Abstract
Cardio-facio-cutaneous syndrome (CFC) is one of the RASopathies that bears many clinical features in common with the other syndromes in this group, most notably Noonan syndrome and Costello syndrome. CFC is genetically heterogeneous and caused by gene mutations in the Ras/mitogen-activated protein kinase pathway. The major features of CFC include characteristic craniofacial dysmorphology, congenital heart disease, dermatologic abnormalities, growth retardation, and intellectual disability. It is essential that this condition be differentiated from other RASopathies, as a correct diagnosis is important for appropriate medical management and determining recurrence risk. Children and adults with CFC require multidisciplinary care from specialists, and the need for comprehensive management has been apparent to families and health care professionals caring for affected individuals. To address this need, CFC International, a nonprofit family support organization that provides a forum for information, support, and facilitation of research in basic medical and social issues affecting individuals with CFC, organized a consensus conference. Experts in multiple medical specialties provided clinical management guidelines for pediatricians and other care providers. These guidelines will assist in an accurate diagnosis of individuals with CFC, provide best practice recommendations, and facilitate long-term medical care.
Keywords: cardio-facio-cutaneous syndrome, BRAF mutation, management guidelines, MEK1 mutation, MEK2 mutation, RASopathy
Cardio-facio-cutaneous syndrome (CFC) is a multiple congenital anomaly disorder that belongs to a group of syndromes known as RASopathies.1 Although CFC has distinctive characteristics, many of the clinical features overlap with 2 other RASopathies, namely Noonan syndrome (NS) and Costello syndrome (CS), therefore making the diagnosis challenging, especially in the newborn period. CFC is an autosomal dominant disorder with the vast majority of cases arising by a new mutation of BRAF, MEK1, MEK2 or rarely, KRAS genes. The most common findings include dysmorphic craniofacial features, congenital heart disease, dermatologic abnormalities, failure to thrive, gastrointestinal dysfunction, neurocognitive delay, and seizures. Although the worldwide prevalence of CFC is unknown, the prevalence of CFC in Japan is estimated at 1 in 810 000 individuals.2
It is often challenging to differentiate CFC from other RASopathies; therefore, CFC International convened an international conference on November 5–6, 2012, composed of health care providers and physician-scientists with expertise in CFC to aid in establishing a correct diagnosis and provide optimum clinical management to patients. The goal of this conference was to review the most current medical and scientific information about CFC, and develop guidelines for its diagnosis and clinical management. With this, we set forth timely recommendations for best practices and comprehensive medical management for individuals with CFC (Table 1).
TABLE 1.
Management Recommendations for CFC
| Clinical Specialty | Recommendations |
|---|---|
| Genetics | At risk for CFC based upon physical examination, developmental history, and medical history. |
| At diagnosis: | |
| • Genetics consultation. | |
| • Genetic testing directed by a geneticist/genetics provider using multigene Ras/MAPK pathway panel testing (if available). Approximately 80% mutation detection rate for CFC. | |
| • Consider sequential gene testing if panel testing is not available: (1) BRAF, (2) MEK1 and MEK2, and (3) KRAS | |
| • Parental testing if variant of uncertain significance is detected. | |
| • Consider high-resolution chromosome microarray if gene testing is negative. | |
| • If the above testing is negative, the geneticist/genetics provider can determine if exome sequencing is appropriate. | |
| Ongoing management: | |
| • Annual follow-up with geneticist/genetics provider or specialty Ras pathway clinic. | |
| Cardiovascular | At risk for pulmonary stenosis, HCM, septal defects. |
| At diagnosis: | |
| • Echocardiogram, electrocardiogram. | |
| • Consultation with a cardiologist if murmur present or if clinical features suggest CFC or other RASopathy. | |
| Ongoing management: | |
| • Cardiology follow-up if cardiac disease found at diagnosis or at each of the age intervals below. Cardiologist will decide on necessity for cardiac catheterization, interventional procedures, or surgical procedures depending on the individual cardiac abnormalities of each patient. | |
| • Infancy up to 1 y: If arrhythmias present, 24-h Holter evaluation. | |
| • Childhood and adolescence (up to 20 y): If no cardiac disease found initially, repeat echocardiogram every 2–3 y. Measurement of blood pressure at each visit. | |
| • Adulthood (>20 y): Echocardiogram every 3–5 y if no previous heart disease found. Measurement of blood pressure at each visit. | |
| Dermatologic | At risk for keratosis pilaris, ulerythema ophryogenes, eczema, progressive multiple pigmented nevi, dystrophic nails, lymphedema, hemangiomas, hyperkeratosis, and generalized hyperpigmentation. |
| At diagnosis: | |
| • Consultation with a dermatologist. | |
| • Evaluation of hemangiomas. | |
| • Evaluation of pigmented nevi. | |
| • If lymphedema present, referral to vascular specialist/clinic. | |
| Ongoing management: | |
| • Frequent dermatology visits for management of xerosis, hyperkeratosis, and eczema. | |
| • Annual evaluation of pigmented nevi. | |
| • Referral to a podiatrist for dystrophic nails or hyperkeratosis if needed. | |
| • Monitor for lymphedema. | |
| • Meticulous skin care and early treatment of skin infection in the context of lymphedema. | |
| • Sun protection as recommended for the general population (ie, sunscreen; hats). | |
| Neurologic | At risk for infantile spasms, seizures, hydrocephalus, type I Chiari malformation, and other structural brain anomalies. |
| At diagnosis: | |
| • Referral to neurologist for a baseline evaluation. | |
| • Families should receive anticipatory guidance about the risk of seizures (infantile spasms, other seizure types) and other neurologic issues. | |
| • Brain MRI should be obtained in cases of rapid increase in head growth, infantile spasms, changes in neurologic examination, and regression of skills. | |
| • EEG if there is a suspicion of seizure activity. | |
| • Accurate seizure classification with clinical history and EEG to help guide medical management. | |
| Ongoing management: | |
| • Continued follow-up with neurologist for seizure management (if present). | |
| • In child with infantile spasms, consult with cardiologist for possible steroid management due to risk of cardiomyopathy. | |
| • If peripheral neuropathy suspected, consult with neurologist for nerve conduction velocities and electromyogram. | |
| Cognitive and behavioral | At risk for intellectual disability, delayed fine and gross motor skills, emotional and behavioral problems, atypical sensory processing, and speech/language impairments. |
| At diagnosis: | |
| • Referral to early childhood services or local school system for needs assessment and intervention. | |
| • Speech and language evaluation including assessment of oral-motor functioning, articulation, and expressive/receptive language ability. Speech and language therapy as indicated based on evaluation. For severe delays, consideration of alternative or augmentative communication systems. | |
| • Physical therapy (specific attention to hypotonia and gross motor delay). | |
| • Occupational therapy (specific attention to hypotonia, sensory integration, and vision concerns). | |
| • Behavioral therapy, mental health services, and/or alternative therapies may be considered to address behavioral, sensory, motor, social, emotional, and/or communication concerns. | |
| Ongoing management: | |
| • Continued evaluation and services by early childhood intervention programs in early childhood. | |
| • Upon school entry, physician referral for a full neuropsychological evaluation. | |
| • Upon school entry, school professionals and families should collaboratively develop an Individualized Education Plan (IEP) and/or other accommodation plan. Clarity should be established regarding medical diagnosis and eligibility for special education services. | |
| • If behavioral concerns exist, a functional behavior assessment may be indicated to assist in development of a behavior intervention plan (specific attention to sensory concerns, communication skills, and attentional ability). | |
| Gastrointestinal | At risk for feeding and/or swallowing difficulties, FTT, constipation, gastroesophageal reflux, and intestinal malrotation. |
