Abstract
Background
The zinc-finger transcription factor CASZ1 is required for differentiation of a distinct population of cardiomyocytes during development. However, expression of Casz1 mRNA is detected throughout the developing heart, suggesting the spatial regulation of CASZ1 occurs at the protein level. Relatively little is known about posttranscriptional regulation of Casz1 in the heart.
Results
We generated antibodies that specifically recognize CASZ1 in developing Xenopus embryos, and performed immunofluorescence analysis of CASZ1 during cardiac development. CASZ1 was detected throughout the developing myocardium. CASZ1 was restricted to terminally differentiated cardiomyocytes, and was down-regulated in cells that re-enter the cell cycle. We determined that CASZ1 expression correlated with terminal differentiation in cardiac muscle cells, skeletal muscle cells, and lymph-heart musculature.
Conclusions
This study indicates that spatially distinct expression of CASZ1 protein may be due to posttranscriptional control of Casz1 mRNA during cardiac development. The results of this study provide insights into the role of Casz1 in cardiac function and in the differentiation of other cell types, including skeletal muscle and lymph heart.
Keywords: Casz1, Xenopus, heart development, myocardium, cell cycle, lymph heart, skeletal muscle
Introduction
The zinc finger transcription factor CASZ1 (CASTOR) is required for proper cardiovascular development in Xenopus along the ventral midline (Christine and Conlon, 2008; Charpentier et al., 2013). Cardiomyocytes lacking CASZ1 display a higher mitotic index, indicating that CASZ1 plays a crucial role in regulating cardiomyocyte proliferation (Christine and Conlon, 2008). The human homolog of CASZ1 (hCASZ1) also is characterized as a tumor suppressor, with higher expression levels during neural tissue differentiation (Liu et al., 2006, 2011a, b). Recent work shows that hCASZ1 inhibits cell-cycle progression in neuroblastoma (Liu et al., 2013). Two independent genome-wide association studies identify a genetic association at the hCasz1 locus with hypertension and high systolic blood pressure (Levy et al., 2009; Takeuchi et al., 2010). These studies implicate a potential link between CASZ1 and cardiac and vascular dysfunction. The roles of CASZ1 in multiple developmental contexts highlight its importance as a key developmental transcription factor.
The tissue-specific expression patterns of Casz1 mRNA in Xenopus and mammals have been determined (Vacalla and Theil, 2002; Liu et al., 2006; Christine and Conlon, 2008), although little is known about its posttranscriptional control or the subcellular localization of CASZ1 protein during development. Casz1 mRNA is expressed throughout the endocardium and myocardium (Christine and Conlon, 2008). However, CASZ1 depletion from heart tissue affects only a small subset of cardiomyocytes. This could be due to the function of CASZ1 in these cells, the regulation of CASZ1 nuclear localization in these cells, or the presence or spatial regulation of CASZ1 cofactors that modulate its activity. To address these questions, we generated antibodies to examine CASZ1 protein levels and localization patterns during cardiac development. We found that CASZ1 protein was expressed throughout the developing myocardium, and was down-regulated during cardiomyocyte proliferation. We also found that CASZ1 protein expression correlated with cellular differentiation of cardiomyocytes, skeletal muscle, and ectoderm derivatives throughout embryonic development. These results provide insights into CASZ1 function in development and its role as a tumor suppressor.
