Abstract
Objective
Input impedance is the frequency-dependent afterload to pulsatile blood flow. Studies of input impedance have been performed as early as the 1960s and have been applied to hypertension (HTN). However, to date, these studies have not been systematically evaluated. This systematic review aims to summarize the literature, interpret existing data from the perspective of impedance theory, and to discuss their potential for generating physiological insights into HTN.
Methods
We identified 11 studies where computed impedance moduli from both HTN and control (CNT) groups were reported. In addition, we performed bivariate analyses of raw data from 3 of these studies.
Results
Major findings include (1) HTN groups had consistently elevated impedance moduli at 0Hz (Z0) and at heart rate frequency (Z1), an increased frequency where impedance phase first crosses 0 (f0), but no consistent pattern in characteristic impedance (Zc), when compared to CNT groups; (2) systolic (SBP) and diastolic (DBP) blood pressure are highly correlated with Z0 and Z1, moderately correlated with f0, less correlated with Zc; and (3) the measurement and calculation methods for Zc are varied and inconsistent, and (4) a not insignificant proportion of hypertensive subjects have “normal” Z0, Z1 and Zc values. These findings are limited by the heterogeneous study populations and small sample sizes.
Conclusions
These findings suggest that Z0, Z1 and f0 are significantly associated with hypertension, while the role of Zc is less clear. Additional studies are needed to evaluate these input impedance variables in order to generate substantial implications in clinic settings.
Keywords: input impedance, characteristic impedance, hypertension, wave reflection
Introduction
Despite the availability of multiple antihypertensive medications, surveys show that only half the patients with hypertension are able to adequately control their blood pressure1. Although factors such as noncompliance, drug side effects, and poor access to health care are important contributors, our limited understanding of the dynamic and spectral features of hypertension may also play a role. Input impedance describes the frequency dependent opposition to blood flow and provides a more complex assessment of blood flow/pressure than peripheral resistance alone. By evaluating impedance across multiple frequencies, input impedance can capture underlying physiological processes - such as wave reflections and aortic (visco)elasticity – that may be important determinants for systemic blood pressure. Input impedance was suggested by McDonald and Taylor as early as in 19592, implemented by O’Rourke3, et al. and explained in detail in text books of Hemodynamics by Milnor4 and MacDonald’s blood flow in arteries by Nichols and O’Rourke5.
In general, the input impedance of the ascending aorta possesses the following characteristics: 1) the modulus (or amplitude) of the input impedance is greatest at zero frequency; 2) with increasing frequency, the modulus decreases in magnitude towards a minimal value which is commonly located between the 2nd to 4th harmonics (i.e. frequencies corresponding to two to four times the heart rate) and is approximately 5-10% of the input resistance (zero frequency impedance); 3) the input impedance moduli settle and fluctuate around a steady positive impedance value (Zc, the characteristic impedance).
Past physiological studies have predominantly focused on two aspects of the aortic input impedance: Z0 and Zc. Z0 is the peripheral vascular resistance and embodies impedance to flow as if the flow were steady and continuous. Zc, on the other hand, is the impedance at higher frequencies and is generally attributed to the local aortic wall stiffness and diameter. Other reported impedance parameters include f0 - the frequency where input impedance reaches its first minimum and its phase first crosses zero – and Z1- the impedance modulus at the heart-rate frequency6. f0 reveals information about wave reflections, with a higher f0 indicating earlier reflections.
Studies of aortic input impedance have been specifically applied to hypertension populations. However, to date, these studies have not been systematically evaluated. This review aims to summarize the existing evidence, to interpret the data from the perspective of impedance theory, and to discuss their potential for generating physiological insights into hypertension.
Method
This systematic review includes 11 articles7-17 from two electronic databases: PubMed and Scientific Citation Index for dates ranging from the database inception to May 2010. The searching keywords were “impedance”, “input resistance”, and “windkessel model” crossed with the keywords “hypertension”, “high blood pressure”, and “hypertensive”. Searches were limited to the English language. We also manually searched the references of all relevant publications. Articles were selected if the studies 1) contained in vivo human data; (2) had primary-collected data and were not review papers, commentaries, or editorials; (3) involved both control normotensive subjects and systemic hypertension subjects; (4) had central input impedance measured/calculated. Exclusion criteria were that the studies (1) had patients with effects of pharmacological drugs (we required the hypertensive patients were either never treated or withdrawn from the drug for at least a week); (2) had patients with other severe cardiovascular comorbidities.
Extracted data includes subjects, age, heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), Z0, Z1, Zc and f0. Other parameters such as input impedance phase, cardiac output, stroke volume, and total/mean work power were evaluated but ultimately excluded due to limited data availability from the papers for comparison. In cases where different units were employed, appropriate unit conversions were performed.
In a number of studies, the published raw data were employed for bivariate analysis. Primary authors of selected papers were also contacted to obtain additional raw data but with no success. Scatter plot matrix was computed from JMP (SAS Corporate, Cary, NC, USA) with Pearson correlation method and density ellipse was displayed with 95% as the confidential interval. Greater narrowing of the ellipse along the diagonal axis indicated greater correlations (coefficient r>0.5) while rounding of the ellipse and absence of a diagonal orientation suggested lack of correlation (r<0.5) between variables.