| At diagnosis: | |
| • Nutrition assessment/growth measurements by primary physician. | |
| • Refer to gastroenterologist in early infancy for feeding difficulties, gastroesophageal reflux, and poor growth. | |
| • If feeding difficulties are present, referral for feeding therapy evaluation and recommendations. | |
| • Evaluate for gastroesophageal reflux and swallowing dysfunction by swallowing studies, pH studies, upper gastrointestinal series, and endoscopy studies as recommended by gastroenterologist. | |
| • Consider treatment with proton pump inhibitors for gastroesophageal reflux. | |
| • Consider assisted feeding for FTT (nasogastric or gastrostomy tube), found to be necessary in 40% to 50% with CFC. Surgical recommendations to be assisted by gastroenterologist. | |
| • If feeding difficulties are present, then refer for feeding therapy evaluation. | |
| Ongoing management: | |
| • Regular follow-up to monitor growth and nutrition. | |
| • Continued feeding therapy if there are persistent feeding difficulties. | |
| • Treatment of gastroesophageal reflux and constipation as needed as children get older | |
| Growth/endocrine | At risk for failure to thrive, short stature, GH deficiency, GH resistance, and delayed puberty. |
| At diagnosis: | |
| • Refer to endocrinologist between ages 2 and 3 y for growth monitoring or earlier if there are concerns about growth. | |
| • Obtain thyrotropin, free thyroxine, IGF-1, and IGF-BP3 levels because thyroid and GH abnormalities are seen in other RASopathies. | |
| • Nutritional assessment/growth measurements by primary physician. | |
| Ongoing management: | |
| • Monitor growth carefully (height, weight, and head circumference at each visit) and refer to appropriate specialists if significant change in growth curves (eg, endocrinologist, gastroenterologist, neurologist, or neurosurgeon). | |
| • Regular follow-up by endocrinologist if growth failure, GH deficiency, or thyroid hormone abnormality. | |
| • If growth failure: thyroid function studies, celiac disease screening, GH stimulation studies to be directed by endocrinologist. | |
| • Monitor pubertal development beginning around age 10 y. | |
| Musculoskeletal | At risk for hypotonia with decreased muscle, scoliosis, pes planus, joint contractures, hip dysplasia, and pectus deformities. |
| At diagnosis: | |
| • Referral to a pediatric orthopedist. | |
| Ongoing management: | |
| • Radiograph of thoracolumbar spine, pelvis and lateral radiograph of cervical spine depending on clinical findings of child. | |
| • For those who are not ambulatory: AP radiographs of pelvis every 2 y to monitor for hip dysplasia. | |
| • Monitor for scoliosis. | |
| • Spine MRI before any spinal surgery. | |
| • Long-term follow-up with orthopedist as appropriate. | |
| • Before orthopedic surgery, see hematologic recommendations. | |
| • Bone density scan in young adults. | |
| Ophthalmology | At risk for ptosis, amblyopia, refractive errors, strabismus, cataracts, optic nerve hypoplasia, optic atrophy, cortical visual impairment, delayed visual maturation, and abnormal depth perception. |
| At diagnosis: | |
| • Referral to a pediatric ophthalmologist. | |
| • Early intervention as appropriate (ie, correction of ptosis, prescription glasses for refractive errors or strabismus, patching for amblyopia). | |
| Ongoing management: | |
| • Follow-up every 6–12 mo or more frequently as recommended by ophthalmologist. | |
| • Visual functional assessment by early childhood programs and vision resources for poor vision and abnormal depth perception. | |
| • In those with optic nerve abnormalities, MRI of brain should be obtained to screen for malformations that could cause optic atrophy. | |
| Otolaryngology/audiology | At risk for narrow ear canals, impacted cerumen, hearing loss, and laryngomalacia. |
| At diagnosis: | |
| • Audiologic evaluation. | |
| • Refer to otolaryngologist for airway evaluation/management in the case of neonatal or early respiratory issues. | |
| • Referral to otolaryngologist for hearing loss management. | |
| Ongoing management: | |
| • Audiologic assessment every 2–3 y, or more frequently if necessary. Hearing aids as needed. | |
| • Refer to otolaryngologist for impacted cerumen, abnormal audiologic testing, and hearing loss. | |
| • Prompt treatment of ear infections to minimize hearing loss. | |
| • Otolaryngology follow-up for management of airway. | |
| Renal/genitourinary | At risk for kidney malformation, vesicoureteral reflux, and cryptorchidism. |
| At diagnosis: | |
| • Renal ultrasound to evaluate for structural renal anomalies. | |
| • Refer to urologist and endocrinologist if cryptorchidism present. | |
| Ongoing management: | |
| • As indicated by urologist. | |
| Hematology/oncology | At risk for easy bruising, von Willebrand disease, and thrombocytopenia. |
| At diagnosis: | |
| • Obtain history regarding easy bruising or bleeding problems. | |
| • If easy bruising or bleeding problems are present, screen with CBC, platelet count, platelet function study, and von Willebrand screen. | |
| • Refer to a hematologist for an abnormal CBC. | |
| Ongoing management: | |
| • If evidence of easy bruising or bleeding appears with time, then screen with CBC, platelet count, platelet function study, and von Willebrand screen. | |
| • If patient requires surgery, screen with the above testing before surgery if not already done. | |
| • For those on divalproex sodium for seizures, obtain platelet count every 6 mo. | |
| Dental | At risk for malocclusion, posterior crossbite, and bruxism. |
| At diagnosis: | |
| • Dental evaluation. | |
| Ongoing management: | |
| • Appropriate hygiene. | |
| • Restorative care. | |
| • Orthodontic treatment as needed. |
CBC, complete blood cell; FTT, failure to thrive.
History
CFC was first reported in 1986 by Reynolds et al3 and by Baraitser and Patton.4 Eight individuals were described with distinct facial features, ectodermal abnormalities, cardiac malformations, and intellectual disability.3 At that time, many believed that the facial features of CFC overlapped with those of NS, and a controversy emerged regarding the delineation of CFC as a new, distinct syndrome versus a severe form of NS.5
In 2001, PTPN11 was found to be one of the causal genes of NS, and it was possible to demonstrate that well-characterized individuals with CFC did not have a mutation in PTPN11.6–8 Furthermore, individuals with CFC did not carry mutations in the HRAS gene, which causes CS, another phenotypically similar condition.9 Identification of these 2 genes for NS and CS suggested that CFC, NS, and CS were separate, distinct conditions, with overlapping phenotypes.
The final proof came in 2006 when 2 groups demonstrated that CFC is a heterogeneous disorder caused by mutations in 4 different genes: BRAF,10,11 MEK1,11 MEK2,11 and KRAS.10 These discoveries led to the recognition that CFC was a distinct syndrome separate from NS and CS and also explained that the similarity between them lay in a common underlying molecular pathway, the Ras/mitogen-activated protein kinase (MAPK) pathway.1,12
Molecular Characterization
The Ras/MAPK pathway is one of the most studied signaling cascades and is well known for its role in oncogenesis and tumor progression. This pathway is critically involved in cell proliferation, differentiation, motility, apoptosis, and senescence. CFC is caused by dysregulation of the Ras/MAPK pathway due to heterozygous activating mutations in protein kinases, BRAF,10,11 MEK1,11 or MEK2.11 Mutations in KRAS, a small GTPase, have been implicated as causing both CFC and NS.10
Heterozygous BRAF mutations are found in ∼75% of mutation-positive CFC individuals.1 BRAF, an onco-protein, is a serine/threonine protein kinase and one of downstream effectors of Ras. Most BRAF mutations are missense and found in exons 6 and 12 and confer an activation of the oncoprotein. The most common BRAF mutations occur in exon 6 (p.Q257R), in exon 12 at p.E501, and in exon 11 (p.G469E). Heterozygous mutations in MEK1 (MAP2K1) and MEK2 (MAP2K2) are present in ∼25% of CFC individuals in which a gene mutation has been identified.1 The most common missense MEK mutation is MEK1 p.Y130C. MEK1 and MEK2 are threonine/tyrosine kinases, with both isoforms having the ability to phosphorylate and activate ERK1 and ERK2. Functional studies of MEK mutant CFC proteins have shown that all are activating.11,13
CFC is typically the result of a de novo heterozygous mutation. It is very rare for individuals with CFC to reproduce.14 Germline mosaicism has yet to be reported.
