coated coverslips (Neuvitro) in a 24-well plate and transfected. After Cu2+ induction, cells were prechilled, incubated with Wg-conditioned medium on ice for 3 h, and processed for immunostaining.
Fluorescence microscopy, imaging, and quantification
All samples were imaged by an Axioimager M2 (Carl Zeiss) equipped with an Apotome system and a camera (AxioCam MRm; Carl Zeiss). Images were acquired using 40×/1.3 NA oil EC Plan-NeoFluor or 63×/1.4 NA oil Plan-Apochromat objective lenses at room temperature. The Axiovision 4.8 software (Carl Zeiss) was used for data acquisition, and projections of z stacks were compiled using the Orthoview functions. Images were exported as 16-bit TIF files and processed with Photoshop CS4 (Adobe). Fluorescence intensities were quantified on selected regions of the germarium using the Measure tool of ImageJ 1.45s (National Institutes of Health). For quantification of Wg levels (Figs. 1 K and 2 F′), total intensity of Wg staining was measured over the area of apical cells and along the basement membrane between the apical cells and the FSCs. Relative intensity was calculated by setting values in control samples to 1. For quantification of fz3-RFP activity (Fig. 2 G′), mean RFP intensity was measured over FSCs and follicle precursor cells in region 2b and relative intensity was calculated by setting the control value to 1. For quantification of Dlp staining (Fig. 3, I and I′), mean intensities were measured over the terminal filament (TF) cell region and over the rest of the germaria, and the ratio of the former to the latter was calculated for each genotype.
Statistics
To determine the number of stalk cells, ovaries were stained with anti-LamC to label the nuclear membrane of stalk cells, then the cell number in the first stalk (posterior to region 3 of the germarium; Fig. 1 A) and the second stalk (posterior to stage 1–2 egg chamber; Fig. 1 A) were counted. To determine the number of dividing follicle cells per germarium, ovaries were stained with anti–phospho-Histone H3, and the number of positively labeled follicle cells in region 2b and region 3 of the germarium (Fig. 1 A) was counted. For lineage analysis in Fig. 2 D, germaria were examined for the presence of GSC or FSC clones positively labeled by lacZ staining, and the percentages containing at least one labeled clone were determined. Mean values were calculated from four independent experiments, and for each experiment, ∼60–100 germaria were scored. Stem cell clones were identified by aging the flies for 7 d after clone induction to allow all transit clones to exit the germaria before dissection. GSC clones were further confirmed by their location (adjacent to cap cells), and FSC clones were confirmed by their low level of Fas3 staining, triangular shape, and location at the border of regions 2a and 2b. A Student’s t test (two-tailed, two-sample equal variance) was used for statistical analysis and a P-value of <0.05 was considered significant.
Online supplemental material
Fig. S1 shows that extracellular anti-Wg staining in the germaria is specific. Fig. S2 shows the assessment of Wg activity-reporter candidates in the germaria. Fig. S3 describes Mmp2 mutant alleles and additional phenotypes. Fig. S4 shows Mmp2-EGFP-GPI structure and expression, GAL4 domains, and Mmp2 knockdown in follicle cells. Fig. S5 show supplementary biochemical analysis in cell culture. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201403084/DC1.
Supplementary Material
Acknowledgments
We thank K. Cadigan, R. DasGupta, D. Drummond-Barbosa, A. Gonzalez-Reyes, N. Perrimon, S. Selleck, G. Struhl, the Bloomington Drosophila Stock Center, TRiP at Harvard Medical School (National Institutes of Health/NIGMS R01-GM084947), National Institute of Genetics Fly Stock Center (Japan), and the Vienna Drosophila RNAi Center for fly stocks; S. Cohen, X. Lin, H. Nakato, and Addgene for plasmids; the Drosophila Genomics Resource Center for cell lines; H. Bellen, I. Duncan, and the Developmental Studies Hybridoma Bank for antibodies. We thank W. Parks, E. Lee, and G. Bhave for suggestions on the biochemistry; K. LaFever for technical support; U. Tepass for ideas; and L. Lee and W. Ramos-Lewis for comments on the manuscript.
