Abstract
Osteoclasts are large polykaryons that have the unique capacity to degrade bone and are generated by the differentiation of myeloid lineage progenitors. To identify the genes involved in osteoclast development, we performed microarray analysis, and we found that carboxypeptidase E (CPE), a prohormone processing enzyme, was highly upregulated in osteoclasts compared with their precursors, bone marrow-derived macrophages (BMMs). Here, we demonstrate a novel role for CPE in receptor activator of NF-κB ligand (RANKL)-induced osteoclast differentiation. The overexpression of CPE in BMMs increases the formation of tartrate-resistant acid phosphatase (TRAP)-positive multinuclear osteoclasts and the expression of c-Fos and nuclear factor of activated T cells c1 (NFATc1), which are key regulators in osteoclastogenesis. Furthermore, employing CPE knockout mice, we show that CPE deficiency attenuates osteoclast formation. Together, our data suggest that CPE might be an important modulator of RANKL-induced osteoclast differentiation.
Keywords: c-Fos, CPE, NFATc1, osteoclast, RANKL
INTRODUCTION
Bone homeostasis is maintained by the balance between bone-resorbing osteoclasts and bone-forming osteoblasts during bone remodeling (Anderson et al., 1997; Lacey et al., 1998). Excessive increases in osteoclastic bone resorption generally cause bone diseases such as osteoporosis and rheumatoid arthritis, and metastatic cancers (Baron and Hesse, 2012; Boyle et al., 2003). Osteoclasts are multinucleated cells that play a specialized role in bone resorption. These polykaryons differentiate from hematopoietic precursors of the monocyte/macrophage lineage in the presence of receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)(Novack and Teitelbaum, 2008; Teitelbaum, 2007; Teitelbaum and Ross, 2003).
Binding of RANKL to its receptor, RANK, which is expressed on osteoclast precursor cells, induces the recruitment of tumor necrosis factor receptor-associated factor (TRAF) molecules. RANKL binding to RANK triggers signal transduction processes involving NF-κB and mitogen-activated protein kinases. The activation of RANKL/RANK system leads to the induction of c-Fos and nuclear factor of activated T cells c1 (NFATc1), which are crucial transcription factors required for osteoclast differentiation. NFATc1, in turn, regulates the expression of osteoclast-specific genes, including tartrate-resistant acid phosphatase (TRAP) and cathepsin K.
Carboxypeptidase E (CPE) is a prohormone processing peptidase that is abundant in brain and endocrine tissues (Fricker and Snyder, 1982; Hook and Loh, 1984). This enzyme cleaves basic residues from the carboxy-terminal end of neuropeptide intermediates to yield mature bioactive forms (Cawley et al., 2012). CPE has also been shown to act as a prohormone sorting receptor for the regulated secretory pathway in endocrine and neuroendocrine cells (Cool et al., 1997). In addition, CPE is implicated in physiological cellular function and many diseases such as diabetes (Cawley et al., 2004; Leiter et al., 1999; Naggert et al., 1995), obesity (Cawley et al., 2004; Leiter et al., 1999), and tumor growth and metastasis (Du et al., 2001; He et al., 2004; Lee et al., 2011; Murthy et al., 2010; Tang et al., 2009), suggesting that CPE is a potential therapeutic drug target. Interestingly, previous studies have suggested a role for CPE in skeletal development. These studies demonstrated abundant expression of CPE in developing skeletal structures (Zheng et al., 1994) and growth plate chondrocytes (Zhang et al., 2008), although its specific role was not determined in these studies. Recently, Cawley et al. (2010) reported that CPE indirectly regulates bone metabolism through the sympathetic nervous system. However, the direct role of CPE in osteoclast differentiation is still poorly understood.
In this study, we identify CPE as a novel osteoclastogenic protein that is upregulated during osteoclast differentiation. We further reveal that CPE has a direct role in osteoclast differentiation induced by RANKL.
MATERIALS AND METHODS
Animals
Heterozygous (Cpe+/−) mice were obtained from The Jackson Laboratory (USA). Mice were bred in our animal facility. Genotyping was performed according to the protocol supplied by The Jackson Laboratory. All experimental procedures were performed with the approval of the Committee on the Ethics of Animal Experiments of the Kyungpook National University(Approval No. KNU-2011-99).
