Abstract
Significant advances have been made in nucleos(t)ide analogue (NA) therapy to treat chronic hepatitis B, and this therapy reduces the risk of hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) in some patients. However, whether NAs can also prevent recurrence after radical resection of HBV-related HCC remains controversial and is an important question, given that most patients will experience recurrence within a few years of curative surgery. Here we systematically reviewed the literature since 2004 on outcomes after administering NAs to patients with HBV-related HCC following radical resection. We focused on treatment indications, duration, effects on recurrence-free survival and overall survival, and the management of NA resistance. We find that patients with HCC should strongly consider NA therapy if they are positive for HBV-DNA, and that the available evidence suggests that postoperative NA therapy can increase both recurrence-free and overall survival. To minimize drug resistance, clinicians should opt for potent analogues with higher resistance barriers, and they should monitor the patient carefully for emergence of NA-resistant HBV.
Keywords: Antiviral therapy, Hepatitis B virus, Hepatocellular carcinoma, Liver resection, Nucleos(t)ide analogue, Survival rate
Core tip: Significant advances have been made in nucleos(t)ide analogue (NA) therapy to treat chronic hepatitis B. However, for patients undergoing radical resection for hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC), a number of important questions remain undefined, including when NA therapy should be initiated, how long the treatment should continue, and whether NAs can prevent recurrence after radical resection. Here we review the available evidence on these questions in the Medline database. We focus on NA treatment indications, duration, effects on recurrence-free survival and overall survival, and management of NA resistance in patients with HBV-related HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most frequent cause of cancer-related death in the world[1]. Hepatic resection, percutaneous ethanol injection, radiofrequency ablation are recognized as radical treatment options for HCC and are highly effective at removing tumors; however, patients’ prognosis after radical resection remains poor, due to the high recurrence rate[1,2]. HCC recurrence occurs in up to 41%-50% of patients within 2 years after resection (early recurrence) and in up to 20% of patients more than 2 years later (late recurrence)[3,4]. Most early recurrence appears to reflect diffusion of primary tumors, while most late recurrence stems from de novo tumors spontaneously arising in the remnant diseased liver[3-5].
In China and Sub-Saharan Africa, the major risk factor for HCC is hepatitis B virus (HBV) infection. Therefore investigators reasoned that the same nucleos(t)ide analogues (NAs) that have been proven so effective against chronic HBV infection may also benefit patients with HBV-related HCC. Indeed, randomized controlled trials (RCTs)[6] and large retrospective studies[7-9] have shown that NAs can dramatically reduce the risk of HCC in patients with chronic HBV infection or cirrhosis. While this suggests that NAs are effective against primary HCC, the question of whether they can also prevent HCC recurrence after radical resection remains controversial[10].
Here we systematically reviewed the literature on this question by searching the Medline database for articles published since 2004 on outcomes of NA therapy in patients with HBV-related HCC. We used the following search terms: “nucleoside analogue”, “nucleoside analog”, “nucleotide analogue”, “nucleotide analog”, “antiviral therapy”, “hepatitis B virus”, “hepatocellular carcinoma”, “liver resection”, and “survival rate”. We focused on treatment indications, duration, effects on recurrence-free survival and overall survival, and the development of NA resistance.
TYPES OF NAS
Five types of oral NAs have been used in clinical practice: lamivudine (LAM), adefovir dipivoxil (ADV), telbivudine (LdT), entecavir (ETV) and tenofovir disoproxil fumarate (TDF). LAM and LdT are L-nucleoside analogues, ADV and TDF are acyclic adenine nucleotide analogues, and ETV is a cyclopentyl guanosine analogue[11]. All 5 of these NA types can be phosphorylated in cells, and subsequently compete with natural nucleotides to be incorporated into viral DNA by HBV polymerase/reverse transcriptase. Since the analogues cannot be extended by HBV polymerase, they cause premature termination of genome replication. Studies suggest that ETV, TDF, and LdT are similarly effective at suppressing HBV-DNA synthesis and are more potent than LAM and ADV[11], although none can completely eradicate HBV due to the persistence of covalently closed circular DNA in the nuclei of infected hepatocytes[12].
INDICATIONS AND DURATION OF NA THERAPY AFTER HCC SURGERY
Nowadays there are Asian-Pacific consensus[11], Chinese Medical Association guideline[13], American Association for the Study of Liver Disease (AASLD) guideline[14], European Association for the Study of Liver (EASL) guideline[15], Treatment Algorithm in the United States[16] and Asian-American guideline[17] related to the treatment of chronic hepatitis B infection. In these guidelines[11,13-17], the criteria for initiating treatment such as ALT level and HBV-DNA amount are different. Current Asian guidelines[11,13] recommend that NA therapy be considered if the ALT level is > 2-fold greater than the upper limit of the normal range, and the HBV-DNA level is either > 20000 IU/mL if the patient is HBeAg-positive or > 2000 IU/mL if the patient is HBeAg-negative. In America, with the same criteria about ALT level, NA therapy is recommended to patients if their HBV-DNA level is > 20000 IU/mL[14]. While a panel of Asian-American physicians with expertise in hepatitis B treatment has suggested[17] that Asia Americans should be considered for treatment when they have HBV-DNA levels above 2000 IU/mL, and serum ALT levels above the upper limit of the normal range, and so did EASL guidelines[15] in the criteria of ALT level and HBV-DNA amount, which are stricter than AASLD guideline[14].
