Abstract
Objective
To examine our experience with managing sporadic bilateral renal masses, focusing on trends in surgical management over time, because as loss of renal function is associated with adverse cardiovascular outcomes, nephron-sparing approaches are increasingly emphasized in the treatment of kidney tumours, creating new challenges for the treatment of bilateral tumours.
Patients and Methods
We identified all patients who underwent partial or radical nephrectomy (PN or RN) at Memorial Sloan-Kettering Cancer Center (MSKCC) during 1989-2008. We compared patients presenting with synchronous bilateral renal masses with those with unilateral tumour and evaluated trends in management using logistic regression.
Results
Of the 2777 patients studied, 73 (3%) presented with synchronous bilateral disease. The overall survival and clinical/pathologica features between groups were similar. Of those patients receiving bilateral operations for synchronous tumours, three had bilatera RN (all before 2003), 28 (38%) had an RN followed by a PN, 10 (14%) had a PN then an RN, and 32 (44%) had bilateral PN. Over time, the proportion of patients receiving bilateral PN increased (P < 0.001); 13 of 14 patients after 2005 had bilateral PN, compared with only 34% (16 of 45) between 1995 and 2004. Forty-five patients (62%) had the larger tumour removed during the first operation. The concordance rate between tumours in a specific histological subtype was 70% (51/73), and concordance for benign vs malignant disease was 90% (66/73).
Conclusion
The use of PN in the management of synchronous bilateral renal masses has increased over time. The contemporary treatment of synchronous bilateral renal masses at MSKCC involves staged PN when feasible, with the more involved kidney (often larger tumour) operated on first.
Keywords: carcinoma, renal cell, kidney, nephrectomy, urological surgery
Introduction
The incidence of renal cancer within the USA has increased alarmingly; in 2008, ≈54 390 new cases of kidney cancer were diagnosed [1]. Patients with bilateral renal tumours (BRTs) account for ≈4% of these cases [2]. BRTs are classified in several ways; they can be hereditary, familial, or sporadic, and their presentation can be synchronous or asynchronous. Von Hippel-Lindau and other hereditary RCC syndromes often result in multifocal bilateral tumours and represent a distinct clinical entity from sporadic BRTs. Here we focus on sporadic synchronous BRTs.
BRTs offer challenging therapeutic dilemmas. Recent evidence suggests that ≈25% of patients with unilateral (U)RTs will have significant preoperative renal dysfunction [3,4], and one can logically hypothesise even greater preoperative renal dysfunction in patients with BRTs. There is increasing awareness that chronic kidney disease and/or a rapid decline in estimated GFR (eGFR) increases the risk of cardiovascular events and death [3,5–8]. Because of the importance of minimizing treatment-related loss of renal function, nephron-sparing treatment has received increased emphasis. The presence of bilateral renal masses is one of the established indications for nephron-sparing surgery (NSS) [9], yet historically this operation has been underused. As long as a portion of one kidney was preserved, avoiding the need for dialysis, radical nephrectomy (RN) was common for the more involved kidney or larger tumour.
To analyse contemporary treatment patterns for sporadic BRTs, we examined our experience over the past two decades of treating these tumours, focusing on preoperative renal dysfunction, concordance of pathological characteristics, overall survival, and the development of surgical management. In addition, we present a contemporary algorithm for managing synchronous BRTs.
Patients and Methods
After obtaining institutional review board approval, the prospective renal surgery database at Memorial Sloan-Kettering Cancer Center (MSKCC) was queried for all patients undergoing partial (PN) or RN for a solid renal cortical tumour between July 1989 and June 2008. We excluded patients with a hereditary renal cancer syndrome, leaving only patients with sporadic renal tumours. Patients were then divided into two groups according to their original presentation. The first group consisted of patients presenting with synchronous cortical BRTs who had staged procedures, while the second group consisted of those presenting with cortical URTs; the second group included 50 patients who went on to develop an asynchronous, contralateral tumour.