Reagents
RANKL and M-CSF were obtained from R&D Systems (USA). Antibodies against CPE, NFATc1, and cathepsinK were purchased from BD Biosciences (USA), BD Pharmingen (USA), and Millipore (USA), respectively.Antibody for c-Fos was purchased from Santa Cruz Biotechnology (USA) and β-actin was obtained from Sigma-Aldrich(USA).
Osteoclast culture
Bone marrow-derived macrophages (BMMs) wereprepared from bone marrow cells as described previously (Kim et al., 2012). To generate osteoclasts, BMMs were cultured for 4 days with α-minimal essential medium (α-MEM) containing 10% fetal bovine serum (FBS) in the presence of RANKL (20 ng/ml) and M-CSF (10 ng/ml). The media were changed every 2 days.
Tartrate-resistant acid phosphatase (TRAP) staining
Cells were fixed in 4% paraformaldehyde for 20 min and subsequently stained for TRAP activity with a 0.1 M acetate solution (pH 5.0) containing 6.76 mM sodium tartrate, 0.1 mg/ml naphthol AS-MX phosphate, and 0.5 mg/ml Fast Red Violet. TRAP-positive multinucleated cells with three or more nuclei were scored.
TRAP solution assay (TRAP activity)
To measure TRAP activity, cells were fixed and incubated with 70 μl (in a 96 well plate) of citrate buffer (50 mM, pH 4.6) containing 10 mM sodium tartrate and 10 mM p-nitrophenylphosphate (Sigma-Aldrich) for 15 min at 37°C. The enzyme reaction mixtures were then transferred into new plates containing an equal volume of 0.1 N NaOH. Absorbance was measured at 405 nm using a microplate reader.
Retroviral transduction
CPE was cloned into the pMX retroviral vector and transfected into Plat-E packaging cells (Morita et al., 2000)using TransIT-LT1 (Mirus, USA). The viral supernatant was collected from the culture media 24 to 48 h after transfection. BMMs were infected with the virus for 24 h in the presence of 4 μg/ml polybrene (Sigma-Aldrich). The cells were selected with 1 μg/ml blasticidin for 3 days and subsequently used for osteoclast generation.
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was performed as described previously (Kim et al., 2012). The following primers were used: CPE, 5′-ATGGTAATG-AGGCGGTTGG-3′ and 5′-TTCTTGCGACAGGGAGGT-3′; TRAP, 5′-ACAGCCCCCCACTCCCACCCT-3′ and 5′-TCAGGGTCTGGGTCTCCTTGG-3′; cathepsin K, 5′-GGAAGAAGACTCACCAGAAGC-3′ and 5′-GTCATATAGCCGCCTCCACAG-3′; RANK, 5′-TTTGTGGAATTGGGTCAATGAT-3′ and 5′-ACCTCGCTGACCAGTGTGAA-3′; TRAF6, 5′-GCTCAAACGGACCATTCGGA-3′ and 5′-GGGATTGTGGGTCGCTGAAA-3′; c-Fms, 5′-GAGCCTCTTGCAGGAGGTG-3′ and 5′-GGTCCAATGGGCAGCTGG-3′; and GAPDH, 5′-ACTTTGTCAAGCTCATTTCC-3′ and 5′-TGCAGCGAACTTTATTGATG-3′.
Real-time PCR
Real-time PCR was performed using an ABI 7500 Real-Time PCR System and SYBR Green dye (Applied Biosystems, USA). The following primers were used: c-Fos, 5′-AGGCCCAGTGGCTCAGAGA-3′ and 5′-GCTCCCAGTCTGCTGCATAGA-3′; NFATc1, 5′-ACCACCTTTCCGCAACCA-3′ and 5′-TTCCGTTTCCCGTTGCA-3′; TRAP, 5′-TCCCCAATGCCCCATTC-3′ and 5′-CGGTTCTGGCGATCTCTTTG-3′; cathepsin K, 5′-GGCTGTGGAGGCGGCTAT-3′ and 5′-AGAGTCAATGCCTCCGTTCTG-3′.
