Abstract
The reuniens nucleus in the midline thalamus projects to the medial prefrontal cortex (mPFC) and the hippocampus, and has been suggested to modulate interactions between these regions, such as spindle–ripple correlations during sleep and theta band coherence during exploratory behavior. Feedback from the hippocampus to the nucleus reuniens has received less attention but has the potential to influence thalamocortical networks as a function of hippocampal activation. We used the retrograde tracer cholera toxin B conjugated to two fluorophores to study thalamic projections to the dorsal and ventral hippocampus and to the prelimbic and infralimbic subregions of mPFC. We also examined the feedback connections from the hippocampus to reuniens. The goal was to evaluate the anatomical basis for direct coordination between reuniens, mPFC, and hippocampus by looking for double-labeled cells in reuniens and hippocampus. In confirmation of previous reports, the nucleus reuniens was the origin of most thalamic afferents to the dorsal hippocampus, whereas both reuniens and the lateral dorsal nucleus projected to ventral hippocampus. Feedback from hippocampus to reuniens originated primarily in the dorsal and ventral subiculum. Thalamic cells with collaterals to mPFC and hippocampus were found in reuniens, across its anteroposterior axis, and represented, on average, about 8 % of the labeled cells in reuniens. Hippocampal cells with collaterals to mPFC and reuniens were less common (~1 % of the labeled subicular cells), and located in the molecular layer of the subiculum. The results indicate that a subset of reuniens cells can directly coordinate activity in mPFC and hippocampus. Cells with collaterals in the hippocampus–reuniens–mPFC network may be important for the systems consolidation of memory traces and for theta synchronization during exploratory behavior.
Keywords: Hippocampus, Prefrontal cortex, Reuniens, Thalamus, Memory consolidation, Theta
Introduction
Coordination of activity between the hippocampus and medial prefrontal cortex (mPFC) has been demonstrated extensively during sleep and wakefulness (Siapas and Wilson 1998; Sirota et al. 2003; Siapas et al. 2005; Jones and Wilson 2005; Hyman et al. 2005, 2010; Peyrache et al. 2011). Interactions between hippocampal and thalamocortical networks during sleep are considered critical for systems memory consolidation, a process by which episodic memory recall progressively becomes independent of the hippocampus and relies more on neocortical regions (Maviel et al. 2004; Quinn et al. 2008). During sleep, ensembles of hippocampal place cells reactivate memory traces that represent spatial trajectories encoded during wakefulness (Wilson and McNaughton 1994; Louie and Wilson 2001; Foster and Wilson 2006). The reactivation of place cells occurs during brief high-frequency oscillations (150–200 Hz) in the hippocampal local field potential (ripples) and is thought to have a direct role in memory consolidation (Girardeau et al. 2009; Ego-Stengel and Wilson 2010). Ripples in the CA1 region of the hippocampus occur in close time proximity to spindles recorded in neocortical regions such as mPFC (Siapas and Wilson 1998; Sirota et al. 2003; Peyrache et al. 2011). Spindles are low-frequency (7–14 Hz) oscillations in the local field potential that occur during drowsiness and sleep, and are generated by rhythmic firing in cells of the thalamic reticular nucleus (TRN). The spikes in TRN evoke rebound bursting in the relay cells of the dorsal thalamus, which can then engage their postsynaptic neocortical networks in the spindle oscillation (Steriade et al. 1987; Halassa et al. 2011). CA1 ripples occur both before spindles (Siapas and Wilson 1998; Peyrache et al. 2011) and locked to the troughs of the spindle oscillation (Sirota et al. 2003; Peyrache et al. 2011). Because of the thalamic role in spindle generation, thalamic cells projecting to CA1 and mPFC could help phase-lock ripples to spindles. In addition, cells in the hippocampus with projections to the thalamus and mPFC could influence spindle occurrence in thalamocortical networks.
Interactions between hippocampus and mPFC are also evident during active wakefulness. The firing of cells in CA1 and mPFC is phase locked to the hippocampal theta rhythm (4–10 Hz), and the firing coherence in the theta band increases during working memory (Siapas et al. 2005; Jones and Wilson 2005; Hyman et al. 2005, 2010). Conversely, a decrease in theta coherence between CA1 and mPFC is associated with poor performance in a working memory task (Sigurdsson et al. 2010). The ventral part of CA1 projects directly to mPFC (Swanson 1981; Jay and Witter 1991; Jay et al. 1992; Hoover and Vertes 2007) and this pathway has been proposed to play a role in CA1–mPFC theta synchronization. Thalamic cells in the nucleus reuniens receive afferents from areas involved in the generation of the theta rhythm, and cells projecting from reuniens to CA1 and mPFC could also contribute to theta synchronization between these two areas (Risold et al. 1997; McKenna and Vertes 2004).
In spite of the evidence from physiological experiments, the anatomical networks that could directly synchronize and modulate activity in hippocampus, mPFC, and thalamus remain largely unexplored. One possibility is that there are cells within one or more of these regions that branch to project to the other two. These collateralized projections could influence the timing of activity in the postsynaptic regions. Additional regions outside the hippocampus, mPFC, and the thalamus could also have a synchronizing effect on these regions by sending collaterals to them. Candidate areas include the entorhinal and perirhinal cortices, involved in a number of memory processes (Steffenach et al. 2005; Suh et al. 2011; Kealy and Commins 2011), and the septum, which has a pivotal role in theta rhythm generation (Lee et al. 1994). mPFC does not project to hippocampus (Vertes et al. 2007) and could not directly affect activity in the thalamus and hippocampus. However, both thalamus and hippocampus have the potential to contain cells with collaterals to the other two structures. The nucleus reuniens has extensive projections to the hippocampus (Herkenham 1978; Wouterlood et al. 1990; Dolleman-Van der Weel and Witter 1996; Vertes et al. 2006). In addition to sending axons to the entorhinal and subicular regions, reuniens contributes a dense projection to CA1's stratum lacunosum moleculare (slm) throughout its dorsoventral axis (Dolleman-Van der Weel and Witter 1996; Vertes et al. 2006). A subpopulation of reuniens cells was recently found to project both to CA1 and mPFC using retrograde tracers and epifluorescence techniques (Hoover and Vertes 2011). We sought to further characterize the existence of collateral branches in reuniens cells projecting to mPFC and hippocampus using stereotaxic injections of the retrograde tracer cholera toxin B, and using both epifluorescence and confocal fluorescence for tissue imaging. Because of evidence that subregions of mPFC have different connectivity and functions (Vertes 2004; Vidal-Gonzalez et al. 2006), we also examined if cells with collaterals are present after injections limited to the prelimbic or the infralimbic subdivisions of mPFC. In addition, we determined the topography of the reuniens population that projects to hippocampus and mPFC, and tested the possibility that feedback projections from hippocampus (Swanson 1981; Jay and Witter 1991; Cenquizca and Swanson 2006) could send axonal branches to both reuniens and mPFC. Finally, we asked if double-labeled cells could be found in regions outside the three areas of interest (hippocampus, mPFC, reuniens), namely, in the entorhinal and perirhinal cortices and in the septal area.
Methods
We used 35 male Long-Evans rats (400–500 g) for these experiments. Animals were housed individually, provided with food and water ad libitum, and monitored in a temperature-controlled room with a 12 h light/dark cycle (lights on/off at 7:00 am/7:00 pm). These experiments were approved by the Committee on Animal Care at the Massachusetts Institute of Technology and conform to US National Institutes of Health guidelines for the care and use of laboratory animals.
Stereotaxic injections of anatomical tracers
Anesthesia was induced by an intraperitoneal injection of a solution of ketamine (25 mg/kg), xylazine (3 mg/kg) and atropine (0.027 mg/kg), followed by maintenance with 1–2 % inhaled isoflurane. We used the subunit B of cholera toxin (CTB) conjugated to Alexa Fluorophores 488 and 594. The CTB was dissolved at a concentration of 1 % in 0.06 M PB, except in one animal in which we used 0.5 % concentration with similar results but weaker intensity of cell labeling. The CTB solution was pressure injected at 0.1 or 0.05 μl/min (for the reuniens injections) using glass micropipettes (average tip diameter 63 μm, range 40–80 μm measured in a sample of five micropipettes) and a stereotaxic injector (Stoelting QSI injector). To minimize tracer diffusion along the pipette track, the pipette was left in place after injection for 10–15 min and then removed slowly.
