Abstract
Gintonin, a novel, ginseng-derived G protein-coupled lysophosphatidic acid (LPA) receptor ligand, elicits [Ca2+]i transients in neuronal and non-neuronal cells via pertussis toxin-sensitive and pertussis toxin-insensitive G proteins. The slowly activating delayed rectifier K+ (IKs) channel is a cardiac K+ channel composed of KCNQ1 and KCNE1 subunits. The C terminus of the KCNQ1 channel protein has two calmodulin-binding sites that are involved in regulating IKs channels. In this study, we investigated the molecular mechanisms of gintonin-mediated activation of human IKs channel activity by expressing human IKs channels in Xenopus oocytes. We found that gintonin enhances IKs channel currents in concentration- and voltage-dependent manners. The EC50 for the IKs channel was 0.05 ± 0.01 μg/ml. Gintonin-mediated activation of the IKs channels was blocked by an LPA1/3 receptor antagonist, an active phospholipase C inhibitor, an IP3 receptor antagonist, and the calcium chelator BAPTA. Gintonin-mediated activation of both the IKs channel was also blocked by the calmodulin (CaM) blocker calmidazolium. Mutations in the KCNQ1 [Ca2+]i/CaM-binding IQ motif sites (S373P, W392R, or R539W)blocked the action of gintonin on IKs channel. However, gintonin had no effect on hERG K+ channel activity. These results show that gintonin-mediated enhancement of IKs channel currents is achieved through binding of the [Ca2+]i/CaM complex to the C terminus of KCNQ1 subunit.
Keywords: ginseng, gintonin, heart, IKs channel, LPA receptor
INTRODUCTION
The KCNQ family of channel proteins (also known as Kv7) form K+-selective, voltage-gated channels (Hille, 2001) that are slowly activating delayed rectifier K+ (IKs) channels. Four members of the KCNQ family are neuronal (KCNQ2-5), and one (KCNQ1) is expressed in cardiac tissue. KCNQ1 as well as the hERG K+ (IKr) channel is responsible for repolarization in the heart, and both are involved in cardiac diseases such as arrhythmia (Sanguinetti et al., 1996). Channels formed from KCNQ proteins consist of homomeric tetramers or heteromeric tetramers containing KCNQ as the α-subunit, and each KCNQ subunit is composed of six α-helical transmembrane segments (S1-S6). KCNQ channel proteins also co-assemble with KCNE1-4 subunits (McCrossan and Abbott, 2004). It has been shown that cells expressing KCNQ1 in the absence of KCNE1 exhibit outward K+ currents with unique activation kinetics (Salata et al., 1998). In addition, the C terminus of KCNQ1 contains binding sites for the intracellular Ca2+/calmodulin (CaM) complex, which regulates KCNQ1 channel activity (Ghosh et al., 2006; Tohse, 1990). It has been reported that LQT syndrome in humans is caused by defective interaction of the Ca2+/CaM complex with the KCNQ1 C terminus (Ghosh et al., 2006; Shamgar et al., 2006). Thus, the Ca2+/CaM interaction sites in KCNQ1 are important for normal cardiac activity.
Panax ginseng root has been commonly used for centuries as a tonic that has pharmacological effects on multiple organs (Attele et al., 1999). For example, ginseng extract has been shown to protect against cardiac ischemia-reperfusion injury (Furukawa et al., 2006) and to shorten action potential duration by enhancing the IKs current (Bai et al., 2003). Bai et al. (2003) also showed that ginsenoside Re, a ginseng saponin, might protect against cardiac ischemia-reperfusion injury; however, the molecular mechanisms for this activity at the level of the cell membrane were not well explained. Recent reports have shown that ginseng also contains a ligand for the G protein-coupled lysophosphatidic acid (LPA) receptor called gintonin (Hwang et al., 2012; Pyo et al., 2011). Gintonin exerts its effects through induction of [Ca2+]i transients, resulting in the regulation of Kv1.2 channel activity (Lee et al., 2013). In the present study, we used the Xenopus oocyte gene expression system to investigate the molecular mechanisms underlying how gintonin-mediated [Ca2+]i transients are coupled to the regulation of IKs channel activity. Next, we also examined gintonin effect on IKs in guinea pig cardiac myocytes to know whether gintonin could directly regulate the intrinsic IKs in mammalian cardiac myocytes. The results show that gintonin enhances IKs channel currents through the interaction of the Ca2+/CaM complex with KCNQ1 IQ motifs and that gintonin also increases IKs currents in guinea pig cardiac myocytes. We further discuss the pharmacological roles and applications of gintonin in the regulation of KCNQ1 channel activity in the heart.
MATERIALS AND METHODS
Materials
Gintonin was prepared from Panaxginseng according to the method described in Pyo et al. (2011). Prior to use, gintonin was dissolved in dimethyl sulfoxide (DMSO), the final concentration of which was less than 0.01%. This stock was then added to the bath medium buffer. The cDNAs for the human KCNQ1 and KCNE1 channels (GenBank ID: NM_000218) were kindly provided by Dr. Pongs (University of Hamburg, Germany). All other agents were purchased from Sigma-Aldrich (USA).
Preparation of Xenopus oocytes and microinjection
Xenopuslaevis frogs were purchased from Xenopus I (USA). Their care and handling were in accordance with the highest standards of the institutional guidelines of Konkuk University. For isolation of oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester, after which the ovarian follicles were removed. The oocytes were treated with collagenase and then agitated for 2 h in Ca2+-free medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin. Stage V–VI oocytes were collected and stored in ND96 medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.5) supplemented with 50 μg/mlgentamicin. The oocyte-containing solution was maintained at 18°C with continuous gentle shaking and renewed every day. Electrophysiological experiments were performed within 5–6 days of oocyte isolation, with gintonin applied to the bath. For K+ channel experiments, cRNAs encoding KCNQ1 + KCNE1 (40 nl) were injected into the animal or vegetal pole of each oocyte one day after isolation, using a 10 μl microdispenser (VWR Scientific, USA) fitted with a tapered glass pipette tip (15–20 μm in diameter) (Lee et al., 2005).