Genotype/Phenotype Correlations
Few genotype–phenotype studies of CFC have been performed to date. One statistically significant genotype–phenotype correlation is the incidence of pulmonic stenosis, which is present in 50% of individuals with CFC with a BRAF gene mutation and 37% of those with a MEK gene mutation.15 It also appears that, while not statistically significant, MEK mutations may be associated with a higher likelihood of prematurity, ventricular septal defects, pectus deformity, urogenital abnormalities, and dermatological abnormalities,15,16 whereas BRAF mutations may be more commonly associated with hypertrophic cardiomyopathy (HCM), atrial septal defects, moderate to severe intellectual disability, and significant feeding difficulties.15
Among the RASopathies as a whole, there are relatively well-established genotype–phenotype correlations with respect to cognitive development. Mutations associated with CFC and CS tend to correspond to greater impairments in intellectual and adaptive functioning than mutations associated with NS.17–19 Although some reports indicate that individuals with MEK mutations have milder intellectual disabilities when compared with those with BRAF mutations, the numbers are still too small to reach statistical significance.15,20 There is some preliminary evidence to suggest that the most common BRAF missense mutation, p.Q257R, may be associated with fewer developmental delays than other BRAF mutations.19 Further studies are required to determine if this will be confirmed.
KRAS mutations can be found in individuals with the clinical diagnosis of both CFC and NS.10,21,22 In general, individuals with a KRAS mutation have mild to moderate intellectual disability, dysmorphic facial features, short stature, dermatologic abnormalities, and ophthalmologic abnormalities. Regardless of phenotypic assignment, it is essential to establish the molecular etiology of all individuals with CFC to provide appropriate medical care. Additionally, the molecular confirmation will be important for genetic counseling and understanding of recurrence risk and natural history.
Genetic Testing for CFC Syndrome
All individuals suspected to have CFC should undergo examination and molecular testing directed by a geneticist who can determine the most appropriate and cost effective type of testing and can provide explanations of the results obtained to the family and other health providers. Current commercial tests for the molecular diagnosis of CFC include the following: (1) next-generation sequencing of multigene panels of the common RASopathy genes, (2) individual single gene sequencing, and (3) chromosome microarray to screen for copy number abnormalities. Multigene panel testing is usually the preferred initial test and will detect a mutation in ∼70% to 90% of individuals with the clinical diagnosis of CFC.15 If panel testing is not available, sequential gene testing is recommended, beginning with BRAF, MEK1 and MEK2, and KRAS. In the event that a variant of uncertain significance is identified, parental testing and physical examination is recommended to determine if the variant was inherited. If molecular testing of the CFC genes is negative, the geneticist can arrange for testing of the other Rasopathy genes if they have not yet been done and consider other conditions in the differential diagnosis (Table 2). If these are negative, the geneticist may consider a chromosome microarray analysis to screen for microdeletions/microduplications that can be associated with phenotypes that partially overlap with CFC.23–26 If all of the above are negative, the geneticist/genetics provider can consider whole exome or genome sequencing for diagnosis.
TABLE 2.
Differential Diagnosis of CFC and Other Conditions
| Syndrome | Common Features With CFC | Differences With CFC |
|---|---|---|
| NS | Hypertelorism, downslanting palpebral fissures, ptosis, short stature, relative macrocephaly, PVS, HCM, atrial septal defect, and some with cognitive delay. | Facial features less coarse, lower incidence of severe feeding problems, fewer cutaneous features such as follicular hyperkeratosis, sparse eyebrows, ulerythema ophryogenes, and less incidence of marked cognitive delay. |
| CS | Coarse facial features, curly hair, broad nasal bridge, downslanting palpebral fissures, epicanthal folds, PVS, HCM, pectus deformity, short stature, and intellectual disability. | Papillomata of the face or perianal regions, multifocal atrial tachycardia, ulnar deviation of wrist and fingers, and loose skin. |
| NS with multiple lentigines (formerly LEOPARD syndrome) | Short stature, hypertelorism, PVS, HCM, and some with cognitive delay. | Multiple skin lentigines, frequent sensorineural deafness, cardiac conduction abnormalities, and less incidence of marked cognitive delay. |
| Baraitser-Winter syndrome | Hypertelorism, ptosis, short stature, and cognitive delay. | Iris coloboma, lissencephaly, pachygyria, aortic valve anomalies, and facial features less coarse. |
| NS with loose anagen hair | Triangular facies, hypertelorism, high forehead, sparse thin hair, short stature, eczema, dry skin, and macrocephaly. | After infancy facial features less coarse, mitral valve dysplasia, and less incidence of marked cognitive delay. |
Clinical Description With Differential Diagnosis
Clinical features of CFC begin in the prenatal period with ultrasound findings of increased nuchal lucency and, sometimes, cystic hygroma.27,28 Polyhydramnios is very common (77%) and premature birth is present in up to a half of pregnancies.29
Craniofacial characteristics, coupled with features such as cardiac disease, feeding difficulty, and developmental delay, are often the first diagnostic clues after birth suggesting the diagnosis of CFC. In 2002, a CFC index was developed based on 82 clinical traits to assist with diagnosis.30 However, at present, there are no pathognomonic or obligatory features uniquely associated with CFC that can aid in making a definitive clinical diagnosis.
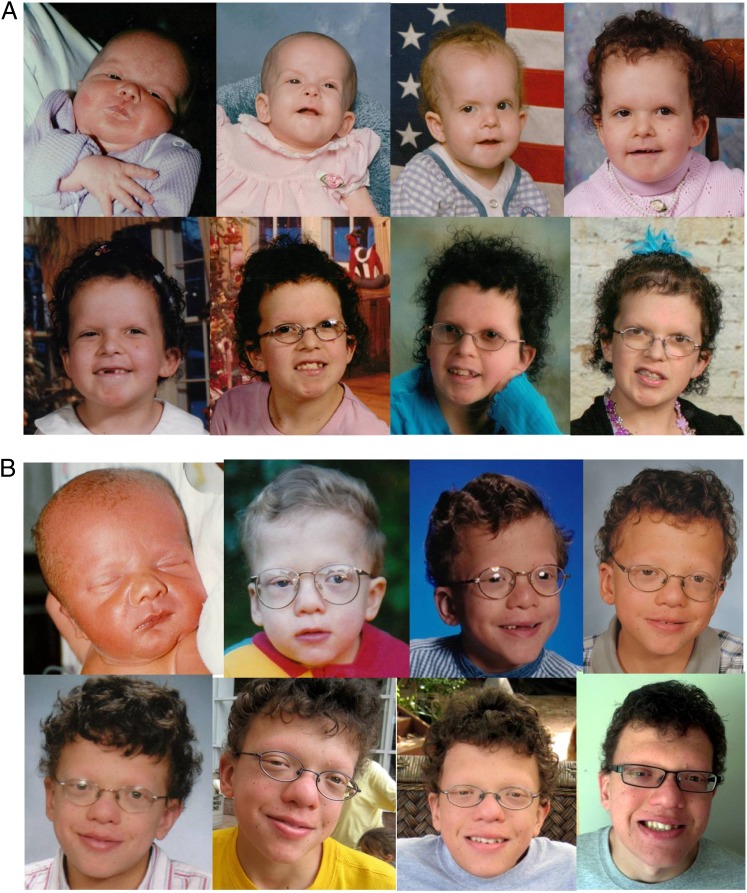
The head and face are characterized by macrocephaly/relative macrocephaly, tall forehead with bitemporal narrowing, increased facial width and depth, coarse facial features, and small chin.3,31–33 Sparse, curly, and friable hair, with sparse or absent eyebrows with hyperkeratosis (ulerythema ophryogenes) are very common in CFC and occur with greater frequency than in other RASopathies.3,16,32 Ocular features include ptosis, hypertelorism, downslanting palpebral fissures, and epicanthal folds. The nose is often short with a broad nasal base, bulbous tip, and anteverted nares.3,31,32 The mouth is wide with a deeply grooved philtrum and Cupid’s bow contour of the upper vermillion border.32 The palate is narrow and high arched with a short, broad or bifid uvula.32 Ears are posteriorly angulated and low set. With age, the face may become coarser and facial characteristics less pronounced (Fig 1 A and B).15,32
FIGURE 1.
A, Patient with CFC (BRAF p.Q257R missense mutation) at (from left to right) 4 days, 3 months, 3 years, 5 years, 7 years, 12 years, 14 years, and 16 years. B, Patient with CFC (MEK2 p.F57C missense mutation) at (from left to right) 1 day, 3 years, 7 years, 9 years, 13 years, 14 years, 16 years, and 20 years.
Many of the clinical features of CFC overlap with those of NS and CS (Table 2). Other conditions to be considered in the differential diagnosis of CFC include NS with multiple lentigines, Baraitser-Winter syndrome, and NS with loose anagen hair. Because of differences in the medical management, long-term prognosis, and genetic risks, obtaining the correct diagnosis is very important among these syndromes with overlapping features.