The eGFR was calculated according to the abbreviated Modified Diet and Renal Disease equation, accounting for patient age, race, and the last serum creatinine level before the renal procedure [10].
Summary statistics were used to describe the study cohort, with continuous variables given as the median (interquartile range, IQR) and absolute numbers with percentages were given for categorical variables. The difference in preoperative creatinine and eGFR between patients with and without synchronous BRTs at presentation was evaluated using the Mann–Whitney U-test. For the synchronous BRT patients, we analysed the time between procedures, the type of procedure, and the order of operations according to tumour size. Univariate logistic regression was used to evaluate trends in the surgical management of synchronous RTs over time, with receiving two PNs (yes or no) as the dependent variable. Overall survival was defined as the time from first operation to last follow-up and was estimated using Kaplan-Meier methods. Six patients with no follow-up (all in the URT group) were excluded from the survival analyses. In all statistical analyses P < 0.05 was considered to indicate statistical significance.
Results
The baseline patient characteristics are summarized in Table 1; of 2777 patients, 2704 (97%) presented with URTs and 73 (3%) with synchronous BRT; 50 (2%) of those with URTs later developed an asynchronous contralateral tumour, but for the purpose of this study they were retained in the URT group. Synchronous BRTs at presentation was more common in men (4% vs 1%) and African-Americans (6% vs 3% of other ethnic groups). The median preoperative creatinine level was 1.2 mg/dL in the BRT group, which was slightly higher than in the URT group (1.1 mg/dL, P = 0.007). However, the median preoperative eGFR did not differ significantly between the groups (69 vs 67 mL/min per 1.73 m2; P = 0.9). Among patients with synchronous BRTs the concordance between the tumours in specific histological subtype was 70% (51/73), and the concordance for benign vs malignant disease was 90% (66/73).
Table 1. The clinical and pathological characteristics of patients who had PN or RN, stratified by presence of bilateral synchronous disease at presentation.
| Median (IQR) or n (%) characteristic | URT | Synchronous BRT (at 1st procedure) 1st/2nd |
|---|---|---|
| No. of patients | 2704 | 73 |
| Age | 62 (53, 70) | 62 (55, 69) |
| Male | 1719 (64) | 64 (88) |
| African-American | 133 (5) | 8 (11) |
| Preoperative: | ||
| Creatinine*, mg/dL | 1.1 (0.9, 1.3) | 1.2 (1.0, 1.4) |
| eGFR†, mL/min/1.73 m2 | 67 (57, 78) | 69 (55, 79) |
| Max tumour size, cm | 4.1 (2.7, 7.0) | 4.0 (3.0, 6.0)/3.1 (2.1, 4.5) |
| pT stage (RCC only) | ||
| T1a | 1028 (43) | 31 (47)/40 (63) |
| T1b | 471 (19) | 15 (23)/7 (11) |
| T2 | 224 (9) | 4 (6)/6 (10) |
| T3a | 412 (17) | 9 (14)/6 (10) |
| T3b | 233 (10) | 3 (5)/3 (5) |
| T3c | 5 (0.2) | 1 (2)/0 |
| T4 | 24 (1) | 0/0 |
| T (unknown) | 21 (1) | 3 (5)/1 (2) |
| Histology | ||
| Conventional (clear cell) | 1736 (64) | 41 (56)/35 (48) |
| Papillary | 343 (13) | 19 (26)/22 (30) |
| Chromophobe | 258 (10) | 4 (5)/3 (4) |
| Collecting duct | 7 (0.3) | 1 (1)/0 |
| RCC unclassified | 74 (3) | 1 (1)/3 (4) |
| Oncocytoma | 245 (9) | 7 (10)/7 (10) |
| Angiomyolipoma | 40 (1) | 0/0/ |
| Adenoma | 1 (0.04) | 0/0 |
| Type of procedure | ||
| PN | 1102 (41) | 42 (58)/60 (82) |
| RN | 1602 (59) | 31 (42)/13 (18) |
Preoperative creatinine available for 2642 URT and 66 BRT patients;
eGFR available for 2613 URT and 66 BRT patients.