Recommended treatment duration also varies depending on these guidelines[11,13-15]. In HBeAg-positive patients who show HBeAg seroconversion and undetectable levels of HBV-DNA, Asian-Pacific guideline[11] recommends that NA treatment can be discontinued after 12 mo of consolidation therapy, while AASLD guideline[14] recommends the duration of consolidation therapy be at least 6 mo. In HBeAg-negative patients, both Asian-Pacific and AASLD guidelines recommend NA treatment should ideally be stopped when HBsAg is no longer detectable[11,14], while Asian-Pacific guideline[11] advises if the patient remains HBsAg-positive, NA treatment can be discontinued after at least 2 years of therapy when test results show undetectable HBV-DNA levels on 3 separate occasions 6 mo apart. EASL guideline[15] suggests that in both HBeAg-positive and HBeAg-negative patients sustained off-treatment HBsAg loss is the ideal end point. Sustained off-treatment virological and biochemical response in HBeAg-negative patients (including HBeaAg-positive patients at baseline with durable anti-HBe seroconversion) is the second, and a maintained undectable HBV-DNA under long-term antiviral therapy in HBeAg-positive patients without anti-HBe seroconversion and in HBeAg-negative patients is the next most desirable end point.
Since these guidelines[11,13-17] were different from each other and were developed for patients whose major disease was chronic HBV infection, it is unclear whether they are optimal for patients with HBV-related HCC. Given the need to reduce HBV replication as much as possible in these patients, particularly before drug resistance emerges, the Chinese Medical Association[18] recommends that the threshold of viremia to initiate NA therapy for patients with HBV-related HCC should be lower than the threshold for patients without HCC, and that patients with HBV-related HCC should take NA therapy as long as they show detectable levels of HBV-DNA, regardless of ALT levels. Going even further, some investigators[19] have suggested routine prophylactic NA therapy for HCC patients with HBV-DNA levels < 2000 IU/mL before liver resection. The aim is to prevent HBV reactivation after liver resection, which occurs in as many as 19% of patients within the first 1 year and which can severely reduce liver function and survival[19].
Since NA therapy cannot completely eradicate HBV, some investigators have advocated lifelong treatment, regardless of undetectable levels of HBV-DNA and HBeAg seroconversion in HBeAg-positive patients or HBsAg loss in HBeAg-negative patients. Those authors argue that long-term therapy may help prevent hepatitis flare-ups and inhibit hepatocarcinogenesis to the greatest extent[20], although there is not sufficient evidence nowadays.
POSTOPERATIVE NA THERAPY AND RECURRENCE-FREE SURVIVAL
Our extensive online search in the Medline database identified 19 studies published since 2004 that investigated outcomes of postoperative NA therapy in patients with HBV-related HCC. These references comprise 17 retrospective studies[21-37] and 2 RCTs[38,39]. Most of studies come from Asia, including Chinese mainland, Japan, Hong Kong and Tai Wan, which reflects HBV epidemiology and the high incidence of HBV-related HCC in this region. One study from the United States has a small number of patients appeared first in 2011[36] and further follow up published in 2014 with more cases and a longer follow up over 12 years[37]. Of the 19 included studies, besides patients who underwent hepatic resection (6705, 96.7%), NA therapy were also applied for patients with ablative procedures as follows: radiofrequency ablation (176, 2.5%), percutaneous ethanol injection (7, 0.1%), and transarterial chemoembolization (49, 0.7%). Patients’ characteristics in these studies are shown in Table 1. The outcomes data are shown in the Table 2.
Table 1.