Western blotting
Cultured cells were lysed in lysis buffer containing 50 mMTris (pH 7.4), 150 mMNaCl, 1% NP-40, 1 mM EDTA, and protease and phosphatase inhibitors. The protein concentration was determined using the Bicinchoninic Acid Kit (Pierce, USA), and equal amounts of proteins were separated on a sodium dodecyl sulfate (SDS)-polyacrylamide gel. The gels were blotted onto nitrocellulose membranes and incubated with primary antibodies. The immunoreactive proteins were detected with enhanced chemiluminescence reagents (ECL-plus, Amersham, GE Healthcare) after incubation with the appropriate secondary antibody.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.1% Triton X-100 for 10 min. After blocking with 1% bovine serum albumin for 10 min, the cells were labeled with anti-CPE antibody (1:100) in blocking solution for 1 h. The samples were rinsed with phosphate-buffered saline (PBS) and incubated with Alexa Fluor 488 goat anti-mouse IgG antibody (1:100, Invitrogen) for 1 h. After washing with PBS, the samples were mounted with 90% glycerol in PBS and observed using a fluorescence microscope (Nikon, Japan).
Statistical analysis
All experiments were performed in triplicate. The data are presented as the means ± S.D. Statistical significance was determined using a two-tailed Student’s t-test.
RESULTS
CPE expression is upregulated during osteoclastogenesis
To identify genes that are regulated during osteoclast development, we performed microarray analysis and compared the gene expression profiles between undifferentiated precursor cells and fully differentiated osteoclasts. The microarray data showed that CPE expression was increased by 43-fold in osteoclasts compared with primary bone marrow-derived macrophages (BMMs) (data not shown). To confirm the relevance of this observation, we examined the expression pattern of CPE during RANKL-induced osteoclast differentiation. BMMs were isolated and subsequently cultured in the presence of osteoclastogenic cytokines. We found that CPE mRNA expression was time-dependently elevated during osteoclastogenesis (Fig. 1A). Similarly, the protein level of CPE was strongly increased as the cells differentiated (Fig. 1B). This pattern of CPE protein expression was further confirmed by immunofluorescent staining. We observed clear staining of CPE in mature osteoclasts; however, we detected very low CPE signals in undifferentiated BMMs (Fig. 1C).
Fig. 1.
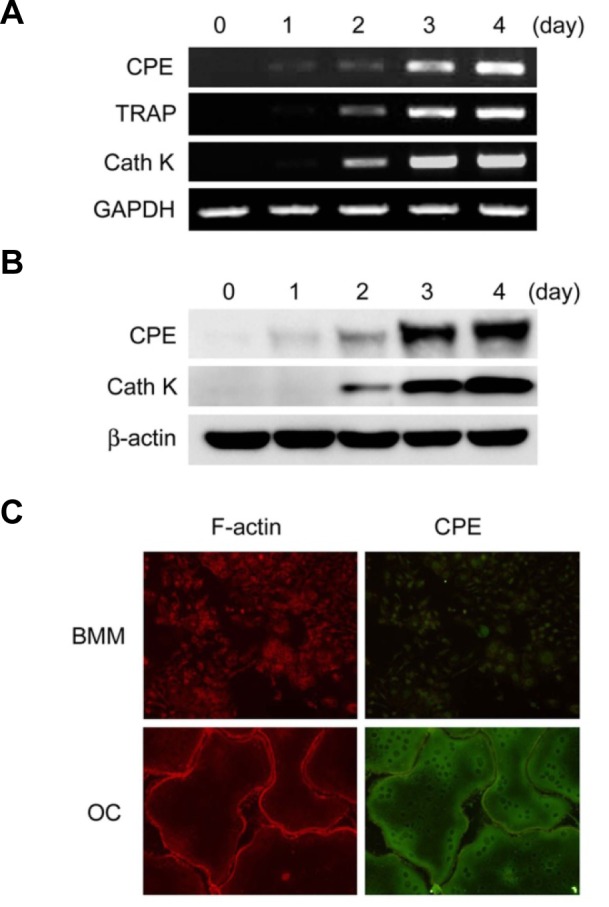
Expression of CPE is enhanced during osteoclast differentiation. BMMs were cultured in M-CSF (10 ng/ml) and RANKL (20 ng/ml) for the indicated number of days. (A) Expression of CPE was determined by RT-PCR. (B) CPE expression was analyzed by immunoblotting. TRAP or cathepsinK (CathK) served as positive controls for osteoclastogenesis, and GAPDH or β-actin served as loading controls. (C) BMMs or osteoclasts (OC) were fixed and permeabilized prior to staining. F-actin and CPE were stained with TRITC-conjugated phalloidin (red) and anti-CPE antibody (green), respectively.