Cardiac Issues
Nearly 75% of individuals with CFC have cardiovascular involvement of various kinds (Table 3).15,32 As soon as clinical findings of CFC are recognized or a diagnosis of CFC is confirmed, a consultation with a pediatric cardiologist and echocardiography should be obtained. The most common cardiac problem is pulmonary valvular stenosis (PVS), present in ∼45% of individuals, sometimes with a dysplastic pulmonary valve.15,29,32 PVS may occur alone or concomitantly (in nearly 20%) with other cardiac defects, such as atrial septal defect or HCM. The cardiologist may consider cardiac catheterization and balloon angioplasty if the PVS is severe, although it is not always successful. Surgery may also be recommended by the cardiologist in preference to balloon angioplasty, especially if there are multiple cardiac defects. The surgery might include pulmonary valvotomy and correction of other defects as necessary.
TABLE 3.
Summary of Occurrence of Specific Clinical Manifestations in CFC
| Common | May Occur | Rare |
|---|---|---|
| Cardiac | ||
| Pulmonary valve stenosis | Supravalvular pulmonary stenosis | Aortic root dilation |
| HCM | Bicuspid aortic valve | Restrictive cardiomyopathy |
| Atrial septal defect | Coarctation of the aorta | Ebstein’s anomaly of the tricuspid valve |
| Ventricular septal defect | Coronary artery abnormality | |
| Mitral valve anomalies | Aortic valve thickening | |
| Rhythm disturbance | ||
| Dermatologic | ||
| Ulerythema ophryogenes | Acanthosis nigricans | Papillomas |
| Keratosis pilaris/hyperkeratosis | Hyperplastic nipples | Multiple café-au-lait macules |
| Acquired melanocytic nevi | Creases on the fingertips | |
| Hemangiomas | Foot callosities | |
| Fast growth of nails | Lymphedema | |
| Sparse arm and leg hair | Generalized hyperpigmentation | |
| Prepubertal axillary odor | ||
| Poor hair growth on scalp | ||
| Ear lobe creases | ||
| Xerosis/eczema | ||
| Neurologic | ||
| Macrocephaly | Myelination abnormality | Gray matter heterotopia |
| Hypotonia with motor delay | Virchow-Robin spaces | Abnormal corpus callosum |
| Ventriculomegaly/ hydrocephalus | Type I Chiari malformation | Arachnoid cyst |
| Neurocognitive delay | Optic nerve hypoplasia | Periventricular leukoencephalopathy |
| Seizures | Corticospinal tract findings | Peripheral neuropathy |
| Gastrointestinal | ||
| Suck/swallowing dysfunction | Intestinal malrotation | Intestinal atrophy |
| Gastroesophageal reflux | Anal stenosis | Antral narrowing |
| Constipation | Inguinal hernias | Anal papilloma |
| Fetal polyhydramnios | Fecal impaction | Hepatomegaly |
| Gastrostomy tube feedings | Functional megacolon | Fatty liver |
| Oral aversion | Umbilical hernia | Crohn disease |
| Musculoskeletal | ||
| Pes planus | Elbow contracture | Abnormal tibial torsion |
| Hand dysfunction | Kyphosis | Lordosis |
| Scoliosis | Femoral retroversion | Torticollis |
| Hip dysplasia | Hip contractures | Hallux valgus |
| Knee contractures | Pectus deformity | Equinus |
| Shoulder dysfunction | Leg pains | Radial head subluxation |
| Dysfunctional gait | Wrist instability | |
| Osteopenia | Short metatarsals | |
| Hypotonia | ||
HCM, present in nearly 40% of individuals with CFC, can occur in infancy and have a wide variation in severity, sometimes rapidly progressive, even resulting in death or cardiac transplantation.34 Others have only mild cardiac thickening that is not progressive and may improve with time. One adult with HCM detected shortly before his death at age 21 years also had pulmonary hypertension and pulmonary vascular changes (Grade 3 Heath/Edwards) on autopsy.32 In general, children and adults with CFC and mild HCM may require only periodic reevaluation with their cardiologist. Those with more significant HCM could require treatment with β-blocker medications or surgical procedures including myomectomy to decrease outflow obstruction.
Other forms of heart disease (Table 3) seen in CFC are atrial sepal defect (18% to 28%), ventricular septal defect (11% to 22%), and other rarer defects such as mitral valve dysplasia, coarctation of the aorta, and subaortic stenosis.29,31,32 Each of these conditions and those in Table 3 are managed by a cardiologist who determines reevaluation frequency and plans interventions as necessary including cardiac catheterization, medication management, device closure for septal defects, and surgical procedures.
Arrhythmias are uncommon in CFC, when compared with CS where they occur more frequently.2,10,15,35–37 Types of arrhythmias previously documented include the following: supraventricular tachycardia, atrioventricular block, and Wolff–Parkinson–White.15 Consultation with a cardiologist is recommended for management of arrhythmias.
Little is known about the natural history of heart disease in CFC and whether heart abnormalities such as myocardial thickening/dysfunction (HCM) can develop at older ages. All patients with CFC, even those who have had previous normal echocardiograms or underwent surgical repair of congenital heart defects as young children, should have periodic cardiac reevaluation by a cardiologist.
Dermatologic Manifestations
Dermatologic manifestations are cardinal features of CFC, important for clinical diagnosis and differentiating CFC from other RASopathies.36 Although the severity and type of ectodermal involvement varies (Table 3), virtually all CFC individuals develop some form of cutaneous involvement and for this reason, dermatologic consultation and follow-up are recommended.16,22,38 Among the manifestations are wavy or curly scalp hair, with sparse hair at the temples, poor hair growth, and sparse arm and leg hair. Ulerythema ophryogenes, characterized by brow erythema with loss of follicles, causes sparse or absent eyebrows with only 10% of individuals having normal eyebrows.16 Numerous acquired melanocytic nevi are striking features of CFC, with this finding less common in other RASopathies.39 It is not uncommon for individuals to develop over 100 nevi as they age, with the nevi not restricted to light-exposed areas.16 To date, there are no reports of malignant transformation of pigmented lesions in CFC.
Keratosis pilaris (follicular hyperkeratosis of the extremities and/or face) is also seen in the majority of cases. Individuals with CFC have a high rate of heat intolerance, may sweat excessively, and develop axillary body odor due to hyperkeratosis. Other dermatologic findings in CFC include dry skin, eczema, dystrophic nails with rapid nail growth, generalized hyperpigmentation, ear lobe creases, acanthosis nigricans, hyperplastic nipples, and creases on the fingertips. Nasal and perianal papillomata, typically seen in CS, are uncommon in CFC. Infantile hemangiomas, seen in 25% of CFC individuals, higher than other RASopathies, have a similar natural history to those in the general population. Some skin manifestations evolve over time and are usually seen in adolescence and adulthood. Palmo-plantar calluses can develop especially in pressure areas, and peripheral lymphedema is occasionally present, most often affecting the lower limbs.32 Those with lymphedema can be referred to vascular/lymphedema clinics for management. Early treatment of skin infections is recommended in those with lymphedema.
Neurologic Findings
Neurologic abnormalities are universally present in CFC and range from mild to severe.40 Hypotonia, motor delay, speech delay, and learning disability can be considered main features (Table 3). Neurologic examination typically will demonstrate macrocephaly, hypotonia, ocular abnormalities, corticospinal tract findings, touch sensitivity, and tactile defensiveness.29,41,42 Peripheral neuropathy has been rarely documented in CFC, but may be underdiagnosed.43,44 Brain MRI studies reveal structural malformations in 9% to 85% of patients with CFC.42 Abnormalities include ventriculomegaly, hydrocephalus, cortical atrophy, prominent Virchow-Robin spaces, and abnormal myelination.40,42 Other structural anomalies such as type I Chiari malformation, arachnoid cyst, gray matter heterotopia, corpus callosum abnormalities, cerebellar calcification, and periventricular leukoencephalopathy have been reported.40–42
It has been suggested that central nervous system malformations are underreported in CFC because only 38% to 50% of children have had brain MRI or computed tomography scans.42,44 The fact that many children with CFC do have structural brain malformations suggests the need for brain imaging in patients with CFC, as finding morphologic brain changes could lead to better understanding of the individual’s neurologic findings or underlying diagnosis. For example, individuals with NS have relatively few central nervous system malformations, and a brain MRI with some of the above structural abnormalities may suggest a diagnosis of CFC rather than NS.