Results
CASZ1 Protein is Expressed Concurrently With Cardiomyocyte Differentiation
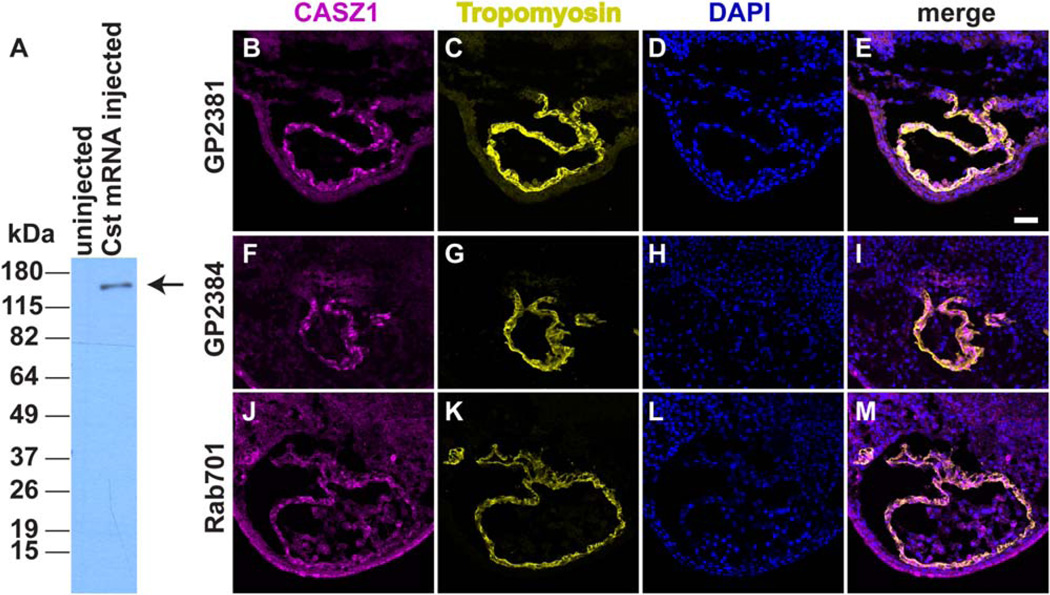
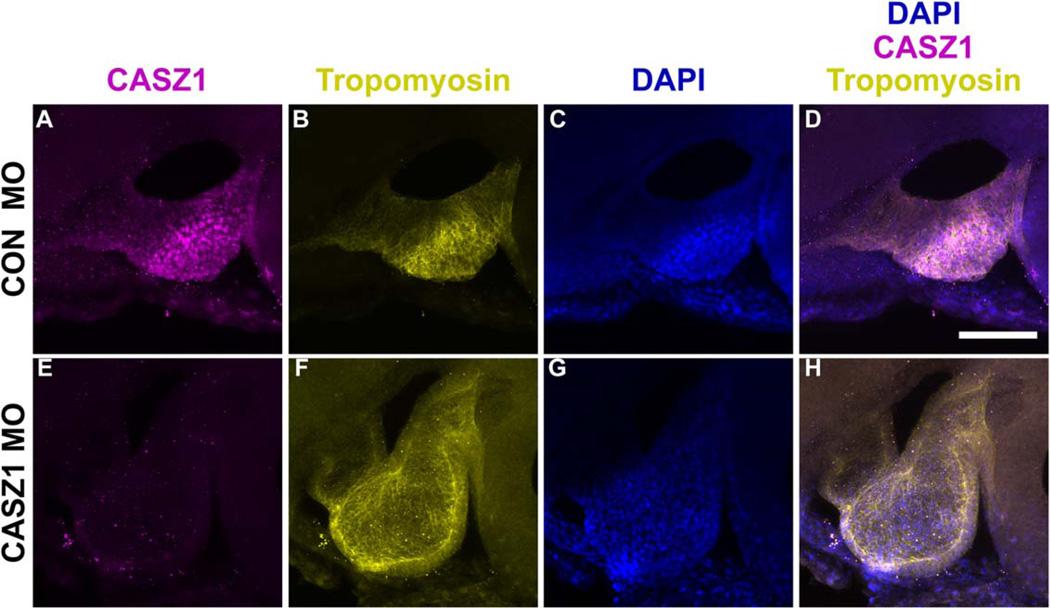
As cardiac cells migrate to the ventral midline and fuse to form the linear heart tube, a subpopulation of these cells differentiates into the myocardium and expresses the muscle protein tropomyosin (Tmy). Our previous work indicated that CASZ1 has a role during early cardiomyocyte differentiation in the Xenopus embryo (Christine and Conlon, 2008). At the mRNA level, Casz1 is expressed throughout the developing heart, yet CASZ1 appears to be required only for a small subset of cardiomyocytes at the ventral midline. To address the possibility that CASZ1 protein expression is posttranscriptionally regulated during cardiac development, we generated and affinity-purified antibodies against Xenopus CASZ1 (see Experimental Procedures). We verified the following parameters to demonstrate that these antibodies were specific for CASZ1: (1) they recognized over-expressed CASZ1 in stage 12 Xenopus embryos (Fig. 1A); (2) three independent CASZ1 antibodies (GP2381, GP2384, Rab701) showed similar immunostaining patterns in thin sections (Fig. 1B–M); and (3) anti-CASZ1 staining was abolished in embryos injected with Casz1-morpholinos (MOs) (Fig. 2). The CASZ1 antibody GP2381 had the highest titer of the three antibodies tested, and was used for the remaining studies.
Fig. 1.
Detection of CASZ1 in Xenopus embryos with anti-CASZ1 antibodies. A: Western blot of crude Xenopus lysates of uninjected and Casz1-V5 mRNA injected embryos (stage 12) demonstrates specificity of GP2381 anti-CASZ1 antibodies. Arrow denotes band corresponding to CASZ1-V5. B–M: Immunofluorescence of stage 40 Xenopus embryos (transverse sections) with three independent anti-CASZ1 antibodies. Both guinea pig antibodies (GP2381 and GP2384) and the single rabbit antibody (Rab701) recognize CASZ1 protein in cardiomyocyte nuclei throughout the heart. Dorsal is to the top. Anti-CASZ1 (B,F,J), anti-Tropomyosin (C,G,K), DAPI (D,H,L), and corresponding merge (E, I, M). Scale bar = 50 µm.
Fig. 2.
CASZ1 protein is depleted in Casz1 morpholino (MO)-injected embryos. Maximum projections of z-stacks (50 µm total) through the linear heart tube (stage 34) of control (A–D) and MO injected embryos (E–H). Anterior to the left, dorsal to the top. Anti-CASZ1 (A, E), anti-Tropomyosin (B, F), DAPI (C,G), and corresponding merge (D,H). Scale bar = 50 µm.
To obtain high spatial resolution of CASZ1 protein expression patterns in Xenopus heart, we performed whole-mount antibody immunostaining using anti-CASZ1 antibodies (GP2381) followed by optical sectioning through intact hearts (see Experimental Procedures). In the linear heart tube, CASZ1 protein was localized to nuclei throughout the differentiated myocardium (Fig. 2A–D, Supp. Movie S1, which is available online). CASZ1 was expressed predominantly in cells expressing Tmy, a marker of differentiated cardiomyocytes (myocardium), but CASZ1 was not observed in cardiac cells before differentiation (data not shown). Casz1 depletion results in a failure of cardiac progenitors at the ventral midline to differentiate into cardiomyocytes (Christine and Conlon, 2008). CASZ1 protein expression was associated with myocardial differentiation and did not appear to be restricted to a specific subset of cardiomyocytes at the ventral midline. These results suggest that CASZ1 function may require specific cofactors within the developing myocardium to regulate differentiation at the ventral midline.