The coordinates for injection were measured from bregma and were chosen according to the Paxinos and Watson atlas (1986). We placed single injections (400–1,000 nl) of CTB into the prelimbic (PL) or the infralimbic (IL) regions of mPFC (anterior 3, lateral 1.5 and depth 3 mm for PL, depth 4 mm for IL). For both mPFC regions, the pipette was lowered at a 16° angle to prevent damage to the superior sagittal sinus. The same animal received one (500–1,000 nl) or two (250 nl each; n = 10 of the animals) injections of CTB conjugated to the other fluorophore into the dorsal or ventral hippocampus (coordinates ranged between: posterior 3.5–5, lateral 2.5–4, depth 2.2–2.4 mm for the dorsal region, and posterior 5–5.7, lateral 5.4, depth 6 mm for ventral), or one injection in the nucleus reuniens. To inject the reuniens, we used a volume of 250–300 nl and the following coordinate range: posterior 1.8–2, lateral 1.7–2.1 and depth 6.7–6.9 mm, with the pipette at a 16° angle. Chemicals were purchased from Invitrogen (cholera-toxin conjugates), Vector Labs (Vectashield® mounting medium with DAPI) and Boston Bio-products (phosphate buffers and paraformaldehyde).
Tissue processing and imaging
Animals were euthanized 7–10 days after surgery with an overdose of sodium pentobarbital (100 mg/kg via IP), then transcardially perfused with 60 ml of 0.01 M PBS followed by 120 ml of 4 % paraformaldehyde. Brains were post-fixed at least overnight before sectioning (50–60 μm) in a coronal plane using a vibratome (Leica VT1000S). Sections were mounted with Vectashield® mounting medium containing 1.5 μg/ml of DAPI.
Pictures were taken using an AxioCam HR camera on an Axio Imager Z1 motorized microscope (Zeiss). Zeiss filter sets number 20 (Rhodamine/TRITC), 49 (DAPI) and 47HE (FITC) were used to observe CTB–AF594, DAPI, and CTB–AF488, respectively.
Confocal images were obtained with Zeiss confocal laser scanning LSM 510 microscope using a 20× plan-apochromat objective (1.0 numerical aperture). A 405 nm diode, a 488 nm krypton-argon, and 543 nm helium-neodymium lasers were used for fluorophore excitation, in combination with bandpass filters at 420–480 nm (for DAPI), 505–530 nm (for Alexa fluorophore-488) and a long-pass filter >560 (for Alexa fluorophore-594). The optical slice was less than 6.5 μm. To estimate the fraction of double-labeled cells in reuniens, we selected tissue from four rats with representative injections in the prelimbic or infralimbic mPFC regions and the dorsal or ventral hippocampus (i.e., one rat each with injections in prelimbic and dorsal hippocampus, prelimbic and ventral hippocampus, infralimbic and dorsal hippocampus, and infralimbic and ventral hippocampus). For each rat, six confocal pictures were taken every 200 μm along the anteroposterior axis of the nucleus reuniens. We used a similar strategy to estimate the number of double-labeled cells in the subicular region of the hippocampus after injections in reuniens and mPFC: five confocal pictures were taken (every 250 μm) along the anteroposterior axis of the subiculum (dorsal and ventral) of four rats with injections in reuniens and prelimbic (two rats) or infralimbic (two rats). The region of interest for cell counting was defined with the help of a library of Nissl-stained sections and another of sections processed for calbindin immunohistochemistry obtained from the tissue from one of the animals. Two experimenters independently counted the number of cells projecting to hippocampus, mPFC or reuniens and the number of those that were double labeled. Cells for which the presence of the two tracers could not be unequivocally confirmed are reported as `unclassified'. The reported values are averages of the counts from the two experimenters.
Cytoarchitecture
Nissl stain and calbindin immunohistochemistry were used to reveal cytoarchitecture and identify regions of interest in the thalamus and hippocampus. The Nissl stain was performed on mounted sections which were first hydrated, and then submerged in 0.5 % of cresyl violet acetate for about 2–3 min; this step was followed by dehydration in solutions of increasing ethanol concentration and mounting with permount.
For the calbindin immunohistochemistry, one of every six sections was collected in PBS during sectioning. The calbindin immunohistochemistry started with 1 h in blocking solution (5 % goat serum and 0.5 % Triton X-100 in 0.01 M PBS) at room temperature. Then, the sections were incubated overnight (at 4 °C) in 1:1,000 primary antibody (Swant mouse anti-calbindin, catalog # 300) in blocking solution. We used a goat anti-mouse secondary antibody conjugated to the Alexa Fluorophore 647 (invitrogen catalog # A-21236) at 1:250 in PBS for 3 h (room temperature). Examples of sections processed for Nissl and calbindin are shown in Supplementary Figure 1.
Results
A total of 35 animals received stereotaxic injections of the subunit B of the cholera toxin (CTB) conjugated to alexa fluorophores 488 and 594. The target regions were the prelimbic or infralimbic areas of the mPFC, the dorsal or ventral hippocampus and the thalamic nucleus reuniens (Table 1 displays the number of rats used in each experiment).
Table 1.
Breakout of the number of rats used for each experiment
| Experiment | Presence of double-labeled cells | Thalamic projections to PL/IL | Thalamic projections to dorsal/ventral HC | Hippocampal feedback to Re | ||
|---|---|---|---|---|---|---|
| mPFC + HC | mPFC + Re | Other regions (septum, EC, PR) | ||||
| Number of rats | 10 | 4 | 11 | 13 | 10 | 10 |
A total of 35 rats were used, some animals were used in multiple experiments
mPFC medial prefrontal cortex; HC hippocampus; Re reuniens; EC entorhinal cortex; PR perirhinal cortex; PL prelimbic; IL infralimbic
Hippocampus-projecting and mPFC-projecting cell populations in the thalamus
Injection sites
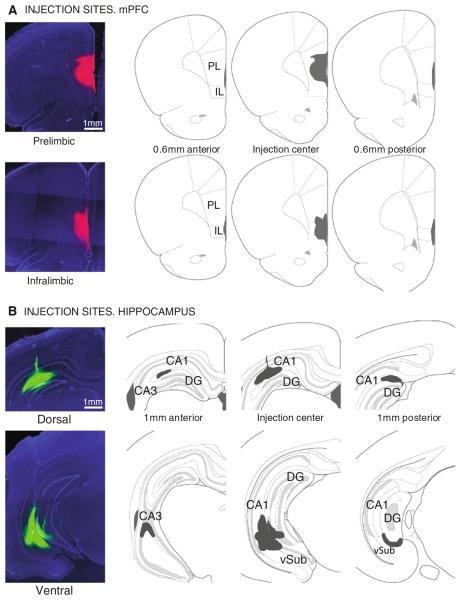
Figure 1 shows representative examples of the injection sites and the spread of the tracer in the anteroposterior axis of mPFC (Fig. 1a) and hippocampus (Fig. 1b). The center of the injection was identified as the brain section with maximum dorsoventral and mediolateral spread of the tracer, which often coincided with signs of light pipette tip damage in the tissue. Pictures were taken every 200 μm in the anterior and posterior directions from the injection center to determine the spread of the tracer. The hippocampal coordinates were chosen to target the stratum lacunosum moleculare (slm) of CA1, where axons from reuniens cells terminate (Herkenham 1978; Wouterlood et al. 1990; Dolleman-Van der Weel and Witter 1996; Vertes et al. 2006). In dorsal hippocampus, the tracer solution diffused along the mediolateral and anteroposterior axis of slm, with less spread in the dorsoventral direction. There was always spread into the upper blade of the dentate gyrus with dorsal CA1 injections. However, given that the projections from reuniens to hippocampus are limited to CA1's slm, the retrograde tracer could only be picked up in this CA1 layer. In fact, control injections in CA2–CA3 or dentate gyrus that did not affect slm, did not result in retrograde labeling in the thalamus (n = 15) and give further support to the specificity of reuniens' projections to CA1's slm. The spread of the tracer in mPFC was more symmetrical than in hippocampus, with no spread into the neighboring striatum or the contralateral mPFC.
Fig. 1.