Unlike other RASopathies, seizures of various types may affect ∼40% to 50% of children with CFC.29,40 Seizure types may include complex partial, generalized tonic-clonic, absence seizures, and/or infantile spasms.40 Infantile spasms, which are relatively rare in the general population, are a common seizure type in CFC.40,45,46 When there is clinical suspicion for infantile spasms or other seizures, an urgent sleep EEG should be obtained along with consultation with a neurologist. Prompt diagnosis and subsequent treatment is critical in infantile spasms to avoid permanent neurodevelopmental sequelae. Seizures in CFC are often refractory to medications, requiring several anticonvulsants.40,46 Polytherapy may lead to an increased risk of medication-related side effects that could impact individuals with CFC who already have learning disability and developmental delay. Long-term neurologic reevaluation can be organized by the neurology consultant on the basis of need for ongoing seizure management.
Cognitive and Behavioral
Relatively few studies have reported on neuropsychological sequelae of CFC. Skills in intellectual, motor, social, communicative, and behavioral functioning vary widely. Intellectual disability is present in 90% to 100% of individuals with CFC,30,40 yet IQs in the low average to average range have been reported among a few individuals with BRAF mutations.47,48 Gross motor skills tend to be more delayed in the early years (ie, birth to age 6 years) than fine motor skills,19 perhaps due to the high incidence of hypotonia. Mean age of walking without assistance is reported to be around 3 years, although ∼18% of individuals with CFC are unable to achieve independent walking.40 Language abilities range from limited nonverbal communication to capacity to speak in full sentences. On average, children with CFC speak their first word around 2 years of age, although 9% to 31% remain nonverbal.19,40 Progress in basic language development can continue well into adolescence. Receptive language ability is typically stronger than expressive language.19 Many families report using simple sign language or assistive technologies to facilitate communication.29
In academic settings, virtually all children with CFC receive special education services such as speech/language therapy, occupational therapy, physical therapy, and/or assistance of a paraprofessional.19 School performance can vary from profound disability (with minimal/static developmental progress) to mild disability (with achievement of basic reading, writing, and math skills).40 Behaviorally, many families report concerns related to irritability, short attention span, stubbornness, and obsessive or aggressive behaviors.43 Sensory processing problems (eg, tactile defensiveness) and a heightened risk for autism spectrum disorder traits have also been reported.29,49 Sleep problems are common, including poor sleeping patterns, night sweating, sleep apnea, and night terrors.29
Although research to evaluate the effectiveness of behavioral treatments in CFC is currently lacking, studies of individuals manifesting similar characteristics suggest likely avenues for successful intervention. Therapies focused on expanding functional communication (eg, interventions incorporating sign language, discrete-trial training or naturalistic [“milieu”] teaching) have demonstrated efficacy in individuals with intellectual disabilities and those with autism spectrum disorders.50,51 Parent-training interventions to manage behavior or promote specific skills have also proven successful.52,53 Approaches such as personalized schedules and functional behavior assessments have demonstrated effectiveness in children with similar neurologic and behavioral impairments.54,55 Finally, treatments targeting difficulties with sensory processing have the potential to improve functioning.52 Given the wide variability in clinical characteristics in CFC, tailoring intervention to each child’s unique developmental trajectory is essential, as is coordinated implementation of services across the home, medical, and educational settings.
Gastrointestinal and Growth Issues
Failure to thrive and poor growth in infancy are almost universal in CFC.2,36 These are usually accompanied by severe feeding problems including gastroesophageal reflux, suck/swallow dysfunction, and oral aversion (Table 3). The swallowing difficulties are reflected early by prenatal polyhydramnios that, after birth, is followed by difficulty in maintaining adequate oral caloric intake.41,56 Prolonged supplemental feedings via nasogastric tube feedings or gastrostomy tube placement are common (40% to 50%) in CFC. Many still require assisted feeding in late childhood and adolescence.29 Respiratory complications such as choking, aspiration pneumonia, and chronic raspy breathing are also related to the swallowing/oropharyngeal difficulties. Oral aversion and sensory integration difficulties with solid foods can persist into adulthood.
Severe feeding difficulties, gastroesophageal reflux, and failure to thrive in an infant with facial features similar to NS along with cardiac disease should suggest the possible diagnosis of CFC. An early evaluation by a gastroenterology specialist is recommended in infancy or at the time of diagnosis to allow guidance in testing and decisions regarding necessity of supplemental feedings or surgeries such as fundoplication. Feeding therapy is suggested at the first sign of oral aversion. Treatment of gastresophageal reflux with proton pump inhibitors may be of some value.
Constipation related to intestinal dysmotility is often seen in children with CFC and can remain a lifelong problem. Gastroenterologists can differentiate the constipation caused by intestinal dysmotility from other less common conditions such as intestinal malrotation, functional megacolon, anal stenosis, or rarely intestinal atrophy.41,44,57 Constipation can continue into adulthood and follow-up by a gastroenterologist may be helpful in planning a satisfactory bowel regimen.
Endocrine Issues
Short stature is a common feature in all RASopathies.1,58,59 This is not surprising since the Ras/MAPK pathway plays a major role in mediating the intracellular signaling of Insulin-like Growth Factor (IGF-1), which mediates postnatal growth effects of growth hormone (GH). In addition, MAPK activation is important in regulating the proliferation of pituitary somatotrophs that synthesize and release GH.60 Therefore, dysregulation of the Ras/MAPK signaling pathway may contribute, along with other intrinsic and extrinsic factors, to growth failure and short stature.59
It is estimated that two-thirds of children with CFC have short stature.1,3,4,58,61,62 The exact cause of short stature is not well established; some individuals may have GH deficiency, whereas some may have GH resistance. In addition, inadequate nutrition, due to the high prevalence of feeding problems in CFC, might contribute to failure to thrive, poor growth, and possibly IGF-1 deficiency. Although GH therapy is approved by the Food and Drug Administration for short stature in NS, the use of GH in CFC has not been studied. For children with NS, GH use may result in short-term improvement in growth velocity and a modest gain in final adult height.63 Studies on the benefits of GH therapy in CFC are needed. Currently, the only approved use of GH in CFC is for those with documented GH deficiency.
Monitoring linear growth throughout infancy and childhood is essential to identify growth concerns early. Individuals with height consistently below the third percentile or with poor growth velocity should be evaluated for growth failure. If weight gain is suboptimal and there are feeding difficulties and poor caloric intake, gastrointestinal evaluation is warranted.
Recent reports have suggested a higher incidence of autoimmunity in those with a RASopathy.64 Because autoimmune thyroiditis is common in the general population, measurements of thyroid function are recommended at diagnosis. Only a few instances of hypothyroidism have been documented in CFC,29 so follow-up thyroid testing can be decided by an endocrinologist. Autoimmunity in CFC is an area that could benefit from future research.
Puberty may be delayed in individuals with CFC. Health care providers should be mindful of monitoring Tanner staging annually beginning at 10 years of age. Endocrine evaluation is appropriate if onset of puberty is delayed beyond age 12 to 13 years.
Musculoskeletal Conditions
Musculoskeletal findings are seen in all RASopathies but are particularly prominent in CFC. Global hypotonia is evident on clinical examination especially in the newborn period and is manifested by delayed motor skills, muscle weakness, and decreased muscle bulk. As children grow older, muscle weakness appears to gradually improve, although individuals still may have gross motor delays.65,66 Skeletal muscle strength does not appear to completely normalize and muscle bulk usually remains underdeveloped, and may actually decrease in older individuals. The origin of hypotonia in CFC is unclear, as it may arise from central nervous system dysfunction, muscle pathology, or a combination of the two. Skeletal muscle biopsies of CFC demonstrate relatively normal architecture, but abnormal muscle fiber size and variability, with a predominance of type 2 muscle fibers.67 In addition, some preliminary observations have described individuals with CFC with oxidative phosphorylation dysfunction and muscular coenzyme Q10 deficiency.68,69
Orthopedic conditions in CFC (Table 3) occur with high frequency and cause significant disability,65 so referral to an orthopedist is recommended at diagnosis. Individuals may have scoliosis and/or kyphosis, pectus excavatum and/or carinatum, joint hyperextensibility, joint contractures, pes planus, and dysfunctional gait.29,65 The need for a walker is not uncommon.