The treatment characteristics of patients who presented with synchronous disease are given in Table 2. All these patients had two staged operations, with the larger of the BRTs removed during the first in most patients (62%). Overall, 44% of patients received bilateral PNs, 38% had a RN then a PN, 14% had a PN then RN, and 4% had bilateral RNs.
Table 2. Treatment characteristics of 73 patients presenting with synchronous BRTs.
| Characteristic | Median (IQR) or N (%) |
|---|---|
| Year of first procedure | |
| 1989–1994 | 14 (19) |
| 1995–1999 | 14 (19) |
| 2000–2004 | 31 (42) |
| 2005–2008 | 14 (19) |
| Months between procedures (IQR) | 3.0 (1.8, 6.1) |
| Type of procedures | |
| PN then PN | 32 (44) |
| PN then RN | 10 (14) |
| RN then PN | 28 (38) |
| RN then RN | 3 (4) |
| Operated on first | |
| Larger tumour | 45 (62) |
| Smaller tumour | 19 (26) |
| Equal-sized tumours | 4 (5) |
| Unknown | 5 (7) |
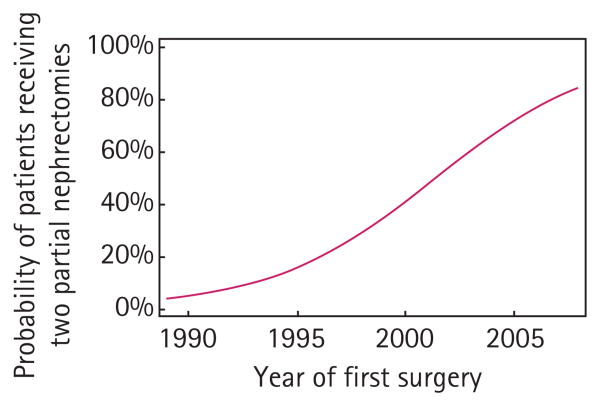
Treatment patterns changed with time, favouring bilateral PNs (Fig. 1, P < 0.001). For example, from 1995 to 1999 only two of 14 patients had a bilateral PN, compared to 14/31 (45%) between 2000 and 2004, and 13 of 14 after 2005. On logistic regression analysis, the odds of being managed with bilateral PNs was higher by a factor of 1.3 for each increasing year of the study (odds ratio 1.30, 95% CI 1.13–1.49, P < 0.001).
Fig. 1.
Predicted probability of receiving two PNs for synchronous disease by year of procedure.
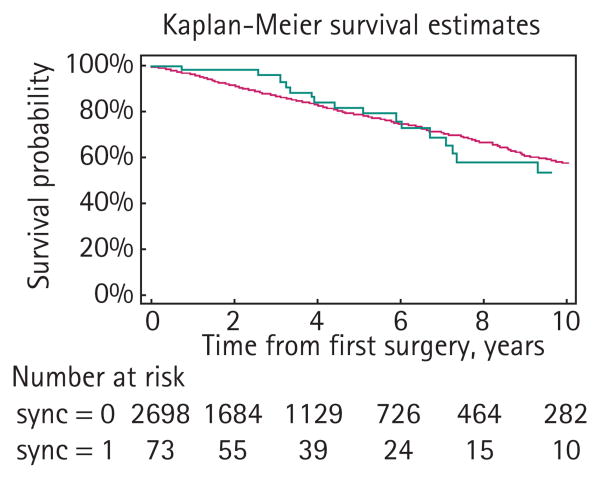
Among the 2771 patients with follow-up, there were 645 deaths, 624 in the URT and 22 in the synchronous BRT groups (Table 3). The median follow-up for survivors was 38 months. There was no obvious evidence of a difference in overall survival between groups (Fig. 2, P = 0.9, log rank test), although the few BRTs prevents definitive conclusions.