Representative examples of CTB injection sites in medial prefrontal cortex (mPFC) in (a), and hippocampus in (b). Coronal sections, 2.5×. PL prelimbic; IL infralimbic; DG dentate gyrus; vSub ventral subiculum
mPFC-projecting thalamic cells
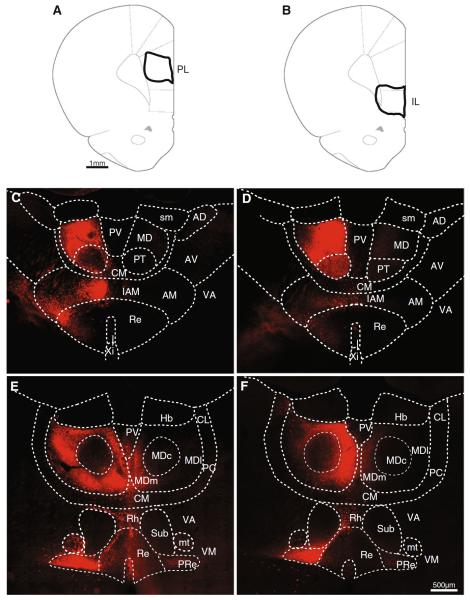
From rats with injections in mPFC and hippocampus, we selected a subset of cases restricted to the prelimbic (n = 8) and infralimbic (n = 5) mPFC subregions to compare the thalamic populations projecting to these areas. Figure 2 shows representative examples of two injections in the prelimbic (Fig. 2a) and infralimbic (Fig. 2b) cortices. The diagrams at the top of Fig. 2 show the outline of the injection sites (at the point of maximum tracer spread). For each case, the retrograde labeling at two anteroposterior levels in the thalamus is shown in the panels below its injection diagram. In the anterior part of the thalamus (Fig. 2c, d), the anterior group of nuclei constitutes a large part of the thalamic tissue, and the reuniens from each hemisphere (the so-called `heads' of the reuniens) progressively converge to join at the midline. At this anterior level, cells were labeled ipsilaterally in the parataenial and the paraventricular nuclei (Fig. 2c, d). Labeling in the paraventricular was moderate in its ventral portion (in between the heads of the nucleus reuniens, not shown) and sparse in the dorsal part. Within the anterior group of thalamic nuclei, cells were found in the medial nucleus ipsilaterally (including its interanteromedial portion in the midline, Fig. 2c, d), few and less intensely labeled cells could also be seen in the contralateral anterior medial nucleus just lateral to the reuniens. The labeled cell population spread into the central medial nucleus as well (Fig. 2c, d). In the nucleus reuniens (Fig. 2c, d), labeled cells were densest in the lateral and ventral part of the ipsilateral nucleus. Sparse cells could also be found directly above the third ventricle and extending into the ventral part of the reuniens, in the area designated as the xiphoid nucleus by Paxinos and Watson (1986). We did not find noticeable differences between the topography and the labeling intensity of prelimbic and infralimbic projecting thalamic populations at this anterior thalamic level.
Fig. 2.
Thalamic populations projecting to mPFC. a, b Schematic diagrams with the outlines of prelimbic (left) and infralimbic (right) injections. c, d Examples of retrogradely labeled cells at two anteroposterior levels in the thalamus (coronal sections, 20×); each example corresponds to the injections indicated with solid lines in the diagrams. c, d Are more anterior than e, f. PL prelimbic; IL infralimbic; PV paraventricular; CM central medial; MD mediodorsal (subdivisions: central, lateral and medial indicated as MDc, MDl and MDm, respectively); PT parataenial; sm stria medullaris; AD anterodorsal; AV anteroventral; AM anteromedial; IAM interanteromedial; VA ventral anterior; VM ventral medial; Re reuniens; PRe perireuniens; Xi xiphoid; Hb habenula; CL central lateral; PC paracentral; Rh Rhomboid; Sub submedial; mt mamillothalamic tract
At more posterior levels, the highest number of prelimbic-projecting cells (Fig. 2e, f) was found in the ipsilateral mediodorsal and reuniens nuclei. Moderate number of labeled cells were also found in the ipsilateral central medial, rhomboid, and ventral medial nuclei (Fig. 2e, f). Labeled cells frequently spread from the rhomboid nucleus laterally around the submedial nucleus and toward the ventral medial nucleus (Fig. 2e). From the ventral medial, scattered labeled cells occasionally extended into the ventral anterior nucleus. Within the reuniens (Fig. 2e, f), two clear regions could be distinguished based on cell density: the lateral part of the nucleus (also known as the `wing' of the reuniens or perireuniens nucleus), contained a high density of intensely labeled cells; instead, in the central part of the nucleus, cells were less dense and the intensity of labeling in individual cells was weaker. The perireuniens region contained high number of labeled cells in both hemispheres, although more cells were labeled in the side ipsilateral to the injection. Ventral to the reuniens–perireuniens area, sparse cells were seen in the ipsilateral zona incerta (Fig. 2e, f). In the contralateral hemisphere labeled cells were observed in the ventral medial and in the perireuniens and, occasionally, in the mediodorsal nucleus (Fig. 2e).
Injections in the prelimbic region labeled cells across a broader area in the thalamus than infralimbic injections. CTB in the infralimbic produced less cell labeling in the central medial and interanteromedial nuclei compared to prelimbic injections (Fig. 2c–f). In addition, the medial and lateral portions of the mediodorsal nucleus were intensely labeled from the CTB injections in the prelimbic area, but only the medial part contained a dense population of labeled cells after infralimbic injections, which gave only weak labeling in the lateral portion (Fig. 2e, f). The central part of the mediodorsal had at most very sparse labeling with both prelimbic and infralimbic injections (notice the dark round area in the center of the mediodorsal in the bottom panels of Fig. 2e, f), this is in agreement with the findings from previous tracing studies (Condé et al. 1990; Hoover and Vertes 2007). Within the reuniens nucleus, the main difference between prelimbic and infralimbic injections was that prelimbic injections labeled a narrow and dense band of cells along the lateral edge of the nucleus (Fig. 2e yellow arrow); fluorescence was always very intense in these cells and they were not seen after infralimbic injections.
At the most posterior levels of the midline thalamus (roughly from the level where the lateral geniculate nucleus appears in coronal sections), the number of cells labeled from the prefrontal or the hippocampal injections decreased substantially, and the results were similar for prelimbic and infralimbic injections (data not shown).
Hippocampus-projecting thalamic cells
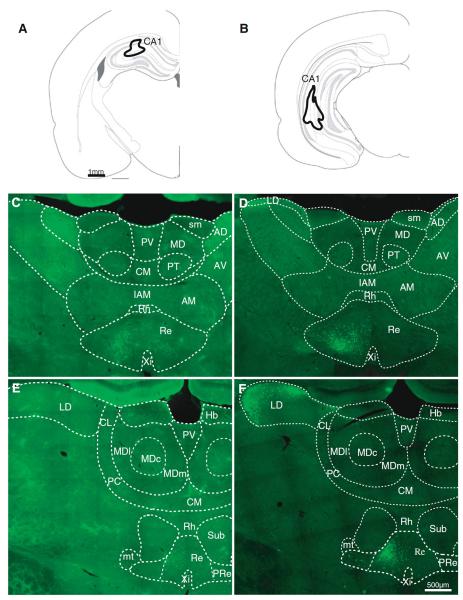
Injections of CTB in dorsal (n = 7) or ventral (n = 3) hippocampus labeled cells in the ipsilateral reuniens throughout its anteroposterior axis, and the number of cells decreased in caudal regions of the nucleus, in parallel to the decrease in mPFC-projecting cells. Figure 3 displays representative examples of retrograde labeling at two anteroposterior levels of the thalamus after injections of CTB–AF488 in dorsal (Fig. 3a) and ventral (Fig. 3b) hippocampus. The diagrams on top represent the spread of the tracer at the injection sites, and the images below show the retrograde cell labeling from these injections. Cells labeled after hippocampal injections were densest in the central and lateral parts of the reuniens nucleus (Fig. 3c through f) and overlapped with the mPFC-projecting population. Hippocampus-projecting cells were more evenly spread than mPFC-projecting cells, and no clear distinction could be made between the perireuniens and the central part of reuniens, although few cells were found in the perireuniens after dorsal hippocampal injections (Fig. 3e). Hippocampus-projecting reuniens cells were largely ipsilateral, as opposed to the bilateral origin of mPFC projections from perireuniens.
Fig. 3.