Site-directed mutagenesis of human KCNQ1
Single amino acid substitutions were made using the QuikChange™ XL Site-Directed Mutagenesis Kit (Stratagene, USA), along with Pfu DNA polymerase and sense and antisense primers encoding the desired mutations. Overlap extension of the target domain by sequential polymerase chain reaction (PCR) was carried out according to the manufacturer’s protocol. The final PCR products were transformed into E. coli DH5α, screened by PCR, and confirmed by sequencing of the target regions. The mutant DNA constructs were linearized at their 3′ ends by digestionwithXhoI, and run-off transcripts were prepared using the methylated cap analog m7G(5′)ppp(5′)G. The cRNAs were prepared using the mMessagemMachinein vitro transcription kit (Ambion, USA) with T7 RNA polymerase. The absence of degraded RNA was confirmed by denaturing agarose gel electrophoresis followed by ethidium bromide staining. Similarly, recombinant plasmids containing human KCNQ cDNA inserts were linearized by digestion with the appropriate restriction enzymes, and cRNAs were obtained using the mMessagemMachine transcription kit with SP6 RNA polymerase or T7 polymerase. The final cRNA products were resuspended at a concentration of 1 μg/μl in RNase-free water and stored at −80°C (Lee et al., 2008).
Guinea pig ventricular myocyteisolation
Ventricular cells were isolated from hearts of guinea pigs (body weights of 250–300 g) using the enzymatic dissociation technique (Fujisawa et al., 2000). All experimental procedures were conducted in accordance with the guidelines of the Sungkyunkwan University School of Medicine Institutional Animal Care and Use Committee (Approval No. IACUC-11-39). Briefly, guinea pigs were injected with heparin (1.0 units/kg) and euthanized by stunning-induced coma with loss of all reflex-responses, followed by cardiac excision. The heart was cannulated by a 18-gauge needle and then retrogradely perfused via the aorta on a Langendorff apparatus. During coronary perfusion all perfusates were maintained at 37°C and equilibrated with 100% O2. Initially the heart was perfused with normal Tyrode solution for 2–3 min to clear the blood. The heart was then perfused with Ca2+-free solution for 2 min. Finally the heart was perfused with enzyme solution for 14–16 min. Enzyme solution contains 1 mg/ml collagenase (Worthington Type 2) and 0.1mg/ml protease (Sigma) in Ca2+-free solution. After perfusion with enzyme solution, the ventricles were separated with the atria and chopped into small pieces. Single cells were dissociated in high K+, low Cl− solution from these small pieces using blunt-tip glass pipette and stored in the same solution at 4°C until use.
Voltage-clamp recording
Membrane currents were recorded from single isolated myocytes in a perforated patch configuration by using nystatin (200 μg/ml; ICN) at 35 ± 1°C. Voltage clamp was performed by using an EPS-8 amplifier (HEKA Instruments) and filtered at 5 kHz. The patch pipettes (World Precision Instruments) were made by a Narishige puller (PP-830; Narishige, Japan). The patch pipettes used had a resistance of 2–3 mega ohms when filled with the below pipette solutions. The bath solution (or normal Tyrode solution) contained (mM): NaCl 140, KCl 5.4, MgCl2 0.5, CaCl2 1.8, glucose 10, HEPES 5, titrated to pH 7.4 with NaOH. Ca2+ free solution contained (mM): NaCl 140, KCl 5.4, MgCl2 0.5, glucose 10, HEPES 5, titrated to pH 7.4 with NaOH. The high-K+ and low-Cl− solution contained (mM): KOH 70, KCl 40, L-glutamic acid 50, taurine 20, KH2PO4 20, MgCl2 3, glucose 10, HEPES 10, EGTA 0.5. The pipette solution for perforated patches contained (mM): KCl 140, HEPES 10, MgCl2 1, EGTA 5, titrated to pH 7.2 with KOH.
Two-electrode voltage clamp recording
A custom-made Plexiglas net chamber was used for two-electrode voltage-clamp recordings as previously reported (Lee et al., 2005). The oocytes were impaled with two microelectrodes filled with 3 M KCl (0.2–0.7 MΩ), and electrophysiological experiments were carried out at room temperature using an Oocyte Clamp (OC-725C, Warner Instruments, USA). Stimulation and data acquisition were controlled with a pClamp 8 (Axon Instruments, USA). For most electrophysiological experiments, oocytes were perfused initially with a Cl−- and Ca2+-free solution (96 mM NaOH, 2 mM KOH, 8 mM Mg-gluconate, 5 mM HEPES, and 5 mM EGTA, pH 7.4 with methanesulfonic acid) in the presence of a Cl− channel blocker (500 μM anthracene-9-carboxylicacid) to inhibit endogenous Cl− channels (Choi et al., 2011). The oocytes were then clamped at a holding potential of −80 mV, the membrane potential was depolarized to +30 mV for 2.5 s at 10-s intervals, and currents were recorded.
Data analysis
To obtain the concentration-response curves showing the effect of gintonin on IKs currents, the peak amplitudes at different concentrations of gintonin were plotted, and Origin software (Origin, USA) was used to fit the plot to the Hill equation y / ymax = [A]nH / ([A]nH + [IC50]nH), where y is the peak current at a given concentration of gintonin, ymax is the maximal peak current, EC50 is the concentration of gintonin producing a half-maximal effect, [A] is the concentration of gintonin, and nH is the Hill coefficient. All values are presented as mean ± S.E.M. The significance of differences between mean control and treatment values was determined using Student’s t-test. P < 0.05 was considered statistically significant.