Overexpression of CPE enhances osteoclast formation
The expression pattern of CPE suggests that CPE may have biological significance in the context of osteoclast development. To examine this possibility, we retrovirally transduced primaryBMMs with CPE or control (pMX empty vector). The overexpression of CPE was confirmed by RT-PCR (Fig. 2A).Transduced BMMs were cultured in the presence of M-CSF and various concentrations of RANKL for 4 days. The cells were then stained for TRAP, which is a cytochemical marker of osteoclasts. The overexpression of CPE significantly increased the number of TRAP- positive multi-nuclear osteoclasts (MNCs) at all concentrations of RANKL (Figs. 2B and 2C). In agreement with the increased numbers of TRAP-expressing cells, TRAP activity, as measured by TRAP solution assay, was also elevated in CPE-transduced osteoclasts (Fig. 2D). We also observed that the overexpression of CPE did not affect precursor proliferation or survival by means of an MTS assay (data not shown).
Fig. 2.
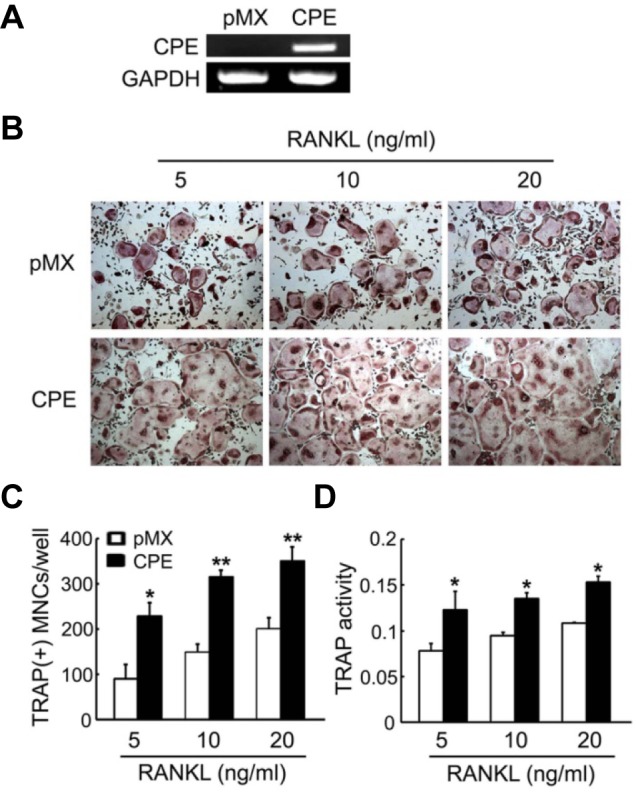
Overexpression of CPE enhances osteoclast formation. BMMs were transduced with pMX vector (control) or CPE (pMX-CPE) retrovirus. (A) CPE expression was analyzed by RT-PCR. (B) BMMs were cultured with M-CSF (10 ng/ml) and the indicated concentrations of RANKL and stained for TRAP after 4 days. (C) The TRAP-positive multinucleated cells (MNCs) were counted. (D) TRAP activity was determined by measuring the OD values at 405 nm on day 4. The data are expressed as the means ± SD. *P < 0.05, **P < 0.001.