Thalamic populations projecting to hippocampus. a, b Diagrams of two injections in dorsal and ventral hippocampus. c–f Examples of retrogradely labeled cells (coronal sections, 20×) at two anteroposterior thalamic levels from the injections shown above; c, d more anterior than e, f. LD lateral dorsal; other abbreviations as in Fig. 2
Ventral hippocampal injections resulted in a higher number of cells labeled in reuniens than dorsal injections. Injections in the dorsal hippocampus resulted in retrograde labeled cells only in the reuniens and occasional scattered cells in the rhomboid nucleus (Fig. 3c, e), whereas ventral hippocampus injections (Fig. 3d, f) labeled cells in reuniens and in the lateral dorsal nucleus (and even less cells were found in rhomboid with ventral injections). At least some of the labeled lateral dorsal cells may have resulted from tracer spread into the subiculum; in one animal with an injection largely confined to CA1, retrogradely labeled cells in the lateral dorsal nucleus were also seen in the most lateral and most medial parts of the nucleus. The population of cells in the lateral dorsal nucleus was less dense than the reuniens population, and was homogeneously distributed in the lateral part of the nucleus with some cells spreading medially toward the habenula (Fig. 3f). Another difference between dorsal and ventral hippocampus injections was that the population projecting to dorsal hippocampus was located in the central part of reuniens, while the population projecting to ventral hippocampus was located more ventrally in the nucleus with some cells found in the perireuniens region (Fig. 3f, see also Fig. 4d).
Fig. 4.
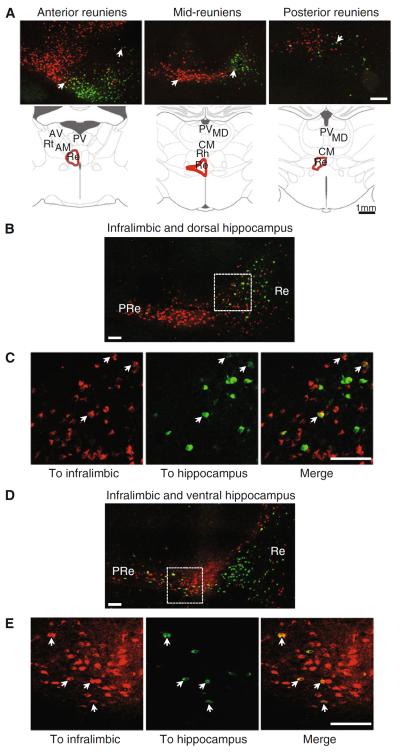
Single and double-labeled cells in reuniens. a Confocal microscope images of the nucleus reuniens at three anteroposterior anatomical levels indicated by the diagrams below, showing the topography of the mPFC-projecting (red) and hippocampus-projecting (green) populations. White arrows indicate examples of double-labeled cells. b Confocal image of the nucleus reuniens after injections in infralimbic (red) and dorsal hippocampus (green); c close-up view of the boxed area in (b) with examples of double-labeled cells indicated by white arrows. d Confocal image of the nucleus reuniens after injections in infralimbic (red) and ventral hippocampus (green). e Close-up views of the area inside the white dashed box arrows indicate double-labeled cells. Re reuniens; PRe perireuniens. Scale bars 200 μm in (a), 100 μm in (b–e)
Reuniens cells with collaterals to hippocampus and mPFC
Double-labeled cells in reuniens
Figure 4 displays confocal micrographs with examples of thalamic double-labeled cells, which, in the thalamus, were only found in reuniens (n = 10 rats). Figure 4a shows the topography of the mPFC and hippocampus-projecting populations within the nucleus reuniens at three different anatomical levels identified by the diagrams below. Double-labeled cells were found in the region of overlap between the mPFC and the hippocampus-projecting populations, along the anteroposterior axis of the nucleus. A few examples of double labeling are indicated by arrows in the micrographs of Fig. 4a.
Double-labeled cells in reuniens were found in all injection combinations (i.e., prelimbic/infralimbic and dorsal/ventral hippocampus); an example of confocal micrographs from tracer injections in the infralimbic and dorsal hippocampus is shown in Fig. 4b–c. The whole nucleus reuniens, with the overlapping mPFC and hippocampus-projecting populations, is shown in b, whereas Fig. 4c shows close-up views of double-labeled cells from the boxed area in Fig. 4b (a few examples of double-labeled cells are indicated by white arrows). Examples of double-labeled cells from an animal injected in the infralimbic and ventral hippocampus are shown in Fig. 4d–e (the overall nucleus is shown in Fig. 4d, and close-up views of the boxed area in that section are shown in Fig. 4e).
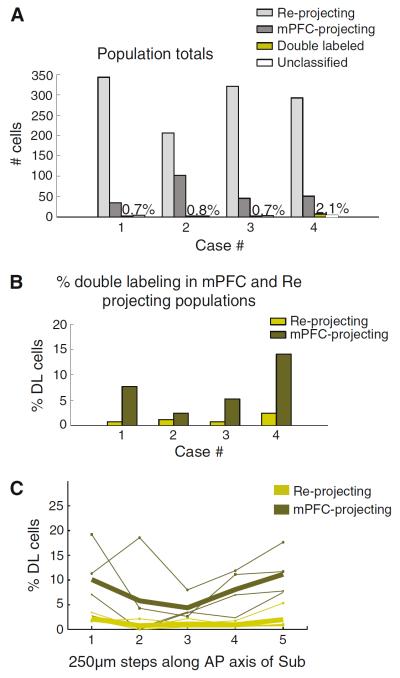
We quantified the fraction of hippocampus-projecting cells that also projected to mPFC, i.e., the double-labeled cells, in confocal images taken at six different levels (200 lm apart) in the anteroposterior axis of reuniens (Fig. 5c, d). The quantification was based on counts from two experimenters. For this quantification, we selected tissue from four rats, each with one combination of representative injections in the prelimbic or infralimbic regions (two rats) and in the dorsal or ventral hippocampus (two rats). Confocal images of these four cases confirmed the presence of double-labeled cells in reuniens independently of the combination of injections (Supplementary Figure 2a displays the injection sites and overall percent of double-labeled cells for all four cases). We found the highest number of double labeling among the hippocampal-projecting population, where double-labeled cells ranged between 19.9 and 30.9 % (average 24.6 % for the four cases; Fig. 5a,b). The fraction of double-labeled cells was lower in the mPFC-projecting population (range between 4.2 and 17.9 %, average 12.12 %). When considering the two populations (projecting to mPFC and hippocampus) together, an average of 7.8 % of reuniens cells were double labeled (range 3.45–10.16 %). The number of double-labeled cells remained fairly stable along the anteroposterior axis of the nucleus (Fig. 5c).
Fig. 5.
Cell population quantification in the reuniens. a Total number of reuniens cells in each group: single labeled (hippocampal-projecting or mPFC-projecting), double labeled or unclassified. b Percentage of double-labeled cells in the hippocampal or mPFC-projecting population for the four cases. c Percent of double-labeled cells as a function of the anteroposterior reuniens axis. Re reuniens; mPFC medial prefrontal cortex; HC hippocampus; DL double labeled; AP anteroposterior
Hippocampal cells projecting to reuniens
Reuniens cells with projections to both mPFC and hippocampus are good candidates to facilitate correlated activity in these two regions. Hippocampal projections to mPFC and to the midline thalamus could also influence interactions between thalamocortical networks. The projections from the ventral hippocampus to mPFC have been previously described (Swanson 1981; Jay and Witter 1991; Jay et al. 1992; Cenquizca and Swanson 2006). We injected CTB in the nucleus reuniens and mPFC (n = 10) to study the feedback projections from hippocampus to reuniens and test for double labeling.