Scoliosis occurs in ∼33% of individuals with CFC and mimics idiopathic or neurogenic scoliosis, without evidence of overt vertebral malformations.65 Type I Chiari malformations have been found on MRI studies, and such malformations have been linked to the development of scoliosis.65,70 Pes planus or planovalgus is seen in two-thirds of individuals with CFC and tends to be more severe than in the general population, with significant forefoot valgus.65 Treatment is generally nonsurgical; however, those with severe deformity and/or significant functional impairment may require surgical correction. Osteopenia has been anecdotally reported, and there is biochemical evidence of increased bone resorption in several RASopathies, including CFC; however, the clinical consequence of increased bone resorption in this population is not yet known.58
Ophthalmologic Features
Ocular manifestations in CFC occur in the majority of individuals.11,33,37,40,71 Commonly reported findings include strabismus, refractive errors, nystagmus, ptosis, and optic nerve hypoplasia.11,33,40 Craniofacial abnormalities related to the eyes include hypertelorism, epicanthal folds, and hypoplastic supraorbital ridges.71 A recent study of a large cohort of mutation-positive individuals with CFC identified the above findings, as well as problems with depth perception and reduced visual acuity.33 A number of children undergo strabismus correction in early infancy or early childhood, with exotropia being more common. Refractive errors include myopia, hyperopia, and astigmatism. Nystagmus is present in a number of individuals, some of whom adopt head postures to dampen the nystagmus that may diminish with age. Amblyopia is a common finding. Cataracts have been reported in one individual who required no intervention.11 Fundus evaluation reveals optic nerve changes ranging from hypoplastic optic disks to small but normal appearing optic disks, tilted and irregular optic disk margins, and peripapillary pigmentation and atrophy.33 Abnormalities of the anterior segment, fovea, macula, peripheral retina, or blood vessels are usually not present.
Although the majority of individuals with CFC have ocular problems, most are amenable to treatment. These include ptosis, refractive errors, and strabismus. Proper treatment depends on early referral for ophthalmologic assessment (Table 1). In those with optic nerve problems, MRI brain imaging is helpful to assess for brain abnormalities such as type I Chiari malformation or hydrocephalus that could result in optic atrophy.
Otolaryngology/Audiology Manifestations
External ear abnormalities such as low set, posteriorly rotated ears with upturned lobes are often seen in CFC.11,33,37 Other features include ear lobe creases, small indentations behind the ears, or ear pits.16,36,71 Narrow external auditory canals, excess cerumen, and necessity for ear tube placements are common.29 Sensitivity to loud sounds and hearing loss have also been reported.15 No systematic evaluation has yet been done to determine the prevalence or type of hearing loss, which affects many patients with CFC. Therefore, audiologic assessment and otolaryngology consultation is recommended on diagnosis. Laryngotracheal abnormalities such as laryngotracheomalacia and laryngeal clefts are also seen in CFC. Some young infants have required tracheostomy because of marked laryngomalacia41 and require long-term care by an otolaryngologist (Table 1).
Renal/Genitourinary Findings
Renal and genitourinary abnormalities occur in ∼17% to 33% of individuals with CFC.15,29 Renal cysts, medullary nephrocalcinosis, nephrolithiasis, hydronephrosis, and hydroureteronephrosis have all been reported.41,56 Cryptorchidism has been found in two-thirds of male patients, similar to the incidence in NS.15
Hematology/Oncology Conditions
Unlike NS, easy bruising and bleeding problems have not been reported as frequently in CFC. There is 1 report of transient thrombocytopenia in a newborn and 1 with a history of frequent epistaxis.32 One individual has been reported with von Willebrand disease.29 If easy bruising and bleeding problems are present on diagnosis or appear with time, screening for platelet abnormality and von Willebrand disease is recommended.
Although the mutations causing CFC are in a well-known oncogenic pathway, it is unclear if individuals with CFC are at an increased risk for malignancies. In ∼200 individuals with CFC reported in the literature, 3 have been found to have acute lymphoblastic leukemia,10,14,72 one with non-Hodgkin lymphoma,73 and one with large B-cell lymphoma. One individual with CFC (BRAF mutation) had a hepatoblastoma, possibly the result of postcardiac transplant immunosuppression.34 Additional studies are needed to accurately assess the risk of malignancy in CFC.74
Oral/Dental Issues
Individuals with CFC have a characteristic dental phenotype including malocclusion with open bite, posterior crossbite, high labial frenal attachment, and high-arched palate.33 Oral habits may include tongue thrusting, bruxism, and open mouth posture. Dental development typically follows the timing present in the general population with normal eruption patterns, although ∼10% of individuals may be delayed. Dental crowding may be seen, but not at a higher frequency than the general population. It is rare to observe hypodontia, gingival hyperplasia, or supernumerary teeth.29 Dental caries are typical though the enamel is clinically normal. Overall, there is not a unique dental pathology requiring specific treatment, but routine dental examinations, appropriate hygiene, restorative care, and orthodontic care if needed are recommended. Due to neurocognitive delay and oral aversion, some may exhibit anxiety during dental examinations. Thus, these individuals should be seen early and often to accustom them to dental treatment.
Summary
This report provides health professionals with current information with regard to the diagnosis and best practices of care for individuals with CFC (Table 1). It is important to understand that CFC is a variable and genetically heterogeneous disorder caused by mutations of genes in the Ras/MAPK pathway. Like other RASopathies, recognizable facial features, congenital heart disease, and short stature characterize CFC. In addition, CFC also has cutaneous and neurologic involvement. Because of the many aspects of CFC described herein, multidisciplinary care is essential. The prognosis of many aspects of CFC is still unknown, and full understanding of care expectations during childhood and adulthood is yet to be established.
Acknowledgments
A consensus conference was organized by CFC International to bring together health care professionals and researchers with particular expertise in the care of individuals with CFC. Funding for the consensus conference was provided by CFC International through donations to their organization for educational efforts. None of the participants received honorariums for the conference. We thank Dr G. Zampino for helpful comments about the gastrointestinal section.