RESULTS
Gintonin increases IKs channel currents in a concentration-and voltage-dependent manner and shifted the steady-state activation curves leftward
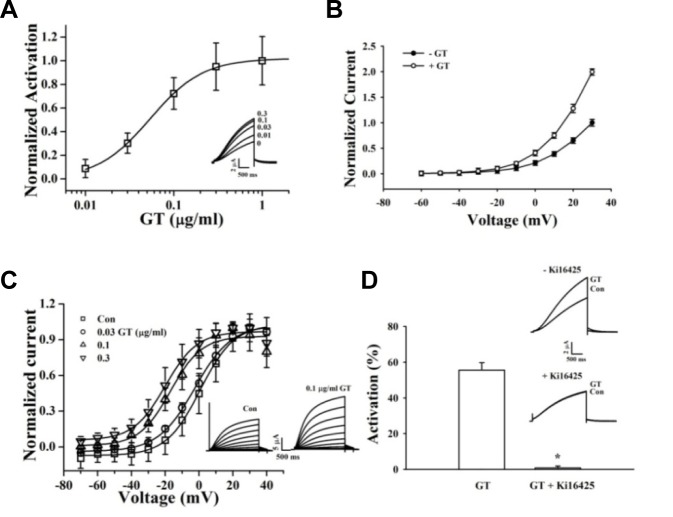
We examined the effect of gintonin on the activities of IKs channels expressed in Xenopus oocytes. The channel currents were elicited by a 2.5-s depolarization to a test potential of +30 mV at10-s intervals from a holding potential of −80 mV. Gintonin activates IKs channels in a concentration-dependent and reversible manner. The maximum mean activation values for IKs are 10.89% ± 8.71%, 32.76% ± 9.64%, 78.78% ± 26.26%, 104.3% ± 22.49%, and 112.12% ± 24.1% at 0.01, 0.03, 0.1, 0.3, and 1 μg/ml, respectively (Fig. 2A). The EC50 for gintonin on the IKs channel is 0.05 ± 0.01 μg/ml (Fig. 2A). The current-voltage relationship curves (10 mV increments from −60 mV to +30 mV) show that the enhancement of IKs channels by gintonin is voltage-dependent (Fig. 2B). To study steady-state activation of these channels, cells were held at −80 mV and then pulsed with voltages ranging from −70 mV to +30 mV. The voltage-activation relationships for the IKs channels were determined from tail current analysis. Tail currents were normalized to the maximal currents and fitted with a Boltzmann equation. A representative trace is shown in Fig. 2C. The steady-state activation V1/2 for IKs is 0.64 ± 0.78 mV in the control, and k for this relationship is 10.03 ± 0.70 mV (Fig. 2C). The higher the gintonin concentration used, the more the activation curves of the IKs channel shift leftward (control: V1/2 = 0.64 ± 0.78 mV, n = 6; 0.03 μg/mlgintonin: −2.61 ± 0.82 mV, n = 7; 0.1 μg/mlgintonin: −16.90 ± 2.08 mV, n = 6; 0.3 μg/ml gintonin: −19.96 ± 1.47 mV, n = 10).
Fig. 2.
Effects of gintonin on IKs channel activity. (A) Gintonin concentration-response curves for IKs channels (mean ± S.E.M; n = 7–10 oocytes each). Inset, representative traces of gintonin-mediated IKs (KCNQ1 + KCNE1) channel activation at various gintonin concentrations. The representative peak outward current amplitude at +30 mV from a holding potential of −80 mV was measured in the absence or presence of gintonin. (B) Effects of gintonin (0.1 and 0.3 μg/ml each) on the current–voltage (I–V) relationship of the IKs channels (mean ± S.E.M; n = 10–12 oocytes each). (C) Voltage-dependence activation curves for the IKs channel. Inset, Left and right current traces are before and after application of 0.1 μg/mlgintoninto IKschannels, respectively. Currents recorded during 3 s depolarizing pulses to membrane potentials of −60 to +50 mV, applied from a holding potential of −80 mV. Tail currents were measured at −70 mV. I–V relationships for normalized IKs tail currents. Data were fitted to a Boltzmann function. (D) Attenuation of gintonin-induced IKs channel activity after treatment with Ki16425. The histogram shows blockage of gintonin-mediated IKs channel activation by the LPA1/3 receptor antagonist, Ki16425. Application of 0.1 and 1 μg/ml gintoninto IKs channels, respectively (mean ± S.E.M; n = 10–12 each) (*P < 0.001, compared to gintonin treatment only). Inset, Currents traces recorded in the absence and presence of 1 μM Ki16425 in oocytes expressing IKs channels; currents were recorded with a 3-s voltage step to +30 mV from a holding potential of −80 mV.
Ki16425 attenuates gintonin-mediated activation of the IKs channels
Because Xenopusoocytes express endogenous LPA receptors (Kimura et al., 2001), oocytes expressing IKs channels were first pretreated with the LPA1/3 antagonist Ki16425 (1 μM, 30 min) and then challenged with gintonin (0.1 or 1 μg/ml). As shown in Fig. 2D, 0.1 μg/mlgintonin activates IKs channels by 55.5%; however, Ki16425 abolishes gintonin-mediated activation of IKs channels (n = 6, P < 0.001). Thus, gintonin activates these potassium channels via endogenous LPA receptors (Fig. 2D).