Table 3. Cause of death, as n (%), among patients followed for survival after renal surgery.
| Cause of death | URT | Synchronous BRT |
|---|---|---|
| N | 2702 | 73 |
| Kidney cancer | 163 (6) | 8 (11) |
| Other cancer | 14 (0.5) | 2 (3) |
| Other causes | 43 (2) | 1 (1) |
| Unknown causes | 404 (15) | 11 (15) |
Fig. 2.
Overall survival estimates. Dark grey curve, patients with URTs; light grey curve, patients with synchronous BRTs.
Discussion
In the present study we focused on the treatment of synchronous, non-hereditary BRTs, analysing 73 synchronous BRTs and 2704 URTs surgically treated at MSKCC. There was a higher preoperative creatinine level in the BRT group, but no evidence of a difference in preoperative eGFR. Although there were relatively few patients analysed, the survival of those with synchronous BRTs was similar to that of those with URTs. The concordance rate of tumour histology for resected synchronous BRTs was 70% and was similar to an earlier report [11]. Most (62%) of patients with synchronous BRTs had the larger tumour excised first. Of the four different nephrectomy combinations, the most common was bilateral PN, undergone by 32 patients (44%). Over the study period there was a significant change toward the use of PN for both procedures.
There is considerable debate about the optimal approach for managing bilateral renal masses. Should the masses be treated with RN, PN, ablation or observation? What is the role for open vs minimally invasive procedures? Should there be a single procedure or staged procedures? There are strong opinions for and against each of these options. At our centre, the preference is for staged PNs and for operating on the more involved kidney first, for reasons detailed below.
The principal reason for preferring PNs is, of course, preservation of renal function. BRTs increase the risk of renal failure because of the disease process itself and the renal loss associated with treatment. Historically, patients with bilateral solid renal masses (of any size) were treated with bilateral RN and placed on haemodialysis or given a renal transplant [12]. Although this approach effectively treated the tumours, we suspect that the patients' quality of life was worsened by the sudden loss of native renal function and need for dialysis. In fact, the high morbidity and mortality associated with dialysis probably portended a worse prognosis than that from small resected or observed renal tumours. With the established oncological efficacy of PN [13], this procedure has become an excellent, and often preferred, treatment option for patients with URTs or BRTs. Other nephron-sparing options for patients with BRTs include ablative or surveillance strategies. Nevertheless, the adoption of NSS has been slow, and it is still underused in the treatment of small renal masses and BRTs [14]. With ≈19 million Americans already living with chronic kidney disease, and the prevalence rising [15], we must strive to preserve kidney volume during the treatment of renal tumours, considering that patients' renal functional outcomes might be just as important as their oncological results for their overall health and survival. However, despite maximal effort, not all BRTs are amenable to PN, and RN might still be required, especially for massive tumours that have replaced most of the renal parenchyma.
There are several important reasons for staged procedures, and for operating on the more involved kidney (and often larger tumour) first. Recent population-based studies have shown that tumour size is related to tumour grade, metastatic potential, and cancer-specific survival [16–18]. Removal and pathological analysis of the larger, more involved, tumour first yields important prognostic information on the presumably more aggressive tumour, thus aiding in future treatment decisions. For example, if during resection of the more significant tumour a positive lymph node is found, then surgery on the contralateral side can be deferred or not done, particularly if rapid disease progression ensues. In addition, given the high concordance in histological subtype between synchronous BRTs (70% in our cohort), a staged procedure often allows for prediction of the subtype of the second tumour. Removing the more involved tumour first might mitigate the risk of metastasis, which is theoretically higher from the larger tumour. A final consideration is that complicated PNs often involve prolonged cold ischaemic times, increasing the risk of acute tubular necrosis. We prefer staged procedures to maximize postoperative kidney function (i.e. the contralateral kidney can help to maintain renal function while the other kidney recovers from its surgical insult). Obviously there are exceptions to this preference, and individual circumstances dictate patient care.