Expression of CASZ1 in Cardiac Tissue is Restricted to Cardiomyocytes
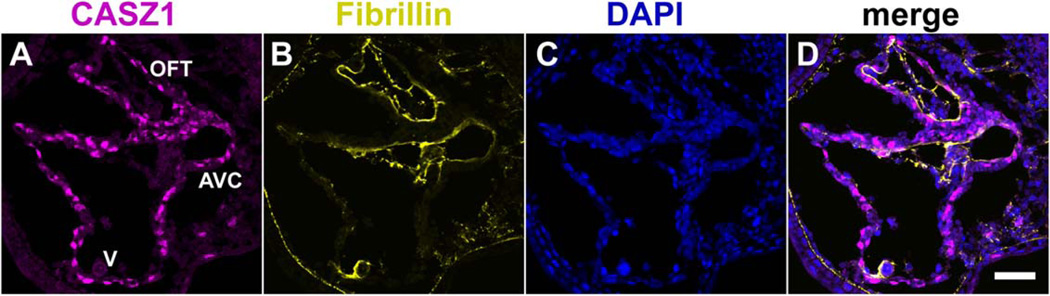
We previously observed Casz1 mRNA expression in the developing endocardium (Christine and Conlon, 2008). Endocardium-specific expression of CASZ1 could contribute to its role in myocardial differentiation by providing specific signals or cofactors to the myocardium. However, our results suggest that CASZ1 protein is not detected in the endocardium of the linear heart tube (Fig. 2A–D, Supp. Movie S1). To determine if Casz1 was posttranscriptionally regulated in the endocardium, we examined CASZ1 protein expression in sectioned Xenopus hearts throughout development. To differentiate myocardium and endocardium in the developing heart at high resolution, we examined the expression of Fibrillin, which is localized to the space between myocardium and endocardium (Kolker et al., 2000). Consistent with the results from our whole-mount expression analysis, we did not detect CASZ1 protein in the endocardium of the linear heart tube (Fig. 3) or at later stages (data not shown). These results suggest that Casz1 mRNA undergoes posttranscriptional modification to prevent protein expression in the endocardium.
Fig. 3.
CASZ1 expression in the heart is restricted to the myocardium. A–D: Transverse section (10 µm) through the heart shows CASZ1 expression (A) is excluded from endocardium in the outflow tract (OFT) and atrioventricular canal (AVC) that is surrounded by Fibrillin (B). DAPI (C) and corresponding merge (D). V, ventricle, dorsal is to the top
Expression of CASZ1 Protein in Cardiomyocytes Persists During Adulthood
We showed that CASZ1 protein was expressed throughout the myocardium of the linear heart tube. Although CASZ1 has a role during early cardiac development, a potential role for CASZ1 at later stages of cardiac development has not been determined. Recent studies suggest that CASZ1 plays a role in human hypertension (Levy et al., 2009; Takeuchi et al., 2010). Therefore, we performed whole-mount antibody staining to detect the localization of CASZ1 and the myocardial marker tropomyosin during development of Xenopus cardiomyocytes. We observed CASZ1 protein throughout the myocardium of the looped, chambered heart (stage 43) and during trabeculation (stage 47) (Fig. 4A–D; Supp. Movies S2, S3). CASZ1 also localized to nuclei of adult myocardium (Fig. 4E–H). Therefore, CASZ1 is expressed in the myocardium throughout development, which is consistent with a role for CASZ1 in the function and differentiation of cardiomyocytes at both earlier and later developmental stages.
Fig. 4.
CASZ1 is expressed throughout the myocardium. A–D: Maximum projections of z-stacks (50 µm total) through the looped heart (stage 43; ventral view). E–H: Transverse section (10 µm) of dorsal region of adult heart ventricle. anti-CASZ1 (A,E), anti-Tropomyosin (B,F), DAPI (C,G), and corresponding merge (D,H). Scale bars = 50 µm.
CASZ1 is Regulated During Cardiomyocyte Proliferation
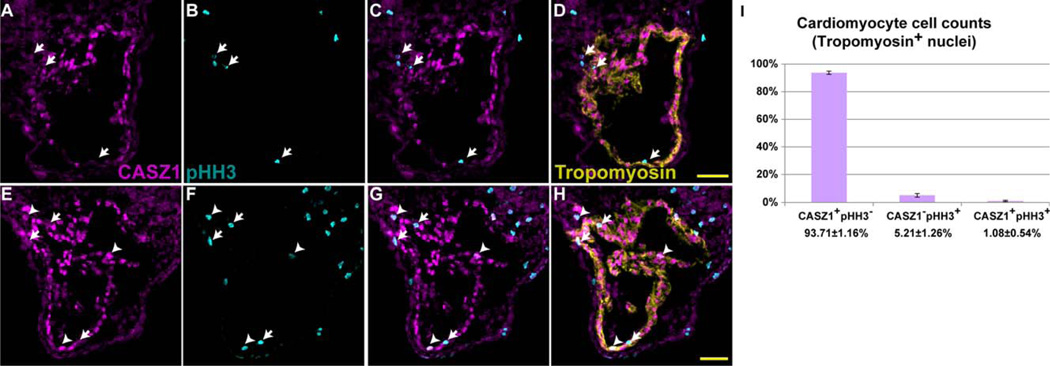
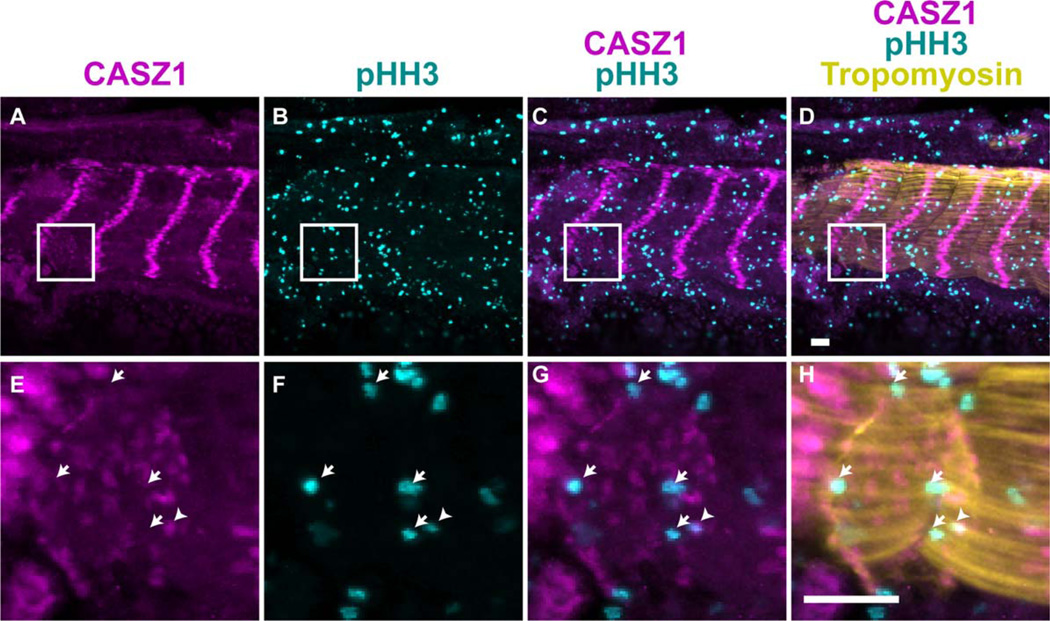
We next sought to understand the functional role of CASZ1 in the myocardium. Depletion of CASZ1 leads to an increase in the cardiomyocyte mitotic index (Christine and Conlon, 2008). Although the majority of cardiomyocytes express CASZ1 during normal cardiac development, we observed that some do not. As development proceeds, the heart grows through the addition of cardiomyocytes from other sources such as the secondary heart field. Existing cardiomyocytes also undergo proliferation to increase the cell number within the heart. We hypothesize that expression of CASZ1 is correlated with terminal differentiation