Characteristics of patients with hepatitis B virus-related hepatocellular carcinoma treated with nucleos(t)ide analogues or not after radical resection
| Ref. | Country or region | No. of patient1 | Mean age (yr)1 | TNM stage (I/II/III/IV) (n) | Multiple tumor (%)1 | Mean tumor size (cm)1 | Portal vein invasion (%)1 | Mean HBV-DNA level (log10 copies/mL)1 | Mean ALT (U/L)1 | Cirrhosis (%)1 | Initial treatment for HCC, (Ope/RFA/PEI/TACE) | NA therapy | Mean antiviral treatment duration (mo) | Mean follow-up duration (mo)1 |
| Piao et al[21] | Japan | 30 vs 40 | 59 vs 58 | 31/25/11/3 | N/A | 2.3 vs 2.52 | N/A | 6.1 vs 6.52 | 88 vs 62 | N/A | 22/16/0/32 | LAM | N/A | 24 |
| Shuqun et al[22] | Chinese mainland | 16 vs 17 | 48.3 vs 48.5 | N/A | N/A | ≥ 5 cm: 56.2% vs 70.6% | 37.5 vs 23.5 | N/A | N/A | 100 vs 94.1 | 33/0/0/0 | LAM | 12 | 12-36 |
| Kuzuya et al[23] | Japan | 16 vs 33 | 59.8 vs 61.1 | 25/19/5/0 | N/A | N/A | N/A | 6.2 vs 4.12 | 56.6 vs 54.2 | N/A | 31/18/0/0 | LAM | 22.7 | 38.0 vs 32.6 |
| Kubo et al[24] | Japan | 14 vs 10 | 55 vs 55 | 5/9/10/0 | N/A | 2.4 vs 2.8 | 28.6 vs 40.0 | 6.0 vs 6.0 | 53 vs 562 | 42.9 vs 40.0 | 24/0/0/0 | LAM | 32 | 36.7 vs 7.32 |
| Hung et al[25] | Hong Kong | 10 vs 62 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 72/0/0/0 | LAM | N/A | 18.92 |
| Yoshida et al[26] | Japan | 33 vs 71 | 57 vs 59 | I + II: 57.6% vs 73.2% | N/A | 2.6 vs 2.8 | N/A | ≥ 3.7: 100% vs 63% | 54 vs 362 | N/A | 0/104/0/0 | LAM | N/A | 33 vs 47 |
| Koda et al[27] | Japan | 30 vs 20 | 59 vs 60 | 19/20/11/0 | N/A | N/A | N/A | 5.7 vs 5.2 | 78 vs 54 | N/A | 12/24/5/9 | 28LAM + 2ETV | 28.6 | 28.6 vs 36.3 |
| Chuma et al[28] | Japan | 20 vs 30 | 55.7 vs 55.6 | 19/27/4/0 | 25.0 vs 23.3 | 1.7 vs 2.1 | N/A | 6.0 vs 5.92 | 43.1 vs 37.7 | 55.0 vs 53.3 | 10/10/0/0 | 15LMA + 5ETV | N/A | 35.5 vs 49.22 |
| Li et al[29] | Chinese mainland | 43 vs 36 | 46 vs 45 | 13/27/39/0 | N/A | 7.1 vs 8.5 | 30.2 vs 27.8 | 6.5 vs 7.3 | 60.8 vs 56.5 | 55.8 vs 69.4 | 79/0/0/0 | LAM | N/A | 12 vs 12 |
| Chan et al[30] | Hong Kong | 42 vs 94 | 57 vs 552 | 39/32/64/0 | N/A | 9.3 vs 9.02 | 11.9 vs 18.1 | N/A | 58.0 vs 42.52 | 73.8 vs 56.4 | 136/0/0/0 | 38LAM + 4ETV | N/A | N/A |
| Wu et al[31] | Tai Wan | 518 vs 4051 | 54.4 vs 54.6 | N/A | N/A | N/A | N/A | N/A | N/A | 48.6 vs 38.7 | 4569/0/0/0 | 159LAM + 292ETV + 36LdT + 31Combined | 17.4 | 31.7 vs 26.2 |
| Urata et al[32] | Japan | 46 vs 13 | 57 vs 58 | N/A | 28.3 vs 61.5 | 2.8 vs 3.4 | 34.8 vs 46.2 | 4.7 vs 6.1 | 46.8 vs 58.0 | 45.7 vs 30.8 | 59/0/0/0 | 22LAM + 24ETV | N/A | 36.22 |
| Ke et al[33] | Chinese mainland | 141 vs 141 | 48.9 vs 49.7 | N/A | 27.7 vs 24.1 | 4.5 vs 5.02 | 7.8 vs 7.1 | 4.9 vs 4.7 | 39 vs 42 | 81.6 vs 81.6 | 282/0/0/0 | LAM | 12 | 24 vs 23 |
| Yin et al[38] | Chinese mainland | 81 vs 82 | 47.9 vs 49.3 | N/A | 12.3 vs 22.0 | ≥ 3 cm: 86.4% vs 93.9% | 3.7 vs 7.3 | 4.9 vs 4.6 | 47.3 vs 37.5 | 24.7 vs 28.0 | 163/0/0/0 | LAM | N/A | 39.92 |
| 215 vs 402 | 50.1 vs 50.2 | N/A | 14.4 vs 12.7 | ≥ 3 cm: 89.3% vs 92.3% | 14.0 vs 15.4 | 4.5 vs 3.8 | > 42: 48.8% vs 36.8% | 47.0 vs 35.8 | 617/0/0/0 | LAM | N/A | 23.82 | ||
| Su et al[34] | Tai Wan | 62 vs 271 | 52 vs 582 | N/A | 22.6 vs 46.9 | 2.7 vs 4.22 | 11.3 vs 20.0 | 5.9 vs 5.52 | 45 vs 422 | 33.7 vs 45.8 | 333/0/0/0 | 40LAM + 19ETV + 3PEG-IFN | N/A | 45.92 |
| Yan et al[35] | Chinese mainland | 35 vs 25 | 45 vs 47 | 22/29/9/0 | N/A | 4.7 vs 5.0 | 65.7 vs 68.0 | > 5: 54.3% vs 72.0% | 41.5 vs 35.8 | N/A | 60/0/0/0 | LAM | N/A | N/A |
| Hann et al[37] | The United States | 16 vs 9 | 57 vs 532 | N/A | 0 vs 0 | 2.7 vs 3.02 | 0 vs 0 | 5.4 vs 6.92 | N/A | N/A | 3/4/2/8/others3 | 8(LAM + TDF) + 3(LAM + ADV) + 2(TLV + TDF) + 2TDF + 1LAM | N/A | 60.2 |
| Huang et al[39] | Chinese mainland | 100 vs 100 | 50.6 vs 50.5 | N/A | 17 vs 16 | 4.9 vs 5.1 | 0 vs 0 | > 3.3: 100% vs 100% | 52.6 vs 51.4 | N/A | 200/0/0/0 | ADV | N/A | 602 |
Patients who received postoperative NA treatment vs patients who received no postoperative NA treatment;
Median values;
Two patients received resection and RFA for their initial treatment; Three patients received RFA and TACE; One patient received RFA, PEI and TACE; Two patients received cryoablation. Boldfaced data come from randomized controlled trials in our review[38,39]. ADV: Adefovir dipivoxil; ETV: Entecavir; LAM: Lamivudine; LdT: Telbivudine; N/A: Not available; Ope: Operation; PEI: Percutaneous ethanol injection; RFA: Radiofrequency ablation; TACE: Transarterial chemoembolization; NA: Nucleos(t)ide analogue.