Other high-volume academic centres have reported their surgical experience with synchronous BRTs. The Mayo Clinic's series of patients with bilateral renal masses was recently described by Boorjian et al. [11]; of 148 patients with synchronous BRTs, 46 had bilateral PNs and 82 had a combination of PN and RN. In their discussion, they state that ‘NSS remains the standard of care for patients with bilateral tumours.’ [11] The Mayo Clinic has traditionally preferred to treat these patients with a single, transperitoneal procedure rather than a staged approach. In earlier reports of the Mayo Clinic's experience with synchronous tumours, Blute et al. [19,20] described their surgical technique and showed that 66 (70%) of 94 patients with synchronous BRTs had both tumours excised with a single procedure, with acceptable morbidity.
Uzzo and Novick [9] described the Cleveland Clinic experience, and favoured staged procedures, operating on the less involved kidney first. Booth et al. [21] explained the M.D. Anderson approach, whereby staged procedures are used, and based on individual patient scenarios. They tend to operate on the ‘easier’ side first if both tumours are of low clinical stage, whereas if one tumour is a higher stage they will approach it first. A consortium of 12 international urological centres reported on treatment, clinicopathological features, and prognosis of 153 patients with synchronous BRTs [22]. Of the patients undergoing bilateral surgical procedures, 46% had a single operation addressing both tumours, while 54% had staged procedures. Of patients with synchronous BRTs, 53% had bilateral PNs. Pahernik et al. [23] described the approach at Johannes Gutenberg University in Mainz, which involves bilateral staged PNs when possible, similar to the M.D. Anderson, and they generally preferred to operate on the more favourable tumour first. Table 4 summarizes the surgical preferences for synchronous BRTs of the cited studies. Although surgical philosophies among these institutions differ slightly, the common theme among all is the importance of nephron preservation.
Table 4. A review of previous reports of the surgical management strategies for synchronous BRTs.
| Reference | Surgical management preference |
|---|---|
| [11,19] (Mayo Clinic) | Single, transperitoneal procedure addressing the more difficult tumour first |
| [9] (Cleveland Clinic) | Staged procedures, operating on the less involved tumour first |
| [21] (M.D. Anderson) | Staged procedures; if tumours similar in stage, then operate on easier tumour first; if tumours discordant in stage, then operate on the higher stage tumour first |
| [22] (International Consortium) | Included 12 centres with various approaches; 46% of patients had single procedure; 54% staged procedure |
| [23] (Mainz, Germany) | Staged procedures, operating on the more favourable tumour first |
| Present series | Staged procedures, operating on the more involved (often larger) tumour first |
All centres stress the importance of nephron preservation when possible. Surgical management preferences for BRTs were extracted from the listed publications. Clearly these strategies are often modified based on individual patients and situations.
We acknowledge the retrospective nature of the present study and its inherent limitations. We opted to not analyse open vs laparoscopic approaches, feeling that NSS is the important point regardless of technique. Our patient population was selected from a tertiary-care centre for surgical procedures and is not representative of all patients presenting with bilateral renal masses. Some patients are unfit for surgery and might be better served with observation, the ultimate nephron-sparing approach. We understand that each patient is an individual and no single treatment approach can be blindly applied to all. Nevertheless, we generally favour our approach for the previously mentioned reasons, but understand that it does not work for all situations.
In conclusion, the preoperative serum creatinine level was worse in patients with synchronous BRTs than in those with URTs. During the study period there was an increase in the use of bilateral PN in the management of synchronous renal masses, as our understanding of the deleterious effects of renal-wasting surgery developed. The contemporary treatment of synchronous BRTs at MSKCC involves, when feasible, staged PNs with the larger (more involved) tumour resected first.