Reuniens-projecting hippocampal cells and double labeling
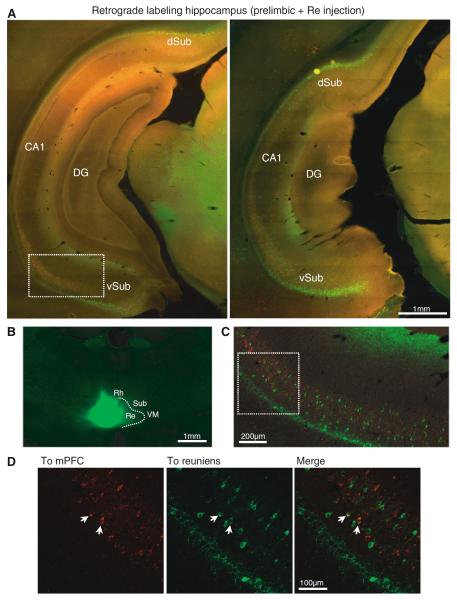
Figure 6 display examples of retrogradely labeled cells after injections in reuniens and mPFC at two different anteroposterior levels of the caudal hippocampus (Fig. 6a). The reuniens injection for this case is shown in Fig. 6b. Injections in the reuniens nucleus (n = 10) labeled cells in the dorsal and ventral subiculum at caudal hippocampal levels. The amount of labeled cells increased toward posterior parts of the subiculum (Fig. 6a), notice the increased labeling in the image on the right, which is more posterior. From the dorsal subiculum, labeled cells could be followed into deep layers of the retrosplenial cortex. Labeled cells (projecting to reuniens) were very sparse in the ventral CA1 region although the number increased in caudal regions. Cells were intensely labeled in the pyramidal cell layer of the subiculum; in the molecular layer, cells were sparser and less intensely labeled (Fig. 6a, c).
Fig. 6.
Subicular populations projecting to reuniens and mPFC. a Retrograde labeling in caudal hippocampus after CTB-488 injection in reuniens and CTB-594 injection in the prelimbic cortex (coronal sections, 5×). b Example of CTB injection in the nucleus reuniens (2.5×). c Confocal micrograph of the boxed area in (a) showing the mPFC (red) and reuniens (green) populations. d Close-up view of the boxed area in (c) with examples of double-labeled cells indicated by arrows. DG dentate gyrus; dSub dorsal subiculum; vSub ventral subiculum; Rh rhomboid; Re reuniens; Sub submedial; VM ventral medial
In agreement with previous reports (Swanson 1981; Jay and Witter 1991), we found mPFC-projecting cells in the caudal part of CA1 and subiculum. In particular, the subicular cells projecting to mPFC were sparse and located in its proximal part (closer to CA1). In caudal regions of the subiculum, the reuniens-projecting population overlapped with mPFC-projecting cells and we found occasional double-labeled cells in the molecular layer of the ventral and dorsal subiculum (n = 4 animals with injections in mPFC -2 in prelimbic and two in infralimbic- and ipsilateral reuniens). Figure 6c and d displays confocal micrographs with two examples of cells in the ventral subiculum that were double labeled from injections in the prelimbic and the reuniens (Fig. 6c is a close-up of the boxed area in Fig. 6a, d is a close-up of the boxed area in Fig. 6c). We calculated the fraction of reuniens and mPFC-projecting cells that were double labeled with a similar approach to that used to estimate double labeling in reuniens. Two experimenters counted the labeled cells in confocal images taken at five different levels (every 250 μm) in the anteroposterior axis of the caudal subiculum of four rats. The rats used for these calculations had injections in reuniens and mPFC (prelimbic-2 rats or infralimbic-2 rats; supplementary Figure. 2b displays the injection sites and overall percent of double-labeled cells for all four cases) and we found double-labeled cells in all injection combinations. The results of these counts are shown in Fig. 7. Double labeling in the subiculum was less common than in the nucleus reuniens, with 1.07 % (range 0.65–2.09 %, n = 4 rats) of double-labeled cells in the overall population of retrogradely labeled subicular cells; Fig. 7a includes the total number of cells in each population for the 4 rats analyzed. We did not find differences between the number of double-labeled cells in the dorsal and ventral subiculum (p > 0.9, Mann–Whitney U test) and the counts for both regions have been pooled together. The highest percentage of double-labeled cells in the subiculum was found among the mPFC-projecting cells (average 7.38 %, range 2.45–14 %), whereas very few of the reuniens-projecting cells were found to also project to mPFC (average 1.3 %, range 0.75–2.46 %). The percentage of double-labeled cells in the anteroposterior axis of the subiculum is shown in Fig. 7c for the two cell projecting populations (to mPFC and to reuniens); the average values of double-labeled cells (indicated by thicker lines) remained stable in this particular axis.
Fig. 7.
Cell population quantification in the subiculum. a Total number of subicular cells in each group: single labeled (reuniens projecting or mPFC projecting), double labeled or unclassified. b Percentage of double-labeled cells in the reuniens or mPFC-projecting population for the four cases. c Percent of double-labeled cells as a function of anatomical level along the anteroposterior subicular axis. Re reuniens; mPFC medial prefrontal cortex; DL double labeled; AP anteroposterior
Double-labeled cells outside the thalamus and hippocampus
Extra-thalamic regions with potential for collaterals to mPFC and CA1
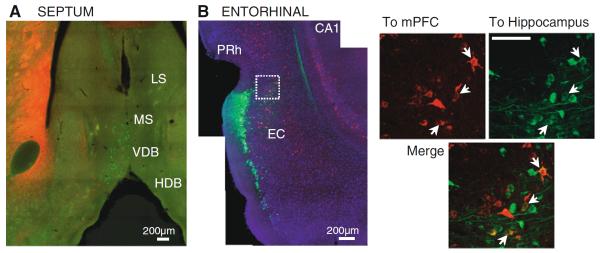
Within the thalamus, we have only found double-labeled cells in the nucleus reuniens. However, other brain regions that project to mPFC and CA1 could include cell populations with collateral axonal branches to the two regions, which would give them the potential to directly affect ongoing activity in both areas. Regions with projections to mPFC and CA1 include the entorhinal and perirhinal cortices, amygdala, septum and diagonal band of Broca, ventral tegmental area, and hypothalamus (Wyss et al. 1979; Oka and Yoshida 1985; Nyakas et al. 1987; Hoover and Vertes 2007). Of these regions, two stand out as particularly relevant because of their involvement in memory and theta rhythm generation. The entorhinal cortex represents the interface between neocortex and hippocampus, providing the main glutamatergic input to CA1 through direct and indirect pathways that are important for memory (Steffenach et al. 2005; Suh et al. 2011). In addition, the entorhinal cortex is a target of reuniens (Herkenham 1978; Vertes et al. 2006). The septal region is crucially involved in the generation of the hippocampal theta rhythm (Lee et al. 1994), an oscillation prominent during exploration and thought to be critical for the encoding of spatial information in the hippocampus (Buzsáki 2002). We tested for the presence of double labeling in these two areas: the septal region, and the entorhinal cortex. From animals with double-labeled cells in reuniens, we selected cases for observation throughout the septum and entorhinal cortex (n = 7, entorhinal cortex; n = 6, septum). Figure 8a shows an example of mPFC (red) and hippocampus (green) projecting populations in the septal area. We found populations of labeled cells in the medial septum and in the vertical and horizontal limbs of the diagonal band of Broca. Double-labeled cells were not found in these regions (medial septum and diagonal band of Broca) with our injections.
Fig. 8.
Septal and entorhinal areas after CTB injections in hippocampus (green) and mPFC (red). a Example of non-overlapping populations in the septal region after injections in hippocampus and mPFC (coronal section, 10×). b Confocal pictures (20×) of the dorsolateral entorhinal and perirhinal cortices after injections in dorsal hippocampus (green) and mPFC (red); DAPI stain in blue. Close-up view of the area inside the white box is shown to the right. Arrows indicate double-labeled cells. LS lateral septum; MS medial septum; VDB vertical limb of the diagonal band of Broca; HDB horizontal limb of the diagonal band; PRh perirhinal cortex; EC entorhinal cortex. Scale bar in the close-up images: 100 μm
In contrast to the segregated populations in the septum, a small number of cells in layers III/IV of the dorsal subdivision of the entorhinal cortex were labeled with the two tracers. An example is shown in Fig. 8b, which displays a mosaic confocal image of the entorhinal cortex of a case with injections in infralimbic and dorsal hippocampus (CTB-488); examples of double-labeled cells in the boxed area are shown in a closer view to the right of the mosaic image. Interestingly, this area of entorhinal cortex receives direct input from the nucleus reuniens (Wouterlood et al. 1990). This raises the possibility of an indirect route for effects of reuniens on CA1 and mPFC.