We also determined the receiver operating characteristic (ROC) curve with the raw data for Z0, Z1, Zc and f0 in regards to the diagnosis of hypertension.
Results
Table 1 summarizes the data extracted from the selected studies. Patients’ characteristics including gender and age are listed and categorized according to the group designations (control [CNT] vs. hypertension [HTN]) if the information was provided. In addition, the type of hypertension (essential/permanent vs. isolated systolic vs. mixed hypertension) is also listed. Overall, seven out of 11 studies7-13 evaluated essential hypertension whereas two studies assessed isolated systolic15,16, one investigated mixed hypertension17 and one study did not indicate the type of hypertension14. With the exception of Ferrier16 and Mitchell17 studies, study subjects were predominantly male. Mitchell’s study17 performed separate analyses according to gender. The HTN subjects within the isolated systolic and mixed hypertension studies were generally older, although two Merillon’s essential hypertension studies7,8 had older hypertensive groups yet statistically younger control groups (bold fonts indicate statistically significant differences between HTN and CNT groups).
Table 1.
Extracted data including paper, subjects, age, heart rates (HR, beat/minute), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), input impedance at 0Hz (Z0, dyn·s·cm−5), first modulus of input impedance (Z1, dyn·s·cm−5), characteristic impedance (Zc, dyn·s·cm−5) and the frequency where input impedance reaches its first minimum and its phase first crosses zero (f0).
| paper | subjects | Age | HR | SBP | DBP | Z0 | Z1 | Zc | f0 |
|---|---|---|---|---|---|---|---|---|---|
| Merillon JP 1982 |
CNTL (11M2F) | 40 | 79 | 109.8 | 73.7 | 1140 | 90 | 4.3 | |
| Essential HTN (12M) | 51 | 83 | 174.4 | 100.8 | 1680 | 114 | 5.2 | ||
| Merillon JP 1983 |
CNTL (10 M 1F) | 38 | 81 | 112 | 73 | 1173 | 100 | ||
| Permant essential HTN (11M) |
50 | 84 | 171 | 100 | 1677 | 104 | |||
| Merillon JP 1985 |
CNTL (25M) | 43 | 75 | 119 | 72 | 1270 | 101 | ||
| Essential HTN (19M1F) | 47 | 85 | 171 | 101 | 1712 | 134 | |||
| Ting CT 1986 |
CNTL (7M1F) | 41.9 | 97.2 | 120.2 | 74.8 | 1713.3 | 165.1 | 93.9 | 2.97 |
| Essential HT (6M5F) | 34.8 | 79.2 | 158.6 | 92.5 | 2294.9 | 300.7 | 145.7 | 4.15 | |
| Ting CT 1991 |
CNTL (7M3F)) | 34.5 | 87.4 | 115.7 | 77.3 | 1268 | 105 | 77.9 | 3.5 |
| essential HTN (8M4F) | 33.8 | 86.8 | 162.4 | 102.5 | 1962 | 200 | 82.5 | 4.6 | |
| Ting CT 1993 |
CNTL (10M4F) | 32.6 | 83.5 | 111.8 | 73.5 | 1239 | 110 | 75.5 | |
| essential HTN (9M3F) | 37 | 79.7 | 156.9 | 96.2 | 1612 | 185 | 60.4 | ||
| Ting CT 1995 |
(CNT is same as Ting CT 1993) |
32.6 | 83.5 | 111.8 | 73.5 | 1239 | 110 | 75.5 | 3.1 |
| Essential HTN (8M4F) | 32.9 | 82.3 | 160.6 | 100.2 | 1863 | 220 | 71.9 | 4.3 | |
| Chang KC 1990 |
CNTL (5M2F) | 46 | 75 | 122 | 74 | 1651 | 177 | 122 | 3.4 |
| HTN (7M2F) | 49 | 79 | 180 | 100 | 2751 | 383 | 193 | 4.8 | |
| Nichols WW 1992 |
CNTL (9) | 58 | 78 | 134 | 80 | 1395 | 210 | 89 | |
| isolated systolic HTN (9) | 58 | 76 | 166 | 84 | 2013 | 329 | 184 | ||
| Ferrier KE 2001 |
CNTL (10M 10F) | 64 | 62 | 119 | <90 | 191.2 | 133.6 | ||
| isolated systolic HTN(10 M10 F) |
64 | 64 | 154 | <90 | 261.6 | 187.2 | |||
| Mitchell GF 2003 |
CNTL (11F) | 56 | 68 | 114 | 64 | 1686 | 236 | 185 | |
| mixed HTN (50 F) | 61 | 65 | 167 | 83 | 2415 | 444 | 268 | ||
| CNTL (19M) | 60 | 62 | 125 | 69 | 1673 | 221 | 159 | ||
| mixed HTN (78 M) | 60 | 63 | 162 | 88 | 2131 | 314 | 208 |
Table 1 also lists the mean values for the heart rate, blood pressure, and input impedance parameters for each of the studies. Across all studies, Z0 and Z1 are significantly increased in the HTN group compared to the CNT group. Zc, however, did not display the same consistency and 5 of the 11 studies showed either no significant difference between the two groups or reduced Zc values in the HTN group. The three studies15-17 involving isolated and mixed hypertension and with older subjects, however, were consistent with relative increases in Zc for HTN patients.