Glossary
- CFC
cardio-facio-cutaneous syndrome
- CS
Costello syndrome
- GH
growth hormone
- HCM
hypertrophic cardiomyopathy
- IGF
insulinlike growth factor
- MAPK
mitogen-activated protein kinase
- NS
Noonan syndrome
- PVS
pulmonary valvular stenosis
Footnotes
Dr M.E. Pierpont was Chair of the CFC Consensus Conference, designed the agenda of the CFC Consensus Conference, invited the participants of the CFC Consensus Conference, participated in the Conference and in writing the manuscript, coassembled the manuscript, edited the manuscript, reviewed and revised the manuscript, and approved the management recommendations; Ms Magoulas assisted in conceptualizing the CFC Consensus Conference, participated in the Conference and in writing the manuscript, coassembled the manuscript, designed the tables, designed the figures, reviewed and revised the manuscript, and approved the management recommendations; Drs Adi, Kavamura, Neri, Noonan, E.I. Pierpont, Reinker, Roberts, Shankar, Sullivan, and Wolford, participated in the CFC Consensus Conference and in writing the manuscript, reviewed and revised the manuscript, and approved the management recommendations; Ms Conger and Ms Santa Cruz were responsible for conceptualizing and planning the CFC Consensus Conference, participated in the CFC Consensus Conference, reviewed and revised the manuscript, and approved the management recommendations; Dr Rauen was Co-Chair of the CFC Consensus Conference, codesigned the agenda of the CFC Consensus Conference, participated in the CFC Consensus Conference and in writing the manuscript, coassembled the manuscript, edited the manuscript, reviewed and revised the manuscript, and approved the management recommendations; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: CFC Consensus Conference (November 5–6, 2012) supported in part by CFC International, Vestal, New York. Dr Rauen is funded in part by National Institutes of Health RO1-ARO62165. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19(3):230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe Y, Aoki Y, Kuriyama S, et al. Costello and CFC syndrome study group in Japan . Prevalence and clinical features of Costello syndrome and cardio-facio-cutaneous syndrome in Japan: findings from a nationwide epidemiological survey. Am J Med Genet A. 2012;158A(5):1083–1094 [DOI] [PubMed] [Google Scholar]
- 3.Reynolds JF, Neri G, Herrmann JP, et al. New multiple congenital anomalies/mental retardation syndrome with cardio-facio-cutaneous involvement—the CFC syndrome. Am J Med Genet. 1986;25(3):413–427 [DOI] [PubMed] [Google Scholar]
- 4.Baraitser M, Patton MA. A Noonan-like short stature syndrome with sparse hair. J Med Genet. 1986;23(2):161–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neri G, Zollino M, Reynolds JF. The Noonan-CFC controversy. Am J Med Genet. 1991;39(3):367–370 [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–468 [DOI] [PubMed] [Google Scholar]
- 7.Kavamura MI, Pomponi MG, Zollino M, et al. PTPN11 mutations are not responsible for the Cardiofaciocutaneous (CFC) syndrome. Eur J Hum Genet. 2003;11(1):64–68 [DOI] [PubMed] [Google Scholar]
- 8.Ion A, Tartaglia M, Song X, et al. Absence of PTPN11 mutations in 28 cases of cardiofaciocutaneous (CFC) syndrome. Hum Genet. 2002;111(4-5):421–427 [DOI] [PubMed] [Google Scholar]
- 9.Estep AL, Tidyman WE, Teitell MA, Cotter PD, Rauen KA. HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am J Med Genet A. 2006;140(1):8–16 [DOI] [PubMed] [Google Scholar]
- 10.Niihori T, Aoki Y, Narumi Y, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38(3):294–296 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Viciana P, Tetsu O, Tidyman WE, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311(5765):1287–1290 [DOI] [PubMed] [Google Scholar]
- 12.Rauen KA. Distinguishing Costello versus cardio-facio-cutaneous syndrome: BRAF mutations in patients with a Costello phenotype. Am J Med Genet A. 2006;140(15):1681–1683 [DOI] [PubMed] [Google Scholar]
- 13.Anastasaki C, Estep AL, Marais R, Rauen KA, Patton EE. Kinase-activating and kinase-impaired cardio-facio-cutaneous syndrome alleles have activity during zebrafish development and are sensitive to small molecule inhibitors. Hum Mol Genet. 2009;18(14):2543–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauen KA, Tidyman WE, Estep AL, et al. Molecular and functional analysis of a novel MEK2 mutation in cardio-facio-cutaneous syndrome: transmission through four generations. Am J Med Genet A. 2010;152A(4):807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allanson JE, Annerén G, Aoki Y, et al. Cardio-facio-cutaneous syndrome: does genotype predict phenotype? Am J Med Genet C Semin Med Genet. 2011;157C(2):129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel DH, McKenzie J, Frieden IJ, Rauen KA. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br J Dermatol. 2011;164(3):521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cesarini L, Alfieri P, Pantaleoni F, et al. Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade. Am J Med Genet A. 2009;149A(2):140–146 [DOI] [PubMed] [Google Scholar]
- 18.Lee BH, Kim JM, Jin HY, Kim GH, Choi JH, Yoo HW. Spectrum of mutations in Noonan syndrome and their correlation with phenotypes. J Pediatr. 2011;159(6):1029–1035 [DOI] [PubMed] [Google Scholar]
- 19.Pierpont EI, Pierpont ME, Mendelsohn NJ, et al. Effects of germline mutations in the Ras/MAPK signaling pathway on adaptive behavior: cardiofaciocutaneous syndrome and Noonan syndrome. Am J Med Genet A. 2010;152A(3):591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nava C, Hanna N, Michot C, et al. Cardio-facio-cutaneous and Noonan syndromes due to mutations in the RAS/MAPK signalling pathway: genotype-phenotype relationships and overlap with Costello syndrome. J Med Genet. 2007;44(12):763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubbert S, Zenker M, Rowe SL, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38(3):331–336 [DOI] [PubMed] [Google Scholar]
- 22.Zenker M, Lehmann K, Schulz AL, et al. Expansion of the genotypic and phenotypic spectrum in patients with KRAS germline mutations. J Med Genet. 2007;44(2):131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhyaya M, Ruggieri M, Maynard J, et al. Gross deletions of the neurofibromatosis type 1 (NF1) gene are predominantly of maternal origin and commonly associated with a learning disability, dysmorphic features and developmental delay. Hum Genet. 1998;102(5):591–597 [DOI] [PubMed] [Google Scholar]
- 24.Shchelochkov OA, Patel A, Weissenberger GM, et al. Duplication of chromosome band 12q24.11q24.23 results in apparent Noonan syndrome. Am J Med Genet A. 2008;146A(8):1042–1048 [DOI] [PubMed] [Google Scholar]
- 25.Graham JM, Jr, Kramer N, Bejjani BA, et al. Genomic duplication of PTPN11 is an uncommon cause of Noonan syndrome. Am J Med Genet A. 2009;149A(10):2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowaczyk MJ, Thompson BA, Zeesman S, et al. Deletion of MAP2K2/MEK2: a novel mechanism for a RASopathy? [published online ahead of print February 4, 2013] Clin Genet.23379592 [Google Scholar]
- 27.Witters I, Denayer E, Brems H, Fryns JP, Legius E. The cardiofaciocutaneous syndrome: prenatal findings in two patients. Prenat Diagn. 2008;28(1):53–55 [DOI] [PubMed] [Google Scholar]
- 28.Terry J, Rauen KA, Nowaczyk MJ. Fetal autopsy findings of cardiofaciocutaneous syndrome with a unique BRAF mutation. Pediatr Dev Pathol. 2014;17(1):59–63 [DOI] [PubMed] [Google Scholar]
- 29.Armour CM, Allanson JE. Further delineation of cardio-facio-cutaneous syndrome: clinical features of 38 individuals with proven mutations. J Med Genet. 2008;45(4):249–254 [DOI] [PubMed] [Google Scholar]
- 30.Kavamura MI, Peres CA, Alchorne MM, Brunoni D. CFC index for the diagnosis of cardiofaciocutaneous syndrome. Am J Med Genet. 2002;112(1):12–16 [DOI] [PubMed] [Google Scholar]
- 31.Allanson J, Opitz JM, Carey JC, et al. Cardio-facio-cutaneous syndrome: a distinct entity. Proc Greenwood Genet Ctr. 2002;21:67 [Google Scholar]
- 32.Roberts A, Allanson J, Jadico SK, et al. The cardiofaciocutaneous syndrome. J Med Genet. 2006;43(11):833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin AF, Oberoi S, Landan M, et al. Craniofacial and dental development in cardio-facio-cutaneous syndrome: the importance of Ras signaling homeostasis. Clin Genet. 2013;83(6):539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Rahawan MM, Chute DJ, Sol-Church K, et al. Hepatoblastoma and heart transplantation in a patient with cardio-facio-cutaneous syndrome. Am J Med Genet A. 2007;143A(13):1481–1488 [DOI] [PubMed] [Google Scholar]