Extra-hippocampal regions with potential for collaterals to mPFC and reuniens
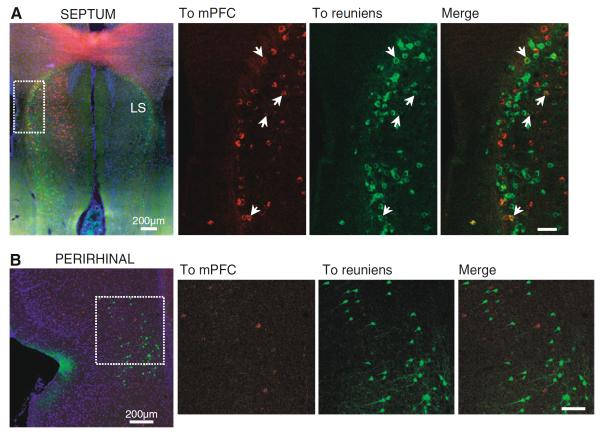
The nucleus reuniens receives afferents from an extensive number of regions (Herkenham 1978; McKenna and Vertes 2004), which could provide collaterals to both reuniens and mPFC with the potential to synchronize activity in this midline thalamocortical network. In four cases with injections in mPFC and reuniens, we looked for double-labeled cells in two regions that project to reuniens and mPFC and are functionally related to the hippocampus: the septum and the perirhinal cortex. The septal population projecting to reuniens was fairly dense and mostly limited to the lateral septum, including a group of reuniens-projecting cells at the most anterior levels of the septum, in the region bordering with the dorsal tenia tecta. mPFC-projecting cells were found in two septal areas: in the horizontal limb of the diagonal band of Broca, with little overlap with the reuniens-projecting population, and in anterior parts of the lateral septum and dorsal tenia tecta, overlapping with cells that project to reuniens. We found double-labeled cells only in this anterior septal region (n = 4 rats). Figure 9a displays examples of this anterior part of the septum; on the left, the mPFC-projecting (red) and reuniens-projecting (green) populations can be seen overlapping at the most lateral border of the lateral septum. To the right of this image, confocal micrographs display examples of double-labeled cells (the micrographs show the area indicated with a dashed white box on the image to the left). In the perirhinal region, the cells projecting to reuniens occupied the deepest layers, whereas mPFC-projecting cells were more superficial. Reuniens and mPFC-projecting populations overlapped to some extent, but we found no double-labeled cells in the perirhinal region (n = 4 rats). An example is shown in Fig. 9b, in this figure confocal images display the mPFC (red) and reuniens (green) projecting populations in the perirhinal cortex; a mosaic of the perirhinal cortex is shown to the left and a closer view of the boxed area is shown to the right. Overall, these results suggest that the subiculum and some portions of the septum, but not the perirhinal cortex, may play a role in modulating activity simultaneously in reuniens and mPFC.
Fig. 9.
Septal and perirhinal areas after CTB injections in reuniens (green) and mPFC (red). a Anterior septal region showing overlapping reuniens and mPFC-projecting populations (epifluorescence image on the left, 10×); right, confocal close-up of the boxed area showing double-labeled cells (arrows). b Confocal images of the perirhinal region showing segregated populations from the mPFC right are close-up views of the area outlined by the white box. Scale bar in the close-up images: 100 μm
Discussion
A number of electrophysiological studies have reported close temporal correlations between activity in the hippocampus and the mPFC. The thalamus is thought to contribute to these correlations. We have used retrograde tracers to study the anatomical networks that underlie direct interactions between these three regions. In agreement with previous reports (Hoover and Vertes 2011), we found that the nucleus reuniens was the only thalamic region where individual cells sent collaterals to both mPFC and hippocampus. About 25 % of the hippocampus-projecting cells in reuniens were double labeled after injections of retrograde tracers in hippocampus and either the prelimbic or the infralimbic regions of mPFC. The double-labeled reuniens cells were present across the anteroposterior axis of the nucleus, including the perireuniens area. Outside of reuniens, double-labeled cells (projecting to both mPFC and hippocampus) were found in the entorhinal cortex, but not in the septum. Caudal parts of the hippocampus, including the dorsal and ventral subiculum, were found to project to reuniens and a few cells in the subiculum and the anterior part of the lateral septum (but not the perirhinal region) also sent collaterals to mPFC.
Thalamic populations projecting to hippocampus and mPFC
Two groups of thalamic nuclei have direct projections to the medial temporal lobe: anterior and midline. Although both groups project to the temporal lobe, each may participate in different functional networks involving the dorsal and ventral hippocampus. The dorsal hippocampus has been suggested to be part of a network, important for spatial navigation and contextual memory, that includes the anterior thalamic nuclei and other regions such as the ventral tegmental area, the substantia nigra pars reticulata, and the retrosplenial and cingulate cortices (Fanselow and Dong 2010). Instead, the ventral part of the hippocampus is thought to be important for neuroendocrine regulation and for the control of stress and emotional responses. The ventral hippocampal network includes olfactory regions, the amygdala, the hypothalamus and the prefrontal cortex, a cortical region that is densely interconnected with the midline nuclei in the thalamus (Vertes et al. 2006; Vertes and Hoover 2008). Nuclei in the midline include the parataenial, paraventricular, rhomboid, reuniens, mediodorsal, and intermediodorsal (Jones 2007; Van der Werf et al. 2002). All project to mPFC, particularly to its prelimbic and infralimbic subdivisions. Projections from midline nuclei to the temporal lobe are in general more widespread than those of the anterior group, which is primarily connected with the subicular complex (Thompson and Robertson 1987; Van Groen and Wyss 1992; Shibata 1993; Van Groen and Wyss 1995). In particular, the nucleus reuniens projects densely to entorhinal cortex, CA1 and the subicular complex (Herkenham 1978; Wouterlood et al. 1990; Dolleman-Van Der Weel and Witter 1996; Risold et al. 1997; Vertes et al. 2006).
Our results from injections in the dorsal and ventral hippocampus indicate that reuniens may participate in both the dorsal and ventral hippocampal networks. We have found that injections of retrograde tracer in dorsal hippocampus labeled cells in reuniens, occasional sparse cells in the rhomboid, and no cells in the rest of the thalamus. Injections of the same tracer in ventral parts of the hippocampus labeled a larger population of cells in reuniens. This could reflect differences in tracer diffusion in dorsal compared to ventral hippocampus due to the orientation of the hippocampal layers relative to the orientation of the glass pipette used for injection, i.e., in ventral injections there could be more diffusion along the axis where the reuniens axons terminate. Anterograde tracers have provided evidence of denser terminal arbors from reuniens in ventral hippocampal regions (Wouterlood et al. 1990; Vertes et al. 2006). The current results suggest that the higher density of reuniens terminals observed in ventral compared to dorsal hippocampus may result from a larger number of reuniens cells projecting to ventral hippocampus, as opposed to a denser ramification of axon terminals from a small hippocampus-projecting population.
In addition to reuniens, ventral hippocampus injections also retrogradely labeled cells in the lateral dorsal nucleus (part of the anterior group). Because the injections in ventral hippocampus often spread to subiculum, the labeled cells in the lateral dorsal nucleus could be the result of lateral dorsal axon terminals picking up tracer in the subiculum (Van Groen and Wyss 1992). Consistent with this, one case with less tracer spread into subiculum resulted in much less labeling in the lateral dorsal nucleus, while still presenting a population of labeled cells in reuniens that was denser than with dorsal hippocampus injections.
Thalamic projections to mPFC originate mainly in the midline and intralaminar groups of thalamic nuclei (Krettek and Price 1977; Condé et al. 1990; Berendse and Groenewegen 1991; Moga et al. 1995; Hoover and Vertes 2007). Comparisons of projections to the infralimbic and prelimbic mPFC have been done (Hoover and Vertes 2007) with overall similar findings to those reported here, i.e., a substantial overlap in the thalamic nuclei that project to infralimbic and prelimbic cortices. There were two differences between the studies. Hoover and Vertes found a projection from the central medial nucleus to infralimbic but not prelimbic mPFC, whereas labeled cells were found in the central medial after injections in the prelimbic region in our tissue. Another difference involved the mediodorsal (MD) nucleus. Only the medial part was found to project to prelimbic and infralimbic regions by Hoover and Vertes. We also found the medial MD to be the only source of projections from this nucleus to the infralimbic cortex; however, after prelimbic injections, labeled cells were found in both the medial and lateral portions of MD, a result in agreement with Condé et al. (1990). A number of factors could account for the variability between studies, among them, the precise anatomical coordinates targeted by the injections, the binding properties of different tracers or the rat strains. Overall, the thalamic nuclei found to project to the infralimbic and prelimbic subregions of mPFC is in agreement across studies.