and its expression must be down-regulated within the myocardium to allow cellular proliferation. To test this, we examined the expression of CASZ1 protein with respect to the mitotic marker phosphohistone H3 (pHH3) in stage 40 Xenopus embryos, in which the myocardium is undergoing active growth. We detected the expression of pHH3 in all cardiomyocytes not expressing CASZ1 (Fig. 5, arrows, 5.21 ± 1.26%; >500 cardiomyocytes counted per animal; n = 3). CASZ1 was detected in only a small subset (1.08 ± 9.54%) of cardiomyocytes that were also positive for pHH3 (Fig. 5, arrowheads). The majority of proliferating cardiomyocytes (83%) do not express CASZ1, whereas the remaining 17% of cardiomyocytes have detectable CASZ1 protein. This population of cells with remnants of CASZ1 may reflect cells entering mitosis that have not yet down-regulated CASZ1 expression. These results show that CASZ1 protein expression occurs in terminally differentiated cardiomyocytes, and suggest that it must be down-regulated for cells to undergo proliferation.
Fig. 5.
CASZ1 expression in the myocardium is correlated to the cell cycle. A–H: Transverse sections (10 µm, dorsal to the top) through the heart of a wild-type stage 40 embryo, with anti-CASZ1 (A,E), anti-phospho-histone H3 (B,F), merge (C,G), overlaid with anti-Tropomyosin staining (D,H). Arrows denote CASZ1− pHH3+ Tmy+ cardiomyocytes; arrowheads denote CASZ1+ pHH3+ Tmy+ cardiomyocytes. Scale bars = 50 µm. I: Quantification of cell counts of Tropomyosin+ cardiomyocyte nuclei from stage 40 embryos (n = 3; >500 cardiomyocytes scored per animal). Bars indicate standard deviation among animals scored.
CASZ1 Protein Expression Correlates With Onset of Muscle Differentiation
CASZ1 expression is concurrent with terminal differentiation of cardiomyocytes. Casz1 mRNA is also detected in somites of developing Xenopus embryos (Christine and Conlon, 2008). To test if CASZ1 protein expression in somites is regulated in a similar manner as that in heart, we performed whole-mount analysis of CASZ1 localization with respect to localization of the muscle marker Tropomyosin. For these studies, we used a well-established transgenic Xenopus line in which green fluorescent protein (GFP) was expressed in all skeletal and cardiac muscles under the control of the cardiac actin promoter (Latinkic et al., 2002; Tandon et al., 2013). We examined somite development from late tail bud embryos (stage 27) through tadpole stages (stage 47). CASZ1 was localized in the nuclei of differentiated skeletal muscle; Tropomyosin and GFP also were detected in these cells (Fig. 6). Next, we examined CASZ1 protein expression in other muscle types, including pharyngeal, jaw, and lymph heart muscle cells. We detected CASZ1 in all differentiated muscle cells (detectable by Tropomyosin and Cardiac Actin GFP) in developing tadpoles (Fig. 6). These results show that CASZ1 protein expression correlates with terminal differentiation of both cardiac and skeletal muscle.
Fig. 6.
CASZ1 is expressed in all differentiated muscle in the embryo. A–E: Maximum projections of z-stacks (620.96 µm total) of stained stage 43 cardiac actin::gfp embryo, anterior half. F–J: Maximum projection of z-stacks (102.04 µm total) of magnified view of somites from embryo in A–E. K–O: Maximum projection of z-stacks (59.41 µm total) of magnified view of lymph heart from embryo in A–E. P–T: Maximum projection of z-stacks (180.29 µm total) of magnified view of heart from embryo in A–E. Anti-CASZ1 staining (A,F,K,P), anti-Tropomyosin (B,G,L,Q), anti-GFP (C,H,M,R), DAPI (D,I,N,S), and corresponding merge (E,J,O,T). Anterior to the left, dorsal to the top. Scale bars = 500 µm in E; 50 µm in J,O,T.