- 35.Lin AE, Alexander ME, Colan SD, et al. Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: a Ras/MAPK pathway syndrome. Am J Med Genet A. 2011;155A(3):486–507 [DOI] [PubMed] [Google Scholar]
- 36.Gripp KW, Lin AE, Nicholson L, et al. Further delineation of the phenotype resulting from BRAF or MEK1 germline mutations helps differentiate cardio-facio-cutaneous syndrome from Costello syndrome. Am J Med Genet A. 2007;143A(13):1472–1480 [DOI] [PubMed] [Google Scholar]
- 37.Narumi Y, Aoki Y, Niihori T, et al. Molecular and clinical characterization of cardio-facio-cutaneous (CFC) syndrome: overlapping clinical manifestations with Costello syndrome. Am J Med Genet A. 2007;143A(8):799–807 [DOI] [PubMed] [Google Scholar]
- 38.Weiss G, Confino Y, Shemer A, Trau H. Cutaneous manifestations in the cardiofaciocutaneous syndrome, a variant of the classical Noonan syndrome. Report of a case and review of the literature. J Eur Acad Dermatol Venereol. 2004;18(3):324–327 [DOI] [PubMed] [Google Scholar]
- 39.Morice-Picard F, Ezzedine K, Delrue MA, et al. Cutaneous manifestations in Costello and cardiofaciocutaneous syndrome: report of 18 cases and literature review. Pediatr Dermatol. 2013;30(6):665–673 [DOI] [PubMed] [Google Scholar]
- 40.Yoon G, Rosenberg J, Blaser S, Rauen KA. Neurological complications of cardio-facio-cutaneous syndrome. Dev Med Child Neurol. 2007;49(12):894–899 [DOI] [PubMed] [Google Scholar]
- 41.Grebe TA, Clericuzio C. Neurologic and gastrointestinal dysfunction in cardio-facio-cutaneous syndrome: identification of a severe phenotype. Am J Med Genet. 2000;95(2):135–143 [PubMed] [Google Scholar]
- 42.Papadopoulou E, Sifakis S, Sol-Church K, et al. CNS imaging is a key diagnostic tool in the evaluation of patients with CFC syndrome: two cases and literature review. Am J Med Genet A. 2011;155A(3):605–611 [DOI] [PubMed] [Google Scholar]
- 43.DeRoos ST, Ryan MM, Ouvrier RA. Peripheral neuropathy in cardiofaciocutaneous syndrome. Pediatr Neurol. 2007;36(4):250–252 [DOI] [PubMed] [Google Scholar]
- 44.Manci EA, Martinez JE, Horenstein MG, et al. Cardiofaciocutaneous syndrome (CFC) with congenital peripheral neuropathy and nonorganic malnutrition: an autopsy study. Am J Med Genet A. 2005;137(A):1–8 [DOI] [PubMed] [Google Scholar]
- 45.Aizaki K, Sugai K, Saito Y, et al. Cardio-facio-cutaneous syndrome with infantile spasms and delayed myelination. Brain Dev. 2011;33(2):166–169 [DOI] [PubMed] [Google Scholar]
- 46.Adachi M, Abe Y, Aoki Y, Matsubara Y. Epilepsy in RAS/MAPK syndrome: two cases of cardio-facio-cutaneous syndrome with epileptic encephalopathy and a literature review. Seizure. 2012;21(1):55–60 [DOI] [PubMed] [Google Scholar]
- 47.Koudova M, Seemanova E, Zenker M. Novel BRAF mutation in a patient with LEOPARD syndrome and normal intelligence. Eur J Med Genet. 2009;52(5):337–340 [DOI] [PubMed] [Google Scholar]
- 48.Pierpont EI, Pierpont ME, Mendelsohn NJ, Roberts AE, Tworog-Dube E, Seidenberg MS. Genotype differences in cognitive functioning in Noonan syndrome. Genes Brain Behav. 2009;8(3):275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adviento B, Corbin IL, Widjala F, et al. Autism traits in the RASopathies. J Med Genet. 2014;51(1):10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldstein H. Communication intervention for children with autism: a review of treatment efficacy. J Autism Dev Disord. 2002;32(5):373–396 [DOI] [PubMed] [Google Scholar]
- 51.Kaiser AP, Roberts MY. Parent-implemented enhanced milieu teaching with preschool children who have intellectual disabilities. J Speech Lang Hear Res. 2013;56(1):295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Case-Smith J, Arbesman M. Evidence-based review of interventions for autism used in or of relevance to occupational therapy. Am J Occup Ther. 2008;62(4):416–429 [DOI] [PubMed] [Google Scholar]
- 53.Lafasakis M, Sturmey P. Training parent implementation of discrete-trial teaching: effects on generalization of parent teaching and child correct responding. J Appl Behav Anal. 2007;40(4):685–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaBelle C, Charlop-Christy M. Individualizing functional analysis to assess multiple and changing functions of severe behavior problems in children with autism. J Posit Behav Interv. 2002;4:231–241 [Google Scholar]
- 55.Mesibov G, Browder D, Kirkland C. Using individualized schedules as a component of positive behavioral support for students with developmental disabilities. J Posit Behav Interv. 2002;4:73–79 [Google Scholar]
- 56.Herman TE, McAlister WH. Gastrointestinal and renal abnormalities in cardio-facio-cutaneous syndrome. Pediatr Radiol. 2005;35(2):202–205 [DOI] [PubMed] [Google Scholar]
- 57.McDaniel CH, Fujimoto A. Intestinal malrotation in a child with cardio-facio-cutaneous syndrome. Am J Med Genet. 1997;70(3):284–286 [DOI] [PubMed] [Google Scholar]
- 58.Stevenson DA, Yang FC. The musculoskeletal phenotype of the RASopathies. Am J Med Genet C Semin Med Genet. 2011;157C(2):90–103 [DOI] [PubMed] [Google Scholar]
- 59.Zenker M. Clinical manifestations of mutations in RAS and related intracellular signal transduction factors. Curr Opin Pediatr. 2011;23(4):443–451 [DOI] [PubMed] [Google Scholar]
- 60.Zeitler P, Siriwardana G. Stimulation of mitogen-activated protein kinase pathway in rat somatotrophs by growth hormone-releasing hormone. Endocrine. 2000;12(3):257–264 [DOI] [PubMed] [Google Scholar]
- 61.Neri G, Sabatino G, Bertini E, Genuardi M. The CFC syndrome—report of the first two cases outside the United States. Am J Med Genet. 1987;27(4):767–771 [DOI] [PubMed] [Google Scholar]
- 62.Bottani A, Hammerer I, Schinzel A. The cardio-facio-cutaneous syndrome: report of a patient and review of the literature. Eur J Pediatr. 1991;150(7):486–488 [DOI] [PubMed] [Google Scholar]
- 63.Lee PA, Ross J, Germak JA, Gut R. Effect of 4 years of growth hormone therapy in children with Noonan syndrome in the American Norditropin Studies: Web-Enabled Research (ANSWER) Program registry. Int J Pediatr Endocrinol. 2012;2012(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quaio CR, Carvalho JF, da Silva CA, et al. Autoimmune disease and multiple autoantibodies in 42 patients with RASopathies. Am J Med Genet A. 2012;158A(5):1077–1082 [DOI] [PubMed] [Google Scholar]
- 65.Reinker KA, Stevenson DA, Tsung A. Orthopaedic conditions in Ras/MAPK related disorders. J Pediatr Orthop. 2011;31(5):599–605 [DOI] [PubMed] [Google Scholar]
- 66.Stevenson DA, Allen S, Tidyman WE, et al. Peripheral muscle weakness in RASopathies. Muscle Nerve. 2012;46(3):394–399 [DOI] [PubMed] [Google Scholar]
- 67.Tidyman WE, Lee HS, Rauen KA. Skeletal muscle pathology in Costello and cardio-facio-cutaneous syndromes: developmental consequences of germline Ras/MAPK activation on myogenesis. Am J Med Genet C Semin Med Genet. 2011;157C(2):104–114 [DOI] [PubMed] [Google Scholar]
- 68.Kleefstra T, Wortmann SB, Rodenburg RJ, et al. Mitochondrial dysfunction and organic aciduria in five patients carrying mutations in the Ras-MAPK pathway. Eur J Hum Genet. 2011;19(2):138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aeby A, Sznajer Y, Cavé H, et al. Cardiofaciocutaneous (CFC) syndrome associated with muscular coenzyme Q10 deficiency. J Inherit Metab Dis. 2007;30(5):827. [DOI] [PubMed] [Google Scholar]
- 70.Krieger MD, Falkinstein Y, Bowen IE, Tolo VT, McComb JG. Scoliosis and Chiari malformation Type 1 in children. J Neurosurg Pediatr. 2011;7(1):25–29 [DOI] [PubMed] [Google Scholar]
- 71.Shankar SP, Young T, Burner E, Rauen K. Visual and Auditory features in cardio-facio-cutaneous syndrome. J Investig Med. 2009;7:115–115 [Google Scholar]
- 72.Makita Y, Narumi Y, Yoshida M, et al. Leukemia in Cardio-facio-cutaneous (CFC) syndrome: a patient with a germline mutation in BRAF proto-oncogene. J Pediatr Hematol Oncol. 2007;29(5):287–290 [DOI] [PubMed] [Google Scholar]
- 73.Ohtake A, Aoki Y, Saito Y, et al. Non-Hodgkin lymphoma in a patient with cardiofaciocutaneous syndrome. J Pediatr Hematol Oncol. 2011;33(8):e342–e346 [DOI] [PubMed] [Google Scholar]
- 74.Kratz CP, Rapisuwon S, Reed H, Hasle H, Rosenberg PS. Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes. Am J Med Genet C Semin Med Genet. 2011;157C(2):83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]