Table 2.
Survival outcomes of patients with hepatitis B virus-related hepatocellular carcinoma treated with nucleos(t)ide analogues or not after radical resection
| Year of publication | Ref. | Group | n |
Overall survival rate (%) |
Recurrence-free survival rate (%) |
||||||
| 1 yr | 3 yr | 5 yr | P | 1 yr | 3 yr | 5 yr | P | ||||
| 2005 | Piao et al[21] | NAs | 30 | 100 | 91.3 | N/A | 0.12 | 75 | 46 | N/A | > 0.05 |
| Control | 40 | 92.4 | 66 | N/A | 58 | 22 | N/A | ||||
| 2006 | Shuqun et al[22] | NAs | 16 | 24 | N/A | N/A | 0.0053 | 19.7 | N/A | N/A | > 0.05 |
| Control | 17 | 0 | N/A | N/A | 4.5 | N/A | N/A | ||||
| 2007 | Kuzuya et al[23] | NAs | 16 | 100 | 100 | N/A | 0.063 | 86.5 | 64.9 | N/A | 0.622 |
| Control | 33 | 86.6 | 46.8 | N/A | 86.6 | 46.8 | N/A | ||||
| 2007 | Kubo et al[24] | NAs | 14 | N/A | N/A | N/A | N/A | 90 | 90 | 78 | 0.0086 |
| Control | 10 | N/A | N/A | N/A | 55 | 28 | 28 | ||||
| 2008 | Hung et al[25] | NAs | 10 | N/A | N/A | N/A | N/A | 90 | N/A | N/A | 0.03 |
| Control | 62 | N/A | N/A | N/A | 75 | N/A | N/A | ||||
| 2008 | Yoshida et al[26] | NAs | 33 | 100 | 80 | 59 | > 0.05 | N/A | N/A | N/A | > 0.05 |
| Control | 71 | 100 | 85 | 70 | N/A | N/A | N/A | ||||
| 2009 | Koda et al[27] | NAs | 30 | 96 | 76 | 76 | 0.02 | 65 | 15 | N/A | > 0.05 |
| Control | 20 | 86 | 48 | 32 | 72 | 30 | N/A | ||||
| 2009 | Chuma et al[28] | NAs | 20 | N/A | N/A | N/A | N/A | 90 | 55 | 45 | > 0.05 |
| Control | 64 | N/A | N/A | N/A | 85.9 | 50 | 43.7 | ||||
| 2010 | Li et al[29] | NAs | 43 | 41.9 | N/A | N/A | 0.0094 | 23.3 | N/A | N/A | 0.072 |
| Control | 36 | 33.3 | N/A | N/A | 8.3 | N/A | N/A | ||||
| 2011 | Chan et al[30] | NAs | 42 | 88.1 | 79.1 | 71.2 | 0.005 | 66.5 | 51.4 | 51.4 | 0.05 |
| Control | 94 | 76.5 | 47.5 | 43.5 | 48.9 | 33.8 | 33.8 | ||||
| 2012 | Wu et al[31] | NAs | 518 | 94 | 81 | 73 | 0.002 | 87 | 66 | 54 | < 0.001 |
| Control | 4051 | 91 | 74 | 62 | 78 | 56 | 47 | ||||
| 2012 | Urata et al[32] | NAs | 46 | 100 | 97.1 | 89.7 | 0.0025 | 71.6 | 56.8 | 42.6 | 0.0478 |
| Control | 13 | 84.6 | 68.4 | 59.8 | 61.5 | 19.2 | 19.2 | ||||
| 2013 | Ke et al[33] | NAs | 141 | 92.1 | 84.4 | 79.1 | 0.009 | 73.1 | 54.7 | 44.5 | 0.503 |
| Control | 141 | 89.6 | 66.3 | 52.1 | 68.8 | 47.8 | 43 | ||||
| 2013 | Yin et al[38] | NAs | 81 | 98 | 88 | N/A | < 0.001 | 81 | 46 | N/A | < 0.001 |
| Control | 82 | 86 | 51 | N/A | 50 | 20 | N/A | ||||
| NAs | 215 | 84 | 60 | N/A | 0.04 | 52 | 37.5 | N/A | < 0.001 | ||
| Control | 402 | 75 | 50 | N/A | 43 | 21 | N/A | ||||
| 2013 | Su et al[34] | NAs | 62 | 99 | 96 | 89 | < 0.001 | 90 | 64 | 58 | < 0.001 |
| Control | 271 | 84 | 64 | 49 | 64 | 44 | 34 | ||||
| 2013 | Yan et al[35] | NAs | 35 | N/A | N/A | N/A | N/A | 74.3 | 11.4 | N/A | 0.283 |
| Control | 25 | N/A | N/A | N/A | 80 | 0 | N/A | ||||
| 2014 | Hann et al[37] | NAs | 16 | 100 | 93.8 | 86.5 | < 0.001 | 81.3 | 81.3 | 81.3 | < 0.001 |
| Control | 9 | 55.6 | 0 | 0 | 11.1 | 0 | 0 | ||||
| 2014 | Huang et al[39] | NAs | 100 | 96 | 77.6 | 63.1 | 0.001 | 85 | 50.3 | 46.1 | 0.026 |
| Control | 100 | 94 | 67.4 | 41.5 | 84 | 37.9 | 27.1 | ||||