CASZ1 may Regulate Cell-Cycle Progression in the Lymph Heart
We detected CASZ1 in cardiac muscle, skeletal muscle, and in lymph heart cells that display characteristics of both types of muscle (Peyrot et al., 2010). We showed previously that depletion of CASZ1 results in edemas, which can be attributed to lymph heart dysfunction (Peyrot et al., 2010). We hypothesized that CASZ1 may play a similar role in the lymph heart as it does in the myocardium, and may prevent entry into the cell cycle. We tested this hypothesis by examining CASZ1 and pHH3 expression in the lymph heart. The results show that CASZ1 levels are lower in the lymph myocardium during cellular proliferation (Fig. 7). Therefore, the regulation of CASZ1 protein expression appears to be cell-cycle dependent in terminally differentiated lymph heart cells.
Fig. 7.
CASZ1 expression in the lymph heart is also correlated to the cell cycle. A-H: Maximal projection of 100 µm of z-stacks through the somites (A–D) and magnified view of lymph heart in white box in panel D (E–H) of a stage 43 embryo, with anti-CASZ1 (A,E), anti-phospho-histone H3, pHH3 (B,F), merge (C,G), and overlaid with anti-Tropomyosin staining (D,H). Arrows denote CASZ1− pHH3+ lymph muscle nuclei. Arrowheads denote CASZ1+ pHH3+ lymph muscle nuclei.
CASZ1 Protein Expression is Regulated in Differentiated Neural Tissue
During mesoderm development, CASZ1 protein expression is restricted to terminally differentiated muscles. It is unclear if it is restricted to terminally differentiated cells in other tissues. Casz1 mRNA is detected in the neural tube and eye, in addition to muscle (Christine and Conlon, 2008). To determine if CASZ1 protein expression in these ectodermal subtypes was restricted to differentiated cells, we examined CASZ1 protein expression in the neural tube and eye. We consistently detected CASZ1 protein localize to the dorsal neural tube (Fig. 8A–C). In the eye, CASZ1 was localized to the layer of photoreceptors in the outermost portions of the retina. To test if CASZ1 was expressed within a specific subset of photoreceptors (i.e., rods or cones), we performed immunostaining with anti-CASZ1 and anti-Gatransducin antibodies. All CASZ1-expressing cells in the retina were also positive for GαTransducin and thus CASZ1 is expressed in differentiated rod photoreceptors in the eye (Fig. 8). Therefore, CASZ1 protein expression correlates with terminal differentiation of both mesodermal and ectodermal cell types.
Fig. 8.
CASZ1 is expressed in differentiated neural tissue during development. A–C: Expression of CASZ1 in the dorsal portions of anterior neural tube. D–K: CASZ1 is present (D,H) in both eyes of stage 42 embryos and colocalizes (G,K) to DAPI-stained nuclei (F,J) of rod photoreceptors marked by Gαtransducin (E,I). Panels H–K are magnified views of eyes in D–G.
Discussion
In this study, we characterized the expression of CASZ1 protein, a transcription factor that plays crucial roles in developmental and cancer biology. We showed that CASZ1 was detected in developing heart, somites, eye, and neural tube. These results were consistent with those of previous in situ hybridization studies of Casz1 mRNA expression. We obtained a high-resolution map of CASZ1 protein expression within these organs and within tissues that were not tested previously during in situ hybridization studies. The localization of CASZ1 to the nucleus is consistent with its putative role as a transcription factor. The spatial and temporal expression of CASZ1 in the heart, neural tube, and eye suggest a general role of CASZ1 in differentiation of tissues during development. We show here the potential role for CASZ1 in the development of additional tissues, namely the lymph heart and skeletal muscle derivatives.
Mechanisms of CASZ1 Function During Cardiac Development
Casz1 mRNA is detected throughout the entire myocardium and endocardium. However, CASZ1 is required for differentiation of only a subset of cardiomyocytes at the ventral midline. This suggests that Casz1 may undergo posttranscriptional regulation in a subset of cardiac progenitor cells at the ventral midline. We did not detect CASZ1 protein within the endocardium, which is consistent with posttranscriptional regulation (Figs. (3 and 4)). We did detect CASZ1 protein throughout the myocardium. This suggests that additional cofactors, which may be spatially restricted to these cardiac progenitor cells, modulate CASZ1 activity. It has been shown that human CASZ1 (hCASZ1) regulates gene expression and that this activity is dependent on zinc fingers 1–4. However, deletion of zinc finger 5 or the entire C-terminus of hCASZ1 does not affect its transcriptional activity (Virden et al., 2012). We hypothesize that these regions may be required to mediate interactions with putative CASZ1 cofactors to spatially regulate CASZ1 function during development. Alternatively, CASZ1 may function redundantly with other cardiac transcription factors to promote the differentiation of cardiac progenitors into cardiomyocytes. The combination of CASZ1 and these other factors may influence cardiomyocyte differentiation and spatial identity (such as atrial vs. ventricular).
CASZ1 protein is expressed during early cardiac development. Expression persists in the myocardium throughout development, including that of the adult heart. This leads to the hypothesis that CASZ1 plays additional roles during cardiac development, perhaps in regulating cardiomyocyte growth or function. This is supported by recent studies associating hCasz1 with hypertension and high systolic blood pressure in humans (Levy et al., 2009; Takeuchi et al., 2010). Further work to determine the role of CASZ1 in adult heart function using conditional deletion mutants will be needed to elucidate the role of CASZ1 in adult heart disease.