The magnitudes of Z0 are six to twelve folds greater than that of Z1 while Zc amplitudes are generally smaller than that of Z1. For the five studies reporting zero crossing frequencies, the mean f0 was uniformly increased in HTN group as compared to the CNT group.
Figure 1 illustrates the aggregated results in graphical form. The HTN to CNT ratios of mean Z0 magnitudes were generally 1.5 across studies and were comparable to the ratios of mean SBP and partially DBP amplitudes. The HTN to CNT ratio for Z1 revealed a much greater range of ratios from 1.4 to 2.2. As noted previously, the ratios of Zc were not consistent across studies, and although mean Zc magnitudes were generally increased in hypertensive patients, some studies - notably Ting’s 1993 study12 - demonstrated reduced Zc.
Figure 1.
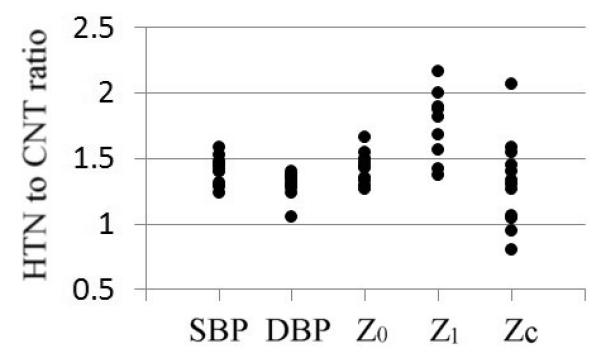
Plot of the variable ratios between hypertension (HTN) and control (CNT) groups. Variables include systolic blood pressure (SBP), diastolic blood pressure (DBP), input impedance at 0Hz (Z0), first modulus of input impedance (Z1) and characteristic impedance (Zc).
Table 2 lists the devices and methods used to acquire blood pressure and flow measurements. In addition, the mathematical methods for calculating Z0 and Zc are also summarized. Most studies used intravascular catheter measurements, whereas Ferrier and Mitchell employed applanation tonometry at the carotid artery and Doppler imaging of the left ventricle outflow tract. Across the selected studies, four different methods were used to calculate Zc: the average modulus of input impedance above 4Hz7,8,10-13; averaged input impedance above 2 Hz14-16; indirect derivation of Zc from pulse wave velocity using the Waterhammer formula (where ρ is the density of blood, C is the wave velocity, r is the diastolic aortic radius) 9; and a time-domain approach where characteristic impedance was calculated as ΔP/ΔQ in early systole17. The methods for obtaining Z1 were not included in Table 2 because Z1 was derived as the ratio of 1st blood pressure harmonic modulus to 1st blood flow harmonic modulus in the seven studies where this information was detailed. No detailed description was provided in Mitchell’s study.
Table 2.
Locations and methods that the blood pressure and flow were measured and the method in the calculation of input impedance at 0Hz (Z0) and characteristic impedance (Zc).
| Paper | Measurement | Device | Z0 calculation method | Zc calculation method |
|---|---|---|---|---|
| Merillon JP 1982 |
Ascending aorta pressure and blood flow |
Catheters with micromanometer and flowmeter sensor |
P0/Q0 (P0: pressure harmonic modulus at 0 Hz, Q0: flow harmonic modulus at 0 Hz) |
Averaging of all moduli of input impedance above 4 Hz |
| Merillon JP 1983 |
Same as Merillon JP 1982 |
Same as in Merillon JP 1982 |
Same as Merillon JP 1982 |
Same as in Merillon JP 1982 |
| Merillon JP 1985 |
Left ventricular and ascending aortic pressure and aortic flow velocity |
Mcromanometer mounted on catheter |
80×P/CO (P: mean aortic pressure, CO: cardiac output) |
ρC/πr2 (r: diastolic aortic radius, C:pulse wave velocity, ρ: density of blood |
| Ting CT 1986 |
Left ventricular and ascending aortic pressure and aortic flow velocity |