Activation of the G protein-coupled LPA receptor by gintonin up-regulates IKs channel currents by activating the PLC-IP3-[Ca2+]i pathway
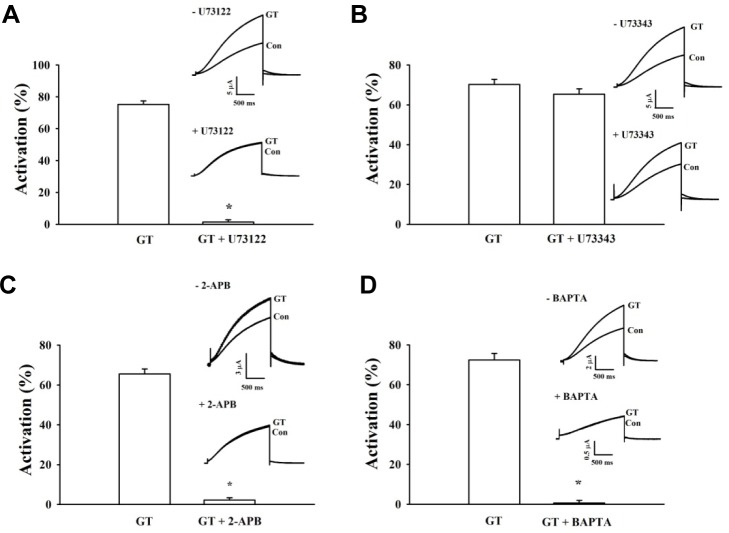
Next, we pretreated cells with U73122, an active inhibitor of phospholipase C. As a result of this treatment, gintonin-mediated activation of IKs channels is completely eliminated. In contrast, when we pretreated cells with U73343, an inactive analog, gintonin-mediated activation is not significantly affected (Figs. 3A and 3B). These data indicate that the Gαq/11-PLC pathway is involved in gintonin-mediated activation of IKs channels.
Fig. 3.
Signal transduction pathways of gintonin-mediated IKs channel activation. (A, B) Representative recordings of IKs (A) channel currents following application of gintonin (GT) for 30 s in the presence of U73122, an active PLC inhibitor. U73343, an inactive PLC inhibitor, in oocytes expressing IKs channels. Inset, the representative peak outward current amplitude at +30 mV from a holding potential of −80 mV was measured in the presence of gintonin. The active or inactive PLC inhibitor was pretreated for 5 min before gintonin application. (C, D) Time-current relationship after application of gintonin (GT) for 30 s in the presence of 2-APB, an IP3 receptor antagonist, or BAPTA-AM, a membrane permeable Ca2+chelator, in oocytes expressing IKs channels. Inset, the representative peak outward current amplitude at +30 mV from a holding potential of −80 mV was measured in the presence of gintonin. The application of 2-APB or BAPTA preceded the gintonin application by 2 h. Summary histograms show the peak outward IKs channel currents (mean ± S.E.M; n = 13-14 oocytes each) recorded in oocytes expressing the IKs channel in the absence or presence of the indicated agents (*P < 0.001, compared to gintonin alone).
To investigate the role of IP3 receptors on gintonin action, the effect of the IP3 receptor inhibitor 2-APB on gintonin-mediated activation of IKs channels was examined (Fig. 3C). We found that gintonin-mediated activation of IKs channels is blocked completely by 2-APB. These data also demonstrate that gintonin-mediated activation of IKs channels requires signaling through the IP3 receptor. To determine whether [Ca2+]i transients from intracellular Ca2+ stores play a role in gintonin-mediated activation of IKs channels, we used the calcium chelator BAPTA. As shown in Fig. 3D, we treated oocytes with 10 μM BAPTA-AM, a membrane permeable Ca2+chelator, to chelate free cytosolic Ca2+ and almost abolishes gintonin action on IKs channels. These results indicate that gintonin-mediated [Ca2+]i transients are required for activation of IKs channels.
Involvement of Ca2+/CaM in gintonin-mediated activation of IKs channels
KCNQ1 has two CaM-binding IQ motifs (Fig. 1; Yus-Najera et al., 2002). CaM regulates the activity of IKs channels through interactions with two CaM-binding IQ motifs (Ghosh et al., 2006; Shamgar et al., 2006). To examine the role of CaM in gintoninmediated activation of IKs channels, we first treated cells with calmidazolium, a CaM inhibitor. As shown in Fig. 4A, gintonin has almost no effect on either channel current in the presence of calmidazolium. Next, we constructed channels using KCNQ1 subunits that had been mutated in the C-terminalCaM-binding IQ motifs: S373P or W392R in the IQ1 region and R539W in the IQ2 region. Mutations of CaM-binding IQ motifs reduced the amplitude of both the IKs channel currents. Gintonin-mediated activation of the IKs channels is also attenuated (Fig. 4B, insets). The EC50 values for the wild-type, S373P, W392R, and R539W IKs channels are 0.06 ± 0.01, 3.66 ± 0.41, 4.36 ± 0.67, and 2.30 ± 0.05 μg/ml, respectively (Fig. 4B). Mutations in the KCNQ1 C terminus cause a rightward shift in the concentration-response curves, indicating that gintonin-mediated activation of IKs channels is achieved through Ca2+/CaM binding to the IQ motifs in KCNQ1. Interestingly, gintonin has no effect on hERG K+ (IKr) channel activity in IhERG and Itail, although gintonin slightly inhibits Ideactivating-tail compared to the control (Fig. 5). Thus, gintonin selectively regulates IKs but not IKr channels.
Fig. 1.
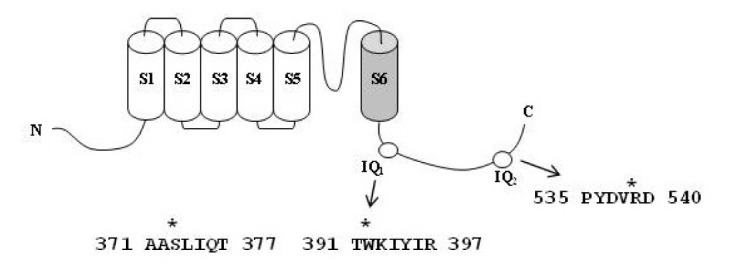
Schematic of the human KCNQ1 subunit topology showing amino acidmutations. (A) A sequence alignment of the KCNQ1 channel protein and the amino acid residues that were mutated CaM-binding sites IQ1 (373, 392) and IQ2 (539).
Fig. 4.