CASZ1 Expression in Muscle Development
An intriguing aspect of CASZ1 protein expression is its presence in the lymph heart musculature. Lymph hearts are pulsatile organs that propel lymph into the venous system to mediate fluid homeostasis. The lymphatic system regulates immunity and metastasis. The Xenopus tadpole was established as a model to study gene function during formation and development of the lymphatic system (Ny et al., 2005). Recent work shows that the lymph heart musculature develops under distinct control from the lymph endothelium (Peyrot et al., 2010). A pair of anterior lymph hearts emerges during tail bud stages, whereas an additional four pairs emerge in the posterior during late tadpole stages. The lymph heart musculature has characteristics of skeletal and heart muscle. However, lymph heart myotubes have greater structural and functional similarity with cardiac muscle than skeletal muscle, with a thin branched structure (Fig. 6K–O) that beats rhythmically. The lymph heart expresses skeletal muscle markers (12/101 and myoD), but not markers of cardiac progenitors (GATA-4/5/6) or cardiomyocytes (troponin) (Peyrot et al., 2010). This has led to the hypothesis that lymph heart muscle cells may be skeletal muscle cells with properties similar to cardiac muscle cells. We report here that the transcription factor CASZ1 is expressed in differentiated lymph muscle cells. Therefore, this could be a nuclear transcription factor linking development of skeletal and heart muscle in the lymph musculature.
The transcription factor engrailed-1 (En-1) regulates gene expression in myoblasts between the third and fourth anterior somites beginning at stage 28. En-1-expressing myoblasts eventually coalesce into a region where the lymph heart forms. These cells eventually differentiate into the anterior pair of lymph hearts by stage 39, and express 12/101, myoD, and CASZ1 (Fig. 6) (Peyrot et al., 2010). This process is dependent on Hedgehog (Hh) signaling, because blocking Hh signaling results in the loss of En-1-positive cells and a failure to form lymph heart muscle. Recent studies in Drosophila show that Hh signaling functions upstream of Castor (the Drosophila homolog of CASZ1) to regulate follicle stem-cell maintenance during oogenesis and cell-cycle exit in neuroblasts (Chai et al., 2013; Chang et al., 2013). Lymph heart dysfunction leads to the development of edema, as does CASZ1 depletion. However, because CASZ1 depletion in the early embryo affects both cardiac and vascular development (Christine and Conlon, 2008; Charpentier et al., 2013), it is difficult to determine if the edema is due to lymphatic dysfunction. In this study, we show that CASZ1 expression is mutually exclusive with expression of the mitosis marker phospho-histone H3 in the lymph heart (Fig. 7). Therefore, we propose that CASZ1 regulates cell proliferation within the lymph heart, and that this is dependent on Hh signaling. Targeted deletion of CASZ1 in the lymph musculature would be needed to further determine the role of CASZ1 in lymph heart development and function.
CASZ1 and the Cell Cycle
We showed previously that CASZ1 was required for the proper differentiation of a subset of cardiac progenitor cells at the ventral midline of Xenopus embryos (Christine and Conlon, 2008). CASZ1 depletion leads to an increased mitotic index within cardiomyocytes during development. In this study, we show that CASZ1 protein is detected in several differentiated tissues, including rod photoreceptors in the eye and various muscle types. We also show that CASZ1 expression is tightly linked to the cell cycle in cardiomyocytes and lymph heart, because CASZ1 protein is not detected in the majority of mitotic cells. A recent report showed that Casz1 expression in neuroblastomas activated pRb in the G1 phase of the cell cycle and inhibited the G2/M regulators cyclin B1 and Chk1, thereby increasing the length of the cell cycle and decreasing cell proliferation (Liu et al., 2013). The Drosophila homolog of CASZ1 functions downstream of Hh signaling to regulate cell fate in neuroblasts and stem-cell maintenance in follicles (Chai et al., 2013; Chang et al., 2013). It is unknown if Hh signaling regulates Casz1 expression, thereby inhibiting proliferation and suppressing tumor formation. Our observations of CASZ1 protein expression in the lymph heart, where Hh signaling is required, suggests that vertebrate Casz1 may be regulated at some level by Hh signaling, similar to that in Drosophila. Our studies also provide insights that can help to clarify how CASZ1 affects cardiac and neural development. For example, studies in Xenopus and mouse show that Hh signaling promotes cell-cycle progression and exit in the retina (Locker et al., 2006; Sakagami et al., 2009). In Drosophila, engrailed is a Hh target in the eye. This suggests that CASZ1 may function downstream of Hh/En-1 during photoreceptor differentiation in the eye. Future studies should examine the relationship between Hh signaling and CASZ1 during muscle and neural development, and its potential role in tumor suppression.
Experimental Procedures
Xenopus Embryo Collection and Manipulation
Embryos were obtained and cultured as described previously (Mandel et al., 2010). Embryos were injected with 80 ng CASZ1 morpholinos as described previously (Christine and Conlon, 2008), and collected at stage 34 to test for antibody specificity. Full-length CASZ1-V5 protein was overexpressed in embryos by injecting the corresponding capped mRNA into embryos at the one-cell stage, and culturing as described previously (Christine and Conlon, 2008).
Plasmid Construction and Antibody Production
Xenopus Casz1 (amino acids 304–836) was amplified by PCR using primers NMA-7 (5′-gcggagctcCCTGGAATTGAGTCAGG-CAT-3′) and NMA-8 (5′-cgcctcgagATGCCCACTTATAATTGGGA-3′), and cloned in-frame with a C-terminal 6x–His tag in the pET-21a vector (Novagen) to generate plasmid UNC202. UNC202 was used to generate recombinant CASZ1 protein in BL21-CodonPlus (Stratagene) cells. The CASZ1–6xHis fusion proteins were bound to TALON Metal Affinity Resin (Clontech) and purified under denaturing conditions. Gel slices containing purified CASZ1 protein were used to immunize three guinea pigs (Pocono Rabbit Farm, PA). Two animals (GP2381 and GP2384) displayed an immune response to the antigen and were used for affinity purification against CASZ1-6xHis bound to a nitrocellulose membrane (Olmsted, 1981; Smith and Fisher, 1984). GP2381 and GP2384 antisera displayed similar immunostaining patterns to a rabbit anti-CASZ1 (Rab701) (Charpentier et al., 2013) antibody generated previously, with higher titer and less background.