All 19 studies reported data on recurrence-free survival after radical surgery. Several retrospective studies[21-23,26-29,33,35] showed that NA treatment did not lead to significantly higher recurrence-free survival than non-NA treatment, while other retrospective studies[24,25,30-32,34,36,37] and the RCTs[38,39] showed that NA therapy was associated with significantly higher recurrence-free survival than non-NA treatment.
To synthesize these findings quantitatively, we generated bubble plots of 1-, 3-, and 5-year recurrence-free survival, with bubble size proportional to the size of the study cohort (Figure 1). We also compared median recurrence-free survival between NA and non-NA groups using the Mann-Whitney U test. The NA group (1468 patients) showed a median recurrence-free survival of 85.0% (range 19.7%-90.0%) at 1 year, 57.0% (range 11.4%-90.0%) at 3 years, and 54.0% (range 42.6%-81.3%) at 5 years. These median survival rates were significantly higher than the corresponding values in the non-NA group (5541 patients): 78.0% (range 4.5%-86.6%) at 1 year, 56.0% (range 0%-56.0%) at 3 years, and 47.0% (range 0%-47.0%) at 5 years (all P < 0.001).
Figure 1.
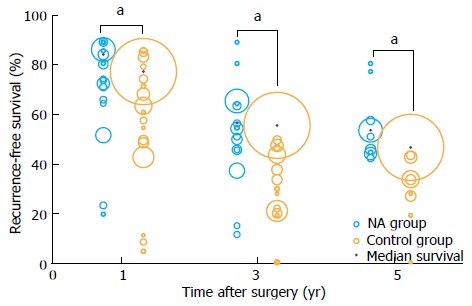
Bubble plot of recurrence-free survival in patients receiving nucleos(t)ide analogue therapy or not after radical resection to treat hepatitis B virus-related hepatocellular carcinoma. Bubble size reflects relative cohort size. aP < 0.05: NA group vs Control group. NA: Nucleos(t)ide analogue.
Next we examined whether, based on the available evidence, NA therapy prevents early recurrence, late recurrence, or both. Studies have shown that tumor factors are associated with early HCC recurrence, while high viral loads and hepatic inflammatory activity are associated with late HCC recurrence[3,4]. NAs can suppress HBV-DNA replication and promote ALT normalization but cannot affect tumor factors directly, so in theory NAs may prevent late HCC recurrence but have minimal effect on early HCC recurrence. Several retrospective studies and a RCT[27,33,35,39] support this idea. However, the other RCT[38] in our review found that NA therapy significantly decreased early HCC recurrence, while it did not report outcomes on late HCC recurrence. NA therapy may inhibit early HCC recurrence, which usually arises due to diffusion of the primary tumor, by reducing high HBV load and HBV mutations, all of which are associated with HCC metastasis and growth[40-42], as well as by inhibiting HBxAg, which promotes HCC invasiveness and metastatic potential[43,44]. Further studies are urgently needed to clarify whether and how NA therapy affects risk of HCC recurrence, since the results of RCT[38] in our review may overestimate the NA efficacy because the control group at baseline had significantly higher rates of cirrhosis, lower rates of tumor encapsulation, and higher rates of HBeAg positivity than the NA group, as well as poorer tumor differentiation and higher AFP levels.
POSTOPERATIVE NA THERAPY AND OVERALL SURVIVAL
A total of 15 studies reported data on overall survival after radical surgery. Twelve of them, including the RCTs[22,27,29-34,36-39], concluded that NA treatment leads to significantly higher overall survival than non-NA treatment, but 3 studies[21,23,26] concluded that NA therapy does not lead to significantly higher overall survival.