After mPFC injections (both prelimbic and infralimbic), two clear regions could be distinguished within the reuniens nucleus based on the density of labeled cells and the intensity of the signal. The lateral portion of the reuniens, the perireuniens region, contained a dense cell population with intense fluorescence; cells medial to this area (in the reuniens `proper') were less densely packed and less intensely labeled. These two populations within reuniens may reflect different axonal terminations in cortex; for example, the intensely labeled cells could have larger terminal boutons or a broader spatial spread of their axon terminal arbors, being able to pick up larger amounts of tracer. Reuniens terminals target superficial and deep layers of mPFC (Vertes et al. 2006). A characterization of the terminal type and layer of termination of cells in each reuniens subregion will help further understand the effect of this nucleus on specific mPFC circuits. The perireuniens region has been reported to project to the perirhinal cortex (Dolleman-Van Der Weel and Witter 1996); the presence of a densely labeled mPFC-projecting population adds another feature that distinguishes perireuniens from reuniens and underlines the need for more functional and anatomical studies to better understand this thalamic midline region.
Double-labeled cells in reuniens
The nucleus reuniens has numerous efferents (Herkenham 1978; Vertes et al. 2006), and the possibility that cells in the nucleus could send branches to multiple postsynaptic regions has been explored for a number of brain areas. Su and Bentivoglio (1990) first described in the rat the existence of reuniens cells projecting to both the hippocampus and the nucleus accumbens (see also Otake and Nakamura 1998), in addition to cells projecting to both the hippocampus and the amygdala. Interestingly, reuniens projections to hippocampal subregions (CA1, subiculum) originate in segregated cell populations (Dolleman-Van Der Weel and Witter 1996). Cells with collaterals to both hippocampus and mPFC were recently reported (Hoover and Vertes 2011). We have been able to confirm this finding with confocal microscopy, which reduces out-of-focus light from the specimen and allows more reliable identification of double-labeled cells. In addition, mPFC injections in the Hoover and Vertes study targeted both prelimbic and infralimbic subregions, leaving open the possibility that the double-labeled population projected to only one of the mPFC regions. We now present evidence that double-labeled cells project to the prelimbic and to the infralimbic cortex. In fact, cells with collaterals to both hippocampus and mPFC were found in reuniens after all injection combinations (dorsal and ventral hippocampus, prelimbic and infralimbic mPFC). Additional steps forward with respect to the Hoover and Vertes study include the quantification of double-labeled cells in the subicular feedback projection to reuniens, as well as the search for double-labeled cells in areas outside of the main network of interest (mPFC–thalamus–hippocampus), such as the entorhinal cortex and the septum (see below).
Our quantifications of the number of labeled cells from confocal images resulted in an average of 7.8 % of double-labeled cells (range 3.45–10.16 %), which is similar to the values reported by the study of Hoover and Vertes (3–9 % of the total population). We found a particular high number of double-labeled cells among the hippocampus-projecting population; 25 % of hippocampus-projecting cells also projected to mPFC, a result that could underline the critical role of mPFC co-activation whenever thalamo-hippocampal projections are active.
Double-labeled cells in hippocampus
A few studies have reported projections from caudal parts of the hippocampus to the nucleus reuniens (Risold et al. 1997; McKenna and Vertes 2004; Cenquizca and Swanson 2006). Projections were weak from CA1 and denser from dorsal and ventral subiculum; the number of subicular cells projecting to reuniens increased at posterior subicular levels (McKenna and Vertes 2004), something that we have also observed in our sections. Cells in the ventral subiculum were found to send collaterals to the midline thalamus and the lateral septum, and to the midline thalamus and the ventral medial hypothalamus (Namura et al. 1994). However, projections to both reuniens and mPFC have not been specifically tested. In agreement with the previous reports, we have found labeled cells in dorsal and ventral subiculum after CTB injections in reuniens, and only scattered cells in the ventral part of CA1. In addition, we report the presence of sparse cells in the molecular layer of the subiculum that send collaterals to both mPFC and the nucleus reuniens. Double-labeled cells represented about 1 % of the overall population of retrogradely labeled cells, and the fraction was highest among the mPFC-projecting cells (7.38 % on average also projected to reuniens). Anatomical tracers have occasionally been reported to preferentially bind to certain projections (Schofield et al. 2007; Deng and Rogers 1999) and we cannot completely rule out that the differences in the percentage of double-labeled cells in reuniens compared to the subiculum do not result from biases in the binding of CTB-AF conjugates. Nonetheless, CTB is considered a sensitive retrograde tracer (Conte et al. 2009) and enters cells by binding to gangliosides in the cell membrane (Stoeckel 1977; Joseph et al. 1978). One advantage of CTB conjugates for collateralization studies is that using the same compound minimizes the variability in the spread, uptake and transport that would result from using multiple tracers in the same animal (Conte et al. 2009).
The dorsal subiculum has been suggested to be part of the dorsal hippocampus network involved in spatial navigation. Instead, the ventral subiculum network would be involved in stress and emotional responses (Fanselow and Dong 2010). Our data suggest that reuniens participates in both the dorsal and ventral networks, and that coordinated activity in reuniens and mPFC may be important for the functions implemented by these networks, which contain cells that branch projections to both reuniens and mPFC. More information is needed to understand the function of reuniens in these circuits. A follow-up experiment would be to determine if reuniens represents a point of convergence for dorsal and ventral subicular afferents, or if it contains segregated populations that form part of the dorsal and ventral subicular networks.
Other regions with double-labeled cells
The observation of double-labeled cells in reuniens and subiculum is a first step in identifying regions with potential to directly synchronize activity between hippocampus, mPFC, and the thalamus. This finding does not exclude the possibility of additional populations in other brain areas that could have a simultaneous effect on hippocampus, mPFC, and/or the thalamus. In fact, some cells in the entorhinal cortex were found to project to both hippocampus and mPFC, and cells in anterior parts of the septal region projected to both mPFC and reuniens. The presence of double-labeled cells in the dorsolateral entorhinal cortex is particularly intriguing, given that this is a major target of reuniens and is involved in spatial memory (Steffenach et al. 2005). Therefore, reuniens could have an influence on hippocampus and mPFC both directly and indirectly, through the entorhinal projection. More work is needed to determine if the subpopulation of entorhinal cells that provides axon terminals to hippocampus and mPFC contributes to spatial memory, perhaps by synchronizing activity in its postsynaptic targets.
In the septal region, double-labeled cells were only found in the most anterior part of the lateral septum after injections in mPFC and reuniens. Conversely, the medial septum and diagonal band of Broca contained segregated populations of cells projecting to hippocampus and mPFC. This does not eliminate the possibility that other septal cells could directly modulate activity in hippocampus and mPFC. For example, septal projections to CA1 are denser to areas not targeted by our injections (e.g., from the medial septum to CA1 stratum oriens; Oka and Yoshida 1985; Nyakas et al. 1987; Yamano and Luiten 1989). Septal afferents to mPFC are also stronger to areas that may not have been reached by our injections (e.g., the rostral part of the prelimbic; Hoover and Vertes 2007). Injections directed to other hippocampus or prefrontal subregions could reveal double labeling in the septum as well as other brain regions. Nonetheless, the results suggest that the specific regions targeted by the tracer can be directly modulated by reuniens collaterals, while receiving segregated input from regions such as the septum. In the thalamus, only reuniens appears to have a cell population that could directly influence mPFC and hippocampus. Within the hippocampus, only the subiculum contains a sparse cell population that could have a similar direct influence on the reuniens–mPFC thalamocortical network.
Functional relevance
Hippocampus and mPFC have a critical role in both short and long-term memory. During awake exploratory behavior, subsets of mPFC cells fire spikes locked to preferential phases of the hippocampus theta (4–10 Hz) and the degree of phase locking correlates with behavioral performance on working memory tasks (Siapas et al. 2005; Jones and Wilson 2005; Hyman et al. 2010). Although the response properties of reuniens cells are currently unknown, the nucleus receives substantial afferents from regions involved in the generation of theta (Vertes et al. 2004) as well as from visual and motor regions (McKenna and Vertes 2004), indicating a potential role in navigation. Reuniens cells with collaterals to mPFC and hippocampus could be important during exploration to synchronize the activity of both structures.