Catheter with pressure and velocity sensors |
(Pv-Pa)/Q (Pv: average ventricular pressure; Pa: average atrial pressure; Q: mean blood flow) |
Same as in Merillon JP 1982 |
| Ting CT 1991 |
same as Ting CT 1986 |
Same as Ting CT 1986 |
Same as Ting CT 1986 | Same as in Merillon JP 1982 |
| Ting CT 1993 |
same as Ting CT 1986 |
Same as Ting CT 1986 |
Same as Ting CT 1986 | Same as in Merillon JP 1982 |
| Ting CT 1995 |
Same as Ting CT 1986 |
Same as Ting CT 1986 |
Same as Ting CT 1986 | Same as in Merillon JP 1982 |
| Chang KC 1990 |
Ascending aorta flow velocity and pressure |
Multisensor catheter |
Quotient of mean aortic pressure and cardiac output |
Averaging of all moduli of input impedance above 2 Hz |
| Nichols WW 1992 |
Ascending aortic blood flow velocity and aortic and left ventricular pressure |
Catheter mounted with multisensors |
P/Q (P: mean pressure, Q: mean flow) |
Averaging of all moduli of input impedance above 2 Hz |
| Ferrier KE 2001 |
Carotid artery pressure, volumetric aortic flow |
Applanation tonometry and handheld doppler velocimeter |
Cardiac output derived from velocity flow was used to calculate total peripheral resistance |
Averaging of all moduli of input impedance above 2 Hz |
| Mitchell GF 2003 |
Carotid artery pressure and left ventricular outflow tract flow |
Tonometry and pulsed doppler |
Peripheral resistance (No method was mentioned) |
ΔP/ΔQ (ΔP and ΔQ: pressure and flow difference in the early systolic period) |
To further explore the detailed relationships between input impedance and blood pressure parameters (SBP, DBP, mean BP [MBP], and pulse pressure [PP]), the primary data from three of these 11 papers7,10,14 were concatenated (Merillon’s paper7 lacked Z1 data) and are listed in Table 3. We also compared age distributions in the CNT (41.82±10.68) and HTN groups (44.97±12.68) which are not significantly different. The bivariate relationships are plotted in Figure 2. The figure shows scatter plots with coefficient correlations and an overlying density ellipse with 95% as the confidential interval. There are strong correlations (r>0.5) between Z0 and SBP, MBP, PP and between Z1 and SBP, MBP, PP. Weak correlations exist between Zc and SBP, DBP, MBP, PP. The correlation between f0 and blood pressure parameters are weak but otherwise strongest between f0 and Z1.
Table 3.
Raw data from 3 of the 11 reviewed paper. MBP and PP were calculated from SBP and DBP as MBP=SBP/3+2*DBP/3, PP=SBP-DBP.
| paper | subjects | age | SBP | DBP | MBP | PP | Z0 | Z1 | Zc | f0 |
|---|---|---|---|---|---|---|---|---|---|---|
| Merillon JP 1982 |
N1 | 47 | 96.8 | 65.3 | 79.5 | 31.5 | 840 | 42 | 3.1 | |
| N2 | 30 | 100.5 | 65.3 | 82.5 | 35.3 | 1140 | 90 | 3.9 | ||
| N3 | 22 | 106.5 | 69.0 | 87.8 | 37.5 | 1140 | 108 | 3.4 | ||
| N4 | 45 | 87.8 | 62.3 | 76.5 | 25.5 | 1260 | 126 | 3.6 | ||
| N5 | 41 | 126.0 | 81.8 | 102.0 | 44.3 | 1080 | 78 | 4.7 | 26 | |
| N6 | 94.5 | 65.3 | 81.0 | 29.3 | 960 | 54 | 3.8 | |||
| N7 | 58 | 117.8 | 75.8 | 93.8 | 42.0 | 1200 | 60 | 5.8 | ||
| N8 | 25 | 114.0 | 84.8 | 100.5 | 29.3 | 1080 | 120 | 4.0 | ||
| N9 | 53 | 108.0 | 69.0 | 84.0 | 39.0 | 780 | 72 | 3.3 | ||
| N10 | 32 | 121.5 | 87.0 | 102.8 | 34.5 | 1260 | 72 | 5.7 | ||
| N11 | 56 | 120.8 | 78.8 | 97.5 | 42.0 | 1260 | 150 | 4.6 | ||