This work was supported by National Institutes of Health grants R03 HD074834 and R01 GM073883 to A. Page-McCaw.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- Arm
- Armadillo
- BAC
- bacterial artificial chromosome
- BMP
- bone morphogenetic protein
- Dally
- division abnormally delayed
- Dlp
- Dally-like protein
- DSHB
- Developmental Studies Hybridoma Bank
- EMS
- ethyl methanesulfonate
- FSC
- follicle stem cell
- fz3
- frizzled 3
- GAG
- glycosaminoglycan
- GPI
- glycosylphosphatidylinositol
- GSC
- germline stem cell
- Hh
- Hedgehog
- hsFLP
- heat shock-induced Flippase
- MMP
- matrix metalloproteinase
- Ptc
- Patched
- RFP
- red fluorescent protein
- TF
- terminal filament
- Timp
- tissue inhibitor of metalloproteinase
- ts
- temperature sensitive
- Wg
- Wingless
References
- Alexandre, C., Baena-Lopez A., and Vincent J.P.. 2014. Patterning and growth control by membrane-tethered Wingless. Nature. 505:180–185 10.1038/nature12879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg, G.H., Selva E.M., Goodman R.M., Dasgupta R., and Perrimon N.. 2004. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev. Biol. 276:89–100 10.1016/j.ydbio.2004.08.023 [DOI] [PubMed] [Google Scholar]
- Belenkaya, T.Y., Han C., Yan D., Opoka R.J., Khodoun M., Liu H., and Lin X.. 2004. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 119:231–244 10.1016/j.cell.2004.09.031 [DOI] [PubMed] [Google Scholar]
- Bolívar, J., Pearson J., López-Onieva L., and González-Reyes A.. 2006. Genetic dissection of a stem cell niche: the case of the Drosophila ovary. Dev. Dyn. 235:2969–2979 10.1002/dvdy.20967 [DOI] [PubMed] [Google Scholar]
- Broderick, S., Wang X., Simms N., and Page-McCaw A.. 2012. Drosophila Ninjurin A induces nonapoptotic cell death. PLoS ONE. 7:e44567 10.1371/journal.pone.0044567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro, M.I., Xiang Y.Y., Lobe C., and Filmus J.. 2005. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 65:6245–6254 10.1158/0008-5472.CAN-04-4244 [DOI] [PubMed] [Google Scholar]
- Clevers, H., and Nusse R.. 2012. Wnt/β-catenin signaling and disease. Cell. 149:1192–1205 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Cox, R.T., Pai L.M., Kirkpatrick C., Stein J., and Peifer M.. 1999. Roles of the C terminus of Armadillo in Wingless signaling in Drosophila. Genetics. 153:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun, M.R., Ess J., and Saunders S.. 2001. Simpson Golabi Behmel syndrome: progress toward understanding the molecular basis for overgrowth, malformation, and cancer predisposition. Mol. Genet. Metab. 72:279–286 10.1006/mgme.2001.3150 [DOI] [PubMed] [Google Scholar]
- De Cat, B., Muyldermans S.Y., Coomans C., Degeest G., Vanderschueren B., Creemers J., Biemar F., Peers B., and David G.. 2003. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J. Cell Biol. 163:625–635 10.1083/jcb.200302152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decock, J., Thirkettle S., Wagstaff L., and Edwards D.R.. 2011. Matrix metalloproteinases: protective roles in cancer. J. Cell. Mol. Med. 15:1254–1265 10.1111/j.1582-4934.2011.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejima, K., Kanai M.I., Akiyama T., Levings D.C., and Nakato H.. 2011. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J. Biol. Chem. 286:17103–17111 10.1074/jbc.M110.208082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau, W., Barker N., and Clevers H.. 2007. WNT signaling in the normal intestine and colorectal cancer. Front. Biosci. 12:471–491 10.2741/2076 [DOI] [PubMed] [Google Scholar]
- Desbordes, S.C., and Sanson B.. 2003. The glypican Dally-like is required for Hedgehog signalling in the embryonic epidermis of Drosophila. Development. 130:6245–6255 10.1242/dev.00874 [DOI] [PubMed] [Google Scholar]