Western Blotting
Embryos were collected at stage 12. Proteins were extracted and denatured as described previously (Amin et al., 2014). GP2381 anti-CASZ1 antibodies were used at 1:1,000 dilution. Horseradish peroxidase (HRP)-labeled donkey-anti-guinea pig secondary antibodies (Jackson Immuno) were used at 1:10,000 dilution.
Immunostaining and Imaging
Embryos were fixed in 4% paraformaldehyde before immunostaining. Before whole-mount staining, pericardial cavities were punctured and embryos were treated with 1 mg/ml bovine testicular hyaluronidase (Sigma). Embryos were rinsed with phosphate buffered saline (PBS) and blocked in 10% fetal bovine serum, 1% Triton-X100, 1 × PBS for 4 hr at room temperature. Guinea pig GP2381 anti-CASZ1 (1:50), mouse anti-tropomyosin (CH1, 1:10; Developmental Studies Hybridoma Bank), rabbit anti-phospho-histone H3 (product no. 06–570, 1:100; Millipore), and rabbit anti-GFP (A6455, 1:500; Molecular Probes) antibodies were applied overnight at 4°C. Animals were washed 6 times with PBS-T (1 × PBS and 0.1% Triton-X100) and incubated with Cy3-donkey anti-guinea pig (1:200; Jackson Immuno), Alexa647-donkey anti-mouse IgG (1:500; Molecular Probes), and Alexa488-donkey anti-rabbit (1:500; Molecular Probes) overnight at 4°C. Embryos were washed 6 times with PBS-T and incubated with 500 µg/ml DAPI (4’,6-diamidine-2-phenylidole-dihydrochloride) in PBS for 2 hr at room temperature. Embryos were dehydrated gradually in methanol and cleared in Murray’s solution to visualize staining (Kolker et al., 2000).
Sectioned immunohistochemistry was performed as described previously (Brown et al., 2005; Goetz et al., 2006; Langdon et al., 2007), with the addition of an antigen-retrieval step before blocking. In this step, slides containing cryosections were hydrated in 1 × PBS, placed in sodium citrate buffer (10 mM sodium citrate, 0.5% Tween-20, pH 6.0) with constant heating for 20 min, and allowed to gradually cool for an additional 15 min. Slides were rinsed quickly in 1 × PBS and blocked as above. Guinea pig anti-CASZ1 (1:200), mouse anti-tropomyosin (1:50), rabbit anti-phospho-histone H3 (1:500); and rabbit anti-transducin (1:100; product no. sc-389; Santa Cruz Biotechnology, Santa Cruz, CA) (Choi et al., 2011) antibodies were applied overnight at 4°C in a humidity chamber. Cy3-donkey anti-guinea pig (1:500; Jackson Immuno), Alexa647-donkey anti-mouse IgG (1:1,000), and Alexa488-donkey anti-rabbit (1:1,000) were used for 2 hr at room temperature. Slides were mounted with coverslips with Pro-Long Gold Antifade Reagent with DAPI (Invitrogen) before imaging.
Whole-mount and sectioned staining samples were visualized on a Zeiss 700 confocal microscope with MyZen software. The z-stacks were flattened using maximum projection (ImageJ) to generate images representing whole-mount staining. To generate 3D rendering, isosurfaces, and movies demonstrating CASZ1 staining in the heart, z-stacks were imported to Imaris x64 6.1.5 software (Bitplane AG, St. Paul, MN) as described previously (Doherty et al., 2010).
Cardiomyocyte Cell Counting
Cryosectioned embryos were stained with a combination of anti-CASZ1, anti-phospho-histone H3, anti-tropomyosin, and DAPI. Sequential transverse sections of the heart were imaged and false-colored to reflect all markers. Tropomyosin-positive (Tropomyosin+) nuclei (DAPI+) were counted for the presence of CASZ1 and phospho-histone H3 staining. A minimum of 500 cardiomyocytes (>7 sections) were counted from a total of three stage 40 embryos.
Supplementary Material
Acknowledgments
The monoclonal antibodies against Tropomyosin (CH1) and Fibrillin (JB3) developed by Jim Jung-Ching Lin and Charles D. Little, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. We thank Dr. Michael Zuber for his helpful discussions on the retinal expression of CASZ1 and the University of North Carolina Microscopy Services Lab for their support with imaging and training on IMARIS software. F.L.C. was funded by the NIH/NHLBI and N.M.A. was supported by an AHA Postdoctoral Fellowship.