| N12 | 56 | 117.8 | 76.5 | 94.5 | 41.3 | 900 | 60 | 5.7 | ||
| N13 | 30 | 114.0 | 79.5 | 102.8 | 34.5 | 1620 | 138 | 3.7 | ||
| Ting CT 1986 |
N1 | 30 | 126.9 | 76.0 | 100.7 | 50.9 | 2288 | 272 | 238 | 3.51 |
| N2 | 56 | 139.6 | 73.0 | 100.7 | 66.6 | 1741 | 234 | 68 | 1.96 | |
| N3 | 37 | 106.1 | 72.2 | 88.2 | 33.9 | 1393 | 108 | 138 | 2.49 | |
| N4 | 39 | 126.9 | 102.9 | 114.3 | 24.0 | 935 | 41 | 44 | 3.07 | |
| N5 | 40 | 113.0 | 69.0 | 90.3 | 44.0 | 2168 | 243 | 147 | 2.89 | |
| N6 | 37 | 115.2 | 75.4 | 93.9 | 39.8 | 1434 | 118 | 95 | 3.10 | |
| N7 | 45 | 113.5 | 80.5 | 97.2 | 33.0 | 1794 | 121 | 56 | 2.92 | |
| N8 | 51 | 125.9 | 77.5 | 100.6 | 48.4 | 1855 | 181 | 113 | 3.30 | |
| Chang KC 1990 |
N1 | 56 | 137 | 82 | 108 | 55 | 1994 | 234 | 147 | 3.9 |
| N2 | 37 | 118 | 79 | 98 | 39 | 1182 | 101 | 98 | 2.9 | |
| N3 | 45 | 122 | 64 | 89 | 58 | 1358 | 204 | 116 | 4.1 | |
| N4 | 40 | 111 | 66 | 88 | 45 | 1979 | 232 | 162 | 3.4 | |
| N5 | 55 | 130 | 77 | 103 | 53 | 1824 | 238 | 119 | 2.5 | |
| N6 | 36 | 111 | 67 | 89 | 44 | 1274 | 156 | 123 | 4.1 | |
| N7 | 46 | 124 | 82 | 101 | 42 | 1949 | 74 | 87 | 2.7 | |
| Merillon JP 1982 |
H1 | 44 | 165.8 | 85.5 | 118.5 | 80.3 | 1260 | 60 | 6.1 | |
| H2 | 58 | 176.3 | 105.8 | 139.5 | 70.5 | 1800 | 222 | 5.5 | ||
| H3 | 59 | 204.8 | 94.5 | 133.5 | 110.3 | 1680 | 42 | 4.4 | ||
| H4 | 53 | 243.8 | 117.0 | 164.3 | 126.8 | 2520 | 36 | 4.6 | ||
| H5 | 59 | 152.3 | 94.5 | 120.0 | 57.8 | 1680 | 48 | 5.1 | ||
| H6 | 52 | 162.8 | 96.8 | 126.0 | 66.0 | 1260 | 60 | 6.1 | ||
| H7 | 29 | 136.5 | 96.0 | 114.8 | 40.5 | 1440 | 102 | 4.9 | ||
| H8 | 51 | 170.3 | 111.8 | 135.0 | 58.5 | 1800 | 96 | 4.6 | ||
| H9 | 59 | 164.3 | 82.5 | 117.8 | 81.8 | 1680 | 126 | 5.7 | ||
| H10 | 36 | 192.8 | 112.5 | 144.8 | 80.3 | 1920 | 204 | 5.5 | ||
| H11 | 60 | 156.8 | 103.5 | 127.5 | 53.3 | 1320 | 162 | 4.7 | ||
| H12 | 52 | 163.5 | 103.5 | 129.8 | 60.0 | 1920 | 240 | 5.2 | ||
| Ting CT 1986 |
H1 | 30 | 165.7 | 98.0 | 128.3 | 67.7 | 1379 | 160 | 113 | 4.05 |
| H2 | 25 | 142.8 | 93.6 | 118.8 | 49.2 | 2068 | 195 | 121 | 4.89 | |
| H3 | 35 | 211.6 | 123.0 | 159.8 | 88.6 | 2721 | 377 | 154 | 4.41 | |
| H4 | 34 | 151.8 | 92.3 | 122.1 | 59.5 | 1481 | 165 | 126 | 2.26 | |
| H5 | 35 | 153.4 | 87.1 | 118.2 | 66.3 | 2968 | 425 | 173 | 3.98 | |
| H6 | 44 | 160.8 | 90.9 | 121.6 | 69.9 | 4768 | 656 | 361 | 3.47 | |
| H7 | 53 | 161.9 | 90.4 | 119.7 | 71.5 | 2127 | 278 | 145 | 3.86 | |
| H8 | 46 | 167.1 | 92.1 | 123.2 | 75.0 | 2327 | 362 | 185 | 3.69 | |
| H9 | 30 | 206.6 | 128.0 | 162.1 | 78.6 | 2774 | 349 | 169 | 4.11 | |
| H10 | 25 | 172.3 | 95.3 | 129.7 | 77.0 | 3175 | 518 | 148 | 5.20 | |
| H11 | 26 | 148.5 | 93.5 | 121.1 | 55.0 | 1797 | 199 | 128 | 4.53 | |
| Chang KC 1990 |
H1 | 31 | 151 | 90 | 120 | 61 | 3174 | 412 | 131 | 5.7 |
| H2 | 33 | 151 | 100 | 126 | 51 | 1942 | 219 | 154 | 4.4 | |
| H3 | 58 | 176 | 88 | 125 | 88 | 2164 | 396 | 288 | 3.5 | |
| H4 | 62 | 163 | 72 | 108 | 91 | 2567 | 516 | 279 | 4.9 | |
| H5 | 65 | 203 | 100 | 146 | 103 | 3875 | 518 | 197 | 6.6 | |
| H6 | 40 | 222 | 132 | 173 | 90 | 3817 | 459 | 157 | 5.4 | |
| H7 | 56 | 177 | 90 | 125 | 87 | 2454 | 428 | 216 | 4.4 | |
| H8 | 56 | 208 | 115 | 154 | 93 | 2424 | 299 | 218 | 4.5 | |
| H9 | 43 | 165 | 117 | 137 | 48 | 2344 | 203 | 99 | 4.2 |
Figure 2.