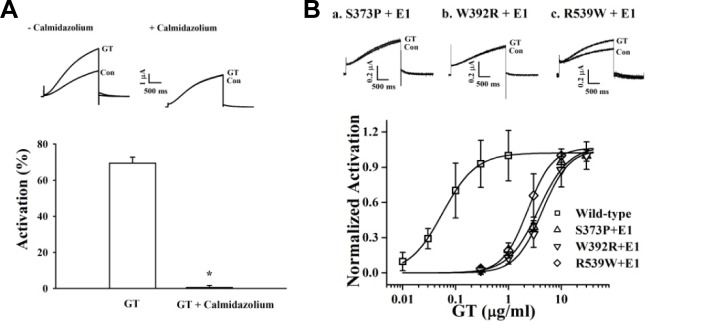
Involvement of CaM in gintonin-mediated IKs channel activation. (A) Oocytes expressing IKs channels were incubated in the absence or presence of calmidazolium (1.5 μM) for 10 min. Insets, the representative gintonin-mediated mediated peak outward current amplitude at +30 mV from a holding potential of −80 mV was measured in the absence or presence of calmidazolium. Summary histograms show peak outward IKs channel currents recorded in the absence or presence of calmidazolium (mean ± S.E.M; n = 13–14 oocytes each; *P < 0.001, compared to gintonin alone). (B) Oocytes expressing IKs channels mutated at the Ca2+/CaM-binding sites (S373P, W392R, or R539W) were treated with gintonin for 60 s. Mutation of Ca2+/CaM-binding sites resulted in a rightward shift of the gintonin concentration-response curve (mean ± S.E.M; n = 10–12 oocytes each). Insets, the representative peak outward current amplitude at +30 mV from a holding potential of −80 mV was measured in the presence of gintonin. Gintonin-mediated peak outward IKs channel currents recorded in oocytes expressing mutant channels were significantly attenuated (mean ± S.E.M; n = 10–12 oocytes each; *P < 0.001, compared to the wild type).
Fig. 5.
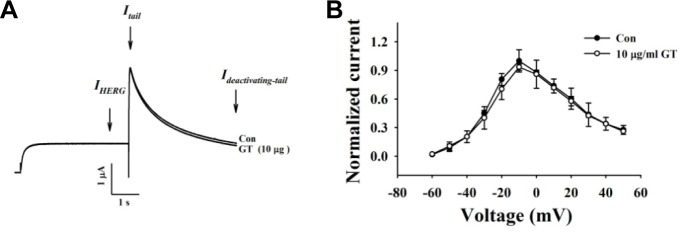
Effects of gintonin on IhERG, Itail, and slow Ideactivating-tail. (A) Representative current trace showing hERG K+ channel enhancement by gintonin (10 μg/ml). Currents were in response to 4-s voltage steps to 0 mV from a holding potential of −90 mV, followed by repolarization to −60 mV. (B) I–V relationship for hERG K+ currents measured at the end of the 4-s test pulse before and after application of 10 μg/ml gintonin (n = 5). Currents were normalized to the control current at 0 mV for each oocyte. Data are represented as mean ± S.E.M. (n = 7).
IKs of guinea-pig ventricular myocytes is modulated by gintonin
Finally, we investigated whether gintonin could modulate IKs in guinea-pig ventricular myocytes. Myocytes were depolarized from −50 to +50 mV for 500 ms at 0.05 Hz. Under these experimental conditions, the time-dependent outward current developed during depolarization and the outward tail current that deactivated during repolarization was almost exclusively composed of IKs (Missan et al., 2006). We confirmed that the amplitude of the tail current was unaffected by acute application of the rapidly activating K+ channel blocker E4031 (50 μM, data not shown). As indicated by the representative data in the time plot of Fig. 6A, the tail IKs current was significantly increased by gintonin (3 μg/ml). In the present study, The average of gintonin-mediated amplitude increase of the tail current was 55.34 ± 3.21% (n = 10). We applied a family of voltage steps from a holding potential of −50 mV, and measured the amplitude of the tail current at −30 mV following each test current. As indicated by the current records obtained during the example gintoinin experiment and mean I–V relationships, gintonin increased IKs without altering I–V relationships (Fig. 6B).
Fig. 6.
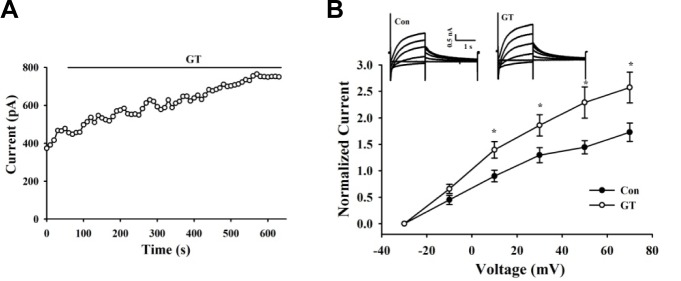
Gintonin increases IKs of guinea pig ventricular myocytes. (A) Time course of changes in the amplitude of the IKs tail during applications of gintonin (3 μg/ml). (B) I–V relationships obtained before and after application of gintonin. Currents were elicited by voltage steps from −30 mV to +70 mV with a subsequent step to −30 mV for the tail current. Representative recordings are shown in the inset. Data are represented as mean ± S.E. (n = 8). *p < 0.05 versus control condition (paired t test)
To confirm that the LPA receptor was involved in gintonin effecton IKs in guinea-pig ventricular myocytes, we repeated similar experiments in the presence of LPA1/3 antagonist Ki16425 (10 μM). As shown in Fig. 7A, Ki16425 almost completely blocked gintonin-mediated modulation of IKs in guinea-pig ventricular myocytes. Summarized data in Fig. 7B showed that in the presence of Ki16425, the IKs was not enhanced but rather reduced (25.13 ± 13.64%, n = 3) by subsequent bath application of gintonin. Taken together, these data suggest that gintonin modulates IKs in native cardiac myocytes possibly via endogenous LPA receptors.
Fig. 7.