Overexpression of CPE enhances the induction of c-Fos and NFATc1
Because ectopic expression of CPE increased osteoclast formation, we next examined the impact of CPE overexpression onthe expression of osteoclastogenic markers. As shown in Fig. 3A, the mRNA expression of c-Fos and NFATc1, key transcriptionfactors for osteoclastogenesis, was significantly increased in CPE-transduced osteoclasts compared with vector-infected cells.Reflecting the enhanced NFATc1 expression, the mRNA expression of NFATc1 target genes, TRAP and cathepsinK, was also increased by the overexpression of CPE (Fig. 3A). This CPE-mediated upregulation of c-Fos, NFATc1, and cathepsinK was further confirmed by immunoblotting analysis (Fig. 3B). On the other hand, the overexpression of CPE did not affect the mRNA expression of RANK, c-Fms, or TRAF6 in response to RANKL (Fig. 3C). Overall, these results indicate that CPE overexpression enhances RANKL-induced osteoclast differentiation.
Fig. 3.
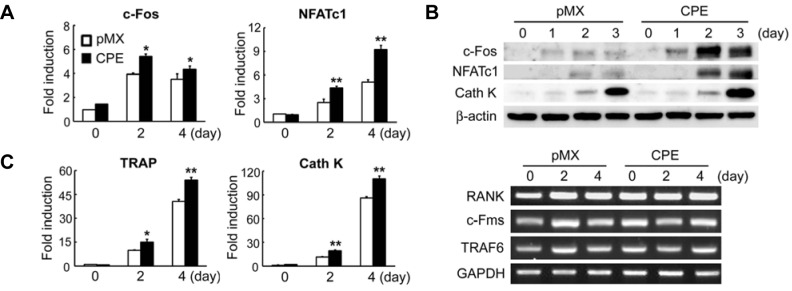
Overexpression of CPE increases the expression of c-Fos and NFATc1. BMMs were transduced with pMX control or CPE retrovirus and cultured with M-CSF (10 ng/ml) and RANKL (20 ng/ml) for the indicated number of days. Real-time PCR (A), immunoblotting (B), or RT-PCR (C) was performed to assess the expression of the indicated genes. GAPDH and β-actin served as loading controls. The data are expressed as the means ± SD. *P < 0.05, **P < 0.001.
Deficiency of CPE attenuates osteoclast formation
We next investigated the physiologic role of CPE in osteoclast differentiation. BMMs from WT or CPE knockout (KO) mice were cultured with M-CSF and two different concentrations of RANKL. In both concentrations of RANKL, the deficiency of CPE attenuated osteoclast formation (Figs. 4A and 4B). Reflecting the decreased numbers of TRAP-positive cells, TRAP activity wasalso reduced in CPE KO mice compared with WT mice (Fig. 4C). These results indicate that CPE acts as a positive modulator in RANKL-mediated osteoclastogenesis.
Fig. 4.
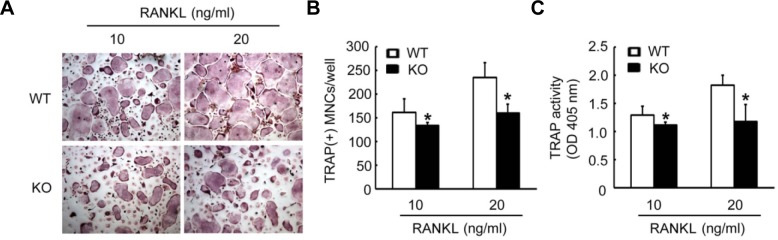
Deficiency of CPE decreases osteoclast formation. BMMs derived from WT or CPE KO mice were cultured for 4 days with 10 ng/ml of MCSF and the indicated concentrations of RANKL. (A) Cells were fixed and stained for TRAP. (B) Numbers of TRAP-positive MNCs were counted. (C) TRAP activity was determined. The data are expressed as the means ± SD. *P < 0.05.
DISCUSSION
Osteoclast development is a sequential multistep event involvinghematopoietic progenitor generation, differentiation, fusion, andactivation. This process requires several biomolecules; however, many molecules involved in osteoclast development remain unidentified. To identify genes regulated by RANKL, we performed microarray analysis using primary BMMs after stimulation with RANKL. We observed that CPE expression was abundant in bone-resorbing osteoclasts. The expression of CPE was very low in undifferentiated progenitor cells and was increased during the osteoclastogenic process. The expression pattern of CPE implies that CPE may have an important role in osteoclast development. Indeed, we found that CPE positively regulates osteoclast differentiation.