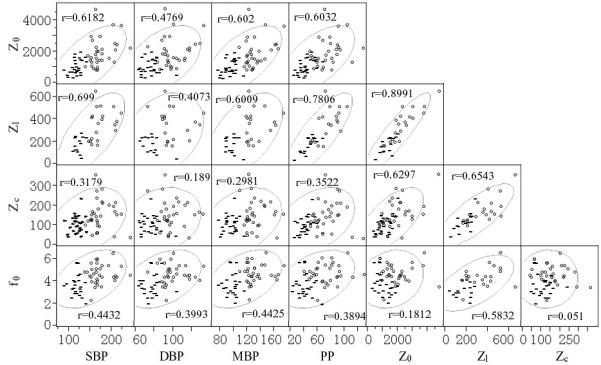
Scatter plot of the raw data with group designation markers. “-” denotes for the control subjects and “o” is for the hypertensive subjects. The figure also showed the density ellipses depicting the 95% of the confidential interval.
The scatter plots in the figure also have group designation markers: CNT in “-“ and HTN in “O”. A clear distinction is noted between CNT and HTN subjects with respect to SBP, DBP and MBP, since these parameters were used as inclusion/exclusion criteria. However, the differences in PP values between the two groups are less distinct and even much less so for the input impedance parameters Z0, Z1, Zc, and f0. For these latter variables, substantial overlap between the two groups exists – particularly for Zc. A not insignificant proportion of hypertensive subjects have “normal” input impedance parameters (based on the ranges of values for the normal subjects). Conversely, some normal subjects have elevated input impedance values although such cases are comparatively less common.
With this subset of subjects, the area under Receiver Operating Characteristic (ROC) curves for Z0, Z1, Zc and f0 in regards to the diagnosis of hypertension were 0.80, 0.88, 0.72, and 0.80, respectively.
Discussion
This systematic review identified 11 studies that reported input impedance data for both normotensive (total N=147) and hypertensive patients (N=256). Collectively, the existing evidence suggests that (1) based on mean input impedance and blood pressure values, HTN groups had consistently elevated Z0, Z1, and f0 values compared to the CNT groups but no consistent pattern with Zc amplitudes, (2) based on individual, raw data analysis of three studies, SBP and DBP are highly correlated with input impedance parameters Z0 and Z1, somewhat correlated with f0, but less correlated with Zc, (3) the measurement and calculation methods for Zc are varied and inconsistent, and (4) a not insignificant proportion of hypertensive subjects have “normal” Z0, Z1 and Zc values. From a physiological standpoint, these data imply that peripheral vascular resistance (Z0), input impedance at the heart beat frequency (Z1), and wave reflection (f0) are important factors associated with hypertension, while the role of aortic stiffness and aortic diameter (Zc) is less clear in these hypertensive subjects.
The significant correlation between peripheral vascular resistance (Z0) and hypertension is not a new finding and consistent with the understanding that vasoconstriction (possibly from enhanced sympathetic nervous activity) plays a prominent role in hypertension pathophysiology. Whether this vasoconstriction plays a causative, pathogenic role or is an abnormal response to persistently elevated blood pressure remains unclear18,19.
In our raw data analyses, Z1 demonstrated the strongest correlation with SBP and PP than any other reported input impedance parameter. Moreover, Z1 was associated with greatest area under the ROC curve for the diagnosis of hypertension compared to Z0, Zc, and f0. However, the total number of studies evaluating Z1 in hypertension remains low, and the physiological implication of Z1 is itself ambiguous. Based on the bivariate data, Z1 also has the unique feature of being highly correlated with all other input impedance parameters reported here: Z0 (peripheral resistance), Zc (aortic stiffness and diameter), and f0 (wave reflection). From a mechanistic standpoint, this may be observed because the frequency is low enough to be influenced by peripheral resistance and possibly high enough to be influenced by aortic stiffness. Additionally, Z1 resides in the time domain where wave reflections have taken effect (1st harmonic frequency [~1 Hz] < minimum modulus frequency [~2-4 Hz]). As a consequence, Z1 may reflect the composite properties that personify the vascular system and thereby be a useful global marker for the impedance confronting the pulsating heart – particularly for hypertension. Naturally, additional studies should be performed before a definitive conclusion can be made.
The zero-crossing frequency (f0) was significantly increased in hypertensive patients compared to normotensive controls in all 5 studies reporting this information. Furthermore, by the bivariate data, a moderate positive correlation (r>0.4) between f0 and SBP is seen. An increased f0 occurs when either the pressure/flow wave velocity increases or the effective distance to reflection sites are decreased. According to past studies, the former scenario is more likely and can be explained by the aortic distention resulting from elevated pressures and the accompanying increase in elastance (stiffness) which subsequently increases pulse wave velocities20. This early reflection generates a temporal (earlier) shift in the retrograde wave at the ascending aorta to coincide with the outgoing systolic pressure wave, thus explaining for the moderate correlation between SBP and f0.