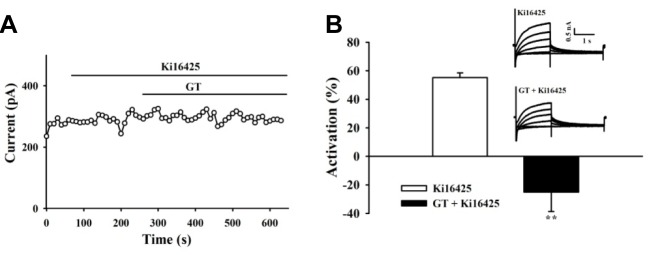
Effects of gintonin on ventricular IKs depend on LPA receptor. (A) LPA1/3 antagonist, Ki16425 (10 μM) significantly blocked the activation of IKs by gintonin (3 μg/ml). (B) Summary of the percent activation of IKs. Values are expressed as means ± S.E. **p < 0.01 versus control condition (n = 3). Representative recordings obtained beforeand after gintonin in Ki16425-pretreated cellsare shown in the inset.
DISCUSSION
KCNQ1 (IKs) and hERG K+(IKr) channels are the main K+ channels in cardiac myocytes and both contribute to heart repolarization after cardiac action potential and shorten the action potential duration (Robbins, 2001). Although both channels are closely associated with cardiac activities, regulation of the two channels differs. For example, the C terminus of KCNQ1 contains Ca2+/CaMcomplex interaction sites whereas the hERG K+ (IKr) channel subunit does not (Ghosh et al., 2006; Tohse, 1990); it has been shown that mutations that disrupt the KCNQ1-Ca2+/CaMcomplex interactions cause LQT syndrome (Ghosh et al., 2006; Shamgar et al., 2006). It has been reported that ginseng root has a beneficial effect on a variety of cardiovascular diseases, including hypertension (Attele et al., 1999). In previous reports, we have shown that gintonin but not ginsenosides, another class of active ingredient in ginseng, elicits [Ca2+]i transients via G protein-coupled LPA receptors (Hwang et al., 2012) and that gintonin regulates Ca2+-dependent K+ (BKCa) and Kv1.2 channels (Choi et al., 2013; Lee et al., 2013). However, thus far, little was known about the molecular mechanisms through which gintonin regulates IKs activity.
In this study, we have demonstrated that gintonin activates IKs channels through the LPA receptor-Gαq/11-PLC-IP3-Ca2+/CaM signal pathwayusing human IKs channels in Xenopus oocytes. We also demonstrated a similar gintonin/LPA receptor-dependent regulation of IKs in guinea-pig ventricular myocytes (Figs. 6 and 7). Gintonin-mediated activation of IKs channels is concentration- and voltage-dependent. In addition, gintonin treatment causes the steady-state activation curves for these channels to shift leftward in a dose-dependent manner. Interestingly, the EC50 for the effect of gintonin on IKs activation was about nine times less than for KCNQ1-only channels (data not shown). Thus, the KCNE1 subunit might be necessary for full activation of IKs induced by Ca2+/CaM complex after gintonin treatment. We also examined the effect of gintonin on hERG (IKr) channel activity. However, gintonin only has a slight inhibitory effect on Ideactivating-tail (Fig. 5). Consequently, our study shows a possibility that gintonin-mediated [Ca2+]i transients via LPA receptor activation might govern IKs rather than IKrin the regulation of K+ channels in cardiac myocyte repolarization.
The presence of BAPTA, a calcium chelator, or calmidazolium, a CaM inhibitor, attenuated the gintonin-mediated activation of IKs channels. In addition, mutation of the amino acid residues in the Ca2+/CaM-binding sites (IQ1 and IQ2) in the C terminus of KCNQ1 also attenuated gintonin-mediated activation of IKs channels (Fig. 4). These results show that Ca2+, CaM, or binding of Ca2+/CaM complex to the C terminus of KCNQ1 is required to activate IKs. In further studies on the role of these Ca2+/CaM-binding sites, mutations of IQ motifs exhibited differential effects on gintonin-mediated activation of IKs channels. The EC50 in theW392R, S373P, and R539R mutant IKs channels was increased by 72-, 61-, and 38-fold as compared to the wild-type channels, respectively. The EC50 for IQ1 (W393R and S373P) and IQ2 (R539W) mutants was about 1.5-fold less than that of the IQ2 site (Fig. 4B). These results indicate that mutations in individual residues in the Ca2+/CaM-binding sites might affect the conformation of the three-dimensional structure of the KCNQ1 C terminus and change Ca2+/CaM binding in a differential manner.
In a previous study, we demonstrated that ginsenoside Rg3, a ginseng saponin, also enhances IKs channel currents following depolarization (Choi et al., 2010). We found that gintonin differs from ginsenoside Rg3in the regulatory patterns for IKs channel activation. Ginsenoside Rg3-induced enhancement of IKs channel currents is not achieved through receptor-mediated [Ca2+]i transients as shown in schematic diagram in Fig. 6. Thus, ginsenoside Rg3-induced IKs current enhancement does not act via a membrane receptor signaling transduction pathway. Instead, ginsenoside Rg3-induced enhancement of IKs channels currents was abolished by substitution of the K318 and V319 residues located at the channel pore entrance (Choi et al., 2010). Thus, ginsenoside Rg3 regulates IKs activity through direct interaction with channel proteins at the channel pore entrance. In contrast, gintonin amplifies IKs activation through a signaling pathway mediated by a membrane-bound G protein-coupled LPA receptors (Fig. 6). Supporting this observation is that fact that gintonin, even at much lower concentrations than ginsenoside Rg3, induces greater amplitudes of outward IKs channel currents (by 4- to 5-fold) than does ginsenosideRg3 (Fig. 6). The EC50 of gintonin is approximately 35nM (under the assumption that the molecular weight of gintonin is 20 kDa) (Hwang et al., 2012), whereas that of the ginsenoside Rg3 was about 15 μM for IKs activation (Choi et al., 2010). In addition, interruption of the receptor signaling pathway by inhibitors or mutations abolished or attenuated gintonin-mediated but not ginsenoside Rg3-mediated IKs channel activation (data not shown). These results indicate that ginseng contains two agents with two different action modes for the regulation of IKs activity. However, gintonin is more efficient for IKs activation than ginsenoside Rg3 because gintonin exerts its effects through LPA receptors whereas but ginsenoside Rg3 does not (Fig. 6).