- Duncan, D.M., Burgess E.A., and Duncan I.. 1998. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 12:1290–1303 10.1101/gad.12.9.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster, C., Panáková D., Mahmoud A., and Eaton S.. 2007. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev. Cell. 13:57–71 10.1016/j.devcel.2007.04.019 [DOI] [PubMed] [Google Scholar]
- Fico, A., Maina F., and Dono R.. 2011. Fine-tuning of cell signaling by glypicans. Cell. Mol. Life Sci. 68:923–929 10.1007/s00018-007-7471-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fico, A., De Chevigny A., Egea J., Bösl M.R., Cremer H., Maina F., and Dono R.. 2012. Modulating Glypican4 suppresses tumorigenicity of embryonic stem cells while preserving self-renewal and pluripotency. Stem Cells. 30:1863–1874 10.1002/stem.1165 [DOI] [PubMed] [Google Scholar]
- Filmus, J., Capurro M., and Rast J.. 2008. Glypicans. Genome Biol. 9:224 10.1186/gb-2008-9-5-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesken-Nikitin, A., Hwang C.I., Cheng C.Y., Michurina T.V., Enikolopov G., and Nikitin A.Y.. 2013. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 495:241–245 10.1038/nature11979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, A.J., Lin H., Ingham P.W., and Spradling A.C.. 1996a. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 122:1125–1135 [DOI] [PubMed] [Google Scholar]
- Forbes, A.J., Spradling A.C., Ingham P.W., and Lin H.. 1996b. The role of segment polarity genes during early oogenesis in Drosophila. Development. 122:3283–3294 [DOI] [PubMed] [Google Scholar]
- Franch-Marro, X., Marchand O., Piddini E., Ricardo S., Alexandre C., and Vincent J.P.. 2005. Glypicans shunt the Wingless signal between local signalling and further transport. Development. 132:659–666 10.1242/dev.01639 [DOI] [PubMed] [Google Scholar]
- Gallet, A., Staccini-Lavenant L., and Thérond P.P.. 2008. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev. Cell. 14:712–725 10.1016/j.devcel.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Guo, Z., and Wang Z.. 2009. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 136:3627–3635 10.1242/dev.036939 [DOI] [PubMed] [Google Scholar]
- Han, C., Yan D., Belenkaya T.Y., and Lin X.. 2005. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 132:667–679 10.1242/dev.01636 [DOI] [PubMed] [Google Scholar]
- Hanahan, D., and Weinberg R.A.. 2011. Hallmarks of cancer: the next generation. Cell. 144:646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Harrison, D.A., and Perrimon N.. 1993. Simple and efficient generation of marked clones in Drosophila. Curr. Biol. 3:424–433 10.1016/0960-9822(93)90349-S [DOI] [PubMed] [Google Scholar]
- Hayashi, Y., Kobayashi S., and Nakato H.. 2009. Drosophila glypicans regulate the germline stem cell niche. J. Cell Biol. 187:473–480 10.1083/jcb.200904118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, Y., Sexton T.R., Dejima K., Perry D.W., Takemura M., Kobayashi S., Nakato H., and Harrison D.A.. 2012. Glypicans regulate JAK/STAT signaling and distribution of the Unpaired morphogen. Development. 139:4162–4171 10.1242/dev.078055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac, S., and Bilder D.. 2005. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn. 232:559–574 10.1002/dvdy.20286 [DOI] [PubMed] [Google Scholar]