Grant sponsor: NIH/NHLBI; Grant number: RO1 DE018825; Grant number: RO1 HL089641; Grant number: R21 HD073044; Grant sponsor: AHA; Grant number: 11POST6320005.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Amin NM, Greco TM, Kuchenbrod LM, Rigney MM, Chung M, Wallingford J, Cristea IM, Conlon FL. Tissue specific proteomic profiling by Isolation of Nuclei Tagged in Specific Cell Types (INTACT) Development. 2014;141:962–973. doi: 10.1242/dev.098327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Martz SN, Binder O, Goetz SC, Price BM, Smith JC, Conlon FL. Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development. 2005;132:553–563. doi: 10.1242/dev.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai PC, Liu Z, Chia W, Cai Y. Hedgehog signaling acts with the temporal cascade to promote neuroblast cell cycle exit. PLoS Biol. 2013;11:e1001494. doi: 10.1371/journal.pbio.1001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Jang AC, Lin CH, Montell DJ. Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis. Proc Natl Acad Sci U S A. 2013;110:E1734–E1742. doi: 10.1073/pnas.1300725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier MS, Christine KS, Amin NM, Dorr KM, Kushner EJ, Bautch VL, Taylor JM, Conlon FL. CASZ1 promotes vascular assembly and morphogenesis through the direct regulation of an EGFL7/RhoA-mediated pathway. Dev Cell. 2013;25:132–143. doi: 10.1016/j.devcel.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RY, Engbretson GA, Solessio EC, Jones GA, Coughlin A, Aleksic I, Zuber ME. Cone degeneration following rod ablation in a reversible model of retinal degeneration. Invest Ophthalmol Vis Sci. 2011;52:364–373. doi: 10.1167/iovs.10-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine KS, Conlon FL. Vertebrate CASTOR is required for differentiation of cardiac precursor cells at the ventral midline. Dev Cell. 2008;14:616–623. doi: 10.1016/j.devcel.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JT, Conlon FL, Mack CP, Taylor JM. Focal adhesion kinase is essential for cardiac looping and multichamber heart formation. Genesis. 2010;48:492–504. doi: 10.1002/dvg.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Brown DD, Conlon FL. TBX5 is required for embryonic cardiac cell cycle progression. Development. 2006;133:2575–2584. doi: 10.1242/dev.02420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolker SJ, Tajchman U, Weeks DL. Confocal imaging of early heart development in Xenopus laevis. Dev Biol. 2000;218:64–73. doi: 10.1006/dbio.1999.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon YG, Goetz SC, Berg AE, Swanik JT, Conlon FL. SHP-2 is required for the maintenance of cardiac progenitors. Development. 2007;134:4119–4130. doi: 10.1242/dev.009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinkic BV, Cooper B, Towers N, Sparrow D, Kotecha S, Mohun TJ. Distinct enhancers regulate skeletal and cardiac muscle-specific expression programs of the cardiac alpha-actin gene in Xenopus embryos. Dev Biol. 2002;245:57–70. doi: 10.1006/dbio.2002.0639. [DOI] [PubMed] [Google Scholar]
- Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Naranjo A, Thiele CJ. CASZ1b, the short isoform of CASZ1 gene, coexpresses with CASZ1a during neurogenesis and suppresses neuroblastoma cell growth. PLoS One. 2011a;6:e18557. doi: 10.1371/journal.pone.0018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Rader J, He S, Phung T, Thiele CJ. CASZ1 inhibits cell cycle progression in neuroblastoma by restoring pRb activity. Cell Cycle. 2013;12:2210–2218. doi: 10.4161/cc.25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang X, Li Z, McMahon C, Sizer C, Barenboim-Stapleton L, Bliskovsky V, Mock B, Ried T, London WB, Maris J, Khan J, Thiele CJ. CASZ1, a candidate tumor-suppressor gene, suppresses neuroblastoma tumor growth through reprogramming gene expression. Cell Death Differ. 2011b;18:1174–1183. doi: 10.1038/cdd.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang X, Tan F, Cullion K, Thiele CJ. Molecular cloning and characterization of human Castor, a novel human gene upregulated during cell differentiation. Biochem Biophys Res Commun. 2006;344:834–844. doi: 10.1016/j.bbrc.2006.03.207. [DOI] [PubMed] [Google Scholar]
- Locker M, Agathocleous M, Amato MA, Parain K, Harris WA, Perron M. Hedgehog signaling and the retina: insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006;20:3036–3048. doi: 10.1101/gad.391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel EM, Kaltenbrun E, Callis TE, Zeng XX, Marques SR, Yelon D, Wang DZ, Conlon FL. The BMP pathway acts to directly regulate Tbx20 in the developing heart. Development. 2010;137:1919–1929. doi: 10.1242/dev.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, Terclavers S, Ciesiolka M, Kalin R, Man WY, Senn I, Wyns S, Lupu F, Brandli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Olmsted JB. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981;256:11955–11957. [PubMed] [Google Scholar]
- Peyrot SM, Martin BL, Harland RM. Lymph heart musculature is under distinct developmental control from lymphatic endothelium. Dev Biol. 2010;339:429–438. doi: 10.1016/j.ydbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami K, Gan L, Yang XJ. Distinct effects of Hedgehog signaling on neuronal fate specification and cell cycle progression in the embryonic mouse retina. J Neurosci. 2009;29:6932–6944. doi: 10.1523/JNEUROSCI.0289-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, Nabika T, Fujioka A, Ohnaka K, Asano H, Yamori Y, Yamaguchi S, Kobayashi S, Takayanagi R, Ogihara T, Kato N. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation. 2010;121:2302–2309. doi: 10.1161/CIRCULATIONAHA.109.904664. [DOI] [PubMed] [Google Scholar]
- Tandon P, Miteva YV, Kuchenbrod LM, Cristea IM, Conlon FL. Tcf21 regulates the specification and maturation of proepi-cardial cells. Development. 2013;140:2409–2421. doi: 10.1242/dev.093385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacalla CM, Theil T. Cst, a novel mouse gene related to Drosophila Castor, exhibits dynamic expression patterns during neu-rogenesis and heart development. Mech Dev. 2002;118:265–268. doi: 10.1016/s0925-4773(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Virden RA, Thiele CJ, Liu Z. Characterization of critical domains within the tumor suppressor CASZ1 required for transcriptional regulation and growth suppression. Mol Cell Biol. 2012;32:1518–1528. doi: 10.1128/MCB.06039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.