Gintonin comprises about 0.2% of ginseng (Pyo et al., 2011). Gintonin is shown to contain two proteins, ginseng major latex-like protein 151 (GLP151) and ginseng ribonuclease-like storage proteins (Hwang et al., 2012). GLP 151 belongs to the Bet v1 family of proteins and exhibits similar properties to other members of Bet v1 families (Hwang et al., 2012). GLP151 has hydrophobic ligand-binding sites (Hwang et al., 2012). GLP151 contains hydrophobic ligand-binding sites and a glycine-rich region that binds to phosphate groups (Hwang et al., 2012). Currently, we are investigating the possibility that GLP151 could be a main candidate for LPA binding protein of gintonin.
The previous reports have shown that ginseng extract or ginseng components, including ginsenosides, exhibit beneficial effects against cardiovascular diseases by relaxation of blood vessels constricted by adrenergic receptor stimulations (Chen and Zhang, 2009; Kang et al., 1995; Kim et al., 1999). In a study using a single cell, ginsenosides inhibit L-type Ca2+ channel currents in cardiac myocytes (Bai et al., 2003; 2004). We have also shown in a previous study that ginsenoside Rg3 inhibits L-type Ca2+ channel currents through interactions with the amino acid residues L427, N428, and L431 in the transmembrane domain I segment 6 (Choi et al., 2009). In addition to L-type Ca2+ channel regulation by ginsenosides, recent reports showed that ginsenoside Re regulates IKs in cardiac myocytes (Bai et al., 2004), and we have also demonstrated that ginsenoside Rg3 enhances IKs through interaction with K318 and V319 residues (Choi et al., 2010). In the present study, we have demonstrated that gintonin activates IKs channels in cardiac myocytesthrough LPA receptors. Thus, although gintonin and ginsenosides differ from each other for the activation of IKs channel, the enhancing effect of both gintonin and ginsenosides on IKs channel currents might contribute to a facilitation of repolarization of cardiac action potential and shorten action potential duration. These results show the possibility that both ginsenosides and gintonin could be candidates against cardiovascular diseases. However, we do not know currently how the actions of gintonin on IKs currents might be coupled toalleviation of arrhythmia, which is caused by IKs and IKr channel dysfunctions. More investigations might be required for application of gintonin to heart dysfunction.
In summary, we found that gintonin induces activation of IKs channels via a membrane G protein-coupled LPA receptor signaling pathway. Using site-directed mutagenesis, we further confirmed the role of the Ca2+/CaM complex in gintonin-mediated IKs channel regulation. These novel findings provide insight into the molecular basis of the pharmacological effects of ginseng in cardiovascular systems.
Fig. 8.
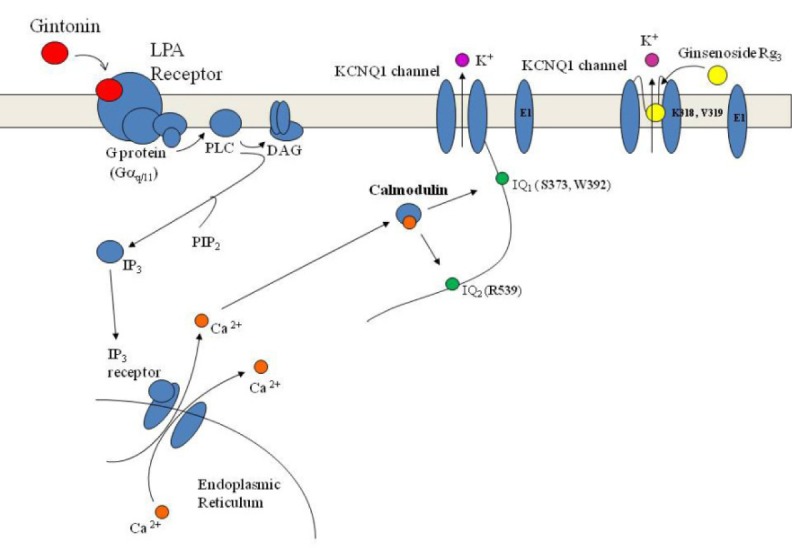
Diagram comparing gintonin- and ginsenoside Rg3-mediated activation of the IKs channel. Gintonin-mediated IKs activation proceeds via Ca2+/CaM binding to IQ motifs via G protein-coupled LPA1 receptors, whereas ginsenoside Rg3 activates IKs through a direct interaction with specific amino acids located at the pore entryway of channel proteins following depolarization (Choi et al., 2010).
Acknowledgments
This work was supported by the Basic Science Research Program (2011-0021144) and the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2012-0006686), Veterinary Science Research Institute of the Konkuk University, and Brain Korea 21 plus to S.-Y. Nah.