According to the raw data, f0 is not correlated with either Z0 or Zc. From a theoretical standpoint, f0 and Zc are expected to be correlated because earlier reflections (as indicated by increased f0) result from an increase in aortic stiffness (which greatly determines Zc). However, Zc is also inversely related to the aortic radius to the power of 2.5, and the increase in radius accompanying hypertension may effectively nullify any increase in Zc attributed to enhanced wall stiffness. As a consequence, wave reflection (f0) appears to be an important, separable variable with respect to systolic hypertension.
As already made evident, characteristic impedance is a complicated variable influenced by not only arterial stiffness but also vessel radius. Its inverse relationship with aorta radius may partially explain for the inconsistencies in HTN to CNT ratios of Zc seen across the selected studies and also for the relatively weak correlation between Zc and blood pressure parameters in the aggregated raw data. This confounding factor has been mentioned by researchers before21. In addition, the data suggests an age-effect as well. The younger hypertension cohort for Ting’s three studies had reduced Zc, whereas the older hypertension groups in Ferrier, Mitchell, and Nichols studies had significantly increased characteristic impedances compared to their respective control groups.
The inconsistencies in Zc ratios across studies may also stem from the variable methods used to derive Zc. As documented in Table 2, four different approaches were used to calculate Zc. Two relied on the frequency-domain approach, albeit with different frequency cutoffs; one invoked a time-domain approach; and one calculated Zc indirectly by using the Water-Hammer formula. Although the technical strengths and limitations for each approach are beyond the scope of this review, a more transparent and consistent approach is needed if Zc is to be broadly applied to the clinical setting as some have advocated22. The need for consistency becomes more poignant in light of past studies showing how different characteristic impedance values can be obtained from the same data3. Moreover, hypertension may be particularly susceptible to variabilities in Zc if Zc is derived in the frequency domain. Due to the increased zero-crossing frequency, a frequency-domain cutoff of 2 Hz, for instance, will likely incorporate the impedance minimum and thus potentially distort the final characteristic impedance results (which should theoretically be without any reflection effects).
The raw bivariate data evaluated in this review were useful in identifying the overlap in input impedance variables between the HTN and CNT groups. The overlap did not occur due to increased incidences of elevated input impedance variables (particularly Z0 and Z1) in CNT subjects. Rather, there were more incidences of HTN subjects having “normal” ranges of input impedance values. Given the limited number of subjects within this raw data sample and the variable analytical methods, this observation will have to be confirmed with additional studies but, if true, will carry substantial implications for the clinician taking care of a hypertensive patient. The elevated pressure may originate from a specific physiological etiology (e.g., elevated peripheral vascular resistance vs. aortic stiffness vs. wave reflection effects), and a dynamic approach such as aortic input impedance may help clinicians determine which specific etiology is involved and subsequently what medication should be prescribed.
This review has identified some suggestive relationships between input impedance and blood pressure parameters, but the existing evidence for aortic input impedance in hypertension is still inadequate. Only 11 studies were identified in this review and the number of subjects per study was generally small (with the exception of Mitchell’s study17). These studies did not report data on body size (i.e. height, weight) – a known contributing factor to impedance measurement23. In addition, the analyzed raw data were a composite of only 3 studies. The level of evidence is insufficient to conclude how input impedance may differ between the types of hypertension (i.e. isolated hypertension vs. essential hypertension), much less understand the effects of age and gender. Importantly, the majority of the studies involved younger subjects with essential hypertension who generally possess different pathophysiologies than older individuals with isolated systolic hypertension. Aside from the aforementioned need for consistent Zc derivations methods, the methods for acquiring pressure and flow data needed for aortic input impedance are also varied (Table 2) and require some level of standardization.
Nevertheless, despite these limitations, aortic input impedance provides a comprehensive, frequency-dependent view of afterload encountered by the heart and may facilitate a more personalized approach to hypertension by delineating what pathophysiological factors are involved in each patient. Thus far, the existing literature lends support for the role of Z0, Z1, and f0 in essential hypertension although additional studies and standardizations are needed before input impedance can be confidently applied to the clinical setting.