During systems memory consolidation, the role of the hippocampus in memory recall decreases gradually (Squire et al. 2001; Maviel et al. 2004). Neocortical regions such as mPFC progressively become important for recall and, over time, memory recall can become fully hippocampus independent (although see Goshen et al. 2011). The close time correlation of CA1 ensemble reactivation and neocortical spindles during sleep is thought to be important in this change from dependence to independence from the hippocampus (Siapas and Wilson 1998; Sirota et al. 2003; Peyrache et al. 2011). Cells with collateral axons to hippocampus and mPFC, such as those in reuniens, could help the consolidation of memories. Manipulation experiments of reuniens (using pharmacology or electrical stimulation) could give insights on the role of reuniens in consolidation (Loureiro et al. 2012); however, these experiments are difficult to interpret because of the likely effects on surrounding nuclei or fibers of passage. Currently available techniques such as optogenetics and pharmacogenetics (Fenno et al. 2011; Rogan and Roth 2011) largely diminish those problems and also make it feasible to genetically target specific populations within the nucleus.
More specifically, hippocampal ensemble reactivation in CA1 (ripples in the local field potential) often precedes neocortical spindles and can also be locked to the troughs of spindles (Siapas and Wilson 1998; Sirota et al. 2003; Peyrache et al. 2011). In addition, ripple events can occur in groups (ripple bursts), in which ensembles of place cells represent extended exploratory behavior (Davidson et al. 2009). The results suggest that whenever a spindle is generated in reuniens, a subset of its cells may simultaneously influence activity in mPFC and CA1, potentially facilitating the transfer of mnemonic information. Because CA1 axons terminate in subiculum, subicular cells projecting to thalamus could contribute to the time relations observed in electrophysiological studies by facilitating thalamic spindle occurrence after CA1 population activation. Cells in the subiculum with branches to reuniens and mPFC could have an additional role in synchronizing thalamocortical activity; in other words, this subicular population could ensure that spindles generated in reuniens engage its postsynaptic cortical network in mPFC. Finally, both the reuniens cells that project to hippocampus and mPFC and the subicular cells that project to reuniens and mPFC could influence the finer time scale of the interactions between the regions. Oscillatory activity in reuniens cells could for example be important in determining the time interval between the individual ripples within ripple bursts, and in locking ripples to certain phases of cortical spindle oscillations. Simultaneous recordings in these areas would help determine the precise time correlations between the three regions and advance our understanding of the mechanisms of memory formation and consolidation.
Supplementary Material
Acknowledgments
We thank K. Rockland for advice and comments throughout the duration of the project and for feedback on the manuscript. We also thank MJ. Galazo and J. Castro for technical advice and the M. Sur and S. Tonegawa laboratories for access to confocal and epifluorescence microscopes. This work was supported by a postdoctoral fellowship from the Caja Madrid Foundation (C.V.) and US National Institutes of Health grant 5R01MH061976 (M.A.W.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-013-0543-5) contains supplementary material, which is available to authorized users.
References
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. An analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–114. doi: 10.1002/cne.20985. doi:10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condé F, Audinat E, Maire-Lepoivre E, Crépel F. Afferent connections of the medial frontal cortex of the rat. A study using retrograde transport of fluorescent dyes. I. Thalamic afferents. Brain Res Bull. 1990;24:341–354. doi: 10.1016/0361-9230(90)90088-h. [DOI] [PubMed] [Google Scholar]
- Conte WL, Kamishina H, Reep RL. The efficacy of the fluorescent conjugates of cholera toxin subunit B for multiple retrograde tract tracing in the central nervous system. Brain Struct Funct. 2009;213:367–373. doi: 10.1007/s00429-009-0212-x. doi:10.1007/s00429-009-0212-x. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. doi:10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Rogers LJ. Differential sensitivities of the two visual pathways of the chick to labelling by fluorescent retrograde tracers. J Neurosci Methods. 1999;89:75–86. doi: 10.1016/s0165-0270(99)00044-8. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van Der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637:AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. doi:10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures. Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. doi:10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. doi:10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. doi:10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, et al. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. doi:10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, et al. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. doi:10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, et al. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–1120. doi: 10.1038/nn.2880. doi:10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. doi:10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. doi:10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct Funct. 2011 doi: 10.1007/s00429-011-0345-6. doi:10.1007/s00429-011-0345-6. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. doi:10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front Integr Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. doi:10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. doi:10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jay TM, Thierry A-M, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci. 1992;4:1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. Cambridge University Press; London: 2007. [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. doi:10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph KC, Kim SU, Stieber A, Gonatas NK. Endocytosis of cholera toxin into neuronal GERL. Proc Natl Acad Sci USA. 1978;75:2815–2819. doi: 10.1073/pnas.75.6.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy J, Commins S. The rat perirhinal cortex: a review of anatomy, physiology, plasticity, and function. Prog Neurobiol. 2011;93:522–548. doi: 10.1016/j.pneurobio.2011.03.002. doi:10.1016/j.pneurobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. doi:10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, et al. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. doi:10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, et al. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci. 2012;32:9947–9959. doi: 10.1523/JNEUROSCI.0410-12.2012. doi:10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. doi:10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. doi:10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Namura S, Takada M, Kikuchi H, Mizuno N. Topographical organization of subicular neurons projecting to subcortical regions. Brain Res Bull. 1994;35:221–231. doi: 10.1016/0361-9230(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Luiten PG, Spencer DG, Traber J. Detailed projection patterns of septal and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CA1 and dentate gyrus. Brain Res Bull. 1987;18:533–545. doi: 10.1016/0361-9230(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Oka H, Yoshida K. Septohippocampal connections to field CA1 of the rat identified with field potential analysis and retrograde labeling by horseradish peroxidase. Neurosci Lett. 1985;58:19–24. doi: 10.1016/0304-3940(85)90322-2. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y. Single midline thalamic neurons projecting to both the ventral striatum and the prefrontal cortex in the rat. Neuroscience. 1998;86:635–649. doi: 10.1016/s0306-4522(98)00062-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; London: 1986. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Battaglia FP, Destexhe A. Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs. Proc Natl Acad Sci USA. 2011;108:17207–17212. doi: 10.1073/pnas.1103612108. doi:10.1073/pnas.1103612108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, et al. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem. 2008;15:368–372. doi: 10.1101/lm.813608. doi:10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. doi:10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Schofield RM, Sorensen KA, Motts SD. On the use of retrograde tracers for identification of axon collaterals with multiple fluorescent retrograde tracers. Neuroscience. 2007;146:773–783. doi: 10.1016/j.neuroscience.2007.02.026. doi:10.1016/j.neuroscience.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol. 1993;330:533–542. doi: 10.1002/cne.903300409. doi:10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, et al. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. doi:10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. doi:10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. doi:10.1002/1098-1063(2001)11:1<50∷AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Steffenach H-A, Witter M, Moser M-B, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. doi:10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Stoeckel K, Schwab M, Thoenen H. Role of gangliosides in the uptake and retrograde axonal transport of cholera and tetanus toxin as compared to nerve growth factor and wheat germ agglutinin. Brain Res. 1977;132:273–285. doi: 10.1016/0006-8993(77)90421-8. [DOI] [PubMed] [Google Scholar]
- Su HS, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol. 1990;297:582–593. doi: 10.1002/cne.902970410. doi:10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, et al. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. doi:10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Robertson RT. Organization of subcortical pathways for sensory projections to the limbic cortex. I. Subcortical projections to the medial limbic cortex in the rat. J Comp Neurol. 1987;265:175–188. doi: 10.1002/cne.902650203. doi:10.1002/cne.902650203. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Projections from the lateral dorsal nucleus of the thalamus to the limbic and visual cortices in the rat. J Comp Neurol. 1992;324:427–448. doi: 10.1002/cne.903240310. doi:10.1002/cne.903240310. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J Comp Neurol. 1995;358:584–604. doi: 10.1002/cne.903580411. doi:10.1002/cne.903580411. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. doi:10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and parataenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. doi:10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. doi:10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, et al. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. doi:10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. doi:10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. doi:10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. doi:10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Swanson LW, Cowan WM. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience. 1979;4:463–476. doi: 10.1016/0306-4522(79)90124-6. [DOI] [PubMed] [Google Scholar]
- Yamano M, Luiten PG. Direct synaptic contacts of medial septal efferents with somatostatin immunoreactive neurons in the rat hippocampus. Brain Res Bull. 1989;22:993–1001. doi: 10.1016/0361-9230(89)90011-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.