REFERENCES
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Bai CX, Sunami A, Namiki T, Sawanobori T, Furukawa T. Electrophysiological effects of ginseng and ginsenoside Re in guinea pig ventricular myocytes. Eur J Pharmacol. 2003;476:35–44. doi: 10.1016/s0014-2999(03)02174-5. [DOI] [PubMed] [Google Scholar]
- Bai CX, Takahashi K, Masumiya H, Sawanobori T, Furukawa T. Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in guinea-pig cardiomyocytes. Br J Pharmacol. 2004;142:567–575. doi: 10.1038/sj.bjp.0705814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J, Cui J, McDonald TV. HERG K+ channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ Res. 2001;89:1168–1176. doi: 10.1161/hh2401.101375. [DOI] [PubMed] [Google Scholar]
- Chen CX, Zhang HY. Protective effect of ginsenoside Re on isoproterenol-induced triggered ventricular arrhythmia in rabbits. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:384–388. [PubMed] [Google Scholar]
- Choi SH, Lee JH, Pyo MK, Lee BH, Shin TJ, Hwang SH, Kim BR, Lee SM, Oh JW, Kim HC, et al. Mutations Leu427, Asn428, and Leu431 residues within transmembrane domain-I-segment 6 attenuate ginsenoside-mediated L-type Ca2+ channel current inhibitions. Biol Pharm Bull. 2009;32:1224–1230. doi: 10.1248/bpb.32.1224. [DOI] [PubMed] [Google Scholar]
- Choi SH, Shi TJ, Lee BH, Chu DH, Choe H, Pyo MK, Hwang SH, Kim BR, Lee SM, Lee JH, et al. Ginsenoside Rg3 activates human KCNQ1 K+ channel currents through interacting with the K318 and V319 residues: a role of KCNE1 subunit. Eur J Pharmacol. 2010;637:138–147. doi: 10.1016/j.ejphar.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Choi SH, Shin TJ, Lee BH, Hwang SH, Lee SM, Lee BC, Park CS, Ha TS, Nah SY. Ginsenoside Rg3 enhances large conductance Ca2+-activated potassium channel cur rents: a role of Tyr360 residue. Mol Cells. 2011;31:133–140. doi: 10.1007/s10059-011-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee BH, Hwang SH, Kim HJ, Lee SM, Kim HC, Rhim HW, Nah SY. Molecular mechanisms of large-conductance Ca2+-activated potassium channel activation by ginseng gintonin. Evid Based Complement Alternat Med. 2013;2013323709 doi: 10.1155/2013/323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Ono K, Iijima T. Time-dependent block of the slowly activating delayed rectifier K+ current by chromanol 293B in guinea-pig ventricular cells. Br J Pharmacol. 2000;129:1007–1013. doi: 10.1038/sj.bjp.0703126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Bai CX, Kaihara A, Ozaki E, Kawano T, Nakaya Y, Awais M Sato M, Umezawa Y, Kurokawa J. Ginsenoside Re, a main phytosterol of Panax ginseng, activates cardiac potassium channels via a nongenomic pathway of sex hormones. Mol Pharmacol. 2006;70:1916–1924. doi: 10.1124/mol.106.028134. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Nunziato DA, Pitt GS. KCNQ1 assembly and function is blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circ Res. 2006;98:1048–1054. doi: 10.1161/01.RES.0000218863.44140.f2. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Vol. 814 Sinauer Associates, Inc; Sunderland, MA: 2001. [Google Scholar]
- Hwang SH, Shin TJ, Choi SH, Cho HJ, Lee BH, Pyo MK, Lee JH, Kang J, Kim HJ, Park CW, et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Schini-Kerth VB, Kim ND. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sci. 1995;56:1577–1586. doi: 10.1016/0024-3205(95)00124-o. [DOI] [PubMed] [Google Scholar]
- Kim ND, Kang SY, Park JH, Schini-Kerth VB. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur J Pharmacol. 1999;367:41–49. doi: 10.1016/s0014-2999(98)00898-x. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Schmitt A, Fukushima N, Ishii I, Kimura H, Nebreda AR, Chun J. Two vovelXenopus homologs of mammalian LPA1/EDG-2 function as lysophosphatidic acid receptors in Xenopus oocytes and mammalian cells. J Biol Chem. 2001;276:15208–15215. doi: 10.1074/jbc.M011588200. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Kim DH, Rhim H, Kim SS, et al. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol Pharmacol. 2005;68:1114–1126. doi: 10.1124/mol.105.015115. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee BH, Choi SH, Yoon IS, Pyo MK, Shin TJ, Choi WS, Lim Y, Rhim H, Won KH, et al. Ginsenoside Rg3 inhibits human 1.4 channel currents by interacting with the Lys531 residue. Mol Pharmacol. 2008;73:619–626. doi: 10.1124/mol.107.040360. [DOI] [PubMed] [Google Scholar]
- Lee JH, Choi SH, Lee BH, Hwang SH, Kim HJ, Rhee J, Chung C, Nah SY. Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase α. Neurosci Lett. 2013;26:143–148. doi: 10.1016/j.neulet.2013.05.048. [DOI] [PubMed] [Google Scholar]
- McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Missan S, Linsdell P, McDonald TF. Tyrosine kinase and phosphatase regulation of slow delayed-rectifier K+ current in guinea-pig ventricular myocytes. J Physiol. 2006;1:469–482. doi: 10.1113/jphysiol.2005.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta J, Furukawa T, Marumo F, Sawanobori T, Hiraoka M. Subcellular mechanism for Ca2+-dependent enhancement of delayed rectifier K+ current in isolated membrane patches of guinea pig ventricular myocytes. Circ Res. 1994;74:96–104. doi: 10.1161/01.res.74.1.96. [DOI] [PubMed] [Google Scholar]
- Pyo MK, Choi SH, Hwang SH, Shin TJ, Lee BH, Lee SM, Lim YD, Nah SY. Novel glycoproteins from ginseng. J Gingseng Res. 2011;35:92–103. [Google Scholar]
- Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;54:220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC. Dysfunction of delayed rectifier potassium channels in an inherited cardiac arrhythmia. Ann N Y Acad Sci. 1999;868:406–413. doi: 10.1111/j.1749-6632.1999.tb11302.x. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Shamgar L, Ma L, Schmitt N, Haitin Y, Peretz A, Wiener R, Hirsch J, Pongs O, Attali B. Calmodulin is essential for cardiac IKS channel gating and assembly: impaired function in long-QT mutations. Circ Res. 2006;98:1055–1063. doi: 10.1161/01.RES.0000218979.40770.69. [DOI] [PubMed] [Google Scholar]
- Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990;258:H1200–1207. doi: 10.1152/ajpheart.1990.258.4.H1200. [DOI] [PubMed] [Google Scholar]
- Yus-Najera E, Santana-Castro I, Villarroel A. The identification and characterization of a noncontinuouscalmodulin-binding site in noninactivating voltage-dependent KCNQ potassium channels. J Biol Chem. 2002;277:28545–28553. doi: 10.1074/jbc.M204130200. [DOI] [PubMed] [Google Scholar]