- Jakubovic, B.D., and Jothy S.. 2007. Glypican-3: from the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp. Mol. Pathol. 82:184–189 10.1016/j.yexmp.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Kessenbrock, K., Dijkgraaf G.J., Lawson D.A., Littlepage L.E., Shahi P., Pieper U., and Werb Z.. 2013. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell. 13:300–313 10.1016/j.stem.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.S., Saunders A.M., Hamaoka B.Y., Beachy P.A., and Leahy D.J.. 2011. Structure of the protein core of the glypican Dally-like and localization of a region important for hedgehog signaling. Proc. Natl. Acad. Sci. USA. 108:13112–13117 10.1073/pnas.1109877108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly, D., Wang S., and Xie T.. 2011. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 138:5087–5097 10.1242/dev.067850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick, C.A., Dimitroff B.D., Rawson J.M., and Selleck S.B.. 2004. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev. Cell. 7:513–523 10.1016/j.devcel.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Kreuger, J., Perez L., Giraldez A.J., and Cohen S.M.. 2004. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev. Cell. 7:503–512 10.1016/j.devcel.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Lander, A.D., Kimble J., Clevers H., Fuchs E., Montarras D., Buckingham M., Calof A.L., Trumpp A., and Oskarsson T.. 2012. What does the concept of the stem cell niche really mean today? BMC Biol. 10:19 10.1186/1741-7007-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., and Xie T.. 2005. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21:605–631 10.1146/annurev.cellbio.21.012704.131525 [DOI] [PubMed] [Google Scholar]
- Li, Q., Park P.W., Wilson C.L., and Parks W.C.. 2002. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 111:635–646 10.1016/S0092-8674(02)01079-6 [DOI] [PubMed] [Google Scholar]
- Llano, E., Adam G., Pendás A.M., Quesada V., Sánchez L.M., Santamariá I., Noselli S., and López-Otín C.. 2002. Structural and enzymatic characterization of Drosophila Dm2-MMP, a membrane-bound matrix metalloproteinase with tissue-specific expression. J. Biol. Chem. 277:23321–23329 10.1074/jbc.M200121200 [DOI] [PubMed] [Google Scholar]
- Manseau, L., Baradaran A., Brower D., Budhu A., Elefant F., Phan H., Philp A.V., Yang M., Glover D., Kaiser K., et al. 1997. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev. Dyn. 209:310–322 [DOI] [PubMed] [Google Scholar]
- Margolis, J., and Spradling A.. 1995. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 121:3797–3807 [DOI] [PubMed] [Google Scholar]
- Marois, E., Mahmoud A., and Eaton S.. 2006. The endocytic pathway and formation of the Wingless morphogen gradient. Development. 133:307–317 10.1242/dev.02197 [DOI] [PubMed] [Google Scholar]
- Niehrs, C.2012. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13:767–779 10.1038/nrm3470 [DOI] [PubMed] [Google Scholar]
- Nystul, T., and Spradling A.. 2007. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 1:277–285 10.1016/j.stem.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Nystul, T., and Spradling A.. 2010. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 184:503–515 10.1534/genetics.109.109538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, E.R., Pancratov R., Chatterjee S.S., Changkakoty B., Pervaiz Z., and DasGupta R.. 2011. Yan, an ETS-domain transcription factor, negatively modulates the Wingless pathway in the Drosophila eye. EMBO Rep. 12:1047–1054 10.1038/embor.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw, A., Serano J., Santé J.M., and Rubin G.M.. 2003. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev. Cell. 4:95–106 10.1016/S1534-5807(02)00400-8 [DOI] [PubMed] [Google Scholar]
- Page-McCaw, A., Ewald A.J., and Werb Z.. 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8:221–233 10.1038/nrm2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, K.W., and Schier A.F.. 2011. Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 27:377–407 10.1146/annurev-cellbio-092910-154148 [DOI] [PubMed] [Google Scholar]