The 1-, 3-, and 5-year overall survival rates were summarized using bubble plots (Figure 2), and median rates were compared between NA and non-NA groups using the Mann-Whitney U test. Median survival in the NA group (1468 patients) was 94.0% (range 24.0%-100.0%) at 1 year, 81.0% (range 60.0%-100.0%) at 3 years, and 73.0% (range 59.0%-89.7%) at 5 years. These values were significantly higher than the corresponding ones for the non-NA group (5200 patients): 91.0% (range 0-100.0%) at 1 year, 74.0% (range 0-85.0%) at 3 years, and 62.0% (range 0%-70.0%) at 5 years (all P < 0.001).
Figure 2.
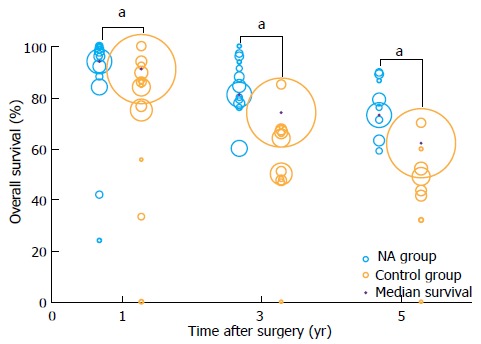
Bubble plot of overall survival in patients receiving nucleos(t)ide analogue therapy or not after radical resection to treat hepatitis B virus-related hepatocellular carcinoma. Bubble size reflects relative cohort size. aP < 0.05: NA group vs Control group. NA: Nucleos(t)ide analogue.
Investigators have attributed this survival benefit to 3 factors. First, NA therapy can efficiently suppress HBV replication and reactivation, ease liver inflammation and fibrosis, impede progression of liver disease, and prevent liver failure[21-23,27,29,33,38,45]. Second, liver function improvement after NA therapy increases the possibility of curative re-treatment and allows surgeons to remove a larger liver region after recurrence, which means lower risk of residual tumors[23,29,33,45]. Third, NA therapy can reduce recurrence, helping to increase overall survival[24,25,30-32,34,36-38].
To define more precisely which patients with HBV-related HCC may benefit from NA therapy, we retrospectively studied its efficacy in patients with HCC in different stages of the Barcelona Clinic Liver Cancer (BCLC) system[33]. We found that NA therapy provided significant survival benefit to patients with BCLC stage A or B disease, but not to patients with BCLC-C disease. These results are similar to those reported in 2 larger retrospective studies[30,34]. This may reflect the poor prognosis of BCLC-C patients, whose short survival provides insufficient time for NA therapy to be effective.
MANAGEMENT OF NA RESISTANCE IN HBV-RELARED HCC PATIENTS
One of the major problems associated with long-term NA therapy is the emergence of NA-resistant HBV strains[21,23,27]. Such resistance increases not only the risk of breakthrough hepatitis and liver failure, but also the difficulty and cost of subsequent treatment. LAM has the worst antiviral resistance profile among NAs, and LAM resistance is caused by mutations of the YMDD region in the active site of the HBV polymerase/reverse transcriptase gene[11]. One study[27] reported YMDD mutations in 11 of 28 patients after 28.6 ± 16.7 mo of LAM administration. Of those 11 patients, 6 exhibited breakthrough hepatitis; fortunately none of them experienced fatal liver failure because they were immediately given ADV or ETV.
To prevent NA resistance and manage its clinical effects in patients with HBV-related HCC, clinicians should obtain a thorough medical history for NA candidates. Patients who previously received NA therapy and developed resistance should receive potent NA not associated with cross-resistance (Table 3) in order to reduce the risk of eliciting multiple drug-resistant viral strains[12]. For patients who have never received any NA therapy, potent drugs with high resistance barriers, such as ETV and TDV, may be the best choice[12]. Clinicians should also not rush to incorrect conclusions about NA resistance, since about 40% of cases of HBV-related breakthrough hepatitis occur simply because of poor patient adherence to NA therapy rather than NA resistance[46]. On the other hand, drug resistance should be considered if regular follow-up tests of HBV-DNA levels and liver function every 2-3 mo give abnormal results and other possible causes can be excluded. In such cases, an appropriate rescue therapy using potent NAs without cross-resistance should be given as soon as genotypic drug resistance is confirmed[11].
Table 3.
Mutations of the hepatitis B virus polymerase gene arising after initial therapy with one nucleos(t)ide analogue and resulting in cross-resistance to other nucleos(t)ide analogues
| Initial NA therapy | Mutational sites after initial NA therapy | Cross-resistance data | ||||
| LAM | LdT | ETV | ADV | TDF | ||
| Wild-type | S | S | S | S | S | |
| LAM or LdT | M204I/V | R | R | I | S | S |
| ADV | N236T | S | S | S | R | I |
| LAM or LdT or ADV | A181T/V | R | R | S | R | I |
| ADV or TDF | A181T/V + N236T1 | R | R | S | R | R |
| ETV | L181M + M204V/I ± I169 ± T184 ± S202 ± M250V2 | R | R | R | S | S |
Resistance to ADV or TDF is associated with the substitution A181T/V and/or N235T in HBV polymerase gene;
Resistance to ETV is associated with substitutions at I169, T184, S202 or M250V, and with the simultaneous substitutions at L181M plus M204V/I in HBV polymerase gene. Data come from ref. [11]. ADV: Adefovir dipivoxil; ETV: Entecavir; I: Intermediate; LAM: Lamivudine; LdT: Telbivudine; NA: Nucleos(t)ide analogue; R: Resistant; S: Sensitive; TDF: Tenofovir disoproxil fumarate.