Acknowledgments
We are indebted to the Stephen Hanson Family Fellowship for their support. This project was also supported by NIH T32 CA82088 and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Abbreviations
- (U)(B)RT
(unilateral) (bilateral) renal tumours
- eGFR
estimated GFR
- IQR
interquartile range
- MSKCC
Memorial Sloan-Kettering Cancer Center
- NSS
nephron-sparing surgery
- PN
partial nephrectomy
- RN
radical nephrectomy
Footnotes
Conflict of Interest: None declared.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Marshall FF, Stewart AK, Menck HR. The National Cancer Data Base: report on kidney cancers. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1997;80:2167–74. [PubMed] [Google Scholar]
- 3.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane BR, Babineau DC, Poggio ED, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008;180:2363–8. doi: 10.1016/j.juro.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors – is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–71. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–8. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Boorjian SA, Crispen PL, Lohse CM, Leibovich BC, Blute ML. The impact of temporal presentation on clinical and pathological outcomes for patients with sporadic bilateral renal masses. Eur Urol. 2008;54:855–63. doi: 10.1016/j.eururo.2008.04.079. [DOI] [PubMed] [Google Scholar]
- 12.Black J, Rotellar C, Rakowski TA, Winchester JF. Bilateral nephrectomy and dialysis as an option for patients with bilateral renal cancer. Nephron. 1988;49:150–3. doi: 10.1159/000185042. [DOI] [PubMed] [Google Scholar]
- 13.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–5. [PubMed] [Google Scholar]
- 14.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254–9. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 15.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen MM, Gill IS. Effect of renal cancer size on the prevalence of metastasis at diagnosis and mortality. J Urol. 2009;181:1020–7. doi: 10.1016/j.juro.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Rothman J, Egleston B, Wong YN, Iffrig K, Lebovitch S, Uzzo RG. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysis. J Urol. 2009;181:29–33. doi: 10.1016/j.juro.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scoll BJ, Wong YN, Egleston BL, Kunkle DA, Saad IR, Uzzo RG. Age, tumor size and relative survival of patients with localized renal cell carcinoma: a surveillance, epidemiology and end results analysis. J Urol. 2009;181:506–11. doi: 10.1016/j.juro.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blute ML, Amling CL, Bryant SC, Zincke H. Management and extended outcome of patients with synchronous bilateral solid renal neoplasms in the absence of von Hippel-Lindau disease. Mayo Clin Proc. 2000;75:1020–6. doi: 10.4065/75.10.1020. [DOI] [PubMed] [Google Scholar]
- 20.Blute ML, Itano NB, Cheville JC, Weaver AL, Lohse CM, Zincke H. The effect of bilaterality, pathological features and surgical outcome in nonhereditary rena cell carcinoma. J Urol. 2003;169:1276–81. doi: 10.1097/01.ju.0000051883.41237.43. [DOI] [PubMed] [Google Scholar]
- 21.Booth J, Matin SF, Ahrar K, Tamboli P, Wood CG. Contemporary strategies for treating nonhereditary synchronous bilateral renal tumors and the impact of minimally invasive, nephron-sparing techniques. Urol Oncol. 2008;26:37–42. doi: 10.1016/j.urolonc.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Klatte T, Wunderlich H, Patard JJ, et al. Clinicopathological features and prognosis of synchronous bilatera renal cell carcinoma: an internationa multicentre experience. BJU Int. 2007;100:21–5. doi: 10.1111/j.1464-410X.2007.06877.x. [DOI] [PubMed] [Google Scholar]
- 23.Pahernik S, Cudovic D, Roos F, Melchior SW, Thuroff JW. Bilateral synchronous sporadic renal cell carcinoma: surgica management, oncological and functiona outcomes. BJU Int. 2007;100:26–9. doi: 10.1111/j.1464-410X.2007.06899.x. [DOI] [PubMed] [Google Scholar]