Previous studies identified CPE as a highly expressed gene in the context of skeletal development. Studies utilizing in situ hybridization during development revealed that CPE was expressed in cartilage primordium in cephalic bones (Zheng et al., 1994). In addition, microarray analysis of perichondral and reserve chondrocytes in the growth plates of long bones demonstrated that CPE was abundant in both zones (Zhang et al., 2008). These studies proposed that CPE may be involved in skeletal development. In the current study, we provide clear evidence for the expression of CPE in osteoclast lineage cells and its role in osteoclast differentiation.
It has been reported that CPE knockout (KO) mice, an obese animal model, have low bone mineral density (Cawley et al., 2010). The study demonstrated that CPE KO mice had reduced levels of the hypothalamic neuropeptides, resulting in an overall increase in the ratio of RANKL/osteoprotegerin. Although this study highlighted the importance of CPE in energy metabolism and bone remodeling, the direct role of CPE in osteoclast differentiation was not investigated. On the other hand, our study shows that CPE directly regulates RANKL-induced osteoclastogenesis. Furthermore, our work also reveals that the deficiency of CPE results in decreased osteoclast formation. Together, both studies clearly indicate that CPE is important for bone metabolism and remodeling.
c-Fos and NFATc1 are key transcription factors that regulate the induction of osteoclastogenic genes. Mice that are deficient in c-Fos exhibit severe osteopetrotic phenotype due to defective osteoclast formation (Johnson et al., 1992; Wang et al., 1992). The important role of NFATc1 in osteoclastogenesis was demonstrated by in vitro experiments. Embryonic stem cells derived from NFATc1-deficient mice cannot differentiate into osteoclasts, and ectopic expression of NFATc1 results in the generation of osteoclasts from precursor cells in the absence of RANKL (Hirotani et al., 2004; Takayanagi et al., 2002). In vivo expression of constitutively active NFATc1 results in osteopenia due to increased osteoclast differentiation (Ikeda et al., 2006). In this study, we found that the overexpression of CPE increases the expression of both c-Fos and NFATc1. Thus, critical for the acceleration of osteoclast differentiation. Further studies will be necessary to clarify the molecular mechanism underlying CPE-mediated induction of these transcription factors by RANKL.
CPE cleaves hormone precursors, including proopiomelanocortin (POMC), and produces several physiologically important peptides (D’Agostino and Diano, 2010). It has been demonstrated that POMC is expressed in osteoclasts (Zhong et al., 2005), suggesting that a POMC-derived peptide may have a direct role in osteoclast development. In agreement with these observations, we also found that POMC was expressed in osteoclast lineage cells (data not shown). One POMC-derived hormone often studied in skeletal cells is alpha-melanocyte stimulating hormone (α-MSH). Cornish et al. (2003) reported the direct effect of α-MSH on osteoclasts, showing that α-MSH stimulates osteoclast formation from BMMs and administration of α-MSH to mice decreased bone volume and trabecular number. In this regard, increased levels of α-MSH by CPE overexpression would likely to contribute to CPE-mediated enhancement of osteoclast formation.
In summary, we report that the metallocarboxypeptidase family member CPE is upregulated during osteoclast development. Our results demonstrate that the overexpression of CPE increases osteoclast differentiation through the induction of c-Fos and NFATc1, key regulators of osteoclastogenesis. Furthermore, we show that CPE deficiency decreases osteoclast generation in response to RANKL. Thus, in addition to its indirect effects on bone metabolism through the regulation of peptide hormone processing, CPE exerts direct effects on osteoclasts and plays a pivotal role in osteoclast differentiation.
Acknowledgments
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2013R1A1A2A10005515).