- Sahai-Hernandez, P., and Nystul T.G.. 2013. A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development. 140:4490–4498 10.1242/dev.098558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail, A., Sun Q., Zhao H., Bernardo M.M., Cho J.A., and Fridman R.. 2008. MT4-(MMP17) and MT6-MMP (MMP25), A unique set of membrane-anchored matrix metalloproteinases: properties and expression in cancer. Cancer Metastasis Rev. 27:289–302 10.1007/s10555-008-9129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X., and Xie T.. 2003. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 130:3259–3268 10.1242/dev.00524 [DOI] [PubMed] [Google Scholar]
- Song, X., Zhu C.H., Doan C., and Xie T.. 2002. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 296:1855–1857 10.1126/science.1069871 [DOI] [PubMed] [Google Scholar]
- Stevens, L.J., and Page-McCaw A.. 2012. A secreted MMP is required for reepithelialization during wound healing. Mol. Biol. Cell. 23:1068–1079 10.1091/mbc.E11-09-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini, M., and Cohen S.M.. 2000. Wingless gradient formation in the Drosophila wing. Curr. Biol. 10:293–300 10.1016/S0960-9822(00)00378-X [DOI] [PubMed] [Google Scholar]
- Uhlirova, M., and Bohmann D.. 2006. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25:5294–5304 10.1038/sj.emboj.7601401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken, K.J., Kasprowicz J., Kuenen S., Yan J., Hassan B.A., and Verstreken P.. 2008. Recombineering-mediated tagging of Drosophila genomic constructs for in vivo localization and acute protein inactivation. Nucleic Acids Res. 36:e114 10.1093/nar/gkn486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken, K.J., Carlson J.W., Schulze K.L., Pan H., He Y., Spokony R., Wan K.H., Koriabine M., de Jong P.J., White K.P., et al. 2009. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat. Methods. 6:431–434 10.1038/nmeth.1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vied, C., Reilein A., Field N.S., and Kalderon D.. 2012. Regulation of stem cells by intersecting gradients of long-range niche signals. Dev. Cell. 23:836–848 10.1016/j.devcel.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, S., Xie Z., Filenova E., and Brew K.. 2003. Drosophila TIMP is a potent inhibitor of MMPs and TACE: similarities in structure and function to TIMP-3. Biochemistry. 42:12200–12207 10.1021/bi035358x [DOI] [PubMed] [Google Scholar]
- Wu, Y., Belenkaya T.Y., and Lin X.. 2010. Dual roles of Drosophila glypican Dally-like in Wingless/Wnt signaling and distribution. Methods Enzymol. 480:33–50 10.1016/S0076-6879(10)80002-3 [DOI] [PubMed] [Google Scholar]
- Xu, T., and Rubin G.M.. 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 117:1223–1237 [DOI] [PubMed] [Google Scholar]
- Yan, D., and Lin X.. 2007. Drosophila glypican Dally-like acts in FGF-receiving cells to modulate FGF signaling during tracheal morphogenesis. Dev. Biol. 312:203–216 10.1016/j.ydbio.2007.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, D., and Lin X.. 2009. Shaping morphogen gradients by proteoglycans. Cold Spring Harb. Perspect. Biol. 1:a002493 10.1101/cshperspect.a002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, D., Wu Y., Feng Y., Lin S.C., and Lin X.. 2009. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev. Cell. 17:470–481 10.1016/j.devcel.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa, S., Lee J.S., and Ishimoto A.. 1998. Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to wingless signaling. J. Biol. Chem. 273:32353–32359 10.1074/jbc.273.48.32353 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., and Kalderon D.. 2001. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 410:599–604 10.1038/35069099 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., You J., Ren W., and Lin X.. 2013. Drosophila glypicans Dally and Dally-like are essential regulators for JAK/STAT signaling and Unpaired distribution in eye development. Dev. Biol. 375:23–32 10.1016/j.ydbio.2012.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.