Acknowledgements
This research was supported by grant number P30AT005895 of the National Center for Complementary Alternative Medicine (NCCAM). The project described was supported by Clinical Translational Science Award UL1RR025758 to Harvard University and Massachusetts General Hospital from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary Alternative Medicine or the National Center for Research Resources or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The manuscript represents our original work and has not been published previously in print or electronic format. All authors for this manuscript meet criteria for authorship. No author has any affiliation with an organization with financial interest in the material presented in this manuscript.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;290(2):197. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.McDonald DA, Taylor MG. The hydrodynamics of the arterial circulation. Prog Biophys Biophys Chem. 1959;9:107–73. [Google Scholar]
- 3.O’Rourke MR. Steady and pulsatile energy losses in the systemic circulation under normal conditions and in simulated arterial disease. Cardiovasc Res. 1967;1(4):313–26. doi: 10.1093/cvr/1.4.313. [DOI] [PubMed] [Google Scholar]
- 4.Milnor WR. Hemodynamics. 2nd ed Williams & Wilkins; Baltimore, MD: 1989. [Google Scholar]
- 5.Nichols WW, O’Rourke MF, Vlachopoulos C. McDonald’s blood flow in arteries. 6th ed: theoretical experimental and clinical principles Hodder Arnold Pulishers; 2011. [Google Scholar]
- 6.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49(6):1248–55. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 7.Merillon JP, Fontenier GJ, Lerallut JF, Jaffrin MY, Motte GA, Genain CP, Gourgon RR. Aortic input impedance in normal man and arterial hypertension: its modification during changes in aortic pressure. Cardiovasc Res. 1982;16(11):646–56. doi: 10.1093/cvr/16.11.646. [DOI] [PubMed] [Google Scholar]
- 8.Merillon JP, Lebras Y, Chastre J, Lerallut JF, Motte G, Fontenier G, et al. Forward and backward waves in the arterial system, their relationship to pressure waves form. Eur Heart J. 1983;4:13–20. doi: 10.1093/eurheartj/4.suppl_g.13. [DOI] [PubMed] [Google Scholar]
- 9.Merillon JP, Masque C, Dahan M, Juliard JM, Azancot I, Motte G, Gourgon R. Changes in left ventricular performance during chronic pressure or volume overload: importance of physical properteis of the arterial system. J Cardiovasc Pharmocol. 1985;7(suppl 2):S36–40. doi: 10.1097/00005344-198507002-00008. [DOI] [PubMed] [Google Scholar]
- 10.Ting CT, Brin KP, Lin SJ, Wang SP, Chang MS, Chiang BN, Yin FC. Arterial hemodynamics in human hypertension. J Clin Invest. 1986;78(6):1462–71. doi: 10.1172/JCI112737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting CT, Chou CY, Chang MS, Wang SP, Chiang BN, Yin FC. Arterial hemodynamics in human hypertension. Effects of adrenergic blockade. Circulation. 1991;84(3):1049–57. doi: 10.1161/01.cir.84.3.1049. [DOI] [PubMed] [Google Scholar]
- 12.Ting CT, Yang TM, Chen JW, Chang MS, Yin FC. Arterial hemodynamics in human hypertension. Effects of angiotensin converting enzyme inhibition. Hypertension. 1993;22(6):839–46. doi: 10.1161/01.hyp.22.6.839. [DOI] [PubMed] [Google Scholar]
- 13.Ting CT, Chen JW, Chang MS, Yin FC. Arterial hemodynamics in human hypertension. Effects of the calcium channel antagonist nifedipine. Hypertension. 1995;25(6):1326–32. doi: 10.1161/01.hyp.25.6.1326. [DOI] [PubMed] [Google Scholar]
- 14.Chang KC, Hsieh KS, Kou TS, Chen HI. Effects of nifedipine on systemic hydraulic vascular load in patients with hypertension. Cardiovasc Res. 1990;24(9):719–26. doi: 10.1093/cvr/24.9.719. [DOI] [PubMed] [Google Scholar]
- 15.Nichols WW, Nicolini FA, Pepine CJ. Determinants of isolated systolic hypertension in the elderly. J Hypertens Suppl. 1992;10(6):S73–7. [PubMed] [Google Scholar]
- 16.Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. 2001;38(2):222–226. doi: 10.1161/01.hyp.38.2.222. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Lacourcière Y, Ouellet JP, Izzo JL, Jr, Neutel J, Kerwin LJ, et al. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertesion: the role of proximal aortic diameter a nd the aortic pressure-flow relationship. Circulation. 2003;108(13):1592–8. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 18.Aalkjaer C, Heagerty AM, Bailey I, Mulvany MJ, Swales JD. Studies of isolated resistance vessels from offspring of essential hypertensive patients. Hypertension. 1987;9(6 Pt 2):155–8. doi: 10.1161/01.hyp.9.6_pt_2.iii155. [DOI] [PubMed] [Google Scholar]
- 19.Bund SJ, West KP, Heagerty AM. Effects of protection from pressure on resistance artery morphology and reactivity in spontaneously hyupertensive Wistar-Kyoto rats. Circ Res. 1991;68(5):1230–40. doi: 10.1161/01.res.68.5.1230. [DOI] [PubMed] [Google Scholar]
- 20.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 21.Nichols WW, O’Rourke MF, Avolio AP, Yaginuma T, Murgo JP, Pepine CJ, Conti CR. Effects of age on ventricular-vascular coupling. Am J Cardiol. 1985;55(9):1179–84. doi: 10.1016/0002-9149(85)90659-9. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptiility-Reykjavik Study. Brain. 2011;134(Pt 11):3398–407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chirinos JA, Rietzshel ER, De Buyzere D, Gillebert TC, Gupta AK, Segers P, Asklepios investigators Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension. 2009;54(3):558–66. doi: 10.1161/HYPERTENSIONAHA.109.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]