REFERENCES
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metabol. 2012;97:311–325. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Cawley NX, Zhou J, Hill JM, Abebe D, Romboz S, Yanik T, Rodriguiz RM, Wetsel WC, Loh YP. The carboxypetidase E Knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology. 2004;145:5807–5819. doi: 10.1210/en.2004-0847. [DOI] [PubMed] [Google Scholar]
- Cawley NX, Yanik T, Woronowicz A, Chang W, Marini JC, Loh YP. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density. Am J Physiol Endocrinol Metab. 2010;299:E189–197. doi: 10.1152/ajpendo.00516.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocrine Rev. 2012;33:216–253. doi: 10.1210/er.2011-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Mountjoy KG, Bava U, Lin JM, Myers DE, Naot D, Reid IR. alpha -melanocyte- stimulating hormone is a novel regulator of bone. American journal of physiology. Endocrinol Metabol. 2003;284:E1181–1190. doi: 10.1152/ajpendo.00412.2002. [DOI] [PubMed] [Google Scholar]
- D’Agostino G, Diano S. Alpha-melanocyte stimulating hormone: production and degradation. J Mol Med. 2010;88:1195–1201. doi: 10.1007/s00109-010-0651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Keegan BP, North WG. Key peptide processing enzymes are expressed by breast cancer cells. Cancer Lett. 2001;165:211–218. doi: 10.1016/s0304-3835(01)00409-8. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Snyder SH. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci USA. 1982;79:3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, Jen J, Gabrielson E, Brambilla E, Travis WD, et al. Identification of carboxypeptidase E and gamma-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Hum Pathol. 2004;35:1196–1209. doi: 10.1016/j.humpath.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Hirotani H, Tuohy NA, Woo JT, Stern PH, Clipstone NA. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem. 2004;279:13984–13992. doi: 10.1074/jbc.M213067200. [DOI] [PubMed] [Google Scholar]
- Hook VY, Loh YP. Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proc Natl Acad Sci USA. 1984;81:2776–2780. doi: 10.1073/pnas.81.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Nishimura R, Matsubara T, Hata K, Reddy SV, Yoneda T. Activation of NFAT signal in vivo leads to osteopenia associated with increased osteoclastogenesis and bone-resorbing activity. J Immunol. 2006;177:2384–2390. doi: 10.4049/jimmunol.177.4.2384. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hong JM, Yoon KA, Kim N, Cho DW, Choi JY, Lee IK, Kim SY. Early growth response 2 negatively modulates osteoclast differentiation through upregulation of Id helix-loop-helix proteins. Bone. 2012;51:643–650. doi: 10.1016/j.bone.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lee TK, Murthy SR, Cawley NX, Dhanvantari S, Hewitt SM, Lou H, Lau T, Ma S, Huynh T, Wesley RA, et al. An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers. J Clin Invest. 2011;121:880–892. doi: 10.1172/JCI40433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Leiter EH, Kintner J, Flurkey K, Beamer WG, Naggert JK. Physiologic and endocrinologic characterization of male sex-biased diabetes in C57BLKS/J mice congenic for the fat mutation at the carboxypeptidease E locus. Endocrine. 1999;10:57–66. doi: 10.1385/endo:10:1:57. [DOI] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Murthy SR, Pacak K, Loh YP. Carboxypeptidase E: elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cell Mol Neurobiol. 2010;30:1377–1381. doi: 10.1007/s10571-010-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Ann Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Tang SS, Zhang JH, Liu HX, Li HZ. PC2/CPE-mediated pro-protein processing in tumor cells and its differentiated cells or tissues. Mol Cell Endocrinol. 2009;303:43–49. doi: 10.1016/j.mce.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- Zhang M, Pritchard MR, Middleton FA, Horton JA, Damron TA. Microarray analysis of perichondral and reserve growth plate zones identifies differential gene expressions and signal pathways. Bone. 2008;43:511–520. doi: 10.1016/j.bone.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Streck RD, Scott RE, Seidah NG, Pintar JE. The developmental expression in rat of proteases furin, PC1, PC2, and carboxypeptidase E: implications for early maturation of proteolytic processing capacity. J Neurosci. 1994;14:4656–4673. doi: 10.1523/JNEUROSCI.14-08-04656.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, Kang B, Xu J, Bollag RJ, Isales CM. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36:820–831. doi: 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]