CONCLUSION
Given the serious clinical consequences of uncontrolled HBV replication, patients with HBV-related HCC should consider taking NA if they are positive for HBV-DNA. Because NA therapy cannot completely eradicate HBV, patients should prepare for the possibility that they may require lifelong treatment. With the currently advanced techniques of the loco-regional ablations such as radiofrequency ablation, microwave ablation and others, NA therapy also applies for HCC patients who underwent such procedures in addition to surgical resection, and a significant body of evidence suggests that postoperative NA therapy in patients with HBV-related HCC improves both recurrence-free survival and overall survival.
Every coin has two sides. Emergence of NA-resistant HBV strains is a significant concern, highlighting the importance of regular monitoring of HBV-DNA levels and liver function during NA therapy. The most potent NAs with high resistance barriers, such as EVT and TDF, may be the best choice for NA-naïve patients. In case of drug resistance, rescue therapy should be carried out using potent NAs not associated with cross-resistance.
Footnotes
P- Reviewer: Gao ZL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhong JH, Li H, Li LQ, You XM, Zhang Y, Zhao YN, Liu JY, Xiang BD, Wu GB. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol. 2012;38:286–295. doi: 10.1016/j.ejso.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Du ZG, Wei YG, Chen KF, Li B. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution’s experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int. 2014;13:153–161. doi: 10.1016/s1499-3872(14)60025-4. [DOI] [PubMed] [Google Scholar]
- 4.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhong JH, Ma L, Li LQ. Postoperative therapy options for hepatocellular carcinoma. Scand J Gastroenterol. 2014;49:649–661. doi: 10.3109/00365521.2014.905626. [DOI] [PubMed] [Google Scholar]
- 6.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–184. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 9.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 10.Zhong JH, Li le Q, Wu LC. Lamivudine with or without adefovir dipivoxil for postoperative hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;(12):CD008713. doi: 10.1002/14651858.CD008713.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 12.Santantonio TA, Fasano M. Chronic hepatitis B: Advances in treatment. World J Hepatol. 2014;6:284–292. doi: 10.4254/wjh.v6.i5.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version) Zhonghua Ganzangbing Zazhi. 2011;19:13–24. doi: 10.3760/cma.j.issn.1007-3418.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 15.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–1341; quiz 1286. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Tong MJ, Pan CQ, Hann HW, Kowdley KV, Han SH, Min AD, Leduc TS. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci. 2011;56:3143–3162. doi: 10.1007/s10620-011-1841-5. [DOI] [PubMed] [Google Scholar]
- 18.Recommendation on antiviral therapy to hepatitis B/C virus related hepatocellular carcinoma. Zhonghua Ganzangbing Zazhi. 2013;21:96–100. [PubMed] [Google Scholar]
- 19.Huang G, Lai EC, Lau WY, Zhou WP, Shen F, Pan ZY, Fu SY, Wu MC. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257:490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 20.Wei Q, Xu X, Ling Q, Zheng S. Indefinite antiviral therapy may be required after surgical resection for hepatocellular carcinoma complicating chronic hepatitis B. J Res Med Sci. 2013;18:726–730. [PMC free article] [PubMed] [Google Scholar]
- 21.Piao CY, Fujioka S, Iwasaki Y, Fujio K, Kaneyoshi T, Araki Y, Hashimoto K, Senoh T, Terada R, Nishida T, et al. Lamivudine treatment in patients with HBV-related hepatocellular carcinoma--using an untreated, matched control cohort. Acta Med Okayama. 2005;59:217–224. doi: 10.18926/AMO/31969. [DOI] [PubMed] [Google Scholar]
- 22.Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Wenming C, Zhengfeng Y, Yuxiang Z, Peijun W. Antiviral therapy using lamivudine and thymosin alpha1 for hepatocellular carcinoma coexisting with chronic hepatitis B infection. Hepatogastroenterology. 2006;53:249–252. [PubMed] [Google Scholar]
- 23.Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929–1935. doi: 10.1111/j.1440-1746.2006.04707.x. [DOI] [PubMed] [Google Scholar]
- 24.Kubo S, Tanaka H, Takemura S, Yamamoto S, Hai S, Ichikawa T, Kodai S, Shinkawa H, Sakaguchi H, Tamori A, et al. Effects of lamivudine on outcome after liver resection for hepatocellular carcinoma in patients with active replication of hepatitis B virus. Hepatol Res. 2007;37:94–100. doi: 10.1111/j.1872-034X.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 25.Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Yoshida H, Goto E, Sato T, Ohki T, Masuzaki R, Tateishi R, Goto T, Shiina S, Kawabe T, et al. Safety and efficacy of lamivudine after radiofrequency ablation in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Int. 2008;2:89–94. doi: 10.1007/s12072-007-9020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koda M, Nagahara T, Matono T, Sugihara T, Mandai M, Ueki M, Ohyama K, Hosho K, Okano J, Kishimoto Y, et al. Nucleotide analogs for patients with HBV-related hepatocellular carcinoma increase the survival rate through improved liver function. Intern Med. 2009;48:11–17. doi: 10.2169/internalmedicine.48.1534. [DOI] [PubMed] [Google Scholar]
- 28.Chuma M, Hige S, Kamiyama T, Meguro T, Nagasaka A, Nakanishi K, Yamamoto Y, Nakanishi M, Kohara T, Sho T, et al. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2009;44:991–999. doi: 10.1007/s00535-009-0093-z. [DOI] [PubMed] [Google Scholar]
- 29.Li N, Lai EC, Shi J, Guo WX, Xue J, Huang B, Lau WY, Wu MC, Cheng SQ. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection. Ann Surg Oncol. 2010;17:179–185. doi: 10.1245/s10434-009-0694-z. [DOI] [PubMed] [Google Scholar]
- 30.Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–681. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- 31.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 32.Urata Y, Kubo S, Takemura S, Uenishi T, Kodai S, Shinkawa H, Sakae M, Kaneda K, Ohata K, Nozawa A, et al. Effects of antiviral therapy on long-term outcome after liver resection for hepatitis B virus-related hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:685–696. doi: 10.1007/s00534-011-0489-z. [DOI] [PubMed] [Google Scholar]
- 33.Ke Y, Ma L, You XM, Huang SX, Liang YR, Xiang BD, Li LQ, Zhong JH. Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol Med. 2013;10:158–164. doi: 10.7497/j.issn.2095-3941.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su CW, Chiou YW, Tsai YH, Teng RD, Chau GY, Lei HJ, Hung HH, Huo TI, Wu JC. The Influence of Hepatitis B Viral Load and Pre-S Deletion Mutations on Post-Operative Recurrence of Hepatocellular Carcinoma and the Tertiary Preventive Effects by Anti-Viral Therapy. PLoS One. 2013;8:e66457. doi: 10.1371/journal.pone.0066457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Q, Ni J, Zhang GL, Yao X, Yuan WB, Zhou L, Zheng SS. Efficacy of postoperative antiviral combined transcatheter arterial chemoembolization therapy in prevention of hepatitis B-related hepatocellular carcinoma recurrence. Chin Med J (Engl) 2013;126:855–859. [PubMed] [Google Scholar]
- 36.Hann HW, Bergin D, Coben R, DiMarino AJ. Prevention of new hepatocellular carcinoma with concomitant antiviral therapy in chronic hepatitis B patients whose initial tumor was successfully ablated. Int J Cancer. 2011;128:739–742. doi: 10.1002/ijc.25382. [DOI] [PubMed] [Google Scholar]
- 37.Hann HW, Coben R, Brown D, Needleman L, Rosato E, Min A, Hann RS, Park KB, Dunn S, DiMarino AJ. A long-term study of the effects of antiviral therapy on survival of patients with HBV-associated hepatocellular carcinoma (HCC) following local tumor ablation. Cancer Med. 2014;3:390–396. doi: 10.1002/cam4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, Guo W, Zhang H, Wang H, Cheng S, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647–3655. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 39.Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, Zhou WP, Wu MC. Antiviral Therapy Improves Postoperative Survival in Patients With Hepatocellular Carcinoma: A Randomized Controlled Trial. Ann Surg. 2014:Jul 28; Epub ahead of print. doi: 10.1097/SLA.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Wang Z, An S, Zhou B, Zhou Y, Chan HL, Hou J. Role of hepatitis B virus genotypes and quantitative HBV DNA in metastasis and recurrence of hepatocellular carcinoma. J Med Virol. 2008;80:591–597. doi: 10.1002/jmv.21117. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Tong S, Tai AW, Hussain M, Lok AS. Hepatitis B virus core promoter mutations contribute to hepatocarcinogenesis by deregulating SKP2 and its target, p21. Gastroenterology. 2011;141:1412–1421, 1412-1421. doi: 10.1053/j.gastro.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mun HS, Lee SA, Kim H, Hwang ES, Kook YH, Kim BJ. Novel F141L pre-S2 mutation in hepatitis B virus increases the risk of hepatocellular carcinoma in patients with chronic genotype C infections. J Virol. 2011;85:123–132. doi: 10.1128/JVI.01524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int J Cancer. 2007;120:1208–1214. doi: 10.1002/ijc.22452. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Xu L, He H, Zhu Y, Liu J, Wang S, Chen L, Wu Q, Xu J, Gu J. Hepatitis B virus X protein promotes hepatoma cell invasion and metastasis by stabilizing Snail protein. Cancer Sci. 2012;103:2072–2081. doi: 10.1111/cas.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JH, Park JW, Koh DW, Lee WJ, Kim CM. Efficacy of lamivudine on hepatitis B viral status and liver function in patients with hepatitis B virus-related hepatocellular carcinoma. Liver Int. 2009;29:203–207. doi: 10.1111/j.1478-3231.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 46.Hongthanakorn C, Chotiyaputta W, Oberhelman K, Fontana RJ, Marrero JA, Licari T, Lok AS. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. Hepatology. 2011;53:1854–1863. doi: 10.1002/hep.24318. [DOI] [PubMed] [Google Scholar]


